Abstract
Key points
While a consensus has now been reached on the effect of motor imagery (MI) – the mental simulation of an action – on motor cortical areas, less is known about its impact on spinal structures.
The current study, using H‐reflex conditioning paradigms, examined the effect of a 20 min MI practice on several spinal mechanisms of the plantar flexor muscles.
We observed modulations of spinal presynaptic circuitry while imagining, which was even more pronounced following an acute session of MI practice.
We suggested that the small cortical output generated during MI may reach specific spinal circuits and that repeating MI may increase the sensitivity of the spinal cord to its effects.
The short‐term plasticity induced by MI practice may include spinal network modulation in addition to cortical reorganization.
Abstract
Kinesthetic motor imagery (MI) is the mental simulation of a movement with its sensory consequences but without its concomitant execution. While the effect of MI practice on cortical areas is well known, its influence on spinal circuitry remains unclear. Here, we assessed plastic changes in spinal structures following an acute MI practice. Thirteen young healthy participants accomplished two experimental sessions: a 20 min MI training consisting of four blocks of 25 imagined maximal isometric plantar flexions, and a 20 min rest (control session). The level of spinal presynaptic inhibition was assessed by conditioning the triceps surae spinal H‐reflex with two methods: (i) the stimulation of the common peroneal nerve that induced D1 presynaptic inhibition (HPSI response), and (ii) the stimulation of the femoral nerve that induced heteronymous Ia facilitation (HFAC response). We then compared the effects of MI on unconditioned (HTEST) and conditioned (HPSI and HFAC) responses before, immediately after and 10 min after the 20 min session. After resting for 20 min, no changes were observed on the recorded parameters. After MI practice, the amplitude of rest HTEST was unchanged, while HPSI and HFAC significantly increased, showing a reduction of presynaptic inhibition with no impact on the afferent‐motoneuronal synapse. The current results revealed the acute effect of MI practice on baseline spinal presynaptic inhibition, increasing the sensitivity of the spinal circuitry to MI. These findings will help in understanding the mechanisms of neural plasticity following chronic practice.
Keywords: H‐reflex, D1 presynaptic inhibition, heteronymous Ia facilitation, triceps surae, soleus
Key points
While a consensus has now been reached on the effect of motor imagery (MI) – the mental simulation of an action – on motor cortical areas, less is known about its impact on spinal structures.
The current study, using H‐reflex conditioning paradigms, examined the effect of a 20 min MI practice on several spinal mechanisms of the plantar flexor muscles.
We observed modulations of spinal presynaptic circuitry while imagining, which was even more pronounced following an acute session of MI practice.
We suggested that the small cortical output generated during MI may reach specific spinal circuits and that repeating MI may increase the sensitivity of the spinal cord to its effects.
The short‐term plasticity induced by MI practice may include spinal network modulation in addition to cortical reorganization.
Introduction
Over the past decades, scientists and physiotherapists have aimed to develop simple, safe, efficient and cost‐effective methods for motor rehabilitation, especially when patients’ functional movement is impaired. Among them, motor imagery (MI), defined as ‘the mental simulation of a movement without concomitant motor output’ (Decety, 1996), has gained in popularity. The use of such a modality in a training‐based approach is well known to enhance motor performance (Lebon et al. 2010; Gentili & Papaxanthis, 2015; Slimani et al. 2016). These positive outcomes are explained by the neural correlates of MI. Indeed, it is now well established that brain areas devoted to motor control and execution, e.g. supplementary motor area (SMA), premotor and primary motor cortices, cerebellum, and parietal cortex, are also activated during MI (Decety et al. 1994; Lotze et al. 1999; Grèzes & Decety, 2001; Ehrsson et al. 2003; Guillot et al. 2009; Munzert et al. 2009; Kilintari et al. 2016). The generation of a subliminal cortical output during MI has also been suggested to impact subcortical structures (Bonnet et al. 1997; Hale et al. 2003; Cowley et al. 2008; Aoyama & Kaneko, 2011; Grosprêtre et al. 2016). While the spinal network could also play an important role in motor performance following MI practice (Grosprêtre et al. 2018), the activation of such structures during MI has not been studied as much as brain areas and a consensus has yet to be reached.
Some authors found an increase of H‐reflex amplitude during MI. This has been attributed to a modulation of motoneuronal excitability due to a small corticospinal command reaching the spinal network (Jeannerod, 1995). Other authors did not find any modulation of the spinal network (Yahagi et al. 1996; Kasai et al. 1997; Aoyama & Kaneko, 2011; Mouthon et al. 2015). Importantly, studies that found H‐reflex modulation during MI acknowledged that this behaviour was present only in about 50% of the participants (Kiers et al. 1997; Hale et al. 2003; Cowley et al. 2008). This observation was associated with high intersubject variability, with increases during MI ranging from +1% to +25% of rest H‐reflex amplitude (Cowley et al. 2008). The absence of H‐reflex modulation does not rule out a possible influence of MI over the spinal network. Aoyama and Kaneko found an effect of MI on the stretch reflex but not on the H‐reflex and therefore suggested a selective effect of this modality on spinal networks (Aoyama & Kaneko, 2011). Other spinal structures, such as interneurons not directly related to the H‐reflex loop, may then also be activated during MI (Hale, 2007; Aoyama & Kaneko, 2011; Grosprêtre et al. 2016). By using an H‐reflex conditioning technique, Hale (2007) suggested an involvement of the spinal presynaptic inhibitory pathway during MI. It is known that interneurons, and more particularly those mediating presynaptic inhibition of the primary afferents, have a lower excitability threshold than α‐motoneurons (Daniele & MacDermott, 2009). The primary afferent depolarization (PAD) interneurons that are controlled by cortical projections (Jankowska, 1992, 2001) may respond to subliminal cortical outputs such as those generated during MI (Grosprêtre et al. 2016). This may explain how MI activates spinal structures, even in the absence of clear H‐reflex changes. Moreover, it has been suggested that, if not observed at first glance during one imagined movement trial, H‐reflex changes might occur with repetitive solicitation of the neural circuits by MI practice (Hale, 2003). Therefore, the impact of MI over spinal excitability may be observed only after a certain amount of practice and the neural pathway involving PAD interneurons may mediate this adaptation.
The first aim of the present study was to examine the modulation of spinal presynaptic circuitry during MI (online effect). We used two main H‐reflex conditioning techniques at rest and during MI: D1 presynaptic inhibition (Pierrot‐Deseilligny & Burke, 2005) and heteronymous Ia facilitation, both recommended to ascertain the evaluation of the presynaptic inhibition level (Crone et al. 1990; Pierrot‐Deseilligny & Burke, 2005). We hypothesized a reduction of presynaptic inhibition and an increase of heteronymous facilitation while imagining that would demonstrate a reduced presynaptic inhibition during MI. The second aim of this study was to test whether the repetition of MI altered baseline spinal excitability. We hypothesized that the H‐reflex would be modulated after a repetitive mental practice, due to reduced baseline presynaptic inhibition level.
Methods
Ethical approval
Each of the participants was informed about the possible risks and discomfort, and gave written consent to participate. The experimental design of the study was approved by the regional ethics committee (CPP EST: approval no. A00064‐49). The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database.
Participants
Thirteen healthy participants (9 males and 4 females; age: 28.1 ± 6.6 years; height: 1.75 ± 0.1 m; weight: 74.4 ± 12.0 kg, mean ± SD), with no history of neurological or muscular disorders participated in two experiments. Participants first completed the revised version of the Movement Imagery Questionnaire (MIQ‐r) to determine their self‐estimation of MI ability (Hall & Martin, 1997). The initial mean MIQ‐R score was 50.5 ± 4.1 (mean ± SD, maximum score: 56), indicating a good imagery capacity. The participants committed not to engage in any unusual training or exercise programme during the whole duration of the study and were requested to avoid any intense exercise prior to and after each session.
Experimental design
During a first visit to the laboratory (20–30 min), the participants were initiated into MI practice and familiarized with the neural stimulation protocols. Then, the main protocol included two experiments of about 3 h each, randomly performed and separated by 48 h to 1 week. Experiment A was designed to assess spinal circuitry modulations during and after MI practice; experiment B was used as a control experiment (see Fig. 1 A). During each experiment, the following guideline was followed. After skin preparation and electrode positioning, participants sat on an isokinetic dynamometer (Biodex System 3, Shirley, NY, USA) used to record instantaneous muscle torque. The axis of the dynamometer was aligned with the external malleolus of the right leg. Participants were placed with hip and knee joints at 90° (180° = full extension) and ankle joint at 90° (angle between the leg and the sole of the foot). Stimulation parameters of the posterior tibial nerve were first determined to record triceps surae test H‐reflexes and maximal M‐waves. The conditioning stimuli delivered over femoral and tibial nerve were then determined. Once the parameters of the several stimulations were fixed, pretests were performed before participants underwent one 20 min training session of MI (experiment A) or stayed at rest for 20 min (experiment B). The electromyographic activity of the triceps surae muscles was recorded throughout both experiments. Right after (POST0) and 10 min after the end of the training/rest session (POST10), measurements were repeated as in pre‐tests.
Figure 1. Overview of the experimental protocol and illustrations of the inhibitory and facilitatory pathways of the soleus H‐reflex.
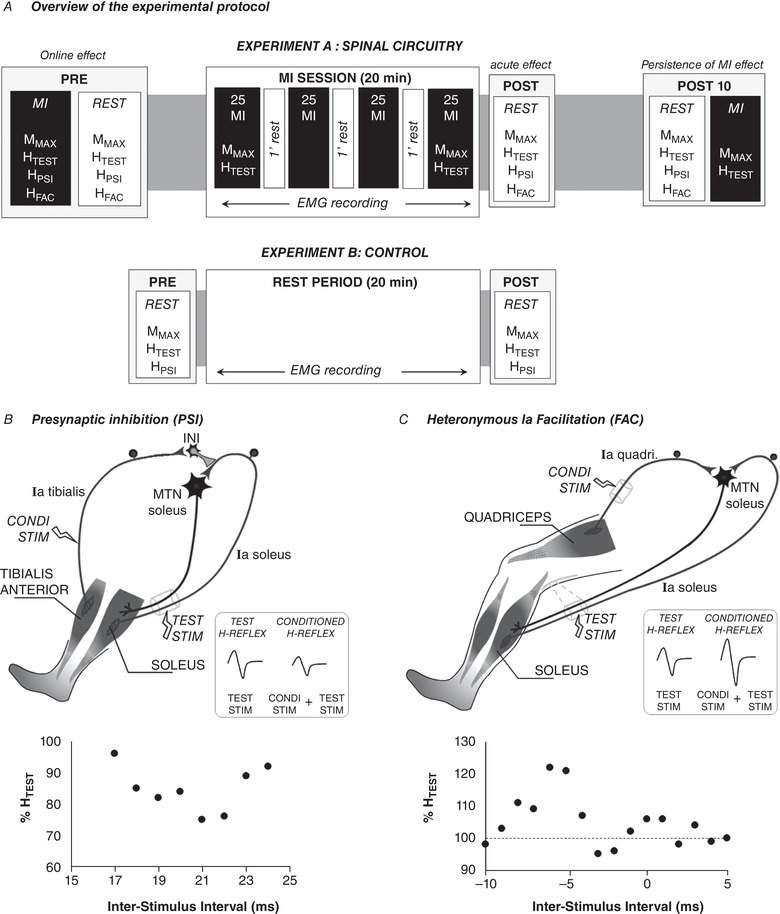
A, the main experiment (experiment A) was designed to assess (i) the online effect of MI in comparison to rest and (ii) the acute effects of a single MI training session. The second experiment (experiment B) was designed as a control condition that aimed at recording the responses before (PRE) and immediately after (POST0) a rest period of similar duration with MI training session. HFAC, H‐reflex conditioned by facilitatory pathway; HPSI, H‐reflex conditioned by presynaptic inhibitory pathway; HTEST, H‐reflex test; MMAX, maximal M‐wave. B, depiction of the neural networks inducing D1 presynaptic inhibition. The soleus unconditioned H reflex, labelled TEST H‐Reflex (HTEST), is elicited by electrical stimulation of the tibial nerve (TEST STIM). A prior conditioning stimulus (Condi stim) applied to the common peroneal nerve reduces the amplitude of the soleus H‐reflex (Conditioned reflex). The Ia afferent of the tibialis muscle connects to inhibitory interneurons that inhibit the Ia terminals of the soleus muscle. C, depiction of the neural networks inducing heteronymous Ia facilitation. A prior conditioning stimulus (Condi stim) applied to the femoral nerve increases the amplitude of the test H‐reflex. This facilitation is monosynaptic between the quadriceps Ia afferents (Ia quadri) and the motoneuronal pool of the soleus (MTN soleus). In B and C, graphs below illustrations depict plots of conditioned responses (as percentage unconditioned HTEST) as a function of the inter‐stimulus intervals for one representative participant. Each point represents an average value of 5 trials. Positive intervals represent conditioned stimulation evoked before the TEST STIM and negative intervals the conditioned stimulation evoked after the TEST STIM.
Experiment A – spinal circuitry
We assessed changes in inhibitory and facilitatory spinal pathways of the soleus and gastrocnemius medialis at rest, during MI (online effect), and following one session of MI training (acute effect). The first step of this experiment consisted of measuring unconditioned (test) and conditioned responses at rest and during MI. Then, participants mentally trained for 20 min (4 blocks of 25 imagined contractions of plantar flexion), with five test H‐reflexes evoked during MI in the first and last block of training. Note that measurements during MI in the last block were considered as POST0 measurements. Rest measurements (test and conditioned H‐reflexes) were performed immediately after (POST0) and 10 min after (POST10) the training session. Test reflexes during MI were also measured at POST10 (see Fig. 1 A for full timeline).
Experiment B – control experiment
This experiment was designed to analyse the time effect on spinal presynaptic circuitry. The time period was similar to that of the MI session in experiment A. Participants were asked to stay relaxed without thinking of any movement or activity. Electromyoraphic signals were monitored during the whole session to ensure that no minor contraction was induced during this rest period. As experiment B is a control condition for which no modulation was expected, we limited the measurements to test H‐reflex and D1 presynaptic conditioned H‐reflex (Fig. 1 A).
Motor imagery session
Participants were instructed to imagine pressing maximally on the pedal of the ergometer and to evoke the corresponding sensation of muscle contraction. They were requested to feel the intensity of muscle contraction normally elicited during actual performance (kinaesthetic modality). Each imagined trial was preceded by two oral signals (get ready, go) given by the experimenter and ended by a stop signal. To ensure that imagined contractions did not modulate background muscular activity, EMG signals of soleus (SOL) and gastrocnemius medialis (GM) were recorded continuously. Background EMG did not change along the four blocks of the MI session (P > 0.05). After each MI series, participants were asked to rate the quality of their imagery on a Likert scale from 1 (poor) to 7 (excellent). This self‐evaluated MI capacity (6.14 ± 0.1 on average over the whole session, mean ± SD) did not change significantly during the MI session (P > 0.05).
Electromyographic recordings
EMG activity was recorded from two muscles of the triceps surae muscle (SOL and GM), from one of the tibial compartment (tibialis anterior; TA), and from one of the quadriceps muscle (vastus medialis; VM). After shaving and dry‐cleaning the skin with alcohol to maintain low impedance (<5 kΩ), EMG signals were recorded by using two silver chloride surface electrodes (8 mm diameter, centre‐to‐centre distance: 2 cm; Controle Graphique S.A., Brie‐Comte‐Robert, France) placed over the muscle bellies. For SOL and GM, the electrodes were positioned 2 cm below the insertions of the gastrocnemii over the Achilles’ tendon and over the mid belly of the gastrocnemii muscles, respectively. For TA, recording electrodes were placed at one‐third of the distance from the fibula proximal head and the tip of the medial malleolus. VM signals were recorded with electrodes placed in a distal position on the muscle belly. The common reference electrode was placed in a central position between the stimulation and recording sites of the triceps surae. Electrode placements were marked on the skin with indelible ink to ensure the same positioning between sessions. EMG signals were amplified and filtered with a bandwidth frequency ranging from 15 to 1 kHz, with a gain of 1000 (Heka amplifier, Heka Elektronik, Lambrecht/Pfalz, Germany). EMG and mechanical signals were digitized on‐line (sampling frequency: 5 kHz) and stored for analysis with Tida software (Heka Elektronik).
Posterior tibial nerve stimulation
To evoke SOL and GM H‐reflexes, single rectangular pulses (1 ms width) were delivered to the posterior tibial nerve with a Digitimer stimulator (model DS7; Digitimer, Welwyn Garden City, UK). A self‐adhesive cathode (8 mm diameter, Ag–AgCl) was placed in the popliteal fossa and an anode (5 × 10 cm; Medicompex SA, Ecublens, Switzerland) was placed over the patella. The optimal stimulation site was first located by a hand‐held cathode ball electrode (0.5 cm diameter). Then, the stimulation electrode was firmly fixed with straps. The intensity of stimulation was then increased from H‐reflex threshold to maximal M‐wave, with 1 mA increment. The intensity was further increased by 20% to ensure that maximal M‐wave amplitude (MMAX) lay in the plateau of its maximal value.
For test reflex recordings (HTEST), the intensity used was set to elicit 50% of the maximal SOL H‐reflex amplitude on the ascending part of the recruitment curve. This stimulation intensity minimizes the risk of antidromic collision between an α‐motoneuron axon and reflex volley that could interfere with conditioning manoeuvres. The small M‐waves (MatH) accompanying these reflexes were used to ensure the consistency of stimulus condition within and between sessions (Grosprêtre et al. 2014). Similar normalized MatH (MatH/MMAX) indicated that the recorded H‐reflex lay in the same portion of the recruitment curve, with stable nerve stimulation conditions. During each experiment, five measures were recorded for each response at each time.
Assessment of presynaptic inhibition
In both experiments, SOL and GM H‐reflexes (HTEST) were conditioned in order to induce D1 presynaptic inhibition of Ia afferents onto α‐motoneurons (Achache et al. 2010). The decrease observed between test (HTEST) and conditioned response amplitude (HPSI) reflects the activation of presynaptic inhibitory mechanisms (Fig. 1 B). The conditioning stimulus was applied to the branch of the common peroneal nerve activating ipsilateral pretibial flexors with two silver chloride surface electrodes (8 mm diameter) placed at the upper part of the antero‐lateral side of the leg, distal to the caput fibulae (Forget et al. 1989). The conditioning stimuli consisted of a triple stimulation of the common peroneal nerve at 300 Hz (1 ms duration), with the intensity set at 120% of TA motor threshold (Achache et al. 2010). The optimal conditioning–test interval that gives the greatest H‐reflex inhibition was determined for each participant by testing several intervals around 20 ms, by steps of 1 ms. Test–conditioning intervals were reassessed at the beginning of each experiment (Fig. 1 B).
Assessment of heteronymous Ia facilitation
To assess spinal facilitation, a conditioning stimulus was applied to the ipsilateral femoral nerve. It is well known that prior activation of femoral nerve facilitates triceps surae (SOL and GM) Ia–α transmission, due to monosynaptic projection on spinal triceps surae α‐motoneurons (Hultborn et al. 1987). Figure 1 C depicts the underlying circuitry of this facilitatory pathway. A cathode ball electrode (0.5 cm diameter) was placed in the femoral triangle laterally to the femoral artery and an anode electrode (5 × 10 cm, Medicompex) was placed under the gluteus maximus muscle belly. Electrical stimulations of 1 ms pulse width were delivered with an electrical stimulator (Digitimer DS7A). At rest, the motor threshold (MT) was first determined as the lowest stimulation intensity that allowed recording of a VM M‐wave. The conditioning intensity was set to 130% of the MT. This intensity ensured consistency of the conditioning stimulus by measuring the small M‐wave amplitude of the VM. The conditioning–test interval was then determined to provide the greatest facilitation of soleus HTEST amplitude, by evoking five conditioned H‐reflexes (HFAC) at several intervals from −10 ms (test before conditioning stimulus, negative interval) to +5 ms (test after conditioning stimulus, positive interval) by steps of 1 ms (Fig. 1 C). The femoral nerve stimulation site being closer to the spinal cord than the posterior tibial nerve site, negative intervals are recommended to ensure a monosynaptic action of the quadriceps Ia afferent over triceps surae motoneuronal pool (Morita et al. 1995). Indeed, facilitations observed with larger conditioning–test intervals can also involve other polysynaptic circuits. It has already been reported that heteronymous Ia facilitation of the H‐reflex could not be observed in all participants, in the lower limb (Bergmans et al. 1978) and the upper limb (Baudry et al. 2010). Therefore, the spinal facilitation was tested at POST0 and POST10 only for participants who showed H‐reflex increase with negative conditioning–test intervals.
Data analysis
The root mean square (RMS) of EMG activity was determined for each muscle and session. For each parameter, peak‐to‐peak amplitudes of the five evoked responses were averaged and analysed. Each averaged response was normalized to the corresponding averaged maximal M‐wave evoked in the same condition (HTEST/MMAX, HPSI/MMAX, HFAC/MMAX).
Statistical analysis
All data are expressed as the mean ± standard deviation. The normality of the data was verified by the Shapiro–Wilk test (P < 0.05) in order to ensure the use of parametric tests. Separate analyses were performed on SOL and GM muscles. Each reflex response was normalized to the corresponding maximal M‐wave (MMAX) and statistical analyses were performed on the H/MMAX ratio.
To assess the effect of both conditioning techniques at rest and the online effect of MI (PRE measurements of experiment A) a two‐way repeated measures ANOVA was used for normalized H‐reflex responses (H/MMAX) with the factors motor imagery (rest, motor imagery) and conditioning (test, inhibition or facilitation). To assess the acute effects of the MI session on conditioned H‐reflexes at rest, two‐way repeated measures ANOVA with factors time (PRE, POST0, POST10) and conditioning (test, inhibition or facilitation) was performed. Acute effects of the MI session on HTEST/MMAX were evaluated by a two‐way repeated measures ANOVA, with factors time (PRE, POST0, POST10) and motor imagery (rest, motor imagery).
Data of the control experiment (experiment B) were analysed with a two‐way repeated measures ANOVA with the factors time (PRE, POST0) and conditioning (test, inhibition).
When a main effect or an interaction was found, a post hoc analysis was conducted using Tukey's HSD (honest significant difference) test. Statistical analysis was performed using STATISTICA (version 8.0, Statsoft, Tulsa, OK, USA). Relationships between the relative changes of the different tested variables were assessed with Pearson's correlations (r coefficient), with P obtained in the Bravais–Pearson table. The effect size was calculated by the partial eta‐squared (). Level of significance was accepted at P < 0.05.
Results
Experiment A: initial checking
A significant interaction was found between MI and presynaptic conditioning for H‐reflexes of the SOL (n = 13; F 1,12 = 7.505, P = 0.017, ) and the GM (n = 13; F 1,12 = 5.126, P = 0.042, ). Post hoc analyses revealed significant differences between both conditioned responses (HPSI and HFAC) and the initial test H‐reflex amplitude in PRE measurements. In all participants (n = 13) the common peroneal nerve conditioning stimulus induced a significantly lower HPSI amplitude as compared to the HTEST reflex in both SOL (−23.2 ± 5.4%, P = 0.007) and GM muscles (−22.6 ± 5.7%, P < 0.001), showing D1 presynaptic inhibition. The conditioning–test interval used to induce spinal inhibition was 21 ± 2 ms on average, as previously demonstrated (Faist et al. 1996).
With regards to H‐reflex facilitation, not all participants exhibited a proper heteronymous Ia facilitation according to the conditioning–test interval. While a significant effect of the conditioning stimulus (femoral nerve stimulation) to increase triceps surae H‐reflex amplitude was found in all participants at PRE, the conditioning–test interval that showed this facilitation varied among participants, from negative (conditioning after test) to positive (test after conditioning) intervals. In the present study, 8 of 13 participants demonstrated H‐reflex increase with a negative conditioning–test interval. Therefore, spinal facilitation at POST and POST10 was tested for these eight participants with negative intervals (mean interval: −4.6 ± 2.1 ms). At PRE test, the conditioning stimulus applied to the femoral nerve significantly increased the rest H‐reflex amplitude, showing a greater HFAC as compared to HTEST reflex of both SOL (+15.1 ± 4.9%, P = 0.029) and GM muscles (+14.2 ± 2.9%, P = 0.010), revealing heteronymous Ia facilitation.
Experiment A: online effect of MI (PRE measurements)
In all participants, HTEST/MMAX was not modulated by MI (SOL: P = 0.528; GM: P = 0.635). On the contrary, HPSI/MMAX was significantly increased by MI in both SOL (P = 0.001) and GM (P = 0.004). Consequently, MI led to a cancellation of presynaptic inhibition (Fig. 2 A and B). HFAC/MMAX was also greater during MI as compared to rest in the considered participants (n = 8), for SOL (P = 0.013) and GM (P < 0.001). This result demonstrates an increase of heteronymous Ia facilitation induced by MI (Fig. 2 C and D). Note that the increase of HPSI amplitude observed during MI was significantly correlated to the increase of HFAC amplitude for SOL (r = 0.768, P < 0.05, Fig. 2 E) and for GM (r = 0.72, P < 0.05, Fig. 2 F).
Figure 2. Initial effect of motor imagery (MI) on test and conditioned responses.
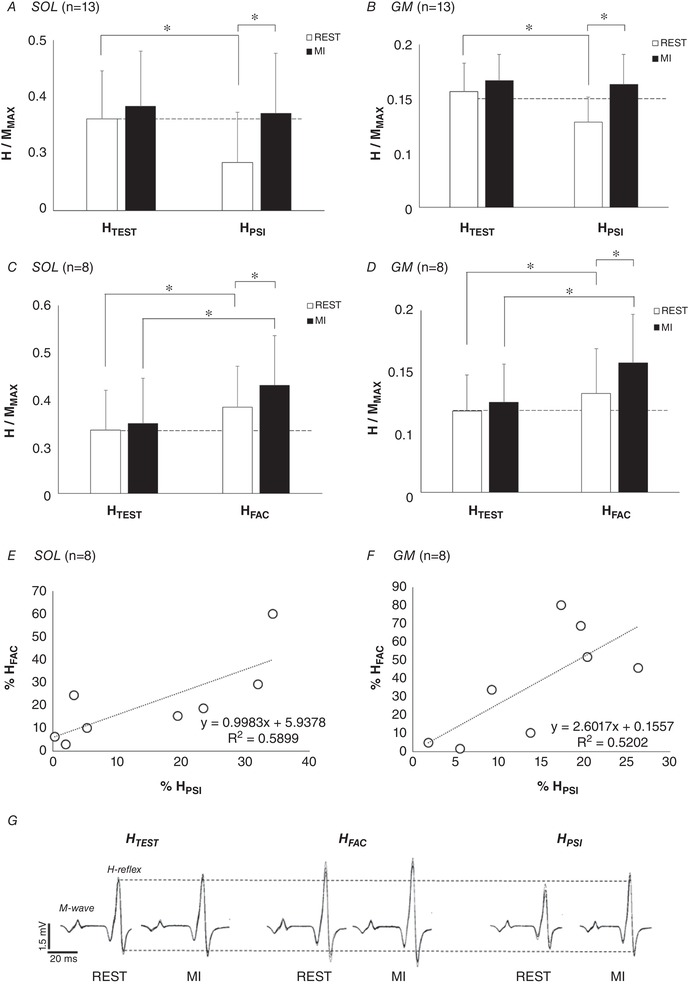
H‐reflexes of soleus (SOL) and gastrocnemius medialis (GM) are depicted at rest (open bars) and during MI (filled bars), normalized by the corresponding maximal M‐wave (MMAX). A and B, the effect of MI on D1 presynaptic inhibition (HPSI) in SOL and GM, respectively. C and D, the effect of MI on heteronymous Ia facilitation (HFAC) in SOL and GM, respectively. E and F, the relationships between the increase of HPSI and HFAC during MI for SOL and GM, respectively. Increases of HPSI and HFAC amplitude induced by MI are expressed as a percentage of the responses at rest (% REST). *Significant difference at P < 0.05. G, EMG signals of soleus responses in one representative participant.
Experiment A: acute effect of MI training (PRE vs. POST measurements)
In all participants (n = 13), no significant main or interaction effects of the factors time (PRE vs. POST0 vs. POST10) and motor imagery (MI vs. rest) were observed for the background RMS (P > 0.05), submaximal (MatH/MMAX) and maximal M‐waves (P > 0.05), for both muscles (Table 1).
Table 1.
Maximal M‐waves and normalized background EMG activity before and after the motor imagery (MI) session for Experiment A
| PRE | POST0 | POST 10 | |||||
|---|---|---|---|---|---|---|---|
| Muscle | Parameter | REST | MI | REST | MI | REST | MI |
| SOL | MMAX (mV) | 5.53 ± 1.77 | 5.57 ± 1.71 | 5.46 ± 1.78 | 5.45 ± 1.74 | 5.81 ± 1.76 | 5.80 ± 1.71 |
| RMS (% MMAX) | 0.10 ± 0.05 | 0.09 ± 0.04 | 0.10 ± 0.05 | 0.10 ± 0.05 | 0.09 ± 0.05 | 0.09 ± 0.05 | |
| GM | MMAX (mV) | 3.51 ± 1.46 | 3.49 ± 1.43 | 3.57 ± 1.58 | 3.50 ± 1.50 | 3.62 ± 1.77 | 3.53 ± 1.70 |
| RMS (% MMAX) | 0.25 ± 0.20 | 0.22 ± 0.19 | 0.19 ± 0.15 | 0.19 ± 0.15 | 0.19 ± 0.15 | 0.20 ± 0.15 | |
Data are presented as mean ± SD for soleus (SOL) and gastrocnemius medialis (GM), at rest and during MI.
A significant interaction effect was found on test spinal responses (HTEST/MMAX) between the factors motor imagery and time, for SOL (n = 13; F 2,24 = 5.337; P = 0.012; ) and GM (n = 13; F 2,24 = 5.185; P = 0.013; ). The MI training session did not modulate HTEST/MMAX at rest, from PRE to POST0 (SOL: P = 0.948; GM: P = 0.837) and from PRE to POST10 (SOL: P = 0.545; GM: P = 0.995). Interestingly, HTEST/MMAX was increased during MI in comparison to rest at POST0 (SOL: +35.1 ± 20.8%, P = 0.019; GM: +45.9 ± 14.1%, P < 0.001) as well as at POST10 (SOL: +26.5 ± 6.5%, GM: +34.7 ± 18.1%, Fig. 3).
Figure 3. Effect of MI on test reflexes.
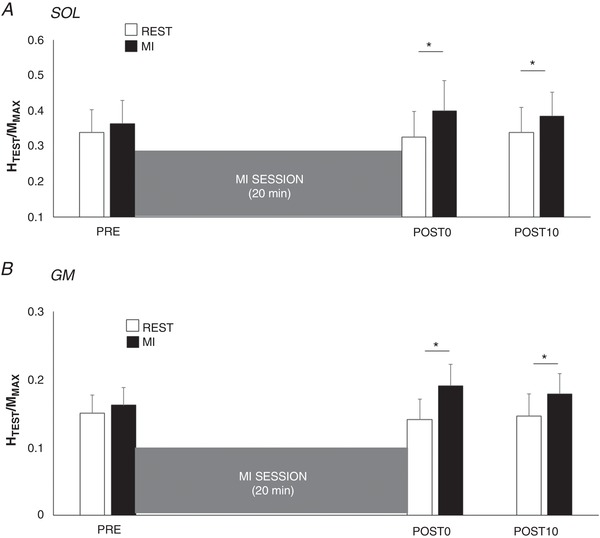
Normalized test H‐reflexes (HTEST/MMAX) of soleus (A, SOL) and gastrocnemius medialis (B, GM) were recorded at rest (open bars) and during MI (filled bars) before (PRE), immediately after (POST0) and 10 min after (POST10) the training session. *Significant difference between MI and rest HTEST/MMAX (P < 0.05).
A significant interaction was found between the factors time and conditioning for HPSI/MMAX at rest (n = 13; SOL: F 2,24 = 4.747, P = 0.018, ; GM: F 2,2 = 5.727, P = 0.009, ). Interestingly, we observed a suppression of presynaptic inhibition at rest after the MI training session, as evidenced by a greater amplitude of HPSI/MMAX at POST0 (SOL: P = 0.026, and GM: P = 0.009) and at POST10 (SOL: P = 0.014; GM: P = 0.014) in comparison to PRE (Fig. 4 A and B).
Figure 4. H‐reflexes test (HTEST), conditioned with presynaptic inhibition (HPSI) and with heteronymous Ia facilitation (HFAC) before and after the MI training session.
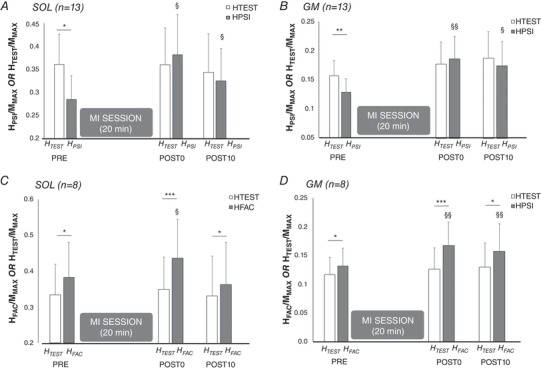
Responses of soleus (SOL) and gastrocnemius medialis (GM) are depicted in A and B, respectively, before (PRE), immediately after (POST0) and 10 min after (POST 10) MI session. TEST responses depict unconditioned reflexes (open bars), while HPSI depicts H‐reflexes conditioned by prior common peroneal nerve (presynaptic inhibition conditioning) and HFAC represents H‐reflexes conditioned by prior femoral nerve stimulation (Heteronymous Ia facilitation) (filled bars for each conditioned responses). Each response (TEST, HPSI and HFAC) is normalized by the corresponding maximal M‐wave (MMAX). *, ** and ***: significant difference with the corresponding TEST response at the same time point (conditioning effect: HTEST vs. HPSI or HFAC), at P < 0.05, P < 0.01 and P < 0.001, respectively. § and §§: significant difference with the corresponding H response at PRE (time effect: PRE vs. POST0 or POST 10) at P < 0.05 and P < 0.01, respectively.
In the considered participants, a significant interaction was also found between the factors time and conditioning for and HFAC/MMAX at rest (n = 8; SOL: F 2,14 = 4.052, P = 0.040, ; GM: F 2,14 = 4.842, P = 0.025, ). An increase of rest heteronymous Ia facilitation after the MI session was evidenced by a greater amplitude of HFAC/MMAX at POST0 in comparison to PRE (SOL: P = 0.028; GM: P = 0.001), and at POST10 in comparison to PRE for GM only (Fig. 4). The facilitation of conditioned reflexes increased from +15.1 ± 4.9% for SOL and +14.2 ± 2.9% for GM at baseline to +32.4 ± 9.5% and +46.8 ± 10.6% at POST0 for SOL and GM, respectively.
Experiment B: control experiment
After the 20 min rest period, no particular variation of background EMG was observed (P > 0.05 for all results). Maximal (MMAX) and submaximal M‐waves (MatH/MMAX) did not show any significant differences pre‐to‐post (SOL: P = 0.49; GM: P = 0.35). The rest period did not modulate spinal excitability, with similar test and conditioned responses at POST0 in comparison to PRE (all P > 0.05). HPSI/MMAX was statistically lower than HTEST/MMAX at any time of the experiment and in a similar extent, showing that presynaptic inhibition did not vary across time (Fig. 5).
Figure 5. Test (HTEST) and conditioned reflexes with presynaptic inhibition (HPSI) before and after the control condition.
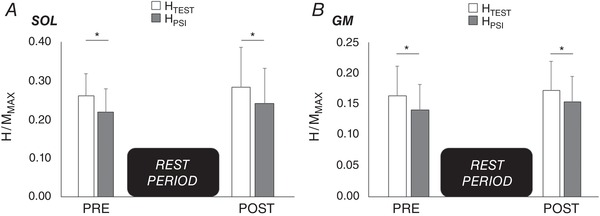
Normalized HTEST (open bars) and HPSI (filled bars) of soleus (A) and gastrocnemius medialis (B) before and after the rest period of 20 min. *Significant difference (P < 0.05) between HTEST and HPSI.
Discussion
In the current study, we assessed the effects of MI on the spinal presynaptic circuitry of the triceps surae muscle, through H‐reflex conditioning techniques, such as Ia presynaptic inhibition (HPSI) and heteronymous facilitation (HFAC). Our main findings revealed that both conditioned responses (HPSI and HFAC) increased during MI (online effect), without any modulation of the unconditioned H‐reflex (HTEST). After a session of MI practice (acute effect), we observed a cancellation of baseline presynaptic inhibition at rest. In addition, H‐test amplitude increased during MI, revealing a modulation of Ia afferent‐motoneuronal transmission efficacy.
Online effect of MI on spinal excitability
It is important to note that during MI of maximal isometric plantar flexions, no significant modulation was observed on background EMG activity, as well as on maximal (MMAX) and submaximal (MatH) M‐waves. This result is in accordance with the majority of previous studies showing no motor output during MI in comparison to rest (e.g. Lotze et al. 1999; Mulder et al. 2005; Rozand et al. 2014) and allows us to neglect a possible influence of the muscular state in the observed neural modulations.
The present study showed that MI did not immediately affect global spinal excitability, as evidenced by a lack of significant change in the test H‐reflex during MI in PRE measurements. Interestingly, we demonstrated here that MI impacted specific spinal structures with lower level of excitability, as previously suggested (Grosprêtre et al. 2016). Accordingly, we found an increase in both conditioned H‐reflexes during MI, reflecting the modulation of presynaptic inhibition level, mediated by an interneuronal network. It is well known that descending inputs influence not only the motoneuronal pool, but also others spinal circuits, in particular the interneurons mediating presynaptic inhibition (Jankowska, 1992, 2001). These interneurons induce primary afferent depolarization (PAD) of sensory fibres, such as Ia afferents, by a GABA‐ergic action on afferent fibre terminals. This neural mechanism reduces the neurotransmitter release in the Ia to α‐motoneuron synapse (Rudomin, 2009). In fact, the modulation of spinal presynaptic inhibition can only be ascertained when data from two H‐reflex conditioning techniques show similar trends: D1 presynaptic inhibition and heteronymous Ia facilitation. The combination of both methods (see Fig. 1 for more details) was recommended to avoid possible drawbacks associated with other mechanisms such as potential changes in the recruitment gain of the H‐reflex (Crone et al. 1990; Pierrot‐Deseilligny & Burke, 2005; Baudry & Enoka, 2009). Therefore, the positive effect of MI on both the presynaptic inhibited (HPSI) and the facilitated H‐reflexes (HFAC) positively correlated with each other, corroborating the involvement of presynaptic circuitry during MI.
The main hypothesis behind the partial or even total cancelation of presynaptic inhibition during MI lay in the influence of the supraspinal command on the PAD interneuronal network (Dyer et al. 2009). Indeed, post‐synaptic modulation of motoneuronal responsiveness would have led to both conditioned and test H‐reflex increase (Grosprêtre et al. 2016). In fact, animal experiments have shown that the activation threshold of such spinal interneurons is lower than that of the motoneurons (Daniele & MacDermott, 2009), allowing them to be more sensitive to small stimuli such as the minor cortical output generated by MI. Accordingly, PAD interneurons might be the first to be affected by MI, due to their lower activation thresholds compared to those of the motoneurons (Rudomin, 1990; Meunier & Pierrot‐Deseilligny, 1998). Contrary to the initial hypothesis of Jeannerod (1995), the current study considered that MI did not result in direct increase of motoneuronal excitability, but rather in a modulation of interneurons’ excitability that regulated the presynaptic inhibition level.
Spinal plasticity after MI practice
Hale and colleagues (2003) suggested that MI effects on spinal excitability would be more pronounced after repetitive MI practice. Accordingly, the present results revealed greater effects after MI practice, evidenced by increased amplitude of conditioned responses at rest, as well as increased amplitude of test H‐reflex during MI.
It was reported that physical activity‐dependent plasticity not only occurred at cortical levels but also impacted the spinal circuitry, first including the motoneuronal pool (Wolpaw, 2007). The present study showed that an acute session of MI was not sufficient to modulate the transmission efficacy between Ia afferent and α‐motoneurons at rest. Minor impulses generated by MI may not be enough to induce either baseline H‐reflex (HTEST) or maximal muscular response (MMAX) modulations, resulting in similar rest H/M ratios after the MI session. However, these ratios while imagining were increased after MI training, demonstrating that the MI condition exacerbated the neural modifications occurring at the spinal level.
To better understand such effects, the conditioning methods aiming at investigating presynaptic circuitry were replicated during POST0 measurements. Interestingly, MI training resulted in transient removal of presynaptic inhibition, corroborated by concomitant increases in the presynaptic inhibited (HPSI) and facilitated H‐reflex (HFAC) (see Fig. 4). Similar modulations of presynaptic inhibition has already been shown after one session of actual isometric dorsi‐flexion (Jessop et al. 2013). This result was attributed to inhibitory influence of the cortico‐spinal descending command onto PAD interneurons in lumbar spinal cord (Iles, 1996; Meunier & Pierrot‐Deseilligny, 1998). Indeed, it is known that cortico‐spinal projections that connect to α‐motoneurons also have collateral projections to the interneuronal network (Chen et al. 2001), which is more sensitive to repetitive subthreshold impulses (Daniele & MacDermott, 2009). Supraspinal descending inputs to the spinal cord were suggested to play a major role in driving plastic changes of this interneuronal network after repetitive physical practice (Kubota et al. 2015). In the present study, the repetition of the minor cortical descending drive during MI may increase excitability of the presynaptic interneuronal network (mechanism I, Fig. 6).
Figure 6.
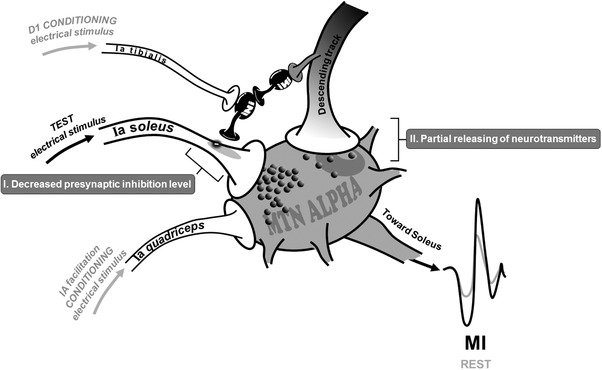
Potential neural mechanisms involved in spinal excitability modulation following MI practice
However, in addition to modulations of presynaptic circuitry, other mechanisms can be involved in the unconditioned H‐reflex increase during MI at POST0. In fact, the HTEST response involves all structures of the reflexive pathway, from Ia activation to α‐motoneuronal discharge. Accordingly, the fact of HTEST increase during MI in comparison to rest at POST measurements cannot exclude a possible action of the subliminal descending output on other structures than PAD interneurons. One of the main neurophysiological mechanisms relies on modulation at the level of the direct pyramidal tract projection to the motoneuronal membrane. I is noteworthy that cervico‐medullar evoked potentials (CMEP) were recently found to be larger during MI than at rest, while the H‐reflex remained unchanged (Grosprêtre et al. 2016). CMEP is evoked at the subcortical level by a transcutaneous electrical stimulus applied over the neck and assesses direct pyramidal cortical projection on the spinal motoneuronal pool (Taylor, 2006). The increase of CMEP during MI may reflect a possible modulation at the cortico‐motoneuronal junction. Therefore, in the present study, this synapse might also be subject to small neurotransmitter release during mental practice, increasing H‐reflex amplitude during MI at POST0 (see mechanism II, Fig. 6). Nevertheless, the POST MI session highlighted that this partial release might not be sufficient to modulate baseline spinal excitability (no increase of rest H‐reflex) but can be added to the global effect of MI observed after practice (mechanisms I + II).
Finally, conditioned responses (HPSI and HFAC) tended to return to baseline 10 min after the end of the MI session, as evidenced by a visual decrease between POST and POST10 (see Fig. 4). The neural changes induced by repetitive execution of actual simple movements are usually found to return to baseline within a few minutes after the end of the training session (Classen et al. 1998). The transient change of the spinal network excitability indicated a short‐term plasticity induced by an acute session of imagined repetitions. This non‐lasting effect might not therefore exclude a long‐term effect of MI at the spinal level after several sessions. Interestingly, we recently found that 1 week of daily MI practice significantly increased baseline spinal excitability (Grosprêtre et al. 2018).
Conclusion
The present study showed that a single session of MI was sufficient to induce transient spinal plasticity. Particularly, presynaptic interneuronal plasticity, which is known for its contribution to the enhancement of skills and performance after actual training (Wang et al. 2006), might also participate to the gains observed after repetitive use of MI. While previous studies mainly focused on cortical reorganization during and after MI practice (Porro et al. 1996; Schnitzler et al. 1997; Gueugneau et al. 2013), the present results demonstrated that MI could significantly impact the spinal level even in the absence of baseline H‐reflex modulation. The minor cortical output generated during MI seems devoted to the most sensitive structures, i.e. the spinal presynaptic network, highlighted by both D1 presynaptic conditioning and heteronymous Ia facilitation methods. In addition, spinal plasticity at rest and during MI was more evidenced following MI practice. If the cortical output observed during MI reflects a lower extent of the voluntary drive generated during actual movement, it would also affect other spinal structures such as reciprocal inhibition. As a perspective, it would be of interest to assess this kind of mechanisms by using a greater range of conditioning–test intervals during and after an MI session.
Additional information
Competing interests
No conflicts of interest, financial or otherwise, are declared by the authors.
Author contributions
S.G. and A.M. designed the work; S.G. and A.M. performed the experiments; S.G. analysed the data; S.G., F.L., C.P. and A.M. interpreted the data. All authors participated in the drafting of the manuscript and in revising the work. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM).
Biography
Sidney Grosprêtre received his PhD in Neurophysiology in 2013 from the University of Burgundy in France. He is now associate professor in EA4660‐C3S Laboratory in Besançon, France. He teaches neurosciences, physiology and biomechanics. His work mainly focuses on neurophysiology, more precisely on the circuitry of the human spinal cord, based on H‐reflex and transcranial magnetic stimulation studies. He won a young investigator award from the European congress of sport science (ECSS) for his research on the effects of motor imagery on spinal circuitry, and hope in the future to further understand the neural plasticity induced by chronic mental practice.

Edited by: Janet Taylor & Dario Farina
References
- Achache V, Roche N, Lamy J‐C, Boakye M, Lackmy A, Gastal A, Quentin V & Katz R (2010). Transmission within several spinal pathways in adults with cerebral palsy. Brain 133, 1470–1483. [DOI] [PubMed] [Google Scholar]
- Aoyama T & Kaneko F (2011). The effect of motor imagery on gain modulation of the spinal reflex. Brain Res 1372, 41–48. [DOI] [PubMed] [Google Scholar]
- Baudry S & Enoka RM (2009). Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199, 83–88. [DOI] [PubMed] [Google Scholar]
- Baudry S, Maerz AH & Enoka RM (2010). Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J, Delwaide PJ & Gadea‐Ciria M (1978). Short‐latency effects of low‐threshold muscular afferent fibers on different motoneuronal pools of the lower limb in man. Exp Neurol 60, 380–385. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Decety J, Jeannerod M & Requin J (1997). Mental simulation of an action modulates the excitability of spinal reflex pathways in man. Brain Res Cogn Brain Res 5, 221–228. [DOI] [PubMed] [Google Scholar]
- Chen D, Theiss RD, Ebersole K, Miller JF, Rymer WZ & Heckman CJ (2001). Spinal interneurons that receive input from muscle afferents are differentially modulated by dorsolateral descending systems. J Neurophysiol 85, 1005–1008. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M & Cohen LG (1998). Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Cowley PM, Clark BC & Ploutz‐Snyder LL (2008). Kinesthetic motor imagery and spinal excitability: the effect of contraction intensity and spatial localization. Clin Neurophysiol 119, 1849–1856. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J & Pierrot‐Deseilligny E (1990). Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81, 35–45. [DOI] [PubMed] [Google Scholar]
- Daniele C & MacDermott AB (2009). Low‐threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci 29, 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J (1996). The neurophysiological basis of motor imagery. Behav Brain Res 77, 45–52. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC & Fazio F (1994). Mapping motor representations with positron emission tomography. Nature 371, 600–602. [DOI] [PubMed] [Google Scholar]
- Dyer J‐O, Maupas E, de Andrade Melo S, Bourbonnais D, Fleury J & Forget R (2009). Transmission in heteronymous spinal pathways is modified after stroke and related to motor incoordination. PLoS One 4, e4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Geyer S & Naito E (2003). Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body‐part‐specific motor representations. J Neurophysiol 90, 3304–3316. [DOI] [PubMed] [Google Scholar]
- Faist M, Dietz V & Pierrot‐Deseilligny E (1996). Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res 109, 441–449. [DOI] [PubMed] [Google Scholar]
- Forget R, Pantieri R, Pierrot‐Deseilligny E, Shindo M & Tanaka R (1989). Facilitation of quadriceps motoneurones by group I afferents from pretibial flexors in man. 1. Possible interneuronal pathway. Exp Brain Res 78, 10–20. [DOI] [PubMed] [Google Scholar]
- Gentili RJ & Papaxanthis C (2015). Laterality effects in motor learning by mental practice in right-handers. Neuroscience 297, 231–242. [DOI] [PubMed] [Google Scholar]
- Grèzes J & Decety J (2001). Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta‐analysis. Hum Brain Mapp 12, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosprêtre S, Jacquet T, Lebon F, Papaxanthis C & Martin A (2018). Neural mechanisms of strength increase after one‐week motor imagery training. Eur J Sport Sci 18, 209–218. [DOI] [PubMed] [Google Scholar]
- Grosprêtre S, Lebon F, Papaxanthis C & Martin A (2016). New evidence of corticospinal network modulation induced by motor imagery. J Neurophysiol 115, 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosprêtre S, Papaxanthis C & Martin A (2014). Modulation of spinal excitability by a sub‐threshold stimulation of M1 area during muscle lengthening. Neuroscience 263, 60–71. [DOI] [PubMed] [Google Scholar]
- Gueugneau N, Bove M, Avanzino L, Jacquin A, Pozzo T & Papaxanthis C (2013). Interhemispheric inhibition during mental actions of different complexity. PLoS One 8, e56973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot A, Collet C, Nguyen VA, Malouin F, Richards C & Doyon J (2009). Brain activity during visual versus kinesthetic imagery: an fMRI study. Hum Brain Mapp 30, 2157–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale BS (2007). The Effects of Motor Imagery on the Hoffmann Reflex and Presynaptic Inhibition, Thesis, ed. Indiana University, Bloomington, IN, USA. [Google Scholar]
- Hale BS, Raglin JS & Koceja DM (2003). Effect of mental imagery of a motor task on the Hoffmann reflex. Behav Brain Res 142, 81–87. [DOI] [PubMed] [Google Scholar]
- Hall CR & Martin KA (1997). Measuring movement imagery abilities: A revision of the Movement Imagery Questionnaire. J Ment Imag 21, 143–154. [Google Scholar]
- Hultborn H, Meunier S, Pierrot‐Deseilligny E & Shindo M (1987). Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF (1996). Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E (1992). Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38, 335–378. [DOI] [PubMed] [Google Scholar]
- Jankowska E (2001). Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 533, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M (1995). Mental imagery in the motor context. Neuropsychologia 33, 1419–1432. [DOI] [PubMed] [Google Scholar]
- Jessop T, DePaola A, Casaletto L, Englard C & Knikou M (2013). Short‐term plasticity of human spinal inhibitory circuits after isometric and isotonic ankle training. Eur J Appl Physiol 113, 273–284. [DOI] [PubMed] [Google Scholar]
- Kasai T, Kawai S, Kawanishi M & Yahagi S (1997). Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res 744, 147–150. [DOI] [PubMed] [Google Scholar]
- Kiers L, Fernando B & Tomkins D (1997). Facilitatory effect of thinking about movement on magnetic motor‐evoked potentials. Electroencephalogr Clin Neurophysiol 105, 262–268. [DOI] [PubMed] [Google Scholar]
- Kilintari M, Narayana S, Babajani‐Feremi A, Rezaie R & Papanicolaou AC (2016). Brain activation profiles during kinesthetic and visual imagery: An fMRI study. Brain Res 1646, 249–261. [DOI] [PubMed] [Google Scholar]
- Kubota S, Hirano M, Koizume Y, Tanabe S & Funase K (2015). Changes in the spinal neural circuits are dependent on the movement speed of the visuomotor task. Front Hum Neurosci 9, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon F, Collet C & Guillot A (2010). Benefits of motor imagery training on muscle strength. J Strength Cond Res 24, 1680–1687. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hülsmann E, Flor H, Klose U, Birbaumer N & Grodd W (1999). Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci 11, 491–501. [DOI] [PubMed] [Google Scholar]
- Meunier S & Pierrot‐Deseilligny E (1998). Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119, 415–426. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H & Yanagisawa N (1995). Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res 104, 167–170. [DOI] [PubMed] [Google Scholar]
- Mouthon A, Ruffieux J, Wälchli M, Keller M & Taube W (2015). Task‐dependent changes of corticospinal excitability during observation and motor imagery of balance tasks. Neuroscience 303, 535–543. [DOI] [PubMed] [Google Scholar]
- Mulder T, De Vries S & Zijlstra S (2005). Observation, imagination and execution of an effortful movement: More evidence for a central explanation of motor imagery. Exp Brain Res 163, 344–351. [DOI] [PubMed] [Google Scholar]
- Munzert J, Lorey B & Zentgraf K (2009). Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev 60, 306–326. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny E. & Burke D. (eds) (2005). The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge University Press, Cambridge. [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M & di Prampero PE (1996). Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci 16, 7688–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozand V, Lebon F, Papaxanthis C & Lepers R (2014). Does a mental training session induce neuromuscular fatigue? Med Sci Sports Exerc 46, 1981–1989. [DOI] [PubMed] [Google Scholar]
- Rudomin P (1990). Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci 13, 499–505. [DOI] [PubMed] [Google Scholar]
- Rudomin P (2009). In search of lost presynaptic inhibition. Exp Brain Res 196, 139–151. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Salenius S, Salmelin R, Jousmäki V & Hari R (1997). Involvement of primary motor cortex in motor imagery: a neuromagnetic study. Neuroimage 6, 201–208. [DOI] [PubMed] [Google Scholar]
- Slimani M, Tod D, Chaabene H, Miarka B & Chamari K (2016). Effects of mental imagery on muscular strength in healthy and patient participants: a systematic review. J Sports Sci Med 15, 434–450. [PMC free article] [PubMed] [Google Scholar]
- Taylor JL (2006). Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol 16, 215–223. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR & Chen XY (2006). Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci 23, 141–150. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR (2007). Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol 189, 155–169. [DOI] [PubMed] [Google Scholar]
- Yahagi S, Shimura K & Kasai T (1996). An increase in cortical excitability with no change in spinal excitability during motor imagery. Percept Mot Skills 83, 288–290. [DOI] [PubMed] [Google Scholar]


