Abstract
More than 40% of HIV infections occur via female reproductive tract (FRT) through heterosexual transmission. Epithelial cells that line the female genital mucosa are the first line of defense against HIV-1 and other sexually transmitted pathogens. These sentient cells recognize and respond to external stimuli by induction of a range of carefully balanced innate immune responses. Previously, we have shown that in response to HIV-1 gp120, the genital epithelial cells (GECs) from upper reproductive tract induce an inflammatory response that may facilitate HIV-1 translocation and infection. In this study, we report that the endometrial and endocervical GECs simultaneously induce biologically active interferon-β (IFNβ) antiviral responses following exposure to HIV-1 that act to protect the epithelial tight junction barrier. The innate antiviral response was directly induced by HIV-1 envelope glycoprotein gp120 and addition of gp120 neutralizing antibody inhibited IFNβ production. Interferon-β was induced by gp120 in upper GECs through Toll-like receptor 2 signaling and required presence of heparan sulfate on epithelial cell surface. The induction of IFNβ was dependent upon activation of transcription factor IRF3 (interferon regulatory factor 3). The IFNβ was biologically active, had a protective effect on epithelial tight junction barrier and was able to inhibit HIV-1 infection in TZM-bl indicator cells and HIV-1 replication in T cells. This is the first report that recognition of HIV-1 by upper GECs leads to induction of innate antiviral pathways. This could explain the overall low infectivity of HIV-1 in the FRT and could be exploited for HIV-1 prophylaxis.
Introduction
Almost 70% of HIV infection occurs via sexual transmission in the intestinal or genital tract. Globally, women constitute more than half of 36.9 million HIV-infected individuals. HIV and AIDS are the leading causes of death worldwide among women of reproductive age. Clinical and experimental studies indicate that HIV can be transmitted through both the upper (oviduct, uterus and endocervix) and lower (ectocervix, vagina) genital tract. In particular, the transformation zone in the cervix is considered to be a highly susceptible site because of the presence of a large number of target cells in the lamina propria, below the epithelium.1 However, despite the relatively large surface area available for HIV-1 exposure and the higher incidence in women, the overall transmission in female reproductive tract (FRT) is relatively inefficient, estimated at 1:200 to 1:2000 per coital act, indicating that the FRT provides a significant barrier to HIV transmission.2
Following HIV-1 exposure, the acute events that determine the outcome of the interaction with the virus in the FRT are still not well understood. HIV needs to cross the epithelial lining of the genital mucosa in order to establish infection in the female genital tract. There is general consensus that the epithelial cells themselves do not get infected.1 However, there is increasing evidence that they play a key role as sensors and first responders for the host innate immune system, in addition to forming a physical and functional barrier against microorganisms.3
The upper genital epithelial cells (GECs) are dynamically active cells that display a carefully orchestrated response to external stimuli. They perform a multitude of tasks in encounters with pathogens, including induction of innate responses, as well as relaying signals to activate other cells of the innate and adaptive immune system. Both the upper and lower genital tract epithelium have been shown to express antimicrobial peptides as well as a repertoire of pathogen pattern recognition receptors like Toll-like receptors (TLRs) that allows them to respond to a wide array of pathogens and initiate innate and adaptive responses.4 We and others have reported the induction of innate antiviral responses in upper GEC cultures following treatment with TLR ligands, resulting in decreased HSV-2 replication. The TLR3 ligand polyinosinic:polycytidylic acid (poly I:C) was shown to induce the greatest antiviral effect, but this was accompanied with enhanced production of inflammatory cytokines.5,6 The antiviral effect by upper GECs was mediated by production of interferon-β (IFNβ) in response to TLR ligands. Other studies have also reported production of interferon-stimulated factors such as MyxA, 2′5′-oligoadenylate synthetase (OAS) and inducible nitric oxide synthase that have direct antiviral effects in primary cultures of endometrial epithelial cells as well as cervical and cervicovaginal cell lines.6,7
Type I interferons (IFNs) play a mixed role in HIV-1 infection. In general, production of IFN in the acute phase is associated with protection against infection and control of viral replication, although it also leads to immune activation and influx of other immune cells that supports viral replication.8,9 The protective role played by type I IFN is associated with inhibition of HIV-1 replication because of upregulation of a number of IFN-stimulated genes (ISGs).10,11,12,13,14 Plasmacytoid dendritic cells (pDCs) in particular have been reported to be a rich source of IFN during HIV-1 infection and studies in elite controllers, HIV-1-infected individuals who maintain undetectable viral load in the absence of antiretroviral therapy, show that pdcs counts correlate with IFN production.15,16
Previously, we have reported that the upper GECs do not get productively infected with HIV-1.17 Primary epithelial cells isolated from endometrium and cervix responded directly to HIV-1, resulting in production of inflammatory cytokines that disrupted the tight junctions forming the epithelial barrier, allowing translocation of the virus and luminal bacteria.18 Further studies revealed that pattern recognition receptors TLR2 and TLR4 present on the cell surface recognized HIV-1 envelope glycoprotein gp120 that activated nuclear factor (NF)-κB pathway and led to production of proinflammatory cytokine production.19 However, no studies have examined whether HIV-1 exposure can activate innate antiviral immunity in the mucosal epithelium of the genital tract and whether this could play a role in inhibiting HIV-1 infection and replication.
In the current study we analyzed the antiviral responses of the genital epithelium from upper reproductive tract and determined the role it may play in preventing HIV infection and replication.
Materials and methods
Cell lines, reagents and antibodies
Vero cell line (ATCC, Manassas, VA, USA) was used for analyzing biological activity of type I IFN. Vero cells were grown in α-minimum essential medium (α-MEM; Invitrogen, Burlington, ON, Canada), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Burlington, ON, Canada). The human embryonic kidney-293 (HEK293) cell line (ATCC) was used for transfection assays. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Burlington, ON, Canada) supplemented with 10% FBS (Invitrogen). The following reagents were used for the study: tri-palmitoylated lipopeptide Pam3CSK (InvivoGen, San Diego, CA, USA), lipopolysaccharide (LPS) from Escherichia coli 026:B6 (Invitrogen; TLR4 ligand), the novel TLR4 ligand FimH, poly I:C (TLR3 ligand; Sigma Aldrich, Burlington, ON, Canada), BX795 (InvivoGen), heparinase III from Flavobacterium heparinum (Sigma Aldrich) and heparan sulfate (Sigma Aldrich). Recombinant HIV-1 gp120 protein (strain ADA and Bal) was obtained through NIH AIDS Research and Reference Reagent program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID) (Bethesda, MD, USA), National Institutes of Health (NIH) (Bethesda, MD, USA), USA. Subsequently, gp120 was bought from a commercial source (Immunodiagnostic Inc., Woburn, MA, USA). Recombinant gp120 from both sources was compared and commercial source gp120 was used exclusively after obtaining comparable results. The following antibodies were used: anti-human CD282/TLR2, clone TL2.1 (eBioscience, San Diego, CA, USA), mouse IgG2a K isotype control antibody (eBioscience), anti-human CD284/TLR4, clone HTA125 (eBioscience), anti-human CD285/TLR5 (InvivoGen), anti-gp120 neutralizing antibody, clone 2G12 (Polymun Scientific, Klosterneuburg, Austria), mouse anti-IRF3 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and rabbit anti-NF-κB p65 (Santa Cruz Biotechnology, Inc.), anti-IFNβ antibody (25 μg/ml; Merck Millipore, Etobicoke, ON, Canada) and anti-TNFα antibody (25 μg/ml; Rockland Immunochemicals Inc., Limerick, PA, USA).
Ethics statement
Primary genital epithelial cells were isolated from cervical and endometrial tissues obtained from women aged 30–59 years (mean age 42.9+7.2) undergoing hysterectomies for benign gynecological reasons at Hamilton Health Sciences Hospital. Informed written consent was obtained in accordance with the approval of the Hamilton Integrated Human Research Ethics Board.
Primary endometrial and cervical epithelial cell cultures
All experiments were conducted on primary epithelial cell cultures isolated from hysterectomy tissues. Detailed protocol for isolation and culture of primary endometrial and endocervical epithelial cultures, referred to as GECs hereon, has been described previously.20,21 Briefly, endometrial and endocervical tissues were separately minced into small pieces and digested in an enzyme mixture at 37 °C. The GECs were isolated by a series of separations through nylon mesh filters (Small Parts Inc., Miramar, FL, USA), resuspended in DMEM/F12 primary growth medium (Invitrogen) and seeded onto MatrigelTM (Becton Dickinson and Company, Mississauga, ON, Canada)-coated tissue culture inserts (BD Falcon, Mississauga, ON, Canada). Epithelial cultures were grown for 5–7 days until confluent monolayers were formed. The confluency was monitored by transepithelial resistance (TER) measured by a volt ohm meter (EVOM; World Precision Instruments, Sarasota, FL, USA). Confluent monolayers showing TER values greater than 1000 Ω/cm were used for further experiments. The purity of epithelial monolayers was between 95 and 98%, with no trace of any hematopoietic cells. The detailed protocol for measuring purity of upper genital epithelial cultures has been previously described.20
Viral strains and GEC exposure
HIV-1 ADA (M-tropic) was prepared by infection of primary adherent monocyte-derived macrophages from human peripheral blood mononuclear cells, followed by virus concentration using the Amicon Ultra-15 filtration system (Millipore, Billerica, MA, USA). Env-deleted HIV-1 (env −) was kindly provided by Dr D. Johnson (NCI, NIH, USA), and was prepared on an NL4-3 backbone. For viral exposure, primary GECs were grown to confluency on transwell inserts and subsequently exposed to 100 μl of HIV-ADA (106 infectious viral unit (IU)/ml, corresponding to a multiplicity of infection (MOI) of 1), ultraviolet (UV)-inactivated HIV (106 IU/ml), HIV-1 env − (at a p24 concentration of 79 ng/ml) or recombinant gp120 (100 ng/ml) for various time points, depending on the experiment.
To explore the role of TLR on antiviral signaling in GECs by HIV-1, confluent primary cells were treated with neutralizing antibodies against TLR2, 4, 5, isotype antibody (all at 10 μg/ml) and heparan sulfate (40 μg/ml) during the course of gp120 exposure or pretreatment with heparinase III (6 mIU/ml) for 1 h before gp120 exposure. The interferon regulatory factor 3 (IRF3)-mediated pathways were also investigated by pretreating cells with BX795 (an inhibitor of TBK-1/IKKɛ phosphorylation, an upstream event in activation of IRF3) at a concentration of 1 μM for 1 h before experimental exposure of HIV-1 or gp120. To determine the role of NF-κB in the induction of IFNβ, GEC monolayers were pretreated with the NF-κB inhibitor pyrrolidine dithiocarbamate (10 μM, Sigma-Aldrich) for 1 h before exposure with gp120.
HIV-1 infection assay in TZM-bl cell line
The TZM-bl indicator cell line (obtained through NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Dr John C Kappes, Dr Xiaoyun Wu and Tranzyme Inc. (Research Triangle Park, NC, USA))22,23,24,25 was maintained in DMEM supplemented with 10% heat-inactivated FBS (Invitrogen). The cell line was used for quantitative analysis of HIV infection using luciferase as a reporter.
Briefly, 60 000 TZM-bl cells were seeded into a 24-well plate in 500 μl of 10% DMEM. Various treatments containing either media alone or recombinant IFNβ (100 pg/ml) or GEC supernatants from mock control or gp120-treated monolayers incubated in the presence or absence of rabbit anti-human IFNβ (AB1431; Millipore, Etobicoke, ON, Canada) or rabbit isotype IgG control (ab172730; Abcam Inc., Toronto, ON, Canada) at 10 μg/ ml concentrations were added to TZM-bl cells in a total volume of 250 μl. Each sample was added to TZM-bl cells in the presence of HIV-1 for 2 h with a gentle shaking every 15 min. After 2 h, 1 ml of 10% DMEM was overlaid and the cells were further incubated for 24 h at 37 °C. After 24 h, the cells were washed once with 1 × phosphate-buffered saline, lysed and the luciferase activity was measured using a Stratagene luciferase assay kit (Agilent Technologies, Mississauga, ON, Canada) as per the manufacturer’s instructions.
Type 1 IFN bioassay
To assess the presence of biologically active IFN, a vesicular stomatitis virus (VSV) plaque reduction assay was used. This method is based on assaying the ability of VSV-GFP, a lytic but IFN-sensitive virus that expresses green fluorescent protein (GFP) under the control of a virus promoter (kindly provided by Dr B. Lichty, McMaster University), to replicate within cultures. This assay has been used previously to assess the presence of biologically active type I IFN. Briefly, the supernatants were collected from primary epithelial cells after treatments and diluted with α-MEM, and added to plates with Vero cells grown to 80% confluency. After 24 h, samples were removed, and Vero cells were challenged with VSV-GFP. After 1 h, the unattached VSV-GFP was removed and Vero cells were overlaid with 2% methylcellulose/2 × F11 MEM medium (1:1 ratio) and incubated for 48 h. Levels of GFP fluorescence were visualized and quantified using a Typhoon scanner (Amersham Bioscience, GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). Fluorescence was inversely proportional to the IFN activity. The fluorescence reading of treated cultures was compared with control cultures (no IFN) and presented as relative fluorescence.
Immunofluorescent staining
GECs were exposed to HIV-1, gp120 or poly I:C (positive control for staining, 25 μg/ml) and were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA). Cells were stained with primary mouse monoclonal anti-human IRF3 (SL-12, Santa Cruz Biotechnology, Inc.) and Alexa Flour 488-conjugated secondary antibody, followed by propidium iodide nuclear counterstaining. Filters were excised from the polystyrene filter supports, and mounted on glass slides in Vecta Shield Hard Set mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA). All samples were imaged on an inverted confocal laser-scanning LSM 510 microscope (Carl Zeiss Canada Ltd, Toronto, ON, USA). Confocal microscope settings for image acquisition and processing were identical between control and treated monolayers and three separate, random images were acquired and analyzed for each experimental condition. Images were analyzed using Image J software for measuring levels of nuclear colocalization of IRF3.
Plasmids and HEK293 transfection assays
Expression vectors for human TLR2, TLR4, CD14, MD2, β-galactosidase (β-gal) and plasmids were kindly provided by Dr Cynthia Leifer (Cornell University Ithaca, NY, USA). Interferon-β-luciferase (IFNβ-Luc) and interferon-stimulated response element (ISRE-Luc)-inducible reporter plasmids were a kind gift from Dr John Hiscott and are designed to monitor the activation of IFNβ pathways.26 All plasmids were amplified and purified using Endo-free Midi Prep columns (Qiagen, Toronto, ON, Canada). Transfections for luciferase assay were carried out in HEK293 cell line. Subconfluent HEK293 cells were transfected with 100 ng of pSV β-gal (internal transfection control for normalizing transfection efficiencies), 100 ng of IFNβ-Luc or ISRE-Luc reporter plasmid (firefly luciferase, experimental reporter), 30 ng of pUNO-hTLR2 or pUNO-hTLR4 with or without 30 ng CD14 and pUNO hMD2 expression plasmids and pBABE (empty vector) for a total of 1 μg DNA/well. Transfections were completed using Gene Juice transfection reagent (EMD Millipore). At 24 h post transfection, cells were treated with gp120, Pam3CSK4 (a TLR2 ligand) and LPS (a TLR4 ligand). The cells were subsequently harvested, lysed and luciferase activity was measured using a Stratagene luciferase assay kit (Agilent Technologies) as per the manufacturer’s instructions. Activity of pSV-β-gal luciferase activity was also measured by reporter assay (Luminescent β-galactosidase detection kit II, Clontech Lab Inc., Mountain View, CA, USA) according to the manufacturer’s instruction. The fold increases in IFNβ-luciferase expression or ISRE-luciferase expression (experimental reporters) were normalized with β-gal expression as an internal control for transfection.
1G5 HIV-1 LTR assay
To measure the effect of various treatments on activation of HIV-1 long terminal repeat (LTR), 106 1G5 cells were treated with phorbol 12-myristate 13-acetate (PMA) at 50 ng/ml (eBioscience) for 24 h at 37 °C.27 The cells were lysed, and luciferase activity was determined using a luciferase assay (Agilent). To measure the effect of epithelial supernatants containing IFNβ on 1G5 LTR activation, supernatants were collected from mock or HIV-1-treated cultures. Supernatants were then incubated with anti-IFNβ antibody (25 μg/ml; Millipore) or anti-TNFα antibody (25 μg/ml; Rockland Immunochemicals) or rabbit isotype IgG control (25 μg/ml; Abcam) for 1 h before addition to PMA-treated 1G5 cells and luciferase activity was measured after 24 h.
Microarray gene expression and data analysis
For microarray analysis, 1 × 105 primary genital epithelial cells were exposed to HIV-1 for 48 h at an MOI of 1 and were processed for RNA isolation using RNAeasy kit (Qiagen). Purified RNA was resuspended in RNase-free water and quantified using a Nanovue spectrophotometer (General Electric, Mississauga, ON, Canada). RNA bioanalysis, microarray chip hybridization and processing were performed by the Center for Applied Genomics Facility at Hospital for Sick Kids (Toronto, Ontario, Canada). DNA microarray analysis was carried out using the Affymetrix Human Genome ST 2.0 array (Affymetrix, Santa Clara, CA, USA) according to the manufacturer’s instructions (Agilent Technologies). The.CEL data files generated by the Affymetrix microarray were analyzed using GeneSpring GX version 13.0 (Agilent Technologies). Fold changes (>2.0) in gene expression and gene ontology annotations were determined.
Quantitative real-time PCR
Quantitative real time-PCR (qRT-PCR) was performed using gene-specific primers listed in Table 1 and SYBR Green PCR mix according to the manufacturer’s manual (FroggaBio, Toronto, ON, Canada). qRT-PCR was performed using StepOne PlusTM Real-Time PCR System (Thermo Fisher, Burlington, ON, Canada). Samples were run in triplicate and all data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression as an internal control.
Table 1.
Real-time PCR primers for different interferon-stimulated genes (ISGs)
Statistical analysis
GraphPad Prism Version 4 (GraphPad Software, San Diego, CA, USA) was used to compare three or more means by one-way analysis of variance (ANOVA) for analyzing different treatments at the same time and two-way ANOVA for comparing treatments with their specific controls. To compare two different treatment means, unpaired t-test was used. When an overall statistically significant difference was seen (P<0.05), Bonferroni post-test was performed to adjust the P-value for multiple comparisons. P-values for each analysis are indicated in figure legends.
Results
Antiviral responses induced by endometrial epithelial cells in response to HIV gp120
To examine the innate antiviral response of epithelial cells from upper genital tract, confluent monolayers of endometrial epithelial cells were exposed to HIV-1 (105/well) or recombinant gp120 protein (100 ng/ml), or an env-deleted mutant that lacks the HIV-1 viral envelope precursor, gp160,18,28 and supernatants were analyzed for IFNβ by enzyme-linked immunosorbent assay (ELISA). The results showed that supernatants collected from monolayers treated with HIV-1 or gp120 contained significantly higher amount of IFNβ as compared with supernatants from mock treatment (Figure 1a). IFNβ level was much higher in basolateral supernatants compared with apical supernatants, demonstrating preferential secretion of IFNβ from the basal interface of the epithelium (Figure 1a). The env-deleted HIV-1 mutant failed to induce IFNβ in endometrial GECs, indicating that HIV envelope is required to induce antiviral responses in endometrial GECs (Figure 1a). The levels of IFNβ produced by different donor tissues was variable based on the tissue: baseline (range 20–80 pg/ml, n=6), apical supernatants following HIV-1/gp120 treatment (140–600 pg/ml, n=6) and basolateral supernatants (200–2185 pg/ml, n=6) but the increased responses to gp120 and HIV-1 was similar in all tissues. To confirm the role of gp120 in IFNβ production by endometrial GECs, viral preparations as well as recombinant gp120 were incubated with anti-gp120 neutralizing antibody (2G12 clone) or isotype antibody before exposure to endometrial GECs. Supernatants collected 24 h post treatment showed that gp120 neutralization reduced induction of IFNβ to baseline levels (Figures 1b–d). All together, these results indicate that HIV-1 gp120 is directly responsible for induction of an IFNβ response in endometrial GECs.
Figure 1.
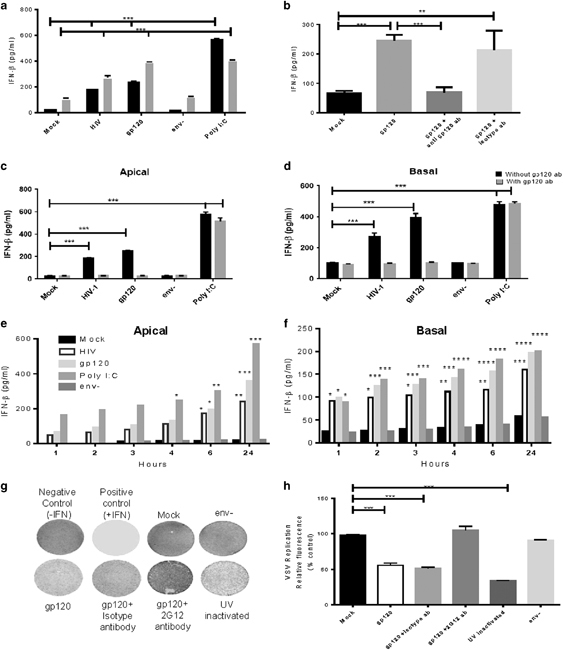
Primary endometrial epithelial cells respond to HIV-1 envelope gp120 by production of IFNβ. (a) Confluent monolayers of primary endometrial GECs were exposed to medium (mock), HIV (105 IU/well), gp120 (100 ng/ml), env − HIV mutant (105 IU/well) or UV-inactivated HIV for 24 h and supernatants were analyzed for IFNβ production. IFNβ was measured by ELISA in both apical (black bars) and basolateral (gray) supernatants. Endometrial GECs produce significant amounts of IFNβ in response to HIV, gp120 and poly I:C when compared with mock-treated cells. N=4. (b) Endometrial GECs were exposed to gp120 alone or with anti-gp120 neutralizing (2G12) or isotype antibody for 24 h, apical supernatants were collected and analyzed for IFNβ production by ELISA. N=2. (c, d) Endometrial GECs were exposed for 24 h to medium (mock), HIV, gp120, env − HIV mutant or poly I:C, with or without anti gp120 neutralizing antibody. IFNβ production in supernatants was determined without gp120 antibody (black bars) or following treatment with anti gp120 antibody (gray bars). (e, f) Time course for IFNβ production was analyzed in supernatants collected from endometrial GECS after exposure to medium (mock), HIV, gp120, poly I:C, env − HIV mutant for 1, 2, 3, 4, 6 and 24 h. IFNβ production in apical (c) and basal (d) supernatants was measured by ELISA. (g) Endometrial GECs were exposed to mock or env − mutant or gp120 alone or in the presence of either isotype antibody or gp120 neutralizing antibody or UV-inactivated HIV-1 for 24 h. Supernatants were tested by VSV-GFP assay on Vero cells to determine biological activity of type I IFNs. VSV-GFP was added to Vero cells without a pretreatment (negative control), recombinant IFNβ (100 pg/ml) added to Vero cells before VSV infection (positive control) or supernatants collected from treatments done in above experiments. GFP fluorescence, an indicator of VSV-GFP infection, was quantified by scanning the plates on a Typhoon Biomolecular imager. Images are representative of three separate experiments conducted in triplicate. (h) Quantitative representation of VSV-GFP fluorescence. Each experimental condition was compared with negative control and calculated as percent of negative control. Data shown represent mean±s.e.m. of three separate experiments from cells isolated from three individual tissues. *P<0.05, **P<0.01 and ***P<0.001. ELISA, enzyme-linked immunosorbent assay; GEC, genital epithelial cell; GFP, green fluorescent protein; IFNβ, interferon-β; poly I:C, polyinosinic:polycytidylic acid; UV, ultraviolet; VSV, vesicular stomatitis virus.
Next, we examined the time kinetics of induction of IFNβ in response to HIV-1 and gp120. Poly I:C was used as a positive control. Endometrial GECs were exposed to different treatments and supernatants were collected hourly for 1–6 h and at 24 h. IFNβ was significantly induced in apical supernatants at 6 h time point in response to HIV-1 and gp120, but significantly higher induction was observed in basolateral supernatants at earlier time points (Figures 1e and f).
In order to examine biological activity of gp120-mediated IFN, we used a VSV-GFP assay that assesses the presence of biologically active IFN.5,29 Using this assay, we measured the ability of VSV-GFP to replicate in Vero cells, in the presence of supernatants from UV-inactivated HIV-1 or gp120-treated endometrial GEC cultures. The presence of biologically active type I IFN was confirmed in supernatants from UV-inactivated HIV-1 and gp120-treated GEC cultures by the significant decrease in replication of VSV-GFP (Figures 1g and h). Neutralization of recombinant gp120 with neutralizing antibodies completely abrogated type I IFN-mediated protection against VSV-GFP replication, whereas addition of isotype antibody did not influence the induction of biologically active type I IFN production (Figures 1g and h).
Antiviral responses induced in primary endocervical cells by HIV-1
To examine whether HIV-1 also induced antiviral responses in primary endocervical epithelial cells, similar to endometrial epithelial cells, we exposed primary endocervical epithelial cultures to medium (mock) or HIV-1 (105 IU/well) for 24 h and measured TER as a measure of epithelial barrier function tumor necrosis factor-α (TNFα) and IFNβ production. Similar to the previously reported effect of HIV-1 in endometrial epithelial cells, HIV-1 exposure impaired the barrier function in endocervical epithelial cells and induced significant production of TNFα and IFNβ, although induction of IFNβ appeared to be less potent compared with endometrial GECs (Supplementary Figures 1a–d). As the effects of HIV-1 on barrier disruption, TNFα and IFNβ production were analogous in primary endometrial and endocervical epithelial cells, further experiments were conducted on primary endometrial epithelial cells that were more abundantly available and because the larger amount of tissue provided bigger cultures.
Role of IFNβ in HIV-mediated barrier impairment
Previously, we reported that exposure to HIV in endometrial and cervical epithelial cells resulted in impairment of epithelial barrier primarily because of the upregulation of inflammatory cytokines, particularly TNFα46. However, the effect of IFNβ on gp120-mediated barrier disruption has not been examined. Therefore, we next examined the role of IFNβ in HIV-1 gp120-mediated barrier disruption. Confluent monolayers of endometrial GECs were treated with medium (mock) or HIV-1 in the presence or absence of human anti-IFNβ antibody, human anti-TNFα antibody or isotype antibody. At 24 h post treatment TER was measured and compared with baseline values. As expected, HIV-1 significantly decreased the TER, indicating barrier disruption, but the presence of TNFα neutralizing antibodies ameliorated the HIV-mediated barrier disruption, whereas addition of anti-IFNβ neutralizing antibody significantly exacerbated the HIV-mediated barrier disruption (Figure 2a). Supernatants collected from these cultures confirmed the protective role of IFNβ, as neutralization of IFNβ resulted in upregulation of TNFα (Figure 2b) that correlated with increase in barrier impairment compared with effect of HIV-1 alone. Neutralization of TNFα resulted in upregulation of IFNβ production that correlated with enhanced protection of barrier (Figure 2c). These studies indicate that IFNβ produced by GECs opposes the effect of TNFα and plays a protective role on HIV-1-mediated barrier disruption.
Figure 2.
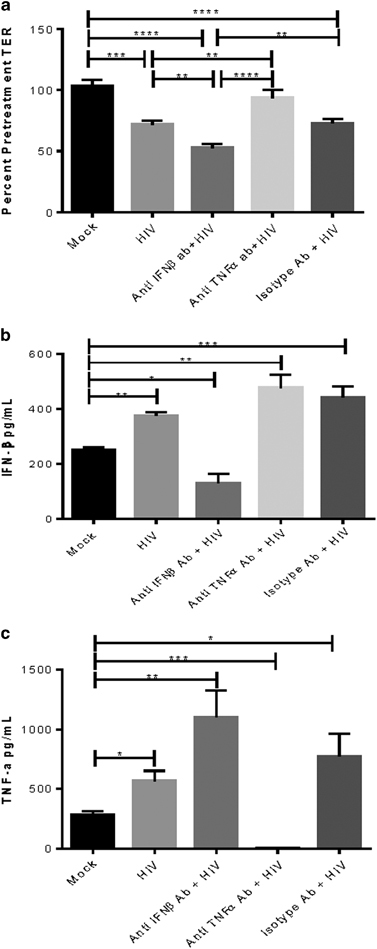
Role of IFNβ in HIV-mediated barrier impairment. Confluent monolayers of primary endometrial GECs were exposed to medium (mock) and HIV-1 (105 IU/well) in the absence or presence of human anti-IFNβ antibody, anti-TNFα antibody or isotype control antibody. (a) Epithelial barrier function was determined by measurement of TER at 24 h post treatment and shown as ratio of pretreatment values. Supernatants were collected after 24 h of treatments and analyzed for (b) TNFα and (c) IFNβ by ELISA. Data shown are representative of two separate experiments done on tissues taken from two different donors and show mean±s.e.m. of treatments run in duplicate. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. ELISA, enzyme-linked immunosorbent assay; GEC, genital epithelial cell; IFNβ, interferon-β; TER, transepithelial resistance; TNFα, tumor necrosis factor-α.
Induction of IFNβ by HIV-1 gp120 is mediated through IRF3 activation
Induction of IFNβ is typically mediated through activation of transcription factor, IRF3. Therefore, we next examined the involvement of IRF3 in IFNβ production by GECs in response to HIV-1. GEC monolayers were exposed to HIV-1 and gp120 for different lengths of time and fixed and stained for IRF3. Nuclear translocation of IRF3 was observed at 1 h post exposure to HIV-1 or gp120. Exposure to gp120 in the presence of anti-gp120 antibodies (2G12) inhibited IRF3 translocation (Figures 3a and b). Peak translocation of IRF3 was observed at 2 h post exposure with HIV-1 or gp120 (Figure 3c). The TLR3 ligand poly I:C, which is known to activate IRF3, was used as a positive control. These results indicate that activation and translocation of IRF3 is a direct consequence of exposure to HIV-1 viral envelope protein, gp120.
Figure 3.
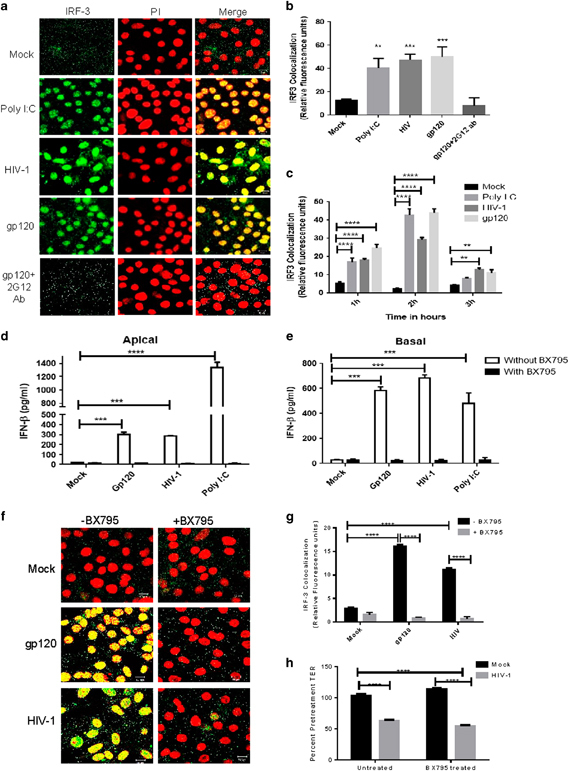
Induction of IFNβ in endometrial GECs by HIV-1 gp120 is mediated through IRF3. Endometrial GECs were exposed to medium or poly I:C, HIV-1 (105 IU/well) or gp120 (100 ng/ml alone or with anti-gp120 neutralizing antibody) for 1–3 h. Cells were fixed and stained for the IRF3 (green fluorescence). Nuclear counterstaining (red fluorescence) was achieved using PI. Images were captured by a laser-scanning confocal microscopy. (a) Representative images are shown at 2 h time point from one of three separate experiments. Magnification × 1260. (b) IRF3 translocation and nuclear colocalization was measured by Image J software and presented as relative light units. (c) Time kinetics of IRF3 colocalization following treatment of endometrial GECs with medium or poly I:C (positive control), HIV-1 or gp120. (d, e) Endometrial GECs were incubated with the IRF3 inhibitor, BX795 (1 μM) for 1 h, before exposure with gp120, HIV-1 or poly I:C (positive control). Supernatants were collected after 24 h and assayed by ELISA. Results showed IFNβ production in apical (d) and basolateral supernatants (e). (f) Endometrial GECs were treated with BX795 for 1 h before gp120 or HIV-1 exposure for 2 h. The cells were fixed and stained for IRF3 and nuclei. Images were captured by laser-scanning confocal microscopy. Magnification: × 1260. (g) Colocalization was measured by image J software and represented in a bar diagram. h Endometrial GECs were preincubated with BX795 or media (mock) for 1 h and TERs were measured pretreatment and after 24 h of treatment with mock or HIV-1 to check whether BX795 was affecting HIV-1-mediated barrier disruption. Images are representatives of three separate experiments from cells isolated from three individual tissues. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. ELISA, enzyme-linked immunosorbent assay; GEC, genital epithelial cell; IFNβ, interferon-β; IRF3, interferon regulatory factor 3; PI, propidium iodide; poly I:C, polyinosinic:polycytidylic acid; TER, transepithelial resistance.
HIV-1 or gp120-mediated IFNβ production is blocked by inhibition of IRF3
As IFNβ can be induced through transcription factors other than IRF3, we next examined the contribution of IRF3 pathway towards gp120-mediated induction of IFNβ by inhibiting IRF3. BX795, a specific inhibitor of TBK-1/ IKKɛ phosphorylation, an upstream event critical in activation of IRF3, was used for inhibiting the IRF3 pathway.30 GEC monolayers were pretreated with medium or BX795 for 1 h before exposure with gp120 for 24 h. Supernatants were collected and analyzed for IFNβ production by ELISA. BX795 almost completely inhibited gp120-mediated induction of IFNβ production (Figures 3d and e), indicating that activation of IRF3 was necessary for the induction of IFNβ by gp120. Interestingly, whereas IFNβ was almost completely inhibited by BX795, the levels of proinflammatory cytokines, including TNF-α and IL-1α, were increased in the presence of BX795, a likely effect of cross-regulation between IFNβ and pro-inflammatory cytokine pathways31 (Supplementary Figure 2).
To confirm that BX795 was inhibiting IRF3 activation, endometrial GEC monolayers were stained for IRF3 after treatment with and without BX795 before HIV-1 and gp120 treatment. Confocal microscopy results revealed that BX795 inhibited HIV-mediated and gp120-mediated IRF3 activation and nuclear translocation (Figures 3f and g). Treatment with BX795 in mock and HIV-1-treated cultures did not have any effect on the GEC viability, as seen by TER (Figure 3h), a measurement of epithelial tight junctions used as indicators of the barrier function and overall integrity of monolayer. As expected, HIV-1 treatment decreased TERs significantly compared with mock treatment and the presence of BX795 exacerbated this effect (Figure 3h).
Previous studies have shown that in addition to IRF transcription factors (IRF3/7), NF-κB may also play a critical role in IFNβ expression.32. Previously, we have shown that gp120 treatment leads to NF-κB translocation in GECs and blocking it with pyrrolidine dithiocarbamate inhibits production of inflammatory cytokines including TNFα.19 When GEC monolayers were treated with pyrrolidine dithiocarbamate, before treatment with gp120, there was a small but significant decrease in IFNβ produced by GECs in response to gp120 that indicated that NF-κB likely enhanced the gp120-mediated IFNβ production (Supplementary Figure 3a). However, blocking NF-κB with pyrrolidine dithiocarbamate did not have any effect on IRF3 translocation (Supplementary Figure 3b). These results indicate that IRF3 activation is the primary pathway for IFNβ induction in the GECs in response to gp120, but NF-κB may also play a minor role in enhancing IFNβ production.
HIV-1 gp120-mediated IRF3 activation and IFNβ induction occurs via TLR2 signaling pathway
Previously, we showed that HIV-1 can induce proinflammatory cytokines by signaling through TLR2 and TLR4.19 Therefore, we next examined whether TLR2 and 4 played any role in IFNβ induction. TLR5 was included as a control in these experiments, as these three TLRs are primarily responsible for recognizing pathogen-associated glycoproteins at the surface of cells and inducing intracellular signaling.33 TLRs 1 and 6 are also associated with cell-surface protein recognition, but act in association with TLR2. We first determined whether TLRs 2, 4 or 5 were associated with gp120-mediated induction of IFNβ. GECs were pretreated with neutralizing antibodies against TLR2, TLR4 and TLR5 before gp120 exposure. Supernatants were collected and analyzed by IFNβ ELISA. IFNβ production was completely blocked in the presence of only TLR2 antibody (Figure 4a). In contrast, pretreatment of GECs with isotype control, TLR4 or TLR5 neutralizing antibody did not have any effect on gp120-mediated IFNβ production (Figure 4a). Pam3CSK4 (synthetic triacylated lipopeptide), a TLR2 ligand, and FimH (Fimbriae protein), a TLR4 ligand, that are known to induce these pathways in GECs were used as controls to verify the specificity of the antibodies.34,35
Figure 4.
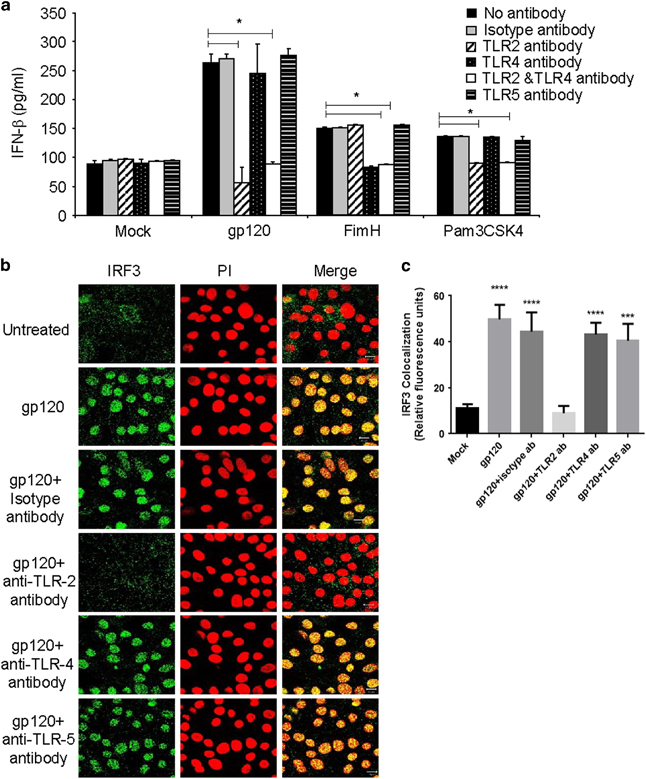
Neutralization of TLR2 blocks gp120-mediated IFNβ production and IRF3 activation. (a) Endometrial GECs were pretreated with neutralizing antibodies against TLR2, TLR4, TLR5 or isotype control antibodies (all at 10 μg/ml) before exposure to gp120 (100 ng/ml) or mock treatment (media). FimH and Pam3CSK4 were used as positive controls for activation of TLR4 and TLR2, respectively. Supernatants were collected after 24 h and analyzed by ELISA for IFNβ production. Data shown are mean+s.d. and representative of three separate experiments done on cells isolated from three different tissues. (b) Epithelial monolayers were fixed after 2 h of exposure of gp120 with and without pretreatment with neutralizing antibodies against TLR2, TLR4, TLR5 or isotype control antibody and stained for IRF3. Propidium iodide was used to stain nuclei. Images were captures by a laser-scanning confocal microscopy. Magnification × 1260. Images are representative of one of three separate experiments done on cells isolated from three different tissues. (c) Quantitation of IRF3 colocalization were done by Image J software and presented in the graph. Significance was calculated by one-way ANOVA and IRF3 colocalization in all treatments were compared with mock treatment. *P<0.05, ***P<0.001 and ****P<0.0001. ANOVA, analysis of variance; ELISA, enzyme-linked immunosorbent assay; GEC, genital epithelial cell; IFNβ, interferon-β; IRF3, interferon regulatory factor 3; TLR, Toll-like receptor.
HIV-1 gp120-mediated IRF3 translocation is blocked in the presence of TLR2 antibody
As the induction of IFNβ production in GECs was seen to be mediated through TLR2 pathways, we next determine whether neutralizing antibodies to TLR2 abrogated gp120-mediated activation and nuclear translocation of IRF3. TLR4 and TLR5 antibodies were used as controls to confirm the specificity of the interaction. GEC monolayers were incubated with antibodies against TLR2, TLR4, TLR5 or isotype control for 1 h before treatment with gp120. After 1 h of gp120 exposure, cells were fixed and stained for IRF3. IRF3 nuclear translocation was found to be blocked following gp120 treatment in the presence of TLR2 antibody, but not TLR4, TLR5 or isotype antibodies (Figures 4b and c). These results confirm that gp120-mediated IFNβ production and IRF3 activation was mediated via TLR2 signaling pathway.
Gp120 can induce IFNβ and ISRE genes through TLR2
Our previous results had shown that gp120 activates proinflammatory cytokines and NF-κB pathway via both TLR2 and TLR4, yet experiments in primary endometrial GEC monolayers indicated that HIV-1 gp120 induced IFNβ via TLR2 pathway alone. Therefore, to confirm the primary epithelial cell results, we used a cell line-based expression system in the kidney embryonic cell line, HEK293, that has been used extensively for testing TLR functioning.36,37 HEK293 cells were either transfected with an IFNβ-luciferase or ISRE-luciferase reporter plasmid and a TLR2 or TLR4 expression plasmid and subsequently stimulated with gp120 or with known TLR ligands. TLR4 plasmid was also cotransfected with MD2 and CD14 plasmids as these proteins are required for LPS-mediated TLR4 signaling.38 Gp120 activated IFNβ and ISRE expression significantly only in TLR2-transfected cells compared with mock treatment as measured by luciferase activity (Figures 5a, b, e and f), whereas there was no induction of luciferase activity by gp120 in cells transfected by TLR4 expression plasmid with and without MD2 and CD14 (Figures 5c, d, g and h). The absence of gp120 in env − mutant treatment failed to activate IFNβ and ISRE expression in both TLR2 and TLR4 cotransfected cells, confirming the specific role of gp120 in activation of IFNβ through TLR2 pathway.
Figure 5.
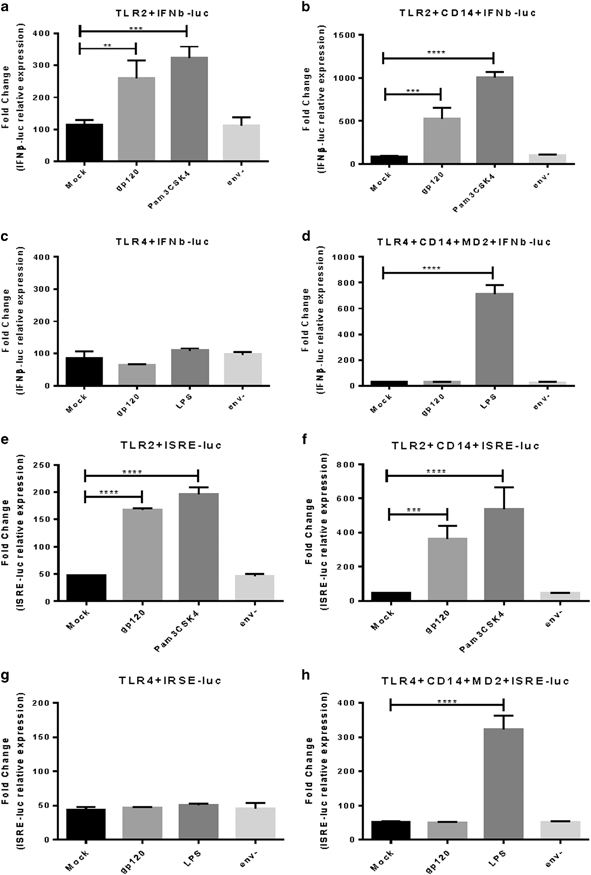
Gp120 induces IFNβ and ISRE through activation of TLR2. HEK293 cells were transiently transfected with either IFNβ-luciferase (a–d) or ISRE-luciferase (e–h) reporter in combination with TLR2 (a, b, e, f) or TLR4 (c, d, g, h) expression plasmid alone or in combination with CD14 and or MD2 expression plasmid. At 24h after transfection, cells were stimulated with gp120 (100 ng/ml), or env − mutant (105 IU/well) or 10 μg/ml Pam3CSK4 (positive control for TLR2), or (0.1 mg LPS (positive control for TLR4) or with medium (mock, negative control). Cells were disrupted, and luciferase activity was measured 16 h after stimulation and normalized to β-gal-luciferase activity. Data shown are representative of three individual experiments, each performed in triplicate. Data are represented as mean±s.d. **P<0.01, ***P<0.001 and ****P<0.0001. β-gal, β-galactosidase; GEC, genital epithelial cell; IFNβ, interferon-β; IRF3, interferon regulatory factor 3; ISRE, interferon-stimulated response element; LPS, lipopolysaccharide; TLR, Toll-like receptor.
Cell surface heparan sulfate is required for gp120-mediated IFNβ production
Previously, we have shown that HIV-1 gp120-mediated induction of inflammatory response was activated through TLR2 and TLR4 pathway and required presence of heparan sulfate on cell surface.19 Therefore, we next examined whether heparan sulfate was also required for the induction of IFNβ by gp120. Confluent GEC monolayers were first treated with heparinase III to remove heparan sulfate from the cell surface or exogenous heparan sulfate was added before treating cells with HIV-1 gp120 or poly I:C, a TLR3 ligand that does not require presence of heparan sulfate to activate the TLR3 pathway and leads to induction of IFNβ. GEC cultures that were treated with media instead of gp120 acted as negative controls. The results indicated that gp120 required the presence of heparan sulfate on the cell surface in order to induce IFNβ production as removal of heparan sulfate by heparinase III decreased the IFNβ production in GECs to baseline (Figures 6a and b). Cells treated with heparinase III followed by addition of exogenous heparan sulfate restored induction of IFNβ production in response to gp120, confirming that heparan sulfate is required for gp120 to initiate the IFNβ signaling pathway.
Figure 6.
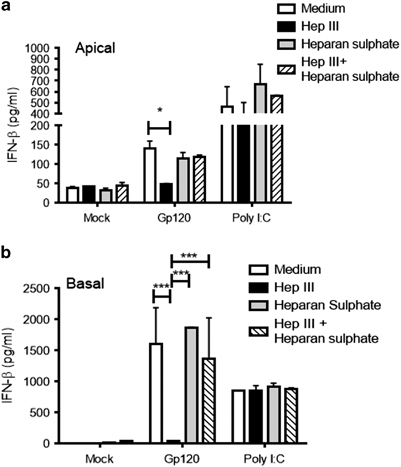
Cell surface heparan sulfate is necessary for gp120-mediated induction of IFNβ. Confluent endometrial epithelial monolayers were pretreated with heparinase III for 1 h before exposure with medium (mock), gp120 (100 ng/ml) or treated with heparan sulfate in the presence of gp120 treatment. Apical (a) and basolateral (b) supernatant were collected and analyzed for IFNβ production. Data are representative of three experiments from three different tissues, each performed in triplicate. Data are represented as mean±s.d. *P<0.05, ***P<0.001. IFNβ, interferon-β.
Gp120-mediated IFNβ responses by endometrial GECs reduces HIV-1 infection
Thus far, our results indicated that HIV-1 gp120 was inducing upregulation of IFNβ from GECs from upper genital tract via TLR2 pathway through activation of IRF3. However, we have previously reported that gp120 also activates the production of proinflammatory cytokines via the NF-κB pathway through TLR2 and TLR4 activation.19 As the proinflammatory cytokines are known to enhance HIV-1 infection and replication whereas the IFNβ pathway has antiviral effects, we next examined the functional significance of IFNβ production in response to HIV-1 gp120 with respect to HIV-1 infection and replication. We tested the effect of IFNβ produced by GECs on HIV-1 infection by examining the effect of supernatants collected from primary female GECs on HIV-1 infection of TZM-bl indicator cells. Supernatants collected from mock and gp120-exposed GECs were added to pre-seeded TZM-bl cells before HIV-1 infection and rate of infection was measured by luciferase activity. The recombinant human IFNβ (100 pg/ml; n=6) was used as a positive control. The results showed that adding recombinant IFNβ and supernatants collected from GEC exposed to gp120 significantly reduced luciferase activity, an indicator of decreased HIV-1 infection in TZM-bl cells because of antiviral activity (Figure 7a). To confirm that the decreased infection was due to IFNβ present in epithelial supernatants, TZM-bl cells were treated with supernatants from GECs exposed to gp120 in the presence or absence of IFNβ antibody or isotype control before infection with HIV-1. Neutralizing IFNβ with anti-IFNβ antibody in both recombinant IFNβ and gp120-exposed GEC supernatant resulted in loss of antiviral activity, resulting in increased infection similar to that seen in controls where TZM-bl cells were directly infected with HIV-1 (Figure 7a). Mock-infected GECs did not show any significant antiviral activity and treatment with isotype control antibody instead of anti-IFNβ antibody did not have any effect on luciferase activity.
Figure 7.
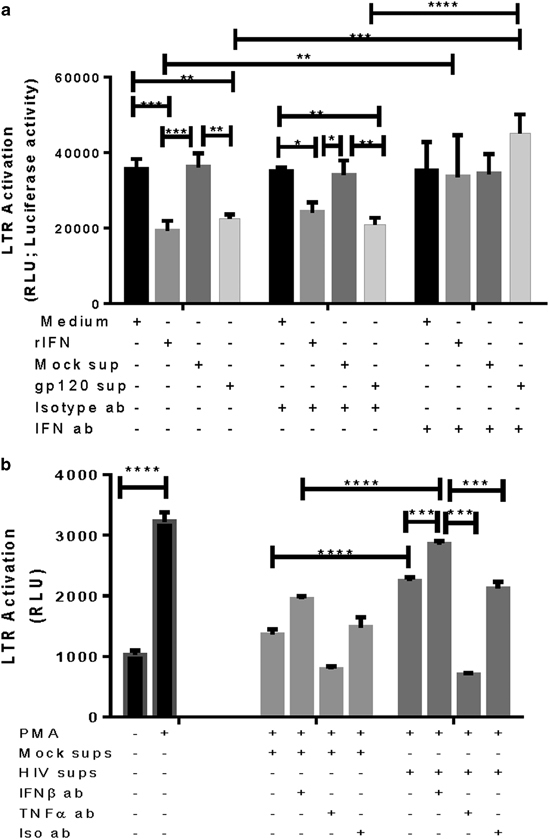
HIV-mediated IFNβ production in endometrial GEC supernatants decreases HIV-1 infection and HIV replication. (a) Supernatants were collected from GECs exposed to medium (mock) or gp120, added either alone or in combination with anti-IFNβ antibody or isotype antibody to TZM-bl indicator cells 1 h before infection with HIV-1. (b) 1G5 cells were activated for 24 h with PMA and GEC supernatants were added in the presence of either anti-IFNβ or anti-TNFα or isotype antibody individually. After 24 h of treatment, cells were lysed and luciferase activity was measured. Data are representative of three separate experiments done on three different tissues, run in duplicate. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. GEC, genital epithelial cell; IFNβ, interferon-β; PMA, phorbol 12-myristate 13-acetate; TNFα, tumor necrosis factor-α.
These results show that IFNβ produced by epithelial cells from the upper genital tract in response to HIV-1 exposure can exert an antiviral effect by decreasing HIV-1 infection.
Gp120-mediated IFNβ responses by endometrial GECs reduces HIV-1 replication
In addition to measuring the effect of HIV-1-induced IFNβ on HIV-1 infection we also analyzed the effect of IFNβ on HIV-1 replication. To test the ability of epithelial supernatants containing IFNβ to affect infection and replication in T cells, we utilized a 1G5 HIV-1 LTR assay that has previously been used as a proxy assay for measuring HIV-1 replication.39
1G5 Jurkat T cells containing stably transfected HIV-LTR-luciferase were activated by addition of PMA that drives HIV-1 LTR. To measure effect of epithelial supernatants containing IFNβ on HIV-1 LTR activation, supernatants from gp120 or mock-treated GEC cultures were treated with anti-IFNβ or anti-TNFα or isotype control antibody before addition to 1G5 cells activated by PMA. HIV-1 LTR activation was increased in 1G5 cells incubated with supernatants from gp120-treated cultures compared with mock treatment, as expected. Furthermore, HIV-1 LTR activation was significantly enhanced in the presence of anti-IFNβ antibody and reduced to background level in the presence of anti-TNFα antibody, whereas isotype antibody did not have any effect on HIV-1 LTR activity (Figure 7b). These results indicate that IFNβ present in epithelial cells treated with gp120 has an effect on decreasing HIV-1 LTR activation, a proxy for HIV-1 replication, in T cells.
HIV-1 exposure of GECs induces interferon stimulatory gene expression
As IFNβ produced by the GECs in response to HIV-1 was able to create an antiviral restrictive state, likely through the induction of various ISGs, we therefore next sought to determine the gene expression profiles of GECs exposed to HIV-1 by human genome ST 2.0 array. Microarray data analysis showed that GECs induced various ISGs in response to HIV-1 at 48 h (Table 2). Of the various ISGs reported in Table 2, ISG15 was upregulated by 2.75-fold by microarray and when validated by real-time PCR (qPCR) it was up by 7.80-fold in HIV-1-exposed GECs. Other ISGs were upregulated from 2.29- to 4.12-fold when compared with mock controls by microarray. Gene expression pattern observed in microarray analysis was further validated by qPCR and showed similar pattern as the microarray data (Table 2).
Table 2.
HIV-1 mediated upregulation of different interferon-stimulated genes (ISGs) in primary genital epithelial cells (GECs)
| Name | Gene | Microarray (fold changes a) | Real-time PCR (fold changes a) | Regulation |
|---|---|---|---|---|
| MX1 | Myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 | 2.60 | 5.44 | Up |
| ISG15 | Interferon-stimulated gene 15 | 2.75 | 7.80 | Up |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 | 3.82 | 8.37 | Up |
| IFIT3 | Interferon-induced protein with tetratricopeptide repeats 3 | 2.59 | 2.87 | Up |
| IFI44L | Interferon-induced protein 44-like | 2.29 | 5.57 | Up |
| RSAD2 | Radical S-adenosylmethionine domain containing 2 | 2.82 | 7.95 | Up |
| OAS1 | 2'-5'-Oligoadenylate synthetase 1 | 2.80 | 3.47 | Up |
| OAS2 | 2'-5'-Oligoadenylate synthetase 1 | 4.12 | 23.82 | Up |
| OAS3 | 2'-5'-Oligoadenylate synthetase 3 | 2.39 | 10.80 | Up |
| BST2 | Bone marrow stromal cell antigen 2 | 2.46 | 6.90 | Up |
aFold change values in HIV-1-exposed GECs compared with unexposed control.
Discussion
The genital epithelial cells are the first sensors and responders in the reproductive tract mucosa.3 They are the gatekeepers that determine whether immune responses will be initiated against a certain antigen and, if so, the magnitude and quality of the response. These unique abilities are conferred on the GECs, to a large extent, by the expression of a full range of pattern recognition receptors, including TLRs.1 Previous studies, including ours, have shown that activation of GECs by TLR ligands, for example, TLR3 activation by poly I:C, can induce production of both inflammatory cytokines through NF-κB pathways as well as IFNβ through IRF3 pathway.5,6 Our current findings demonstrate that GECs from endometrium and cervix were also activated by HIV-1 gp120 to induce IFNβ, although the induction appeared to be less potent in endocervix. The IFNβ was shown to be biologically active and capable of decreasing both HIV infection and replication. Most interestingly, the IFNβ had a protective effect on the tight junction barrier and was seen to counteract the disruptive effect of TNF-α also induced by HIV-1 (Figure 2). The induction was dependent upon recognition of envelope glycoprotein, as no increase in IFNβ production was seen in the absence of gp120 (env − mutant) or following antibody mediated neutralization of gp120. We also showed that TLR2 was responsible for recognition of gp120, as only neutralization of TLR2, but not TLR4 or TLR5, both of which are also expressed on cell surface and can bind microbial glycoproteins, resulted in abrogation of IFNβ production by GECs. Finally, we demonstrated the downstream activation of ISGs that has been shown to have a broad range of antiviral effects on the mucosal surfaces.
Although a number of other studies have shown the activation of type I IFN by HIV-1 in immune cells, particularly pDCs, to the best of our knowledge, this is the first report of activation of IFNβ pathway in epithelial cells by HIV-1 envelope glycoprotein.9,8 Previous studies have shown robust production of IFNα by pDCs in response to HIV-1.15 This interaction was shown to require two distinct interactions between the virus and cell. The first was between viral envelope and CD4 that led to endocytosis of HIV-1 and the second was the recognition of viral RNA delivered by the endosome and consequent activation of a cytoplasmic TLR pathway leading to production of IFNα. Monocyte-derived dendritic cells, on the other hand, had poor recognition and response to HIV-1, producing low levels of IFN and failing to produce proinflammatory cytokines.40 Other immune cells including macrophages also have poor IFN responses to HIV-1 despite sensing HIV-1, likely because of viral immune evasion mechanisms.41 The present study shows that unlike pDCs, activation of IFNβ in GECs was mediated through cell surface TLR2 and the responses in the primary epithelial cultures were quite robust, as seen by the ability of supernatants to significantly decrease HIV-1 infection in an indicator cell line HIV-1 LTR activation in 1G5 T cells and viral replication in chronically HIV-1-infected T cells. Similar to pDCs, the activation took place in the absence of infection, as previous studies have shown that upper GECs do not productively get infected by HIV-1.17
In addition to TLR-mediated activation of type I IFN pathway, we have also previously reported that gp120 leads to induction of proinflammatory cytokines, including TNFα, interleukin (IL)-8, IL-6 and IL-1α.18 TNFα, in particular, was associated with disruption of tight junctions and consequent impairment of mucosal epithelial barrier. The disruption of mucosal barriers during HIV-1 infection has been implicated in microbial translocation and immune activation. Indeed, in our studies, bacteria were observed to translocate across disrupted epithelial monolayers following exposure to HIV-1 gp120. The findings from the present study indicate that in addition to proinflammatory cytokines, the HIV envelope glycoprotein also induces IFNβ, a potent innate antiviral factor. The supernatants from upper genital tract epithelial cells activated by gp120 exerted a significant inhibitory effect on both HIV-1 infection and replication in experiments conducted to specifically assess these outcomes (Figures 7), even in the presence of proinflammatory factors. Thus, the overall net outcome appears to favor an antiviral environment, as related to HIV infection and replication. This is supported by the low rates of transmission seen during heterosexual transmission showing that innate defense mechanisms in FRT under normal conditions are quite robust. However, pre-existing inflammation and/or increased number of target T cells could easily alter this equation. We recently combined mathematical modeling with an in vivo humanized mouse model to demonstrate that the number of target cells present in the reproductive tract are the predominant determinant in the outcome of exposure.42 Viral load was also an important criterion. Ultimately, the outcome of exposure may be decided by a number of factors in the microenvironment including number of target cells, viral load, inflammation, hormone environment and so on. Where antiviral innate immune responses fit into this equation remains to be determined.
We also examined what effect the IFNβ had on barrier functions of the epithelial cells. A number of studies have shown a mixed role for type I IFNs in HIV-1 infection, acting as a potent inhibitor for viral replication, but at the same time playing a supporting role in immune activation and pathogenesis.9,8 Our results show that IFNβ produced by GECs from the upper genital tract opposes the effects of TNFα and acts to protect the epithelial tight junction barrier from the disruptive effects of HIV-1 (Figure 2). A number of previous studies, including experimental and clinical ones done mostly in the context of inflammatory diseases, have shown that TNFα and IFNβ cross-regulate each other.31 Whether IFNβ has any direct effect on GEC tight junction barrier is currently being examined. Also, whether other proinflammatory cytokines such as IL-6 and IL-1α play any role in this system and whether IFNβ modulates expression of these cytokines is not currently known. Further studies are needed to examine these complex interactions in vivo.
One surprising observation in the current study was that IFNβ was activated exclusively through TLR2, even though our previous studies show that gp120 binds to both TLR2 and TLR4 and proinflammatory cytokines are activated through both TLRs.19 Although TLR2 is known primarily for recognition of bacterial cell wall component, a number of studies have also reported that it can play a role in innate immune response to viruses.43,44 Viral glycoproteins from vaccinia, measles and gamma herpes virus have been shown to elicit TLR2-dependent type I IFN responses, primarily in cells of myeloid lineage.43 The intracellular pathways involved in TLR2-mediated induction of type I IFN are not completely clear, although it appears that endosomal localization might be involved following ligand binding.44 To the best of our knowledge this is the first report of TLR2-mediated activation of IFNβ in epithelial cells. Further work is needed to elucidate the detailed intracellular mechanism involved in this pathway. It is interesting to note that the overall IFNβ levels were 2–3-fold higher in the basolateral compartment of the GEC cultures compared with apical side, although there was no difference in the biological activity. As HIV-1 target cells are located primarily in the lamina propria of the genital tract underneath the epithelium, an innate IFN-mediated immune response induced by GECs would be beneficial for creating an antiviral milieu in the genital tract.1 The IFNβ secreted from the basolateral side of GECs could lead to induction of ISGs in immune cells such as macrophages, DCs and T cells, making the likelihood of successful HIV-1 infection more unlikely. This inference is supported by the fact that the probability of HIV-1 transmission in the female genital tract is relatively low (1:200–1:2000 per coital act).2 Indeed, in our studies we found that IFN-β production by GECs results in downstream signaling in the epithelial cells themselves, inducing various ISGs such as MX1, OAS1, OAS2, OAS3, ISG15 and RSAD2 as determined by DNA microarrays and confirmed by qPCR (Table 2). The ISGs reported herein has been shown to exhibit antiviral activity exerted through different mechanisms.45 Given the antiviral properties of these ISGs, it is likely that an IFN- mediated innate antiviral response mounted by GECs is beneficial in initial virus clearance as well as mucosal protection. However, the exact role of these ISGs in GECs is not known. Further in vivo studies would be needed to assess the relative contribution of GECs in mounting an effective antiviral response that decreases susceptibility to HIV-1. If this plays a significant role, it could be exploited for antiviral prophylaxis.
Although our studies provide evidence for the first time that HIV-1 gp120 can induce type I IFN pathway in upper genital epithelial cells, the in vivo significance of these studies needs to be confirmed in clinical studies. Our primary epithelial cells are isolated from endocervix and endometrial hysterectomy tissues and demonstrate the induction of IFNβ by HIV gp120 in upper genital tract epithelium. Whether this pathway is also activated in the vaginal epithelium, which is the site of primary exposure from infected semen, needs to be further examined. Whether viral or gp120 concentrations reach sufficient levels in the upper genital tract to initiate this type of response remains to be proved, although there is growing evidence that sufficient amounts of virus does reach the upper genital tract to initiate infection.46 Non-human primate studies have shown that following intravaginal inoculation with SIV, endocervix is the preferred site for initiation of infection in macaques.47 More recent studies have shown simian immunodeficiency virus (SIV) infection in ovaries and fallopian tubes of macaques, following intravaginal inoculation.48 The amount of virus present in seminal plasma has been shown to vary widely, ranging from 102 to 107 copies/ml, based on stage of infection and antiretroviral treatment, with most studies reporting a median range of 3–4 log 10 copies/ml.49,50,51,52 However, based on these concentrations in semen, it is difficult to estimate per cell exposure in vivo. Finally, the evidence regarding the amount of gp120 in body fluids is controversial and remains unresolved. The assumption that gp120 should equate to viral counts is simplistic, given that gp120 can be present not only on the virions but also in soluble form secreted by infected cells.53 One study measured much higher concentration of gp120 in tissues such as lymph nodes and spleen (>300 ng/ml) in the absence of plasma viral load than previously estimated.54 However, others have estimated that the range of gp120 in serum of an HIV-1-infected individual is likely between 500 ng/ml and 5 μg/ml when concentrations of soluble gp120, cell-associated gp120 and virion-associated gp120 are added.55 Given the above caveats, the studies summarized here demonstrate mechanistic feasibility and more evidence needs to be gathered from clinical studies to validate their in vivo relevance. A recent study showed that type I IFN-related antiviral factors (APOBEG-3G, TRIM5-a, SAMDH-1, STING, TBk1) were upregulated in the oral epithelium of exposed seronegative individuals, indicating a role for type I IFN-mediated protection against HIV-1 infection in oral mucosa.56 More clinical studies are needed to examine this association in other mucosal tracts.
In summary, we have provided novel experimental evidence that HIV-1 envelope glycoprotein is recognized by TLR2 expressed on primary human epithelial cells of the female upper genital tract, leading to activation of an innate anti-viral IFN response that is biologically relevant, acts to protect the mucosal barrier in the genital tract and can significantly decrease HIV-1 infection and replication.
Electronic supplementary material
Acknowledgements
We thank the Pre-Op Clinic Staff for their assistance in obtaining informed consents and Clinical Pathology Staff at Hamilton Health Sciences Centre for their assistance in providing genital tract tissues. We thank the women who donated their tissues for this study. We are also thankful for the reagents obtained from NIH AIDS Research and Reference Reagent program, Division of AIDS, NIAID, NIH: TZM-bl from Dr John C Kappes, Dr Xiaoyun Wu and Tranzyme Inc. This research was supported by grants from the Canadian Institutes of Health Research (CIHR Operating Grant FRN 126019 (to CK); CIHR Team Grant on Mucosal Immunology of HIV Vaccine Development FRN 138657 (to CK); and the Ontario HIV Treatment Network (OHTN) Applied HIV Research Chair AHRC 779 (to CK). VHF was supported by CIHR Banting Scholarship.
Author contributions
AN and CK conceptualized the study, designed the experiments and wrote the paper; AN, SD, MAZ, VHF and JK performed the experiments and analyzed results in Figures 1, 3, 4, 5, 6 and Supplementary Figures 2 and 3; SD and AN performed the experiments and analyzed results for Figure 2. MAZ and AN performed experiments and analyzed results in Figure 7 and Supplementary Figure 1. MWW worked on revisions to the paper. MO and MJT constructed HIV-1 env deleted clone and provided technical assistance. AAA purified and provided FimH for Figure 4. DMEB provided plasmids and technical assistance for transfection experiments in Figure 5. CK coordinated and supervised the study. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2017.168
References
- 1.Ferreira VH, Kafka JK, Kaushic C. Influence of common mucosal co-factors on HIV infection in the female genital tract. Am J Reprod Immunol. 2014;71:543–554. doi: 10.1111/aji.12221. [DOI] [PubMed] [Google Scholar]
- 2.Shattock R, Moore J. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 3.Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. doi: 10.1111/j.1600-0897.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 4.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Nazli A, Yao X-D, Smieja M, Rosenthal KL, Ashkar AA, Kaushic C. Differential induction of innate anti-viral responses by TLR ligands against Herpes simplex virus, type 2, infection in primary genital epithelium of women. Antiviral Res. 2009;81:103–112. doi: 10.1016/j.antiviral.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 7.Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 8.Bosinger SE, Utay NS. Type I interferon: understanding its role in HIV pathogenesis and therapy. Curr HIV/AIDS Rep. 2015;12:41–53. doi: 10.1007/s11904-014-0244-6. [DOI] [PubMed] [Google Scholar]
- 9.Sivro A, Su RC, Plummer FA, Ball TB. Interferon responses in HIV infection: from protection to disease. AIDS Rev. 2014;16:43–51. [PubMed] [Google Scholar]
- 10.Meylan PR, Guatelli JC, Munis JR, Richman DD, Kornbluth RS. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virol. 1993;193:138–148. doi: 10.1006/viro.1993.1110. [DOI] [PubMed] [Google Scholar]
- 11.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 13.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasr N, Maddocks S, Turville SG, Harman AN, Woolger N, Helbig KJ, et al. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood. 2012;120:778–788. doi: 10.1182/blood-2012-01-407395. [DOI] [PubMed] [Google Scholar]
- 15.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machmach K, Leal M, Gras C, Viciana P, Genebat M, Franco E, et al. Plasmacytoid dendritic cells reduce HIV production in elite controllers. J Virol. 2012;86:4245–4252. doi: 10.1128/JVI.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira VH, Dizzell S, Nazli A, Kafka JK, Mueller K, Nguyen PV, et al. Medroxyprogesterone acetate regulates HIV-1 uptake and transcytosis but not replication in primary genital epithelial cells, resulting in enhanced T-cell infection. J Inf Dis. 2015;211:1745–1756. doi: 10.1093/infdis/jiu832. [DOI] [PubMed] [Google Scholar]
- 18.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, et al. HIV-1 gp120 induces TLR2- and TLR4 mediated innate immune activation in human female genital epithelium. J Immunol. 2013;191:4246–4258. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald EM, Savoy A, Gillgrass A, Fernandez S, Smieja M, Rosenthal KL, et al. Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection. Biol Reprod. 2007;77:1049–1059. doi: 10.1095/biolreprod.107.063933. [DOI] [PubMed] [Google Scholar]
- 21.Kaushic C, Nazli A, Ferreira VH, Kafka JK. Primary human epithelial cell culture system for studying interactions between female upper genital tract and sexually transmitted viruses, HSV-2 and HIV-1. Methods. 2011;55:114–121. doi: 10.1016/j.ymeth.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi Y, McClure MO, Pizzato M. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol. 2008;82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakhaei P, Mesplede T, Solis M, Sun Q, Zhao T, Yang L, et al. The E3 ubiquitin ligase Triad3A negatively regulates the RIG-I/MAVS signaling pathway by targeting TRAF3 for degradation. PLoS Pathog. 2009;5:e1000650. doi: 10.1371/journal.ppat.1000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira VH, Nazli A, Dizzell SE, Mueller K, Kaushic C. The antiinflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE. 2015;1:e0124903. doi: 10.1371/journal.pone.0124903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott DE, Chertova EN, Busch LK, Coren LV, Gagliardi TD, Johnson DG. Mutational analysis of the hydrophobic tail of the human immunodeficiency virus type 1 p6(Gag) protein produces a mutant that fails to package its envelope protein. J Virol. 1999;73:19–28. doi: 10.1128/jvi.73.1.19-28.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meager A. Biological assays for interferons. J Immunol Methods. 2002;261:21–36. doi: 10.1016/s0022-1759(01)00570-1. [DOI] [PubMed] [Google Scholar]
- 30.Clark K, Plater L, Peggie M, Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem. 2009;284:14136–14146. doi: 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantaert T, Baeten D, Tak PP, van Baarsen LG. Type I IFN and TNFalpha cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res Ther. 2010;12:219–229. doi: 10.1186/ar3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Basagoudanavar SH, Wang X, Hopewell E, Albrecht R, Garcia-Sastre A, et al. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J Immunol. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich N, Lienenklaus S, Weiss S, Gekara NO. Murine Toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS ONE. 2010;5:e10250. doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossman KL, Mian MF, Lauzon NM, Gyles CL, Lichty B, Mackenzie R, et al. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J Immunol. 2008;181:6702–6706. doi: 10.4049/jimmunol.181.10.6702. [DOI] [PubMed] [Google Scholar]
- 36.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 37.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 38.Park BS, Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira VH, Nazli A, Khan G, Firoz Mian M, Ashkar AA, Gray-Owen S, et al. Endometrial epithelial cell responses to co-infecting viral and bacterial pathogens in the genital tract can activate the HIV-1 LTR in an NFκB and AP-1 dependent manner. J Infect Dis. 2011;204:299–308. doi: 10.1093/infdis/jir260. [DOI] [PubMed] [Google Scholar]
- 40.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. PNAS. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsili G, Remoli AL, Sgarbanti M, Perrotti E, Fragale A, Battistini A. HIV-1, interferon and the interferon regulatory factor system: an interplay between induction, antiviral responses and viral evasion. Cytokine Growth Factor Rev. 2012;23:255–270. doi: 10.1016/j.cytogfr.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen PV, Wessels JM, Mueller K, Vahedi F, Anipindi V, Verschoor CP, et al. Frequency of human CD45+ target cells is a key determinant of intravaginal HIV-1 infection in humanized mice. Sci Rep. 2017;7:15263–15278. doi: 10.1038/s41598-017-15630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stack J, Doyle SL, Connolly DJ, Reinert LS, O'Keeffe KM, McLoughlin RM, et al. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J Immunol. 2014;193:6090–6102. doi: 10.4049/jimmunol.1401605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, et al. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog. 2014;10:e1004440. doi: 10.1371/journal.ppat.1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stekler J, Sycks BJ, Holte S, Maenza J, Stevens CE, Dragavon J, et al. HIV dynamics in seminal plasma during primary HIV infection. AIDS Res Hum Retroviruses. 2008;24:1269–1274. doi: 10.1089/aid.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman JC, Anton PA, Baldwin GC, Elliott J, Anisman-Posner D, Tanner K, et al. Seminal plasma HIV-1 RNA concentration is strongly associated with altered levels of seminal plasma interferon-gamma, interleukin-17, and interleukin-5. AIDS Res Hum Retroviruses. 2014;30:1082–1088. doi: 10.1089/aid.2013.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachet A, Dulioust E, Salmon D, De Almeida M, Rivalland S, Finkielsztejn L, et al. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 53.Klasse PJ, Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323:1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Santosuosso M, Righi E, Lindstrom V, Leblanc PR, Poznansky MC. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis. 2009;200:1050–1053. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- 55.Cummins NW, Rizza SA, Badley AD. How much gp120 is there? J Infect Dis. 2010;201:1273–1274. doi: 10.1086/651434. [DOI] [PubMed] [Google Scholar]
- 56.Cervantes CA, Oliveira LM, Manfrere KC, Lima JF, Pereira NZ, Duarte AJ, et al. Antiviral factors and type I/III interferon expression associated with regulatory factors in the oral epithelial cells from HIV-1-serodiscordant couples. Sci Rep. 2016;6:25875. doi: 10.1038/srep25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


