Abstract
The plant hormone ethylene regulates ripening in climacteric fruits. The phytohormone abscisic acid (ABA) affects ethylene biosynthesis, but whether ethylene influences ABA biosynthesis is unknown. To explore this possibility, we investigated the interactions between the ABA biosynthesis genes PpNCED2/3 and the ethylene response transcription factor PpERF3 in peach fruit. The ABA content increased during fruit maturation and reached a peak at stage S4 III. The increase was greatly inhibited by the ethylene inhibitor 1-MCP, which also suppressed PpERF3 expression. PpERF3 shared a similar expression profile with PpNCED2/3, encoding a rate-limiting enzyme involved in ABA biosynthesis, during fruit ripening. A yeast one-hybrid assay suggested that the nuclear-localized PpERF3 might bind to the promoters of PpNCED2/3. PpERF3 increased the expression of PpNCED2/3 as shown by dual-luciferase reporters, promoter-GUS assays and transient expression analyses in peach fruit. Collectively, these results suggest that ethylene promotes ABA biosynthesis through PpERF3’s regulation of the expression of ABA biosynthesis genes PpNCED2/3.
Subject terms: Plant signalling, Transcriptional regulatory elements
Fruit ripening: Hormones go hand in hand
Two hormones that regulate fruit ripening are more closely linked than previously thought, according to a study of ripening in peaches. Ethylene is a key ripening hormone in many fruits, and high ethylene levels turn on ethylene response factors (ERFs), genes that trigger production of sugars, pigments, and flavor compounds associated with ripening. Another hormone, abscisic acid (ABA), has recently been found to affect ripening, but its interaction with ethylene is unclear. Zhiqiang Wang and Guohuai Li at the Chinese Academy of Agricultural Sciences and coworkers investigated how ethylene and ABA interact during ripening. They found that as ethylene levels increased, ABA production was stimulated. Further investigation showed that ethylene directly triggered the ABA increase via ERFs. These results illuminate the fruit ripening process, and may help in finding ways to prolong fruit shelf life.
Introduction
Ethylene is the key plant hormone that regulates the fruit ripening process. This regulation is mediated through ethylene-responsive genes, particularly those encoding transcription factors. These transcription factors regulate multiple biochemical pathways that underpin fruit ripening traits, including the levels of sugars, acids, pigments, and flavor and aroma compounds and fruit firmness and texture1,2. In the past few decades, our understanding of the mechanisms by which plants respond to ethylene has greatly increased3,4. Ethylene biosynthesis is catalyzed by two key enzymes: ACC synthase and ACC oxidase. The expression of the genes encoding these two ethylene enzymes is modulated by ethylene-responsive transcription factors, thereby forming a feedback loop. Depending whether the transcription factor acts as a repressor or activator of transcription, the feedback loop may be autoinhibitory or autostimulatory. The autoinhibitory loop, also known as system 1 ethylene production, functions during the early stage of fruit development and is responsible for producing basal levels of ethylene. The autostimulatory loop, also known as system 2 ethylene production, functions during climacteric fruit ripening, and is responsible for producing high levels of ethylene5.
Abscisic acid (ABA) is another plant hormone that has regulatory roles during fruit development and ripening6, although it also controls plant wilting and stomatal closure7. ABA biosynthesis requires the cleavage of C40 carotenoids by 9-cis-epoxycarotenoid dehydrogenase (NCED) to form its direct precursor, xanthoxin. This cleavage process is a key rate-limiting step in ABA biosynthesis8. ABA levels sharply increase preceding the release of ethylene. The process of fruit ripening can be induced by the application of exogenous ABA9. Transgenic tomato (Solanum lycopersicum) fruits containing the SlNCED1-RNAi construct (to silence SlNCED1) exhibited inhibited cell wall degradation due to reduced levels of endogenous ABA, indicating that ABA has crucial roles in fruit ripening10,11. Many studies suggest that ABA affects ethylene production, likely by regulating the levels of ACC synthase (ACS) and ACC oxidase (ACO)12,13. However, little is known about whether ethylene affects ABA biosynthesis.
Ethylene response factors (ERFs) are plant-specific transcription factors. Many of them can mediate the transcription of ethylene-dependent genes3,14,15. Because ERFs comprise a family of transcription factors, different members of the family allow diverse and specific ethylene responses to occur in different plants16. ERF proteins can bind to DNA cis-acting elements such as a DRE (CCGAC), a GCC box (AGCCGCC), (A/G)CC(G/C)AC, and AA(T)TTCAAA motifs through its conserved ERF domain. These elements are found in the promoters of many ethylene-responsive genes, confirming that ERFs are involved in ethylene signaling or biosynthesis3,4,16,17. For example, tomato LeERF interacts with the GCC box and activates the expression of ethylene biosynthesis genes18. In banana (Musa acuminata), MaERF11 represses the expression of MaACO1 and expansin genes via recruiting the histone deacetylase MaHDA14. Apple (Malus domestica) MdERF2 interacts with the DRE motif in the promoter of the MdACS1 gene and suppresses its transcription, thereby inhibiting ethylene biosynthesis in ripening fruit3. Although it well known that ERFs regulate fruit ripening through ethylene1, it is unclear whether they also control fruit ripening by the transcriptional regulation of ABA biosynthesis genes.
The peach (Prunus persica) gene PpERF3 (Prupe.7G194400) shares similar expression patterns with PpACS1 and is regulated by 1-MCP19, but the target genes of PpERF3 are unknown. In the present study, we found that PpERF3 directly bound to the promoters of PpNCED genes and enhanced their transcription. We also found that PpNCED promoter activity is positively regulated by ethylene. Our results show that ERFs regulate ABA biosynthesis in ripening peach fruit by targeting PpNCED promoters.
Results
ABA levels and PpNCED2/3 expression during peach fruit ripening
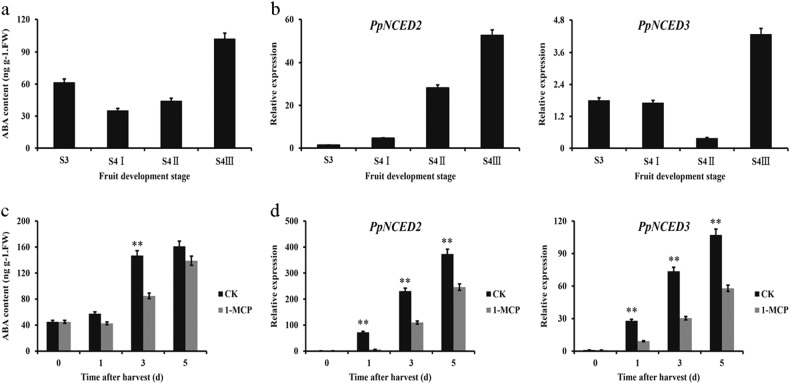
During the maturation of ‘CN13’ peach fruit, the ABA content decreased slowly from S3 to S4 I, which was followed by a gradual increase from S4 I to S4 II and a marked increase from S4 II to S4 III (Fig. 1a). Since PpNCED functions in a rate-limiting step in ABA biosynthesis, we analyzed the transcript profiles of PpNCED genes using transcriptome data and verified the results by qRT-PCR. Three NCED genes were found in peach, Prupe.1G061300, Prupe.4G082000, Prupe.4G150100, which were named PpNCED1, PpNCED2, and PpNCED3, respectively. The mRNA transcript level of PpNCED2 remained low at stage S3 and then increased sharply from S4 I to S4 III. The mRNA transcript level of PpNCED3 decreased at stage S4 II and then increased markedly at stage S4 III (Fig. 1b). In contrast, the PpNCED1 transcript level remained low throughout fruit ripening (Supplementary Table S1).
Fig. 1. The effects of 1-MCP on ABA levels and PpNCED2/3 expression in peach fruit.
a ABA levels in peach fruit. b Expression levels of PpNCED2/3 during peach fruit ripening. c ABA contents in fruit harvested at stage S4 II and treated with 0 or 10 µL L−1 of 1-MCP. d The expression levels of PpNCED2/3 in peach fruit treated as in c. As a control (CK) treatment, 0 µL L−1 of 1-MCP was used. Values are means ± SD of three biological replicates
We analyzed the correlation between ABA content and PpNCED2/3 expression level in peach fruits subjected to 1-MCP treatment. We treated ‘CN13’ peach fruit harvested at stage S4 II with 1-MCP (0, 10 µL L−1) at 20 °C for 1 d to delay fruit ripening. ABA content was lower in fruit treated with 10 µL L−1 1-MCP than in control fruit receiving 0 µL L−1 on days 1, 3, and 5, and a significant difference in ABA content was detected on day 3 (Fig. 1c). To identify the possible roles of PpNCED2/3 in ABA biosynthesis, we analyzed their expression profiles in peach fruit after treatment with 1-MCP. Consistent with the above results, the expression levels of PpNCED2/3 were strongly suppressed in ‘CN13’ fruit under 1-MCP treatment on days 1, 3, and 5 (Fig. 1d).
Promoter elements identified using bioinformatics analysis
The cis-elements in the promoter sequences (i.e., in the 2, 2.9 kb region upstream of the translation start site of PpNCED2 and PpNCED3 genes, respectively) involved in plant hormone (especially ethylene) responses were identified using PlantCARE and a manual search to understand the transcriptional regulation of the PpNCED2/3 genes. ERF (ethylene response factor) binding site, MeJA-responsive element, and abscisic acid-responsive element were found in the promoter of PpNCED2. These elements were also found in the promoter of PpNCED3, in addition to auxin-responsive element, gibberellin-responsive element, and MYB binding site (Table 1).
Table 1.
Cis-acting regulatory elements were predicted in the promoter regions of PpNCED2/3 related to fruit development and ripening in peach
| Genes | cis-elementZ | Sequence | Probable function |
|---|---|---|---|
| PpNCED2 | CRT/DRE | A/GCCGAC | ERF (ethylene response factor) binding site |
| CGTCA-motif | CGTCA | MeJA-responsive element | |
| TGACG-motif | TGACG | MeJA-responsive element | |
| CACGTG | |||
| TACGTG | |||
| ABRE | GCAACGTGTC | Abscisic acid-responsive element | |
| GCCACGTACA | |||
| ACGTGGC | |||
| ACGTGGC | |||
| PpNCED3 | ERE | ATTTCAAA | ERF (ethylene response factor) binding site |
| CRT/DRE | A/GCCGAC | ERF (ethylene response factor) binding site | |
| TGA-element | AACGAC | Auxin-responsive element | |
| P-box | CCTTTTG | Gibberellin-responsive element | |
| TATC-box | TATCCCA | Gibberellin-responsive element | |
| GARE-motif | TCTGTTG | Gibberellin-responsive element | |
| ABRE | T/CACGTG | Abscisic acid-responsive element | |
| CGTCA-motif | CGTCA | MeJA-responsive element | |
| TGACG-motif | TGACG | MeJA-responsive element | |
| MBS | CGGTCA | MYB binding site | |
| MBSI | aaaAaaC(G/C)GTTA | MYB binding site |
PpNCED2/3 promoter activity assays
To further demonstrate that PpNCED2/3 expression levels were enhanced by ethylene, we fused the PpNCED2/3 promoters with the GUS reporter gene and transiently expressed these genes in tomato fruits. After 3 days, we treated the transiently transformed tomato fruits with ethylene and used untreated fruits as controls. As expected, GUS driven by the PpNCED2/3 promoter was highly expressed in fruits treated with ethylene and not strongly expressed in untreated fruits. By contrast, CaMV35Spro::GUS was ubiquitously expressed in fruits regardless of ethylene treatment (Fig. 2). These observations suggested that PpNCED2/3 expression is affected by ethylene.
Fig. 2. Analysis of the activity of PpNCED2/3 promoters in tomato fruit.
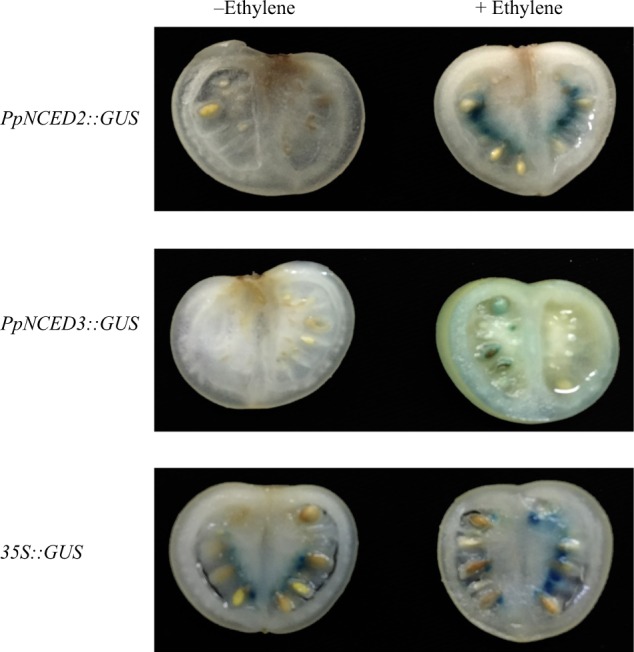
Tomato fruit at the breaker stage was infiltrated with Agrobacterium cells containing either PpNCED2/3::GUS or 35S::GUS construct. At 3 days after infiltration, the fruit was sliced and tested for GUS activity using a GUS-staining solution with or without 10 mM ethylene
Phylogenetic tree analysis of PpERF3 and other ERFs
ERFs comprise one of the largest families of plant transcription factors. Phylogenetic analysis of PpERF3 and ERFs from other fruits, including tomato (Solanum lycopersicum), banana (Musa acuminata), kiwifruit (Actinidia deliciosa), melon (Cucumis melo), and apple (Malus domestica), revealed that PpERF3 clustered with MaERF9 from banana (Supplementary Fig. S1a). PpERF3 aligned with many functionally characterized AP2/ERF-type proteins from various plant species, revealing high homology within the AP2/ERF domain (Supplementary Fig. S1b).
PpERF3 is localized to the nucleus
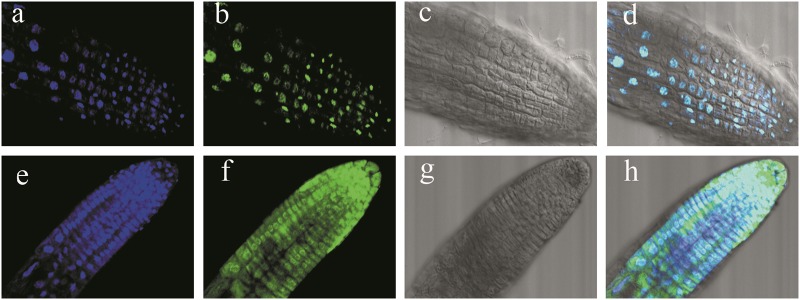
To examine the subcellular localization of PpERF3 in vivo, we cloned PpERF3 and fused the full-length coding sequence without the termination codon in-frame with GFP. We transformed Arabidopsis thaliana plants with the PpERF3::GFP vector via Agrobacterium-mediated transformation and found that the fluorescent signals from the PpERF3::GFP fusion protein were exclusively localized to the nucleus. By contrast, fluorescent signals from the GFP control were ubiquitously distributed throughout the cell (Fig. 3).
Fig. 3. Subcellular localization of PpERF3::GFP protein in transformed Arabidopsis cells.
Fluorescent signals from green fluorescent protein (GFP) were mainly detected in the nuclei of Arabidopsis cells harboring the PpERF3::GFP reporter construct. a, e DAPI; b, f GFP fluorescence; c, g bright field; d, h merged image
PpERF3 might bind to PpNCED2/3 promoters
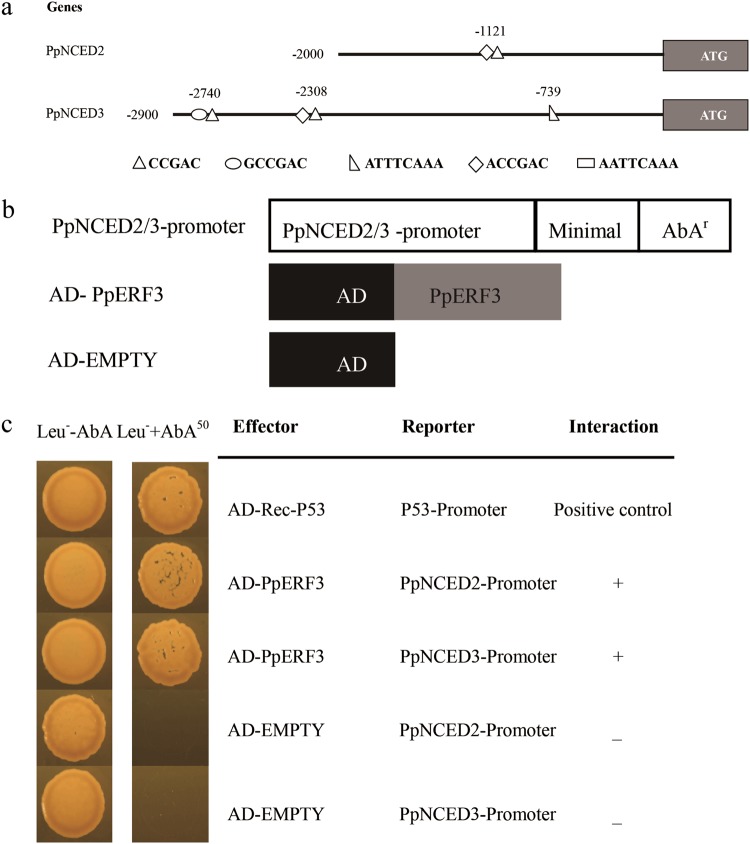
Because the expression levels of PpERF3 and PpNCED2/3 could be enhanced by ethylene treatment and because all of these genes have important roles in fruit ripening, we investigated whether PpERF3 regulates the expression of PpNCED2/3 during fruit ripening. First, we performed a search for cis-acting regulatory motif in the PpNCED2/3 promoters. We found an ERF-binding motif ACCGAC (CCGAC) located at −1121 bp in the PpNCED2 promoter and four ERF-binding motifs, ATTTCAAA, ACCGAC (CCGAC), and GCCGAC (CCGAC) located at −739, −2308, and −2740 bp, respectively, in the PpNCED3 promoter (Fig. 4a).
Fig. 4. PpNCED2/3 promoters contain ERF-binding motifs.
PpERF3 might bind to the PpNCED2/3 promoters. a The promoters of PpNCED2/3 genes are represented by lines (showing promoter length); various motifs in the promoters are indicated. The exact locations of cis-acting elements are marked by numbers that indicate the nucleotide distance from the translation start site. b The CDS of PpERF3 was cloned into the pGADT7 vector, whereas the promoters of PpNCED2/3 were cloned into the pAbAi vector. c The growth status of yeasts on two different types of media after they were transformed with a combination of effector and reporter vectors is shown. Normal yeast growth on the defective medium containing the antibiotic aureobasidin A (−Leu + AbA50) indicates the binding of protein PpERF3 to the promoter sequences of PpNCED2 and PpNCED3
We further performed a Y1H experiment. The CDS of PpERF3 were cloned into the pGADT7 vector for the effector construct, and the PpNCED2 or PpNCED3 promoter fragment was cloned into the pAbAi vector immediately in front of the reporter gene, AUR1-C, for the reporter construct (Fig. 4a). When plasmids carrying cassettes constitutively expressing the PpERF3 effectors were transformed into the Y1H reporter strains, yeast cells harboring the PpNCED2/3 promoters grew well with aureobasidin A (50 ng mL−1) (Clontech, San Francisco, USA), whereas cells co-transformed with empty pGADT7 vector did not (Fig. 4b). These results suggested that PpERF3 binds to the promoters of PpNCED2/3.
PpERF3 enhanced the activity of PpNCED2/3 promoters
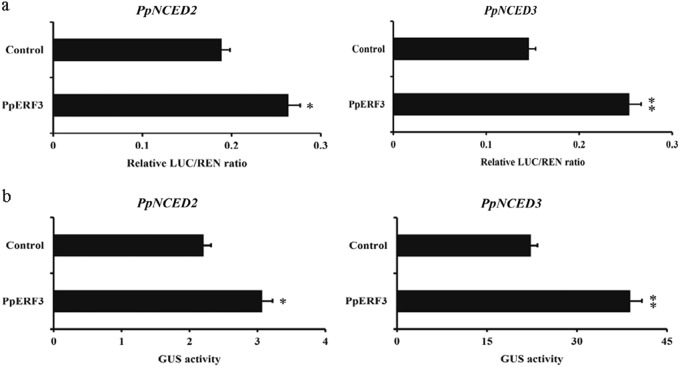
To determine whether PpERF3 can enhance PpNCED2/3 promoter activity given they can bind together, we carried out transient expression assays in tobacco leaves using dual-luciferase reporters. The assays showed that the interaction of PpERF3 with the PpNCED2 promoter lead to a nearly 1.5-fold increase in the relative LUC/REN ratio, and the interaction of PpERF3 with the PpNCED3 promoter lead to a nearly twofold increase in the relative LUC/REN ratio (Fig. 5a). These results suggest that PpERF3 enhances the transcription of PpNCED2/3 by directly interacting with their promoters.
Fig. 5. PpERF3 enhanced the activity of PpNCED2/3 promoters in transient expression assays in tobacco leaves.
a Agrobacterium tumefaciens having PpNCED2/3 or PpERF3 plasmids was infiltrated into tobacco leaves to analyze the activity enhancement of PpNCED2/3 promoters by PpERF3. Significantly higher LUC/REN ratios were obtained with the PpERF3 effect vector than with the non-PpERF3 control vector, indicating that PpERF3 enhanced PpNCED2/3 promoter activity. b The PpERF3 effector with plasmid having the PpNCED2/3 promoters was infiltrated into tobacco leaves. The effector and empty pK2GW7 plasmids that were co-transformed into tobacco were used as controls. Values are means ±SD (n=3), * and ** represent significance at p<0.05 and p<0.01, compared to control based on t-test, respectively
We further performed a GUS transactivation experiment by infiltrating the 35S::PpERF3 and PpNCED2/3::GUS plasmids into tobacco leaves. We observed significantly higher GUS activities driven by PpNCED2/3 promoters in the assays with the 35S::PpERF3 construct than in the control assays without the 35S::PpERF3 construct (Fig. 5b). These results suggest that PpERF3 is a transcriptional activator that regulates the expression of PpNCED2/3.
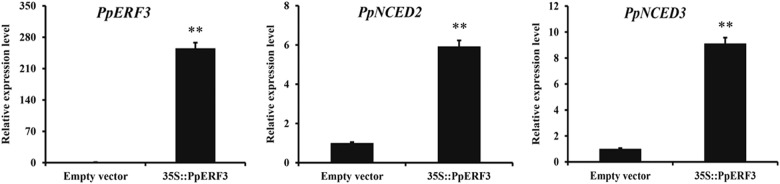
Transient over-expression PpERF3 in peach fruit
To verify the function of PpERF3 in regulating the expression of PpNCED2/3 in peach, ‘CN13’ fruit at stage S3 were transiently transformed using the 35S::PpERF3 construct or a control vector without PpERF3. The transcript of PpERF3 was not detectable in fruit transformed with the control vector, indicating that PpERF3 was not expressed in the fruit at stage S3. However, the PpERF3 transcript level was significantly increased in same-stage fruit transformed with the 35S::PpERF3 construct, indicating the over-expression of PpERF3. The transcript levels of PpNCED2 and PpNCED3 were increased sixfold and ninefold, respectively, in fruit transformed with 35S::PpERF3 relative to their levels in fruit transformed with the control vector (Fig. 6). This result indicated that PpERF3 can activate the expression of both PpNCED2 and PpNCED3 genes in peach fruit.
Fig. 6. Transient over-expression of PpERF3 in peach fruit enhanced the transcript levels of PpNCED2/3.
Transcript levels of PpERF3, PpNCED2, PpNCED3 in peach fruit infiltrated with the empty (control) and 35 S::PpERF3 vectors. Values are means ± SD (n=3), ** represent significance at p<0.01, compared to control based on t-test
Discussion
ABA levels and the expression profiles of PpNCED2/3 in peach fruit
ABA has critical roles in early fruit development and ripening9–13. Depending on the physiological stage of the fruit, the use of exogenous ABA affects the progress of fruit ripening, leading to less or more ripe fruit13. When exogenous ABA was applied to peach fruit at stage S4, the expression levels of ethylene- (ACS1, ACO1), cell wall- (endo-polygalacturonase, pectin methylesterases inhibitors) and auxin-related genes were upregulated, suggesting an acceleration of ripening13. In our study, a marked increase in ABA concentration during peach fruit ripening was observed, suggesting that ABA accumulation at stage S4 III might accelerate the softening and ripening of peach fruit.
In fruit, the concentration of endogenous ABA is determined by the kinetic equilibrium of biosynthesis (NCED), catabolism (ABA 8′-hydroxylase), and reactivation (β-glucosidases/glucosyltransferases) genes. The spatio-temporal expression of these genes is regulated at the transcriptional level by ABA content6. Since the gene encoding NCED was first separated from the maize vp14 mutant20, it has been researched in various climacteric fruit species, including peach13, tomato20, apple21, and melon22, and non-climacteric fruits, such as orange23 and grape24. NCED is a key rate-limiting step in ABA biosynthesis8. In transgenic tomato fruits with SlNCED1 silenced, cell wall degradation is inhibited due to the reduction in endogenous ABA levels. The shelf life of these fruits is longer than those of control fruits10,11. In strawberry, fruit ripening is affected by the downregulation of FaNCED genes25. In this study, we identified three NCED genes (Prupe.1G061300, Prupe.4G082000 and Prupe.4G150100) in the peach genome, and we examined the expression levels of two PpNCED genes in parallel with ABA content. The results suggest that these two genes may be important participants in ABA accumulation during fruit ripening. Compared with the PpNCED2 promoter, the PpNCED3 promoter contained many more binding sites for ERFs and other hormone-responsive proteins. This suggests that PpNCED3 may be regulated by more complicated signal transduction pathways than is PpNCED2.
PpERF3 directly actives the expression of PpNCED2/3
ERF transcription factors function as downstream components of ethylene signaling by regulating the expression of ethylene-responsive genes3. ERFs can direct regulate the expression of ripening-related genes by binding to cis-elements, such as DRE (CCGAC), (A/G)CC(G/C)AC, GCC box (AGCCGCC), and AA(T)TTCAAA, and affect the fruit ripening process3,4,16,17. There are few reports addressing whether ERFs can regulate ABA biosynthesis. Accumulating evidence supports the idea that ABA regulates ethylene biosynthesis and signaling by regulating the expression of LeACS4 and LeACO112,13. Kumar speculated that ethylene may affect the biosynthesis of ABA via regulating the expression of NCED26. In this study, phylogenetic analysis showed that PpERF3 clustered with MaERF9 (Supplementary Fig. S1a). MaERF9 from banana activates MaACO1 promoter activity, and its expression is strongly correlated with the increase in ethylene production associated with climacteric fruit ripening15. We have shown here that PpNCED2/3 promoters contain ERF-binding sites (Table 1) and that PpERF3 can bind to the PpNCED2/3 promoter and enhance both promoter activity in tobacco leaves and the transcript levels of PpNCED2/3 in peach fruit. In addition, PpNCED2/3 expression is responsive to ethylene signaling. Taken together, our results suggest that ethylene may affect ABA biosynthesis via regulating the expression of PpNCED2/3 genes by PpERF3 in peach fruit.
Crosstalk between ABA and ethylene signaling
Fruit ripening is regulated by a complicated network of feedback and crosstalk among various phytohormone signaling pathways26. Increasing evidence suggests that the combined actions of auxin, gibberellins (GAs), cytokinin, ethylene, and ABA have crucial roles in the regulation of fruit ripening26. The present study showed that ABA content was markedly reduced by the ethylene inhibitor 1-MCP (Fig. 1c) and that the promoter activity of the ABA biosynthesis gene PpNCED2/3 was strongly enhanced in tomato fruits by ethylene (Fig. 2). These results suggest that ethylene can regulate the biosynthesis of ABA. It is generally accepted that ethylene and ABA have important roles during fruit ripening. Fruit ABA content increased during ripening, and the initiation of fruit ripening was promoted by exogenous application of ABA but was delayed by fluridone (ABA inhibitor), indicating that ABA has a major role in fruit ripening process18. ABA, synergistically with ethylene, promotes softening in banana27. Overall, ABA and ethylene have important roles in the fruit ripening process, and the feedback regulation between ABA and ethylene biosynthesis may contribute to rapid fruit softening and a short shelf life of peach fruit.
In conclusion, PpNCED2/3 share the same expression pattern with PpERF3, and the expression of these three genes can be greatly inhibited by 1-MCP treatment. PpERF3, as a transcriptional activator, regulates PpNCED2/3 expression by binding to ERF response elements in the promoter region of PpNCED2/3 genes, thereby increasing ABA levels. Our findings indicate that PpERF3 regulates fruit ripening by controlling the transcription of PpNCED2/3 and provide insight into the regulatory network linking ethylene and ABA signaling.
Materials and methods
Fruit tissue collection
The peach cultivar ‘CN13’ grown in the research orchard of Zhengzhou Fruit Research Institute, Zhengzhou, China, was used in this study. ‘CN13’ (melting flesh; MF) fruits were harvested at 87, 90, 93, and 96 DAFB (days after full bloom). These time points corresponded to fruit development stages S3, S4 I, S4 II, and S4 III. At stage S3, the fruit is still green and at the end of the second exponential growth phase. At S4 I, the fruit is no longer inflated and does not release ethylene. At S4 II, the fruit releases low amounts of ethylene. At S4 III, the fruit release much higher levels ethylene and begins to soften rapidly28. Twenty fruits were collected from five different trees at each time point. Fruits collected at S4 II were treated with a solution containing 0 or 10 µl L−1 1-MCP at 20 °C for 24 h. The treated fruits were then stored at 20 °C for 1, 3, and 5 days. Mesocarp tissues of the fruits were directly collected into liquid nitrogen, and stored in a −80 °C freezer for future DNA, RNA, and ABA extraction.
Quantitation of ABA content in ‘CN13’ fruit
ABA was extracted from ‘CN13’ fruit tissues collected at the four stages using the method described by He29. In brief, extraction was carried out in a solution of methanol (80%, v/v) and butylated hydroxytoluene (1 mmol L−1) overnight at 4 °C. The extracts were centrifuged at 10,000×g (4 °C) for 20 min, passed through a C 18 Sep-Pak cartridge (Waters, Milford, MA) and dried in N2. The dried samples were dissolved in PBS buffer (0.01 mol L−1, pH 7.4). ABA content was determined using an enzyme-linked immunosorbent assay (ELISA), and the ELISA data were analyzed according to Weiler et al.30 In brief, the samples, an ABA standard, and ABA antibodies were loaded into the wells of a microtitration plate (Nunc). The plate was maintained at 37 °C for 45 min before horseradish peroxidase-labeled goat anti-rabbit immunoglobulin was added. The plate was then maintained at 37 °C for 1 h before buffered substrate (orthophenylenediamine) was applied. The enzymatic reaction was carried at 37 °C for 15 min in the dark, and then 3 mol L−1 H2SO4 was added to terminate the reaction. The plate was then read at 490 nm.
RNA extraction and cDNA synthesis
RNA was extracted from the collected peach fruit tissues using a Total RNA Kit (Sangon, Shanghai, China) according to the manufacturer’s instructions. RNA samples were evaluated in 1% agarose gels to determine the levels of degradation and contamination and then analyzed using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, USA) to determine RNA quantity and quality. First-strand cDNA was synthesized from the RNA samples with a Reverse Transcriptase kit (Tiangen, Beijing, China). The cDNA products were diluted to 20 ng µL−1 for subsequent quantitative reverse-transcription PCR (qRT-PCR) analyses.
Gene expression analysis by qRT-PCR
The relative expression levels of the NCED and AP2/ERF genes were analyzed together with an Actin gene as a reference31 using qRT-PCR. The analyses were performed with three independent biological replicates. All data were analyzed using the 2−ΔΔCt method32. PCR primers were designed based on the sequences of the 3′-UTRs or 5′-UTRs of individual genes to ensure gene-specificity using Primer 5 software. The designed primers are listed in Supplementary Table S2. These primers produced 60 to 200 bp DNA fragments that were then cloned into pTOPO-blunt vectors and sequenced to confirm the correct amplicons of each primer pair33.
Gene cloning and promoter analysis
DNA was extracted from peach leaves using a DNeasy Plant Mini Kit (Tiangen, Beijing, China). From this extracted DNA, the promoter regions of PpNCED genes were amplified by PCR using Phanta HS Super-Fidelity DNA Polymerase (Vazyme, Nanjing, China), cloned into the pTOPO-blunt vector (Aidlab, Beijing, China) and sequenced. DNA sequence alignments of the cloned promoters were performed using MEGA 5.0 software. The motifs or cis-elements in the promoter sequences were identified using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)34.
Promoter activity assay
The promoter DNA fragment (~3 kb) of PpNCED2/3, amplified as described above, was ligated into pCAMBIA1301 to replace the cauliflower mosaic virus (CaMV) 35S promoter. The resulting PpNCED2/3 pro::GUS constructs or empty vector (pCAMBIA1301) was transferred into Agrobacterium tumefaciens strain GV3101 by electroporation. Then, a liquid culture of A. tumefaciens containing the PpNCED2/3 pro::GUS construct or control vector was injected into Micro Tom tomato fruit at the breaker stage through the fruit stalk until the whole fruit was infiltrated. After infiltration, the transformed fruits were sliced into discs that were then immersed in a GUS-staining solution (50 mM Na-phosphate, pH 7.2, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6, and 0.5% Triton X-100, 0.5 mg L−1 x-gluc) with or without 10 mM ethephon overnight at 37 °C.
Gene characteristics and structural analysis
Neighbor-joining phylogenetic trees were constructed from the protein sequences of PpERF3 and other ERFs affecting ethylene biosynthesis and fruit ripening using the ClustalW tool in conjunction with MEGA 5.035 software with 1000 bootstrap replicates. The AP2/ERF domains of these genes were aligned together with several other functionally characterized AP2/ERF-domain proteins. The deduced amino acid sequences of the AP2/ERF genes used to perform the phylogenetic analysis are listed in Table S3.
Subcellular localization of PpERF3
To investigate the subcellular localization of PpERF3, a PpERF3::GFP fusion protein was constructed. The PCR product of the PpERF3 coding sequence was cloned into the pBI121::GFP36 vector after digestion with the XbaI and BamHI restriction endonucleases. The resulting fusion construct, pBI121-PpERF3::GFP, harbored PpERF3::GFP driven by the 35S promoter. The pBI121-PpERF3::GFP vector was transferred into Agrobacterium tumefaciens strain GV3101. Arabidopsis was transformed by the floral dip method as described previously37. 4′,6-diamidino-2-phenylindole (DAPI) was used to stain the nuclei. The subcellular localization of PpERF3-GFP was observed under a confocal laser-scanning fluorescence microscope.
Yeast one-hybrid assay
The CDS region of PpERF3, amplified as described above, was cloned into the pGADT7 vector. The promoter fragment of PpNCED2 or PpNCED3, amplified as described above, was ligated into the pAbAi vector. A transcription factor and promotor interaction assay was conducted using a Matchmaker™ Gold Yeast One-Hybrid Library Screening System Kit (Clontech, San Francisco, USA).
GUS analysis
The effector construct was constructed by recombining the CDS of PpERF3 with the flanking attB sites into the attP site of pDONR201 using GATEWAY™ BP Clonase™ Enzyme Mix (Invitrogen), followed by moving the PpERF3 fragment from pDONR201 to the pK2GW7 destination vector containing the attR sites by mixing both plasmids using GATEWAY™ LR Clonase™ Enzyme Mix (Invitrogen). The pK2GW7-PpERF3 fusion was driven by the 35S promoter. The reporter constructs were the PpNCED2/3 pro::GUS constructs described in the promoter activity assay. The reporter and effector constructs were transferred into Agrobacterium tumefaciens strain GV3101 and co-infiltrated into tobacco leaves. Each reporter-effector combination was infiltrated at least three times. Three days after infiltration, the leaves were used for GUS activity analyses. A fluorimeter (SpectraMax® i3x Platform, USA) was used to measure fluorescence after the proteins were extracted from the infected tobacco leaves38.
Dual-luciferase reporter assay
The promoter sequences of PpNCED2 and PpNCED3 were cloned into the pGreenII 0800-LUC39 vector, and the CDS of PpERF3 was cloned into the pK2GW7 vector driven by the 35S promoter as an effector. A dual-luciferase assay kit was used to measure LUC and REN luciferase activity, and analysis was performed using the SpectraMax® i3x Platform (USA) at 560 and 465 nm, respectively. The LUC to REN ratio was calculated. No fewer than six measurements were performed per assay.
Transient expression in peach
The expression constructs pK2GW7-PpERF3 were transferred into A. tumefaciens GV3101, and the empty vector (pK2GW7) was used as a control. Peach fruits were harvested at the S3 stage and used for transient expression assay as described by Liu at al.40 Briefly, infiltration was performed by submerging peach flesh cubes (1 cm thick) into Agrobacterium suspension and applying a vacuum (−70kPa) for 30 min. Then, the flesh cubes were sampled for RNA and subsequent expression analysis after culture on MS medium for 2 days.
Electronic supplementary material
genes used to construct the phylogenetic trees
Acknowledgements
We would like to acknowledge the financial support of the National Natural Science Foundation of China [No.31501732] and the Agricultural Science and Technology Innovation Program (ASTIP) [CAAS-ASTIP-2018-ZFRI].
Authors contributions’
W.X. and Z.W. conceived the experiments; Y.D. and Y.W. collected plant materials and conducted the experiments; L.N., L.P., Z.L. and G.C. performed phenotyping; G.H. and W.Z. analyzed the data; W.X., J.L. and Z.W. wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaobei Wang, Wenfang Zeng
Contributor Information
Guohuai Li, Phone: +86-0371-55001909, Email: liguohuai@mail.hzau.edu.cn.
Zhiqiang Wang, Email: wangzhiqiang@caas.cn.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41438-018-0094-2).
References
- 1.Klee HJ, Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 2.Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ. The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol. 2011;157:1568–1579. doi: 10.1104/pp.111.181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T, et al. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016;88:735–748. doi: 10.1111/tpj.13289. [DOI] [PubMed] [Google Scholar]
- 4.Han, Y. et al. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. (2016). 10.1104/pp.16.00301. [DOI] [PMC free article] [PubMed]
- 5.McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature. 1972;237:235. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- 6.Leng P, Yuan B, Guo Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014;65:4577–4588. doi: 10.1093/jxb/eru204. [DOI] [PubMed] [Google Scholar]
- 7.Wright STC, Hiron RWP. (+)-Abscisic acid, the growth inhibitor induced in detached wheat leaves by a period of wilting. Nature. 1969;224:719. doi: 10.1038/224719a0. [DOI] [Google Scholar]
- 8.Estrada-Melo AC, Reid MS, Jiang CZ. Overexpression of an ABA biosynthesis gene using a stress-inducible promoter enhances drought resistance in petunia. Hortic. Res. 2015;2:15013. doi: 10.1038/hortres.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giribaldi M, Gény L, Delrot S, Schubert A. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. J. Exp. Bot. 2010;61:2447–2458. doi: 10.1093/jxb/erq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, et al. Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol. 2012;158:283–298. doi: 10.1104/pp.111.186866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Yuan B, Zhang M, Wang L, Cui M. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and β-carotene contents in tomato fruit. J. Exp. Bot. 2012;63:3097–3108. doi: 10.1093/jxb/ers026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mou W, et al. Comprehensive analysis of ABA effects on ethylene biosynthesis and signaling during tomato fruit ripening. PLoS ONE. 2016;11:e0154072. doi: 10.1371/journal.pone.0154072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto A, Ruiz KB, Ravaglia D, Costa G, Torrigiani P. ABA may promote or delay peach fruit ripening through modulation of ripening-and hormone-related gene expression depending on the developmental stage. Plant. Physiol. Biochem. 2013;64:11–24. doi: 10.1016/j.plaphy.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Shin S, Lv J, Fazio G, Mazzola M, Zhu Y. Transcriptional regulation of ethylene and jasmonate mediated defense response in apple (Malus domestica) root during Pythium ultimum infection. Hortic. Res. 2014;1:14053. doi: 10.1038/hortres.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao YY, et al. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J. Exp. Bot. 2013;64:2499–2510. doi: 10.1093/jxb/ert108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuang JF, et al. The transcriptional regulatory network mediated by banana (Musa acuminata) dehydration‐responsive element binding (MaDREB) transcription factors in fruit ripening. New Phytol. 2017;214:762–781. doi: 10.1111/nph.14389. [DOI] [PubMed] [Google Scholar]
- 17.Yin XR, Allan AC, Chen KS, Ferguson IB. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 2010;153:1280–1292. doi: 10.1104/pp.110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang H, Quan R, Wang XC, Huang R. Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol. 2009;150:365–377. doi: 10.1104/pp.109.135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. Genes involved in ethylene signal transduction in peach (Prunus persica) and their expression profiles during fruit maturation. Sci. Hortic. 2017;224:306–316. doi: 10.1016/j.scienta.2017.06.035. [DOI] [Google Scholar]
- 20.Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR. Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maizeVp14. Plant J. 1999;17:427–431. doi: 10.1046/j.1365-313X.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 21.Lara I, Vendrell M. Changes in abscisic acid levels, ethylene biosynthesis, and protein patterns during fruit maturation of Granny Smith’ apples. J. Am. Soc. Hortic. Sci. 2000;125:183–189. [Google Scholar]
- 22.Sun Y, et al. Transcriptional regulation of genes encoding key enzymes of abscisic acid metabolism during melon (Cucumis melo L.) fruit development and ripening. J. Plant. Growth Regul. 2013;32:233–244. doi: 10.1007/s00344-012-9293-5. [DOI] [Google Scholar]
- 23.Rodrigo MJ, Alquezar B, Zacarías L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck) J. Exp. Bot. 2006;57:633–643. doi: 10.1093/jxb/erj048. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler S, Loveys B, Ford C, Davies C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009;15:195–204. doi: 10.1111/j.1755-0238.2008.00045.x. [DOI] [Google Scholar]
- 25.Ji K, et al. Non-climacteric ripening in strawberry fruit is linked to ABA, FaNCED2 and FaCYP707A1. Funct. Plant Biol. 2012;39:351–357. doi: 10.1071/FP11293. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Khurana A, Sharma AK. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014;65:4561–4575. doi: 10.1093/jxb/eru277. [DOI] [PubMed] [Google Scholar]
- 27.Lohani S, Trivedi PK, Nath P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 2004;31:119–126. doi: 10.1016/j.postharvbio.2003.08.001. [DOI] [Google Scholar]
- 28.Tonutti P, Bonghi C, Ruperti B, Tornielli GB, Ramina A. Ethylene evolution and 1-aminocyclopropane-1-carboxylate oxidase gene expression during early development and ripening of peach fruit. J. Am. Soc. Hortic. Sci. 1997;122:642–647. [Google Scholar]
- 29.He, Z. in Guidance to Experiment on Chemical Control in Crop Plants (ed He, Z. P.) 60–68 (Beijing Agricultural University Publishers, Beijing, 1993).
- 30.Weiler EW, Jourdan PS, Conrad W. Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta. 1981;153:561–571. doi: 10.1007/BF00385542. [DOI] [PubMed] [Google Scholar]
- 31.Tatsuki M, et al. Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch) J. Exp. Bot. 2013;64:1049–1059. doi: 10.1093/jxb/ers381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, et al. Differential expression within the LOX gene family in ripening kiwifruit. J. Exp. Bot. 2006;57:3825–3836. doi: 10.1093/jxb/erl151. [DOI] [PubMed] [Google Scholar]
- 34.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Zhao A, Zhu P, Li J, Han L. Characterization and expression of genes involved in the ethylene biosynthesis and signal transduction during ripening of mulberry fruit. PLoS ONE. 2015;10:e0122081. doi: 10.1371/journal.pone.0122081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Q, et al. A wheat MYB transcriptional repressor TaMyb1D regulates phenylpropanoid metabolism and enhances tolerance to drought and oxidative stresses in transgenic tobacco plants. Plant Sci. 2017;265:112–123. doi: 10.1016/j.plantsci.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 38.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellens RP, et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant. Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, et al. UV‐B irradiation differentially regulates terpene synthases and terpene content of peach. Plant Cell Environ. 2017;40:2261–2275. doi: 10.1111/pce.13029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
genes used to construct the phylogenetic trees