Abstract
B cells home to the lymph nodes (LNs) via high endothelial venules (HEVs) under the guidance of chemokines, particularly CXCL13. However, as CXCL13 is not directly made in HEVs, the molecular mechanism mediating B-cell homing to LNs has remained unclear. We show here that nuclear factor (NF)-κB-inducing kinase (NIK), a kinase mediating activation of the noncanonical NF-κB pathway, functions in lymphatic endothelial cells (LECs) to regulate B-cell homing to LNs. LEC-conditional deletion of NIK in mice did not affect the integrity or global function of lymphatic vessels but caused a severe reduction in the frequency of B cells in LNs. The LEC-specific NIK deficiency did not affect the survival of B cells or the frequency of B cells in the spleen. B-cell adoptive transfer studies revealed that the LEC-specific NIK deletion impairs the ability of LNs to recruit B cells. We further show that NIK mediates expression of the chemokines CXCL13 and CCL19 in LECs. Although CCL19 is also expressed in blood endothelial cells (BECs), CXCL13 is not produced in BECs. These results suggest that NIK regulates naive B-cell homing to LNs via mediating production of the B-cell homing chemokine CXCL13 in LECs.
Introduction
Lymphocytes continuously circulate among blood, lymph and secondary lymphoid organs, including spleen and lymph nodes (LNs).1 To enter LNs, naive lymphocytes adhere to and transmigrate through specific blood vessels known as high endothelial venules (HEVs).2,3,4 Lymphocyte homing to LNs is a multistep process mediated by interaction between circulating lymphocytes and specialized vascular endothelium through adhesion molecules, including chemoattractant receptors, selectins and integrins.5,6 Different chemokines produced in and around HEVs play a crucial role in the specificity of lymphocyte trafficking to LNs. The interaction between CCL21/CCL19 and their receptor CCR7, which is expressed by naive T cells, is crucial for T-cell homing to LNs through adhesion to HEVs.7,8,9 The migration of B cells into LNs is only slightly affected in CCL21/CCL19-deficient mice8 but is significantly reduced in CXCL13-deficient mice,10 suggesting a critical role of CXCL13 in regulating B-cell homing. Indeed, naive recirculating B cells express a high level of CXCR5, the receptor for CXCL13.11 Unlike CCL21, which is expressed by the endothelial cells of HEVs, CXCL13 is produced by non-HEV cells and transported to the luminal surface of HEVs.10
Effective circulation is achieved by two specialized vascular systems: the blood vasculature and the lymphatic vasculature. One of the most specific markers for lymphatic endothelial cells (LECs) is lymphatic endothelial hyaluronan receptor 1 (Lyve1) that has been widely used for the detection and isolation of LECs.12,13,14 The lymphatic vascular system plays a crucial role in fluid homeostasis, immune surveillance and lipid absorption.15,16 During immune responses, dendritic cells (DCs) uptake antigens in peripheral tissues and migrate through afferent lymphatic vessels to regional LNs, where they present specific antigens to T cells to initiate an immune response. Emerging evidence suggests that lymphatic vessels also play an active role in regulating different aspects of immune functions, such as lymphocyte trafficking, antigen presentation and immune tolerance.17 In particular, LECs interact with both innate immune cells and lymphocytes and, thereby, regulate their migration and functions.17 Malfunction of lymphatic vessels can lead to many diseases, including lymphedema, inflammation and tumor metastasis.15,16 The molecular mechanism regulating the function of lymphatic vessels is incompletely understood.
Nuclear factor-κB (NF-κB) proteins function as dimeric transcription factors that regulate a broad range of biological processes including inflammation, lymphoid organogenesis and immune responses.18 The activation of NF-κB family of transcription factors occurs via two major signaling pathways: the canonical and the noncanonical NF-κB pathways. The noncanonical NF-κB pathway activates upon the processing of p100 that is tightly controlled in a signal-induced manner.19,20 One of the major noncanonical NF-κB-inducing receptors is lymphotoxin-β receptor (LTβR) that is expressed on stromal organizer cells that mediates lymphoid organ development by inducing specific chemokines including CCL19, CCL21, CXCL13 and adhesion molecules to recruit lymphoid tissue-inducer cells and lymphocytes.21 NF-κB-inducing kinase (NIK), which activates the kinase IKKα and induces p100 phosphorylation, is a crucial component of the noncanonical NF-κB signaling pathway.22,23 Alymphoplasia (Aly) mice carry a loss-of-function mutation in NIK and the Aly/Aly homozygous mice show impaired development of secondary lymphoid organs and B cells.24 Similar phenotypes were also reported in NIK-knockout (KO) mice,25 indicating that NIK plays a critical role in maintaining intact LNs and B-cell population. NIK is also expressed in endothelial cells in synovial tissue of rheumatoid arthritis,26 although the functional significance is elusive and the role of NIK in normal endothelial cells is also unknown.
To study the function of NIK in lymphatic vessels, we generated conditional KO mice in which NIK was specifically deleted in LECs. We demonstrated that although LEC-specific deletion of NIK had no effect on the global function of lymphatic vessels, it unexpectedly caused reduced B-cell frequency and numbers in LNs because of impaired capacity to recruit B cells. We further obtained evidence that NIK was required for LEC expression of CXCL13, a chemokine that is crucial for B-cell homing. These findings suggest that NIK expressed by lymphatic vessels is important for the homing of B cells to LNs.
Materials and methods
Mice
The NIK-flox mice, provided by Genentech (South San Francisco, CA, USA), were generated using LoxP system targeting exon 2 of the NIK gene.25 To create LEC-conditional NIK-KO (NIKLEC-KO) mice, the NIK-flox mice were crossed with Lyve1 EGFP-hCre mice that express Cre and enhanced green fluorescent protein (EGFP) driven by the Lyve1 promoter27 (The Jackson Laboratory, Bar Harbor, ME, USA) to generate WT (NIK+/+Lyve1+/Cre), heterozygous (NIK+/flLyve1+/Cre) and homozygous NIKLEC-KO (NIKfl/flLyve1+/Cre) mice. Wild-type (WT) and NIKLEC-KO littermates were used for experiments. The mice were maintained in a specific pathogen-free facility, and the animal experiments were performed in accordance with protocols approved by the institutional animal care and use committee of the University of Texas MD Anderson Cancer Center.
Antibodies and reagents
Fluorescence-labeled antibodies for CD3 (17A2), B220 (RA3-6B2), CD4 (RM4-5), MHCII (M5/114.15.2), CD21 (4E3), CD23 (B3B4), CD11c (N418), CXCR5 (SPRCL5), GL7 (GL-7), PD1 (RMP1-30), CD45.2 (104), CD31 (390) and CD95 (15A7) were purchased from Thermo Fisher (Waltham, MA, USA). Anti-ICAM-1 (YN1/1.7.4), anti-LTβR (5G11) and anti-gp38 (8.1.1) were purchased from Biolegend (San Diego, CA, USA). CellTrace carboxyfluorescein succinimidyl ester (CFSE) Cell Proliferation Kit was from Thermo Fisher Scientific.
Flow cytometry and cell sorting
Flow cytometric analyses and cell sorting were performed using a LSRII FACSFortessa (BD, San Jose, CA, USA) and FACSAria (BD), respectively. A single-cell suspension was prepared and incubated with fluorescence-labeled antibodies for 30 min at 4 °C. Cells were then washed twice with phosphate-buffered saline with 3% fetal bovine serum and resuspended for flow cytometry analysis.
In vivo homing assay
After lysis of the red blood cells, a single-cell suspension was prepared from the spleen of WT mice. Splenocytes were labeled with CFSE and injected intravenously into WT and NIKLEC-KO recipient mice. The CFSE+ donor cells recruited to the spleen and LNs of recipient mice were analyzed by flow cytometry 4 h after injection.28
FITC painting assay
WT and NIKLEC-KO mice were shaved and painted on the posterior flank skin over inguinal LNs with 10 μl of 1% fluorescein isothiocyanate (FITC) solution.29 1% FITC solution was prepared in acetone and dibutyl phthalate mixed in a 1:1 volume. FITC+ DCs from inguinal LNs were analyzed by flow cytometry 18 h after painting to identify skin-derived DCs.
Measurement of lymph flow in the ear
To measure the lymph flow in the ear, 3 μl of 1% Evans blue dye solution was injected into the dermis of each mouse ear.30 At 24 h after injection, mouse ears were removed and incubated in 500 μl of formamide at 55 °C with constant agitation for 2 days to extract the remaining Evan blue dye. The amount of extracted dye was determined by measuring the absorbance at 620 nm.
Isolation of lymphatic endothelial cells
The isolation of lymphatic endothelial cells was performed as previously described.31 Briefly, inguinal, axillary and brachial LNs were dissected from mice, opened with needles and pooled together. Pooled LNs were digested with 1 mg/ml collagenase IV and 40 μg/ml DNAse I in 2 ml RPMI-1640 medium with gentle stirring for 30 min at 37 °C. The remaining fragments were further digested with 1 mg/ml collagenase D and 40 μg/ml DNAse I in 2 ml RPMI-1640 medium for 40 min with gentle pipetting every 10 min to break up the aggregates. EDTA was then added to a final concentration of 5 mM and cells were washed with RPMI-1640 medium and resuspended.
Real-time quantitative RT-PCR
Total RNA was isolated from LECs and BECs with an Absolutely RNA Nanoprep Kit (Agilent, Santa Clara, CA, USA) and subjected to complementary DNA synthesis using MMLV (Moloney murine leukemia virus) reverse transcriptase (Invitrogen, Thermo Fisher) and oligo (dT) primers. Real-time quantitative PCR (qRT-PCR) was performed using iCycler Sequence Detection System (Bio-Rad, Hercules, CA, USA) and iQ SYBR Green Supermix (Bio-Rad). The expression of individual genes was normalized to the expression of β-actin (Actb). The primers used in qRT-PRC assays are shown below. β-Actin forward, 5′--3′; and reverse, 5′--3′. CCL19 forward, 5′--3′; and reverse, 5′--3′. CXCL12 forward, 5′--3′; and reverse, 5′--3′. CXCL13 forward, 5′--3′; and reverse, 5′--3′. CD31 forward, 5′--3′; and reverse, 5′--3′. VE-cadherin, 5′--3′; and reverse, 5′--3′.
Mice immunization
Equal volumes of 2 mg/ml NP-KLH and 2 mg/ml Complete Freund’s Adjuvant (CFA) were mixed vigorously for several minutes to form a white emulsion. WT and NIKLEC-KO mice were injected with 100 μl NP-KLH and CFA mixture subcutaneously near inguinal LN (iLN). Serum was collected 7 days after immunization and analyzed by enzyme-linked immunosorbent assay to determine the antigen-specific immunoglobulins M and G (IgM and IgG). Immunized mice were killed in the meantime and iLNs were removed and analyzed by flow cytometry or processed for hematoxylin and eosin staining.
Statistical analysis
Two-tailed unpaired t-test statistical analysis was performed using Prism software (La Jolla, CA, USA). The P-values of <0.05 were considered significant, and the level of significance was indicated as *P<0.05, **P<0.01 and ***P<0.001.
Results
LEC-specific NIK is dispensable for global function of lymphatic vessels
To study the specific role of NIK in LECs, we generated LEC-conditional NIK KO (NIKLEC-KO) mice by crossing the NIK-flox mice with Lyve1 EGFP-hCre mice (Figure 1a) that express the EGFP-hCre transgene from the LEC-specific Lyve1 promoter.25,27 qRT-PCR analysis of purified cell populations revealed specific loss of NIK expression in LECs, but not in BECs, T cells, B cells or DCs (Figures 1b and c). The NIK deletion did not affect the expression of LTβR (Supplementary Figure 1), a well-defined noncanonical NF-κB-stimulating receptor in lymphoid stromal cells.19 To assess whether the function of lymphatic vessel was affected, we examined the capacity of lymphatic vessels to drain fluid and transport DCs from peripheral tissue. We measured the lymph flow in the ear by injecting 3 μl of 1% Evans blue dye solution into the dermis of mouse ears and extracted remaining dye from the ears 24 h later. We then determined the amount of extravasated dye by measuring the absorbance at 620 nm.30 Total Evans blue dye remaining in the ears of WT and NIKLEC-KO mice was comparable (Figure 1d), suggesting normal lymphatic drainage of fluid in NIKLEC-KO mice. As Lymphatic vessels are crucial for DC migration from peripheral tissues to regional LNs, we next investigated the role of LEC-specific NIK in regulating DC migration. Mice were shaved and painted on the posterior flank skin over inguinal LNs with 10 μl of 1% FITC solution.29 FITC+ DCs from inguinal LNs were analyzed 18 h after painting to identify skin-derived DCs. The frequency of FITC+ DCs in inguinal LNs of NIKLEC-KO mice was not significantly different from that of WT mice (Figure 1e), indicating that LEC-specific deletion of NIK did not affect DC migration from skin to LNs. Collectively, these results suggested a dispensable role of LEC-specific NIK in regulating the function of lymphatic vessels, including fluid drainage and DC migration.
Figure 1.
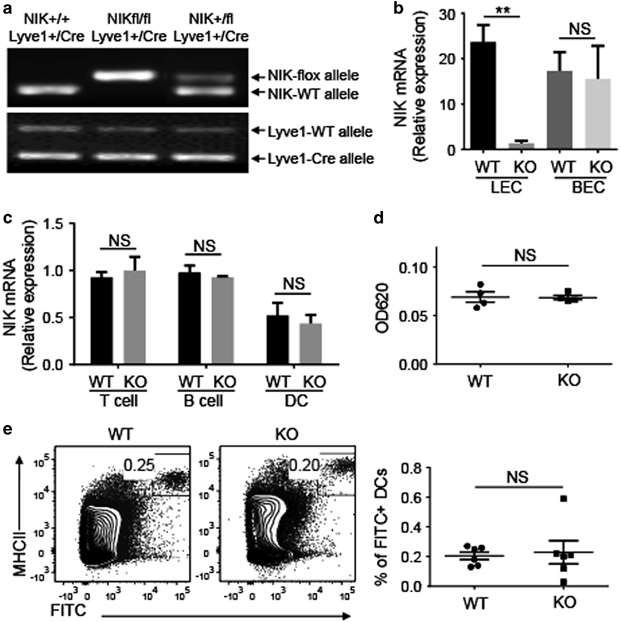
LEC-specific NIK is dispensable for global function of lymphatic vessels. (a) Genotyping PCR analysis of NIK-flox, NIK-WT, Lyve1-Cre and Lyve1-WT alleles using tail DNA to identify wild-type (WT, NIK+/+Lyve1+/Cre), heterozygous (NIK+/flLyve1+/Cre) and homozygous LEC-conditional NIK-KO (NIKLEC-KO, NIKfl/flLyve1+/Cre) mice. (b, c) The qRT-PCR analysis of Nik expression was performed using total RNA from LECs and BECs (b) or T cells, B cells and DCs (c) sorted from WT and NIKLEC-KO (KO) mice by flow cytometry. Data are presented as summary graphs for the expression of Nik normalized to the expression of the housekeeping gene Actb. (d) Measurement of lymph flow in the ear with Evan blue dye. Evan blue dye was injected to the ears of WT and NIKLEC-KO (KO) mice and the remaining dye was extracted 24 h after injection. The amount of extracted dye was determined by measuring the absorbance at 620 nm. (e) Flow cytometry analysis of the frequency of skin-derived DCs (FITC+MHCII+) in iLNs from WT and NIKLEC-KO (KO) mice 18 h after FITC painting. Data are presented as representative plots (left) and summary graphs (right). Data are representative of two independent experiments and are presented as means±s.e.m. BEC, blood endothelial cell; DC, dendritic cell; FITC, fluorescein isothiocyanate; iLN, inguinal lymph node; KO, knockout; LEC, lymphatic endothelial cell; Lyve1, lymphatic endothelial hyaluronan receptor 1; NIK, nuclear factor-κB-inducing kinase; NS, not significant, qRT-PCR, real-time quantitative PCR; WT, wild type. **P<0.01.
LEC-specific NIK deletion reduces the number of B cells in LNs
Although lymphatic vessels are known to mediate antigen uptake and DC migration from peripheral tissue, whether LECs play additional roles in the immune system is poorly understood. We thus examined the effect of LEC-specific NIK deletion on the homeostasis of immune cells in the spleen and LNs. The NIKLEC-KO mice did not have obvious abnormalities in the size of the spleen (Figure 2a). The size of LNs was also normal, with the exception of iLNs that was substantially smaller with significantly reduced cellularity in the NIKLEC-KO mice (Figures 2a and b). A more striking phenotype of the NIKLEC-KO mice was the reduced frequency and absolute numbers of B cells in both the iLNs and other LNs (Figure 2c). In contrast, the frequency of T cells in LNs was elevated in the NIKLEC-KO mice compared with WT control mice (Figure 2c), likely because of the reduction in B cells as the absolute number of T cells was comparable in cervical LNs (cLNs) of NIKLEC-KO and control mice and only slightly increased in mesenteric LNs (mLNs) of NIKLEC-KO mice (Figure 2c). The absolute number of T cells significantly decreased in iLNs of the NIKLEC-KO mice (Figure 2c). However, this was likely because of the overall reduction of cellularity but not a defect in T-cell homing, as the frequency of T cells was not reduced but rather increased in the NIKLEC-KO iLNs (Figure 2c). The frequency of naive and memory-like populations of CD4+ and CD8+ T cells was also comparable between the WT and NIKLEC-KO iLNs, suggesting normal T-cell homeostasis (Supplementary Figure 2). It is currently unclear how the LEC-specific NIK deletion selectively reduces the size and cellularity of iLNs, but this phenotype is highly reproducible.
Figure 2.
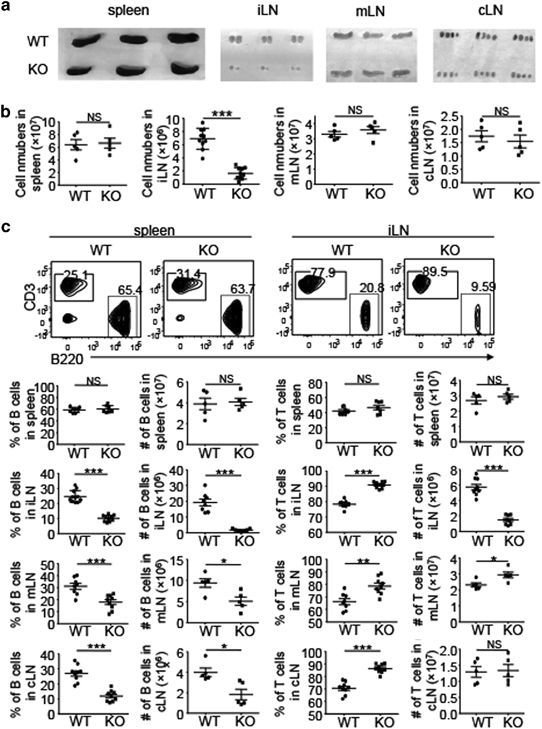
LEC-specific NIK deletion reduces the number of B cells in LNs. (a, b) Representative images (a) and total cell number (b) of spleen, iLNs, mLNs and cLNs of WT and NIKLEC-KO (KO) mice. (c) Flow cytometry analysis of the frequency and absolute cell number of T cells (CD3+) and B cells (B220+) in the spleen, iLN, mLN and cLN of WT and NIKLEC-KO (KO) mice. Data are presented as representative plots (upper panel) and summary graphs (lower panel). Data are representative of at least three independent experiments and are presented as means±s.e.m. *P<0.05; **P<0.01; ***P<0.001. cLN, cervical lymph node; DC, dendritic cell; iLN, inguinal lymph node; KO, knockout; LEC, lymphatic endothelial cell; LN, lymph node; mLN, mesenteric lymph node; NIK, nuclear factor-κB-inducing kinase; NS, not significant, WT, wild type.
To examine whether LEC-specific NIK had a general function in maintaining B-cell abundance in peripheral lymphoid organs, we examined the B- and T-cell frequencies in the spleen of NIKLEC-KO and control mice. Although LEC-specific deletion of NIK significantly reduced the number of B cells in LNs, the frequency and absolute number of B and T cells in the spleen remained unchanged in the NIKLEC-KO mice (Figure 2c). Collectively, these findings suggested that NIK expressed by LECs plays a crucial role in regulating the abundance of B cells in LNs but not in the spleen.
LEC-specific NIK regulates B-cell homing to LNs
To understand the mechanism by which the LEC-specific NIK regulates B-cell frequencies in LNs, we examined the maturation of B cells in the spleen and LNs. Although B-cell development initiates in the bone marrow, the final steps of B-cell maturation occur in the periphery.32 The majority of immature B cells ultimately differentiate into two subsets of mature peripheral B cells: follicular and marginal zone B cells. Both follicular (CD21intCD23+) and marginal zone (CD21highCD23low) B cells in the spleen showed similar frequencies and absolute numbers in WT and NIKLEC-KO mice (Figure 3a). The vast majority of B cells in the iLNs were follicular (CD21intCD23+) B cells, and the frequencies were comparable in WT and NIKLEC-KO mice, although the absolute number of follicular B cells was decreased in NIKLEC-KO mice because of reduced cellularity of iLN (Figure 3a).
Figure 3.
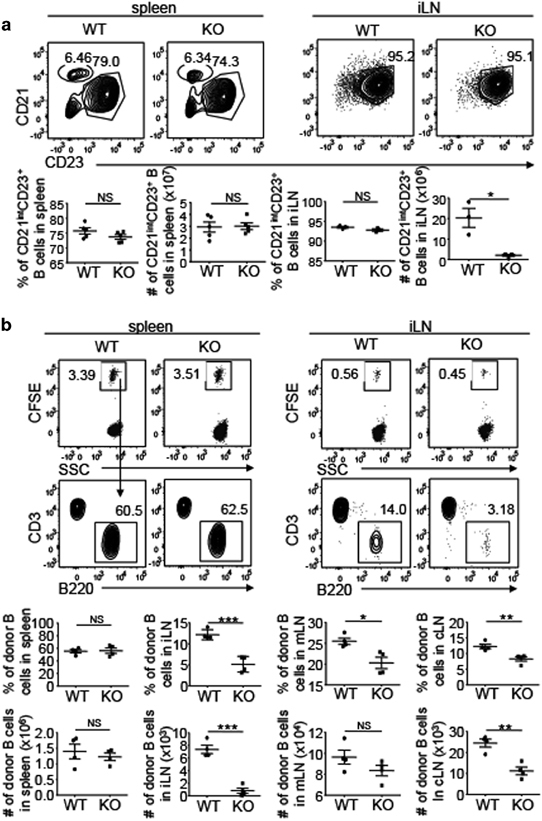
LEC-specific NIK regulates B-cell homing to LNs. (a) Flow cytometry analysis marginal zone (CD21highCD23low) and follicular (CD21intCD23+) B cells in the spleen and follicular B cells in iLNs of WT (NIK+/+Lyve1+/Cre) and NIKLEC-KO (KO, NIKfl/flLyve1+/Cre) mice. Plots were gated on B220+ B cells. Summary graphs of the frequency and absolute cell number of follicular B cells in the spleen and iLNs are shown in the lower panels (each symbol represents a mouse). (b) Flow cytometry analysis of the frequency and absolute cell number of CFSE+ donor B cells (B220+) migrating to the spleen, iLN, mLN and cLN of WT and NIKLEC-KO (KO) recipient mice 4 h after adoptive transfer with CFSE-labeled WT splenocytes. Data are presented as representative plots (upper panel) and summary graphs (lower panel). Data are representative of two independent experiments and are presented as means±s.e.m. *P<0.05; **P<0.01; ***P<0.001. CFSE, carboxyfluorescein succinimidyl ester; cLN, cervical lymph node; DC, dendritic cell; iLN, inguinal lymph node; KO, knockout; LEC, lymphatic endothelial cell; LN, lymph node; mLN, mesenteric lymph node; NIK, nuclear factor-κB-inducing kinase; NS, not significant, WT, wild type.
The results described above prompted us to examine the migration of B cells to the LNs of WT and NIKLEC-KO mice. We labeled splenocytes from WT mice with CFSE and adoptively transferred them into WT or NIKLEC-KO mice followed by measuring the frequency of the transferred B cells in the spleen and LNs of the recipient mice 4 h later. The WT and NIKLEC-KO recipient mice had similar frequencies and absolute numbers of CFSE+ B cells in the spleen (Figure 3b). However, the NIKLEC-KO recipients had a significantly reduced frequency and absolute number of CFSE+ B cells in the LNs (Figure 3b). The total cell counts of the spleen, mLN and cLN were comparable between the WT and NIKLEC-KO recipient mice, although the NIKLEC-KO iLN had significantly reduced cell numbers compared with the WT iLN (Supplementary Figure 3a) that was obviously because of the smaller size of the NIKLEC-KO iLN (Figure 2a). Consistently, the number of T cells was also reduced in the NIKLEC-KO iLN. However, this was not because of homing defect, as the frequency of T cells in NIKLEC-KO iLN was even higher than in WT iLN (Supplementary Figure 3b). The frequency of T cells was also higher in other NIKLEC-KO LNs than WT LNs (Supplementary Figure 3b). These results suggested a crucial role for LEC-specific NIK in regulating the homing of B cells, but not T cells, to the LNs.
NIK regulates expression of CXCL13 in LECs
B cells home to the LNs via HEVs and rely on the chemokine CXCL13, although the underlying mechanism is incompletely understood. To examine how LEC-specific NIK regulated B-cell homing to the LNs, we analyzed different subsets of stromal cells from LNs, including BECs (CD31+gp38−), LECs (CD31+gp38+), fibroblast reticular cells (FRCs, CD31−gp38+) and double negative stromal cells (CD31−gp38−).33 The frequencies of different subsets of stromal cells were similar in the LNs of WT and NIKLEC-KO mice (Figure 4a). LECs, but not BECs, expressed Lyve1, as determined based on expression of the reporter GFP (Figure 4b), confirming that Lyve1 was a specific marker for LECs and NIK was disrupted specifically in LECs of the NIKLEC-KO mice.
Figure 4.
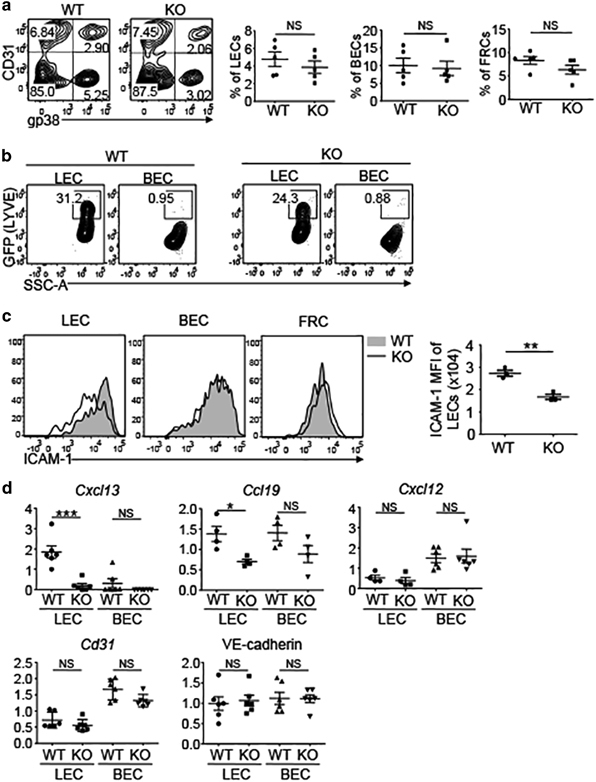
NIK regulates the expression of CXCL13 in LECs. (a) Flow cytometry analysis of the frequency of LECs (CD31+gp38+), BECs (CD31+gp38−) and FRCs (CD31-gp38+) in the LNs of WT and NIKLEC-KO (KO) mice. Plots were gated on CD45.2− stromal cells. Data are presented as representative plots (left) and summary graphs (right). (b) Flow cytometry analysis of the expression of Lyve1 (represented by GFP expression) by LECs and BECs in the LNs of WT and NIKLEC-KO (KO) mice. (c) Flow cytometry analysis of the expression of ICAM-1 by LECs, BECs and FRCs in the LNs of WT and NIKLEC-KO (KO) mice. Data are presented as representative plots (left) and summary graphs for the MFI of ICAM-1 (right). (d) qRT-PCR analysis was performed using total RNA from LECs and BECs that were sorted from LNs of WT and NIKLEC-KO (KO) mice by flow cytometry. Data are presented as summary graphs for the expression of indicated genes normalized to the expression of the housekeeping gene Actb. Data are representative of at least three independent experiments and are presented as means±s.e.m. *P<0.05; **P<0.01; ***P<0.001. BEC, blood endothelial cell; FRC, fibroblast reticular cell; ICAM-1, intercellular adhesion molecule 1; LN, lymph node; KO, knockout; LEC, lymphatic endothelial cell; Lyve1, lymphatic endothelial hyaluronan receptor 1; MFI, mean fluorescence intensity; NIK, nuclear factor-κB-inducing kinase; NS, not significant, qRT-PCR, real-time quantitative PCR; WT, wild type.
It has been reported that noncanonical NF-κB pathway mediates the lymphoid organ development by regulating the expression of various adhesion molecules and chemokines.18 Therefore, we next investigated whether the deletion of NIK affects the expression of these molecules on LECs. LEC-specific deletion of NIK resulted in reduced expression of intercellular adhesion molecule 1 (ICAM-1) on LECs, but had no effect on ICAM-1 expression in BECs or FRCs (Figure 4c). In term of chemokines, LECs, but not BECs, abundantly expressed CXCL13, and the LEC-specific NIK deficiency blocked the expression of CXCL13 (Figure 4d). NIK was also partially required for the expression of CCL19 in LECs; however, CCL19 was also expressed in BECs (Figure 4d). NIK was dispensable for the expression of the chemokine CXCL12 and the cell adhesion molecules CD31 and VE-cadherin (Figure 4d). Collectively, these finding identified LECs as a source of CXCL13 expression and demonstrated a crucial role of NIK in regulating the expression of this B-cell homing chemokine on LECs.
NIK deletion impairs homing of CXCR5+ DCs
DCs from peripheral tissues migrate to regional draining LNs through lymphatic vessels to present foreign antigens to naive T cells. In addition, DCs from blood enter LNs crossing HEVs.34 Chemokines play a crucial role in the trafficking of DCs from both periphery and blood to LNs. It has been reported that antigen-bearing mature DCs localize in the T-cell zone of LNs, a process dependent on the chemokine CCL19.35,36 However, emerging studies have also identified a population of CXCR5-expressing DCs that migrate to LNs in a CXCL13-dependent manner and localize near B-cell follicles.37–39 As NIK regulated the expression of CXCL13 and CCL19 in LECs (Figure 4d), we next examined the effect of LEC-specific deletion of NIK on DCs in the LNs. The WT and NIKLEC-KO mice had similar frequencies and absolute numbers of total DCs in the spleen (Figure 5a). However, the NIKLEC-KO mice had a significantly reduced frequency and absolute number of total DCs in the LNs (Figure 5a). This result was not because of the reduction in total cell counts, as the WT and NIKLEC-KO mice had comparable total cell numbers in the different LNs with the exception of the iLNs (Figure 2b). We then investigated which subsets of DCs in iLNs were influenced by the deletion of NIK in LECs. Consistent with the result of FITC painting assay, which indicated normal migration of DCs from skin to iLNs through lymphatic vessels (Figure 1b), the WT and NIKLEC-KO mice had similar frequencies of MHCIIhigh DCs, a population of DCs derived from the skin40 (Figure 5b). In contrast, MHCIIint DCs significantly decreased in the iLNs of NIKLEC-KO mice (Figure 5b). Interestingly, further analysis of MHCIIint DCs revealed reduced CXCR5+ DCs in the LNs, but not the spleen, of NIKLEC-KO mice (Figure 5c). Collectively, these data suggested a crucial role of LEC-specific NIK in regulating DCs, particularly CXCR5+ DCs, in the LNs, likely through CXCL13.
Figure 5.
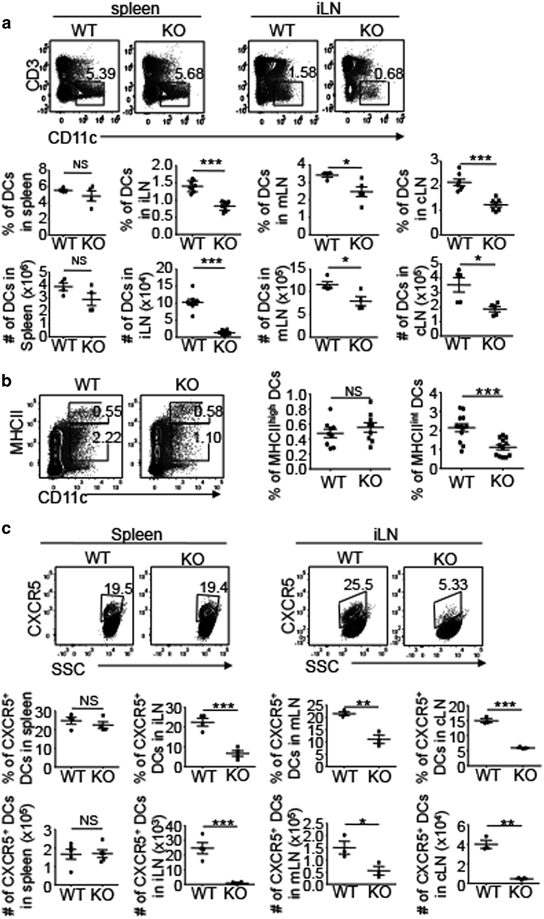
NIK deletion in LECs impairs the homing of CXCR5+ DCs. (a) Flow cytometry analysis of the frequency and absolute cell number of DCs (CD3-CD11c+) in the spleen, iLN, mLN and cLN of WT and NIKLEC-KO (KO) mice. Data are presented as representative plots (upper panel) and summary graphs (lower panel). (b) Flow cytometry analysis of the frequency of MHCIIhighCD11c+ and MHCIIintCD11c+ DCs in the iLN of WT and NIKLEC-KO (KO) mice. Data are presented as representative plots (left) and summary graphs (right). (c) Flow cytometry analysis of the frequency and absolute cell number of CXCR5+ DCs in the spleen, iLN, mLN and cLN of WT and NIKLEC-KO (KO) mice. Plots are gated on MHCIIintCD11c+ DCs. Data are presented as representative plots (upper panel) and summary graphs (lower panel). Data are representative of at least three independent experiments and are presented as means±s.e.m. *P<0.05; **P<0.01; ***P<0.001. cLN, cervical lymph node; DC, dendritic cell; iLN, inguinal lymph node; KO, knockout; LEC, lymphatic endothelial cell; mLN, mesenteric lymph node; NIK, nuclear factor-κB-inducing kinase; NS, not significant.
Effect of LEC-specific NIK deficiency on immune responses
Humoral immune response depends on the formation of germinal centers (GCs) within LNs in response to T cell-dependent antigens, resulting in the generation of plasma cells and memory B cells.41 Upon activation by antigens, B cells interact with cognate T cells to promote differentiation of the T cells into T follicular helper (TFH) cells that in turn stimulate proliferation of the antigen-specific B cells within the GCs. To investigate the effect of LEC-specific NIK deficiency on humoral immune responses, we immunized WT and NIKLEC-KO mice with the hapten NP (4-hydroxy-3-nitrophenylacetyl) linked to keyhole lympet hemocyanin (NP-KLH) that represents a T cell-dependent antigen. Under immunized conditions, the iLN in NIKLEC-KO mice still had smaller size and reduced cell counts than the iLN in WT mice (Supplementary Figure 4a). Furthermore, compared with unimmunized mice, the frequencies of B cells in iLNs elevated in both WT and NIKLEC-KO mice after immunization (Figures 6a and 2c, and Supplementary Figure 4b); however, NIKLEC-KO mice still had lower frequencies and absolute numbers of B cells than WT mice (Figure 6a). Further analysis of B-cell population revealed comparable percentages of CD95+GL7+ GC B cells in WT and NIKLEC-KO mice, although the number of GC B cells decreased in NIKLEC-KO mice because of reduced cellularity and total B cells of iLNs (Figure 6b). In addition, NIKLEC-KO mice had a reduced frequency and absolute number of CXCR5+PD1+ TFH cells (Figure 6c), likely because of reduced numbers of cognate B cells in the LNs.
Figure 6.
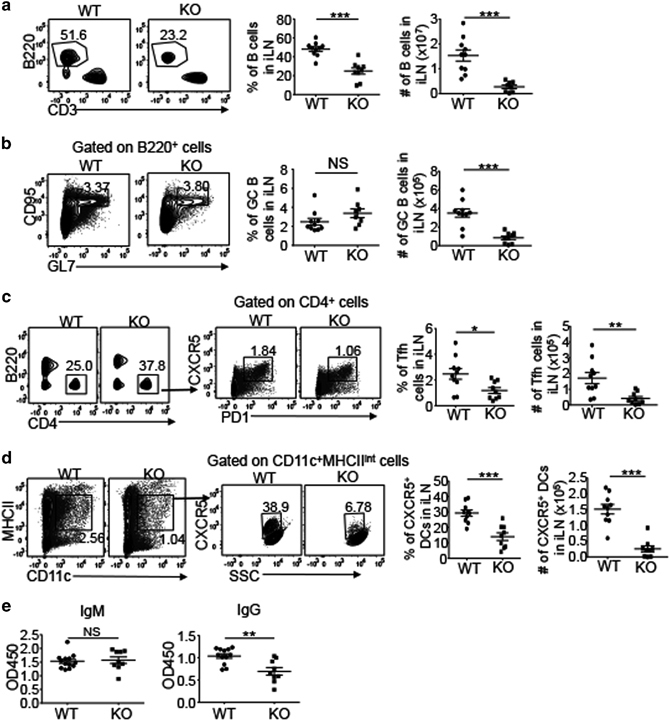
Effect of LEC-specific NIK deficiency on immune responses. (a) Flow cytometry analysis of the frequency and absolute cell number of B cells (B220+) in the iLNs of WT and NIKLEC-KO (KO) mice immunized with NP-KLH. Data are presented as representative plots (left) and summary graphs (right). (b–d) Flow cytometry analysis of the frequency and absolute cell number of germinal center B cells (b, CD95+GL7+), TFH cells (c, PD1+CXCR5+) and CXCR5+ DCs (d) in the iLNs of WT and NIKLEC-KO (KO) mice immunized with NP-KLH. Plots were gated on B220+ (b), CD4+ (c) and CD11c+MHCIIint (d) cells. Data are presented as representative plots (left) and summary graphs (right). (e) ELISA of NP-specific IgM and IgG in the sera of WT and NIKLEC-KO (KO) mice immunized with NP-KLH. Data are representative of two independent experiments and are presented as means±s.e.m. *P<0.05; **P<0.01; ***P<0.001. DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; iLN, inguinal lymph node; KO, knockout; LEC, lymphatic endothelial cell; NIK, nuclear factor-κB-inducing kinase; NP-KLH, hapten NP (4-hydroxy-3-nitrophenylacetyl) linked to keyhole lympet hemocyanin; NS, not significant, TFH, T follicular helper; WT, wild type.
CXCR5+ DCs have been reported to migrate to B-cell follicles to interact with B cells during humoral immune responses,37,39 and we thus examined CXCR5+ DCs in WT and NIKLEC-KO mice after immunization. Compared with unimmunized mice, immunized WT and NIKLEC-KO mice had increased frequencies and absolute numbers of CXCR5+ DCs in iLNs (Figures 6d and 5c, and Supplementary Figure 4c); however, NIKLEC-KO mice still had lower frequencies and absolute numbers of CXCR5+ DCs than WT mice under immunized conditions (Figure 6d and Supplementary Figure 4c). Histological analysis revealed that although the iLN of the NIKLEC-KO mice was smaller than that of WT mice, the overall structural pattern of lymphoid follicles was similar between the WT and KO iLNs (Supplementary Figure 4d). During humoral immune responses, B cells are activated by antigens and become plasma cells within GCs that secret immunoglobulins to protect the host. We measured antigen-specific IgM and IgG in the serum of immunized mice and found that the production of antigen-specific IgG, although not IgM, was significantly reduced in NIKLEC-KO mice compared with WT mice (Figure 6e). These findings together suggested that although the LEC-specific NIK deficiency did not influence antigen-stimulated activation and differentiation of B cells, the attenuated B-cell homing to LNs compromised the magnitude of humoral immune responses after immunization.
Discussion
Lymphatic vessels are known to mediate tissue fluid homeostasis and serve as a route for peripheral tissue DCs to migrate to the LNs during an immune response. The data presented in this study have demonstrated a novel function of lymphatic vessels in the regulation of B-cell homing to LNs. This function relied on expression of the noncanonical NF-κB-inducing kinase NIK in LECs, in which NIK mediated expression of the B-cell homing chemokine CXCL13. Although CXCL13 is known to mediate B-cell homing to LNs via the HEVs, the cells producing CXCL13 have been enigmatic. Our data revealed that LECs were capable of producing CXCL13 in a NIK-dependent manner. In addition to B cells, a population of DCs expresses the CXCL13 receptor CXCR5. As seen with B cells, the homing of CXCR5+ DCs to LNs was attenuated upon deletion of LEC-specific NIK. We further demonstrated that the NIKLEC-KO mice had compromised humoral immune responses, especially in the production of IgG. These findings suggest that the NIK-dependent CXCL13 expression in LECs play an important role in mediating B-cell homing to LNs and humoral immune responses.
The role of noncanonical NF-κB pathway in regulating B-cell development and function has been studied using conventional NIK KO mice and the Aly/Aly homozygous mice that carry a loss-of-function mutation in NIK.24,25 These mice exhibit disrupted lymphoid organ architecture, lack of LNs, reduced B cells and immunoglobulin production.24,25,42–45 In addition, NIK-deficient B cells do not respond to B-cell-activating factor (BAFF) stimulation, suggesting that NIK plays a crucial role in regulating BAFF-mediated B-cell survival.25 However, it has been difficult to fully elucidate the function of noncanonical NF-κB pathway using the conventional NIK KO or mutant mice because of their impaired lymphoid organogenesis. In the present study, we took advantage of conditional NIK KO mice to specifically delete NIK in LECs that allowed the study of NIK function in lymphatic vessels. In contrast to the whole-body NIK KO and mutant mice, the NIKLEC-KO mice had no obvious abnormalities in the spleen and LNs, with the exception of reduced size of iLNs. These conditional KO mice also have normal B-cell maturation and homeostasis in the spleen. Taking advantage of NIKLEC-KO mice, we discovered an unexpected role of NIK in regulating CXCL13 expression in LECs and B-cell homing into LNs.
The continuous migration of immune cells into LNs is crucial for immune surveillance of foreign pathogens. Naive T and B cells extravasate through HEVs, the site of lymphocytes trafficking from the blood to the LNs, via a multistep adhesion cascade.46 Stromal cell networks and chemokines produced by stromal cells play an essential role in regulating the homing of immune cells into and within LNs.47 T cells enter the T-cell areas in the paracortex of LNs, whereas B cells migrate into the B-cell follicles in the cortex that is regulated by CCL19, CCL21 and CXCL13, respectively.4 The sources of these chemokines are poorly identified, although it has been reported that CCL21 is abundantly expressed by the endothelial cells of HEVs, whereas CXCL13 is mainly produced by non-HEV cells and transported to the luminal surface of HEVs.10,48 Also, FRCs in the T-cell zone secrete CCL19 and CCL21, whereas follicular dendritic cells (FDCs) in B-cell follicles produce CXCL13.49–51 Lymphatic vessels are the major sites of migration of DCs and T cells from peripheral tissue to the regional LNs; however, it has remained unclear whether lymphatic vessels are involved in the chemokine production and the regulation of migration of immune cells from the blood. We found in this study that LECs in the LNs express CXCL13 and CCL19 that are significantly reduced in NIK-deficient LECs. In contrast, BECs produce CCL19 but not CXCL13. In addition, the homing of B cells to the LNs is severely impaired in NIKLEC-KO mice, indicating that NIK-expressing-LECs play a crucial role in regulating B-cell migration into the LNs, likely through CXCL13. Similar to our results, a prior in vitro study using mouse embryonic fibroblasts also demonstrates that the noncanonical NF-κB pathway regulates the production of CCL19 and CXCL13 in response to LTβR signals.52 Our present study suggests abundant expression of LTβR in LECs, and it is known that immune cells, such as DCs and T cells, express ligands of LTβR.53,54 It is thus likely that NIK may mediate CXCL13 induction in the LTβR pathway in LECs. We found that the NIKLEC-KO mice had reduced, but not blocked, B-cell homing to LNs, despite the efficient deletion of NIK in LECs using the Lyve1-Cre. The partial block of B-cell homing might be because of expression of CXCL13 by other subsets of stromal cells, such as the FDCs, of NIKLEC-KO mice.
DCs migrate into the LNs by two routes: the afferent lymphatic vessels and the HEVs.34 DCs from peripheral tissues uptake foreign antigens and traffic through afferent lymphatic vessels into regional LNs, where DCs present antigens to and activate naive T cells. In addition, plasmacytoid DCs and the precursors of conventional DCs enter LNs from the blood through HEVs.55,56 CCR7 has been identified as a key chemokine receptor regulating the migration of DCs into LNs.35 CCL19 and CCL21, the ligands for CCR7, are involved in guiding DC migration into and within the LNs to the T cell zone, where T-cell priming takes place.57,58 In addition, CXCR5+ DCs have recently been identified in the spleen, LNs and the dermis of the skin.37–39 CXCR5+ DCs in the LNs increase after infection with Heligmosomoides polygyrus and regulate type 2 helper T-cell responses.37 CXCL13, the ligand for CXCR5, plays an essential role in the localization of CXCR5+ DCs near B-cell follicles.37–39 However, the role of lymphatic vessels in regulating the migration of CXCR5+ DCs has remained unclear. We demonstrated here that CXCR5+ DCs significantly reduced in the LNs, whereas they remained unchanged in the spleen of NIKLEC-KO mice, suggesting that lymphatic vessels are involved in regulating the homing of CXCR5+ DCs to the LNs, likely through LEC-expressing-CXCL13 that is regulated by NIK. In contrast, the frequencies of skin-derived MHCIIhighDCs in the LNs were comparable between WT and NIKLEC-KO mice under both steady-state and inflammatory conditions, indicating that LEC-specific NIK is dispensable for MHCIIhigh DC migration from the skin to the LNs.
We found that LEC-specific NIK deficiency did not influence antigen-stimulated B-cell activation or germinal center B-cell formation. However, in response to immunization by a protein antigen, the NIKLEC-KO mice produced reduced amount of IgG, although not IgM, compared with the WT mice. This result was consistent with the profound reduction in the number of B cells in the LNs of NIKLEC-KO mice. In addition, we found that the iLNs of immunized NIKLEC-KO mice had a reduced frequency and absolute number of TFH that may also contribute to the reduction in antibody production.
In summary, our findings have provided insight into the mechanism by which lymphatic vessels regulate immune cell homing. Our work has demonstrated a role for NIK in regulating the expression of CXCL13 by LECs and the homing of B cells and the CXCR5+ DCs to the LNs. Notably, our findings have implications for the development of potential therapeutic approaches targeting lymphatic vascular system for manipulating humoral immune responses.
Electronic supplementary material
Acknowledgements
We thank Genentech Inc. for providing the NIK-flox mice. This work was supported by grants from the National Institutes of Health (GM84459, AI057555, AI104519 and AI64639). This study also used the NIH/NCI-supported resources under award number P30CA016672 at The MD Anderson Cancer Center. SZ was supported by a scholarship from the China Scholarship Council (CSC) under the Grant CSC 201506210393. We also thank Professor Wei He for his support.
Author contributions
JY and SZ designed and performed the experiments, and JY prepared the figures and wrote the manuscript. LZ, XX, HW, ZJ, MG, J-YY and XC contributed to the performance of the experiments. S-CS supervised the work and wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2017.167
References
- 1.Gowans JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959;146:54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–370. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 4.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 5.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 6.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 7.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/S0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 8.Nakano H, Tamura T, Yoshimoto T, Yagita H, Miyasaka M, Butcher EC, et al. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur J Immunol. 1997;27:215–221. doi: 10.1002/eji.1830270132. [DOI] [PubMed] [Google Scholar]
- 9.Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T, et al. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol. 2003;171:1642–1646. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 11.Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein—coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 12.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 14.Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 15.Kesler CT, Liao S, Munn LL, Padera TP. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2013;5:111–124. doi: 10.1002/wsbm.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo KP, Angeli V. Bidirectional crosstalk between lymphatic endothelial cell and T cell and its implications in tumor immunity. Front Immunol. 2017;8:83. doi: 10.3389/fimmu.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 22.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 23.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/S1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 24.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 25.Brightbill HD, Jackman JK, Suto E, Kennedy H, Jones C, 3rd, Chalasani S, et al. Conditional deletion of NF-kappaB-inducing kinase (NIK) in adult mice disrupts mature B cell survival and activation. J Immunol. 2015;195:953–964. doi: 10.4049/jimmunol.1401514. [DOI] [PubMed] [Google Scholar]
- 26.Maijer KI, Noort AR, de Hair MJ, van der Leij C, van Zoest KP, Choi IY, et al. Nuclear factor-kappaB-inducing kinase is expressed in synovial endothelial cells in patients with early arthritis and correlates with markers of inflammation: a prospective cohort study. J Rheumatol. 2015;42:1573–1581. doi: 10.3899/jrheum.150245. [DOI] [PubMed] [Google Scholar]
- 27.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 29.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight. 2016;1:e85096. doi: 10.1172/jci.insight.85096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MD, Srivastava B, Allman D. Regulation of peripheral B cell maturation. Cell Immunol. 2006;239:92–102. doi: 10.1016/j.cellimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol. 2011;2:35. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol 2009, 31–49. [DOI] [PubMed]
- 35.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 36.Cyster JG. Leukocyte migration: scent of the T zone. Curr Biol. 2000;10:R30–R33. doi: 10.1016/S0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 37.Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeki H, Wu MT, Olasz E, Hwang ST. A migratory population of skin-derived dendritic cells expresses CXCR5, responds to B lymphocyte chemoattractant in vitro, and co-localizes to B cell zones in lymph nodes in vivo. Eur J Immunol. 2000;30:2808–2814. doi: 10.1002/1521-4141(200010)30:10<2808::AID-IMMU2808>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH, et al. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol. 2002;168:5117–5123. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]
- 40.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 41.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada T, Mitani T, Yorita K, Uchida D, Matsushima A, Iwamasa K, et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 43.Shinkura R, Matsuda F, Sakiyama T, Tsubata T, Hiai H, Paumen M, et al. Defects of somatic hypermutation and class switching in alymphoplasia (aly) mutant mice. Int Immunol. 1996;8:1067–1075. doi: 10.1093/intimm/8.7.1067. [DOI] [PubMed] [Google Scholar]
- 44.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 45.Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel RM. Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur J Immunol. 2000;30:2799–2807. doi: 10.1002/1521-4141(200010)30:10<2799::AID-IMMU2799>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 47.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 51.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/S1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 53.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 54.Kumar V, Dasoveanu DC, Chyou S, Tzeng TC, Rozo C, Liang Y, et al. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity. 2015;42:719–730. doi: 10.1016/j.immuni.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1171243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seth S, Oberdorfer L, Hyde R, Hoff K, Thies V, Worbs T, et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol. 2011;186:3364–3372. doi: 10.4049/jimmunol.1002598. [DOI] [PubMed] [Google Scholar]
- 57.Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


