The Xpert HIV-1 Qual demonstrated excellent performance for point-of-care human immunodeficiency virus early infant diagnosis (EID) testing using whole blood at health facilities in Tanzania. EID sensitivity at week 6 was affected, possibly due to viral suppression under nevirapine prophylaxis.
Keywords: HIV, early infant diagnosis, point-of-care testing, Africa, mother-to-child transmission
Abstract
Background
Point-of-care (PoC) systems for early infant diagnosis (EID) may improve timely infant human immunodeficiency virus (HIV) management. Experiences within African public health settings are limited.
Methods
We evaluated the accuracy and operational feasibility of the Xpert HIV-1 Qual for PoC-EID testing, using fresh blood and dried blood spots (DBS) samples at obstetric health facilities in Tanzania at birth and at postpartum weeks 1, 2, 3, and 6 in HIV-exposed infants. Test results were confirmed using TaqMan DBS HIV-deoxyribonucleic acid and/or plasma HIV-ribonucleic acid (RNA) testing.
Results
At week 6, 15 (2.5%) out of 614 infants were diagnosed with HIV; 10 (66.7%) of them at birth (median HIV-RNA 4570 copies/mL). At birth, the Xpert-PoC and Xpert-DBS were 100% sensitive (95% confidence intervals: PoC, 69.2–100%; DBS, 66.4–100%) and 100% specific (PoC, 92.1–100%; DBS, 88.4–100%). By week 3, 5 infants with intra/postpartum HIV-infection (median HIV-RNA 1 160 000 copies/mL) were all correctly diagnosed by Xpert. In 2 cases, Xpert-PoC testing correctly identified HIV-infection when DBS tests (Xpert and TaqMan) were negative, suggesting a greater sensitivity. In 2 infants with confirmed HIV at birth, all tests were negative at week 6, possibly because of viral suppression under nevirapine prophylaxis. Problems were reported in 183/2736 (6.7%) of Xpert-PoC tests, mostly related to power cuts (57.9%).
Conclusions
We demonstrated excellent Xpert HIV-1 Qual performance and good operational feasibility for PoC-EID testing at obstetric health facilities. Week 6 sensitivity issues were possibly related to nevirapine prophylaxis, supporting additional birth PoC-EID testing to avoid underdiagnosis.
Clinical Trials Registration
Despite a significant decrease of infant human immunodeficiency virus (HIV) infections globally, 160 000 new pediatric HIV infections were estimated in 2016 [1]. Most infant HIV infections in sub-Saharan Africa occur through vertical mother-to-child transmission (MTCT) during the perinatal and breastfeeding periods. Without treatment, infant mortality peaks at 2–3 months of age [2], and almost half of HIV-infected infants die if untreated during the first 2 years of life [3]. The World Health Organization recommends HIV nucleic acid testing for HIV-exposed infants at 4 to 6 weeks after birth [4]. Early infant diagnosis (EID) is widely performed by collecting dried blood spots (DBS), which are then sent to centralized laboratories for HIV–deoxyribonucleic acid (DNA) polymerase chain reaction (PCR). These procedures involve multiple linkage steps, which are often associated with high turnaround times and result in delays in communicating results to the mother and initiating antiretroviral therapy (ART) [5–9]. By 2015, only 49% of HIV-exposed infants received EID procedures, of which less than 50% were performed within the recommended first 2 months of life [10, 11]. In a comparative analysis of national programs in 4 African countries, only 30% of perinatally-infected infants were effectively linked to services and started ART in a timely manner [12].
Novel point-of-care (PoC) technologies have the potential to decentralize bedside testing, providing HIV results within less than 2 hours. Hence, PoC EID should enable immediate ART initiation, resulting in further reduction of infant HIV morbidity, mortality, and seeding of viral reservoirs [10, 13]. Several studies from Africa investigating different HIV PoC platforms demonstrated high accuracy for EID; however, most of these studies were performed under laboratory conditions, not under typical field settings [14, 15]. In 2016, 2 qualitative PoC HIV-1 nucleic acid testing systems received World Health Organization prequalifications (Cepheid Xpert HIV-1 Qual and Alere q HIV-1/2 Detect), and within their latest guidelines, the World Health Organization’s recommendation for the use of PoC nucleic acid testing technologies for EID is only conditional, indicating that there is a lack of operational experience in the field [4].
METHODS
Study Design
This prospective diagnostic cohort study in infants born to HIV-infected mothers evaluated the accuracy and operational feasibility of the Xpert HIV-1 Qual assay on the GeneXpert system (Cepheid, Sunnyvale, CA) using fresh, whole blood (Xpert-PoC) collected at different postpartum times in Mbeya, Tanzania. The study was conducted by the National Institute for Medical Research, Mbeya Medical Research Centre and sponsored by the University of Munich. Ethical clearance was granted by the Mbeya Medical Research and Ethics Committee, the Medical Research Coordinating committee in Tanzania, and the ethics committee at the University of Munich in Germany. Regulatory approval was granted by the Tanzania Food and Drugs Authority. This study was registered with ClinicalTrials.gov (NCT02545296).
Participants
The study included HIV-infected pregnant women above 18 years of age and, after delivery, their newborn babies. All recruited women provided written informed consent for themselves and their babies after receiving verbal and written study information. Informed consent was not obtained in a state of full labor or when participants were experiencing birth-related stress, pain, or emotional distress. Women and infants were excluded from study participation if immediate maternal or infant medical assistance was required; in the case of a stillbirth or severe congenital malformation; if the birth was >48 hours prior to enrollment; or if the participant was unlikely to comply with the protocol, as judged by the investigator.
Procedures
Infants were tested for HIV at birth and at postpartum weeks 1, 2, 3 and 6, using 100 µL of fresh blood collected via a heel prick and and analyzed using the Xpert-PoC test at their health facilities, which provided maternity and postnatal clinic services during all visits. Testing was performed by trained nurses/midwives, who documented the date and time of the sample collections and the start of testing, and who informed the mothers about the test results. HIV-PoC results were reported as HIV either being undetected or detected; problems related to system handling, errors, or invalid results were recorded. At each testing point, DBS samples were collected for qualitative HIV-DNA confirmation using the COBAS TaqMan V2 (Roche Molecular Systems, Branchburg, NJ); the confirmation tests were performed at week 6 for all infants, according to the routine Tanzanian infant HIV testing algorithm, and immediately for all infants with positive Xpert-PoC results. All infants with positive Xpert-PoC results for plasma HIV–ribonucleic acid (RNA) analysis (TaqMan V2) and CD4 counting (FACSCount system, BD Bioscience, San Jose, CA) at both the time of the first positive Xpert-PoC result and at the final study visit were targeted for phlebotomy. In the case of positive Xpert-PoC results, PoC testing was continued at all subsequent visits. Retrospective Xpert HIV-1 Qual testing was performed from stored DBS (Xpert-DBS) for all HIV-infected infants at each point in time, as well as in a subset of non-infected infants for comparison of the Xpert-DBS and the Xpert-PoC. DBS HIV-DNA analyses were performed at the laboratory of the Mbeya Zonal Referral Hospital; HIV-RNA, CD4-count, and retrospective Xpert-DBS tests were performed at the College of American Pathologists–accredited research laboratory at the National Institute for Medical Research, Mbeya Medical Research Centre. All HIV-exposed infants were offered nevirapine prophylaxis for 6 weeks. Following discussion with the pediatric HIV care and treatment center and the local ethics committee, it was decided to continue nevirapine in infants with positive Xpert-PoC test results until HIV confirmation, as the Xpert-PoC was still considered investigational. All confirmed HIV-infected infants were referred to the pediatric HIV care and treatment center for ART initiation.
Outcomes
The primary endpoint of this study was identification of HIV infections in newborns, as diagnosed by the Xpert-PoC test and confirmed by standard DBS-EID procedures and plasma viral load (pVL) testing at birth and until 6 weeks postpartum. Secondary endpoints included identification of neonatal HIV infection at any of the postpartum times using Xpert-PoC and Xpert-DBS test results.
Statistical Analyses
All data were recorded on paper, double entered into an OpenClinica database, and corrected for data entry errors. The sample size was based on the primary study endpoint to correctly identify infant HIV infections developed up to week 6 by Xpert-PoC EID, with 80% power and a lower 95% confidence limit of the positive predictive value of 70% or above. To assess diagnostic performance, only valid Xpert results were considered, and binary Xpert-PoC and Xpert-DBS test results were analyzed for sensitivities, specificities, and positive and negative predictive values against the reference standard HIV tests (TaqMan DBS qualitative HIV-DNA and/or pVL). Xpert test results were considered (1) true positive if HIV was confirmed at the same time, or when an HIV infection was already diagnosed in the context of previous visits; (2) true negative when all Xpert tests were negative and HIV negativity was confirmed at least once at the last study visit; (3) false positive if confirmatory tests were negative at the same time and HIV negativity was confirmed during the following visits; or (4) false negative if confirmatory tests were positive at the same time or HIV positivity was already confirmed during previous visits.
Categorical data were characterized by proportions and continuous data by their medians and ranges. Turnaround times of Xpert-PoC test procedures were extracted from nurse records, except the durations of sample analyses, which were exported from the Xpert analyzer’s software. All statistical analyses were performed using Stata statistics software (V14, StataCorp, College Station, TX).
RESULTS
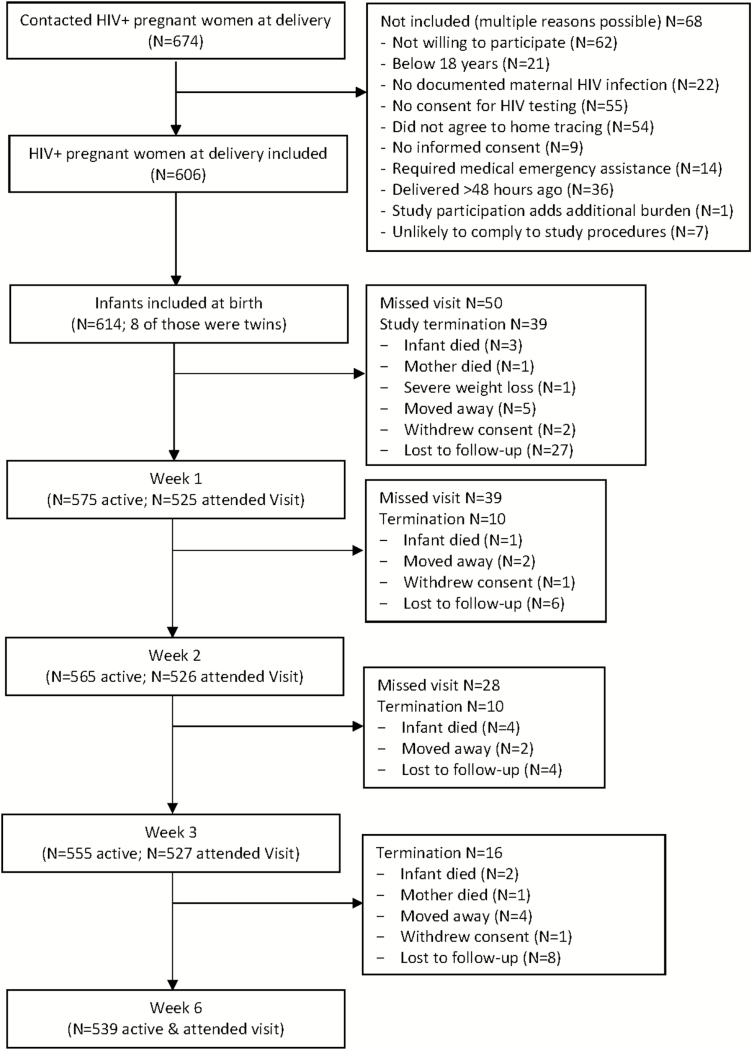
Between June 2015 and September 2016, we screened 674 HIV-infected pregnant mothers who were close to delivery, and enrolled 614 HIV-exposed, live-born infants—16 of them twins—from 606 mothers (Table 1). Reasons for maternal exclusions and early study terminations are shown in Figure 1. The 6-week study period was not completed by 75 (12%) infants, of which 45 (60%) were lost to follow-up and 4 (5.3%) had consent withdrawn by the mother. The majority of early terminations occurred between birth and the second follow-up visit. There were 10 infants who died during the study period due to suspected neonatal sepsis, fever, and respiratory distress (n = 5; 1 of which was an HIV-infected infant), severe pneumonia (n = 1), sudden infant death (n = 2), probable aspiration after feeding with cow’s milk (n = 1), and suspected obstructive bowel disease without passing stool (n = 1). Additionally, 3 infants were hospitalized—1 HIV-infected infant due to pneumonia, 1 not-infected to neonatal sepsis, and not-infected 1 to bullous impetigo—all 3 infants recovered and completed the study.
Table 1.
Infant’s Characteristics (N = 614)
| n (%) | |
|---|---|
| Health facility | |
| Mbeya Zonal Referral Hospital (tertiary level) | 198 (32.3) |
| Mbeya Regional Hospital (secondary level) | 80 (13.0) |
| Ruanda Health Centre (primary level) | 126 (20.5) |
| Kiwanjampaka Health Centre (primary level) | 52 (8.5) |
| Igawilo Health Centre (secondary level) | 158 (25.7) |
| Gender | |
| Female | 299 (48.7) |
| Male | 315 (51.3) |
| Twins | 16 (2.6) |
| Birth weight | |
| Normal (2.5 to 4.5 kg) | 567 (92.3) |
| Low (<2.5 to 1.5 kg) | 47 (7.7) |
| Apgar score at 5 minutes | |
| 8 to 10 | 595 (96.9) |
| Missing | 19 (3.1) |
| Started nevirapine prophylaxis | |
| Yes | 611 (99.5) |
| No | 3 (0.5) |
| Mode of delivery | |
| Vaginal at hospital | 498 (81.1) |
| Vaginal at home | 25 (4.1) |
| Elective caesarean section | 62 (10.1) |
| Emergency caesarean section | 27 (4.4) |
Figure 1.
Study flow. Abbreviation: HIV, human immunodeficiency virus.
Infant HIV Infection and Clinical Presentation
HIV infection was diagnosed in 15/614 (2.5%) infants by 6 weeks post-delivery. Of those, 10 (66.7%) were diagnosed at birth, suggesting intra-uterine transmission. In 5 (33.3%) infants with negative HIV results at birth, intra- or postpartum infection seemed likely. Another 2 infants were first diagnosed at week 1, 1 at week 2 (this infant missed the week 1 assessment and might have been diagnosed earlier otherwise), and 2 at week 3. No new HIV infection was diagnosed at week 6. Viral load results at birth or week 1 were available for 9 HIV-infected infants with a median HIV-RNA of 4570 copies/mL (range 1050 to 53 100). The 5 infants who first tested positive in the postpartum weekly tests had much higher pVLs, with a median of 1 160 000 copies/mL (range 116 000 to >107). For 3 of these infants, clinical symptoms were reported: these were unspecific (vomiting and rhinitis) for 2 babies, while the third developed symptoms consistent with an acute retroviral syndrome (oral thrush, rash, and lymphadenopathy).
Infant HIV Diagnoses
Overall, 2733 Xpert-PoC tests were performed from fresh, whole blood during study visits by nurses at the obstetric health facilities, and 381 retrospective Xpert-DBS tests from 297 infants (including all HIV-infected infants at each testing time) were analyzed at the centralized laboratory. Matched confirmatory tests (TaqMan DBS and/or pVL) were available 676 times, and were performed for all visits in infants who had at least 1 positive Xpert-PoC result, as well as at least once for all infants with negative Xpert-PoC results throughout. Infants with negative Xpert results throughout plus a final negative confirmatory test result were regarded as HIV-uninfected, and all their Xpert results were considered as true negatives.
The diagnostic performance of Xpert testing at birth was excellent, both as PoC and in DBS samples, which both had a sensitivity of 100% (95% CI: PoC, 69.2–100%; DBS, 66.4–100%) and a specificity of 100% (95% CI: PoC, 92.1–100%; DBS, 88.4–100%; Table 2). To evaluate the Xpert-PoC test during follow-up, we interpreted test results in the context of all Xpert and confirmatory test results throughout all visits. The Xpert-PoC test correctly identified all infected infants and, in addition, provided a positive result in 1 incident case at week 1, when confirmatory tests were still negative but turned positive during the following visits (Tables 2 and 3, case 1). In 2 cases (Tables 2 and 3, cases 12 and 15), HIV infection was correctly identified at birth, but some tests turned negative by week 2 and all HIV tests for both cases were negative (or below the threshold of quantifiable HIV-RNA) at week 6, which we interpreted as a result of viral suppression below detection limits in the context of nevirapine prophylaxis. Repeated HIV tests at around week 10 (1 case received triple ART) revealed very low viral replication in both cases and, except for the Xpert-PoC in 1 case, all other HIV tests remained non-reactive (see Supplementary Table 1 for all HIV-infected infant outcome information). When considering HIV infection in the context of all study visits as the reference standard, the overall test performance can be summarized as follows for the Xpert-PoC: sensitivity, 94.7% (85.4–98.9%); specificity, 100% (99.9–100); positive predictive value, 100% (93.4–100); and negative predictive value, 99.9% (99.7–100). The overall test performance can be summarized for the Xpert-DBS as: sensitivity, 88.6% (75.4–96.2); specificity, 100% (98.9–100); positive predictive value, 100% (91.0–100); and negative predictive value, 98.5% (96.6–99.5).
Table 2.
Infant Human Immunodeficiency Virus (HIV) Test Results at Birth Until Week 6 Postpartum for (1) the Xpert-Point of Care Test, Performed from Whole Blood at Obstetric Health Facilities and (2) Retrospective Analysis of Xpert-Dried Blood Spots (DBS) at the Centralized Laboratory Compared to the Standard TaqMan HIV Assay (Either Qualitative HIV-DNA Analysis in DBS and/or Quantitative HIV-RNA Analysis in Plasma)
| TaqMan | TaqMan | ||||||
|---|---|---|---|---|---|---|---|
| Xpert-PoC | Negative | Positive | Xpert-DBS | Negative | Positive | ||
| Birth n = 614 |
Negative | 588 | 0 | Birth n = 68 |
Negative | 59 | 0 |
| Positive | 0 | 10 | Positive | 0 | 9 | ||
| Error/not valid | 16 | 0 | ... | ... | ... | ||
| Week 1 n = 525 |
Negative | 499 | 0 | Week 1 n = 38 |
Negative | 29 | 0 |
| Positive | 1a | 9 | Positive | 0a | 9 | ||
| Error/not valid | 15 | 1 | ... | ... | ... | ||
| Week 2 n = 526 |
Negative | 503 | 0 | Week 2 n = 35 |
Negative | 23 | 1c |
| Positive | 1b | 11 | Positive | 1b | 10 | ||
| Error/not valid | 11 | 0 | ... | ... | ... | ||
| Week 3 n = 527 |
Negative | 504 | 0 | Week 3 n = 0 |
Negative | ... | ... |
| Positive | 0 | 11 | Positive | ... | ... | ||
| Error/not valid | 12 | 0 | ... | ... | ... | ||
| Week 6 n = 539 |
Negative | 521d | 0 | Week 6 n = 239 |
Negative | 229d | 0 |
| Positive | 0 | 10 | Positive | 0 | 10 | ||
| Error/not valid | 8 | 0 | ... | ... | ... | ||
| Post Week 6 n = 2 (false negatives from week 6) |
Negative | 0 | 1d | Post week 6 n = 1 (false negative from week 6) |
Negative | 0 | 1d |
| Positive | 0 | 1 | Positive | 0 | 0 | ||
| Error/not valid | 0 | 0 | ... | ... | ... | ||
Abbreviations: DBS, dried blood spots; PoC, point of care.
aA true positive Xpert-PoC test with false negative TaqMan and Xpert-DBS tests, as confirmed in the following visits (also refer to Table 3, case 1)
bTrue positive Xpert-PoC and Xpert-DBS tests with a false negative TaqMan test, as confirmed in the previous and following visits, (also refer to Table 3, case 15)
cA false negative Xpert-DBS test with true positive Xpert-PoC and TaqMan tests, as confirmed in the previous and following visits (also refer to Table 3, case 12)
dThere were 2 cases with false negatives for all tests (Xpert-PoC, Xpert-DBS, and TaqMan) at week 6, as confirmed in the previous and following visits (also refer to Table 3, cases 12 and 15)
Table 3.
Human Immunodeficiency Virus (HIV) Test Results and Antiretroviral Treatment: Outcomes of Infant Xpert-Point of Care from Whole Blood and Xpert-Dried Blood Spots (DBS) Test Results, Confirmatory HIV Test Results by HIV-DNA in DBS and HIV-RNA in Plasma Associated With Infant Antiretroviral Treatment/Prophylaxis, for 3 Selected Cases at Different Times
| Case | Maternal VL at Birth | Infant HIV Diagnostic Tests | Birth | Week 1 | Week 2 | Week 3 | Week 6 | Post Week 6 |
|---|---|---|---|---|---|---|---|---|
| #1 | 41 900 c/mL | Xpert-PoC | neg | pos | pos | pos | pos | ... |
| Xpert-DBS | neg | neg | pos | pos | pos | |||
| TaqMan DBS HIV-DNA | neg | neg | pos | pos | pos | ... | ||
| TaqMan plasma HIV-RNA | ND | <34 c/mL | ND | ND | 116 000 c/mL | ... | ||
| Infant ARVs | NVP | NVP | NVP | NVP | ABC+3TC+LPV/r | ... | ||
| #12 | 15 100 c/mL | Xpert-PoC | pos | pos | pos | pos | neg | pos |
| Xpert-DBS | pos | pos | neg | ND | neg | ND | ||
| TaqMan DBS HIV-DNA | pos | pos | pos | pos | neg | ND | ||
| TaqMan plasma HIV-RNA | 53 100 c/mL | ND | ND | ND | <34 c/mL | 292 c/mL | ||
| Infant ARVs | NVP | NVP | NVP | NVP | NVP | Not knowna | ||
| #15 | 181 000 c/mL | Xpert-PoC | pos | pos | pos | pos | neg | neg |
| Xpert-DBS | pos | pos | pos | pos | neg | neg | ||
| TaqMan DBS HIV-DNA | pos | pos | neg | pos | neg | neg | ||
| TaqMan plasma HIV-RNA | 1570 c/mL | ND | ND | ND | ND | 75 c/mL | ||
| Infant ARVs | NVP | NVP | NVP | NVP | ZDV+3TC+LPV/r | ZDV+3TC+LPV/r |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ARVs, antiretrovirals; c/mL, copies per milliliter; DBS, dried blood spots; HIV, human immunodeficiency virus; LPV/r, ritonavir boosted lopinavir; ND, not done; neg, negative; NVP, nevirapine; PoC, point of care; pos, positive; VL, viral load; ZDV, zidovudine.
aProbable start date at 3 months postpartum.
Operational Characteristics
The median time from delivery to Xpert-PoC birth testing was 16 hours (range 0.5–58) after delivery in the 593 cases with available information. In 74.8% of babies, testing occurred within 24 hours, in 20.5% between 24 and 48 hours, and in 1.3% >48 hours after birth (3.4% missing information). The main reasons for delayed testing were deliveries at home or during weekends. The median duration of a sample analysis for valid results throughout all visits was 92 minutes (range 91–95). The median time from sample collection to communication of results to the mother was 110 minutes (range 94 minutes to 7 days); in 98.7% of cases, results were disseminated on the same day. Delays were mainly due to mothers having left the clinic already or to technical problems that meant tests had to be performed at other clinics.
In 2670 of 2736 (97.7%) instances, Xpert-PoC testing provided valid results; in 63 cases (2.3%), no HIV diagnosis was obtained, due to invalid test result, error messages, or other technical reasons. However, 132 tests (4.8%) had to be repeated; in 210 cases (7.7%), samples were transferred to other sites for analysis, mainly due to power cuts; and in 183 cases (6.7%), nurses reported problems related to Xpert-PoC procedures (Table 4), most often due to power cuts. Confirmatory HIV tests in Xpert-PoC–positive cases were usually received after 2–3 weeks. Infant ART was initiated by week 6 in 11 out of the 14 infants who were still alive: in 2 cases ART initiation was delayed beyond the study period and in 1 case ART information was missing.
Table 4.
Operational Performance of the Xpert-Point of Care System at 6 Obstetric Health Facilities, Based on Nurses Reports
| Overall Xpert-PoC Test Performance | N = 2736 |
|---|---|
| Valid test result | 2673 (97.7%) |
| Time between sample collection and result communication to the mother of valid test results, median (range) | 110 minutes (94 minutes to 7 days) |
| No final valid test result | 63 (2.3%) |
| Xpert-PoC test problem reported by nurses | 183 (6.7%) |
| Early termination of analysis because of power cut | 106 (57.9%) |
| Error/invalid result | 61 (33.3%) |
| Problem with computer or analyzer handling | 4 (2.2%) |
| Clotted blood | 2 (1.1%) |
| No reason indicated | 10 (5.5%) |
| Xpert-PoC testing had to be repeated | 132 (4.8%) |
| Xpert-PoC testing performed at other site (sample transferred) | 210 (7.7%) |
Abbreviation: PoC, point of care.
DISCUSSION
Our data demonstrated excellent test performance and good operational feasibility of the Xpert HIV-1 Qual for testing on fresh blood samples at birth at public obstetric health facilities and on DBS samples in HIV-exposed neonates in Tanzania. Our findings are comparable to recently-published data from South Africa (100% sensitivity, 99.9% specificity from fresh, wholeblood) [16] and from Botswana (93.3% sensitivity, 100% specificity from DBS) [17] for at-birth testing using the same diagnostic platform. The diagnostic performance in our study was slightly better than for the Alere PoC test at birth (90% sensitivity, 100% specificity in South Africa) [18] or for older infants (98.5% sensitivity, 99.9% specificity in Mozambique) [19]. A possible reason for the greater sensitivity of the Xpert compared to the Alere system might be the different sample volumes (100µL versus 25µL).
In our study, we had the opportunity to observe HIV transmission during the first weeks of life at very close intervals and using study-associated changes in the results of different HIV diagnostics. In 2 cases, we found that the Xpert-PoC had a higher sensitivity to detect neonatal HIV infection compared to Xpert-DBS or TaqMan HIV-DNA testing, either for incident cases following intrapartum transmission or when viral loads were very low, possibly due to nevirapine prophylaxis. The limit of detection for the Xpert HIV-1 Qual assay in fresh, whole blood is 203 copies/mL (95% CI: 181–225) and 531 copies/mL in DBS (95% CI: 474–587) according to the manufacturer [20]. However, in both cases, pVL were below these thresholds. We therefore would recommend caution in interpreting positive Xpert-PoC results as false positives when confirmatory DBS tests do not indicate HIV infection, and instead recommend close follow-up testing in these cases. Higher sensitivities are often associated with lower specificities, which we did not observe in our study, although the South African study, with its larger sample, indeed had a lower specificity [16]. False-positive test results in the absence of confirmatory testing leading to ART-initiation were assumed by authors for more than 10% of infants in settings with low MTCT rates, like in South Africa [21]. We would support the recommendation, given by the authors of these studies, that positive PoC results should immediately be confirmed.
In 2 out of 10 infants with confirmed HIV at birth, we found that HIV was not detected at week 6 by any diagnostic test, possibly due to very low pVL under nevirapine prophylaxis. In the absence of at-birth testing, these cases would not have been detected during 6 weeks of routine EID procedures, which is especially important for intra-uterine HIV-infected babies, with their higher risk for early mortality than infants who get infected during breastfeeding [3]. Viral loads in infants diagnosed at birth can be low (median 4570 copies/mL in our study), which might affect the test sensitivity at birth, especially when using DBS, as seen in the study from Botswana [17]. At later times, suppression of pVL under nevirapine prophylaxis might affect testing results for a significant proportion of neonates, as shown in the French Perinatal Cohort [22] and in South Africa [23, 24]. Our data, therefore, strongly support at-birth testing to reduce missed HIV diagnoses for intra-uterine–infected infants and suggest subsequent week 10 testing (ie, 4 weeks after stopping nevirapine, as recommended in the South African HIV Guidelines [25]).
During birth, the risk for MTCT is high, and in our study we investigated the earliest time at which intra- or postpartum HIV transmission might be detected. All 5 infants who were diagnosed postpartum were identified by Xpert-PoC and Xpert-DBS by week 3. Week 3 EID provides an additional option, especially in cases with high-risk MTCT criteria, to quickly identify neonatal HIV infection for immediate ART initiation, to reduce mortality and the establishment of the viral reservoirs associated with possible later, sustained remission, as previously discussed [26].
A drawback to at-birth testing is that mothers might not return for later EID procedures once a negative infant HIV test is provided. In our study, the majority of losses to follow-ups occurred early, and none of those cases had a positive HIV test at birth. We can speculate that these infants did not receive a second HIV assessment, which implies the need for maternal counseling and potential visit reminder mechanisms.
The operational Xpert-PoC test performance is in line with data from South Africa [16]; errors or invalid results were, however, reported less often in our study (2.2% vs. 5%). The greatest obstacles in our setting were the frequent power cuts, since early terminations of analyses or sample transfers to other sites with more reliable power facilities were the most frequently-reported problems; this is the reality in rural Africa. This problem should be resolved by introducing analyzers with integrated batteries, which would provide ongoing functionality for several hours and bridge any periods of power cuts.
In conclusion, our data are limited by the relatively low sample size, but we found excellent test performance and good operational feasibility of the Xpert HIV-1 Qual test for the use of EID from birth to postpartum week 6, especially when directly performed from fresh blood samples. Sensitivity issues during week 6 EID affected all evaluated HIV test systems, which rather reflects the timing of the currently-recommended EID procedures related to nevirapine -prophylaxis.
In an era of infant antiretrovirals prophylaxis and rapid ART initiation, the accuracy of infant HIV testing and the decision-making for lifelong ARTs are complex. In our view, infant HIV PoC testing at birth, and repeated confirmatory testing as applicable, provide important opportunities to increase diagnostic accuracy, earlier detection, and treatment of infant HIV infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections from 13–16 February 2017 in Seattle, Washington.
Acknowledgments. The authors thank all patients and their partners and families. They thank all the nurses and institutional staff involved in this study from the Mbeya Zonal Referral Hospital Hospital (Sarah Mlelwa, Happy Mtovisala, Dora Simwaba, Ruth Fungo, and Adelina Thomas); the Mbeya Regional Referral Hospital (Anna Sakasumba, Upendo Ngatunga, Veronica Sowoki, and Christina Livigha); the Ruanda Health Centre (Sakina Myefu, Helena Macha, Eledia Mandalasi, and Salome Mwalyezi); the Kiwanjampaka Health Centre (Nyemo Mwajeka, Neema Kapungu, and Sophia Hyera); and the Igawilo Hospital (Debora Kikaro, Neema Mwambene, Ruth Twamzihirwa, and Nitike Kilindu). They further thank Mr Abisai Kisinda, Dr Mkunde Chachage, and their team from the National Institute for Medical Research, Mbeya Medical Research Center (NIMR-MMRC) main laboratory; Mr Yohana Fungo from the Mbeya Zonal Referral Hospital laboratory and his team for confirmatory HIV testing; and Mr Dickens Kowuor and Mr Peter Agrea from the NIMR-MMRC and Dr Friedrich Rieß and Mrs Fidelina Zekoll from the Medical Center of the University of Munich data management unit for database programming and data management. The authors especially thank Mrs Dipti Lallubhai and Mrs Gwynn Stevens, PhD, from Cepheid South Africa for their tireless support regarding infrastructure, test reagents and analyzers, training, and maintenance.
Financial suppport. This work was supported by the German Centre for Infectious Diseases (grant numbers TTU 04.903 and TTU 04.703). Cepheid South Africa provided in-kind support for the GeneXpert analyzer; the Xpert HIV-1 Qual test kits; training and maintenance; and financial support for site infrastructure upgrades. Funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2017 Available at: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. Accessed 22 January 2018.
- 2. Bourne DE, Thompson M, Brody LL, et al. . Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS 2009; 23:101–6. [DOI] [PubMed] [Google Scholar]
- 3. Marston M, Becquet R, Zaba B, et al. . Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol 2011; 40:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2nd edn 2016. Available at: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1. Accessed 22 January 2018.
- 5. Gill MM, Hoffman HJ, Mokone M, et al. . Assessing very early infant diagnosis turnaround times: findings from a birth testing pilot in Lesotho. AIDS Res Treat 2017; 2017:2572594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diallo K, Kim AA, Lecher S, et al. . Early diagnosis of HIV infection in infants - one Caribbean and six Sub-Saharan African countries, 2011-2015. MMWR Morb Mortal Wkly Rep 2016; 65:1285–90. [DOI] [PubMed] [Google Scholar]
- 7. Tiam A, Gill MM, Hoffman HJ, et al. . Conventional early infant diagnosis in Lesotho from specimen collection to results usage to manage patients: where are the bottlenecks?PLoS One 2017; 12:e0184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thiha S, Shewade HD, Philip S, et al. . Early infant diagnosis of HIV in Myanmar: call for innovative interventions to improve uptake and reduce turnaround time. Glob Health Action 2017; 10:1319616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wexler C, Cheng AL, Gautney B, Finocchario-Kessler S, Goggin K, Khamadi S; HITSystem Team Evaluating turnaround times for early infant diagnosis samples in Kenya from 2011-2014: a retrospective analysis of HITSystem program data. PLoS One 2017; 12:e0181005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J 2013; 32:1080–5. [DOI] [PubMed] [Google Scholar]
- 11. Joint United Nations Programme on HIV/AIDS. UNAIDS 2015 progress report on the global plan Available at: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf. Accessed 22 January 2018.
- 12. Chatterjee A, Tripathi S, Gass R, et al. . Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health 2011; 11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Persaud D, Palumbo PE, Ziemniak C, et al. . Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS 2012; 26:1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceffa S, Luhanga R, Andreotti M, et al. . Comparison of the Cepheid GeneXpert and Abbott M2000 HIV-1 real time molecular assays for monitoring HIV-1 viral load and detecting HIV-1 infection. J Virol Methods 2016; 229:35–9. [DOI] [PubMed] [Google Scholar]
- 15. Hsiao NY, Dunning L, Kroon M, Myer L. Laboratory evaluation of the Alere q point-of-care system for early infant HIV diagnosis. PLoS One 2016; 11:e0152672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Technau KG, Kuhn L, Coovadia A, Murnane PM, Sherman G. Xpert HIV-1 point-of-care test for neonatal diagnosis of HIV in the birth testing programme of a maternity hospital: a field evaluation study. Lancet HIV 2017; 4:e442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ibrahim M, Moyo S, Mohammed T, et al. . Brief report: high sensitivity and specificity of the Cepheid Xpert HIV-1 qualitative point-of-care test among newborns in Botswana. J Acquir Immune Defic Syndr 2017; 75:e128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunning L, Kroon M, Fourie L, Ciaranello A, Myer L. Impact of birth HIV-PCR testing on the uptake of follow-up early infant diagnosis services in Cape Town, South Africa. Pediatr Infect Dis J 2017; 36:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jani IV, Meggi B, Mabunda N, et al. . Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr 2014; 67:e1–4. [DOI] [PubMed] [Google Scholar]
- 20. Word Health Organization. WHO prequalification of in vitro diagnostics: Xpert® HIV-1 Qual assay. June2016. Available at: http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160613PQPublicReport_0259-0700-00_XpertQualHIV_v2.pdf. Accessed 22 January 2018.
- 21. Dunning L, Francke JA, Mallampati D, et al. . The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: a cost-effectiveness analysis. PLoS Med 2017; 14:e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burgard M, Blanche S, Jasseron C, et al. . Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. J Pediatr 2012; 160:60–6 e1. [DOI] [PubMed] [Google Scholar]
- 23. Maritz J, Maharaj JN, Cotton MF, Preiser W. Interpretation of indeterminate HIV-1 PCR results are influenced by changing vertical transmission prevention regimens. J Clin Virol 2017; 95:86–9. [DOI] [PubMed] [Google Scholar]
- 24. Haeri Mazanderani AF, Du Plessis NM, Thomas WN, Venter E, Avenant T. Loss of detectability and indeterminate results: challenges facing HIV infant diagnosis in South Africa’s expanding ART programme. S Afr Med J 2014; 104:574–7. [DOI] [PubMed] [Google Scholar]
- 25. Department of Health, Republic of South Africa. National consolidated guideline for PMTCT and the management of HIV in children, adolescents and adults April 2015. Available at: http://www.sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf. Accessed 22 January 2018.
- 26. Persaud D, Gay H, Ziemniak C, et al. . Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.