In Human Immunodeficiency Virus–1 treatment-naive adults, a fixed combination of doravirine/lamivudine/tenofovir disoproxil fumarate demonstrated non-inferior antiretroviral efficacy to efavirenz/emtricitabine/tenofovir disoproxil fumarate at week 48, with similar immunologic effects, low viral drug resistance rates, and significantly fewer neuropsychiatric adverse events.
Keywords: doravirine, efavirenz, HIV-1, treatment-naive, randomized controlled trial
Abstract
Background
Doravirine (DOR), a novel non-nucleoside reverse-transcriptase inhibitor (NNRTI), is active against wild-type Human Immunodeficiency Virus (HIV)-1 and the most common NNRTI-resistant variants, and has a favorable and unique in vitro resistance profile.
Methods
DRIVE-AHEAD is a phase 3, double-blind, non-inferiority trial. Antiretroviral treatment–naive adults with ≥1000 HIV-1 RNA copies/mL were randomized (1:1) to once-daily, fixed-dose DOR at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate (TDF) at 300 mg (DOR/3TC/TDF) or to efavirenz at 600 mg, emtricitabine at 200 mg, and TDF at 300 mg (EFV/FTC/TDF) for 96 weeks. The primary efficacy endpoint was the proportion of participants with <50 HIV-1 RNA copies/mL at week 48 (Food and Drug Administration snapshot approach; non-inferiority margin 10%).
Results
Of the 734 participants randomized, 728 were treated (364 per group) and included in the analyses. At week 48, 84.3% (307/364) of DOR/3TC/TDF recipients and 80.8% (294/364) of EFV/FTC/TDF recipients achieved <50 HIV-1 RNA copies/mL (difference 3.5%, 95% CI, -2.0, 9.0). DOR/3TC/TDF recipients had significantly lower rates of dizziness (8.8% vs 37.1%), sleep disorders/disturbances (12.1% vs 25.2%), and altered sensorium (4.4% vs 8.2%) than EFV/FTC/TDF recipients. Mean changes in fasting low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C) were significantly different between DOR/3TC/TDF and EFV/FTC/TDF (−1.6 vs +8.7 mg/dL and −3.8 vs +13.3 mg/dL, respectively).
Conclusions
In HIV-1 treatment-naive adults, DOR/3TC/TDF demonstrated non-inferior efficacy to EFV/FTC/TDF at week 48 and was well tolerated, with significantly fewer neuropsychiatric events and minimal changes in LDL-C and non–HDL-C compared with EFV/FTC/TDF.
Clinical Trials Registration
Human Immunodeficiency Virus (HIV)-1 infection has been successfully treated with a wide array of antiretroviral therapies, which can vastly improve life expectancy and reduce the risk of HIV-1 transmission, but cannot eradicate the infection. Because lifelong treatment is required, safeguarding against toxicity and comorbidity is important. Adverse events that may lead to treatment discontinuation include neuropsychiatric toxicities, skin rashes, gastrointestinal toxicities, abnormal serum lipid levels, abnormal renal or bone parameters, and drug-drug interactions [1]. Therefore, new antiretroviral therapies with fewer unwanted side effects are needed.
Doravirine (DOR), a novel non-nucleoside reverse-transcriptase inhibitor (NNRTI) of HIV-1, is active in vitro against both wild-type virus and the most common NNRTI-resistant variants at concentrations achieved with 100 mg once-daily (QD) dosing [2, 3] and has a favorable in vitro resistance profile that is unique among NNRTIs [4]. Because it is not a metabolic inducer or inhibitor [5], DOR is not a perpetrator of pharmacokinetic drug-drug interactions. No clinically meaningful interactions were observed when DOR was co-administered with atorvastatin, an oral contraceptive, a magnesium-based antacid, or a proton-pump inhibitor in healthy volunteers [6–8]. As a substrate of cytochrome P450 (CYP)3A4, exposure to DOR is reduced in the presence of moderate or strong inducers of CYP3A4 [9]. DOR can be taken once daily without regard to food [10], and its bioavailability is not affected by age, gender, or moderate hepatic impairment [11, 12].
In a phase 2b study in treatment-naive adults, DOR at 100 mg QD with emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) demonstrated comparable efficacy to efavirenz with FTC/TDF and had a favorable safety profile, with lower rates of drug-related adverse events and central nervous system (CNS) events than efavirenz [13–15]. In the phase 3 DRIVE-FORWARD trial, DOR at 100 mg QD demonstrated non-inferior efficacy and a superior lipid profile compared with darunavir-ritonavir after 48 weeks of combination treatment with 2 nucleoside reverse-transcriptase inhibitors (NRTIs) [16].
DOR is in development as both a single-entity tablet and a combination tablet containing DOR at 100 mg, lamivudine at 300 mg, and TDF at 300 mg (DOR/3TC/TDF; MK-1439A). This report presents the results of a Phase 3 trial comparing the combination tablet, DOR/3TC/TDF, to ATRIPLA (efavirenz at 600 mg, FTC at 200 mg, and TDF at 300 mg; EFV/FTC/TDF) in adults with previously-untreated HIV-1 infections.
METHODS
Study Design
DRIVE-AHEAD (MK-1439A Protocol 021; NCT02403674) is a phase 3, randomized, double-blind, non-inferiority trial conducted at 126 sites worldwide (see Supplementary Appendix) and in conformance with International Conference on Harmonisation, Good Clinical Practice Guidelines and applicable statutes and regulations regarding the protection of human participants in biomedical research. The study protocol was approved by the Independent Ethics Committee for each study site, and all participants provided written informed consent before any study procedures were performed. The double-blind trial continued for 96 weeks; the data cutoff for this report (week 48) was 20 March 2017.
Men and women ≥18 years of age with plasma HIV-1 RNA of ≥1000 copies/mL (within 45 days before study treatment) who were naive to antiretroviral therapy were eligible for the trial if they had no documented or known resistance to any of the study drugs (see Supplementary Appendix) and had calculated creatinine clearance of ≥50 mL/min. Participants were randomly assigned (1:1) to either DOR/3TC/TDF (plus placebo for EFV/FTC/TDF) or EFV/FTC/TDF (plus placebo for DOR/3TC/TDF), stratified by screening HIV-1 RNA (either ≤ or >100000 copies/mL) and chronic hepatitis B and/or C co-infection (either yes or no). DOR/3TC/TDF (and matching placebo) were taken orally QD, without regard to food, at approximately the same time each day. EFV/FTC/TDF (and matching placebo) were taken orally QD, at bedtime, on an empty stomach. No dose modifications were permitted during the study.
Procedures/Measurements
Plasma HIV-1 RNA was measured at all study visits by the central laboratory using the Abbott RealTime HIV-1 assay (lower limit of quantification [LLoQ] of 40 copies/mL). Protocol-defined virologic failure (PDVF) consisted of virologic rebound (confirmed HIV-1 RNA of ≥50 copies/mL after initial response of HIV-1 RNA of <50 copies/mL at any time during the study) or non-response (either confirmed HIV-1 RNA of ≥200 copies/mL at week 24 or 36 or confirmed HIV-1 RNA of ≥50 copies/mL at week 48). In all cases, confirmation required 2 consecutive measures of HIV-1 RNA at least 1 week apart. Participants who met PDVF criteria were discontinued from the study, regardless of their adherence to the study therapy.
Testing for viral resistance to the study drugs was performed for participants with PDVF and for participants who discontinued the trial for any reason, if HIV-1 RNA was >400 copies/mL. Post-baseline genotypic viral resistance to DOR was defined as any of the following mutations in the RT gene: L100I, K101E, V106A, V106I, V106M, V108I, E138K, Y188L, G190A, G190S, H221Y, P225H, F227C, F227L, F227V, M230I, M230L, L234I, P236L, and Y318F. Post-baseline genotypic resistance to EFV, FTC, 3TC, and TDF was assessed by Monogram Biosciences, LabCorp Specialty Testing Group. Phenotypic viral resistance to EFV and the NRTIs was defined by Monogram based on the difference (fold change) between the half-maximal inhibitory concentration (IC50) values for a participant’s virus and wild-type virus. Since no threshold for defining phenotypic resistance to DOR has yet been clinically defined, a 2.5-fold change in IC50 versus wild-type virus was used as a broad assay-reproducibility threshold for potential phenotypic resistance to DOR.
Safety was monitored by adverse event (AE) reporting, treatment-emergent abnormalities in laboratory tests, and physical examinations. AEs were assessed by the investigator for intensity (mild, moderate, severe), seriousness, and relationship to study therapy. Laboratory values were graded according to the Division of AIDS (DAIDS) Criteria [17].
Statistical Analysis
The primary efficacy population was the full analysis set (FAS), which consisted of all randomized participants who received at least 1 dose of a study drug. The primary efficacy endpoint was the proportion of participants achieving HIV-1 RNA of <50 copies/mL at week 48 using the Food and Drug Administration’s “snapshot” approach [18], which treats all missing values as failures, regardless of the reason. The difference between treatment groups at each time point and the associated 95% confidence intervals (CIs) were calculated using the stratum-adjusted Mantel-Haenszel method. The prespecified non-inferiority margin was -10% [18]. With 340 participants per treatment arm, the trial had 90% power to demonstrate that DOR/3TC/TDF is non-inferior to EFV/FTC/TDF on the primary endpoint, at the 1-sided 2.5% alpha-level, assuming a true response rate of 80% for both arms.
Secondary and exploratory efficacy endpoints included HIV-1 RNA of <40 copies/mL, HIV-1 RNA of <200 copies/mL (analyzed using the same approach as described for the primary endpoint), a change from baseline in CD4+ T-cell counts, development of viral drug resistance (as described above), and efficacy by subgroup. The treatment difference in change from baseline in CD4+ T-cell count was estimated using the observed failure (OF) approach, with baseline values carried forward for participants who discontinued due to lack of efficacy. To assess the consistency of the treatment effect, the between-group difference for the primary endpoint (with nominal, unadjusted 95% CI) was calculated within subgroups based on demographic and prognostic factors, using the OF approach.
Safety analyses included all randomized participants who received at least 1 dose of a study medication. The primary safety endpoint was the proportion of participants with neuropsychiatric AEs in 3 pre-specified categories (dizziness, sleep disorders/disturbances, and altered sensorium), which represent the neuropsychiatric events most commonly reported in the phase 2b study of doravirine [13, 14]. The proportion of participants with neuropsychiatric AEs in 2 additional categories (depression/suicide/self-injury and psychosis/psychotic disorders) was a secondary endpoint. All preferred terms from the Medical Dictionary for Regulatory Activities (version 19.1) that fall into these categories were grouped for statistical analysis. Treatment differences and 95% CIs were calculated using Miettinen and Nurminen’s method [19], with P values provided for dizziness, sleep disorders/disturbances, and altered sensorium only. The change from baseline in fasting lipid levels was a secondary safety endpoint and was analyzed using analysis of covariance (ANCOVA) models adjusted by baseline lipid level and treatment group; inferential testing for statistical significance for between-treatment comparisons was pre-specified for low-density lipoprotein cholesterol (LDL-C) and non–high-density lipoprotein cholesterol (HDL-C) only.
RESULTS
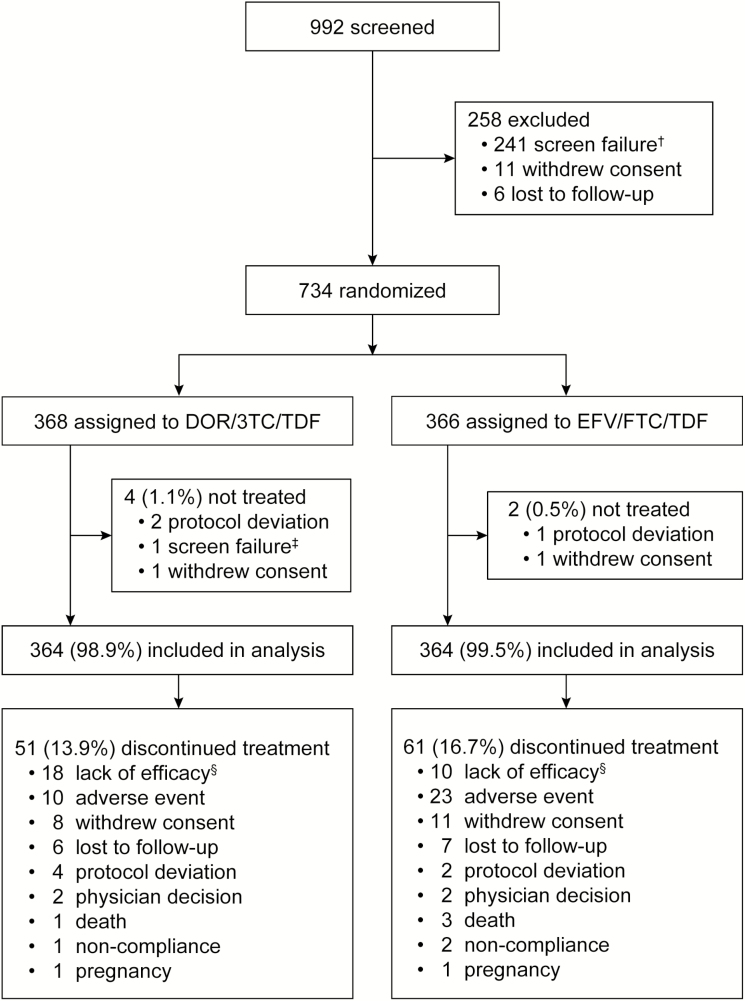
Of the 734 participants assigned to DOR/3TC/TDF (n = 368) or EFV/FTC/TDF (n = 366), 364 in each group received study therapy and were included in the analyses. Demographics and baseline characteristics were generally similar between the treatment groups (Table 1). By week 48, 14% of the DOR/3TC/TDF group and 17% of the EFV/FTC/TDF group had discontinued study treatment, primarily due to lack of efficacy, adverse events, withdrawal of consent, and loss to follow-up (Figure 1).
Table 1.
Demographic and Baseline Characteristics
| DOR/3TC/TDF | EFV/FTC/TDF | Total | |
|---|---|---|---|
| (N = 364) | (N = 364) | (N = 728) | |
| Age (years), Median (range) | 32.0 (18, 70) | 30.0 (18, 69) | 31.0 (18, 70) |
| Male, n (%) | 305 (84%) | 311 (85%) | 616 (85%) |
| Race, n (%) | |||
| White | 177 (49%) | 170 (47%) | 347 (48%) |
| Black or African American | 67 (18%) | 68 (19%) | 135 (19%) |
| Asian | 59 (16%) | 65 (18%) | 124 (17%) |
| Othera | 61 (17%) | 61 (17%) | 122 (17%) |
| Hispanic or Latino Ethnicity | 126 (35%) | 120 (33%) | 246 (34%) |
| Region, n (%) | |||
| Africa | 37 (10%) | 27 (7%) | 64 (9%) |
| Asia/Pacific | 59 (16%) | 62 (17%) | 121 (17%) |
| Europe | 88 (24%) | 94 (26%) | 182 (25%) |
| Latin America | 89 (24%) | 87 (24%) | 176 (24%) |
| North America | 91 (25%) | 94 (26%) | 185 (25%) |
| CD4+ T-Cell Count | |||
| Median (range), cells/mm3 | 414 (19, 1399) | 388 (19, 1452) | 397 (19, 1452) |
| ≤200 cells/mm3, n (%) | 44 (12%) | 46 (13%) | 90 (12%) |
| >200 cells/mm3, n (%) | 320 (88%) | 318 (87%) | 638 (88%) |
| Plasma HIV-1 RNA | |||
| Median (range), log10 copies/mL | 4.4 (2.4, 6.1) | 4.5 (2.6, 6.4) | 4.4 (2.4, 6.4) |
| ≤100000 copies/mL, n (%) | 291 (80%) | 282 (77%) | 573 (79%) |
| >100000 copies/mL, n (%) | 73 (20%) | 82 (23%) | 155 (21%) |
| History of AIDS, n (%) | 46 (13%) | 53 (15%) | 99 (14%) |
| Hepatitis B and/or C,b n (%) | 11 (3%) | 9 (2%) | 20 (3%) |
| HIV-1 Subtype B, n (%) | 232 (64%) | 253 (70%) | 485 (67%) |
Abbreviations: DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg; HIV, human immunodeficiency virus; RNA, ribonucleic acid.
aOther race includes multiracial, American Indian, or Alaska Native.
bEvidence of hepatitis B surface antigen or hepatitis C virus RNA.
Figure 1.
Disposition of participants through week 48. Abbreviations: DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz 600 at mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg. †The most common reasons for screen failure were a documented or known resistance to any study drug (n = 139) and plasma Human Immunodeficiency Virus–1 RNA of <1000 copies/mL at screening (n = 62). Of 992 participants screened, ~2.4% were excluded due to doravirine-associated mutations (Y188L and V106I) and ~4.0% were excluded due to efavirenz-associated mutations (Y188L, K103N, L100I, K101, V108I, G190, and Y181C). ‡In error, 1 participant was randomized who did not meet inclusion/exclusion criteria. §Lack of efficacy was determined by investigator assessment (not a specific HIV-1 RNA level).
Efficacy
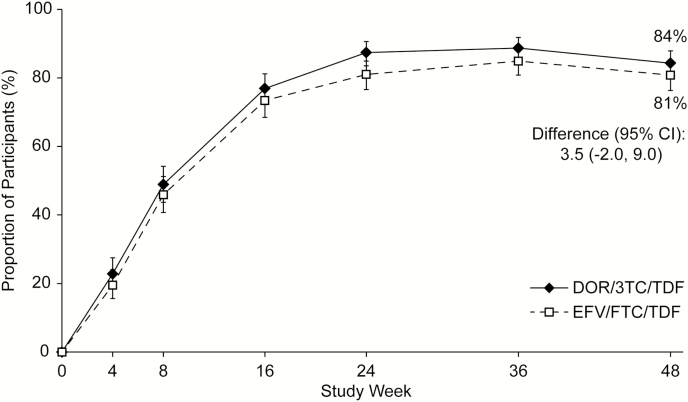
DOR/3TC/TDF was non-inferior to EFV/FTC/TDF on the primary efficacy endpoint, with 84.3% (307/364) and 80.8% (294/364) of participants, respectively, achieving HIV-1 RNA of <50 copies/mL at week 48 (difference, 3.5%; 95% CI, -2.0, 9.0). Virologic response rates were similar between the treatment groups at each time point (Figure 2) and across all baseline prognostic and demographic factors except age (see Supplementary Appendix), with response rates favoring EFV/FTC/TDF in participants ≤31 years old and DOR/3TC/TDF in those older than 31. Among participants with high baseline HIV-1 RNA (>100000 copies/mL), 56/69 (81.2%) in the DOR/3TC/TDF group and 59/73 (80.8%) in the EFV/FTC/TDF group achieved HIV-1 RNA of <50 copies/mL at week 48.
Figure 2.
Proportion of participants with Human Immunodeficiency Virus–1 RNA of <50 copies/mL over time (Food and Drug Administration snapshot approach). Abbreviations: CI, confidence interval; DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg.
Similar results were observed for the virologic endpoints of HIV-1 RNA of <40 copies/mL (83.8% for DOR/3TC/TDF vs 79.7% for EFV/FTC/TDF; difference, 4.1%; 95% CI, -1.5, 9.7) and HIV-1 RNA of <200 copies/mL (Table 2). The mean change in CD4+ T-cell count from baseline to week 48 was similar in the DOR/3TC/TDF and EFV/FTC/TDF groups (198 vs 188 cells/mm3; difference, 10.1; 95% CI, -16.1, 36.3).
Table 2.
Virologic Outcomes at Week 48
| DOR/3TC/TDF (N = 364) | EFV/FTC/TDF (N = 364) | |||
|---|---|---|---|---|
| Primary Analysis (FDA Snapshot Approach) | n | (%) | n | (%) |
| HIV-1 RNA <50 copies/mL | 307 | (84.3) | 294 | (80.8) |
| HIV-1 RNA ≥50 copies/mLa | 39 | (10.7) | 37 | (10.2) |
| No virologic data in week 48 window | 18 | (4.9) | 33 | (9.1) |
| Discontinued study due to AE or deathb | 9 | (2.5) | 24 | (6.6) |
| Discontinued study for other reasonsc | 9 | (2.5) | 8 | (2.2) |
| On study but missing data in window | 0 | (0.0) | 1 | (0.3) |
| Secondary and exploratory endpoints | n/N | (%) | n/N | (%) |
| HIV-1 RNA <50 copies/mL (observed failure) | 307/346 | (88.7) | 294/331 | (88.8) |
| HIV-1 RNA <40 copies/mL (FDA snapshot) | 305/364 | (83.8) | 290/364 | (79.7) |
| HIV-1 RNA <200 copies/mL (FDA snapshot) | 313/364 | (86.0) | 301/364 | (82.7) |
Abbreviations: AE, adverse event; DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg; HIV, human immunodeficiency virus; FDA, Food and Drug Administration.
aIncludes participants who changed any component of background therapy before week 48, participants who discontinued study drug before week 48 for lack or loss of efficacy, and participants with HIV-1 RNA of ≥50 copies/mL in the week 48 window (relative day 295–378).
bIncludes participants who discontinued because of adverse event (AE) or death at any time point from day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
cOther reasons include: lost to follow-up, non-compliance with study drug, physician decision, pregnancy, protocol deviation, screen failure, withdrawal by participant.
Viral Drug Resistance
Only 22 participants (6.0%) in the DOR/3TC/TDF group and 14 (3.8%) in the EFV/FTC/TDF group met the criteria for confirmed PDVF, which was viral rebound in most cases (16/22 and 10/14, respectively). Participants with PDVF generally had low-level viremia at the time of failure. Among those with viral rebound, 10/16 in the DOR/3TC/TDF group and 4/10 in the EFV/FTC/TDF group had HIV-1 RNA between 50 and 200 copies/mL at the viral failure confirmation visit.
Results from viral drug-resistance testing are available for 13 DOR/3TC/TDF recipients and 10 EFV/FTC/TDF recipients who met PDVF criteria, and for 9 and 13, respectively, who discontinued early without PDVF (Table 3). In the DOR/3TC/TDF group, isolates from 7 participants (6 with non-response; 1 rebound) had mutations associated with DOR resistance: 6 isolates had both genotypic and phenotypic resistance to DOR (conferred through V106 in 4 cases, in combination with other mutations such as F227C) and to EFV; and 1 isolate had a DOR-associated resistance mutation (Y318Y/F), but no phenotypic resistance to DOR or EFV. Five of the 7 isolates also had genotypic resistance to 3TC.
Table 3.
Treatment-emergent Drug Resistance, Week 48
| DOR/3TC/TDF (N = 364) | EFV/FTC/TDF (N = 364) | |||||
|---|---|---|---|---|---|---|
| PDVFa | Discontinued Without PDVF | Total | PDVFa | Discontinued Without PDVF | Total | |
| Number (%) of participants, n (%) | 22 (6.0) | 35 (9.6) | 57 (15.7) | 14 (3.8) | 50 (13.7) | 64 (17.6) |
| Genotype test reported, n (%) | 13 (3.4) | 9 (2.5) | 22 (6.0) | 10 (2.5) | 13 (3.4) | 23 (6.3) |
| Phenotype test reported, n (%) | 13 (3.4) | 9 (2.5) | 22 (6.0) | 9 (2.5) | 12 (3.3) | 21 (5.8) |
| Genotypic NNRTI resistance, n (%) | 7 (1.9) | 0 | 7 (1.9) | 9 (2.5) | 3 (0.8) | 12 (3.3) |
| Phenotypic NNRTI resistance, n (%) | 6 (1.6) | 0 | 6 (1.6) | 8 (2.2) | 3 (0.8) | 11 (3.0) |
| Genotypic NRTI resistance, n (%) | 5 (1.4) | 0 | 5 (1.4) | 5 (1.4) | 0 | 5 (1.4) |
| Phenotypic NRTI resistance, n (%) | 5 (1.4) | 0 | 5 (1.4) | 4 (1.1) | 0 | 4 (1.1) |
| Specific NNRTI resistance mutations detected | Y188L; Y318Y/F; V106I, F227C; V106V/I, H221H/Y, F227C; F227C; V106A, P225H, Y318Y/F; V106M/T, F227C/R | K103N; K103N, E138E/G; K103N; K103N; G190E; K103N; K103N, M230L; G190E; K103N, V108V/I, T369T/A/I/V; K103N; K103N; K101K/N, K103N, P225P/H | ||||
| Specific NRTI resistance mutations detected | M41L, M184V; M184V; M184V; K65R; K65K/R, M184V | V118I, M184V; M184V; M184V; M184V, K219K/E; K65K/R, M184M/I | ||||
Abbreviations: DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PDVF, protocol-defined virologic failure.
aPDVF was defined as either confirmed HIV-1 RNA of ≥50 copies/mL after initial response of HIV-1 RNA of <50 copies/mL at any time during the study; confirmed HIV-1 RNA of ≥200 copies/mL at week 24 or week 36; or confirmed HIV-1 RNA of ≥50 copies/mL at week 48.
In the EFV/FTC/TDF group, isolates from 12 participants (4 with non-response, 5 rebound, and 3 early discontinuation) had mutations associated with EFV resistance: 11 isolates had both genotypic and phenotypic resistance to EFV (conferred through K103N in 10 and G190E in 1); and one isolate had genotypic resistance to EFV (G190E), but phenotypic testing failed. Two isolates with EFV resistance did not retain phenotypic sensitivity to DOR: 1 had G190E and 1 had K103N/M230L. Five of the 12 isolates also had genotypic resistance to FTC.
Safety
Overall rates of any AE, drug-related AE, and discontinuation due to an AE were lower in the DOR/3TC/TDF group compared with the EFV/FTC/TDF group (Table 4). The most common reasons for discontinuation were rashes (10 EFV/FTC/TDF, 0 DOR/3TC/TDF) and CNS-related events (9 EFV/FTC/TDF, 4 DOR/3TC/TDF). The most common drug-related AEs for DOR/3TC/TDF and EFV/FTC/TDF were dizziness (7% vs 32%, respectively), abnormal dreams (5% vs 9%), nausea (5% vs 7%), and rash (2% vs 9%). Among the most common AEs of any causality, the largest treatment differences were observed for dizziness, abnormal dreams, and rash (Table 4).
Table 4.
Summary of Clinical Adverse Events
| DOR/3TC/TDF QD | EFV/FTC/TDF QD | Treatment Difference | |||
|---|---|---|---|---|---|
| (N = 364) | (N = 364) | % (95% CI) | |||
| Any AE | 301 | (83%) | 330 | (91%) | -8.0 (-13.0, -3.1) |
| Drug-relateda AE | 113 | (31%) | 229 | (63%) | -31.9 (-38.6, -24.8) |
| Serious AE | 13 | (4%) | 21 | (6%) | -2.2 (-5.5, 0.9) |
| Drug-related serious AE | 1 | (<1%) | 4 | (1%) | -0.8 (-2.5, 0.5) |
| Deathsb | 1 | (<1%) | 3 | (1%) | -0.5 (-2.2, 0.8) |
| Discontinued due to AE | 11 | (3%) | 24 | (7%) | -3.6 (-6.9, -0.5) |
| Discontinued due to drug-related AE | 8 | (2%) | 21 | (6%) | -3.6 (-6.7, -0.8) |
| Neuropsychiatric AE (pre-specified) | 86 | (24%) | 207 | (57%) | -33.2 (-39.8, -26.4) |
| Most common adverse events (incidence ≥5% in either treatment group)c | |||||
| Gastrointestinal disorders | 120 | (33%) | 136 | (37%) | -4.4 (-11.3, 2.5) |
| Diarrhea | 39 | (11%) | 49 | (13%) | -2.7 (-7.6, 2.0) |
| Nausea | 28 | (8%) | 39 | (11%) | -3.0 (-7.3, 1.2) |
| Vomiting | 15 | (4%) | 27 | (7%) | -3.3 (-6.9, 0.1) |
| General disorders | 56 | (15%) | 53 | (15%) | 0.8 (-4.4, 6.1) |
| Fatigue | 21 | (6%) | 22 | (6%) | -0.3 (-3.8, 3.3) |
| Infections and infestations | 183 | (50%) | 174 | (48%) | 2.5 (-4.8, 9.7) |
| Nasopharyngitis | 39 | (11%) | 31 | (9%) | 2.2 (-2.1, 6.6) |
| Pharyngitis | 20 | (5%) | 15 | (4%) | 1.4 (-1.8, 4.7) |
| Upper respiratory tract infection | 33 | (9%) | 23 | (6%) | 2.7 (-1.2, 6.8) |
| Nervous system disorders | 95 | (26%) | 177 | (49%) | -22.5 (-29.3, -15.6) |
| Dizziness | 32 | (9%) | 135 | (37%) | -28.3 (-34.0, -22.5) |
| Headache | 47 | (13%) | 45 | (12%) | 0.5 (-4.3, 5.4) |
| Somnolence | 12 | (3%) | 27 | (7%) | -4.1 (-7.6, -0.9) |
| Psychiatric disorders | 62 | (17%) | 122 | (34%) | -16.5 (-22.7, -10.2) |
| Abnormal dreams | 17 | (5%) | 42 | (12%) | -6.9 (-11.0, -3.0) |
| Insomnia | 19 | (5%) | 32 | (9%) | -3.6 (-7.4, 0.1) |
| Skin/subcutaneous tissue disorders | 61 | (17%) | 95 | (26%) | -9.3 (-15.3, -3.4) |
| Rash | 17 | (5%) | 44 | (12%) | -7.4 (-11.6, -3.5) |
Abbreviations: AE, adverse event; CI, confidence interval; DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg; QD, once daily.
aDetermined by the investigator to be related to study therapy.
bNone of the deaths were considered related to study therapy.
cCategory totals included all adverse events in the category, regardless of incidence.
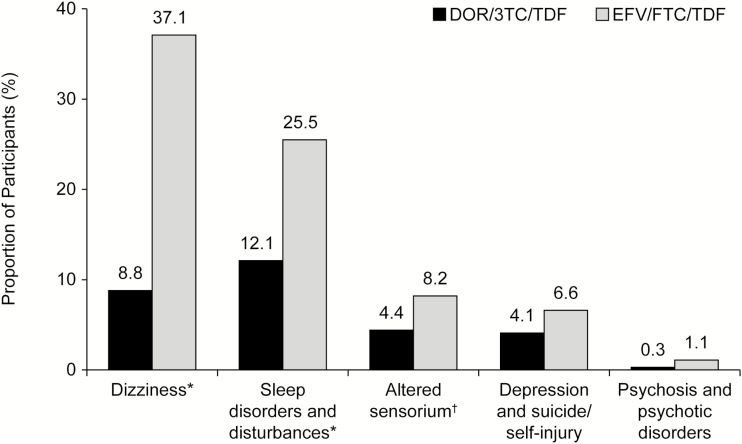
On the primary safety endpoint, the proportion of participants with pre-specified neuropsychiatric events by week 48 was significantly lower in the DOR/3TC/TDF group compared with the EFV/FTC/TDF group for the categories of dizziness (8.8% vs 37.1%, respectively; P ≤ .001), sleep disorders/disturbances (12.1% vs 25.2%; P ≤ .001), and altered sensorium (4.4% vs 8.2%; P = .033). For the secondary categories, the proportion of participants with depression/suicide/self-injury or psychosis/psychotic disorders was numerically lower for DOR/3TC/TDF than EFV/FTC/TDF (Figure 3). These neuropsychiatric events were mild in the majority of cases in each group (DOR/3TC/TDF, 72%; EFV/FTC/TDF, 75%).
Figure 3.
Proportion of participants with neuropsychiatric adverse events in pre-specified categories. Abbreviations: DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg. *P < .001, †P = .033. aStatistical testing was not pre-specified for the secondary categories (depression and suicide/self-injury; psychosis and psychotic disorders).
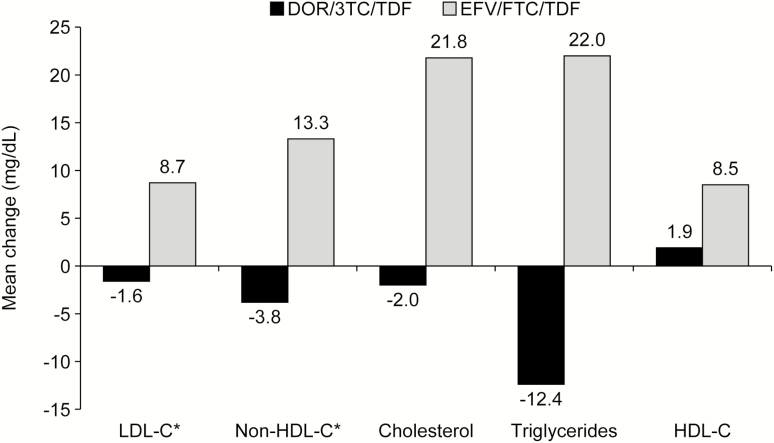
Figure 4 displays the mean change in fasting lipid levels from baseline to week 48. For LDL-C and non–HDL-C, the treatment difference was statistically significant (1-sided P < .0001), with minimal changes (-1.6 and -3.8 mg/dL, respectively) in the DOR/3TC/TDF group versus mean increases (8.7 and 13.3 mg/dL, respectively) in the EFV/FTC/TDF group. The mean change from baseline in the total cholesterol/HDL-C ratio was -0.23 for DOR/3TC/TDF and -0.18 for EFV/FTC/TDF (difference, -0.07; 95% CI, -0.21, 0.07). Lipid-lowering therapy was modified during the study by 2.2% (8/364) of DOR/3TC/TDF recipients and 2.5% (9/364) of EFV/FTC/TDF recipients (difference, 0.3%; 95% CI, -2.7, 2.1). The incidence of DAIDS grade 3–4 laboratory abnormalities, including serum creatinine, was low and similar between the treatment groups (see Supplementary Appendix). Specific bone and renal parameters were not evaluated in this trial; however, fractures and drug-related renal AEs occurred in <1% of each treatment group.
Figure 4.
Mean change in fasting lipid levels from baseline to week 48. Abbreviations: DOR/3TC/TDF, doravirine at 100 mg, lamivudine at 300 mg, and tenofovir disoproxil fumarate at 300 mg; EFV/FTC/TDF, efavirenz at 600 mg, emtricitabine at 200 mg, and tenofovir disoproxil fumarate at 300 mg; HDL-C, high-density lipoprotein cholestoral; LDL-C, low-density lipoprotein cholestoral. *P < .0001. aStatistical testing was not pre-specified for cholesterol, triglycerides, or HDL-C.
DISCUSSION
This Phase 3 trial evaluated the efficacy and safety of DOR/3TC/TDF compared to the widely-used regimen of EFV/FTC/TDF in treatment-naive HIV-1–infected adults. The efficacy of DOR/3TC/TDF was non-inferior to that of EFV/FTC/TDF at week 48 (84.3% vs 80.8%, respectively) and was similar across baseline characteristics of gender, race, baseline viral load, and viral subtype. In both treatment groups, the virologic response rate was lower among participants with baseline HIV-1 RNA of >100000 copies/mL (81.2% for DOR/3TC/TDF; 80.8% for EFV/FTC/TDF) compared to those with baseline HIV-1 RNA of ≤100000 copies/mL (90.6% and 91.1%, respectively). Lower response rates in participants with high baseline viral loads has been observed in other clinical trials and across various antiretroviral classes [20, 21]. Response rates based on HIV-1 RNA thresholds of <40 and <200 copies/mL were consistent with the primary endpoint, and there was no notable difference between treatment groups in the change from baseline in CD4+ T-cell counts at week 48.
The virologic response rate in the EFV/FTC/TDF group (80.8%) was similar to rates reported for this regimen in previous clinical trials: 81% [21] and 81.6% [22]. In both trials, discontinuation due to AEs was more frequent in the EFV/FTC/TDF group than in the comparator group (10% vs 2% and 8.7% vs 2.5%, respectively), which also occurred in our study (6.3% vs 2.7%). These discontinuations reduced the observed response rate by the Food and Drug Administration snapshot approach, which treats all missing data as failures. When missing data are excluded (using the observed failure approach), higher virologic response rates were observed for both treatment groups (DOR/3TC/TDF, 88.7%; EFV/FTC/TDF, 88.8%).
Response rates were also influenced by the conservative definition of PDVF used in this study, which required discontinuation of participants with HIV-1 RNA of ≥50 copies/mL at week 48, as well as those who became suppressed but later had confirmed HIV-1 RNA of ≥50 copies/mL, regardless of time point. Some participants had re-suppressed between the virologic failure confirmation visit and the discontinuation visit, but were required to discontinue per protocol. Other participants who were discontinued due to PDVF criteria but had very low-level viremia may have re-suppressed if they had continued on treatment.
Through 48 weeks of treatment, genotypic resistance to DOR developed in 7 participants in the DOR/3TC/TDF group (1.9% overall; 32% of those with resistance testing), and genotypic resistance to EFV developed in 12 participants in the EFV/FTC/TDF group (3.3% overall; 52% of those with resistance testing). The rate and pattern of resistance observed in the EFV/FTC/TDF group was consistent with previous reports [21–25]. Resistance to DOR was primarily conferred by a substitution in RT V106, in combination with 1 or more mutations, which is consistent with in vitro data showing that DOR has a unique mechanism of resistance requiring multiple mutations or base-pair changes [4]. In a recent report, the prevalence of doravirine-associated mutations was lower than other NNRTI mutations among treatment-naive patients in Europe [26]. With in vitro activity against commonly-transmitted NNRTI mutations, and multiple mutations needed for resistance in most cases, doravirine is likely to be effective in the majority of treatment-naive patients with transmitted NNRTI resistance. However, the DRIVE-AHEAD trial was not designed to address this question, since patients with NNRTI resistance were excluded.
DOR/3TC/TDF was generally well-tolerated, with fewer drug-related AEs and discontinuations due to AEs than EFV/FTC/TDF. DOR/3TC/TDF exhibited favorable neuropsychiatric tolerability and was superior to EFV/FTC/TDF in the analysis of dizziness, sleep disorders/disturbances, and altered sensorium, showing lower event rates in each of these categories. The incidence of rashes was also lower in the DOR/3TC/TDF group (5% vs 12% in the EFV/FTC/TDF group) and was similar to that reported previously for doravirine (7%) [16]. Furthermore, no participants in the DOR/3TC/TDF group discontinued treatment due to rashes. DOR/3TC/TDF demonstrated a favorable overall profile for plasma lipid levels, including significant differences between treatment groups for LDL-C and non–HDL-C (minimal changes in the DOR/3TC/TDF group versus mean increases in the EFV/FTC/TDF group). A similar favorable lipid profile was observed for doravirine in the DRIVE-FORWARD trial [16].
Our study had several limitations. Participants took 2 pills per day (1 active drug and 1 placebo), which could be taken together or at different times; since some participants may have taken the study medications at different times, resulting in a twice-daily regimen, the effects of improved adherence with a 1-pill, once-daily regimen [27] could not be fully captured in this trial. Although the response rates appeared similar across most subgroups, several of these groups were relatively small, such as women (15.4%), Blacks/African Americans (18.5%), and those with high baseline viral loads (>100000 copies/mL, 21.3%), low CD4+ T-cell counts (≤200/mm3, 12.4%), or hepatitis B/C co-infections (2.7%). The small difference in treatment responses by age (favoring EFV/FTC/TDF in younger participants and DOR/3TC/TDF in older participants) was not observed in the DRIVE-FORWARD trial [13] and may be due to chance, since no adjustment was made for multiple comparisons and the trial was not powered to detect statistically-significant differences within subgroups [28].
In summary, DOR/3TC/TDF demonstrated robust antiretroviral efficacy that was statistically non-inferior to that of EFV/FTC/TDF, immunologic efficacy similar to that of EFV/FTC/TDF, and a low rate of treatment-emergent viral drug resistance. DOR/3TC/TDF was superior to EFV/FTC/TDF for neuropsychiatric AEs and changes in LDL-C and non–HDL-C, and was associated with lower rates of rashes, drug-related AEs, and discontinuation due to AEs. The favorable safety profile of DOR/3TC/TDF may reduce the likelihood of non-compliance and treatment discontinuation. Overall, DOR/3TC/TDF is a promising new treatment option for adults with HIV-1 infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all the patients who participated in this study. The contributions of the investigators and their staff are also gratefully recognized. Medical writing, graphic support, and editorial assistance were provided by Kim M. Strohmaier, Danielle Mancaruso, and Carol Zecca, who are employees of Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc.
Financial support. This work was supported by Merck & Co., Inc., Kenilworth, NJ, USA, which provided financial support and investigational drug supplies for the study.
Potential conflicts of interest. C. O. has received research grants, personal fees, and non-financial support for lectureships and serving on advisory boards from Gilead, Merck Sharp & Dohme, Bristol-Myers Squibb, ViiV Healthcare, Abbvie. and Janssen. J.-M. M. has received honoraria for participating in advisory boards with Gilead Sciences, Merck, Bristol Myers Squibb, ViiV, Janssen, and Teva; and has received grant support to his institution from Gilead. P. E. Sax is a Scientific Advisory Board member for Gilead, GlaxoSmithKline/ViiV Healthcare, Merck, and Janssen; and has received grant support to his institution from BMS, Gilead, Merck, and GSK/ViiV. K. E. S., L. L., A. R., X. X., G. L., S. K., P. Sklar, B.-Y. N., G. J. H., C. H., and E. M. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and may own stock and/or stock options in the company. K. E. S. reports grants and other support from Gilead Sciences; other support from Merck, ViiV, Bristol Myers Squibb, and Janssen, outside the submitted work; was Professor of Medicine in the Department of Medicine at Sidney Kimmel Medical College of Thomas Jefferson University at the time the study was conducted; is still a faculty member at Thomas Jefferson University; and is currently a full-time employee of Merck & Co., Inc. E. M. is a shareholder in Pfizer, Bristol Myers Squibb, and Novartis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Johnson JA, Sax PE. Beginning antiretroviral therapy for patients with HIV. Infect Dis Clin North Am 2014; 28:421–38. [DOI] [PubMed] [Google Scholar]
- 2. Feng M, Sachs NA, Xu M, et al. . Doravirine suppresses common nonnucleoside reverse transcriptase inhibitor-associated mutants at clinically relevant concentrations. Antimicrob Agents Chemother 2016; 60:2241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai MT, Feng M, Falgueyret JP, et al. . In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother 2014; 58:1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng M, Wang D, Grobler JA, Hazuda DJ, Miller MD, Lai MT. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother 2015; 59:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez RI, Fillgrove K, Hafey M, et al. . In vitro evaluation of doravirine potential for pharmacokinetic drug interactions. Drug Metab Rev2016; 48(suppl 1):73. [Google Scholar]
- 6. Khalilieh S, Yee KL, Sanchez RI, et al. . Results of a doravirine-atorvastatin drug-drug interaction study. Antimicrob Agents Chemother 2017; 61(2):e01364–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson MS, Kaufman D, Castronuovo P, et al. . Effect of doravirine (MK-1439) on the pharmacokinetics of an oral contraceptive (Ethinyl Estradiol and Levonorgestrel). Reviews in Antiviral Therapy & Infectious Diseases 2015; 4:63–4. [Google Scholar]
- 8. Khalilieh S, Yee KL, Sanchez RI, et al. . Co-administration of doravirine with an aluminum/magnesium containing antacid or pantoprazole, a proton pump inhibitor, does not have a clinically meaningful effect on doravirine pharmacokinetics. Presented at 9th IAS Conference on HIV Science, 23–26 July 2017, Paris. Available at: http://programme.ias2017.org/PAGMaterial/eposters/3996.pdf. Accessed 18 July 2018.
- 9. Yee KL, Khalilieh SG, Sanchez RI, et al. . The effect of single and multiple doses of rifampin on the pharmacokinetics of doravirine in healthy subjects. Clin Drug Investig 2017; 37:659–67. [DOI] [PubMed] [Google Scholar]
- 10. Behm MO, Yee KL, Liu R, Levine V, Panebianco D, Fackler P. The effect of food on doravirine bioavailability: results from two pharmacokinetic studies in healthy subjects. Clin Drug Investig 2017; 37:571–9. [DOI] [PubMed] [Google Scholar]
- 11. Behm MO, Yee KL, Fan L, Fackler P. Effect of gender and age on the relative bioavailability of doravirine: results of a Phase I trial in healthy subjects. Antivir Ther 2017; 22:337–44. [DOI] [PubMed] [Google Scholar]
- 12. Khalilieh S, Yee KL, Liu R, et al. . Moderate hepatic impairment does not affect doravirine pharmacokinetics. J Clin Pharmacol 2017; 57:777–83. [DOI] [PubMed] [Google Scholar]
- 13. Gatell JM, Morales-Ramirez JO, Hagins DP, et al. . Forty-eight-week efficacy and safety and early CNS tolerability of doravirine (MK-1439), a novel NNRTI, with TDF/FTC in ART-naive HIV-positive patients. J Int AIDS Soc 2014; 17(suppl 3):19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gatell J, Raffi F, Plettenberg A, et al. . Efficacy and safety of doravirine 100mg QD vs efavirenz 600mg QD with TDF/FTC in ART-naive HIV-infected patients: week 24 results. J Int AIDS Soc 2015; 18(Suppl. 4):36–7. [Google Scholar]
- 15. Gatell JM, Raffi F; Plettenberg A, et al. . Doravirine 100mg QD vs Efavirenz +TDF/FTC in ART-Naive HIV+ patients: week 48 results. Topics in Antiviral Medicine 2016; 24:E–1(183). [Google Scholar]
- 16. Molina JM, Squires K, Sax PE, et al. . Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV 2018; 5(5):e211–20. [DOI] [PubMed] [Google Scholar]
- 17. Division of AIDS, National Institutues of Health, US Department of Health and Human Services. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, version 2.0. Available at: https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2 nov2014.pdf. Accessed 27 March 2018. [Google Scholar]
- 18. US Food and Drug Administration. Human immunodeficiency virus-1 infection: developing anti-retroviral drugs for treatment, guidance for industry. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm355128.pdf. Accessed 27 March 2018. [Google Scholar]
- 19. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213–26. [DOI] [PubMed] [Google Scholar]
- 20. Stephan C, Hill A, Sawyer W, van Delft Y, Moecklinghoff C. Impact of baseline HIV-1 RNA levels on initial highly active antiretroviral therapy outcome: a meta-analysis of 12,370 patients in 21 clinical trials. HIV Med 2013; 14:284–92. [DOI] [PubMed] [Google Scholar]
- 21. Walmsley SL, Antela A, Clumeck N, et al. ; SINGLE Investigators Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 22. Cohen C, Wohl D, Arribas JR, et al. . Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. AIDS 2014; 28:989–97. [DOI] [PubMed] [Google Scholar]
- 23. Lennox JL, DeJesus E, Lazzarin A, et al. ; STARTMRK investigators Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 24. Sax PE, DeJesus E, Mills A, et al. ; GS-US-236-0102 study team Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 25. Gilead Sciences, LLC. Prescribing information for ATRIPLA (efavirenz/emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use (Revised 01/2018). Available at: https://www.gilead.com/-/media/files/pdfs/medicines/hiv/atripla/atripla_pi.pdf?la=en. Accessed 18 July 2018. [Google Scholar]
- 26. Marcelin A-G, Santoro MM, Charpentier C, et al. . Epidemiological study of doravirine associated resistance mutations in HIV-1-infected treatment-naive patients from two large databases in France and Italy. Reviews in Antiviral Therapy & Infectious Diseases 2017; 4:10–11. [Google Scholar]
- 27. Nachega JB, Parienti J-J, Uthman OA, et al. . Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58:1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007; 357:2189–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.