Abstract
Supercritical fluid chromatography (SFC) meets with great favor due to its high efficiency, low organic solvent consumption, and the specialty for the identification of the isomeric species. This review describes the advances of SFC in targeted and untargeted lipid profiling. The advancement of the SFC instruments and the stationary phases are summarized. Typical applications of SFC to the targeted and untargeted lipid profiling are discussed in detail. Moreover, the perspectives of SFC in the lipid profiling are also proposed. As a useful and promising tool for investigating lipids in vitro and in vivo, SFC will predictably obtain further development.
Keywords: Supercritical fluid chromatography, Online SFC technique, Untargeted lipid profiling, Targeted lipid profiling, Lipidomics
1. Introduction
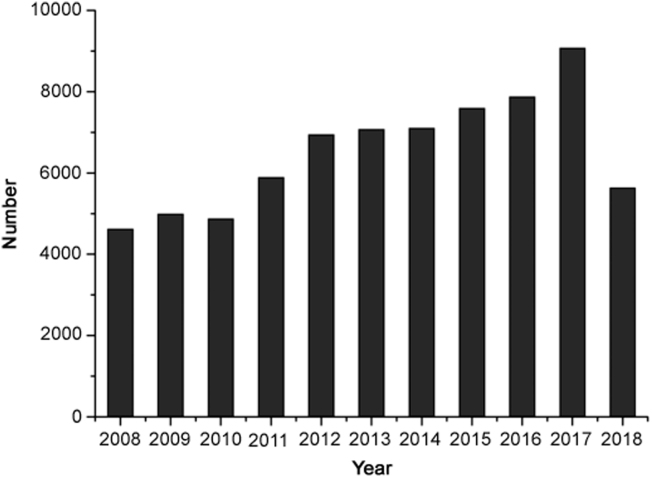
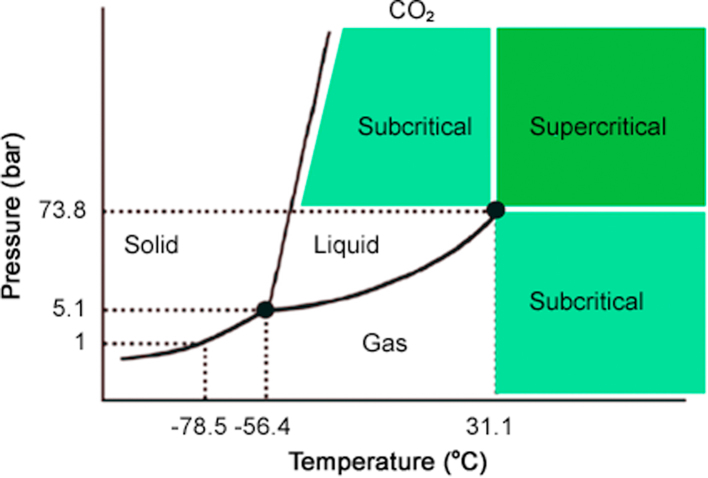
Recently, supercritical fluid chromatography (SFC), as an alternative technique to liquid chromatography (LC) and an extension of gas chromatography (GC), has aroused extensive attention due to its high efficiency and environment protecting [1]. Fig. 1 shows the number of articles on SFC published each year from 2008 to 2018. SFC uses supercritical fluid as the mobile phase, which is a kind of fluid with low viscosity and high diffusivity, which incorporates the features of both liquid and gas [2]. Carbon dioxide (CO2) is most commonly used as the preferred supercritical fluid (SF), which could be easily pressurized and heated beyond its critical points (31 °C, 74 bar) (Fig. 2 shows the phase diagram of CO2). The viscosity and diffusivity of the supercritical fluid are very close to those of gas, which results in high separation efficiency at a high mobile phase velocity. Meanwhile, the density and diffusivity of the supercritical fluid are similar to those of liquid, providing a good solubility for the analytes [3]. Incorporating both the features of liquid and gas, SFC is regarded as hybrid of GC and high-performance liquid chromatography (HPLC) [4] and exhibits a lot of advantages such as high separation efficiency, low organic solvent consumption and short separation time [5]. Moreover, SFC has essentially the same polarity profile as normal phase chromatography, which makes it well-suited for the analysis of the compounds with middle and low polarities, such as lipids.
Fig. 1.
The number of publications on the applications of supercritical fluid chromatography. Based on a Google Scholar search performed on August 14, 2018.
Fig. 2.
Pressure-temperature phase diagram of CO2. Reproduced from reference [15], with the permission of MDPI.
Lipids are a class of representative hydrophobic metabolites in a biological system. Based on the classification system from LIPIDMAPS (http://www.lipidmaps.org/), lipids have been divided into eight categories: (i) fatty acyls, (ii) glycerolipids, (ii) glycerophospholipids, (iv) sphingolipids, (v) sterol lipids, (vi) prenol lipids, (vii) saccharolipids and (viii) polyketides. Lipids are one of the three principal macronutrients in the living organisms. In addition, lipids are the main structural components of cell membranes, which play a vital role in many biological processes such as energy storage and cellular signaling [6]. Thus, a lot of attention has been paid to the detection and quantification of lipids, such as the endogenous lipid analysis in vivo [7], and the nutritious lipid analysis in oils [8] or plants [9]. What's more, lipidomics, defined as the systems-level analysis and characterization of lipids and their interacting moieties, which has been widely applied to the biomarker discovery, the mechanism exploration and the new drug research, is an emerging field of current research.
The separation of lipids has generally been carried out by GC, HPLC and SFC. For GC analysis of the lipids, a derivatization step is needed and thermal degradation of analytes would happen due to the high temperature. HPLC-MS based methods without derivatization are widely used in the analysis of lipids. The main advantage of HPLC over GC is the greater sensitivity as well as the enhanced chromatographic selectivity achieved with a rich variety of packed HPLC columns. However, HPLC for the lipids analysis always takes a long time and is organic solvent consuming. In recent years, SFC has met with great favor in targeted and untargeted lipid profiling due to its high efficiency, low organic solvent consumption, and the specialty for unambiguous identification of the isomeric species of some lipids [10].
This paper outlines the recent advances of SFC in targeted and untargeted lipid profiling. First, the development of SFC is summarized. Then the targeted and untargeted lipid profiling by SFC is described and discussed in detail. Furthermore, the online SFC technologies applied in lipid profiling are summarized. And finally, the perspectives of SFC applied in lipid profiling are also proposed.
2. Development of SFC
2.1. Advancement of SFC instruments
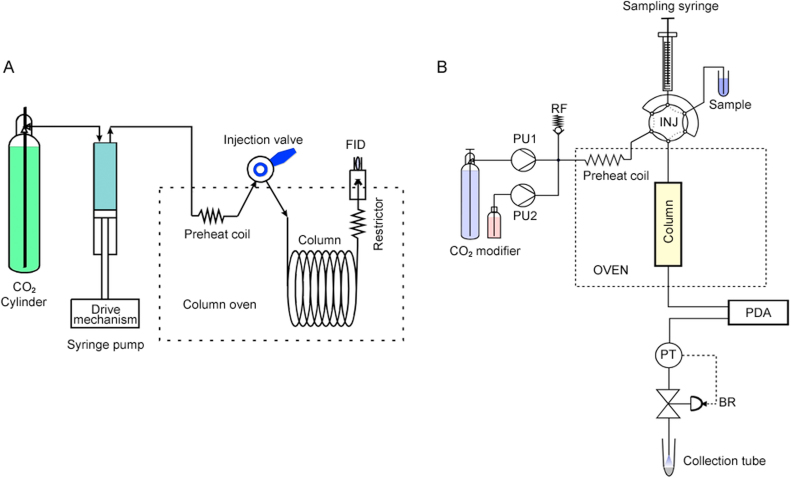
SFC was first reported by Klesper et al. [11] in 1962. In the initial period, the terminology “SFC” was named as high-press gas chromatography or dense gas chromatography. Traditional SFC technique acquired attention from the 1960s to the 1970s [12] and the heat faded because of the rapid growth of HPLC. In the 1980s, the development of SFC met with its second act. The development of instruments and columns led to the commercialization of SFC [1]. Open tubular capillary SFC (cSFC) was first introduced by Novotny [13], which was developed from GC and was usually coupled with flame ionization detectors (FID) [14]. Fig. 3A shows a schematic diagram of a typical GC-like open tubular column SFC system. Even though cSFC was popular in the 1980s as the extension of GC, it disappeared in the 1990s because of its limit use for hydrophobic compounds only [15].
Fig. 3.
(A) Schematic diagram of typical GC-like open tubular column SFC system. Since the flow rate is very low, a screw-driven syringe pump is used. Backpressure is applied by a restrictor that has a certain flow resistance to keep the system pressure above the critical pressure of the fluid. Pressure was controlled by changing the mobile phase flow rate. (B) Schematic diagram of typical LC-like packed-column SFC system with automated backpressure regulator. PU1: liquefied CO2 delivery reciprocating pump with chilled pump heads; PU2: modifier solvent delivery pump; RF: safety relief valve that prevents over pressure; INJ: injection valve; PDA: photodiode array UV detector; PT: pressure transducer; and BR: backpressure regulator. The pressure transducer monitors the pressure real time and the backpressure regulator compares the set pressure and actual pressure and control the flow resistance of the regulator so that the actual pressure becomes equal to the set pressure. Reproduced from reference [1], with the permission of Elsevier.
In 1982, Gere et al. [16] transformed a Hewlett-Packard (HP) HPLC system into an SFC system by adding a back pressure regulator (BPR, in order to keep pressure constant whatever the mobile phase flow rate) and other devices, which was the first commercial SFC instrument. Fig. 3B shows a schematic diagram of a typical LC-like packed-column SFC system with an automated backpressure regulator. Different from cSFC, packed SFC (pSFC) is very similar to an HPLC system for the introduction of a binary pump to directly modify the SF with organic solvents. A backpressure regulator that keeps the fluid pressure above the critical pressure and an oven that keeps the fluid temperature above the critical temperature are vital devices specific to pSFC. As a result, pSFC showed better selectivity and broader range of analysis. In the 1980s, Okamoto et al. [17] developed high efficient and versatile chiral stationary phases (CSPs) and published a series of articles. Later, these CSPs were commercialized by Daicel Corporation (Osaka, Japan) and were rapidly spread throughout the world, which were first used in LC and then extended to SFC [18]. Nevertheless, the application of pSFC was limited in the 1990s due to the poor quantitative performance and the lack of repeatability and robustness [19].
The unwillingness of the analysts to use pSFC was mainly solved by the introduction of new state-of-the-art systems by some instrument manufacturers (Waters, Agilent Technologies, and Shimadzu, in particular). These modified systems equipped with novel BPR were designed based on the recent ultra-high performance liquid chromatography (UHPLC) instruments. What's more, dead volumes of the new SFC systems have been lowered while the reliability, quantitative performance and sensitivity have been improved. These modern systems were compatible with the columns made of modern stationary phases (sub-3 µm core-shell and fully porous sub-2 µm particles) to get the highest kinetic performance (high efficiency and throughput), which brought a substantial revival to pSFC.
2.2. Stationary phases of SFC
The stationary phases of a very wide range are currently available for SFC operation, although very few of them have been purposely created for SFC. The wide choice of the stationary phases could be explained by the fact that modern SFC covers the application of different chromatographic modes, which has been applied to a wide range polarity of analytes. The column category of SFC includes alkyl bonded phases and polar phases [20]. By using silica or hybrid silica stationary phases, SFC could be used for the analysis of biologically active substances which display high hydrophilicity [21]. As for lipophilic compounds, SFC has been considered as a powerful tool for lipids analysis using both alkyl bonded phases and polar phases [22]. BEH C18 and HSS C18 (Waters) are the typical columns exhibiting alkyl bonded moieties which show the reversed phase chromatographic separation. For example, Qu et al. [23] proposed an ultra-high performance supercritical fluid chromatograph (UHPSFC) -MS-based method using BEH C18 column for determination of 8 free fatty acids (FFAs) in edible oils within only 3 min and without any pretreatment. Correspondently, BEH Silica and BEH 2-EP (Waters) are the typical columns which exhibit polar moieties to show the normal phase chromatographic separation. Bamba et al. [24] established a pSFC- ultraviolet (UV)-based method using BEH Silica column for the determination of natural polyprenols.
SFC has been successfully applied to both chiral purity assessment and chiral separation to provide enantiomerically pure compounds. The CSP chemistry for the SFC-based analysis has been developed in these years [25]. SFC has been proven to provide at least two times faster separation than normal-phase HPLC on the chromatographic resolution of chiral compounds [5]. Wang et al. [26] achieved a separation of two demethylated nobiletin metabolites, 3′-demethyl-NOB and 4′-demethyl-NOB, via SFC/UV with a Chiralpak AD column, which provided a 10 min retention time difference between the nobiletin regio-isomers. An SFC system coupled with atmospheric pressure chemical ionization (APCI)-MS using a chiral packed-column was reported for the simultaneous determination of (R, S)-propranolol and (+)-pindolol in mouse blood samples, requiring only 3 min per sample at a low nanogram per milliliter region [27]. Wu et al. [28] successfully separated a series of racemic 2, 2-dimethyl-3-aryl-propanoic acids and the extended structurally similar racemic acids by SFC on a matrix of chiral columns including AD-H, OD-H, AS-H, OJ-H, Lux-cellulose-2, Lux-amylose-2, and Whelk-O1, and AD-H among all the columns showed a greater resolution within 8 min.
According to the Van Deemter equation, reducing particle size of the packing materials is a direct and effective way to enhance the column efficiency. And the high backpressure caused by the small particle size could be solved by adopting a shorter column. Nowadays, a series of columns with small particles (sub-2 µm) developed by Waters are applied in the SFC analysis, e.g., Acquity HSS C18 SB, Acquity BEH (hybrid silica), Acquity BEH 2-EP and Acquity BEH RP18 Shield [29]. Many other attractive stationary phases with different or unique selectivity have also been developed, such as Synergi polar RP (Phenomenex), Acclaim Polar Advantage I (Thermo), Ace C18-PFP (ACT), and Luna HILIC (Phenomenex) with the drawback of their unavailability in sub-2 µm.
3. Targeted lipid profiling by SFC
For the targeted lipid profiling, endogenous lipid analysis in vivo and the nutritious lipid analysis in oils or plants have been widely concerned. It is noteworthy that SFC has apparent advantages over LC and GC for the analysis of polar lipids, providing a fast separation and an identification, especially for the isomeric species. The research work about targeted lipid profiling by SFC is summarized in Table 1.
Table 1.
Research on targeted lipid profiling by SFC.
| Source | Target compounds | Sample size | Analytical column characteristics | SFC conditions (Temperature, pressure) | SFC mobile phases | Detector | Analysis time | Ref. |
|---|---|---|---|---|---|---|---|---|
| Phospholipids/sphingolipids | ||||||||
| Mouse liver | 19 classes of phospholipids, lysophospholipids, and sphingolipids | 10 mg | Inertsil ODS-4 (250 mm × 4.6 mm; 5 µm d.p) | 37 °C, 10 MPa, | A: CO2 | ESI-QqQ-MS | 6 min | [7] |
| B: CH3OH (0.1% Ammonium formate) | ||||||||
| Lettuce and ground beef | Lipid A | 1 g | A cyanopropyl phase column (30 mm × 4.6 mm; 5 µm d.p) | 40 °C, 150 bar, | A: CO2 | DAD and an ion trap MSn | 2 min | [33] |
| B: CH3OH (0.2% DEA) | ||||||||
| Mouse plasma | PCs and PEs | 20 μL | Inertsil ODS-EP (250 mm × 4.6 mm; 5 µm d.p) | 35 °C, 10 MPa | A: CO2 | Orbitrap Fourier transform MS | 15 min | [36] |
| B: CH3OH (0.1% Ammonium formate) | ||||||||
| Fatty acids | ||||||||
| Valeriana officinalis L. | Valerenic Acids and Valepotriates | 10 g | S3-Nitrile Spherisorb (CN; 150 mm × 4.6 mm; 3 µm d.p) | 40 °C, 30 MPa | A: CO2 | UV | 20 min | [39] |
| B: CH3OH: H2O= 9:5 | ||||||||
| Fish oil | 31 free fatty acids | 1 μL | ACQUITY UPC2 HSS C18 SB (150 mm × 3.0 mm; 1.8 µm d.p) | 25 °C, 1500 psi | A: CO2 | ELSD & Q-TOF MS | 12 min | [8] |
| B: CH3OH (0.1% formic acid) for ELSD; CH3OH (10 mM NH4OAc) for MS. | ||||||||
| Edible oils | 8 fatty acids | 10 mg | UPC2 HSS C18 SB column (100 mm × 3.0 mm; 1.8 µm d.p) | 40 °C, 1500 psi | A: CO2 | ESI-QqQ-MS | 3 min | [23] |
| B: methanol/acetonitrile (50:50, v/v) with 0.1% formic acid | ||||||||
| Triacylglycerols | ||||||||
| Pharmaceutical excipients | Mono-, di- and triglycerides | 10 mg | SB-Octyl 50 open tubular capillary column (10 m × 50 µm; 0.25 µm d.p) | 90 °C, 94 bar | Pure CO2 | FID | 45 min | [42] |
| Black currant and alpine currant seed oils | Triacylglycerols containing γ-linolenic (18:3n−6) and α-linolenic acid (18:3n−3) | 10 mg | SB-Cyanopropyl−25 open tubular capillary column (10 m × 50 µm) | 135 °C | A: CO2 | APCI-QqQ-MS | 16.4 min | [43] |
| B: Cyanopropyl | ||||||||
| 15 vegetable oils | 30 triglycerides | – | Hypersil ODS (105 cm × 0.46 cm) | 16 °C, 12 MPa | A: CO2 | UV | 80 min | [44] |
| B: ACN: MeOH= 9:1 | ||||||||
| Various vegetable oils (perrila, soybean, sesame, palm oil), animal fats (lard and beef tallow), and fish oil | More than hundred triglycerides | 1%–3% concentration | L-column ODS (25 cm × 4.6 mm; 5 µm d.p) | First column:0–25 °C | Pure CO2 | UV | 700 min | [45] |
| ODS packing materials (5 cm × 4.6 mm; 5 µm d.p) | Second column:40–70 °C | |||||||
| Sterol lipids | ||||||||
| Human serum | Free cholesterol and cholesteryl esters | 500 μL | SBOctyl-50 (10 m × 50 µm; 0.25 µm d.p) | 65 °C, 137.90–275.79 bar | CO2 | FID | 110 min | [47] |
| Standards | 16 classes of steroids | 0.08–6.4 mg/mL | Brownlee Spherisorb Phenyl cartridge (10 cm × 4.6 mm) | 50 °C | A: CO2 | UV, MS and ELSD | 9 min | [48] |
| B: Organic modifier | ||||||||
| Finnish boars fat tissue samples | Androstenone | 0.8 g | Deltabond Cyano or ODS C18 column (100 mm × 1 mm; 5 µm d.p) | 100 °C | Pure CO2 | QqQ-MS | 20 min | [49] |
| Prenol lipids | ||||||||
| Fresh carrots and tomatoes | Carotenoids | 200 g | SB-phenyl-50 (10 m × 50 µm; 0.25 µm d.p) | 45 °C | A: CO2 | UV | 30 min | [50] |
| B: ethanol | ||||||||
| Alpha-carotene | Two SB-cyanopropyl-50 (10 m × 50 µm; 0.25 µm d.p) | 50 °C | ||||||
| Beta-carotene | SB-cyanopropyl-25 (7 m × 50 µm; 0.25 µm d.p) | 50 °C | ||||||
| Tochu leaves | Octadecaprenol and nonadecaprenol | 2 g | Inertsil ODS3 (250 mm × 4.6 mm; 5 µm d.p) | 130 °C, 19.6 MPa | A: CO2 | UV | 30 min | [24] |
| B: ethanol | ||||||||
| Lyophilized C. reinhardtii | 7 structural classes of carotenoids | 2 mg | Hibar Purospher STAR RP-18e (monomeric ODS, 250 mm × 4.6 mm) | 35 °C, 10 MPa | A: SCCO2 | QqQ-MS | 15 min | [51] |
| B: CH3OH (0.1% ammonium formate) | ||||||||
| Soybean oil | 4 tocopherols and 3 tocotrienols | 10 μL | Amine Luna NH2 (150 mm × 2 mm; 3 µm d.p) | 30 °C, 130 bar | A: CO2 | UV and APCI-Q-TOF MS | 5 min | [52] |
| B: ethanol (0.1% formic acid) | ||||||||
| Polyketides | ||||||||
| Standards | 11 classes of flavonoid | 1 μL | BPI (12 m × 0.1 mm; 0.1 µm d.p) and DB5 (15 m × 0.1 mm; 0.4 µm d.p) | P1:100 °C, 73.3–324.2 bar | Pure CO2 | FT-IR and FID | 40 min | [54] |
| P2:150 °C, 79.0–318.5 bar | ||||||||
| Mouse urine | Nobiletin and its metabolites | 400 μL | Chiralpak AD-H (250 mm × 4.6 mm; 5 µm d.p) | 30 °C, 100 bar | A: CO2 | UV | 25 min | [55] |
| B: CH3OH | ||||||||
| Sweet orange (Citrus sinensis) peel | 4 Polymethoxyflavones | 132 mg | DAICEL AD chiral column (30 mm × 250 mm; 5 µm d.p) | 30 °C, 100 bar | A: CO2 | UV | 6.5 min | [9] |
| B: methanol (0.25% diethylamine) | ||||||||
DAD: diode array detector; ELSD: evaporative light scattering detectors; TOF: time of flight; QqQ-MS: triple quadrupole mass spectrometry detection.
3.1. Phospholipids/sphingolipids
Phospholipids (PLs) and sphingolipids (SPs) belong to polar lipids according to the different types of attached hydrophilic groups. PLs, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE), are the main lipid type in cell membranes [30]. Lyso-phospholipids (LPLs) are the hydrolysis products of PLs by phospholipase that contribute to signal transduction in various pathophysiological processes [31]. SPs, as the structural component of all eukaryotic cell membranes, play important biological roles in membrane fluidity, signal transduction and cell-cell interactions [32]. SFC-MS-based analytical method has been applied to the separation of polar lipids in recent years. A simultaneous and fast determination of 19 polar lipids including PLs, LPLs, and SPs by SFC-MS was performed in 6 min and applied to the analysis of mouse liver [7]. With trimethylsilyldiazomethane as the derivatization reagent used to improve the peak shapes and increase the detection sensitivity, the proposed method was well suited for the analysis of both the high-abundance species and the low-abundance species in these polar lipids. Chen et al. [33] proposed a simple and fast SFC-MS/MS method for the analysis of large and polar lipids (lipid A) within only 2 min.
The separation of PLs by SFC enables the accurate identification and quantitation for the individual molecular, especially for the identification of the isomeric species of PLs (e.g. PC (20:0/18:2) and PC (20:1/18:1), PC (35:6) and PE (18:1/20:5)) and some PLs with low abundances of product ions. In normal-phase liquid chromatography (NPLC), PLs are separated based on their polar head groups, and the isomers of the same lipid class are eluted at the same time [34]. Conversely, in reverse-phase liquid chromatography (RPLC), PLs are separated based on their fatty acyl groups, so the isomers with the same or similar fatty acyl groups may be co-eluted although they belong to different lipid classes [35]. Recently, Yamada et al. [36] developed an SFC coupled with an Orbitrap Fourier transform MS (Orbitrap FT-MS) method using a single octadecylsilyl (ODS) column to analyze various polar lipids in mouse plasma, and isomeric molecules of PLs were successfully separated and identified based on not only their fatty acyl moieties but also their polar head groups. The retention behavior for some positional isomers of polar lipids is also different between HPLC and SFC [37]. For example, 1-Lyso-PC and 2-Lyso-PC are difficult to be separated by HPLC; however, the positional isomers such as 1-Lyso-PC/2-Lyso-PC and 1-Lyso-PE/2-Lyso-PE can be well resolved using SFC. This behavior provides convenience for the unambiguous identification of lipids using SFC. To sum up, compared with HPLC-MS, SFC-MS can achieve a more comprehensive and faster analysis with an efficient separation and unambiguous identification of polar lipids.
3.2. Fatty acids
Fatty acids (FAs) are important building blocks of complex lipids. FFAs are usually derived from triglycerides (TGs) or phospholipids (PLs) in organism. FFAs have a variety of essential functions. For example, FFAs serve as a major provider of physiological energy needs during fasting, and constitute cell membranes, and in some cases act as the key regulators [38]. SFC is commonly used for the effective separation of FFAs and fatty acid methyl esters in oils. Bicchi et al. [39] used SFC with UV detector for the analysis of different fatty acids with the analysis time 50% shorter than that of the corresponding HPLC method. Ashraf-Khorassani et al. [8] applied the UHPSFC coupled with evaporative light scattering (ELSD) and MS to measure 31 FFAs in fish oils with good compatibility. The results also showed that both UHPSFC MS and GC-MS analysis for FFAs had similar detection limits. However, the separation of FFAs via UHPSFC (7 min) was much faster than GC (30 min), and UHPSFC-MS required no derivatization known to be time and expensive chemicals consuming. Qu et al. [23] proposed a UHPSFC-MS method for the determination of 8 FFAs in edible oils with satisfactory correlation coefficients (R2 > 0.994) and good reproducibility (RSD < 15.0%) within only 3 min and no pretreatment was needed. Additionally, a multidimensional approach was used for the analysis of FAs in fish oil [40]. In this study, respectively, silver-ion (SI)-SFC and RPLC were applied in the first and the second dimensions and the detectors of UV coupled with ELSD were employed for data acquisition. Due to the high degree of orthogonality, FAs were clearly separated compared with one-dimensional system and the structure elucidation of FAs was also enhanced.
3.3. Triacylglycerols
Triacylglycerols (TGs) are esters derived from glycerol and three fatty acids. TGs are present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils [41]. In an early study, the analysis of monoglycerides, diglycerides and triglycerides (TGs) mixtures in several pharmaceutical excipients was achieved by capillary SFC (cSFC) coupled to an FID in less than 1 h [42]. Manninen et al. [43] applied cSFC-MS with an APCI source to the quantitative analysis of TGs in berry oils. The method provided fragment information of the fatty acid residues to improve the identification which was not available with SFC-FID, and presented a higher sensitivity than SFC-FID. Using pSFC with the ODS columns, Lesellier et al. [44] studied the retention behavior of 30 TGs from 15 vegetal oils by SFC-MS. The results showed that the retention order of TGs depended on their unsaturation levels and carbon number, the retention increased following the unsaturation number when the opposite effect was obtained in reverse phase HPLC. Moreover, a comprehensive, two-dimensional SFC system with the ODS columns has been developed for the analysis of TGs in fats and oils, which achieved more efficient separations [45].
3.4. Sterol lipids
Sterol lipids (STs) are ringed lipids that play a role in the membrane integrity of eukaryotes. The most familiar type of animal sterol lipids is cholesterol, which is vital to cell membrane structure, and acts as a precursor to fat-soluble vitamins and steroid hormones. STs in plants commonly occur as mixtures, such as sitosterol, stigmasterol and campesterol [46]. The analytical methods of cSFC-FID, cSFC-UV and cSFC-ELSD were applied to the measurement of STs about twenty years ago. Kim et al. [47] employed cSFC-FID for the individual determination of cholesterol and cholesteryl esters in human serum, which achieved an acceptable average relative standard deviation of 2.6% and a detection limit of 4–6 pg. In another work, cSFC with ELSD detector was used for the separation of steroids, which allowed the SFC separations developed for the polar, UV transparent compounds that have been ignored [48]. With the development of packed column and MS, the technology of pSFC-MS was widely used for the analysis of STs. Tuomola et al. [49] applied the pSFC-MS with an APCI source to the analysis of androstenone in pig fat samples. The limit of quantification (LOQ) for androstenone was 0.25 mg/g, demonstrating a superior sensitivity of this method.
3.5. Prenol lipids
Prenol lipids (PRs) mainly consist of fat-soluble vitamins (i.e. vitamins A, E and K), carotenoids and ubiquinones (i.e. coenzyme Q9). In previous research, cSFC-UV has demonstrated significant potential for the improvement of the separation of carotenoids and their isomers from carrots and tomatoes relative to HPLC-UV [50]. The technology of pSFC-MS has been used for the analysis of PRs in recent years. For example, Bamba et al. [24] established a pSFC-UV-based method for the determination of natural polyprenols, which allowed the advantage of baseline separation of polyprenols and increased the resolution of separation two times higher than conventional RPLC-UV. Likewise, Matsubara et al. [51] proved the advantage of pSFC-MS in carotenoids separation with high speed in 15 min and high resolution compared with RPLC-MS. Using a standard-addition method, Méjean et al. [52] applied pSFC-MS to the detection and quantification of seven vitamin E congeners in soybean oil. The analysis was less than 5 min and as sensitive as LC–MS when using similar column settings.
3.6. Polyketides
Polyketides (PKs) are structurally a very diverse family of natural products with diverse biological activities and pharmacological properties, such as polyketide antibiotics, antifungals, cytostatics, anticholesteremic, antiparasitics and coccidiostats [53]. The analysis of some hydroxy- and methoxy-flavones by cSFC with two types of detectors, FID and Fourier transform infrared (FT-IR) spectroscopy, was presented without the use of modifier in the 1990s [54]. Li et al. [55] conducted an investigation into nobiletin's metabolics in mouse urine by various analytical techniques such as RPLC, NPLC and pSFC. Due to the structural similarities of 3′-demethylnobiletin and 4′-demethylnobiletin, only pSFC could achieve a clear separation of these two metabolites. A later work by the same group compared the analytical methods of RPLC, NPLC and pSFC with non-chiral as well as CSPs for the separation of polymethoxyflavones from orange extracts [9]. The results showed that SFC technology had the best separation efficiency, especially under the chiral mode.
4. Untargeted lipid profiling by SFC
Untargeted lipidomics analysis encounters several challenges. First, the samples always contain diverse lipids with different polarity so that it poses obstacles in establishing an appropriate method for simultaneous lipid analysis [56], [57]. Secondly, lots of lipids in the biological samples are low-abundance or labile [58]. Furthermore, quite a few kinds of lipids have similar structures. Recently, SFC coupled with tandem MS shows an excellent performance on lipidomics researches, which enables comprehensive and exhaustive lipid detection [3], [10]. In addition, compared with HPLC, the separation of SFC for various lipids is less time-consuming [37].
Bamba et al. [59], [60] underwent plenty of work to determine the SFC conditions and MS conditions while analyzed diverse lipids with different polarity. Based on the previous studies, they also employed SFC-MS to separate numerous lipids extracted from twelve soybean cultivars with an ODS column [61]. In this study, triacylglycerol and PC, which were largely observed in the soybean extract, were separated and identified precisely, suggesting the outstanding performance of SFC on simultaneously lipid profiling. Lisa et al. [62] established a UHPSFC-ESI-MS method which successfully separated and identified 30 nonpolar and polar lipid classes within 6 min covering 6 main lipid categories. Furthermore, 24 lipid classes including 436 lipid species were identified in porcine brain extract using the same method. Yang et al. [63] carried out untargeted lipidomics research based on the UHPSFC coupling with ion-trap and time-of-flight tandem mass spectrometry (UHPSFC -IT-TOF/MS) to investigate the lipid profiles of stroke rats with STV-Na treatment. This UHPSFC -IT-TOF/MS-based method achieved a fast separation of various lipids within 9 min with a qualified repeatability. The results of pathway analysis suggested that the protective effects of STV-Na might be related to the regulation of several metabolic pathways including glycerophospholipid metabolism, arachidonic acid metabolism and sphingolipid metabolism. In brief, UHPSFC -MS is a useful tool to investigate lipid profiling during the untargeted lipidomics research.
5. Online SFC technology in lipid profiling
5.1. Online coupling of sample preparation techniques with SFC
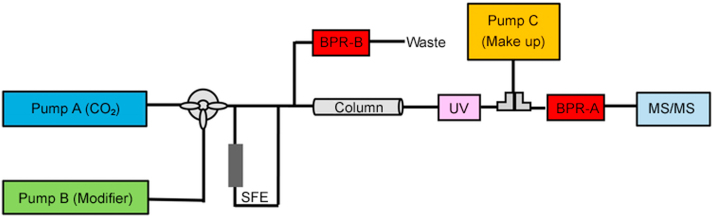
Along with the commercialization of a new generation of instruments, SFC gains improved performance, reliability and robustness, and the online coupling SFC system has been booming recently [64]. So far, supercritical fluid extraction (SFE) and solid phase extraction (SPE) are the two major sample preparation techniques which have been online coupled with SFC. Fig. 4 shows the schematic diagram of the SFE-SFC-MS/MS system. The SFE-SFC online system consists of an SFE module and an SFC module. The supercritical extractant is injected directly to the chromatographic column without any pre-concentration. SFE is a physical separation and purification method, which changes the solubility of supercritical CO2 by regulating the pressure and the temperature. The extraction process is composed of extraction and separation. Under the supercritical state, CO2 is contacted with the substance to be separated, and the components are extracted successively according to their polarities, so as to achieve the purpose of separation and purification [65].
Fig. 4.
The schematic diagram of the SFE-SFC-MS/MS system. BPR-backpressure regulator.
The SPE-SFC system consists of an SPE module and an SFC module. The configuration of the SPE-SFC system is quite similar to that of the common online SPE-UHPLC system because both of them use the valve switching strategy [66]. The system contains two six-port valves, one valve is in charge of SPE procedure while the other is in charge of SFC, and the SPE column and the chromatographic column are connected when the elution begins by the switching of these valves. However, the stability of SFC system might be disturbed by the water matrix after SPE. Thus, the development of the online SPE-SFC system is limited in this decade.
5.2. Online SFE-SFC in lipid profiling
In recent years, there have been only a few studies about the online SFE–SFC system which were applied to the lipid profiling. Suzuki et al. [67] used online SFE-SFC to analyze the disease biomarkers in dried serum spots, which were expected to be applied to disease diagnosis. In this study, four hydrophilic metabolites and 17 hydrophobic metabolites were simultaneously detected within 15 min, and they exhibited comparable diagnostic performance to the serum analysis using LC-MS-MS. Zoccali et al. [68] developed an online method coupling SFE and SFC for a detailed targeted native carotenoids characterization in red habanero peppers, and twenty-one targeted analytes were extracted and identified by the developed methodology in less than 17 min. Uchikata et al. [69] established an online SFE-SFC-MS system for phospholipids profiling of dried plasma spot. Using this system, only 3 μL of plasma could be extracted in 5 min and was analyzed within 15 min. A total of 134 phospholipids, including phosphatidylcholine, lysophosphatidylcholine, sphingomyelin, phosphatidylethanolamine and lysophosphatidylethanolamine, were detected, and 74 phospholipids were analyzed with good repeatability.
6. Perspectives
In conclusion, SFC is considered as a powerful tool for the analysis of the lipid profiling in biological samples. Owing to the advantages of a hybrid of GC and LC, SFC incorporates many features of these two techniques and shows outstanding separation efficiency. And the modifier can flexibly adjust the polarity of the mobile phase in SFC, providing the convenience for simultaneous analysis of various lipids with a wide range of polarities. In recent years, UHPSFC using columns with sub-2 µm particles shows a great potential as the comprehensive and high-throughput method for the analysis of the lipid profiling. The development of new stationary phase is conducive to the separation of the specific analytes, especially for the natural isomers in biological samples. Recently, Takeda et al. [70] have developed widely-targeted quantitative lipidomics methodology using SFC-MS/MS, which represents a potentially useful tool for in-depth studies focused on complex lipid metabolism and biomarker discovery. Moreover, the online SFC technologies could greatly shorten the analysis time and simplify the pretreatment, so they show broad prospects in the lipid profiling. It is believed that the SFC-based method as a promising strategy could provide us many useful insights into lipid metabolism in physiological or pathological studies.
Acknowledgments
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21775047), Pearl River S and T Nova Program of Guangzhou, China (Grant No. 201806010055) and the Fundamental Research Funds for the Central Universities (Grant No. 2018MS55).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Ting Zhou, Email: tingzhou@scut.edu.cn.
Li Gongke, Email: cesgkl@mail.sysu.edu.cn.
References
- 1.Saito M. History of supercritical fluid chromatography: instrumental development. J. Biosci. Bioeng. 2013;115:590–599. doi: 10.1016/j.jbiosc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Tyśkiewicz K., Dębczak A., Gieysztor R. Determination of fat- and water-soluble vitamins by supercritical fluid chromatography: a review. J. Sep. Sci. 2018;41:336–350. doi: 10.1002/jssc.201700598. [DOI] [PubMed] [Google Scholar]
- 3.Desfontaine V., Guillarme D., Francotte E. Supercritical fluid chromatography in pharmaceutical analysis. J. Pharm. Biomed. 2015;113:56–71. doi: 10.1016/j.jpba.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y., Tang G., Zhang T. Supercritical fluid chromatography in traditional Chinese medicine analysis. J. Pharm. Biomed. 2018;147:65–80. doi: 10.1016/j.jpba.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Kalíková K., Šlechtová T., Vozka J. Supercritical fluid chromatography as a tool for enantioselective separation; a review. Anal. Chim. Acta. 2014;821:1–33. doi: 10.1016/j.aca.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Spector A.A., Yorek M.A. Membrane lipid composition and cellular function. J. Lipid Res. 1985;26:1015–1035. [PubMed] [Google Scholar]
- 7.Lee J.W., Nishiumi S., Yoshida M. Simultaneous profiling of polar lipids by supercritical fluid chromatography/tandem mass spectrometry with methylation. J. Chromatogr. A. 2013;1279:98–107. doi: 10.1016/j.chroma.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Ashraf-Khorassani M., Isaac G., Rainville P. Study of ultrahigh performance supercritical fluid chromatography to measure free fatty acids with out fatty acid ester preparation. J. Chromatogr. B. 2015;997:45–55. doi: 10.1016/j.jchromb.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Li S., Lambros T., Wang Z. Efficient and scalable method in isolation of polymethoxyflavones from orange peel extract by supercritical fluid chromatography. J. Chromatogr. B. 2007;846:291–297. doi: 10.1016/j.jchromb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Bamba T., Lee J.W., Matsubara A. Metabolic profiling of lipids by supercritical fluid chromatography/mass spectrometry. J. Chromatogr. A. 2012;1250:212–219. doi: 10.1016/j.chroma.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 11.Klesper E., Corwin A.H., Turner D.A. High pressure gas chromatography above critical temperatures. J. Org. Chem. 1962;27:700–701. [Google Scholar]
- 12.Giddings J.C., Myers M.N., Mclaren L. High pressure gas chromatography of nonvolatile species. Compressed gas is used to cause migration of intractable solutes. Science. 1968;162:67–73. doi: 10.1126/science.162.3849.67. [DOI] [PubMed] [Google Scholar]
- 13.Novotny M. Capillary supercritical fluid chromatography. J. Chromatogr. Libr. 1985;30:105–120. doi: 10.1016/s0021-9673(00)94280-8. [DOI] [PubMed] [Google Scholar]
- 14.Taylor L.T. Supercritical fluid chromatography for the 21st century. J. Supercrit. Fluid. 2009;47:566–573. [Google Scholar]
- 15.Laboureur L., Ollero M., Touboul D. Lipidomics by supercritical fluid chromatography. Int. J. Mol. Sci. 2015;16:13868–13884. doi: 10.3390/ijms160613868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gere D.R., Board R., Mcmanigill D. Supercritical fluid chromatography with small particle diameter packed columns. Anal. Chem. 1982;54:736–740. [Google Scholar]
- 17.Okamoto Y., Kawashima M., Hatada K. Chromatographic resolution: xi. Controlled chiral recognition of cellulose triphenylcarbamate derivatives supported on silica gel. J. Chromatogr. A. 1986;363:173–186. [Google Scholar]
- 18.Mangelings D., Vander H.Y. Chiral separations in sub- and supercritical fluid chromatography. J. Sep. Sci. 2008;31:1252–1273. doi: 10.1002/jssc.200700564. [DOI] [PubMed] [Google Scholar]
- 19.Nováková L., Perrenoud A.G., Francois I. Modern analytical supercritical fluid chromatography using columns packed with sub-2μm particles: a tutorial. Anal. Chim. Acta. 2014;824:18–35. doi: 10.1016/j.aca.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 20.West C., Lesellier E. Characterisation of stationary phases in subcritical fluid chromatography with the solvation parameter model IV: aromatic stationary phases. J. Chromatogr. A. 2006;1115:233–245. doi: 10.1016/j.chroma.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 21.Patel M.A., Riley F., Ashraf-Khorassani M. Supercritical fluid chromatographic resolution of water soluble isomeric carboxyl/amine terminated peptides facilitated via mobile phase water and ion pair formation. J. Chromatogr. A. 2012;1233:85–90. doi: 10.1016/j.chroma.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Bernal J.L., Martín M.T., Toribio L. Supercritical fluid chromatography in food analysis. J. Chromatogr. A. 2013;1313:24–36. doi: 10.1016/j.chroma.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Qu S., Du Z., Zhang Y. Direct detection of free fatty acids in edible oils using supercritical fluid chromatography coupled with mass spectrometry. Food Chem. 2015;170:463–469. doi: 10.1016/j.foodchem.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 24.Bamba T., Fukusaki E., Kajiyama S. High-resolution analysis of polyprenols by supercritical fluid chromatography. J. Chromatogr. A. 2001;911:113–117. doi: 10.1016/s0021-9673(00)01250-4. [DOI] [PubMed] [Google Scholar]
- 25.Speybrouck D., Lipka E. Preparative supercritical fluid chromatography: a powerful tool for chiral separations. J. Chromatogr. A. 2016;1467:33–55. doi: 10.1016/j.chroma.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z., Li S., Jonca M. Comparison of supercritical fluid chromatography and liquid chromatography for the separation of urinary metabolites of nobiletin with chiral and non-chiral stationary phases. Biomed. Chromatogr. 2006;20:1206–1215. doi: 10.1002/bmc.686. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Hsieh Y., Cook J. Supercritical fluid chromatography-tandem mass spectrometry for the enantioselective determination of propranolol and pindolol in mouse blood by serial sampling. Anal. Chem. 2006;78:1212–1217. doi: 10.1021/ac0516178. [DOI] [PubMed] [Google Scholar]
- 28.Wu D., Yip S.H., Li P. Additive free preparative chiral SFC separations of 2,2-dimethyl-3-aryl-propanoic acids. J. Pharm. Biomed. 2016;131:54–63. doi: 10.1016/j.jpba.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Lesellier E., West C. The many faces of packed column supercritical fluid chromatography-A critical review. J. Chromatogr. A. 2015;1382:2–46. doi: 10.1016/j.chroma.2014.12.083. [DOI] [PubMed] [Google Scholar]
- 30.Bochkov V.N., Oskolkova O.V., Birukov K.G. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Sign. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wymann M.P., Schneiter R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 32.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signaling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Lehotay S.J., Moreau R.A. Supercritical fluid chromatography-tandem mass spectrometry for the analysis of lipid A. Anal. Methods. 2013;5:6864–6869. [Google Scholar]
- 34.Okazaki Y., Kamide Y., Hirai M.Y. Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics. 2013;9S:S121–S131. doi: 10.1007/s11306-011-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogiso H., Suzuki T., Taguchi R. Development of a reverse-phase liquid chromatography electrospray ionization mass spectrometry method for lipidomics, improving detection of phosphatidic acid and phosphatidylserine. Anal. Biochem. 2008;375:124–131. doi: 10.1016/j.ab.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Yamada T., Uchikata T., Sakamoto S. Supercritical fluid chromatography/Orbitrap mass spectrometry based lipidomics platform coupled with automated lipid identification software for accurate lipid profiling. J. Chromatogr. A. 2013;1301:237–242. doi: 10.1016/j.chroma.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Lísa M., Cífková E., Khalikova M. Lipidomic analysis of biological samples: comparison of liquid chromatography, supercritical fluid chromatography and direct infusion mass spectrometry methods. J. Chromatogr. A. 2017;1525:96–108. doi: 10.1016/j.chroma.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Binienda Z.K., Sarkar S., Silvaramirez S. Role of free fatty acids in physiological conditions and mitochondrial dysfunction. Food Nutr. Sci. 2013;4:6–15. [Google Scholar]
- 39.Bicchi C., Binello A., Rubiolo P. Packed column SFC/UV versus HPLC/UV analysis of valerenic acids and valepotriates in extracts of Valeriana officinalis L. Phytochem. Anal. 2000;11:179–183. [Google Scholar]
- 40.François I., Sandra P. Comprehensive supercritical fluid chromatography × reversed phase liquid chromatography for the analysis of the fatty acids in fish oil. J. Chromatogr. A. 2009;1216:4005–4012. doi: 10.1016/j.chroma.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 41.Fahy E., Subramaniam S., Murphy R.C. Update of the lipid maps comprehensive classification system for lipids. J. Lipid Res. 2009;50:S9–S14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giron D., Link R., Bouissel S. Analysis of mono-, di- and triglycerides in pharmaceutical excipients by capillary supercritical fluid chromatography. J. Pharm. Biomed. Anal. 1992;10:821–830. doi: 10.1016/0731-7085(91)80087-p. [DOI] [PubMed] [Google Scholar]
- 43.Manninen P., Laakso P. Capillary supercritical fluid chromatography--atmospheric pressure chemical ionization mass spectrometry of gamma- and alpha-linolenic acid containing triacylglycerols in berry oils. Lipids. 1997;32:825–831. doi: 10.1007/s11745-997-0105-1. [DOI] [PubMed] [Google Scholar]
- 44.Lesellier E., Tchapla A. Retention behavior of triglycerides in octadecyl packed subcritical fluid chromatography with CO2/modifier mobile phases. Anal. Chem. 1999;71:5372–5378. doi: 10.1021/ac990539j. [DOI] [PubMed] [Google Scholar]
- 45.Hirata Y., Hashiguchi T., Kawata E. Development of comprehensive two-dimensional packed column supercritical fluid chromatography. J. Sep. Sci. 2003;26:531–535. [Google Scholar]
- 46.Yeagle P.L. Membranes of Cells. Academic Press; 2016. Chapter 9-Cholesterol related sterols: roles in membrane structure and function; pp. 189–218. [Google Scholar]
- 47.Kim D.H., Lee K.J., Heo G.S. Analysis of cholesterol and cholesteryl esters in human serum using capillary supercritical fluid chromatography. J. Chromatogr. B. 1994;655:1–8. doi: 10.1016/0378-4347(94)00032-8. [DOI] [PubMed] [Google Scholar]
- 48.Loran J.S., Cromie K.D. An evaluation of the use of supercritical fluid chromatography with light scattering detection for the analysis of steroids. J. Pharm. Biomed. 1990;8:607–611. doi: 10.1016/0731-7085(90)80088-7. [DOI] [PubMed] [Google Scholar]
- 49.Tuomola M., Hakala M., Manninen P. Determination of androstenone in pig fat using packed column supercritical fluid chromatography mass spectrometry. J. Chromatogr. B. 1998;719:25–30. doi: 10.1016/s0378-4347(98)00409-5. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz H.H., Artz W.E., Poor C.L. High-performance liquid chromatography and capillary supercritical-fluid chromatography separation of vegetable carotenoids and carotenoid isomers. J. Chromatogr. 1989;479:261–268. doi: 10.1016/s0021-9673(01)83342-2. [DOI] [PubMed] [Google Scholar]
- 51.Matsubara A., Bamba T., Ishida H. Highly sensitive and accurate profiling of carotenoids by supercritical fluid chromatography coupled with mass spectrometry. J. Sep. Sci. 2009;32:1459–1464. doi: 10.1002/jssc.200800699. [DOI] [PubMed] [Google Scholar]
- 52.Méjean M., Brunelle A., Touboul D. Quantification of tocopherols and tocotrienols in soybean oil by supercritical-fluid chromatography coupled to high-resolution mass spectrometry. Anal. Bioanal. Chem. 2015;407:5133–5142. doi: 10.1007/s00216-015-8604-7. [DOI] [PubMed] [Google Scholar]
- 53.Surhone L.M., Tennoe M.T., Henssonow S.F. Betascript Publishing; 2010. Polyketide. [Google Scholar]
- 54.Hadj-Mahammed M., Badjah-Hadj-Ahmed Y., Meklati B.Y. Behaviour of polymethoxylated and polyhydroxylated flavones by carbon dioxide supercritical fluid chromatography with flame ionization and fourier transform infrared detectors. Phytochem. Anal. 2010;4:275–278. [Google Scholar]
- 55.Li S.M., Wang Z.Y., Sang S.M. Identification of nobiletin metabolites in mouse urine. Mol. Nutr. Food Res. 2006;50:291–299. doi: 10.1002/mnfr.200500214. [DOI] [PubMed] [Google Scholar]
- 56.Jurowski K., Kochan K., Walczak J. Analytical techniques in lipidomics: state of the art. Crit. Rev. Anal. Chem. 2017;47:418–437. doi: 10.1080/10408347.2017.1310613. [DOI] [PubMed] [Google Scholar]
- 57.Li M., Yang L., Bai Y. Analytical methods in lipidomics and their applications. Anal. Chem. 2014;86:161–175. doi: 10.1021/ac403554h. [DOI] [PubMed] [Google Scholar]
- 58.Rustam Y.H., Reid G.E. Analytical challenges and recent advances in mass spectrometry based lipidomics. Anal. Chem. 2018;90:374–397. doi: 10.1021/acs.analchem.7b04836. [DOI] [PubMed] [Google Scholar]
- 59.Bamba T., Shimonishi N., Matsubara A. High throughput and exhaustive analysis of diverse lipids by using supercritical fluid chromatography-mass spectrometry for metabolomics. J. Biosci. Bioeng. 2008;105:460–469. doi: 10.1263/jbb.105.460. [DOI] [PubMed] [Google Scholar]
- 60.Lee J.W., Yamamoto T., Uchikata T. Development of a polar lipid profiling method by supercritical fluid chromatography/mass spectrometry. J. Sep. Sci. 2011;34:3553–3560. doi: 10.1002/jssc.201100539. [DOI] [PubMed] [Google Scholar]
- 61.Lee J.W., Uchikata T., Matsubara A. Application of supercritical fluid chromatography/mass spectrometry to lipid profiling of soybean. J. Biosci. Bioeng. 2012;113:262–268. doi: 10.1016/j.jbiosc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Lisa M., Holcapek M. High-throughput and comprehensive lipidomic analysis using ultrahigh-performance supercritical fluid chromatography-mass spectrometry. Anal. Chem. 2015;87:7187–7195. doi: 10.1021/acs.analchem.5b01054. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y., Zhong Q., Zhang H. Lipidomics study of the protective effects of isosteviol sodium on stroke rats using ultra high-performance supercritical fluid chromatography coupling with ion-trap and time-of-flight tandem mass spectrometry. J. Pharm. Biomed. 2018;157:145–155. doi: 10.1016/j.jpba.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Grand-Guillaume Perrenoud A., Veuthey J., Guillarme D. Comparison of ultra-high performance supercritical fluid chromatography and ultra-high performance liquid chromatography for the analysis of pharmaceutical compounds. J. Chromatogr. A. 2012;1266:158–167. doi: 10.1016/j.chroma.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Da Silva R.P.F.F., Rocha-Santos T.A.P., Duarte A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016;76:40–51. [Google Scholar]
- 66.Bernal J.L., Jiménez J.J., Rivera J.M. On-line solid-phase extraction coupled to supercritical fluid chromatography with diode array detection for the determination of pesticides in water. J. Chromatogr. A. 1996;754:145–157. [Google Scholar]
- 67.Suzuki M., Nishiumi S., Kobayashi T. Use of on-line supercritical fluid extraction-supercritical fluid chromatography/tandem mass spectrometry to analyze disease biomarkers in dried serum spots compared with serum analysis using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2017;31:886–894. doi: 10.1002/rcm.7857. [DOI] [PubMed] [Google Scholar]
- 68.Zoccali M., Giuffrida D., Dugo P. Direct online extraction and determination by supercritical fluid extraction with chromatography and mass spectrometry of targeted carotenoids from red Habanero peppers (Capsicum Chinese Jacq.) J. Sep. Sci. 2017;40:3905–3913. doi: 10.1002/jssc.201700669. [DOI] [PubMed] [Google Scholar]
- 69.Uchikata T., Matsubara A., Fukusaki E. High-throughput phospholipid profiling system based on supercritical fluid extraction-supercritical fluid chromatography/mass spectrometry for dried plasma spot analysis. J. Chromatogr. A. 2012;1250:69–75. doi: 10.1016/j.chroma.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 70.Takeda H., Izumi Y., Takahashi M. Widely-targeted quantitative lipidomics method by supercritical fluid chromatography triple quadrupole mass spectrometry. J. Lipid Res. 2018;59:1283–1293. doi: 10.1194/jlr.D083014. [DOI] [PMC free article] [PubMed] [Google Scholar]