Abstract
Viral hepatitis is still a public health problem affecting several million people around the world. Neutrophils are polymorphonuclear cells that have a critical role in antibacterial infection. However, the role of neutrophils in viral infection is not fully understood. By using a mouse model of lymphocytic choriomeningitis virus infection-induced viral hepatitis, we observed increased neutrophil recruitment in the liver accompanied by enhanced CD8+ T-cell responses. Liver neutrophils expressed high levels of immunomodulatory cytokines, such as C-X-C chemokine ligand 2, arginase-1, inducible nitric oxide synthase and interleukin (IL)-10, demonstrating immunosuppressive properties. Depletion of neutrophils in vivo by a neutralizing antibody resulted in the exacerbation of liver injury and the promotion of T-cell responses at the immune contraction stage. IL-33 significantly induced neutrophil recruitment in the liver and attenuated liver injury by limiting effector T-cell accumulation. Mechanistically, we found that IL-33 promoted the expression of arginase-1 in neutrophils through the type 2 innate lymphoid cell (ILC2)-derived IL-13. Additionally, IL-13 increased the inhibitory effect of neutrophils on CD8+ T-cell proliferation in vitro, partially through arginase-1. Finally, we found that IL-13 induced arginase-1 expression, depending on signal transducer and activator of transcription factor 6 (STAT6) signaling. Therefore, IL-33 induced immunosuppressive neutrophils via an ILC2/IL-13/STAT6 axis. Collectively, our findings shed new light on the mechanisms associated with IL-33-triggered neutrophils in the liver and suggest potential targets for therapeutic investigation in viral hepatitis.
Introduction
Viral hepatitis is a major public health problem affecting millions of people worldwide. Acute infection with hepatitis A, B and C viruses (HAV, HBV and HCV, respectively) can cause fulminant hepatic failure, characterized by massive necrosis of liver cells.1 Despite these acute symptoms, persistent infections with HBV and HCV lead to chronic hepatitis that is associated with life-threatening liver damage, including fibrosis, cirrhosis and hepatocellular carcinoma.2 The activity of cytotoxic T lymphocytes (CTLs) is a key factor that determines the clinical outcome of viral hepatitis.3 Activated CD8+ T cells can protect against viral infections by secreting antiviral cytokines (for example, interferon (IFN)-γ and tumor necrosis factor (TNF)-α) and eliminate infected hepatocytes by perforin/granzyme and Fas ligand-mediated killing.4 Because CTLs can also damage uninfected bystander parenchymal cells and cause liver injury, their functions need to be tightly regulated.5
The chromatin-associated nuclear factor IL-33 is the IL-1-like cytokine ligand for the ST2 receptor.6 Owing to the broad expression of the ST2 receptor, IL-33 functions in both innate and adaptive immune cells, including granulocytes, dendritic cells, macrophages, natural killer cells, innate lymphoid cells (ILCs) and T cells.7 As an alarmin molecule, IL-33 synergizes with T-cell receptor and IL-12 signaling to promote the effector function of CD8+ T cells.8 IL-33 can expand myeloid-derived suppressor cells (MDSCs) and Foxp3+ regulatory T (Treg) cells in transplant models 9,10 or impair the established immunological tolerance in the lungs by inhibiting the immunosuppressive function of Treg cells.11 Therefore, IL-33 can have distinct roles, depending on the different organs and animal models. In viral infection, deficiency in IL-33/ST2 signaling results in impaired antiviral CTL responses and viral persistence,12 while IL-33 treatment promotes IFN-γ expression and protects the liver against injury.13 However, the underlying mechanism by which IL-33 regulates immune cells and protects the liver during viral infection is not entirely clear.
The role of neutrophils in antibacterial immunity is well defined; however, its role in antiviral immunity is less clear.14 Increased neutrophils were observed in lymphoid organs and blood after 7 days postinfection (dpi) in a mouse model of persistent viral infection but not in infection with an acute viral strain.15 In a clinical study, the reduction of neutrophil counts was observed in HCV patients with completed peginterferon and ribavirin treatment.16 Increasing evidence has demonstrated that, beyond their antibacterial roles, neutrophils function as decision shapers for both innate and adaptive immune responses.17 Interestingly, neutrophils share similarities with granulocytic myeloid-derived suppressor cells (G-MDSCs) and display immunosuppressive properties toward immune cells.18 Recently, neutrophils were demonstrated to inhibit CD8+ T-cell proliferation and facilitate metastasis in a mouse tumor model.19 These findings indicate that neutrophils may have an immunosuppressive action in viral infection by regulating CD8+ T-cell responses. Notably, IL-33 can enhance neutrophil influx to the site of bacterial infection.20 Similar findings were also reported in a mouse model of rheumatoid arthritis.21 Moreover, IL-33 can potently induce type 2 innate lymphoid cells (ILC2) in viral hepatitis,13 and ILC2-derived type 2 cytokines (for example, IL-13) are essential for the education of immunosuppressive leukocytes, including MDSCs and granulocytes.22,23 These immunosuppressive cells can inhibit T-cell proliferation and responses in several ways, such as the release of reactive oxygen species and upregulation of arginase-1 (Arg-1).17 Therefore, we speculated that IL-33 induces immunosuppressive cells through ILC2-derived type 2 cytokines and limits excessive T-cell responses in viral hepatitis.
In this study, we infected mice with the lymphocytic choriomeningitis virus (LCMV), which causes T-cell-mediated hepatitis.24,25,26 We demonstrated that neutrophils have a hepatoprotective role at the immune contraction stage. IL-33 treatment increased neutrophil recruitment in the liver and protected the liver by limiting excessive T-cell responses. We further revealed that IL-33 promoted Arg-1 expression in neutrophils through an ILC2/IL-13/signal transducer and activator of transcription factor 6 (STAT6) axis. This research could be beneficial in translational research and clinical therapeutics for liver diseases.
Materials and methods
Animals, infection and treatment
C57BL/6 (B6) mice from the Jackson Laboratory (Bar Harbor, ME, USA) were bred and maintained under specific pathogen-free conditions in the University of Texas Medical Branch (UTMB, Galveston, TX, USA) animal care facility and were used at 7–10 weeks of age. All procedures were approved by UTMB’s Institutional Animal Care and Use Committee and were performed according to NIH Guidelines. In all cases, mice were intravenously injected with 2 × 106 plaque-forming unit of LCMV strain Clone 13 (Cl 13) to induce acute hepatitis. Some infected mice were intraperitoneally injected with recombinant IL-33 (rIL-33, 1 μg/mouse; Biolegend, San Diego, CA, USA) or phosphate-buffered saline (PBS) daily starting on 1 dpi. For neutrophil depletion, infected mice were treated with the anti-Ly6G antibody (Ab; 300 μg/mouse) every other day.27 The isotype Ab was used as a control.
Hematoylin and eosin (H&E), histological scores and immunohistochemistry (IHC)
Liver specimens were fixed in 10% buffered formalin. Paraffin-embedded sections were stained with H&E for histological evaluation using a modified Knodell scoring system.28 For IHC staining, the sections were treated and incubated overnight with rat-anti-mouse Ly6G Ab, followed by staining with goat anti-rat IgG Ab (1:200, Vector Labs, Burlingame, CA, USA) for 30 min at room temperature. The sections were then stained with alkaline phosphatase-conjugated streptavidin (1:200, Vector Labs) and Vector Red alkaline phosphatase substrate and counterstained with hematoxylin (Sigma, St Louis, MO, USA).
Isolation of lymphocytes from tissues
Intrahepatic lymphocytes (IHLs) were isolated according to our previous method.29 Briefly, the liver was perfused and digested with 0.05% collagenase IV (Roche, Indianapolis, IN, USA) at 37 °C for 30 min. Cell suspensions were passed through a 70-μm nylon cell strainer to yield single-cell suspensions. IHLs were purified by centrifugation (400g) at room temperature for 30 min over a 30/70% discontinuous Percoll gradient (Sigma). For neutrophil isolation, bone marrow cells, splenocytes and IHLs were incubated with phycoerythrin (PE)-anti-Ly6G Ab, followed by incubation with anti-PE magnetic beads (Miltenyi Biotec, Auburn, CA, USA). Cell purification was performed by using positive selection in the LS column. The purity of neutrophils was confirmed by flow cytometry.
Cell culture
ILC2 were isolated and cultured as in our previous report.13 The supernatant from 5-day-cultured ILC2 was collected for in vitro experiments. Anti-IL-13 Ab (2 μg/ml) was used to block the IL-13 signals in vitro. For the ILC2 and neutrophil co-culture experiments, transwell inserts with a pore size of 0.4-μm were used. Briefly, neutrophils (1 × 106) were cultured in the lower chambers with ILC2 in the upper chambers for 16 h. After culture, the neutrophils were collected for transcript level analysis. The inhibitors were added to the cell culture 2 h prior to stimulation with cytokines and the ILC2 soup.
For the proliferation experiment, carboxyfluorescein succinimidyl ester-labeled splenic CD8+ T cells from naive mice (2 × 105) were incubated with neutrophils (2 × 105) in an anti-CD3 Ab (5 μg/ml)-coated plate with soluble anti-CD28 Ab (4 μg/ml). After 48 h, CD8+ T-cell proliferation was evaluated by flow cytometry.
Flow cytometry
For surface staining, cells were first incubated with FcγR blocker (CD16/32), followed by fluorochrome-labeled Abs of surface markers. For intracellular staining, GP33 and GP61 peptides were used in the presence of GolgiStop (BD Biosciences, San Jose, CA, USA) to stimulate virus-specific CD8+ and CD4+ T-cell responses, respectively. Phorbol myristate acetate (50 ng/ml) and ionomycin (750 ng/ml) from Sigma were also used in some experiments. After incubation, the cells were stained for surface markers first, then fixed by using an IC fixation buffer and finally stained for intracellular cytokines (eBioscience, San Diego, CA, USA). The phosflow experiments were performed according to the protocol from BD Biosciences. Briefly, the cells were stimulated with cytokines or the supernatants at the indicated times, followed by immediate fixation using a prewarmed Cytofix Fixation Buffer at 37 °C for 12 min. The cells were permeabilized using chilled Perm Buffer III for 1 h on ice and then were washed and stained with BD phosflow Abs. The samples were processed using an LSRII FACSFortessa system (Becton Dickinson, San Jose, CA, USA) and were analyzed by using the FlowJo software (TreeStar, Ashland, OR, USA).
Recombinant cytokines, antibodies and inhibitors
Carrier-free rIL-33 and rIL-13 were purchased from Biolegend, and rIL-7 for ILC2 culture was obtained from Peprotech (Rocky Hill, NJ, USA). The following Abs were obtained from eBioscience: PE-anti-Ly6G (1A8), fluorescein isothiocyanate (FITC)-, and allophycocyanin-anti-IFN-γ (XMG1.2), PerCP-Cy5.5-anti-CD11b (M1/70), PerCP-efluor 710-anti-TNF-α (MP6-XT22), FITC-anti-CD107a (eBio1D4B), PE-anti-Granzyme B (NGZB), PE-anti-F4/80 (BM8), allophycocyanin-anti-CD11c, and Fixable Viability Dye eFluor 506. The mouse lineage markers were FITC labeled, including CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD11b (M1/70), CD11c (N418), Gr-1 (RB6-8C5), Ter-119 (Ter-119), Fcϵ R1 (MAR-1), CD49b (DX5), NK1.1 (PK136) and B220 (RA3-6B2). The following Abs were purchased from Biolegend: PE-Cy7-anti-CD3 (17A2), APC-Cy7-anti-CD8 (53-6.7), Pacific Blue-anti-CD4 (GK1.5), and purified anti-CD16/32 (2.4G2). The anti-Ly6G Ab (1A8) and isotype control Ab (2A3) were from Bio X Cell (West Lebanon, NH, USA). The following phosflow Abs were purchased from BD Biosciences: Alexa Fluor 647 mouse anti-STAT3 (Py705), anti-STAT5 (pY694), and anti-STAT6 (pY641). The STAT5 inhibitor (Cat. no. 573108) was from Calbiochem (La Jolla, CA, USA). The STAT6 inhibitor AS 1517499 was from Axon Medchem (Reston, VA, USA). The Arg-1 inhibitor Nor-NOHA was from Cayman Chemical (Ann Arbor, MI, USA).
Quantitative reverse transcriptase-PCR (qRT-PCR)
Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Germantown, MD, USA) and was digested with DNase I (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was prepared from 1 μg of RNA by using an iScriptTM Reverse Transcription Kit (Bio-Rad, Hercules, CA, USA). qRT-PCR assays were performed using the iTaq SYBR Green Supermix and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The relative quantity of mRNA expression was calculated using the 2−ΔΔCT method. The primers are listed in Supplementary Table S1.
Bio-plex assay
Liver cytokine profiles were characterized using the ProcartaPlex Mouse Cytokine Panel (eBioscience). The samples were read using a Bio-Rad Bio-Plex 200 System. Raw data were measured as the relative fluorescence intensity and then converted to the concentration according to the standard curve.
Propagation and quantitation of virus
The LCMV stocks were prepared and titrated according to a previous report.30 Briefly, virus was incubated with baby hamster kidney cells for 72 h. The culture fluid was centrifuged for 10 min at 350g and 4 °C and were stored at −70 °C. For quantitation of the virus, Vero cells were cultured with a series of 10-fold virus dilutions for 90 min, followed by a 0.5% agarose overlay. After 4 days of culture, immunofluorescence was performed using a mouse anti-LCMV polyclonal Ab (gift from Dr Robert Tesh, University of Texas Medical Branch),29 and the positive clusters were counted, followed by the calculation of viral titers.
Statistical analyses
The data are shown as the means±s.e.m. and were analyzed by using two-tailed Student’s T-test for comparisons between two groups. One-way analysis of variance was used for the statistical analysis of more than two groups. For analysis of the histological scores, the nonparametric Kruskal–Wallis test was used. *, ** or *** represents P-value<0.05, <0.01 or <0.001, respectively. Statistical analyses were performed by the GraphPad Prism software 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
LCMV infection recruits neutrophils in the liver
To determine whether neutrophils are involved in LCMV-induced viral hepatitis, we first examined the dynamic patterns of neutrophils in the spleen and liver during infection. Compared with uninfected mice, LCMV-challenged mice displayed extensive hepatitis at 7 dpi, as evidenced by increased pathology, serum alanine transaminase (ALT) levels and pro-inflammatory cytokines in the liver (Supplementary Figure S1). The IFN-g+TNF-a+CD8+ T cells started to increase at 5 dpi, spiked at 9 dpi and tended to fade after 15 dpi in both the spleen and liver (Figures 1a and b). The numbers of granzyme B+ and CD107a+ CTLs reached their peaks in the spleen and liver at 9 and 15 dpi, respectively, and remarkably decreased at 21 dpi (Figures 1a and b). As reported previously,15 neutrophils were significantly increased in the spleen when the CLT response shrank at 15 dpi (Figure 1a). However, the liver neutrophils showed a similar pattern to liver CTLs after infection (Figure 1b).
Figure 1.
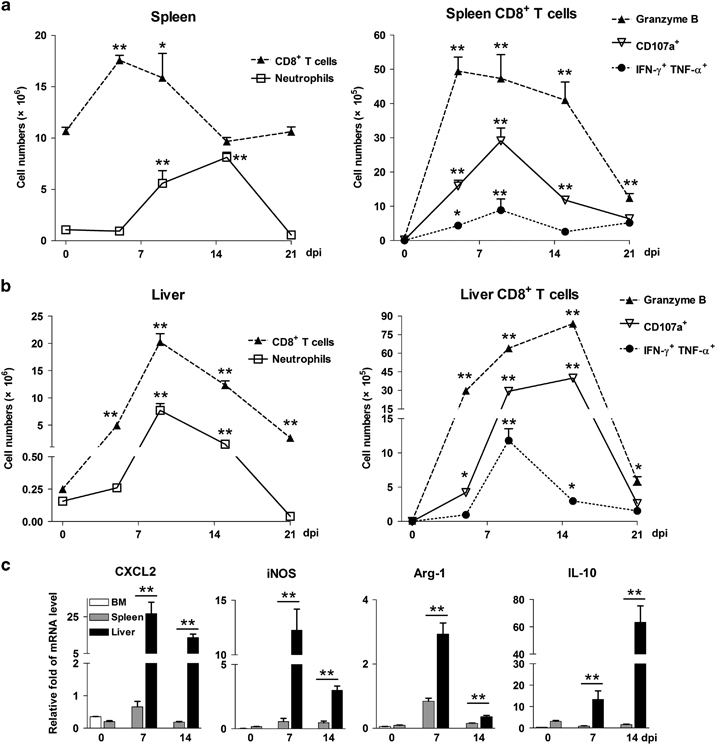
LCMV infection recruits neutrophils in the liver. Mice were i.v. injected with LCMV CL13 (2 × 106 pfu/each mouse) and were killed at 5, 9, 15 and 21 days postinfection (dpi). Uninfected mice were used as 0 dpi. Lymphocytes were isolated from the (a) spleens and (b) livers, followed by analysis using flow cytometry. (c) Neutrophils were purified from the bone marrow, spleens and livers at different time points. The gene transcript levels of CXCL2, iNOS, Arg-1, and IL-10 were measured using real-time PCR. The data are shown as the means±s.e.m. of at least three mice per group from a single representative experiment. The experiment was repeated independently three times. A two-tailed t-test was used to compare the two groups. *P<0.05, **P<0.01. CXCL2, C-X-C chemokine motif ligand 2; IL, interleukin; iNOS, inducible nitric oxide synthase; i.v., intravenous; LCMV, lymphocytic choriomeningitis virus; pfu, plaque-forming unit.
In humans, the CD11bhiCD62Ldim neutrophil subset is considered immunosuppressive because they inhibit T-cell proliferation ex vivo.23,31 In our virus-infected mouse model, we found higher numbers of CD62Ldim neutrophils than CD62Lhi neutrophils in the liver after infection (Supplementary Figure S2). Interestingly, the CD62Ldim subset was the predominant neutrophil subset at the contraction stage (15 dpi). To further characterize liver neutrophils during viral infection, we purified neutrophils from the infected livers. The purity of neutrophils is high with very little contamination of macrophages and dendritic cells (Supplementary Figure S3). We found that liver neutrophils expressed higher levels of C-X-C chemokine motif ligand 2 (CXCL2), inducible nitric oxide synthase (iNOS), Arg-1 and IL-10 at 7 and 14 dpi than spleen neutrophils (Figure 1c). Therefore, liver neutrophils displayed an immunosuppressive phenotype in LCMV-induced hepatitis.
Neutrophil depletion exacerbates liver injury at the contraction phase of T-cell responses
Evidence has been accumulating for the heterogeneity and plasticity of neutrophils, which exhibit both protective and pathological functions in viral infection.14 To assess the role of neutrophils in viral hepatitis, we depleted neutrophils in LCMV-infected mice with anti-Ly6G Ab (1A8), which is a neutralizing Ab specific for neutrophils but not for monocytes.27 The efficiency of depletion was confirmed by Ly6G or Gr-1 staining. No Ly6G+ cells can be detected after neutrophil depletion, while the percentages of CD11b+Gr-1hi cells, which are considered neutrophils, were only 1–2% (Supplementary Figure S4). We found that neutrophil depletion resulted in higher levels of serum ALT and aspartate aminotransferase (AST), increased lymphocyte infiltration and decreased the body weight at 14 but not at 7 dpi (Figures 2a–c). Elevated serum viremia was detected in neutrophil-depleted mice compared with that in control mice (Figure 2d). Although neutrophil depletion did not modify hepatic CD8+ T-cell responses at 7 dpi, it significantly increased the numbers of activated cytotoxic T cells (IFN-γ+, granzyme B+ and CD107a+) in the liver at 14 dpi (Figure 2e). Together, our results suggested that neutrophils have a hepatoprotective role in the liver at the immune contraction stage.
Figure 2.
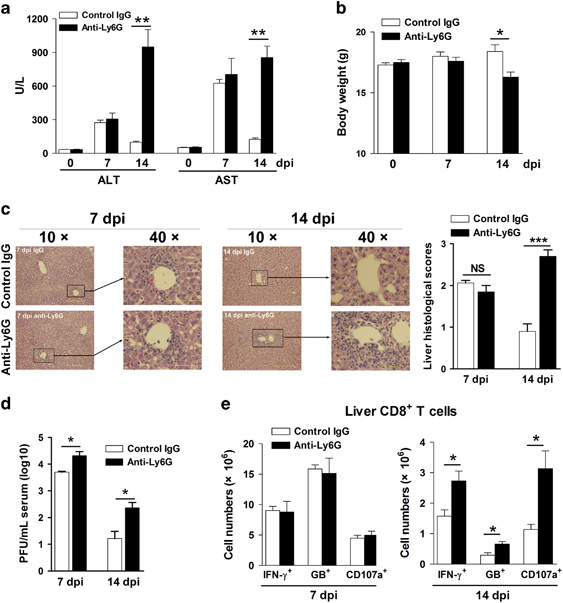
Hepatic neutrophils exhibit immunomodulatory effects and protect the liver against injury in LCMV infection. Mice were i.v. injected with LCMV CL13 (2 × 106 pfu/each mouse). Anti-Ly6G Ab (300 μg/mouse) was i.p. treated every other day after infection starting at 1 dpi. Isotype IgG was used as a control. Mice were killed at 7 and 14 dpi. (a) Serum ALT and AST. (b) Body weight. (c) Liver H&E staining and histological scores. (d) Serum viremia. (e) Numbers of IFN-γ+, granzyme B+ and CD107a+ CD8+ T cells in the liver at 7 and 14 dpi. The data are shown as the means±s.e.m. of at least three mice per group from a single representative experiment. The experiment was repeated twice independently. A two-tailed t-test was used to compare the two groups. *P<0.05, **P<0.01. Ab, antibody; ALT, alanine transaminase; AST, aspartate aminotransferase; dpi, days postinfection; H&E, hematoxylin and eosin; IFN, interferon; IgG, immunoglobulin G; i.p., intraperitoneal; i.v., intravenous; LCMV, lymphocytic choriomeningitis virus; NS, no significance; pfu, plaque-forming unit.
IL-33 recruits neutrophils and protects the liver against injury
The protective roles of the IL-33/ST2 axis have been documented in mouse hepatitis models.13,32 Consistent with our previous observation that IL-33 protects the liver in adenovirus infection,13 we found that IL-33 treatment reduced serum ALT and AST levels, as well as liver injury, in LCMV-infected mice at 6 dpi (Figures 3a and b). In addition, reduced T-cell infiltration in the liver was observed in IL-33-treated mice (Figure 3c). Although IL-33 treatment significantly increased the percentages of IFN-γ-, CD107a- and granzyme B-expressing CD8+ T cells in the liver (Figure 3d), lower levels of antivirus-associated cytokines and a higher viral load were observed in whole-liver tissue of the IL-33-treated group (Figure 3e and Supplementary Figure S5). This reduction in the levels of cytokines and chemokines in liver tissues was probably due to the decreased lymphocyte infiltration and alleviated liver damage by IL-33 treatment (Figures 3a–c).
Figure 3.
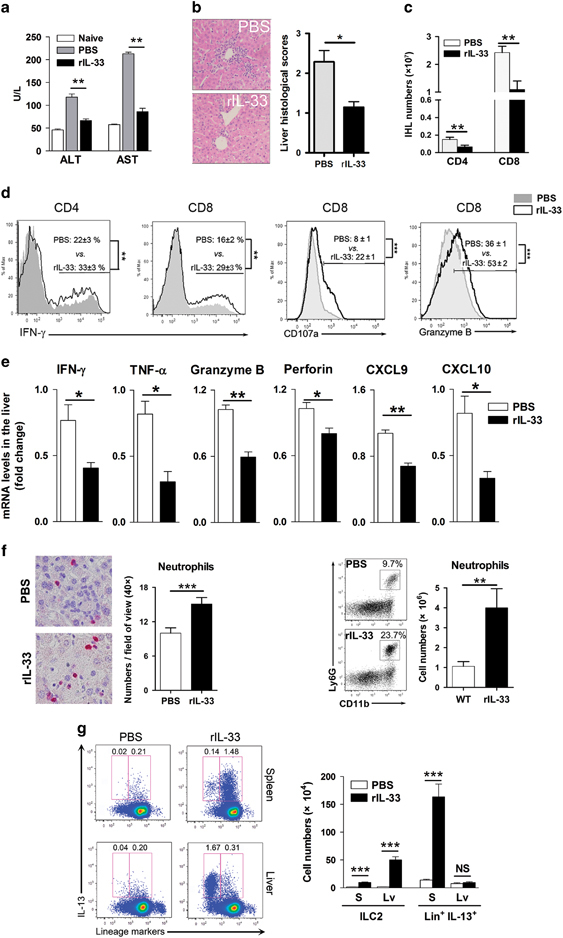
IL-33 attenuates liver injury by limiting T-cell accumulation and recruiting neutrophils in virus-infected mice. Mice were i.v. injected with LCMV CL13 (2 × 106 pfu/each mouse) and were treated daily with rIL-33 (1 μg/mouse). Mice were killed at 6 dpi. (a) Serum ALT and AST levels. (b) Liver H&E staining and histological evaluation. (c) Numbers of hepatic CD4+ and CD8+ T cells. (d) Liver CD4+ and CD8+ T cells were gated first, and the expression of IFN-γ, CD107a and granzyme B were analyzed. (e) Liver mRNA relative folds of IFN-γ, TNF-α, granzyme B, Perforin, CXCL9 and CXCL10. (f) Neutrophils in the liver were examined by IHC (Ly6G staining) and flow cytometry. (g) Cells were stimulated with PMA and ionomycin in the presence of GolgiStop for 4 h. The percentages and numbers of ILC2 and IL-13+ cells in the spleen and livers were measured. The data are shown as the means±s.e.m. of three to four mice per group from a single representative experiment. The experiment was repeated independently three times. Two-tailed t-test was used to compare the two groups. *P<0.05; **P<0.01; ***P<0.001. ALT, alanine transaminase; AST, aspartate aminotransferase; CXCL2, C-X-C chemokine motif ligand 2; dpi, days postinfection; H&E, hematoxylin and eosin; IFN, interferon; IHC, immunohistochemistry; IL, interleukin; ILC2, type 2 innate lymphoid cell; i.v., intravenous; LCMV, lymphocytic choriomeningitis virus; NS, no significance; pfu, plaque-forming unit; PMA, phorbol myristate acetate; rIL-33, recombinant IL-33; TNF, tumor necrosis factor.
In addition to its effect on T cells, IL-33 can behave as a strong chemokine for neutrophil recruitment in bacterial infection.20 Because we have observed the hepatoprotective role of neutrophils (Figure 2), we speculated that IL-33 may attenuate liver injury by promoting neutrophil recruitment in viral hepatitis. Here we provided evidence that neutrophil influx was significantly increased in the liver by IL-33 treatment, as judged by IHC staining and flow cytometry (Figure 3f). In addition, IL-33 treatment increased the percentage of neutrophils in the bone marrow (Supplementary Figure S6). Moreover, IL-33 treatment significantly increased the number of ILC2 in both the spleen and liver (Figure 3g). Interestingly, we found that the predominant type 2 immune cells in the spleen were the lineage marker-positive cells, probably T helper 2 (Th2) cells or alternative macrophages. However, IL-33 preferred to induce ILC2 rather than lineage+ IL-13+ cells in the liver (Figure 3g).
IL-33 induces immunosuppressive neutrophils through ILC2-derived IL-13
To investigate how IL-33 regulates neutrophil activity, we compared the mRNA transcript levels of intrahepatic neutrophils between PBS- and IL-33-treated mice at 6 dpi. We found elevated Arg-1 expression by IL-33 treatment, but other molecules (IFN-γ, TNF-α, IL-10, IL-17, CXCL1, CXCL2 and iNOS) were not changed (Figure 4a). To ascertain how IL-33 induces Arg-1 expression in neutrophils, we stimulated neutrophils with IL-33 in vitro for 6 and 16 h. Unexpectedly, IL-33 failed to increase the Arg-1 transcript levels (Figure 4b). Because IL-33 can potently induce ILC2 in the liver (Figure 3g),13 we speculated that IL-33 promoted Arg-1 expression in the liver through ILC2-derived IL-13. To test this hypothesis, we stimulated neutrophils with IL-13 and found that IL-13 could potently induce Arg-1 expression in neutrophils. Moreover, IL-13 synergized with IL-33 to further amplify its effect (Figure 4b). To verify this finding, we established a transwell system for ILC2 and neutrophil co-culture and found that ILC2 increased Arg-1 expression of neutrophils in a dose-dependent manner (Figure 4c). We also incubated neutrophils with ILC2 culture supernatant and detected markedly increased the Arg-1 expression in neutrophils. However, the effect of the ILC2 supernatants was significantly inhibited by the depletion of IL-13 (Figure 4d). Finally, incubation of CD8+ T cells with neutrophils resulted in decreased T-cell proliferation in vitro. Interestingly, IL-13 significantly inhibited T-cell proliferation in the CD8 T-cell/neutrophil co-culture system, while the arginase inhibitor could partially restore this inhibition (Figure 4e). Taken together, these data confirmed that IL-33 induced immunosuppressive neutrophils via ILC2-derived IL-13, resulting in restrained CD8+ T-cell responses.
Figure 4.
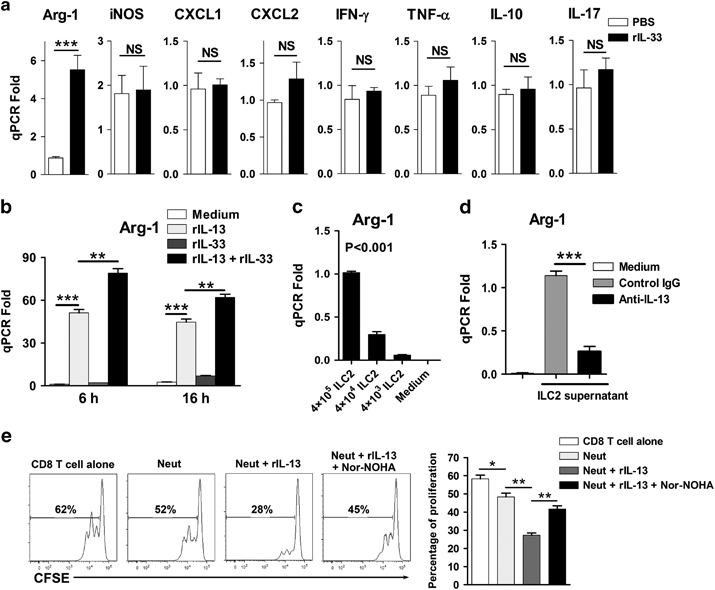
IL-33 induces arginase-1 (Arg-1) expression of neutrophils through the ILC2/IL-13 axis. Mice were i.v. injected with LCMV CL13 (2 × 106 pfu/each mouse). Recombinant IL-33 (1 μg/mouse) was i.p. treated daily starting from 1 dpi. PBS was used as a control. The mice were killed at 6 dpi. (a) Neutrophils were purified from the livers of infected mice with PBS or rIL-33 treatment, and then the transcript levels were analyzed. (b) Neutrophils were cultured in the presence of rIL-13 and rIL-33 for 6 and 16 h. Gene expression of Arg-1 in neutrophils was detected by qPCR. (c) Neutrophils were co-cultured with ILC2 for 16 h, and Arg-1 expression of neutrophils was examined. (d) Neutrophils were cultured with ILC2 supernatant in the presence of anti-IL-13 Ab or control IgG for 16 h, and the gene expression of Arg-1 was analyzed. (e) Bone marrow-derived neutrophils (2 × 105) were incubated with CFSE-labeled splenic CD8+ T cells (2 × 105) from naive mice in an anti-CD3 Ab (5 μg/ml)-coated plate with soluble anti-CD28 Ab (4 μg/ml). Recombinant IL-13 (40 ng/ml) and arginase inhibitor Nor-NOHA (50 μM) were added at the beginning of the culture. After 48 h, CD8+ T-cell proliferation was measured by flow cytometry. The data in panel (a) are shown as the means±s.e.m. of at least three mice per group from a single representative experiment. The experiment was repeated independently three times. The in vitro experiment in panels (b–e) was repeated at least four times independently. Two-tailed t-test was used to compare the two groups. One-way ANOVA was used to compare more than two groups. *P<0.05, **P<0.01, ***P<0.001. Ab, antibody; ANOVA, analysis of variance; CFSE, carboxyfluorescein succinimidyl ester; dpi, days postinfection; IgG, immunoglobulin G; IL, interleukin; ILC2, type 2 innate lymphoid cell; i.p., intraperitoneal; i.v., intravenous; LCMV, lymphocytic choriomeningitis virus; NS, no significance; PBS, phosphate-buffered saline; pfu, plaque-forming unit; qPCR, quantitative PCR; rIL-13, recombinant IL-13.
ILC2-derived IL-13 induces Arg-1 expression in neutrophils through STAT6
Th2 cytokines can activate cells through several STAT signaling pathways, including STAT3, STAT5 and STAT6.33,34,35 To determine the pathway involved in ILC2/IL-13-induced Arg-1 expression, we analyzed the level of STAT phosphorylation in neutrophils by stimulation with IL-13 or the ILC2 supernatant. Both significantly increased the phosphorylated STAT6 (p-STAT6) levels, and the effect of the ILC2 culture supernatant was completely abolished in the presence of anti-IL-13-neutralizing Ab (Figures 5a and b). IL-13 also induced p-STAT5 levels, but not p-STAT3 levels, in neutrophils (Supplementary Figure S7A). To assess the role of STAT5 and STAT6 in regulating the Arg-1 expression, we applied STAT5 and STAT6 inhibitors in cell culture. These inhibitors potently suppressed the phosphorylation of the target molecules in the presence of IL-13 or ILC2 supernatant (Figure 5c and Supplementary Figure S7B). We further found that the inhibition of STAT6, but not STAT5, significantly decreased the Arg-1 transcript levels (Figure 5d and Supplementary Figure S7C). Collectively, ILC2-derived IL-13 promotes Arg-1 expression in neutrophils through the STAT6 signaling pathway.
Figure 5.
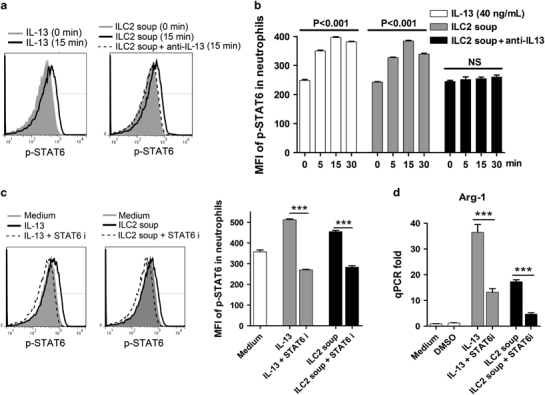
IL-13/ILC2 induces Arg-1 expression of neutrophils through STAT6. (a) Neutrophils were stimulated with rIL-13 (40 ng/ml) or ILC2 supernatant for the indicated times. Anti-IL-13 neutralization Ab (2 μg/ml) was also used. The levels of p-STAT6 in neutrophils were analyzed by phosflow cytometry. (b) The mean fluorescence intensities (MFIs) are shown. (c) STAT6 inhibitor (100 nM) was used in the cell culture and the p-STAT6 was analyzed. (d) Neutrophils were cultured for 8 h with or without stimulation. The transcript levels of Arg-1 in neutrophils were measured by qPCR. The data are shown as the means±s.e.m., and the experiment was repeated independently three times. A two-tailed t-test was used to compare the two groups. One-way ANOVA was used to compare more than two groups. ***P<0.001. Ab, antibody; ANOVA, analysis of variance; IL, interleukin; ILC2, type 2 innate lymphoid cell; NS, no significance; qPCR, quantitative PCR; rIL-13, recombinant IL-13; STAT6, signal transducer and activator of transcription factor 6.
Discussion
Neutrophils are polymorphonuclear leukocytes and were previously described as pro-inflammatory cells during infections. As the first responders to infections, neutrophils are recruited rapidly to the infection site and eliminate pathogens in several ways, including phagocytosis, degranulation and neutrophil extracellular traps (NETs).17,36 In recent years, neutrophils have been considered an important immune regulator for both innate and adaptive immune responses rather than simple pro-inflammatory cells.37 In persistent LCMV infection, neutrophils expand and remain sustained even after 7 dpi, and they have an immunosuppressive role in antiviral T-cell function in the spleen.15 However, the role of neutrophils in viral hepatitis is not entirely clear. In this study, we induced T cell-mediated hepatitis in mice by LCMV infection and observed vigorous recruitment of neutrophils into the liver. The concurrent pattern of neutrophils and CD8+ T cells suggested that neutrophils may have a role in shaping adaptive immune responses in viral hepatitis (Figure 1). The recruitment and function of neutrophils is orchestrated differently within specific organs, depending on tissue-resident cells and the immune environment.38,39,40 Notably, we observed that liver neutrophils were likely to exhibit an immunosuppressive phenotype as characterized by surface CD62dim expression, especially at the immune contraction stage in viral infection (Supplementary Figure S2). Further evidence showed that neutrophils in the liver expressed higher levels of CXCL2, iNOS, Arg-1 and IL-10 than those in the spleen. These findings indicated that liver neutrophils may have an immunosuppressive role by the secretion of immunomodulatory cytokines.
Neutrophils have been reported to inhibit CD8+ T-cell proliferation and cytokine production in persistent infections.15 Here we found that the depletion of neutrophils promoted hepatic CTL responses and exacerbated T-cell-mediated liver injury at 14 dpi, suggesting that neutrophils have an immunosuppressive role in the liver during the immune contraction phase of viral infection (Figure 2). In fact, neutrophil depletion did not influence liver injury and CTL responses at 7 dpi. At the acute stage, nearly half of the neutrophils displayed the CD62Lhi-activated phenotype and may contribute to tissue damage and viral clearance.41 However, at the contraction stage, almost all of the neutrophils showed a CD62dim-immunosuppressive feature (Supplementary Figure S2). Therefore, the distinct outcomes of neutrophil depletion at the acute (7 dpi) and contraction (14 dpi) phases might be due to the unique composition of neutrophil subpopulations at different infection stages. Of note, the timing of neutrophil depletion is critical for the disease outcome.42 Depletion prior to infection may lead to reduced viremia in the host because neutrophils can facilitate viral spread by shaping the local pro-inflammatory responses and attracting unique pathogen-carrier cells to the infection site.43 However, neutrophil depletion after systemic infection may cause increased viral load in the host because viral infection induces the release of NETs, which protect host cells from viral infection.41 Additionally, our data suggested the indispensable role of neutrophils for viral clearance because the depletion of neutrophils caused an increased viral load in the liver during infection (Figure 2d).
IL-33 is a new member of the IL-1 family with a growing number of target cells and diverse functions.7,44 IL-33 deficiency resulted in decreased antiviral immunity in both acute and persistent viral infection.12,45 We found that, although IL-33 treatment enhanced the cytotoxic activity of CTLs, it reduced the number of T cells and attenuated liver injury (Figure 3). This result indicated that exogenous IL-33 negatively regulated CD8+ T-cell proliferation in the liver indirectly. Consistent with a previous report on bacterial and fungal infection,20,46,47 IL-33 triggered neutrophil recruitment in the liver in our model (Figure 3f). In addition, the percentage of neutrophils in the bone marrow was increased by IL-33 treatment (Supplementary Figure S6). This finding suggests that IL-33 may also regulate neutrophil generation in the bone marrow. L-arginine is important for metabolic fitness and the survival capacity of T cells.48,49 Granulocytes are the main producers of arginase, which can inhibit T-cell functions by reducing the bioavailability of L-arginine.50,51,52 In leishmaniasis, arginase depletes L-arginine and suppresses local T-cell responses.53 In HBV transgenic mice, myeloid-derived suppressor cells inhibit HBV-specific CD8+ T-cell responses through arginase.54 We found that IL-33 promoted these liver-infiltrated neutrophils to uniquely express higher Arg-1 (Figure 4a). Unexpectedly, although IL-33 can induce neutrophil recruitment, it was not capable of directly inducing Arg-1 in neutrophils in vitro (Figure 4b). Recently, type 3 innate lymphoid cells were reported to regulate neutrophil responses through the production of cytokines, including granulocyte macrophages colony-stimulating factor.55 Given that IL-33 induces ILC2 in the liver during viral infection,13 we speculated that IL-33 may regulate neutrophil function through ILC2 induction. Interestingly, unlike in the spleen, IL-33 treatment predominantly amplified ILC2 but not the IL-13+ lineage+ cells in the liver (Figure 3g). Therefore, the IL-33-induced type 2 immune response in the liver was attributed to ILC2, but other cells (for example, Th2) in lymphoid organs could contribute to the systemic type 2 immune responses. We further found that ILC2 upregulated Arg-1 expression in neutrophils in a dose-dependent manner, and this process was attributed to ILC2-derived IL-13 (Figures 4b–d). Moreover, IL-13-treated neutrophils suppressed CD8+ T-cell proliferation in vitro through Arg-1 (Figure 4e). We suspected that type 2 immune responses may limit the excessive T-cell activity in viral infection by modulating the function of myeloid cells.56 However, long-lasting type 2 cytokine production is detrimental and may lead to liver fibrosis in chronic infection.57 We previously reported that adoptive transfer of ILC2 did not exhibit a hepatoprotective effect in LCMV infection similar to that of IL-33.58 It is possible that, unlike IL-33, ILC2 may not be able to efficiently recruit neutrophils into the liver. Additionally, viral infection mounts strong type 1 immune responses that can negatively regulate ILC2 function in vivo.59 Therefore, IL-33 can not only recruit neutrophils but can also educate them to obtain immunosuppressive properties through ILC2. Collectively, our results demonstrated that IL-33 inhibited liver CD8+ T-cell responses by ILC-2-educated neutrophils.
IL-13 is an activator of the STAT pathway60 and can induce arginase in myeloid cells.52 We found that IL-13 induced the phosphorylation of both STAT5 and STAT6, but not STAT3, in neutrophils. Moreover, the IL-13-induced Arg-1 expression was dependent on STAT6 but not on STAT5 (Figure 5 and Supplementary Figure S7). Similarly, the ILC2 supernatant increased Arg-1 expression in neutrophils similar to rIL-13, and this induction was abolished by the neutralization of IL-13 (Figure 5). Therefore, IL33 not only recruits neutrophils in the liver but also molds them into immunosuppressive phenotypes through the ILC2/IL-13/STAT6 axis.51 It is likely that IL-33 might also regulate neutrophil function in other ways, such as the IL-33/ILC2/IL-5 axis.61 Additionally, PD-L1 expression in ILC2 and neutrophils may contribute to IL-33-mediated liver protection.62,63 In addition to neutrophils, IL-33 may limit excessive T-cell responses by facilitating other types of immune cells, such as Treg cells and G-MDSCs.10,64 These interesting questions are not involved in this study and may need to be addressed in the future.
In summary, our study provides new insights into the role of neutrophils in viral infection-induced hepatitis. We also demonstrated that IL-33 induces immunosuppressive neutrophils via ILC2 and protects the liver against T cell-mediated injury. A better understanding of this immune regulation will be beneficial for translational research and clinical therapeutics in infectious diseases.
Electronic supplementary material
Acknowledgements
We thank Ms Mardelle Susman and Dr Linsey Yeager for assistance with manuscript preparation. We express our gratitude to other members of the UTMB Joint Immunology Working Group (Dr Stephens, Dr Rajsbaum and Dr Hu, as well as their trainees) for many helpful discussions. This work was supported, in part, by grants from the NIH (AI109100 and AI126371 to JS). PY was a visiting scientist partially supported by the Department of Infectious Diseases, Xiangya Hospital, China and the Natural Science Foundation of Hunan Province (no. 14JJ6003). DMKY and ZK were recipients of summer internships from an NIAID T35 training grant (AI078878, PI: LS).
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Yuejin Liang, Email: yu2liang@utmb.edu.
Jiaren Sun, Email: jisun@utmb.edu.
Electronic supplementary material
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2017.147
References
- 1.Farci P, Alter HJ, Shimoda A, Govindarajan S, Cheung LC, Melpolder JC, et al. Hepatitis C virus-associated fulminant hepatic failure. N Engl J Med. 1996;335:631–634. doi: 10.1056/NEJM199608293350904. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712–721. doi: 10.1002/hep.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 4.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 5.Gremion C, Grabscheid B, Wolk B, Moradpour D, Reichen J, Pichler W, et al. Cytotoxic T lymphocytes derived from patients with chronic hepatitis C virus infection kill bystander cells via Fas-FasL interaction. J Virol. 2004;78:2152–2157. doi: 10.1128/JVI.78.4.2152-2157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajardo T, Morales RA, Campos-Mora M, Campos-Acuna J, Pino-Lagos K. Exogenous interleukin-33 targets myeloid-derived suppressor cells and generates periphery-induced Foxp3(+) regulatory T cells in skin-transplanted mice. Immunology. 2015;146:81–88. doi: 10.1111/imm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CC, Kobayashi T, Iijima K, Hsu FC, Kita H. IL-33 dysregulates regulatory T cells and impairs established immunologic tolerance in the lungs. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed]
- 12.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8 T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, et al. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol. 2013;190:5666–5675. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galani IE, Andreakos E. Neutrophils in viral infections: current concepts and caveats. J Leukoc Biol. 2015;98:557–564. doi: 10.1189/jlb.4VMR1114-555R. [DOI] [PubMed] [Google Scholar]
- 15.Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity. 2013;38:309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez-Uria G, Day JN, Nasir AJ, Russell SK, Vilar FJ. Reduction in neutrophil count during hepatitis C treatment: drug toxicity or predictor of good response? Dig Dis Sci. 2010;55:2058–2062. doi: 10.1007/s10620-009-0969-z. [DOI] [PubMed] [Google Scholar]
- 17.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 18.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr., Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 21.Verri WA, Jr., Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69:1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- 22.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohler J, Gossmann J, Kratzberg T, Lehmann-Grube F. Murine hepatitis caused by lymphocytic choriomeningitis virus. I. The hepatic lesions. Lab Invest. 1994;70:263–278. [PubMed] [Google Scholar]
- 25.Zinkernagel RM, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Garde MD, Movita D, van der Heide M, Herschke F, De Jonghe S, Gama L, et al. Liver monocytes and kupffer cells remain transcriptionally distinct during chronic viral infection. PLoS One. 2016;11:e0166094. doi: 10.1371/journal.pone.0166094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 28.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 29.Jie Z, Liang Y, Hou L, Dong C, Iwakura Y, Soong L, et al. Intrahepatic innate lymphoid cells secrete IL-17A and IL-17F that are crucial for T cell priming in viral infection. J Immunol. 2014;192:3289–3300. doi: 10.4049/jimmunol.1303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh RM, Seedhom MO. Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. Curr Protoc Microbiol. 2008;Chapter 15:Unit 15A 11. doi: 10.1002/9780471729259.mc15a01s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamp VM, Pillay J, Lammers JW, Pickkers P, Ulfman LH, Koenderman L. Human suppressive neutrophils CD16bright/CD62Ldim exhibit decreased adhesion. J Leukoc Biol. 2012;92:1011–1020. doi: 10.1189/jlb.0612273. [DOI] [PubMed] [Google Scholar]
- 32.Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, et al. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol. 2012;56:26–33. doi: 10.1016/j.jhep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Lischke A, Moriggl R, Brandlein S, Berchtold S, Kammer W, Sebald W, et al. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J Biol Chem. 1998;273:31222–31229. doi: 10.1074/jbc.273.47.31222. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei LH, Jacobs AT, Morris SM, Jr., Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C248–C256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- 36.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 38.Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015;36:547–555. doi: 10.1016/j.it.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. 2013;5:348–357. doi: 10.1159/000345943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Zhu L, Chu Z, Yang T, Sun HX, Yang F, et al. Characterization and biological significance of IL-23-induced neutrophil polarization. Cell Mol Immunol. 2017;14:1–13. doi: 10.1038/cmi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, et al. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pingen M, Bryden SR, Pondeville E, Schnettler E, Kohl A, Merits A, et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity. 2016;44:1455–1469. doi: 10.1016/j.immuni.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 45.Carriere V, Arshad MI, Le Seyec J, Lefevre B, Farooq M, Jan A, et al. Endogenous IL-33 deficiency exacerbates liver injury and increases hepatic influx of neutrophils in acute murine viral hepatitis. Mediators Inflamm. 2017;2017:1359064. doi: 10.1155/2017/1359064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lan F, Yuan B, Liu T, Luo X, Huang P, Liu Y, et al. Interleukin-33 facilitates neutrophil recruitment and bacterial clearance in S. aureus-caused peritonitis. Mol Immunol. 2016;72:74–80. doi: 10.1016/j.molimm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J Immunol. 2012;189:287–295. doi: 10.4049/jimmunol.1103564. [DOI] [PubMed] [Google Scholar]
- 48.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842 e813. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 50.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 51.Nascimento DC, Melo PH, Pineros AR, Ferreira RG, Colon DF, Donate PB, et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Modolell M, Choi BS, Ryan RO, Hancock M, Titus RG, Abebe T, et al. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis. 2009;3:e480. doi: 10.1371/journal.pntd.0000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong X, Sun R, Chen Y, Wei H, Tian Z. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol. 2014;193:1645–1653. doi: 10.4049/jimmunol.1303432. [DOI] [PubMed] [Google Scholar]
- 55.Croxatto D, Micheletti A, Montaldo E, Orecchia P, Loiacono F, Canegallo F, et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol. 2016;9:1372–1383. doi: 10.1038/mi.2016.10. [DOI] [PubMed] [Google Scholar]
- 56.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2012;116:3311–3320. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 57.Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 2017. [DOI] [PubMed]
- 58.Liang Y, Jie Z, Hou L, Yi P, Wang W, Kwota Z, et al. IL-33 promotes innate IFN-gamma production and modulates dendritic cell response in LCMV-induced hepatitis in mice. Eur J Immunol. 2015;45:3052–3063. doi: 10.1002/eji.201545696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, et al. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000;105:1063–1070. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- 61.Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translateon study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. doi: 10.1371/journal.pmed.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz C, Khan AR, Floudas A, Saunders SP, Hams E, Rodewald HR, et al. ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. J Exp Med. 2017;214:2507–2521. doi: 10.1084/jem.20170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog. 2014;10:e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


