Abstract
PES1, a BRCT domain-containing protein, has been shown to play a role in modulating the balance and ratio between ERα and ERβ protein, which is involved in the occurrence and development of breast and ovarian cancer. However, its role in connection with the balance and ratio between ERα and ERβ protein in papillary thyroid cancer (PTC) remains unclear. Here, we found that ERα and ERβ were co-expressed in human PTC tissues and cells. ERα promoted and ERβ inhibited the proliferation, invasion and migration of PTC cells. PES1 modulated the balance between ERα and ERβ by elevating the ERα protein level and simultaneously reducing the ERβ protein level, then upregulating the ERα/ERβ protein ratio and promoting the proliferation, invasion and migration of PTC cells. In PTC tissues, PES1 protein level was positively correlated with the ERα protein level and negatively correlated with the ERβ protein level. The PES1 and ERα protein levels were gradually increased and the ERβ protein level was decreased by degree in the occurrence and development of PTC. Increased PES1 and ERα protein levels and decreased ERβ protein level were correlated with the aggressive behaviors of PTC patients such as large tumor size, extrathyroidal extension (ETE), lymph node metastasis (LNM), high BRAFV600E expression and high TNM stage. It is suggested that PES1 promotes the occurrence and development of PTC by elevating the ERα protein level and reducing the ERβ protein level, and then upregulating the ERα/ERβ protein ratio.
Introduction
Papillary thyroid cancer (PTC) is three times more frequent in women than in men, with the greatest gender difference observed during reproductive years and the decreased incidence after menopause1,2. The elevated risk was also reported in women who used estrogen for gynecological problems and in women who used postmenopausal hormone replacement therapy or oral contraception3–5. It is suggested that estrogen may be involved in the occurrence and development of PTC, as has been shown in breast, endometrial and ovarian cancer6.
Estrogen exerts its physiological and pathophysiological actions largely through two estrogen receptors, ERα and ERβ, which belong to the steroid hormone receptor family7,8. ERα and ERβ are architecturally similar with three functional domains: N-terminal domain (NTD), DNA binding domain (DBD) and ligand binding domain (LBD). The two ERs share 97% similarity in their DBD and 59% in LBD, whereas the NTD is merely 16% similar9. The differences in their structures suggest that ERα and ERβ may have different functions. It is well known that ERα expression is associated with aberrant proliferation and the development of malignancy, in contrast, ERβ has been shown to inhibit cell proliferation, migration and invasion10,11. Although there is a controversy regarding the prognostic and predictive roles of ERβ expression, most of the studies that have analyzed a large number of samples have demonstrated a correlation of ERβ expression with a better clinical outcome in estrogen related cancer12,13. Lots of studies have shown that ERα promotes cell proliferation, invasion and migration and has been shown to have tumor-promoting effects, whereas ERβ may play an inhibitory role against the ERα-mediated tumor-promoting effects, especially when co-expressed with ERα14–16. The ERα/ERβ protein ratio would be critical in defining the overall response. Therefore, the imbalance between ERα and ERβ protein levels and the elevated ERα/ERβ protein ratio may be implicated in the occurrence and development of tumor in estrogen responsive organ17,18. Previous studies have shown that like the typical estrogen responsive organ such as breast, uterus and ovary, both ERα and ERβ are co-expressed in the normal and tumor tissues of the thyroid19,20. Moreover, like in breast, endometrial and ovarian cancer, ERα protein is increased, ERβ protein is decreased and finally the ERα/ERβ protein ratio is upregulated, which is involved in the occurrence and development of PTC21–24. However, how the protein levels of ERα and ERβ are modulated and how the ERα/ERβ protein ratio is upregulated in PTC remain unclear.
PES1, a breast cancer–associated gene 1 (BRCA1) C-terminal (BRCT) domain-containing protein, has been shown to play important roles in normal embryonic development, ribosome biogenesis, DNA replication, chromosomal stability and cell cycle progression25–28. Previous studies have demonstrated that PES1 is widely expressed in developing tissues, but is not observed in any adult tissues except for the ovary26,27. However, the subsequent studies have revealed that PES1 is over-expressed in some cancers such as stomach cancer29, prostatic cancer30,31, breast cancer32,33, head and neck squamous cell cancer34, colon cancer35, malignant astrocytomas and glioblastomas36,37 and ovarian cancer38. High PES1 expression is associated with the worse overall and relapse-free survival of patients with malignant tumor. The increased expression of PES1 transforms both mouse and human fibroblasts39, while the repression of PES1 inhibits the proliferation and tumorigenicity of breast cancer cells32,33. These data suggest that PES1 promotes the proliferation and malignant transformation of cells and may contribute to the occurrence and development of tumor.
Recently, Cheng et al.33 and Li et al.38 reported a novel function of PES1 that modulates the balance between ERα and ERβ protein levels through the ubiquitin-proteasome pathway, which contributes to the growth of breast and ovarian cancer cells. However, its role in connection with the balance between ERα and ERβ protein levels and the ERα/ERβ protein ratio in PTC has not been studied yet. Here, we examined PES1 protein level in human PTC tissues and cells and assessed the correlations of PES1 protein level with ERα and ERβ protein levels, with various clinicopathological features of PTC patients and with the ERα/ERβ protein ratio and the proliferation, invasion and migration of PTC cells.
Results
PES1 and ERα protein levels are significantly upregulated and ERβ protein level is significantly downregulated in PTC tissues
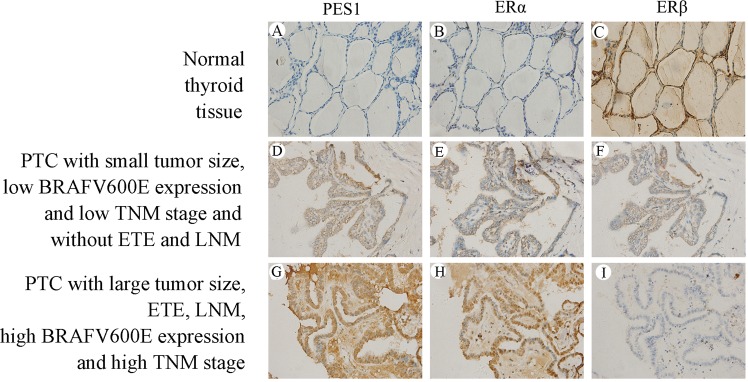
PES1, ERα and ERβ protein levels in PTC and normal thyroid tissues were examined using immunohistochemical (IHC) staining and the examples of IHC staining for the three molecules were shown in Fig. 1A–C is an example of normal thyroid tissues showing almost no follicular cells with staining for PES1 (A) and a few of follicular cells with weak staining for ERα (B), however, a lot of follicular cells with strong staining for ERβ (C). D–F is an example of PTC tissues with small tumor size, low BRAFV600E expression and TNM stage I and without ETE and LNM, showing quite a few of tumor cells with moderate staining for PES1 (D), ERα (E) and ERβ (F). G–I is an example of PTC tissues with large tumor size, ETE, LNM, high BRAFV600E expression and TNM stage IV, showing a lot of tumor cells with strong staining for PES1 (G) and ERα (H), however, a few of tumor cells with weak staining for ERβ (I). It was indicated that PES1 and ERα protein levels are gradually increased and ERβ protein level is decreased by degree in the occurrence and development of PTC. As shown in Table 1, the majority of normal thyroid tissues were negative or had an IHC score 1 for PES1 and were negative or had an IHC score 1, 2, 3 or 4 for ERα, whereas none of cases showed high expression (IHC score ≥5) of PES1 and only 19 cases displayed high expression of ERα. On the other hand, however, only 6 normal thyroid tissues were negative for ERβ and the majority of cases had an IHC score 1–8, whereas 112 cases exhibited high expression of ERβ. High expression rates in normal thyroid tissues were 0%, 9.5% and 56% for PES1, ERα and ERβ, respectively. By contraries, the majority of the PTC tissues were positive and had an IHC score 1–8 for PES1 and ERα, whereas 104 and 103 of the PTC cases exhibited high expression (IHC score ≥5) of PES1and ERα, respectively. On the other hand, however, a significant number of PTC cases were negative for ERβ and only 30 PTC cases showed high expression of ERβ. High expression rates in PTC tissues were 52%, 51.5% and 15% for PES1, ERα and ERβ, respectively. It was indicated that PES1 and ERα protein levels are significantly upregulated and ERβ protein level is significantly downregulated in PTC tissues when compared with those in normal thyroid tissues (P < 0.001) (Table 2).
Figure 1.
IHC staining of PES1, ERα and ERβ. The first row (A–C) is the IHC staining of an example of normal thyroid tissues, showing almost no follicular cells with staining for PES1 (A), a few of follicular cells with weak staining for ERα (B) and a lot of follicular cells with strong staining for ERβ (C). The second row (D–F) is the IHC staining of an example of PTC tissues with small tumor size, low BRAFV600E expression and TNM stage I and without ETE and LNM, showing quite a few of tumor cells with moderate staining for PES1 (D), ERα (E) and ERβ (F). The third row (G–I) is the IHC staining of an example of PTC tissues with large tumor size, ETE, LNM, high BRAFV600E expression and TNM stage IV, showing a lot of tumor cells with strong staining for PES1 (G) and ERα (H), however, a few of tumor cells with weak staining for ERβ (I).
Table 1.
The IHC scores of PES1, ERα and ERβ in 200 PTC and 200 normal thyroid tissues according to the scoring system.
| Score | PES1 | ERα | ERβ | |||
|---|---|---|---|---|---|---|
| Normal thyroid tissue (n) | PTC (n) | Normal thyroid tissue (n) | PTC (n) | Normal thyroid tissue (n) | PTC (n) | |
| 0 | ||||||
| Negative | 176 | 11 | 69 | 9 | 6 | 71 |
| + | ||||||
| 1 | 23 | 18 | 39 | 14 | 12 | 37 |
| 2 | 1 | 21 | 33 | 20 | 19 | 26 |
| 3 | 0 | 23 | 21 | 26 | 24 | 19 |
| 4 | 0 | 23 | 19 | 28 | 27 | 17 |
| ++ | ||||||
| 6 | 0 | 25 | 12 | 30 | 30 | 18 |
| 8 | 0 | 27 | 6 | 39 | 41 | 12 |
| +++ | ||||||
| 9 | 0 | 35 | 1 | 22 | 27 | 0 |
| 12 | 0 | 17 | 0 | 12 | 14 | 0 |
The IHC scores in PTC and normal thyroid tissues were determined as the multiplication of proportion score and intensity score.
Table 2.
Correlations of PES1, ERα and ERβ protein levels with clinicopathological characteristics in 200 PTC patients.
| Characteristics | Case (n) | PES1 | ERα | ERβ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P value | Low | High | P value | Low | High | P value | ||
| Tissue type | ||||||||||
| Normal thyroid tissue | 200 | 200 | 0 | 181 | 19 | 88 | 112 | |||
| PTC | 200 | 96 | 104 | <0.001 | 97 | 103 | <0.001 | 170 | 30 | <0.001 |
| Classic PTC | 142 | 69 | 73 | 0.165 | 70 | 72 | 0.286 | 124 | 18 | 0.222 |
| Follicular Variant of PTC | 21 | 12 | 9 | 13 | 8 | 18 | 3 | |||
| Tall Cell Variant of PTC | 24 | 7 | 17 | 8 | 16 | 17 | 7 | |||
| Oncocytic Variant of PTC | 13 | 8 | 5 | 6 | 7 | 11 | 2 | |||
| Age (years) | ||||||||||
| <45 | 24 | 14 | 10 | 0.280 | 15 | 9 | 0.143 | 18 | 6 | 0.144 |
| ≥45 | 176 | 82 | 94 | 82 | 94 | 152 | 24 | |||
| Gender | ||||||||||
| Male | 46 | 26 | 20 | 0.187 | 27 | 19 | 0.115 | 36 | 10 | 0.145 |
| Female | 154 | 70 | 84 | 70 | 84 | 134 | 20 | |||
| Tumor size (cm) | ||||||||||
| T1 ≤ 2 | 62 | 39 | 23 | 0.005 | 41 | 21 | 0.003 | 46 | 16 | 0.007 |
| 2 < T2 ≤ 4 | 71 | 34 | 37 | 31 | 40 | 61 | 10 | |||
| T3 > 4 | 67 | 23 | 44 | 25 | 42 | 63 | 4 | |||
| Extrothyroid extension (ETE) | ||||||||||
| Absent | 161 | 86 | 75 | 0.002 | 87 | 74 | 0.001 | 131 | 30 | 0.003 |
| Present | 39 | 10 | 29 | 10 | 29 | 39 | 0 | |||
| Lymph node metastasis (LNM) | ||||||||||
| Absent | 110 | 63 | 47 | 0.004 | 63 | 47 | 0.006 | 86 | 24 | 0.003 |
| Present | 90 | 33 | 57 | 34 | 56 | 84 | 6 | |||
| BRAFV600E | ||||||||||
| Low | 67 | 41 | 26 | 0.008 | 43 | 24 | 0.002 | 50 | 17 | 0.004 |
| High | 133 | 55 | 78 | 54 | 79 | 120 | 13 | |||
| TNM stage | ||||||||||
| I-II | 100 | 59 | 41 | 0.002 | 60 | 40 | 0.001 | 77 | 23 | 0.002 |
| III-IV | 100 | 37 | 63 | 37 | 63 | 93 | 7 | |||
P-values derived using Chi-square test to compare the protein levels of PES1, ERα and ERβ between subgroups defined by each clinicopathological parameter; P < 0.05 was considered to be statistically significant.
High PES1 and ERα protein levels and low ERβ protein level are correlated with the aggressive behaviors of PTC patients
To explore whether and how PES1, ERα and ERβ protein levels are correlated with the clinicopathological features of PTC patients, we systematically assessed the correlations of PES1, ERα and ERβ protein levels with various clinicopathological characteristics of the PTC patients using Chi-square test. As shown in Table 2, there were no statistically significant correlations of PES1, ERα and ERβ protein levels with histologic subtype (P = 0.165, 0.286, 0.222), age (P = 0.280, 0.143, 0.144) and gender (P = 0.187, 0.115, 0.145) of PTC patients. However, PES1, ERα and ERβ protein levels were significantly correlated with tumor size (P = 0.005, 0.003, 0.007), ETE (P = 0.002, 0.001, 0.003), LNM (P = 0.004, 0.006, 0.003), BRAFV600E mutation (P = 0.008, 0.002, 0.004) and TNM stage (P = 0.002, 0.001, 0.002). PTC patients with large tumor size, ETE, LNM, high BRAFV600E expression and high TNM stage (III-IV) had higher rates of high PES1 and ERα protein expression and low ERβ protein expression. It was indicated that high PES1 and ERα protein levels and low ERβ protein level are correlated with the aggressive behaviors of PTC patients.
PES1 protein level is positively correlated with ERα protein level and negatively correlated with ERβ protein level in PTC tissues
To explore whether and how PES1 protein level is correlated with ERα and ERβ protein levels, the correlations of PES1 protein level with ERα and ERβ protein levels in PTC tissues were assessed using Spearman rank test. As shown in Table 3, 81/200 cases showed high PES1 protein level associated with high ERα protein level. PES1 protein level was positively correlated with ERα protein level (rs = 0.549, P < 0.001). Conversely, 101/200 cases displayed high PES1 protein level associated with low ERβ protein level. PES1 protein level was negatively correlated with ERβ protein level (rs = −0.353, P < 0.001). In addition, high ERα protein level associated with low ERβ protein level was present in 98/200 cases. A significantly negative correlation (rs = −0.293, P < 0.001) was also present between ERα and ERβ protein levels. It was indicated that PES1 protein level is positively correlated with ERα protein level and negatively correlated with ERβ protein level in PTC tissues.
Table 3.
Correlations of PES1, ERα and ERβ protein levels between each other in 200 PTC tissues.
| Protein | PES1 | ERα | ||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | r s | P value | Low | High | r s | P value | |
| ERβ | ||||||||
| Low | 69 | 101 | −0.353 | <0.001 | 72 | 98 | −0.293 | <0.001 |
| High | 27 | 3 | 25 | 5 | ||||
| ERα | ||||||||
| Low | 74 | 23 | 0.549 | <0.001 | ||||
| High | 22 | 81 | ||||||
P-values for Spearman rank test; PES1, ERα and ERβ were tested pairwise. P < 0.05 was considered to be statistically significant.
PES1 protein level and ERα/ERβ protein ratio are upregulated in human PTC cells
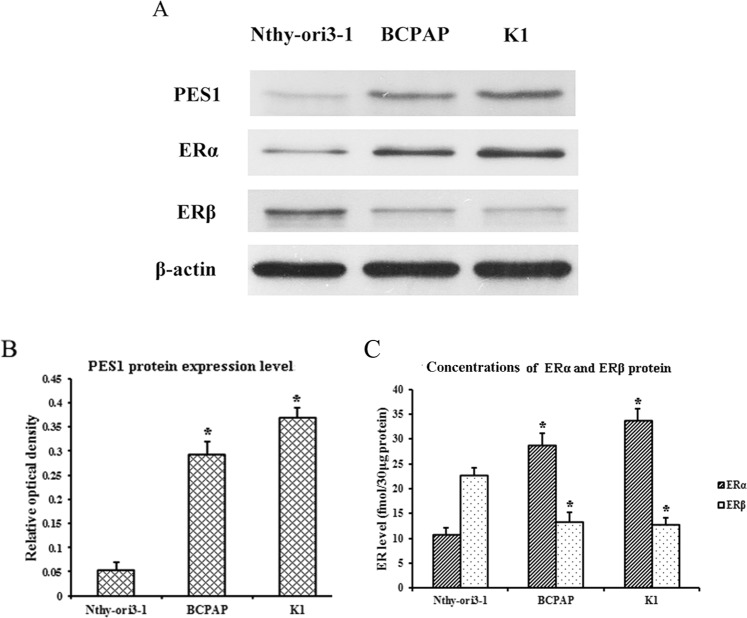
To examine the PES1 protein level in human PTC and normal thyroid cells, we preformed Western blotting using human PTC-derived BCPAP and K1 cells with BRAFV600E mutation and normal thyroid-derived Nthy-ori3-1 cells without BRAFV600E mutation40. As shown in Fig. 2A,B, the PES1 protein level was much higher in BCPAP and K1 cells than that in Nthy-ori3-1 cells. To quantify the ERα/ERβ protein ratio in BCPAP, K1 and Nthy-ori3-1 cells, we measured the concentrations of ERα and ERβ protein in these cells by Western blotting as our previous method41. As shown in Fig. 2C, ERα and ERβ protein concentrations were 28.67 and 13.33 fmol/30 μg cell protein in BCPAP, 33.67 and 12.67 fmol/30 μg cell protein in K1 and 10.67 and 22.67 fmol/30 μg cell protein in Nthy-ori3-1 cells. The ERα/ERβ protein ratio was about 2.15:1 in BCPAP, 2.66:1 in K1 and 1:2.12 in Nthy-ori3-1cells, respectively. It was indicated that the PES1 protein level and the ERα/ERβ protein ratio are upregulated in human PTC cells when compared with those in normal thyroid cells.
Figure 2.
PES1 protein level and ERα/ERβ protein ratio in human PTC-derived BCPAP and K1 cells and normal thyroid-derived Nthy-ori3-1 cells. BCPAP, K1 and Nthy-ori3-1 cells were cultured, then the total protein of these cells was extracted and the protein levels of PES1, ERα and ERβ were assessed by Western blotting. β-actin served as an internal calibrator. (A) Blot examples of PES1, ERα and ERβ protein levels in BCPAP, K1 and Nthy-ori3-1 cells. (B) Bar diagrams of relative PES1 protein level in BCPAP, K1 and Nthy-ori3-1 cells. (C) The concentrations of ERα and ERβ protein in BCPAP, K1 and Nthy-ori3-1 cells. Data presented represent the mean of three independent experiments. Statistical differences between two groups were examined using Students t-test. *P < 0.05, compared with Nthy-ori3-1 normal thyroid cells.
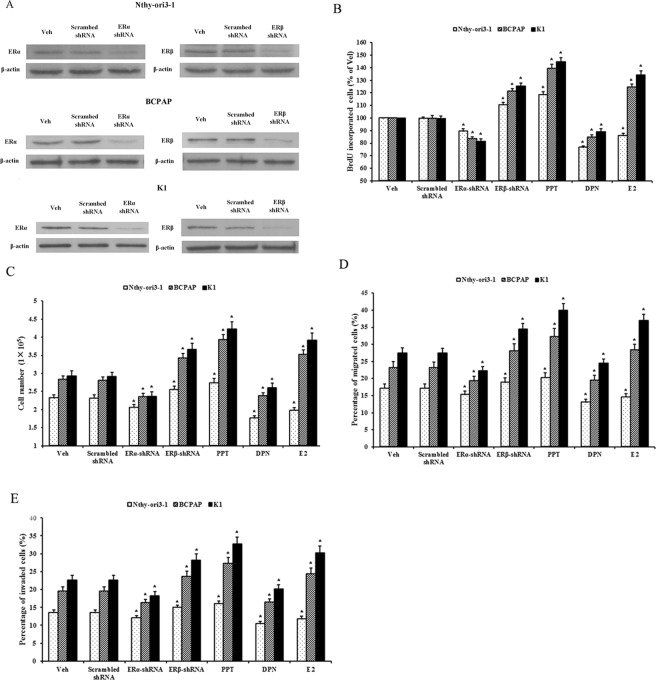
ERα promotes and ERβ inhibits the proliferation, invasion and migration of human PTC cells and normal thyroid cells
To explore the effects of ERα and ERβ on the proliferation, invasion and migration of human PTC and normal thyroid cells, ERα-shRNA and ERβ-shRNA expression vectors were used to generate the stable transfected cells with knockdown of ERα and ERβ. Meanwhile, PPT (ERα-selective agonist) and DPN (ERβ-selective agonist) were used to stimulate ERα and ERβ, respectively. As shown in Fig. 3, ERα-shRNA and ERβ-shRNA expression vectors effectively silenced ERα and ERβ expression, respectively, in BCPAP, K1 and Nthy-ori3-1cells. Compared with scrambed shRNA, ERα-shRNA decreased and ERβ-shRNA increased the proliferation, invasion and migration of human PTC-derived BCPAP and K1 cells and normal thyroid-derived Nthy-ori3-1 cells. Compared with the control (vehicle, Veh), ERα agonist PPT increased and ERβ agonist DPN decreased the proliferation, invasion and migration of these cells. It was indicated that ERα promotes and ERβ inhibits the proliferation, invasion and migration of human PTC cells and normal thyroid cells. Interestingly, E2 increased the proliferation, invasion and migration of human PTC-derived BCPAP and K1 cells with an ERα/ERβ protein ratio of >1, however, decreased the proliferation, invasion and migration of normal thyroid-derived Nthy-ori3-1 cells with an ERα/ERβ protein ratio of <1. It was indicated that the balance between ERα and ERβ protein levels and the ERα/ERβ protein ratio are crucial to the effects of E2 on the proliferation, invasion and migration of these thyroid cells.
Figure 3.
ERα promotes and ERβ inhibits the proliferation, invasion and migration of human PTC cells and normal thyroid cells. BCPAP, K1 and Nthy-ori3-1 cells were stably transfected with the expression vectors of ERα-shRNA, ERβ-shRNA and scrambled shRNA or were exposed to 10 nM of E2, PPT and DPN for 72 h, respectively. (A) Knockdown of ERα and ERβ protein levels by ERα-shRNA and ERβ-shRNA. (B and C) The proliferation of these cells was assessed by BrdU incorporation and cell count assays. (D and F) The migration and invasion of these cells were evaluated by Transwell assay. Data presented represent the mean of three independent experiments. Statistical differences between two groups were examined using Students t-test. *P < 0.05, compared with non treatment (Veh).
PES1 upregulates the ERα/ERβ protein ratio and promotes the proliferation, invasion and migration of human PTC cells and normal thyroid cells
To explore the effects of PES1 on the ERα/ERβ protein ratio and the proliferation, invasion and migration of human PTC cells and normal thyroid cells, PES1-shRNA expression vector was used to generate the stable transfected PTC cells with knockdown of PES1 and PES1expression vector was used to generate the stable transfected normal thyroid cells with increased PES1 expression. As shown in Fig. 4, compared with scrambed shRNA, PES1-shRNA reduced the ERα protein level and simultaneously elevated the ERβ protein level, and then resulted in a decrease of the ERα/ERβ protein ratio and a decrease of the promotion effects of E2 on the proliferation, invasion and migration of human PTC-derived BCPAP and K1 cells. Conversely, compared with empty vector, PES1 expression vector elevated the ERα protein level and simultaneously reduced the ERβ protein level, and then resulted in an increase of the ERα/ERβ protein ratio and an inhibition-promotion transition of the effects of E2 on the proliferation, invasion and migration in normal thyroid-derived Nthy-ori3-1 cells. It was indicated that PES1 elevates the ERα protein level and simultaneously reduces the ERβ protein level, i.e., upregulates the ERα/ERβ protein ratio, and then promotes the proliferation, invasion and migration of human PTC cells and normal thyroid cells.
Figure 4.

Effects of PES1 on the ERα/ERβ protein ratio and the proliferation, invasion and migration of human PTC and normal thyroid cells. The normal thyroid-derived Nthy-ori3-1 cells were stably transfected with the PES1 expression vector or empty vector and the PTC-derived BCPAP and K1 cells were stably transfected with the expression vector of PES1-shRNA or scrambed shRNA. The protein levels of PES1, ERα and ERβ in these stable transfected cells were assessed by Western blotting. β-actin served as an internal calibrator. The proliferation, invasion and migration of these stable transfected cells were assayed after exposure to E2 for 72 h. (A) Blot examples of PES1, ERα and ERβ protein levels in these stable transfected cells. (B) Bar diagrams of relative PES1 protein level in these stable transfected cells. (C) The concentrations of ERα and ERβ protein in these stable transfected cells. (D and E) The proliferation of these stable transfected cells was assessed by BrdU incorporation and cell count assays. (F and G) The migration and invasion of these stable transfected cells were assessed by Transwell assay. Data presented represent the mean of three independent experiments. Statistical differences between two groups were examined using Students t-test. *P < 0.05, compared with non treatment (Veh). #P < 0.05, compared with E2 treatment alone.
Discussion
Studies have shown that estrogen may be involved in the occurrence and development of PTC1–5, as has been shown in breast, endometrial and ovarian cancer6. Estrogen exerts its effects mainly via ERα and ERβ7,8. ERα and ERβ are often co-expressed and contribute to the physiological and pathophysiological responses of estrogen42,43. In this study, we detected ERα and ERβ protein levels in PTC tissues and cells using IHC staining and Western blotting, respectively. Compared with normal thyroid tissues and cells, the ERα protein level was upregulated and the ERβ protein level was downregulated in PTC tissues and cells. PTC tissues had higher rates of high ERα and low ERβ protein expression. PTC cells had more than two times higher protein level of ERα than that of ERβ. This result is in line with the previous data showing that ERα and ERβ are co-expressed in normal and tumor tissues of the thyroid20,21, compared with normal thyroid tissues, the level of ERα is relatively higher than that of ERβ in PTC tissues21–24.
Although ERα and ERβ are architecturally similar with three functional domains of N-terminal domain (NTD), DNA binding domain (DBD) and ligand binding domain (LBD), there are obvious differences in their structures of NTD and LBD. The differences in their structures suggest that ERα and ERβ may have different functions9. Cell-based assays have shown that ERβ is generally less active than ERα and may influence ERα activity. Despite a small number of data associating ERβ with pro-growth and pro-survival when present alone in ERα-negative estrogen related cancer tissues and cells, a large number of data both in vitro and in vivo support that ERβ acts as an anti-proliferative and pro-apoptotic factor, especially when co-expressed with ERα44,45. Most studies have revealed that ERα promotes cell proliferation, invasion and migration and has been shown to have tumor-promoting effects, whereas ERβ, when co-expressed with ERα, may play an inhibitory role against the ERα-mediated tumor-promoting effects14–16. Thus, the complement of ER isoform could influence the biological responses and the balance and ratio between ERα and ERβ protein levels would be critical in defining the overall response14–18. In this study, we showed that compared with normal thyroid tissues, the ERα protein level was upregulated and the ERβ protein level was downregulated in PTC tissues. The ERα protein level was positively correlated with the aggressive behaviors of PTC patients, conversely, the ERβ protein level was negatively correlated with the aggressive behaviors of PTC patients. PTC patients with large tumor size, ETE, LNM, high BRAFV600E expression and high TNM stage (III-IV) had higher rates of high ERα and low ERβ protein expression. Moreover, cell-based assays showed that the ERα/ERβ protein ratio was greater than one (>1) in human PTC-derived BCPAP and K1 cells with the BRAFV600E mutation, conversely, smaller than one (<1) in normal thyroid-derived Nthy-ori3-1 cells without the BRAFV600E mutation. ERα promoted and ERβ inhibited the proliferation, invasion and migration of these PTC cells and normal thyroid cells. Interestingly, E2 increased the proliferation, invasion and migration of human PTC cells with an ERα/ERβ protein ratio of >1, however, decreased the proliferation, invasion and migration of normal thyroid cells with an ERα/ERβ protein ratio of <1. It was indicated that the ERα/ERβ protein ratio is crucial to the effects of E2 on the proliferation, invasion and migration of these thyroid cells.
PES1, a BRCT domain-containing protein, is essential for ribosome biogenesis, nucleologenesis and cell growth, which are all important components that determine the cell proliferation rate25,28. The increased expression of PES1 is involved in the proliferation and malignant conversion of cells and may contribute to the occurrence and development of some human cancers such as prostate, head and neck, stomach, colon, breast and ovarian cancer29–39. Recently, Cheng et al.33 and Li et al.38 reported a novel function of PES1 that regulates the balance between ERα and ERβ protein levels. They found that PES1 enhances the stability of ERα while simultaneously targeting ERβ for proteasome degradation, thereby increasing the protein level of ERα and decreasing that of ERβ, which contributes to the occurrence and development of breast cancer and ovarian cancer. As some ERs regulators have been found to have tissue specificity that modulate their activities46,47, in this study, we examined PES1 protein level in PTC tissues and cells and assessed the correlations of its protein level with the ERα and ERβ protein levels and with the occurrence and development of PTC. We found that PES1 protein level was positively correlated with ERα protein level and negatively correlated with ERβ protein level in human PTC tissues and cells. Compared with normal thyroid tissues, PES1 protein level was significantly increased in PTC tissues and was associated with the aggressive behaviors of PTC patients. PTC patients with large tumor size, ETE, LNM, high BRAFV600E expression and high TNM stage (III-IV) had higher rates of high PES1 and ERα protein levels and low ERβ protein level. Increased PES1 and ERα protein levels and decreased ERβ protein level were associated with the occurrence and development of PTC. Cell-based assays showed that PES1 elevated ERα protein level and simultaneously reduced ERβ protein level and resulted in an upregulated ERα/ERβ protein ratio, and then promoted the proliferation, invasion and migration of human PTC cells.
In summary, ERα and ERβ were co-expressed in human PTC tissues and cells. ERα promoted and ERβ inhibited the proliferation, invasion and migration of PTC cells. PES1 modulated the balance between ERα and ERβ by elevating the ERα protein level and simultaneously reducing the ERβ protein level, then upregulated the ERα/ERβ ratio and promoted the proliferation, invasion and migration of PTC cells. In PTC tissues, PES1 protein level was positively correlated with ERα protein level and negatively correlated with ERβ protein level. PES1 and ERα protein levels were gradually increased and ERβ protein level was decreased by degree in the occurrence and development of PTC. Increased PES1 and ERα protein levels and decreased ERβ protein level were correlated with the aggressive behaviors of PTC patients such as large tumor size, ETE, LNM, high BRAFV600E expression and high TNM stage. It was suggested that PES1 promotes the occurrence and development of PTC by elevating the ERα protein level and reducing the ERβ protein level, and then upregulating the ERα/ERβ protein ratio.
Materials and Methods
Case selection and tissue samples
Tumor tissue samples were obtained from 200 PTC patients who underwent initial thyroidectomy in the Department of Surgery, the First Affiliated Hospital, Chongqing Medical University, between January 2015 and January 2017. At initial thyroidectomy, cervical lymph node dissection (CLND) was performed, tumor size was assessed, histologic subtype was defined and extrathyroidal extension (ETE) and BRAFV600E mutation were evaluated. Of the 200 cases, 142 were classic PTC, 21 follicular variant of PTC, 24 tall cell variant of PTC and 13 oncocytic variant of PTC. There were 39 patients with ETE, 90 patients with lymph node metastasis (LNM), 133 patients with high BRAFV600E expression, 62 patients with tumor size of ≤2 cm, 71 patients with tumor size of >2 and ≤4 cm and 67 patients with tumor size of >4 cm. Of this patient cohort, 46 were men and 154 women; 24 patients were aged <45 years and 176 were aged ≥45 years. According to the seventh edition of thyroid cancer tumor-node-metastasis (TNM) staging system by American Joint Committee on Cancer, 50 patients were stage I, 50 patients stage II, 50 patients stage III and 50 patients stage IV. For statistical analysis, stage I and II were combined into low TNM stage (I-II) and stage III and IV were combined into high TNM stage (III-IV). Besides, 200 normal thyroid tissues were taken from the contralateral lobes of PTC tissues. The study protocol was approved by the Ethics Committee of Chongqing Medical University and informed consent was obtained from all of the patients.
Tissue microarray and IHC staining
Tissue microarray construction and IHC staining were performed as described previously48. Rabbit polyclonal anti-PES1 (ab72539), mouse monoclonal anti-ERα (ab1104) and mouse monoclonal anti-ERβ (ab1103) were purchased from Abcam (Abcam, Cambridge, MA, USA). Mouse monoclonal anti-BRAFV600E (26039) was obtained from NewEast Bioscience (NewEast Bioscience, Malvern, PA, USA). These antibodies were used as primary antibodies at 1:50 dilution. Biotinylated goat-anti-rabbit and goat-anti-mouse IgG (ZB-2010 and ZB-2305, Zhongshan Golden Bridge Biotechnology, China) were used as secondary antibodies at 1:500 dilution.
IHC scoring
A semi-quantitative IHC scoring assessment was performed by two observers blinded to the diagnosis. IHC score was assigned based on the percentage of positive cells and the staining intensity. The percentage was evaluated and assigned a score of 0−4: 0, <5% positive cells; 1, 6–25% positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells; and 4, >75% positive cells. The staining intensity was evaluated and assigned a score of 0−3: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The IHC score was then assigned to each sample by multiplying the percentage score and the staining intensity score, and thus the score ranged from 0 to 12. For statistical analysis, a final IHC score of 0 (negative) or 1–4 (+) was defined as low expression and a final IHC score of 5–8 (++) or 9–12 (+++) as high expression.
Cell culture and treatment
Human PTC-derived BCPAP and K1 cells with BRAFV600E mutation and normal thyroid-derived Nthy-ori3-1 cells without BRAFV600E mutation40 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI-1640 medium supplemented with 10% charcoal-stripped fetal bovine serum (FBS) (GBICO Co., Ltd., Grand Island, NY, USA). The cells were incubated in phenol-red free and serum free medium for 48 h before stimulation with 17β-estradiol (E2), propyl-pyrazole-triol (PPT) and diarylpropionitrile (DPN) (Sigma, St Louis, MO, USA). Cell treatments were performed as indicated in the respective figure legends.
Construction of shRNA expression vectors for PES1, ERα and ERβ
For a vector-based RNA interference (RNAi) approach, short hairpin RNA (shRNA) was cloned into the BamHI-HindIII sites of the pRNAT-U6.1/neo vector (GenScript Corp. Piscataway, NJ, USA). RNAi target sequences were selected from the human PES1, ERα and ERβ sequences (GenBank accession NM_001282328.1 for PES1, NM_001122741.1 for ERα and NM_001291723.1 for ERβ). The candidate target sequences were analyzed by BLAST search to ensure that the knockdown would be unique to PES1, ERα and ERβ. The specific shRNA sequences were 5′-GGATTCCCGGCCAGAAGATCATGTTTGGCAATTCAAGAGATTGCCAAACATGATCTTCTGGTTTTTGAAGCTT-3′ for PES1, 5′-GGATTCCCGGCTACAGGCCAAATTCAGATAA TTCAAGAGATTATCTGAATTTGGCCTGTAGTTTTTGAAGCTT-3′ for ERα and 5′-GGATTC CCGGGCGAGTAACAAGGGCATGGAATTCAAGAGATTCCATGCCCTTGTTACTCGCTTTTTGAAGCTT-3′ for ERβ. A nonsilencing RNAi control vector comprising a scrambled shRNA sequence was generated with the following oligonucleotide 5′-GGATTCCCGGCCTAAGGTTAAGTCGCCC TCGTTCAAGAGACGAGGGCGACTTAACCTTAGGTTTTTGAAGCTT-3′. The underlined, boldface and italic letters denote the hairpin loop, terminal signal and target sites of BamHI and HindIII restriction enzymes, respectively. These shRNA sequences were digested with BamHI and HindIII restriction enzymes (Takara Biotechnology Co., Ltd., Dalian, China) and cloned into the pRNAT-U6.1/neo vector with T4 ligase (Takara). The resulting constructs were verified by direct sequencing (Sangon Biotech Co., Ltd., Shanghai, China).
Construction of PES1 expression vector
Full-length PES1 cDNA was amplified by PCR using human universal QUICK clone cDNA (Clonetech Laboratories Inc., Mountain View, CA, USA) as a PCR template and specific primers based on PES1 cDNA sequence (GenBank Accession: NC_000022.11). The specific primers were as follows: forward 5′-ATAATTGGATTCGCCACCATGGGAGGCCTTGAGAAGA-3′ and reverse 5′-ATTATAAAGCTTCTCCGGCCTTGCCTTCTTGGCCTTC-3′. The underlined and italic letters denote the target sites of BamHI and HindIII restriction enzymes and the additional sequence, respectively. The PCR product containing the PES1 open-reading frame sequence was digested with BamHI and HindIII restriction enzymes (Takara) and cloned into the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA, USA) with T4 ligase (Takara). The resulting construct was verified by direct sequencing (Sangon Biotech Co., Ltd, Shanghai, China).
Transfection
A single day prior to transfection, cells were seeded in 6-well culture plates at a density of 5.0 × 105 cells per well (2.0 ml/well). Transfection was performed using Lipofectamine 2000 reagent (Invitrogen) in accordance with the standard protocol of the manufacturer. In brief, transfection was initiated when the cells were 70–80% confluent. For each well, 5 μg of plasmid DNA was added into 250 μl of Opti-MEM (Invitrogen), 5 μl of lipofectamine 2000 into 250 μl of Opti-MEM, and then mixed plasmid DNA with Lipofectamine 2000. The mixture was added to cells in the 6-well culture plates, giving an end volume of 1 ml. The Opti-MEM medium containing complex was incubated for 8 h at 37 °C, then replaced with 2 ml of standard growth media and cultured for 48 h at 37 °C. Stable transfected cells were selected in the presence of 400 μg/ml of G418 (GIBCO) for 3 weeks with medium change on every 4th or 5th day.
Protein extraction and Western blotting
Cells were harvested and gently lysed for 30 min in 5 volumes of ice-cold RIPA buffer (Sigma) supplemented with Complete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany) at a ratio of 1000:1. Cell lysates were centrifuged at 10 000 × g for 10 min at 4 °C to obtain the supernatants. The protein content of cell lysates was quantified by the Bio-Rad Dc Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Western blotting was performed as described previously. Briefly, 30 μg of total cell protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The transferred membranes were probed with anti-PES1 (ab72539, Abcam), anti-ERα (ab1104, Abcam), anti-ERβ (ab1103, Abcam) and anti-β-actin (ab8227, Abcam) primary antibodies overnight at 4 °C and at 1:500 dilution. Subsequently, the membranes were washed and reacted with peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (sc-2005 and sc-2004, Santa Cruz Biotechnology, CA, USA) for 1 h at room temperature and at 1:2000 dilution. The immunoreactive bands were visualized using an enhanced chemioluminescent ECL reagent (Amersham Biosciences, Piscataway, NJ, USA) and quantified using the TINA version 2.09 program package by normalizing to β-actin signal.
BrdU assay
Cells were seeded in 96-well culture plates at a density of 5 × 103 cells per well, incubated for 24 h, serum-deprived for 48 h and treated as indicated in the figure legends. Then a 5-bromo-2′-deoxyuridine (BrdU) incorporation colorimetric ELISA kit (Roche Diagnostics) was used to assay the cell proliferation in accordance with the standard protocol of the manufacturer.
Cell count assay
Cells were seeded in 6-well culture plates at a density of 2 × 105 cells per well, incubated for 24 h, serum-deprived for 48 h and treated as indicated in the figure legends. Then cells were harvested using Trypsin-EDTA, resuspended in the fresh medium and diluted in 0.4% trypan blue at a volume ratio of 1:1. The cell number was manually counted using a haemocytometer under microscope.
In vitro migration and invasion assays
In vitro migration and invasion assays were performed using transwell chambers (8-μm pore size, 24-well insert, Corning Inc., Corning, NY, USA) according to the manufacturer’s instructions. Briefly, 3 × 104 serum-starved cells were resuspended and seeded in the upper chambers in phenol-red free and serum-free medium supplemented with or without E2, PPT or DPN. The lower chambers were filled with phenol-red free medium supplemented with 10% charcoal-stripped FBS as a chemoattractant. For the invasion assay, the inserts were pre-coated with extracellular matrix gel (BD Biosciences, Bedford, MA, USA). Following 72 h incubation, MTT solution (0.5 mg/mL) was added to the upper and lower chambers and incubated for another 4 h. After this, the cells in the upper membrane surface (residual cells) and the cells in the lower membrane surface (migrated or invaded cells) were scraped off with a cotton swab and dissolved in 400 μl of DMSO, respectively. Then 100 μl of the dissolved solution was taken and the absorbance was measured at 570 nm. Data were expressed as a percentage of migrated and invaded cells, i.e., A/(A + B) × 100, where A is the absorbance of the migrated or invaded cells and B the absorbance of the residual cells.
Statistics
Statistical analyses were performed using the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Data are presented as percentages and mean plus standard deviation, according to distribution. Significance was assessed using Chi-square, Spearman rank and Student’s t-tests, as appropriate, to compare groups. A P-value of < 0.05 was considered to be statistically significant.
Statements for Materials and Methods
The study protocol was approved by the Ethics Committee of Chongqing Medical University and informed consent was obtained from all of the patients. All experiments were performed in accordance with the relevant guidelines and regulations.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81272937).
Author Contributions
Z.M.L., Y.B.Q. and L.Y.L. conceived the experiments. Y.B.Q., R.J. and L.W.X. conducted the experiments. Z.M.L., Y.B.Q., L.Y.L. and M.X. analyzed the data. L.W.X., L.Y.L. and M.X. collected tissue samples and provided patient information. Z.M.L. wrote the manuscript. G.G.C. revised the manuscript. All authors have read and approved the final manuscript.
Data Availability
All data generated or analyzed during this study are included in this article (and its Supplementary Information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-Bo Qiu and Ling-Yao Liao contributed equally.
References
- 1.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. 2014;21:T273–283. doi: 10.1530/ERC-14-0053. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6:1771–1779. doi: 10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Przybylik-Mazurek E, Hubalewska-Dydejczyk A, Fedorowicz A, Pach D. Factors connected with the female sex seem to play an important role in differentiated thyroid cancer. Gynecol Endocrinol. 2012;28:150–155. doi: 10.3109/09513590.2011.563909. [DOI] [PubMed] [Google Scholar]
- 4.Schonfeld SJ, et al. Hormonal and reproductive factors and risk of postmenopausal thyroid cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. 2011;35:e85–90. doi: 10.1016/j.canep.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sungwalee W, Vatanasapt P, Kamsa-Ard S, Suwanrungruang K, Promthet S. Reproductive risk factors for thyroid cancer: a prospective cohort study in Khon Kaen, Thailand. Asian Pac J Cancer Prev. 2013;14:5153–5155. doi: 10.7314/APJCP.2013.14.9.5153. [DOI] [PubMed] [Google Scholar]
- 6.Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23:6379–6391. doi: 10.1038/sj.onc.1207899. [DOI] [PubMed] [Google Scholar]
- 7.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 8.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 9.Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 11.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 12.Honma N, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 13.Marotti JD, Collins LC, Hu R, Tamimi RM. Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2010;23:197–204. doi: 10.1038/modpathol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 15.Williams C, Edvardsson K, Lewandowski SA, Ström A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–1032. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 16.Liu MM, et al. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J. Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 17.Zannoni GF, et al. The expression ratios of estrogen receptor α (ERα) to estrogen receptor β1 (ERβ1) and ERα to ERβ2 identify poor clinical outcome in endometrioid endometrial cancer. Hum Pathol. 2013;44:1047–1054. doi: 10.1016/j.humpath.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Nadal-Serrano M, et al. The ERalpha/ERbeta ratio determines oxidative stress in breast cancer cell lines in response to 17beta-estradiol. J. Cell Biochem. 2012;113:3178–3185. doi: 10.1002/jcb.24192. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Chen G, Meng XY, Liu ZH, Dong S. Serum levels of sex hormones and expression of their receptors in thyroid tissue in female patients with various types of thyroid neoplasms. Pathol Res Pract. 2014;210:830–835. doi: 10.1016/j.prp.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, et al. Differential expression patterns and clinical significance of estrogen receptor-α and β in papillary thyroid carcinoma. BMC Cancer. 2014;14:383. doi: 10.1186/1471-2407-14-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Vito M, et al. Overexpression of estrogen receptor-α in human papillary thyroid carcinomas studied by laser- capture microdissection and molecular biology. Cancer Sci. 2011;102:1921–1927. doi: 10.1111/j.1349-7006.2011.02017.x. [DOI] [PubMed] [Google Scholar]
- 22.Yi JW, et al. Upregulation of the ESR1 Gene and ESR Ratio (ESR1/ESR2) is Associated with a Worse Prognosis in Papillary Thyroid Carcinoma: The Impact of the Estrogen Receptor α/β Expression on Clinical Outcomes in Papillary Thyroid Carcinoma Patients. Ann Surg Oncol. 2017;24:3754–3762. doi: 10.1245/s10434-017-5780-z. [DOI] [PubMed] [Google Scholar]
- 23.Magri F, et al. Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient’s risk. Endocr Relat Cancer. 2012;19:463–471. doi: 10.1530/ERC-11-0389. [DOI] [PubMed] [Google Scholar]
- 24.Rubio GA, Catanuto P, Glassberg MK, Lew JI, Elliot SJ. Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause. Surgery. 2018;163:143–149. doi: 10.1016/j.surg.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Haque J, Boger S, Li J, Duncan SA. The murine Pes1 gene encodes a nuclear protein containing a BRCT domain. Genomics. 2000;70:201–210. doi: 10.1006/geno.2000.6375. [DOI] [PubMed] [Google Scholar]
- 26.Allende ML, et al. Insertional mutagenesis in zebrafish identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Genes Dev. 1996;10:3141–3155. doi: 10.1101/gad.10.24.3141. [DOI] [PubMed] [Google Scholar]
- 27.Gessert S, Maurus D, Rössner A, Kühl M. Pescadillo is required for Xenopus laevis eye development and neural crest migration. Dev Biol. 2007;310:99–112. doi: 10.1016/j.ydbio.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Lerch-Gaggl A, et al. Pescadillo is essential for nucleolar assembly, ribosome biogenesis, and mammalian cell proliferation. J Biol Chem. 2002;277:45347–45355. doi: 10.1074/jbc.M208338200. [DOI] [PubMed] [Google Scholar]
- 29.Kim B, et al. Expression profiling and subtype specific expression of stomach cancer. Cancer Res. 2003;63:8248–8255. [PubMed] [Google Scholar]
- 30.Li Y, et al. Gene expression profiling revealed novel molecular targets of docetaxel and estramustine combination treatment in prostate cancer cells. Mol Cancer Ther. 2005;4:389–398. doi: 10.1158/1535-7163.MCT-04-0244. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132:3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 32.Li J, et al. Down-regulation of pescadillo inhibits proliferation and tumorigenicity of breast cancer cells. Cancer Sci. 2009;100:2255–2260. doi: 10.1111/j.1349-7006.2009.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L, et al. PES1 promotes breast cancer by differentially regulating ERα and ERβ. J. Clin Invest. 2012;122:2857–2870. doi: 10.1172/JCI62676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber A, et al. Protein microarrays for the detection of biomarkers in head and neck squamous cell carcinomas. Hum Pathol. 2007;38:228–238. doi: 10.1016/j.humpath.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Xie W, et al. Transcriptional regulation of PES1 expression by c-Jun in colon cancer. PLoS One. 2012;7:e42253. doi: 10.1371/journal.pone.0042253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita Y, et al. Pescadillo, a novel cell cycle regulatory protein abnormally expressed in malignant cells. J Biol Chem. 2001;276:6656–6665. doi: 10.1074/jbc.M008536200. [DOI] [PubMed] [Google Scholar]
- 37.Nakaguro M, et al. Nucleolar protein PES1 is a marker of neuroblastoma outcome and is associated with neuroblastoma differentiation. Cancer science. 2015;106:237–243. doi: 10.1111/cas.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, et al. PES1 differentially regulates the expression of ERα and ERβ in ovarian cancer. IUBMB Life. 2013;65:1017–1025. doi: 10.1002/iub.1228. [DOI] [PubMed] [Google Scholar]
- 39.Maiorana A, Tu X, Cheng G, Baserga R. Role of pescadillo in the transformation and immortalization of mammalian cells. Oncogene. 2004;23:7116–7124. doi: 10.1038/sj.onc.1207916. [DOI] [PubMed] [Google Scholar]
- 40.Schweppe RE, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mo XM, et al. Up-regulation of Hsp27 by ERα/Sp1 facilitates proliferation and confers resistance to apoptosis in human papillary thyroid cancer cells. Mol Cell Endocrinol. 2016;431:71–87. doi: 10.1016/j.mce.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol. 2013;375:121–129. doi: 10.1016/j.mce.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Powell E, et al. Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERα and ERβ. PLoS One. 2012;7:e30993. doi: 10.1371/journal.pone.0030993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ALeygue E, Murphy LC. A bi-faceted role of estrogen receptor β in breast cancer. Endocr Relat Cancer. 2013;20:127–139. doi: 10.1530/ERC-12-0389. [DOI] [PubMed] [Google Scholar]
- 45.Haring J, Schüler S, Lattrich C, Ortmann O, Treeck O. Role of estrogen receptor β in gynecological cancer. Gynecologic Oncology. 2012;127:673–676. doi: 10.1016/j.ygyno.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Tynan SH, Lundeen SG, Allan GF. Cell type-specific bidirectional regulation of the glucocorticoid-induced leucine zipper (GILZ) gene by estrogen. J Steroid Biochem. Mol Biol. 2004;91:225–239. doi: 10.1016/j.jsbmb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, et al. Tissue type-specific modulation of ER transcriptional activity by NFAT3. Biochem Biophys Res Commun. 2007;353:576–581. doi: 10.1016/j.bbrc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 48.Dai YJ, et al. Concomitant high expression of ERα36, EGFR and HER2 is associated with aggressive behaviors of papillary thyroid carcinomas. Sci Rep. 2017;7:12279. doi: 10.1038/s41598-017-12478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its Supplementary Information files).