Abstract
Paget’s disease of bone (PDB) is characterised by focal abnormalities of bone remodelling, with increased osteoclastic resorption the primary feature of the disease. Genetic factors have been shown to play an important role in PDB, and genome-wide association studies (GWAS) have identified 7 genetic loci as associated with PDB at the genome-wide level. Expression quantitative trait locus (eQTL) studies using cell types that are directly relevant to the disease of interest are increasingly being used to identify putative effector genes for GWAS loci. We have recently constructed a unique osteoclast-specific eQTL resource using cells differentiated in vitro from 158 subjects for study of the genetics of bone disease. Considering the major role osteoclasts have in PDB, we used this resource to investigate potential genetic regulatory effects for the 7 PDB genome-wide significant loci on genes located within 500 kb of each locus. After correction for multiple testing, we observed statistically significant associations for rs4294134 with expression of the gene STMP1, and rs2458413 with expression of the genes DPYS and DCSTAMP. The eQTL associations observed for rs4294134 with STMP1, and rs2458413 with DCSTAMP were further supported by eQTL data from other tissue types. The product of the STMP1 gene has not been extensively studied, however the DCSTAMP gene has an established role in osteoclast differentiation and the associations seen between rs2458413 and PDB are likely mediated through regulatory effects on this gene. This study highlights the value of eQTL data in determining which genes are relevant to GWAS loci.
Introduction
Paget’s disease of bone (PDB), first described by Sir James Paget in 1876, is characterised by focal abnormalities of bone remodelling. It is the most common metabolic bone disease after osteoporosis, affecting around 2% of European individuals aged 55 years and over1, although both the incidence and severity of newly diagnosed cases is falling2,3. The prevalence of PDB increases with age and is slightly more common in men than in women. The abnormal bone remodelling seen in PDB disrupts normal bone architecture and structure, leading to deformity, bone pain, secondary arthritis and increased fracture risk4. The primary feature of PDB at the cellular level is increased osteoclastic bone resorption, followed by increased and disorganised bone formation that is thought to be secondary to the increased osteoclast activity. The osteoclasts in affected bone are larger in size, have more nuclei and display characteristic intra-nuclear inclusion bodies.
Genetic factors have been shown to play an important role in the pathogenesis of PDB and are generally considered to be the primary risk-factor for developing the disease, with relatives of an affected individual having around 7-fold greater risk of developing PDB than the general population5. The genetic architecture of the disease is complex, with contributions from rare mutations in the SQSTM1 gene as well as common variants with small effects located in other genes. Mutations in SQSTM1 have been found to cause autosomal dominant PDB with high but incomplete penetrance6–8, and may account for up to 40% of familial PDB cases and 10% of apparently sporadic PDB cases7,9. The common non-SQSTM1 PDB risk-variants are thought to contribute to the disease in an additive manner and interact with SQSTM1 mutations to influence disease severity10.
Genome-wide association studies (GWAS) have successfully identified many of the common genetic variants associated with PDB11,12. The most recent of these, performed by Albagha et al.12 in a study cohort of 2,215 PDB cases without SQSTM1 mutations and 4,370 controls, identified 7 loci as associated with PDB at the genome-wide significance level. Six of the 7 maximally associated variants at these loci are non-coding in nature, located in intronic or intergenic DNA, suggesting that they may have regulatory effects on the expression of nearby genes. Although some strong candidates have been identified, the identity of many of the genes regulated by these GWAS loci is yet to be determined.
Expression quantitative trait locus (eQTL) studies have increasingly been used to identify putative effector genes for GWAS loci by characterising association between genetic variants and the expression of nearby genes. However, complex tissues containing a mixture of multiple cell types have been used for many of these studies and, apart from a study in osteoblasts derived from trabecular bone explants13, there has historically been a lack of information specifically relevant to bone cells. We have recently constructed a unique osteoclast-specific eQTL dataset for study of the genetics of bone disease14. Considering the major role osteoclasts have in PDB, we sought to use this resource to investigate potential genetic regulatory effects for the 7 genome-wide significant loci for PDB.
Results
The demographics of the study cohort are presented in Supplementary Table S1. None of the 158 study participants were found to be closely related (all PI_HAT < 0.1) and principal components analysis of the unimputed genotype data did not detect any outliers. After imputation and QC procedures had been applied, there were 5,373,348 variants in the genotype dataset with an IMPUTE2 info score ≥0.4 and a minor allele frequency (MAF) ≥5%. In the RNA-Seq dataset derived from the differentiated osteoclast-like cells, quantitative expression data was obtained for 15,688 genes. Particularly high expression was observed for several osteoclast marker genes, including ACP5, ATP6V0D2, CA2, CTSK, DCSTAMP, MMP9, OCSTAMP and SPP1 (Supplementary Figure S1), thereby confirming the appropriateness and rigor of the culture protocol.
Association of PDB GWAS variants with gene expression
We analysed the 7 variants identified as significantly associated with PDB at the genome-wide level by Albagha et al.12, rs10494112 (1p13.3), rs4294134 (7q33), rs2458413 (8q22.3), rs1561570 (10p13), rs10498635 (14q32.12), rs5742915 (15q24.1) and rs3018362 (18q21.33), for association with local gene expression (+/−500 kb) in our osteoclast eQTL dataset. All 7 variants were found to have a MAF ≥5% in the eQTL dataset, with a total of 56 expressed gene transcripts identified within the combined analysis windows (mean 8 per variant). After correction for multiple testing within each locus, we observed statistically significant associations for rs4294134 with expression of the gene STMP1 (Table 1 and Fig. 1A), and rs2458413 with expression of the genes DPYS and DCSTAMP (Table 1 and Fig. 1B,C). For rs4294134, the PDB risk-increasing G allele was associated with increased expression of STMP1 (Table 1). For rs2458413, the PDB risk-increasing T allele was associated with reduced expression of DPYS and increased expression of DCSTAMP (Table 1).
Table 1.
PDB GWAS variants demonstrating significant eQTL associations in the osteoclast-like cells.
| Variant | Location | EA | OA | EAF | PDB OR (95% CI) | Gene | Expressiona | Distance to TSS | P | Betab |
|---|---|---|---|---|---|---|---|---|---|---|
| rs4294134 | chr7:135608380 | G | A | 0.82 | 1.45 (1.29–1.63) | STMP1 | 10.44 ± 1.71 | −54,116 | 3.11E-08 | 0.739 |
| rs2458413 | chr8:104347204 | T | C | 0.56 | 1.40 (1.29–1.51) | DPYS | 0.21 ± 0.16 | −119,850 | 2.27E-04 | −0.396 |
| rs2458413 | chr8:104347204 | T | C | 0.56 | 1.40 (1.29–1.51) | DCSTAMP | 110.75 ± 43.60 | 8,117 | 1.76E-05 | 0.468 |
EA: effect allele; OA: other allele; EAF: effect allele frequency (derived from osteoclast eQTL cohort); PDB: Paget’s disease of bone; OR: odds ratio; CI: confidence interval; TSS: transcription start site; variant locations obtained from dbSNP build 150 (GRCh38/hg38); PDB odds ratios obtained from Albagha et al.12. The eQTL associations are statistically significant using a multiple testing corrected FDR of 5% (analysis corrected for the covariates RNA-Seq batch, patient age and 10 principal components).
aExpression levels are stated as mean reads per kilobase million (RPKM) ± standard deviation.
bNormalised effect size on gene expression for the effect allele.
Figure 1.
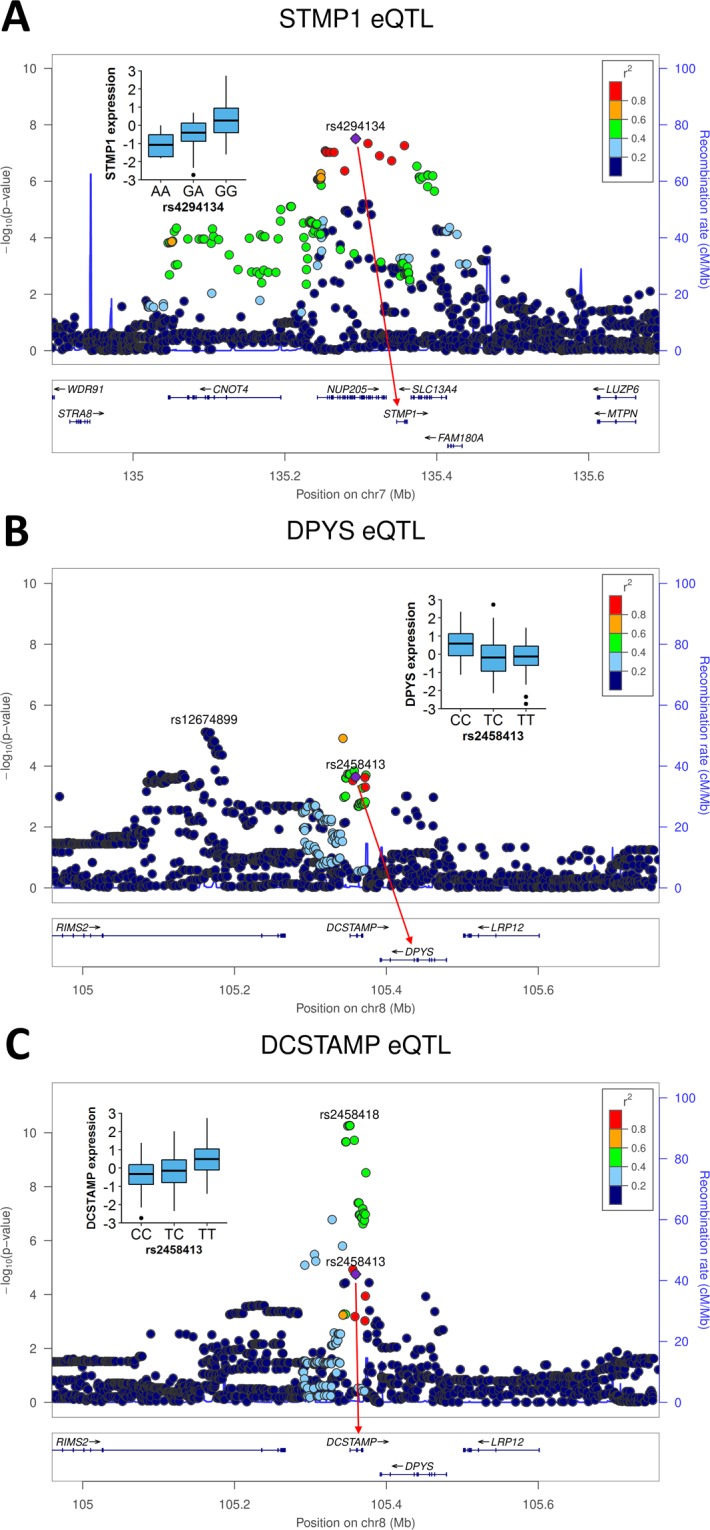
Regional association plots generated using the eQTL association results for (A) STMP1, (B) DPYS and (C) DCSTAMP from the osteoclast-specific dataset. Genetic variants within 400 kb of rs4294134 or rs2458413 are depicted (x axis) along with their eQTL P value (−log10). For DPYS and DCSTAMP, the maximally associated eQTL variant from the region is also indicated (rs12674899 and rs2458418, respectively). Variants are colour coded according to their LD (r2) with PDB GWAS loci rs4294134 or rs2458413 (1000GP Nov 2014 EUR population). The red arrows indicate significant associations between rs4294134 or rs2458413 and expression of nearby genes, with the allelic effects on normalised gene expression presented in the box-and-whisker plots. The recombination rate (blue line) and position of genes, their exons and direction of transcription is also indicated32.
Top eQTL variants for the STMP1, DPYS and DCSTAMP genes
We next examined all variants located within +/−500 kb of the STMP1, DPYS and DCSTAMP transcription start sites (TSS) to identify the top cis-eQTL variant for each gene. For STMP1, the PDB GWAS variant rs4294134 was found to demonstrate the strongest association with expression of the gene (Fig. 1A). For the DPYS gene, rs12674899 was identified as the top eQTL variant from the region, with the more common G allele associated with increased expression of the gene (P = 7.67 × 10−6, Fig. 1B). This variant was found to exhibit a low level of linkage disequilibrium (LD) with the PDB GWAS variant rs2458413 (r2 = 0.13), suggesting that the two may represent independent eQTL association signals. For the DCSTAMP gene, rs2458418 was identified as the maximally associated eQTL variant in the region, with the more common C allele associated with increased expression of the gene (P = 5.36 × 10−11, Fig. 1C). This variant was found to exhibit a moderate degree of LD with the PDB GWAS variant rs2458413 (r2 = 0.43), suggesting that the two may be part of the same eQTL association signal.
Replication in other cell/tissue types
The 2 PDB GWAS/eQTL variants identified in this study, rs4294134 and rs2458413, were queried using the publicly available GTEx Portal15 for evidence of eQTL effects in other tissues. These variants were checked against pre-calculated eQTLs from the GTEx Portal, which were identified for tissues with ≥70 samples using a q-value threshold of 0.05 corrected for multiple-testing. Supporting evidence for regulatory effects was found for rs4294134 with the gene STMP1 in 38 tissue types (P = 4.5 × 10−6 – 6.6 × 10−42) and rs2458413 with DCSTAMP in lung tissue (P = 1.1 × 10−10). For all of these significant associations, the allelic effect seen for rs4294134 and rs2458413 on gene expression was consistent with that observed in this study of osteoclasts.
The top eQTL variants identified for the DPYS and DCSTAMP genes in this study, rs12674899 and rs2458418, were also queried using the GTEx Portal for evidence of replication. No significant associations were seen between the variant rs12674899 and expression of DPYS in any of the GTEx tissues. However, the variant rs2458418 was found to be significantly associated with expression of the DCSTAMP gene in 5 tissue types (P = 2.0 × 10−5 – 6.8 × 10−45), and demonstrated the second strongest association of all variants in the region behind rs2514662, with which it is in strong LD (r2 = 0.97). For each of these tissues, the more common C allele for rs2458418 was associated with higher DCSTAMP expression, consistent with the allelic effect observed in this study.
Bioinformatics analysis
Analysis of rs4294134 using HaploReg v4.116 revealed that it is in strong LD (r2 ≥ 0.8) with 10 other variants in the region. Of these, the variant rs6467603 is a strong candidate for having a regulatory role it lies within a variety of predicted regulatory elements, including promoter histone marks (8 tissues), enhancer histone marks (15 tissues), DNase hypersensitivity sites (10 tissues) and 7 regulatory motifs. The variant rs3110823 also presents strongly from a regulatory perspective, being located in promoter histone marks (2 tissues), enhancer histone marks (13 tissues), DNase hypersensitivity sites (9 tissues), 3 regulatory motifs and a GERP (Genomic Evolutionary Rate Profiling) and SiPhy conserved region.
Analysis of rs2458413 suggested that it is in strong LD (r2 ≥ 0.8) with 6 other variants, of which rs2669448 may have a potential regulatory role. The variant is located within several predicted regulatory elements, including a promoter histone mark (1 tissue), enhancer histone marks (10 tissues), DNase hypersensitivity sites (10 tissues), and is predicted to alter 2 regulatory motifs.
Discussion
We have used our unique osteoclast-specific eQTL dataset to identify a potential regulatory role for the PDB GWAS variant rs4294134 on expression of the gene STMP1 (short transmembrane mitochondrial protein 1). This finding was strongly supported by publicly available eQTL data in multiple tissue types from the GTEx Portal15. The G allele at rs4294134, which is associated with an increased risk of PDB12, was found to be associated with increased expression of STMP1 in our osteoclast eQTL dataset, consistent with the allelic effect observed in the GTEx tissues. The fact that rs4294134 demonstrated the strongest association with expression of STMP1 of all tested variants in our dataset situated within 500 kb of the gene TSS strongly suggests that the association between this variant and PDB is mediated through regulatory effects on STMP1. The product of the STMP1 gene is a short trans-membrane protein that may have a role as a mitochondrial respiratory complex subunit17. This gene has not been extensively studied and has no obvious role in bone biology, although knockdown of the STMP1 gene in zebrafish resulted in a series of mild morphological defects, including abnormal shape of the head and jaw17. Albagha et al.12 initially suggested that the association between rs4294134 and PDB may be mediated through the gene NUP205, which encodes one of the main subunits of the nuclear pore complex involved in the regulation of transport between the cytoplasm and nucleus18. However, that was based primarily on the location of rs4294134 within NUP205 and not on functional evidence, with the authors acknowledging that no genes in the region were known to affect bone metabolism. Our data showed no evidence of a role for rs4294134 in regulating expression of NUP205 in this cell type.
We have identified an eQTL association between the PDB GWAS variant rs2458413 and expression of the gene DPYS in this study. A potentially independent eQTL association signal relevant to the DPYS gene and led by rs12674899 also was identified in the region. The DPYS gene encodes the enzyme dihydropyrimidinase, which has a role in pyrimidine metabolism. Mutations in the DPYS gene have been implicated in dihydropyrimidinase deficiency (OMIM #222748), a very rare autosomal recessive condition characterised by accumulation of dihydrouracil and dihydrothymine in the urine, blood and cerebrospinal fluid. Clinical features of this condition are highly variable and primarily include gastrointestinal abnormalities, hypotonia, seizures and neurological abnormalities19. The product of the DPYS gene has no obvious role in bone biology and it should be noted that this gene was expressed at a relatively low level in the osteoclast-like cells, suggesting that the eQTL association between rs2458413 and expression of DPYS may be more relevant in other cell types and disorders unrelated to PDB.
The PDB GWAS variant rs2458413 was also identified as significantly associated with expression of the DCSTAMP (dendrocyte expressed seven transmembrane protein) gene in this study. The PDB risk-increasing T allele at rs2458413 was found to be associated with increased expression of DCSTAMP, consistent with the allelic effect observed for this gene in lung tissue from the GTEx Portal. The product of the DCSTAMP gene is a multi-pass transmembrane protein that is considered to be a master regulator of osteoclastogenesis, as it is essential for the cell fusion that occurs between osteoclast precursors. Kukita et al.20 demonstrated that expression of the murine Dcstamp gene is upregulated in mouse osteoclast precursors when cultured in the presence of the osteoclastogenic cytokine receptor activator of nuclear factor kappa-B ligand (RANKL). They also found that knockdown of Dcstamp inhibited the formation of multinucleated mouse osteoclast-like cells, while overexpression enhanced osteoclastogenesis in the presence of RANKL. Yagi et al.21 subsequently demonstrated that Dcstamp-knockout mice display an osteopetrotic phenotype due to an absence of functional multinucleated osteoclasts. In humans, osteoclast-like cells derived from PDB patients that are carriers for a rare non-synonymous coding variant in the DCSTAMP gene (rs62620995) present with increased nuclei number compared to cells derived from healthy controls22. These cells also demonstrated increased expression of the DCSTAMP gene compared to those obtained from PDB patients that do not carry the variant22. In the study by Albagha et al.12, DCSTAMP was identified as the likely effector gene from the PDB genome-wide significant locus at 8q22.3. Our data supports this by suggesting that the PDB GWAS variant rs2458413 is exerting an effect on PDB susceptibility though regulatory effects on the DCSTAMP gene. Considering the established role of DCSTAMP in osteoclastogenesis, it seems likely that increased expression of DCSTAMP leads to enhanced osteoclast fusion, thereby contributing to the focal lesions of PDB.
There are some potential limitations to this study. One of these is that the osteoclast-like cells used in these experiments were cultured and differentiated in vitro, and therefore potentially exhibit somewhat different gene expression profiles to osteoclasts in vivo which are subject to the local bone microenvironment. Other potential limitations include the difficulties associated with using cells derived from the circulatory system to study a disease like PDB with a focal nature, as well as the fact that the eQTL effects identified in this study could be more relevant to females due to the absence of males in the study cohort. It should also be noted that the research materials in this study were not focused on individuals with PDB. However, considering the complex nature of the genetics of PDB and the fact that these PDB GWAS variants have a common MAF in the general population, we consider that our findings are relevant and representative of true contributing factors to both osteoclast biology in general and also to this disease. In the case of DCSTAMP, it is possible that increased expression of this gene has minimal effects in the general population, but in individuals at increased risk of PDB due to environmental or other genetic factors the increased expression facilitates enhanced fusion of osteoclast precursors and subsequently formation of the abnormally large, overactive osteoclasts that are characteristic of the disease.
In conclusion, we have identified regulatory effects for the PDB GWAS variants rs4294134 (STMP1) and rs2458413 (DPYS and DCSTAMP) in human osteoclast-like cells. Our data do not support a role for rs4294134 in the regulation of NUP205 in this cell type, as has been proposed previously. The eQTL associations observed for rs4294134 with STMP1, and rs2458413 with DCSTAMP were supported by publicly available eQTL data from other tissue types. The STMP1 gene has not been extensively studied to date, however the DCSTAMP gene has an established role in osteoclast differentiation and the associations seen between rs2458413 and PDB are likely mediated through regulatory effects on this gene.
Methods
Subject recruitment
Recruitment of participants and laboratory procedures has been previously described14. In brief, 158 female participants aged 30–70 were recruited for the study. These individuals had been referred for dual-energy X-ray absorptiometry (DXA) BMD scanning (Hologic, Bedford, MA, USA) at the Bone Density Unit at Sir Charles Gairdner Hospital in Western Australia. Only females were recruited for the study to control for gender-specific differences in osteoclast gene expression. Patients completed a medical history questionnaire, with a number of exclusion criteria applied including the presence of medical conditions or use of medications likely the influence the ability of their cells to differentiate into osteoclasts or resorb bone. To minimise potential issues with population stratification, only subjects with self-reported European ancestry were included in the study. A blood sample was obtained from each patient, which consisted of two 6 ml lithium heparin and one 4 ml ethylenediaminetetraacetic acid (EDTA) sample. Approval for the study was granted by the Sir Charles Gairdner and Osborne Park Health Care Group Human Research Ethics Committee and all participants provided written informed consent. The study was performed in accordance with applicable regulations and guidelines.
Isolation of peripheral blood mononuclear cells and osteoclastogenesis
The lithium heparin blood tubes obtained from each patient were used for isolation of peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation as described previously14 using well-established protocols in our laboratory23. Triplicate cultures were grown for each sample in 24-well cell culture plates, with each well seeded with 1.5 × 106 cells suspended in complete α-MEM (minimum essential medium) supplemented with 25 ng/ml macrophage colony stimulating factor (M-CSF). After 2 days, the culture medium was replaced with complete α-MEM supplemented with 25 ng/ml M-CSF and 100 ng/ml RANKL. The cells were grown in this medium formulation for another 12 days, during which time they underwent osteoclastogenesis. We have previously demonstrated that osteoclast-like cells cultured using this protocol are capable of resorbing bone in an in vitro pit assay14. Staining of the differentiated cells for tartrate resistant acid phosphatase (TRAP) was performed as an additional characterisation of the osteoclast phenotype.
Nucleic acid extraction
The QIAamp DNA Blood Mini Kit (QIAGEN) was used to extract genomic DNA from 200 μl of each EDTA blood sample. Each set of triplicate osteoclast-like cell cultures was harvested at day 14 of culture and combined into a single aliquot before genomic DNA and RNA were extracted using the AllPrep DNA/RNA Mini Kit (QIAGEN). An on-column DNase digestion step was performed for the RNA fraction of each sample to prevent DNA contamination of the RNA samples. Each RNA sample was subjected to quality assessment using the Agilent 2100 Bioanalyzer, with all samples generating RNA integrity numbers (RINs) ≥9.7.
Genotyping and imputation
Each genomic DNA sample extracted from EDTA blood was subjected to genotyping using the Illumina Infinium OmniExpress-24 BeadChip array. Pre-imputation QC criteria were applied to the genotype data, including removal of individuals and variants with a call rate <90%, removal of variants that were monomorphic, unmapped, had a MAF <5% or Hardy-Weinberg equilibrium P < 5 × 10−8. After these QC criteria had been applied, 572,898 variants remained in the genotype dataset. The Sanger Imputation Service was then used to perform genotype imputation using the Haplotype Reference Consortium (HRC) release 1.1 reference panel24. Post-imputation QC included removal of variants with an IMPUTE2 info score <0.4.
Relatedness testing and principal components analysis
Principal components analysis and identity-by-descent (relatedness) testing were performed on the genotype data using Plink v1.925. We generated 10 principal components for the study cohort, which were used to check for outliers and were included as covariates in the eQTL analysis to correct for any population stratification that may be present.
Gene expression analysis
Gene expression was quantitated in the RNA samples extracted from the osteoclast-like cells using 50 bp single-end RNA-Seq as described previously14. Trimmed mean of M-values (TMM) normalisation and correction of the data for total read count by conversion to counts per million (CPM) was performed using the edgeR package26. This software was also used to calculate reads per kilobase million (RPKM) values to allow comparison of expression levels between different genes and with other tissues.
eQTL analysis
The TMM normalised CPM values were used for the eQTL analysis, which was performed using the FastQTL software27. This software efficiently implements linear regression to identify associations between genotypes and gene expression phenotypes. A hypothesis-driven association analysis was performed for the quantile-normalised gene expression levels (generated using the rntransform function of the GenABEL package28) with imputed genotype probabilities for the 7 PDB GWAS variants. The covariates RNA-Seq batch, patient age and 10 principal components were included in the eQTL analysis. All expressed genes with a TSS located within 500 kb of a PDB GWAS variant were included in the analysis (cis-eQTLs). Each locus was corrected for multiple testing using the Benjamini-Yekutieli procedure29 with a false discovery rate (FDR) of 5%.
Bioinformatics analysis
HaploReg v4.116 and RegulomeDB30 were used to perform bioinformatics analysis of individual eQTL-variants to identify other variants in strong LD and to characterise potential regulatory effects. Analysis of LD between eQTL variants within the same locus was performed using LDlink (1000 G Phase 3 EUR population)31.
Supplementary information
Acknowledgements
This study would not have been possible without the women who kindly participated and the staff involved including bone density technologists, nursing and clerical staff. This work was supported by the Australian National Health and Medical Research Council (NHMRC) (Project Grants 1048216, 1127156), the Sir Charles Gairdner Osborne Park Health Care Group (SCGOPHCG) Research Advisory Committee (Grant 2016-17/017) and the iVEC/Pawsey Supercomputing Centre (with funding from the Australian Government and the Government of Western Australia; Project Grants: Pawsey0162 (S.G.W.), Director2025 (S.G.W.)). The salary of B.H.M. was supported by a Raine Medical Research Foundation Priming Grant.
Author Contributions
B.M., K.Z., J.W. and S.W. conceived and designed the study. B.M., K.Z. and S.M. performed the patient recruitment and cell culture. B.M., S.B. and F.D. performed the data analysis. B.M., K.Z., J.X., S.B., J.T., N.P., F.D., J.W. and S.W. contributed to the interpretation of the data. B.M. and S.W. drafted the manuscript. B.M., K.Z., J.X., S.B., S.M., J.T., N.P., F.D., J.W. and S.W. contributed to revising the content of the manuscript.
Data Availability
The osteoclast eQTL data described in this study relevant to the STMP1, DPYS and DCSTAMP genes are available online at https://research-repository.uwa.edu.au/.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37609-0.
References
- 1.Cooper C, et al. The epidemiology of Paget’s disease in Britain: is the prevalence decreasing? Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1999;14:192–197. doi: 10.1359/jbmr.1999.14.2.192. [DOI] [PubMed] [Google Scholar]
- 2.Britton C, et al. The Changing Presentation of Paget’s Disease of Bone in Australia, A High Prevalence Region. Calcified tissue international. 2017;101:564–569. doi: 10.1007/s00223-017-0312-1. [DOI] [PubMed] [Google Scholar]
- 3.Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Ralston S. Epidemiology of Paget’s disease of bone: a systematic review and meta-analysis of secular changes. Bone. 2013;55:347–352. doi: 10.1016/j.bone.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 4.van Staa TP, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17:465–471. doi: 10.1359/jbmr.2002.17.3.465. [DOI] [PubMed] [Google Scholar]
- 5.Siris ES, Ottman R, Flaster E, Kelsey JL. Familial aggregation of Paget’s disease of bone. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1991;6:495–500. doi: 10.1002/jbmr.5650060511. [DOI] [PubMed] [Google Scholar]
- 6.Visconti MR, et al. Mutations of SQSTM1 are associated with severity and clinical outcome in paget disease of bone. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25:2368–2373. doi: 10.1002/jbmr.132. [DOI] [PubMed] [Google Scholar]
- 7.Hocking LJ, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Human molecular genetics. 2002;11:2735–2739. doi: 10.1093/hmg/11.22.2735. [DOI] [PubMed] [Google Scholar]
- 8.Rea SL, Walsh JP, Layfield R, Ratajczak T, Xu J. New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of bone. Endocrine reviews. 2013;34:501–524. doi: 10.1210/er.2012-1034. [DOI] [PubMed] [Google Scholar]
- 9.Rea SL, et al. Sequestosome 1 mutations in Paget’s disease of bone in Australia: prevalence, genotype/phenotype correlation, and a novel non-UBA domain mutation (P364S) associated with increased NF-kappaB signaling without loss of ubiquitin binding. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2009;24:1216–1223. doi: 10.1359/jbmr.090214. [DOI] [PubMed] [Google Scholar]
- 10.Albagha OM. Genetics of Paget’s disease of bone. BoneKEy reports. 2015;4:756. doi: 10.1038/bonekey.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albagha OM, et al. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nature genetics. 2010;42:520–524. doi: 10.1038/ng.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albagha OM, et al. Genome-wide association identifies three new susceptibility loci for Paget’s disease of bone. Nature genetics. 2011;43:685–689. doi: 10.1038/ng.845. [DOI] [PubMed] [Google Scholar]
- 13.Grundberg E, et al. Population genomics in a disease targeted primary cell model. Genome research. 2009;19:1942–1952. doi: 10.1101/gr.095224.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullin, B. H. et al. Expression quantitative trait locus study of bone mineral density GWAS variants in human osteoclasts. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research, 10.1002/jbmr.3412 (2018). [DOI] [PubMed]
- 15.Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis multitissue gene regulation in humans. Science (New York, N.Y.) 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic acids research. 2012;40:D930–934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, et al. Functional prediction and physiological characterization of a novel short trans-membrane protein 1 as a subunit of mitochondrial respiratory complexes. Physiological genomics. 2012;44:1133–1140. doi: 10.1152/physiolgenomics.00079.2012. [DOI] [PubMed] [Google Scholar]
- 18.Grandi P, et al. Nup93, a vertebrate homologue of yeast Nic96p, forms a complex with a novel 205-kDa protein and is required for correct nuclear pore assembly. Molecular biology of the cell. 1997;8:2017–2038. doi: 10.1091/mbc.8.10.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kuilenburg AB, et al. Dihydropyrimidinase deficiency: Phenotype, genotype and structural consequences in 17 patients. Biochimica et biophysica acta. 2010;1802:639–648. doi: 10.1016/j.bbadis.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Kukita T, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. The Journal of experimental medicine. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagi M, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. The Journal of experimental medicine. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurier E, Amiable N, Gagnon E, Brown JP, Michou L. Effect of a rare genetic variant of TM7SF4 gene on osteoclasts of patients with Paget’s disease of bone. BMC medical genetics. 2017;18:133. doi: 10.1186/s12881-017-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullin BH, Mamotte C, Prince RL, Wilson SG. Influence of ARHGEF3 and RHOA knockdown on ACTA2 and other genes in osteoblasts and osteoclasts. PloS one. 2014;9:e98116. doi: 10.1371/journal.pone.0098116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy S, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nature genetics. 2016;48:1279–1283. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson MD, McCarthy DJ, Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England) 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ongen H, Buil A, Brown AA, Dermitzakis ET, Delaneau O. Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics (Oxford, England) 2016;32:1479–1485. doi: 10.1093/bioinformatics/btv722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics (Oxford, England) 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001;29:1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 30.Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome research. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics (Oxford, England) 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruim RJ, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics (Oxford, England) 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The osteoclast eQTL data described in this study relevant to the STMP1, DPYS and DCSTAMP genes are available online at https://research-repository.uwa.edu.au/.


