Abstract
Buprenorphine is currently being studied for treatment-resistant depression because of its rapid effect, relative safety, and unique pharmacodynamics. To understand the neural impact of buprenorphine in depression, we examined acute limbic and reward circuit changes during an intervention with low-dose buprenorphine augmentation pharmacotherapy. Mid and late-life adults with major depression (N = 31) who did not completely respond to an adequate trial of venlafaxine were randomized to augmentation with low-dose buprenorphine or matching placebo. We investigated early neural changes using functional magnetic resonance imaging (fMRI) from pre-randomization to 3 weeks using both an emotional reactivity task and a gambling task. We tested if: 1) there were significant neural changes acutely per intervention group, and 2) if acute neural changes were associated with depressive symptom change over 8 weeks using both the total score and the dysphoria subscale of the Montgomery Asberg Depression Rating Scale. Participants in both the buprenorphine and placebo groups showed similar changes in depressive symptoms. Neither the emotional reactivity nor gambling task resulted in significant neural activation changes from pre-randomization to 3-weeks. In both groups, increases in rostral anterior cingulate (rACC) and ventromedial prefrontal cortex (vmPFC) activation during the emotional reactivity task were associated with overall symptom improvement. In the buprenorphine but not the placebo group, increased activation in left anterior insula (aINS) and bilateral middle frontal gyrus (MFG) was associated with improvement on the dysphoria subscale. Activation changes in the reward task were not associated with buprenorphine. This is the first study to show an association between acute neural changes during emotion reactivity and changes in depression severity with buprenorphine treatment.
Keywords: Treatment-resistant depression, Geriatric, Buprenorphine, Placebo, fMRI, Clinical trial
Highlights
-
•
Adults with depression randomized to augmentation with buprenorphine or placebo.
-
•
Increased activation during emotional reactivity were associated with improvement.
-
•
Increased insula/middle frontal activation associated with dysphoria improvement.
-
•
Insula/middle frontal activation associations depended on treatment group.
-
•
Results may highlight different pathways to response that depend on treatment group.
1. Introduction
Persistent depressive symptoms in mid- and late-life contribute to poor psychosocial functioning, worsened medical comorbidities, and increased all-cause mortality and suicide (Blazer, 2003). Treatment with selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) lead to remission in less than half of patients with depression (Smagula et al., 2015; Tedeschini et al., 2011). In case of non-response, augmentation strategies are only effective in 20–44% of cases (Kaneriya et al., 2016; Lenze et al., 2015; Rush et al., 2006). Thus, there is a need for novel pharmacologic strategies for treatment-resistant depression (TRD) that are safe, well-tolerated, and have a rapid onset of clinical benefit.
In this context, low-dose buprenorphine has emerged as a promising augmentation agent. It has an established safety profile (e.g., limited addiction risk, low risk of respiratory suppression, and pharmacokinetics not influenced by renal dysfunction) (Karp et al., 2014). Our pilot data also suggest that it is well-tolerated, has a rapid clinical effect, and may be effective in mid- and late-life adults with TRD (Karp et al., 2014). These pilot data have been corroborated by a recent randomized double-blind placebo-controlled trial of buprenorphine (combined with samidorphan) in younger adults (Fava et al., 2016).
Buprenorphine is a partial agonist at the mu opiate receptor (MOR) and antagonist at the kappa opiate receptor (KOR), though this affinity is complex (Falcon et al., 2015; Robinson et al., 2017). Cortical and limbic regions rich in opioid receptors are associated with emotional dysregulation and with clinical response to antidepressant pharmacotherapy (Lutz and Kieffer, 2013). However, the specific brain circuits involved in these effects, and the mechanism of action of buprenorphine in treatment-resistant depression are still unknown. Given the complexity of the opioid receptors in buprenorphine and the overlapping distribution of different opioid receptors in the brain (Peciña et al., 2018), we decided to explore the down-stream changes in emotional processing and reward circuitry as these are two characteristically dysfunctional neural systems in major depressive disorder (Kupfer et al., 2012). Therefore, we investigated: (1) low-dose buprenorphine's effect on emotional processing, (2) its effects on neural reward circuitry, and (3) whether acute changes predicted overall changes in depression severity. Our previous work (Karim et al., 2016; Karim et al., 2018) has shown that acute neural changes are associated with treatment response and may guide early clinical decisions. Measuring acute change in brain activity may inform which neural targets should be engaged in efforts to hasten treatment response.
A recently completed multi-site intervention development trial (clinicaltrials.gov Identifier NCT02176291) will help determine clinical efficacy of antidepressant augmentation with low-dose buprenorphine. The goal for this report is focused on describing neural target engagement that may be modulated by the drug. We employed two fMRI behavioral tasks (an emotional reactivity task (Hariri et al., 2002a) and a gambling task (Delgado et al., 2000)) during an 8-week clinical trial of low-dose buprenorphine or matching placebo augmentation in mid- and late-life adults with TRD. These two tasks probe the brain activity in the limbic and reward systems, respectively (Lutz and Kieffer, 2013). We assessed whether acute activation changes in the limbic and reward systems were associated with improvement in depressive symptoms, evaluated by both the total score and the dysphoria subscale (Parker et al., 2003) from the Montgomery Asberg Depression Rating Scale (Montgomery and Asberg, 1979). We explored dysphoria as we aimed to investigate the core antidepressant effect of buprenorphine and because it has been associated with KOR/dynorphin system in animals (Land et al., 2008) and humans (Pietrzak et al., 2014). Thus, the KOR/dynorphine system is a possible mechanism underlying buprenorphine's antidepressant effect. We defined early symptom improvement, henceforth “acute,” as following 3 weeks of treatment, as there exists literature that suggests that clinical response within 2–4 weeks of treatment is predictive of remission (Andreescu et al., 2008; Joel et al., 2014). Thus, we expected a significant neural change by 3 weeks after initiation of treatment.
We expected that buprenorphine may alter limbic neural activation during the emotion reactivity task. Improvement of depressive symptoms may be associated with either an increased neural activation associated with improvement in emotion context insensitivity (Rottenberg et al., 2005) or decreased neural activation associated with lower cognitive bias toward negative stimuli (Disner et al., 2011). Because of the low-doses in this study and reduced addictive profile of low-dose buprenorphine, we did not predict differences in reward network activation between those exposed to buprenorphine and placebo (Koob and Mason, 2016; Tzschentke, 2002). However, increase in reward network activation was anticipated in those who improved clinically, irrespective of the buprenorphine or placebo groups.
2. Methods
2.1. Participants and study design
Participants were at least 50 years old and met DSM-IV criteria for major depressive disorder (MDD) with a minimum Montgomery-Åsberg Depression Rating Scale [MADRS] ≥ 15 (Montgomery and Asberg, 1979). Participants were excluded if they had a major neurocognitive disorder (e.g., dementia), psychosis, bipolar disorder, substance or alcohol use disorder, or contraindications to MRI. We also excluded those with chronic pain, because we were interested in buprenorphine's antidepressant effect without contribution from its analgesic effect. The Institutional Review Board at the University of Pittsburgh approved the study, and all participants provided written informed consent.
This study consisted of two phases and was part of a multi-site intervention development trial investigating antidepressant augmentation with buprenorphine. The data reported here are from the Pittsburgh site, which also collected fMRI data during the augmentation phase.
During the first 12-weeks, participants with depression were treated openly with venlafaxine XR (up to 300 mg/day). Non-responders were randomized under double blind conditions to augmentation with either sublingual buprenorphine or matching placebo (2:1 allocation) for an additional 8 weeks. Buprenorphine or placebo was started at 0.2 mg/day and increased by 0.2 mg/day each week based on depression severity and tolerability up to a maximum of 1.2 mg/day. These dosages were based on previous open-label trials using buprenorphine for treatment resistant depression in younger (0.15–1.8 mg/day) and older adults (0.2–1.6 mg/day) (Bodkin et al., 1995; Karp et al., 2014). Participants had two fMRI scans: pre-randomization and after three weeks of exposure to buprenorphine/placebo, to explore the association between acute changes in activation and 8-week treatment response (Kay et al., 2016).
2.2. Assessments
The MADRS was administered weekly by raters blind to treatment assignment. Response was defined as a MADRS ≤10 for two consecutive weeks. We chose this definition of response (instead of 50% improvement from baseline) to include individuals in phase 2 who may have responded to venlafaxine, but were still quite symptomatic. To detect a depression-specific effect, all analyses also used the five-item dysphoria subscale of the MADRS comprising apparent sadness, reported sadness, lassitude, reduced concentration, and inability to feel (Parker et al., 2003).
Side effects were assessed weekly with the self-reported Antidepressant Side-Effect Checklist (ASEC) (Uher et al., 2009). Euphoria was assessed with a 0–10 visual analog scale (Krystal et al., 2005) to identify a euphoric effect that may promote addictive behavior (Dackis and O'Brien, 2005).
2.3. Emotional reactivity: face/shapes task (Hariri et al., 2000)
During the emotional reactivity task participants either matched faces or shapes. They were instructed to respond with an MR-compatible glove (left or right index finger) by identifying one of two images in the lower row that matches a “cue image” presented in the top row. Only negative emotional faces (angry or fearful) were shown; shapes were geometric forms. There were five shape-matching blocks (24 s) interleaved with four face-matching blocks. For the faces block, six images (balanced by sex and emotion [angry or fearful]) were presented. Before each block, participants were visually instructed to either “match emotion” or “match form” (2 s). Through this implicit emotional reactivity manipulation, the amygdala, anterior insula (aINS), rostral cingulate, and other associated areas are activated (Fitzgerald et al., 2008; Hariri et al., 2000), which may serve as a proxy of brain changes in limbic circuitry during early treatment. Faces were presented from a set of 12 different images and are derived from a standard set of pictures of facial affect. We used E-Prime to present stimuli and record responses (Psychology Software Tools, Inc., Pittsburgh, PA. 2002). While this task does not have happy or neutral faces, the goal was to understand limbic reactivity (amygdala activation), as well as the regulatory components involved, which has been demonstrated best via angry and fearful faces (Hariri et al., 2002b). Notably, happy faces do not elicit a similar limbic response and those with depression can subjectively perceive neutral faces as more negative – thus shapes instead of neutral faces are a good visual and motor control.
2.4. Reward: gambling task
This task (Delgado et al., 2000) activates striatal and other reward-related brain regions (Delgado et al., 2003; Wagner et al., 2007). In this card-guessing game, a question mark appears on the screen as a cue. Participants guess whether the number of the hidden card is greater or lower than 5. Then the actual number is revealed, followed by an upward green, red, or yellow arrow for correct, incorrect, or tie guesses. Participants are told they will receive $1.00 on a win trial and lose $0.50 on a loss trial and that the hidden numbers are generated randomly from 1 to 9. Classic decision-making literature suggests that the impact of negative outcomes, such as losses, is larger than that of positive outcomes, such as gains; thus similar to past studies we have chosen a 2:1 win to loss ratio (Tversky and Kahneman, 1981). In reality, the hidden numbers are picked by the computer based on the number chosen by the participant to fulfill a pre-determined sequence of win, loss, or neutral trials. A total of two blocks were run (38 s), with each consisting of 9 trials (3 of each trial). By comparing the activation for buprenorphine or placebo, this task probes the reward effect of buprenorphine (e.g., abuse potential).
2.5. Image acquisition
All scanning was done on a 3 Tesla Trio TIM MRI scanner (Siemens, Berlin, Germany) using a 12-channel custom head coil located at the MR Research Center at the University of Pittsburgh. Magnetization-prepared rapid gradient-echo (MPRAGE), fluid attenuated inversion recovery (FLAIR), and T2*-weighted blood‑oxygen level dependent (BOLD) sequences were collected during both tasks. Detailed parameters are described in the Supplementary Methods section (Image Acquisition). Participants also had a resting state BOLD scan, however this was not analyzed but will be given special attention in future work.
2.6. Image preprocessing
Image preprocessing was conducted via Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) as detailed in the Supplementary Methods section (Image Preprocessing). Briefly, functional scans were motion corrected, normalized to a standard anatomical space (using the structural scans), and then smoothed. FLAIR scans were used to automatically extract white matter hyperintensity (WMH) burden.
2.7. Modeling task activation
For both tasks, we performed a mass-univariate regression that modeled the mean of the voxel-wise time series, the six motion parameters, and the onset and duration of the task (faces and shapes for the emotion reactivity task; win, loss, and neutral for the reward task) convolved with the canonical hemodynamic response function. The model used a series of temporal cosines to model low frequency noise (1/128 Hz) and an autoregressive [AR(1)] filter was used to model aliased biorhythms and unmodelled activity/noise. The primary contrast for the emotional reactivity task was emotional faces minus shapes; while for the reward task the contrast was reward minus loss (other possible contrasts, including reward minus neutral and loss minus neutral, were reported in the Supplement).
2.8. Biochemistry analytical methods
Buprenorphine and metabolite (Brown et al., 2011) concentrations were determined in venous plasma collected at the time of the second MRI. Three of the metabolites (norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide) (Brown et al., 2011) are biologically active with equal or higher plasma levels than the parent drug (Lutfy and Cowan, 2004). Thus, we measured the plasma level of buprenorphine and these three metabolites. Plasma was stored at −80 °C until analyzed. Concentrations of buprenorphine and the major metabolites were determined by liquid chromatography-mass spectrometry as described previously (Regina and Kharasch, 2013), except that an ABSciex 6500 mass spectrometer was used.
2.9. Statistical analysis
Demographic and psychometric data were analyzed using the Statistical Package for the Social Sciences version 19.0 (SPSS19.0). All data were expressed as mean and standard deviations (SD), and the significance level was set at 0.05. Treatment assignment had been unblinded at the time of statistical analysis.
We performed voxel-wise statistical analyses using statistical non-parametric mapping (SnPM13; http://warwick.ac.uk/snpm) (Nichols and Holmes, 2002) with permutation testing (10,000 permutations per analysis). To adjust for multiple comparisons, we controlled the cluster-wise (uncorrected cluster forming threshold at p < .001) family-wise error (FWE) rate at 0.05. Due to the limited sample sizes and limited approaches available voxel-wise, we have used a difference-based analysis rather than other statistical approaches (e.g., mixed-effects modeling – which would have required a significantly greater sample size and number of observations).
We now describe the analyses conducted for both tasks separately. After conducting an independent samples t-test on baseline imaging comparing the two groups (i.e., buprenorphine and placebo) to verify that there were no differences in activation at baseline, we created a difference image for each participant by subtracting the pre-treatment contrast map from the week 3 (acute post-treatment) contrast map. We performed an independent samples t-test on this difference image to examine changes in activation between the two treatment groups (buprenorphine and placebo).
We also conducted a set of analyses with respect to associations between changes in depression severity and neural activation. Considering the sample size, we did not expect that there would be significant changes in activation, however these associations would be an important step to determining the neural impact of buprenorphine and placebo. We examined the association between early changes (at three weeks compared to pre-treatment) in activation and changes in depression during the 8-week treatment trial, and its interaction with treatment group (i.e., to examine whether early neural changes predicted changes in depression severity and if this was dependent on treatment group). Relative changes in depression severity were calculated for both the total MADRS or its dysphoria subscale based on the score at week 8 minus baseline score, divided by baseline score. Since we had defined response as a 50% improvement in symptoms (see Assessments section), we decided to use a ratio rather than the difference as this was more clinically meaningful whereas the raw difference does not indicate whether this was a meaningful change (e.g., a decrease in raw score of 15 may seem like a lot of improvement, but given a pre-treatment score of 40 – this would not be considered a strong response).
As a post-hoc analysis, we also extracted the activation (measured as the value of the contrast in those regions) within the significant clusters, to assess their association with the plasma levels of buprenorphine and its metabolites. Finally, in additional exploratory analyses, we investigated the association between baseline activation and baseline symptom severity or end-of-trial symptom improvement (supplement Table S3 and Figs. S2–3). Similarly, we investigated the association between euphoria and activation during the gambling task.
3. Results
In the buprenorphine group, 3 participants exited the trial prematurely and only had baseline fMRI scans (due to non-compliance with the protocol, withdrawal of consent, and death from pre-existing cardiac disease unrelated to study participation); one was excluded due to corrupted imaging data; and one exited after only 6 weeks (due to high depression severity) but the data were included in the analysis carrying forward the last depression score. Thus, 16 participants randomized to buprenorphine and 11 randomized to placebo that completed two fMRIs are included in the analysis (Supplemental Fig. S1). These 27 participants did not significantly differ from the three who exited the study in terms of age, sex, or baseline depression severity.
3.1. Clinical measures
In this multi-site treatment development project, the clinical data from the Pittsburgh site indicated no differences between the buprenorphine and placebo groups for any of the demographic (age, sex, ethnicity, education), baseline depression scores, or other clinical characteristics (Supplemental Table S1). The final mean (SD) buprenorphine dosage was 0.5 (0.2) mg/day. All participants showed some improvement in depression severity during the trial. There was no significant group (placebo vs. buprenorphine) difference in: improvement of depressive symptoms (with either the total MADRS or dysphoria subscale); side effects (ASEC); or euphoria (visual analog scale) (Supplemental Table S2). We further confirmed no significant differences between the two groups by performing a mixed-ANOVA using weekly MADRS, where we found that there was no significant interaction between group and time [F(8,168) = 0.44, p = .898], no significant group differences [F(1,21) = 0.62, p = .439], but there was a significant decrease in MADRS across time independent of group [F(8,168) = 3.46, p < .005].
3.2. Emotional reactivity task: face/shapes
We found no group differences in activation at baseline or in the change in activation but found that there existed significant associations between neural changes and symptom changes. Specifically, we found that independent of group, an increase in rostral anterior cingulate gyrus (rACC)/ventral medial prefrontal gyrus (vmPFC) activation was associated with overall improvement on both total MADRS and dysphoria subscale (Table 1 and Fig. 1a/b). In the buprenorphine, but not the placebo group, an increase in left anterior insula (aINS) and bilateral middle frontal gyrus (MFG) activation was associated with improvement on the dysphoria subscale (Table 1 and Fig. 2). Changes in these regions were not associated with levels of BPN or its metabolites (Table 2).
Table 1.
Association between changes in fMRI brain activity after three weeks and depressive symptom improvement.
| Region |
BA |
MNI coordinates |
Cluster size⁎ | Peak t |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Emotional reactivity task | ||||||
| MADRS total score in all participants | ||||||
| rACC/vmPFC | 32,24 | −2 | 32 | 6 | 588 | 4.9 |
| Dysphoria subscale in all participants | ||||||
| rACC/vmPFC | 32,10,24 | 26 | 42 | −2 | 2259 | 5.5 |
| Left MFG/DLPFC | 10,9,46 | −38 | 42 | 10 | 1651 | 6.0 |
| Dysphoria subscale in buprenorphine group | ||||||
| Left aINS, MFG | 10 | −36 | 46 | 8 | 552 | 5.3 |
| Right MFG | 10 | 22 | 50 | 6 | 208 | 4.7 |
| Gambling task | ||||||
| MADRS total score in all participants | ||||||
| Left cuneus | 19 | −6 | −88 | 28 | 239 | −5.0 |
rACC, rostral anterior cingulate gyrus; vmPFC, ventromedial prefrontal gyrus; MFG, middle frontal gyrus; DLPFC, dorsolateral prefrontal gyrus; BA, Brodmann area.
SnPM correction (p-uncorrected cluster forming threshold at 0.001) with FWE p-value at 0.05.
Fig. 1.
Three-week increase in rACC/vmPFC activity in emotional reactivity task was associated with end-of-trial symptom improvement across all subjects. 2a. Association with MADRS improvement. 2b. Association with dysphoria improvement.
Fig. 2.
Three-week increase in left anterior insula and bilateral middle frontal gyrus activity in emotional reactivity task was associated with end-of-trial dysphoria improvement in buprenorphine, not in placebo. Note: Even after excluding the two participants in the BPN group that may seem like outliers (far two most points), the association still holds [r(13) = 0.84].
Table 2.
The correlation of three-week drug level, three-week brain activity increase in fMRI task, end-of-trial symptom improvement, and within different metabolites.
| BPN | norBPN | B3G | N3G | |
|---|---|---|---|---|
| Pearson correlation (p-value) | ||||
| Brain activity within the cluster from regression analysis | ||||
| aINS/bilateral MFC in face/shapes task | 0.007(0.981) | 0.094(0.739) | 0.131(0.642) | 0.509(0.053) |
| ACC/vmPFC in face/shapes task (with MADRS) | −0.052(0.853) | 0.042(0.882) | 0.053(0.852) | 0.425(0.114) |
| ACC/vmPFC in face/shapes task (with dysphoria) | −0.090(0.749) | −0.114(0.687) | 0.094(0.739) | 0.305(0.269) |
| Occipital cluster in gambling task | 0.043(0.878) | 0.322(0.242) | 0.029(0.917) | −0.289(0.296) |
| Symptom improvement | ||||
| MADRS (%) | 0.107(0.704) | −0.153(0.586) | 0.357(0.191) | 0.381(0.162) |
| dysphoria subscale (%) | 0.166(0.553) | 0.031(0.913) | 0.18(0.180) | 0.442(0.099) |
| Inter-metabolite association | ||||
| Buprenorphine (BPN) | – | 0.694(0.004) | 0.734(0.002) | 0.456(0.087) |
| Norbuprenorphine (norBPN) | – | 0.460(0.085) | 0.45(0.092) | |
| Buprenorphine-3-Glucuronide (B3G) | – | 0.651(0.009) | ||
| Norbuprenorphine-3-Glucuronide (N3G) | – | |||
Bold numbers represent p < .05; italicized numbers denote p < .1.
3.3. Gambling task
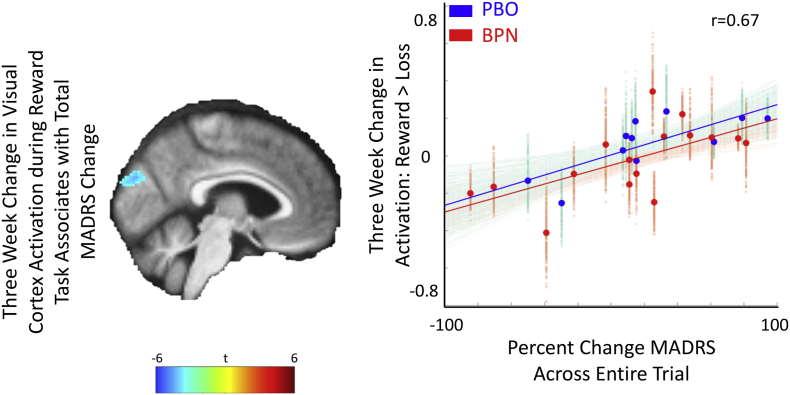
We found no group differences in activation at baseline or in the change in activation, but again found significant associations between neural and symptom changes. An acute increase in visual cortex activation was associated with improvement in total MADRS (Table 1 and Fig. 3). However, no group specific associations were detected, even under different contrasts, including reward minus neutral and loss minus neutral (supplemental Fig. S4). Changes in these regions were not associated with levels of BPN or its metabolites (Table 2).
Fig. 3.
Three-week decrease in visual cortex activity in gambling task was associated with MADRS improvement across all subjects.
4. Discussion
We identified associations between increased emotion reactivity rACC/vmPFC activation and improvement of depressive symptoms independent of treatment (placebo or buprenorphine) and also between increased emotion reactivity left aINS and bilateral MFG activation and improvement of dysphoria in the buprenorphine but not placebo groups. Given the preliminary nature of these results, it is important to replicate this study and validate these results especially given the modest sample size and failure to identify significant changes in neural activation. However, given that this is one of the first studies on buprenorphine in late-life depression, we propose that these results may be related to some existing literature and provide a more general discussion to help identify future work.
During the emotional reactivity task, we observed that an increase in rACC/vmPFC activation was associated with an improvement in depressive symptoms, regardless of the intervention. The increase in rACC/vmPFC activation may reflect top-down control of limbic systems by rACC (Etkin et al., 2006) and vmPFC (Myers-Schulz and Koenigs, 2012). Our finding in the rACC is consistent with the previously reported association in a PET study between increased glucose metabolism of rACC and response to both fluoxetine and placebo (Mayberg et al., 2002). Similarly, rACC activity has been associated with placebo analgesia (Bingel et al., 2006; Petrovic et al., 2002), and modulation of endogenous μ-opioid receptors has been linked to placebo response in pain and antidepressant treatment (Peciña et al., 2015; Zubieta et al., 2005). Within the ACC, both MOR binding (Peciña et al., 2015) and its resting-state functional connectivity (Sikora et al., 2016) correlated with placebo response and predicted subsequent antidepressant response. Since the ACC is densely populated with both MOR and KOR (Henriksen and Willoch, 2008), it is possible that buprenorphine and placebo modulate the opioid system in rACC to improve depressive symptoms.
Increased activation in left aINS and bilateral MFG was associated with improvement on the dysphoria subscale for the buprenorphine but not the placebo group. Both aINS and MFG are associated with negative facial emotional processing (Fusar-Poli et al., 2009), and activation of the aINS has been implicated in the response to antidepressant treatments (Davidson et al., 2003; Langenecker et al., 2007; McGrath et al., 2013). Consistent with our findings, Davidson et al. reported increasing left aINS activation during a negative affect processing fMRI task with successful venlafaxine treatment (Davidson et al., 2003). The aINS functions as a hub in sensing or prioritizing emotional and cognitive salience (Craig, 2002), and successful emotional regulation presumably requires improvement in awareness and differentiation of one's own emotions (Barrett et al., 2001). Thus, our results suggest that the combined effect from the heightened emotional awareness and greater implicit emotional regulation among participants exposed to low-dose buprenorphine (Gyurak et al., 2011; Ochsner and Gross, 2005; Phillips et al., 2008) may be associated with improvement in depression.
It is of interest in our study that the MADRS dysphoria subscale was more sensitive than the overall MADRS scale in suggesting an antidepressant effect of low-dose buprenorphine augmentation. This is supported by a recent PET study using a KOR-selective ligand in trauma patients (Pietrzak et al., 2014). In that study, it was observed that activating the dynorphine/KOR system in the aINS induced dysphoria. Given that we did not find significant changes in activation, but rather associations between changes in activation and changes in depression/dysphoria severity, it is critical to replicate this exploratory finding to validate these results. Ultimately, we were limited by the number of scans at baseline and 3 weeks after beginning treatment, as it is likely that similar neural engagement could be detected in post-treatment with an association beyond the dysphoria subscale as it could take a longer duration for other items in MADRS to improve during depression treatment (Alonzo et al., 2013; Tominaga et al., 2011; Wade and Friis Andersen, 2006).
Although there existed an association between the early increase in cuneus activation during the reward task and symptom improvement across all participants, no expected brain areas involved in reward activation, including ventral striatum, caudate, or insula (Delgado, 2007), were found to be related to symptom improvement. If this lack of greater engagement of the reward system with low-dose buprenorphine compared to placebo is replicated, it may support limited activation of neural reward circuits. However considering the modest sample size, it is unclear if this is just an effect size that we were unable to detect.
We acknowledge that the small sample size does not permit meaningful inferences about clinical effect. We observed neither significant differences between placebo and buprenorphine clinically nor significant neural changes following three weeks of treatment. We did observe that acute changes in activation was associated with changes in depression severity for individuals who received buprenorphine but not placebo. These preliminary associations help improve our understanding of the neural mechanisms of buprenorphine and placebo treatment and requires further investigation. It should be noted however, that only a limited set of regions were associated with buprenorphine specific changes in depression severity. The non-significance of the association in the placebo group may also have to do with the sample size, however the effect size was small (r = −0.16). In addition to the modest sample size, the low dose of buprenorphine may also have reduced our power to detect significant changes or associations. Finally, our analyses did not allow for causative inference; thus, all results are correlational in nature and do not imply causation. Future studies should include multiple assessment points throughout the study to sample fMRI and clinical measures to understand the causative nature of these changes. Further validation of these findings is needed in a sample that includes both clinically significant differences in depression severity and neural activation.
Although speculative, it is possible that we would have observed greater separation from placebo for both antidepressant clinical effect and neural activation with a higher dosage of buprenorphine. For example, in a recent placebo controlled study in which the active treatment was superior to placebo, buprenorphine (combined with samidorphan, a potent MOR antagonist) was prescribed at 2–4 mg/day (Fava et al., 2016). While this is the first study investigating the engagement of depression-relevant neural targets, higher dosages should be considered in future translational studies of buprenorphine.
5. Conclusion
This study found that acute changes in neural activation were associated with changes in symptom improvement in dysphoria in mid- and lafe-life adults with TRD treated with low-dose buprenorphine augmentation. Changes in specific neural activation in the buprenorphine but not the placebo group predicted symptom improvement at 8 weeks. Specifically, this associated increase in left anterior insula and bilateral middle frontal gyrus activation during an emotional reactivity task may explain a unique mechanism of augmentation with this opioid agent. Task-based fMRI may provide a sensitive tool to optimize the dosage of buprenorphine in future studies, balancing the trade-off between clinical efficacy and abuse risk. Future studies are needed to confirm the specific changes we observed in neural activation and to guide dose-finding strategies.
Conflicts of interest
JFK received medication supplies from Indivior to support this investigator initiated trial. He has also received medication supplies from Pfizer for investigator initiated work. JFK receives research funding from NIH and Patient-Centered Outcomes Research Institute and compensation for service on the editorial board of the American Journal of Geriatric Psychiatry and Journal of Clinical Psychiatry. EJL reports research funding (current/past) from Janssen, Alkermes, Acadia, Takeda, Lundbeck,Barnes Jewish Foundation, Patient-Centered Outcomes Research Institute, and Taylor Family Institute for Innovative Psychiatric Research. He receives medication supplies for an investigator-initiated trial from Indivior. BHM currently receives research funding from Brain Canada, the CAMH Foundation, the Canadian Institutes of Health Research, Patient-Centered Outcomes Research Institute, and the US National Institute of Health (NIH). During the last five years, he also received research support from Bristol-Myers Squibb (medications for a NIH-funded clinical trial), Eli-Lilly (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial). He directly own stocks of General Electric (less than $5000). DMB has received research support from the Canadian Institutes of Health Research (CIHR), National Institute of Health (NIH), Brain Canada and the Temerty Family through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Research Institute. He receives research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He also receives in-kind equipment support from Magventure for an investigator-initiated study. He receives medication supplies for an investigator-initiated trial from Indivior. CFR has received research support from the NIH, the Patient Centered Outcomes Research Institute, the Center for Medicare and Medicaid Services, the American Foundation for Suicide Prevention, the Brain and Behavior Research Foundation, and the Commonwealth of Pennsylvania. BristolMeyerSquib and Pfizer have provided pharmaceutical supplies for his NIH sponsored research.
Acknowledgements
This work was funded by R34 MH101371, P30 MH90333, R01 DA025931, and T32 MH019986. The authors thank Sunita Chickering, MA, for providing coordination and clinical management of the subjects, and Alicia Flaker, for analysis of plasma drug and metabolite concentrations. The authors are grateful to the participants and their families, without whom this work could not have been completed, and for whom we will continue to seek methods to ease and prevent depression across the lifespan.
This study was supported in kind by an unrestricted, unsolicited investigator initiated grant (support in the form of medication supplies only) from Indivior Inc. who had no role in study design; collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication, but did review the report for scientific accuracy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101679.
Appendix A. Supplementary data
Supplementary material
References
- Alonzo A., Chan G., Martin D., Mitchell P.B., Loo C. Transcranial direct current stimulation (tDCS) for depression: analysis of response using a three-factor structure of the Montgomery–Åsberg depression rating scale. J. Affect. Disord. 2013;150:91–95. doi: 10.1016/j.jad.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Andreescu C., Mulsant B.H., Houck P.R., Whyte E.M., Mazumdar S., Dombrovski A.Y., Pollock B.G., Reynolds C.F., III Empirically derived decision trees for the treatment of late-life depression. Am. J. Psychiatry. 2008;165:855–862. doi: 10.1176/appi.ajp.2008.07081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Gross J., Christensen T.C., Benvenuto M. Knowing what you're feeling and knowing what to do about it: mapping the relation between emotion differentiation and emotion regulation. Cognit. Emot. 2001;15:713–724. [Google Scholar]
- Bingel U., Lorenz J., Schoell E., Weiller C., Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Blazer D.G. Depression in late life: review and commentary. J. Gerontol. Ser. A Biol. Med. Sci. 2003;58:M249–M265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Bodkin J.A., Zornberg G.L., Lukas S.E., Cole J.O. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Brown S.M., Holtzman M., Kim T., Kharasch E.D. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115:1251–1260. doi: 10.1097/ALN.0b013e318238fea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dackis C., O'Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat. Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W., Anderle M.J., Kalin N.H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am. J. Psychiatr. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Delgado M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado M., Locke H., Stenger V., Fiez J. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn. Affect. Behav. Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12:467. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Falcon E., Maier K., Robinson S.A., Hill-Smith T.E., Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2015;232:907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M., Memisoglu A., Thase M.E., Bodkin J.A., Trivedi M.H., De Somer M., Du Y., Leigh-Pemberton R., DiPetrillo L., Silverman B. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo-controlled trial. Am. J. Psychiatr. 2016;173:499–508. doi: 10.1176/appi.ajp.2015.15070921. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Laird A.R., Maller J., Daskalakis Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Abbamonte M. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34:418. [PMC free article] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognit. Emot. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Bookheimer S.Y., Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D., Egan M.F., Weinberger D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Henriksen G., Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008;131:1171–1196. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel I., Begley A.E., Mulsant B.H., Lenze E.J., Mazumdar S., Dew M.A., Blumberger D., Butters M., Reynolds C.F., 3rd, Team I.G.I. Dynamic prediction of treatment response in late-life depression. Am. J. Geriatr. Psychiatry. 2014;22:167–176. doi: 10.1016/j.jagp.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneriya S.H., Robbins-Welty G.A., Smagula S.F. Predictors and moderators of remission with aripiprazole augmentation in treatment-resistant late-life depression: an analysis of the irl-grey randomized clinical trial. JAMA Psychiatry. 2016;73:329–336. doi: 10.1001/jamapsychiatry.2015.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim H.T., Andreescu C., Tudorascu D., Smagula S.F., Butters M.A., Karp J.F., Reynolds C., Aizenstein H.J. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol. Psychiatry. 2016;22(3):450–457. doi: 10.1038/mp.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim H.T., Wang M., Andreescu C., Tudorascu D., Butters M.A., Karp J.F., Reynolds C.F., 3rd, Aizenstein H.J. Acute trajectories of neural activation predict remission to pharmacotherapy in late-life depression. Neuroimage Clin. 2018;19:831–839. doi: 10.1016/j.nicl.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp J.F., Butters M.A., Begley A.E., Miller M.D., Lenze E.J., Blumberger D.M., Mulsant B.H., Reynolds C.F., III Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J. Clin. Psychiatry. 2014;75:785–793. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D.B., Karim H.T., Soehner A.M., Hasler B.P., Wilckens K.A., James J.A., Aizenstein H.J., Price J.C., Rosario B.L., Kupfer D.J. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep. 2016;39:1779. doi: 10.5665/sleep.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Mason B.J. Existing and future drugs for the treatment of the dark side of addiction. Annu. Rev. Pharmacol. Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Perry E.B., Gueorguieva R., Belger A., Madonick S.H., Abi-Dargham A., Cooper T.B., MacDougall L., Abi-Saab W., D'Souza D.C. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch. Gen. Psychiatry. 2005;62:985–995. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Phillips M.L. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet (London, England) 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B.B., Bruchas M.R., Lemos J.C., Xu M., Melief E.J., Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker S.A., Kennedy S.E., Guidotti L.M., Briceno E.M., Own L.S., Hooven T., Young E.A., Akil H., Noll D.C., Zubieta J.-K. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol. Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze E.J., Mulsant B.H., Blumberger D.M., Karp J.F., Newcomer J.W., Anderson S.J., Dew M.A., Butters M.A., Stack J.A., Begley A.E., Reynolds C.F., III Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–2412. doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K., Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr. Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P.-E., Kieffer B.L. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H.S., Silva J.A., Brannan S.K., Tekell J.L., Mahurin R.K., McGinnis S., Jerabek P.A. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- McGrath C.L., Kelley M.E., Holtzheimer P.E., III Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B., Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol. Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parker R., Flint E.P., Bosworth H.B., Pieper C.F., Steffens D.C. A three-factor analytic model of the MADRS in geriatric depression. Int. J. Geriatr. Psychiatr. 2003;18:73–77. doi: 10.1002/gps.776. [DOI] [PubMed] [Google Scholar]
- Peciña M., Bohnert A.S., Sikora M., Avery E.T., Langenecker S.A., Mickey B.J., Zubieta J.-K. Association between placebo-activated neural systems and antidepressant responses: neurochemistry of placebo effects in major depression. JAMA Psychiatry. 2015;72:1087–1094. doi: 10.1001/jamapsychiatry.2015.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña M., Karp J.F., Mathew S., Todtenkopf M.S., Ehrich E.W., Zubieta J.-K. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P., Kalso E., Petersson K.M., Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Phillips M., Ladouceur C., Drevets13 W. Neural systems underlying voluntary and automatic emotion regulation: toward a neural model of bipolar disorder. Mol. Psychiatry. 2008;13(9):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak R.H., Naganawa M., Huang Y., Corsi-Travali S., Zheng M.-Q., Stein M.B., Henry S., Lim K., Ropchan J., Lin S.-f. Association of in vivo κ-opioid receptor availability and the transdiagnostic dimensional expression of trauma-related psychopathology. JAMA Psychiatry. 2014;71:1262–1270. doi: 10.1001/jamapsychiatry.2014.1221. [DOI] [PubMed] [Google Scholar]
- Regina K.J., Kharasch E.D. High-sensitivity analysis of buprenorphine, norbuprenorphine, buprenorphine glucuronide, and norbuprenorphine glucuronide in plasma and urine by liquid chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013;939:23–31. doi: 10.1016/j.jchromb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.A., Erickson R.L., Browne C.A., Lucki I. A role for the mu opioid receptor in the antidepressant effects of buprenorphine. Behav. Brain Res. 2017;319:96–103. doi: 10.1016/j.bbr.2016.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J., Gross J.J., Gotlib I.H. Emotion context insensitivity in major depressive disorder. J. Abnorm. Psychol. 2005;114:627. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am. J. Psychiatr. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sikora M., Heffernan J., Avery E.T., Mickey B.J., Zubieta J.-K., Peciña M. Salience network functional connectivity predicts placebo effects in major depression. Biol. Psychiatry. 2016;1:68–76. doi: 10.1016/j.bpsc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula S.F., Butters M.A., Anderson S.J. Antidepressant response trajectories and associated clinical prognostic factors among older adults. JAMA Psychiatry. 2015;72:1021–1028. doi: 10.1001/jamapsychiatry.2015.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschini E., Levkovitz Y., Iovieno N., Ameral V.E., Nelson J.C., Papakostas G.I. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J. Clin. Psychiatry. 2011;72:1660–1668. doi: 10.4088/JCP.10r06531. [DOI] [PubMed] [Google Scholar]
- Tominaga K., Okazaki M., Higuchi H., Utagawa I., Nakamura E., Yamaguchi N. Symptom predictors of response to electroconvulsive therapy in older patients with treatment-resistant depression. Int. J. Gen. Med. 2011;4:515–519. doi: 10.2147/IJGM.S21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Tzschentke T.M. Behavioral pharmacology of buprenorphine, with a focus on preclinical models of reward and addiction. Psychopharmacology. 2002;161:1–16. doi: 10.1007/s00213-002-1003-8. [DOI] [PubMed] [Google Scholar]
- Uher R., Farmer A., Henigsberg N., Rietschel M., Mors O., Maier W., Kozel D., Hauser J., Souery D., Placentino A. Adverse reactions to antidepressants. Br. J. Psychiatry. 2009;195:202–210. doi: 10.1192/bjp.bp.108.061960. [DOI] [PubMed] [Google Scholar]
- Wade A., Friis Andersen H. The onset of effect for escitalopram and its relevance for the clinical management of depression. Curr. Med. Res. Opin. 2006;22:2101–2110. doi: 10.1185/030079906X148319. [DOI] [PubMed] [Google Scholar]
- Wagner A., Aizenstein H., Venkatraman V.K., Fudge J., May J.C., Mazurkewicz L., Frank G.K., Bailer U.F., Fischer L., Nguyen V. Altered reward processing in women recovered from anorexia nervosa. Am. J. Psychiatr. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Zubieta J.-K., Bueller J.A., Jackson L.R., Scott D.J., Xu Y., Koeppe R.A., Nichols T.E., Stohler C.S. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J. Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material