Abstract
The regenerative and immunomodulatory characteristics of mesenchymal stem cells (MSCs) make them attractive in the treatment of many diseases. Although they have shown promising preclinical studies of immunomodulation and paracrine effects in inflammatory airway disorders and other lung diseases, there are still challenges that have to be overcome before MSCs can be safely, effectively, and routinely applied in the clinical setting. A good understanding of the roles and mechanisms of the MSC immunomodulatory effects will benefit the application of MSC-based clinical therapy. In this review, we summarize the promises and challenges of the preclinical and clinical trials of MSC therapies, aiming to better understand the role that MSCs play in attempt to treat inflammatory airway disorders.
Keywords: inflammatory airway disorders, MSC therapy mechanisms, mesenchymal stem cells, promise and challenge
Introduction
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a variety of cell types, including bone, fat cells, muscle, cartilage, and connective tissue. MSCs can readily be obtained from adult tissues such as umbilical cord, bone marrow, and fat tissue [1,2]. In addition, MSCs derived from pluripotent stem cells like induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) closely resemble conventional bone marrow or adipose‐tissue derived MSC in terms of both phenotype and function [3–5]. This alternative means of MSC generation could, in theory, provide an indefinite supply of MSCs with well‐defined phenotype and functions. MSCs are self-renewable, easily accessible, and culturally expandable with exceptional genomic stability and only a few ethical issues, which make the cells good candidates for cell therapy, regenerative medicine, and tissue repair [2]. MSCs have been proven to possess anti-inflammatory capacity by secreting various cytokines/chemokines, which suppress T-cell-mediated immune responses [6–8]. Due to their regeneration and anti-inflammatory capacities, MSCs are attractive therapeutic cells for many diseases, such as bone and immune diseases. Here, we review the role of MSCs in the treatment of inflammatory airway disorders, mostly focused on promises and challenges of the preclinical and clinical trials of MSC therapies, aiming to better understand the role that MSCs play in the inflammatory airway disorders.
Status of MSC therapies in airway inflammation
The immunomodulatory properties of MSCs make them a promising candidate for the treatment of inflammatory airway disorders. A growing number of preclinical studies on immunomodulation and paracrine effects of MSCs are being performed to provide evidence of safety and efficacy in animal models of asthma, allergic rhinitis (AR), bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), and other lung diseases [9]. More and more clinical trials are ongoing to examine the MSC therapy in airway inflammation. However, MSC therapies for airway inflammation are still in its infacy.
Allergic airway inflammation
Allergic airway inflammation including asthma and AR is an abnormally exacerbated reaction toward common environmental factors, such as pollen grains, dust mites, or animal dander. The airway inflammation is characterized by wheezing, reversible airway obstruction, airway hyper-responsiveness (AHR), infiltration of eosinophils and type 2 T cells into the airway submucosa, mucus hypersecretion, and airway remodeling [10,11]. Since the discovery of T-helper (Th)1 and Th2 cells in the cluster of differentiation (CD)4+ T-cell subpopulations, it became evident rather quickly that Th2 cells play the crucial role in the development of allergic inflammatory disorders by releasing their specific cytokines interleukin (IL)-4, IL-5, and IL-13. Evidence suggest that a Th type 1/2 imbalance with a Th2-dominant response to inhaled allergens plays a critical role in the pathogenesis of allergic airway inflammation and identified the Th2 cytokines as key effector molecules in this process [12–14]. This concept is well accepted today and has led to the emergence of novel therapeutic approaches interfering with mechanisms of Th2 activation that have entered clinics or showed promising results in clinical studies [15,16]. However, a variety of cell types, including airway epithelial cells, Th2 cells, eosinophils, basophils, mast cells, and dendritic cells (DCs), have been identified that play specific roles in the pathogenesis of allergic airway inflammation.
The airway epithelial cells act as a barrier and provide protection to the lung from inhaled allergens, pathogens, and other noxious agents in the healthy airways [14,17,18]. When exposed to an antigen, allergen-specific immunoglobulin (Ig)E binds to high-affinity Fcε receptors on the surfaces of basophils and mast cells present in the subepithelial layer of the airways, results in the release of leukotrienes, prostaglandins, and histamine. These mediators will lead to narrowed, constricted airways and further stimulate the recruitment of eosinophils, macrophages, neutrophils, and T lymphocytes [10]. For instance, analysis of allergic airway inflammation using an ovalbumin (OVA) immunization and airway exposure model indicates that eosinophils are required for the development of allergic airway inflammation [19]. Further analysis found that the production of eosinophil-regulated chemokines is required to recruit Th2 cells during the development of allergic asthma by causing prolonged bronchoconstriction and epithelial layer damaging [20,21]. DCs were also demonstrated as necessary and sufficient for the induction of allergic airway inflammation [22]. de Heer HJ et al. [23] found that plasmacytoid DCs were able to control exacerbated airway inflammation through the induction of Tregs. CD11b+ classical type 2 DCs (cDC2s) mediated Th2 priming in response to house dust mite (HDM) and Blomiatropicalis mites [24], leading to eosinophilic airway inflammation, mucus production, and AHR. Upon lung fungal infection with Aspergillus fumigatus, CD11b+ cDC2s promote a Th17 response [25], which contribute to neutrophilic inflammation associated with severe asthma [26]. Therefore, although there is a propensity for type 2 cytokines in the lungs, allergic airway inflammation is a multifactorial disease with a variety of immune cell types as described above.
The effects of MSCs on allergic airway inflammation
MSCs have held great promises in the treatment of allergic airway inflammation not only because of their generative ability to repair/replace the injured lung tissues [27], but also due to their immunomodulatory effect that could increase the resistance of the patients to infections and other forms of allergy [28,29]. Recent research has shown that administration of MSCs is associated with reduced symptoms during severe asthma. Bonfield et al. [30] have emphasized the unique therapeutic potential of MSCs in chronic asthma as MSCs decrease airway inflammation and remodeling in the OVA murine model. MSCs participate in decreasing extracellular matrix deposition by reversing excess collagen deposition and changing hyaluronan levels in OVA-induced model [31]. Li et al. [32] showed the potential of human placenta mesenchymal stem cells in suppressing airway inflammation in asthmatic rats. Sun et al. [33] found that MSCs derived from both iPSCs and bone marrow-derived mesenchymal stem cells (BM-MSCs) protected the mouse from OVA-induced allergic inflammation by inhibiting the majority of allergy-specific pathological changes. The local administration of MSCs was able to attenuate airway AHR, inhibit inflammatory cell infiltration and mucus production in the lung, reduce eosinophil infiltration in the nose, and decrease inflammatory cell infiltration in both the bronchoalveolar and nasal lavage fluids in the OVA-induced allergic inflammation in mouse [5,29,33–36]. MSCs significantly reduced serum OVA-specific IgE concentration, inhibited expression of Th2 cytokines and elevated level of Treg cytokines [33,36,37]. Monocytes were also recruited by MSCs to secrete IL-10 that lead to reduced airway inflammation and hyper-responsiveness during the process [38]. In addition, Hong et al. [39] demonstrated that MSCs might also have a role in reducing neutrophilic airway inflammation by down-regulating neutrophil chemokine production. By using a mouse model of neutrophilic airway inflammation, Fang et al. [40] investigated the therapeutic potential of iPSC-MSCs in decreasing neutrophilic airway inflammation through the regulation of Th17 cells, suggesting that the iPSC-MSCs could be applied in the therapy for asthma patients with steroid-resistant neutrophilic airway inflammation.
The effects of MSCs on T cells
Th1/Th2 cell imbalance is involved in the pathogenesis of allergic airway inflammation [41]. There are an increasing number of studies showing that MSCs are able to protect the airways from allergen-driven pathology, by which to reduce airway inflammation and allergen-specific IgE [33,34,42]. MSCs possess potent immunoregulatory properties that could modulate the Th1 cytokine responses in benefit of Th2 types [43]. Administration of BM-MSCs effectively reduced allergic symptoms and inflammatory parameters in the mouse model of AR [44]. BM-MSCs were found to migrate to the nasal and lung tissues following intraperitoneal delivery, they ameliorated the airway remodeling and airway inflammation both in the upper and lower airways via the inhibition of Th2 response. Cho et al. found that adipose-derived mesenchymal stem cells (AD-MSCs) ameliorated allergic airway inflammation and improved lung function through the induction of Treg expansion. The induction of Treg by AD-MSCs involves the secretion of soluble factors such as indoleamine 2,3-dioxygenase (IDO), transforming growth factor-β (TGF-β), and prostaglandin E2 (PGE2) and Treg might be involved in the down-regulation of Th2 cytokines and up-regulation of Th1 cytokine production [45,46]. Fu et al. [47] found that iPSC-MSCs were also capable of modulating T-cell phenotypes, which suppressed Th2 phenotype through inducing Treg expansion. MSCs are able to induce Treg cells in vivo to reduce allergen-driven pathology. Multiple Treg dependent and independent mechanisms of therapeutic action are employed by MSCs [34]. Not only the MSCs but also the secretome of the cells showed the capacity of inhibiting allergic airway inflammation. Yu et al. [48] demonstrated that the culture supernatant of AD-MSCs is able to ameliorate allergic airway inflammation via recruitment of CD4+CD25+Foxp3 T cells in the mouse AR model.
MSCs also showed enhancing effects on the proliferation of PBMCs from patients, and the magnitude of proliferative response differs among allergens [49,50]. Fan et al. found that, when iPSC-MSCs were co-cultured with quiescent T cells, iPSC-MSCs promoted the proliferation of resting lymphocytes and activated CD4+ and CD8+ T cells without any additional stimulation. Treg cells were activated at the same time to balance the biased Th1/Th2 cytokine levels [49].
The effects of MSCs on DCs
DCs, the most potent antigen-presenting cells (APCs) in the immune systems, are critical for initiating and regulating immune responses by modulating antigen (Ag)-specific T-cell activation [51]. DCs have been demonstrated to be necessary for the induction of aberrant immunity to allergens or self-antigens in allergic asthma and autoimmune diseases [52]. Many studies have reported that human BM-MSCs inhibit DC differentiation and maturation and induce differentiation of DCs into regulatory DCs [53–56]. Gao et al. found that iPSC-MSCs exert an inhibitory effect on DC differentiation both by producing IL-10 and by direct cell contact, and induce the generation of an IL-10-producing regulatory DC subset in the progress of Lipopolysaccharide-induced maturation mainly via cell–cell contact [57]. Coculture of MSCs with stimulated DCs will result in decreased expression of C-C motif chemokine receptor 7 (CCR7). Similarly, MSC coculture will lead to DC maturation with significantly less migration to C-C motif ligand 19 (CCL19) [58]. MSCs inhibited the up-regulation of CD1a, CD40, CD80, CD86, and HLA-DR during DC differentiation and prevented the increase of CD40, CD86, and CD83 expression during DC maturation [55]. By inhibiting the activation of mitogen-activated protein kinases (MAPKs) in DCs, MSCs can inhibit the antigen processing and presentation to T cell functions of in vitro cocultured DCs. Furthermore, MSCs are able to down-regulate CCR7 and CD49d, two molecules involved in DC homing to lymphoid organs, in DCs both in vitro and in vivo [59]. Therefore, MSCs play a critical role in the treatment of allergic asthma and allergic rhinitis by regulating DC maturation and differentiation.
The effects of MSCs on epithelial cells
MSCs were found to protect lung epithelial cells exposed to pro-inflammatory cytokines [60–62]. Studies have demonstrated that MSCs and MSC-conditional medium are able to induce repair and protect airway epithelium against cell damage in ex vivo models [63,64]. Furthermore, MSCs reduced apoptosis in pulmonary cell cultures derived from papain-treated mice and in cigarette smoke extract-stimulated endothelial cells [65,66]. This may be due to the engraftment of MSCs in the bronchial epithelium or by paracrine secretions of keratinocyte growth factor (KGF), IL-10, angiopoietin-1, interleukin-1 receptor antagonist, and PGE2 [67]. Islam et al. [67] found that mitochondrial transfer from BM-MSCs to pulmonary alveoli protects against acute lung injury. Li et al. [68] found that iPSC-MSCs decrease the apoptosis of bronchial epithelial cells under hypoxic conditions. Further analysis demonstrated that iPSC-MSCs donate their mitochondria to the dysfunctional mitochondrial epithelial cells, by which they alleviate the asthma inflammation and protect the epithelial cells in the model mouse [29]. Similar results were observed that mitochondrial transfer from MSCs to airway epithelial cells protected against cigarette smoke-induced injury [69]. Therefore, MSCs exert immunomodulative effects on allergic airway inflammation by enhancing epithelial cell proliferation and migration and by reducing epithelial cell apoptosis.
Bronchopulmonary dysplasia (BPD)
In recent years, stem cells have emerged as potential candidates to treat BPD with MSCs being particularly promising [70]. MSCs displayed pleiotropic effects and showed promising results in neonatal rodents in preventing or rescuing lung injury without adverse effects [71]. BM-MSCs showed great potential in migration and homing capacity in BPD models [72,73]. There is a plenty of laboratory evidence demonstrating a protective effect of MSCs in the lung, mostly in hyperoxia-induced lung injury in neonatal rodents [74]. The promising laboratory studies in experimental neonatal lung injury have already led to the first-in-human phase I of allogeneic cord blood-derived MSCs in infants at the risk of developing BPD [75]. On the contrary, Popova et al. [76] showed that the presence of MSC in the tracheal aspirates of preterm infants indicates an increased risk of developing BPD. The role of allogenic MSCs in the pathogenesis BPD is still far away from clear. Although the first MSCs clinical trials in BPD are ongoing, multiple questions still remain.
Cystic fibrosis (CF)
Studies have reported the promising role of stem cells as a potential therapy for CF. MSCs have been proven as an attractive therapeutic modelity in the murine model of CF lung infection and inflammation [77,78]. Allogenic BM-MSCs engrafted into the lungs of cystic fibrosis transmembrane conductance regulator (CFTR) knockout mice; the cells were able to express epithelial phenotypes with CFTR mRNA expression [79,80]. The fight against CF has taken a major step forward. Farrow et al. [81] showed that cells causing the debilitating genetic disorder could be successfully replaced with healthy ones derived from stem cells. In 2017, the first stem cell study in the world for cystic fibrosis opened, allogenic human BM-MSCs from healthy volunteers were infused into a 39-year-old man with CF. The clinical application of stem cells for the treatment of CF certainly warrants further insight into preclinical models, including large animals, organoids, decellularized organs, and lung bioengineering [82,83]. However, engraftment of bone marrow-derived stem cells into the airways is a very inefficient process. Detailed knowledge of the cellular and molecular determinants governing homing to the lung and transformation of MSCs into lung epithelial cells would benefit this process.
Chronic obstructive pulmonary disease (COPD)
Both autologous and allogenic MSCs were widely investigated in the preclinical studies of COPD. Animal model recipients tolerated the injected allogenic MSCs well [84–86]. MSCs were proven able to migrate to the lung and differentiate into type II epithelial cells of the alveoli like cells [87,88]. MSC therapy resulted in the structural repair and functional restoration of the damaged lung in COPD models [60,89,90]. The repair effects of MSCs in the lung with COPD models were mainly caused by multiple paracrine factors secreted by exogenous MSCs. MSCs were also able to replace the damaged structures and contribute to the restoration of lung function [84]. However, the engraftment and survival rate of allogenic MSCs were very low in COPD models [80,91]. Therefore, the successful preclinical studies have not led to the success of clinical trials so far [84]. The source and status of MSCs, dosage issues, patient scales as well as the stage of the disease might contribute to the conflicting results between preclinical studies and clinical trials. Great efforts must be made to figure out the mechanisms that will lead toward a curing solution for COPD patients.
The mechanism of MSC action on airway inflammation
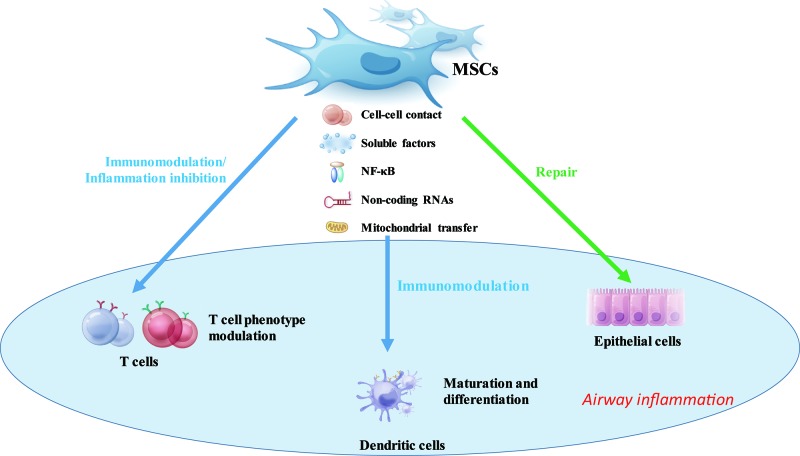
Human MSCs express low levels of major histocompatibility complex (MHC) class I molecules and have no MHC class II molecules or costimulatory molecules, including CD40, CD40L, CD80, and CD86 on cellular surface, which make the cells hypoimmunogenic [92]. Research found that MSCs do not provoke an immunological response even if the MHC class I and MHC class II molecules were up-regulated by the presence of Interferon γ (IFN-γ) [93,94]. Also, the costimulatory molecules are tightly regulated by the inhibitory molecules on MSCs when up-regulated under inflammatory conditions [95,96]. Khattar et al. [97] found that transcriptional reactivation of telomerase reverse transcriptase (TERT) expression in MSCs may directly control immunomodulation related protein synthesis by regulating tRNA gene expression. And the mechanisms of activating transcription from TERT promoters in stem cells could be vastly different depending on the status and microenvironment of the cells [98]. Moreover, MSCs are able to inhibit T-lymphocyte proliferation in mixed lymphocyte culture or in the presence of activators, such as IL-2 and phytohemagglutinin (PHA) [99]. Whereas MSCs were found to support T-cell survival and proliferation when these cells are not activated [49,100,101]. Some studies have shown that MSCs reduced the expression of activation markers on the lymphocytes [102,103], while others found no expression changes of these markers [104,105]. Therefore, the mechanisms involved in the immunomodulatory effects of MSCs on immune cells include different cell–cell contact, soluble factors, genomic regulation, nuclear factor κB (NF-κB) signaling pathway and mitochondrial transfer etc. (see Figure 1 for a schematic overview)
Figure 1. Schematic illustration of mechanisms underlying the MSCs immunomodulatory effects.
MSCs potentially modulate the immune cells (T cells and dendritic cells) and epithelial cells, which are involved in the airway inflammation. The MSCs function their modulatory effects via cell–cell contact, (anti)-inflammatory cytokines and chemokines, genomic regulation, and mitochondrial transfer, to improve lung tissue homeostasis.
Cell–cell contact
MSCs have been demonstrated as expressing integrins (α1-6, αV, and β1-4), intercellular adhesion molecules (ICAM-1, ICAM-2), vascular cell adhesion protein (VCAM)-1, CD72, and CD58 (LFA-3) on their surfaces, which allow them to modulate both autologous and allogeneic T lymphocytes via cell-to-cell contact in vitro [99,106]. Ren et al. demonstrated that ICAM-1 and VCAM-1 on MSCs positively correlate with the immunomodulatory effects of MSCs toward various subtypes of T cells [107,108]. The immunomodulatory effects of MSCs can be significantly reversed by the genetic deletion or functional blocking of adhesion molecules in the cells [109]. In addition, studies claimed that cell–cell contact is necessary for the MSC-mediated inhibition of allogeneic Th17 differentiation, and the expression of programmed death-1 (PD-1) ligand on the surface of MSCs is critical for this [110,111]. Similarly, galectins were found to be constitutively expressed on MSCs and mediated the immunomodulatory effects of MSCs [112]. For instance, CD4+ and CD8+ T cell proliferation were inhibited by galectin-1 and galectin-3 [112,113]. And the expression of galectin-9 was found to be associated with the anti-proliferative effects that MSCs have on T cells [114].
Soluble factors
Although cell–cell contact has been demonstrated as being necessary for the immunodulatory effects of MSCs, transwell membrane experiments showed that MSCs can also exert their immunomodulatory functions through releasing soluble factors [93,99]. The production of suppressive soluble factors is dependent on a cross-talk between MSCs and activated immune cells, inflammatory cytokines secreted by antigen-presenting cells and T cells, including interferon-γ (IFN-γ), IL-1α, IL-1β, and tumor necrosis factor-α (TNF-α) are required for the immunomodulatory activities of MSCs. Researchers found that the use of supernatants from MSCs culture is not enough for T-cell suppression [115]. Whereas the supernatants from MSC-activated T-cell coculture system can suppress T-cell proliferation [92,116]. It suggests that the immunomodulatory effects of MSCs rely on a combination of the complex interplay between soluble factors and specific inflammatory microenvironment.
Many MSC-derived soluble factors have been identified to be associated with the immunomodulatory effects, such as hepatic growth factor (HGF), TGF-β [99], nitric oxide (NO) [117], PGE2 [118], IDO [119], IL-6 [120], and IL-10 [121] etc. These soluble factors are able to ablate the recruitment, activation, and maturation of DCs in the lung and the reactivation of Th2-cytokine–producing CD4+ Th2 cells, by which to ameliorate allergic airway inflammation. The immunomodulatory effects could be monitored by controlling the paracrine effects of MSCs. For example, rap 1 is a key regulator for regulating inflammation [122,123]. Zhang et al. [124] revealed the reduction of pro-inflammatory paracrine cytokines in rap-inhibited MSCs for the MSC-based treatments. Since they have been revealed as a potential link between physiological cues and human ailments [125], nuclear, casein kinase and cyclin-dependent kinase substrate (NUCKS) were also found to be involved in the metabolism and paracrine effects of human MSCs [126,127]. Zhang et al. [128] presented up-regulated paracrine effects in NUCKS knockout MSCs. In addition, it is worth noticing that there are some well acknowledged differences between MSCs derived from different species, which should be considered carefully before interpreting the preclinical data. For instance, human MSCs suppress T-cell proliferation by IDO production, while inducible nitric oxide synthase (iNOS) is employed by mouse MSCs to exert the same function [99,108,129].
Noncoding RNAs
Researches have shown that administration of MSCs after allergen challenge resulted in changes in gene expression in allergic airway inflammation. Tang et al. [130] identified different miRNA and mRNA profiles after asthma induction and BM-MSC treatment, and the miR-21/Acvr2a axis was figured out as an important mechanism for the induction of asthmatic inflammation. In addition, miR-21 was also found mediating the protective effects of iPSC-MSCs in human bronchial epithelial cells under hypoxic conditions [68]. mRNA Ccl11, Ccl24, Il13, Il33, and Ear11 were found being involved in the process of human embryonic stem cell derived MSC immunomodulation in airway allergic inflammation [5]. Furthermore, Wang et al. [131] examined the long noncoding RNA (lncRNA) expression after the induction of asthma, and the differentially expressed lncRNAs after iPSC-MSC treatment. lncRNAsMM9LINCRNAEXON12105+ and AK089315 were finally emphasized as the potential regulators of allergy and the targets of iPSC-MSC treatment. By using various knockout mice, researchers could clearly investigate the genes involved in the MSC immunomodulatory process.
Nuclear factor κB (NF-κB)
MSCs have been demonstrated to sense the inflammatory mediators such as IFN-γ, TNF-α, IL-1, chemokines and leukotrienes, they are able to activate NF-κB to promote the immunomodulatory effects in the inflammatory microenvironment [132–134]. For instance, it has been demonstrated that TNF-α released by activated T cells binds to TNF-R1 on MSCs, activating the NF-κB pathway and contributing to the immunomodulatory effects of MSCs [135–138]. Studies have shown that MSCs inhibit the proliferation of cytotoxic T lymphocytes by B7-H4, which can inhibit the NF-κβ activities [139]. Saldanha-Araujo et al. [140] demonstrated that MSCs are able to induce a shift of NF-κB signaling in T-lymphocytes from canonical to noncanonical, by which they suppress the activation of the T-lymphocytes in the coculture system. Whereas Fan et al. [49] found that MSCs up-regulate the proliferation and activation of quiescent T cells of AR patients via NF-κB signaling pathway. In addition, MSCs promote the differentiation of monocytes to M2 phenotype macrophages through NF-κB and signal transducer and activator of transcription 3 (STAT-3) pathways in the presence of IDO [141–143]. Human MSCs have also been proven to activate peritoneal macrophages via toll-like receptor 2 (TLR2) NF-κB signaling by secreting TNF-stimulated gene 6 (TSG6), which regulates TLR2 NF-κB signaling [144]. Therefore, NF-κB is tightly involved in the immunomodulatory function of MSCs.
Mitochondrial transfer
Mitochondrial transfer is another significant application of MSCs in the treatment of diseases. It has been well-demonstrated that MSCs can deliver function-normal mitochondria to the donor cells or tissues to alleviate the oxidative stresses and cell apoptosis in cardiac diseases with mitochondria damage [145,146]. Recent research has also presented the MSCs-mediated mitochondrial transfer in lung disease treatments. BM-MSCs have shown positive effects in treating mouse models of acute lung injury and allergic airway inflammation via mitochondrial transfer [67,147]. Li et al. [69] presented that MSCs derived from iPSCs protected airway epithelial cells from smoke-caused mitochondria damage by mitochondria transfer, which occurs via the tunneling of nanotubes (TNTs). Yao et al. [29] further demonstrated that iPSC-MSCs attenuate asthma inflammation and protect epithelial cells by transferring their mitochondria via TNTs. Li et al. [28] also presented that mitochondrial transfer prevented the oxidative stresses caused by the damaged mitochondria in the airways. In addition, the transferred mitochondria can alleviate the inflammatory responses of the receptors [29,67]. Several known mechanisms have been revealed for the MSCs mediated mitochondrial transfer in lung disease treatments. Mitochondrial Rho GTPase 1 (MIRO1) was confirmed playing a critical role in the regulation of MSC mitochondrial transfer by MIRO1 overexpression in iPSC-MSCs [147,148]. The microtubule motor protein kinesin family member 5B (KIF5B) is considered to be a regulator of mitochondrial transfer [149]. Furthermore, connexin 43 (CX43) has been found as vital for regulating the TNT formation and mitochondrial transfer efficiency of iPSC-MSCs. Overexpression of CX43 in iPSC-MSCs enhanced the TNT formation between iPSC-MSCs and damaged epithelial cells, the mitochondrial transfer efficiency was also significantly increased. Also, silencing CX43 reduced TNT formation and the immunomodulatory effects of iPSC-MSCs during allergic airway inflammation [29].
Promises and challenges
Current advantages of MSC immunomodulation eventually would lead to a growing exploration of MSCs in clinical trials of allergic airway inflammation. Clinical trials have clearly assessed the safety and feasibility of using MSC treatment for the patients affected by moderate or severe acute respiratory distress syndrome and idiopathic pulmonary fibrosis [150]. There are increasing number of completed and ongoing registered studies examining the effects of MSCs on allergic airway inflammation in human clinical trials on ClinicalTrials.gov database. The number of registered clinical trials testing MSCs has become to 905 internationally, which was only 402 in 2015. However, the major outcomes of clinical trials of MSCs in respiratory disorders have fallen far short of the theoretical potential of these cells in preclinical studies [133]. There is insufficient clinical benefit apparent in early efficacy studies to warrant further commitment of relatively scarce finances [151].
There are still obstacles to be overcome before MSCs becoming realistic in clinics, and the cure to inflammatory airway disorders remains elusive. There is more and more evidence demonstrating the potential of MSC-derived extracellular vesicles (EVs) in respiratory diseases such as asthma [152–155]. However, MSCs and their derived EVs were observed provoking different effects and lung mechanics [154]. More information about the secretome of MSCs is needed before use in therapy. In-depth studies of the mechanisms of MSCs in inflammatory airway disorders are still needed, which to cover cell trafficking, clinical ethics, regulations, and practices in stem cell therapies.
On the other hand, it is still unclear how to develop high-quality, clinical-grade cell products. It is difficult for quality control because different manufacturers produce the products at different sites probably according to different protocols. In addition, even the consistency of the products from the same site is hard to maintain due to problems with the source, batch or derived tissue of the MSCs. It is better for patients to utilize their own stem cell reserves to create robust health and heal from diseases. Therefore, there are still meaningful differences in MSC cell preparation, fitness, and functionality due to the MSC tissue source, culture methods, and expansion levels. It is clear that MSCs are very promising with numerous potential benefits in the treatment of inflammatory airway disorders; however, there are challenges that have to be overcome before stem cells can be safely, effectively, and routinely used in the clinical setting.
Abbreviations
- AD-MSC
adipose-derived mesenchymal stem cell
- Ag
antigen
- AHR
airway hyper-responsiveness
- APC
antigen-presenting cell
- AR
allergic rhinitis
- BM-MSC
bone marrow-derived mesenchymal stem cell
- BPD
bronchopulmonary dysplasia
- CCL19
C-C motif ligand 19
- CCR7
C-C motif chemokine receptor 7
- CD
cluster of differentiation
- cDC2
classical type 2 DC
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- COPD
chronic obstructive pulmonary disease
- CX43
connexin 43
- DC
dendritic cell
- ESC
embryonic stem cell
- EV
extracellular vesicle
- HGF
hepatic growth factor
- HDM
house dust mite
- IDO
indoleamine 2,3-dioxygenase
- Ig
immunoglobulin
- IFN-γ
interferon-γ
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- iPSC
induced pluripotent stem cell
- KGF
keratinocyte growth factor
- KIF5B
kinesin family member 5B
- lncRNA
long noncoding RNA
- MAPK
mitogen-activated protein kinase
- MIRO1
mitochondrial Rho GTPase 1
- MSC
mesenchymal stem cell
- NF-κB
nuclear factor κB
- NO
nitric oxide
- NUCKS
nuclear, casein kinase and cyclin-dependent kinase substrate
- OVA
ovalbumin
- PGE2
prostaglandin E2
- STAT-3
signal transducer and activator of transcription 3
- TGF-β
transforming growth factor-β
- Th
T-helper
- TLR2
toll-like receptor 2
- TNF-α
tumor necrosis factor-α
- TNT
tunneling of nanotube
- TSG6
TNF-stimulated gene 6
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by grants from NSFC for Excellent Young Scholars [81322012 to (Q.-L.F.]; NSFC [81373174, 81471832, 81671882 and 81770984]; the key grant from the Science and Technology Foundation of Guangdong Province of China [2015B020225001]; and the Natural Science Foundation of Guangdong Province [2014A030313051, 2016A030308017 and 2017A030313105].
Author Contribution
X.-L.F., Z.Z., C.Y.M. wrote the manuscript; Q.-L.F. designed and wrote the manuscript.
References
- 1.Ullah I., Subbarao R.B. and Rho G.J. (2015) Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 35, 10.1042/BSR20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C.. et al. (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7, 393–395 10.1080/14653240500319234 [DOI] [PubMed] [Google Scholar]
- 3.Lian Q., Zhang Y., Liang X., Gao F. and Tse H.-F. (2016) Directed differentiation of human-induced pluripotent stem cells to mesenchymal stem cells. Methods Mol. Biol. 1416, 289–298 [DOI] [PubMed] [Google Scholar]
- 4.Soontararak S., Chow L., Johnson V., Coy J., Wheat W., Regan D.. et al. (2018) Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl. Med. 7, 456–467 10.1002/sctm.17-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y.D., Fan X.L., Zhang H., Fang S.B., Li C.L., Deng M.X.. et al. (2018) The genes involved in asthma with the treatment of human embryonic stem cell-derived mesenchymal stem cells. Mol. Immunol. 95, 47–55 10.1016/j.molimm.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 6.Keating A. (2008) How do mesenchymal stromal cells suppress T cells? Cell Stem Cell 2, 106–108 10.1016/j.stem.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Wei W., Huang Y., Li D., Gou H.F. and Wang W. (2018) Improved therapeutic potential of MSCs by genetic modification. Gene Ther., 25, 538–547 10.1038/s41434-018-0041-8 [DOI] [PubMed] [Google Scholar]
- 8.Ding Y., Liang X., Zhang Y., Yi L., Shum H.C., Chen Q.. et al. (2018) Rap1 deficiency-provoked paracrine dysfunction impairs immunosuppressive potency of mesenchymal stem cells in allograft rejection of heart transplantation. Cell Death Dis. 9, 386 10.1038/s41419-018-0414-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss D.J., Bates J.H., Gilbert T., Liles W.C., Lutzko C., Rajagopal J.. et al. (2013) Stem cells and cell therapies in lung biology and diseases: conference report. Ann. Am. Thorac Soc. 10, S25–S44 10.1513/AnnalsATS.201304-089AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broide D.H. (2001) Molecular and cellular mechanisms of allergic disease. J. Allergy Clin. Immunol. 108, S65–S71 10.1067/mai.2001.116436 [DOI] [PubMed] [Google Scholar]
- 11.Agrawal D.K. and Shao Z. (2010) Pathogenesis of allergic airway inflammation. Curr. Allergy Asthma Rep. 10, 39–48 10.1007/s11882-009-0081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias J.A., Lee C.G., Zheng T., Ma B., Homer R.J. and Zhu Z. (2003) New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 10.1172/JCI17748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L.. et al. (1998) Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 10.1126/science.282.5397.2258 [DOI] [PubMed] [Google Scholar]
- 14.Anagnostopoulou P., Dai L., Schatterny J., Hirtz S., Duerr J. and Mall M.A. (2010) Allergic airway inflammation induces a pro-secretory epithelial ion transport phenotype in mice. Eur. Respir. J. 36, 1436–1447 10.1183/09031936.00181209 [DOI] [PubMed] [Google Scholar]
- 15.Pepper A.N., Renz H., Casale T.B. and Garn H. (2017) Biologic Therapy and Novel Molecular Targets of Severe Asthma. J. Allergy Clin Immunol. Pract. 5, 909–916 10.1016/j.jaip.2017.04.038 [DOI] [PubMed] [Google Scholar]
- 16.Garn H. (2018) Is 9 more than 2 also in allergic airway inflammation? J. Allergy Clin. Immunol. 141, 2024–2026 10.1016/j.jaci.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 17.Mall M.A. (2008) Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J. Aerosol. Med. Pulm Drug Deliv. 21, 13–24 10.1089/jamp.2007.0659 [DOI] [PubMed] [Google Scholar]
- 18.Wanner A., Salathe M. and O’Riordan T.G. (1996) Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 154, 1868–1902 10.1164/ajrccm.154.6.8970383 [DOI] [PubMed] [Google Scholar]
- 19.Lee J.J., Dimina D., Macias M.P., Ochkur S.I., McGarry M.P., O’Neill K.R.. et al. (2004) Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 10.1126/science.1099472 [DOI] [PubMed] [Google Scholar]
- 20.Walsh E.R., Sahu N., Kearley J., Benjamin E., Kang B.H., Humbles A.. et al. (2008) Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J. Exp. Med. 205, 1285–1292 10.1084/jem.20071836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen E.A., Ochkur S.I., Pero R.S., Taranova A.G., Protheroe C.A., Colbert D.C.. et al. (2008) Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 205, 699–710 10.1084/jem.20071840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conejero L., Khouili S.C., Martínez-Cano S., Izquierdo H.M., Brandi P. and Sancho D. (2017) Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production. JCI Insight 2, e90420 10.1172/jci.insight.90420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Heer H.J., Hammad H., Soullie T., Hijdra D., Vos N., Willart M.A.. et al. (2004) Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200, 89–98 10.1084/jem.20040035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q., Ho A.W., Schlitzer A., Tang Y., Wong K.H., Wong F.H.. et al. (2014) GM-CSF-licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J. Immunol. 193, 496–509 10.4049/jimmunol.1303138 [DOI] [PubMed] [Google Scholar]
- 25.Schlitzer A., McGovern N., Teo P., Zelante T., Atarashi K., Low D.. et al. (2013) IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 10.1016/j.immuni.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambrecht B.N. and Hammad H. (2015) The immunology of asthma. Nat. Immunol. 16, 45–56 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 27.Royce S.G., Rele S., Broughton B.R.S., Kelly K. and Samuel C.S. (2017) Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J. 31, 4168–4178 10.1096/fj.201700178R [DOI] [PubMed] [Google Scholar]
- 28.Li X., Michaeloudes C., Zhang Y., Wiegman C.H., Adcock I.M., Lian Q.. et al. (2018) Mesenchymal stem cells alleviate oxidative stress–induced mitochondrial dysfunction in the airways. J. Allergy Clin. Immunol. 141, 1634.e5–1645.e5 10.1016/j.jaci.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 29.Yao Y., Fan X.L., Jiang D., Zhang Y., Li X., Xu Z.B.. et al. (2018) Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 11, 1120–1135 10.1016/j.stemcr.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonfield T., Sutton M., Lennon D. and Caplan A. (2015) Mesenchymal stem cells: new directions in treating asthma. J. Immunol. 194 26026056 [Google Scholar]
- 31.Goldstein B.D., Lauer M.E., Caplan A.I. and Bonfield T.L. (2017) Chronic asthma and Mesenchymal stem cells: Hyaluronan and airway remodeling. J. Inflammation 14, 10.1186/s12950-017-0165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Qu T., Tian L., Han T., Jin Y. and Wang Y. (2018) Human placenta mesenchymal stem cells suppress airway inflammation in asthmatic rats by modulating Notch signaling. Mol. Med. Rep. 17, 5336–5343 [DOI] [PubMed] [Google Scholar]
- 33.Sun Y.Q., Deng M.X., He J., Zeng Q.X., Wen W., Wong D.S.. et al. (2012) Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 30, 2692–2699 10.1002/stem.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavanagh H. and Mahon B.P. (2011) Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 66, 523–531 10.1111/j.1398-9995.2010.02509.x [DOI] [PubMed] [Google Scholar]
- 35.Mohammadian M., Boskabady M.H., Kashani I.R., Jahromi G.P., Omidi A., Nejad A.K.. et al. (2016) Effect of bone marrow derived mesenchymal stem cells on lung pathology and inflammation in ovalbumin-induced asthma in mouse. Iran J. Basic Med. Sci. 19, 55–63 [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang Y.C., Liou C.W., Chen S.D., Wang P.W., Chuang J.H., Tiao M.M.. et al. (2017) Mitochondrial Transfer from Wharton’s Jelly Mesenchymal Stem Cell to MERRF Cybrid Reduces Oxidative Stress and Improves Mitochondrial Bioenergetics. Oxid Med. Cell Longev. 2017, 5691215 10.1155/2017/5691215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habibian R., Delirezh N. and Farshid A.A. (2018) The effects of bone marrow-derived mesenchymal stem cells on ovalbumin-induced allergic asthma and cytokine responses in mice. Iran J. Basic Med. Sci. 21, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda K., Webb T.L., Ning F.K., Shiraishi Y., Regan D.P., Chow L.. et al. (2018) Mesenchymal Stem Cells Recruit CCR2(+) Monocytes To Suppress Allergic Airway Inflammation. J. Immunol. 200, 1261–1269 10.4049/jimmunol.1700562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong G.H., Kwon H.S., Lee K.Y., Ha E.H., Moon K.A., Kim S.W.. et al. (2017) hMSCs suppress neutrophil-dominant airway inflammation in a murine model of asthma. Exp. Mol. Med. 49, e288 10.1038/emm.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang S.B., Zhang H.Y., Jiang A.Y., Fan X.L., Lin Y.D., Li C.L.. et al. (2018) Human iPSC-MSCs prevent steroid-resistant neutrophilic airway inflammation via modulating Th17 phenotypes. Stem. Cell Res. Ther. 9, 147 10.1186/s13287-018-0897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzarella G., Bianco A., Catena E., De Palma R. and Abbate G.F. (2000) Th1/Th2 lymphocyte polarization in asthma. Allergy 55, 6–9 10.1034/j.1398-9995.2000.00511.x [DOI] [PubMed] [Google Scholar]
- 42.Gao F., Chiu S.M., Motan D.A., Zhang Z., Chen L., Ji H.L.. et al. (2016) Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 7, e2062 10.1038/cddis.2015.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salek Farrokhi A., Zarnani A.H. and Moazzeni S.M. (2018) Mesenchymal stem cells therapy protects fetuses from resorption and induces Th2 type cytokines profile in abortion prone mouse model. Transpl. Immunol. 47, 26–31 10.1016/j.trim.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 44.Zhao N., Liu Y., Liang H. and Jiang X. (2016) Bone marrow-derived mesenchymal stem cells reduce immune reaction in a mouse model of allergic rhinitis. Am. J. Transl. Res. 8, 5628–5636 [PMC free article] [PubMed] [Google Scholar]
- 45.Cho K.S., Park M.K., Kang S.A., Park H.Y., Hong S.L., Park H.K.. et al. (2014) Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014, 436476 10.1155/2014/436476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai R., Yu Y., Yan G., Hou X., Ni Y. and Shi G. (2018) Intratracheal administration of adipose derived mesenchymal stem cells alleviates chronic asthma in a mouse model. BMC Pulm. Med. 18, 131 10.1186/s12890-018-0701-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu Q.L., Chow Y.Y., Sun S.J., Zeng Q.X., Li H.B., Shi J.B.. et al. (2012) Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy 67, 1215–1222 10.1111/j.1398-9995.2012.02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H.S., Park M.K., Kang S.A., Cho K.S., Mun S.J. and Roh H.J. (2017) Culture supernatant of adipose stem cells can ameliorate allergic airway inflammation via recruitment of CD4(+)CD25(+)Foxp3 T cells. Stem Cell Res. Ther. 8, 8 10.1186/s13287-016-0462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan X.L., Zeng Q.X., Li X., Li C.L., Xu Z.B., Deng X.Q.. et al. (2018) Induced pluripotent stem cell-derived mesenchymal stem cells activate quiescent T cells and elevate regulatory T cell response via NF-kappaB in allergic rhinitis patients. Stem Cell Res. Ther. 9, 170 10.1186/s13287-018-0896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai M., Capitle E., Patel S. and Rameshwar P. (2011) Effects of mesenchymal stem cells on pollen-induced lymphocyte proliferation in subjects with allergic rhinitis: implications of stem cell activity on immune response to pollen. J. Allergy Clin. Immunol. 127, AB99 10.1016/j.jaci.2010.12.397 [DOI] [Google Scholar]
- 51.Banchereau J. and Steinman R.M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 52.van Helden M.J. and Lambrecht B.N. (2013) Dendritic cells in asthma. Curr. Opin. Immunol. 25, 745–754 10.1016/j.coi.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 53.Jiang X.X., Zhang Y., Liu B., Zhang S.X., Wu Y., Yu X.D.. et al. (2005) Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105, 4120–4126 10.1182/blood-2004-02-0586 [DOI] [PubMed] [Google Scholar]
- 54.Spaggiari G.M., Abdelrazik H., Becchetti F. and Moretta L. (2009) MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 113, 6576–6583 10.1182/blood-2009-02-203943 [DOI] [PubMed] [Google Scholar]
- 55.Zhang W., Ge W., Li C., You S., Liao L., Han Q.. et al. (2004) Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 13, 263–271 10.1089/154732804323099190 [DOI] [PubMed] [Google Scholar]
- 56.Beyth S., Borovsky Z., Mevorach D., Liebergall M., Gazit Z., Aslan H.. et al. (2005) Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105, 2214–2219 10.1182/blood-2004-07-2921 [DOI] [PubMed] [Google Scholar]
- 57.Gao W.X., Sun Y.Q., Shi J., Li C.L., Fang S.B., Wang D.. et al. (2017) Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res. Ther. 8, 48 10.1186/s13287-017-0499-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.English K., Barry F.P. and Mahon B.P. (2008) Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol. Lett. 115, 50–58 10.1016/j.imlet.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 59.Chiesa S., Morbelli S., Morando S., Massollo M., Marini C., Bertoni A.. et al. (2011) Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 108, 17384–17389 10.1073/pnas.1103650108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huh J.W., Kim S.Y., Lee J.H., Lee J.S., Van Ta Q., Kim M.. et al. (2011) Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L255–L266 10.1152/ajplung.00253.2010 [DOI] [PubMed] [Google Scholar]
- 61.Li J., Huang S., Zhang J., Feng C., Gao D., Yao B.. et al. (2016) Mesenchymal stem cells ameliorate inflammatory cytokine-induced impairment of AT-II cells through a keratinocyte growth factor-dependent PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 13, 3755–3762 10.3892/mmr.2016.5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broekman W., Amatngalim G.D., de Mooij-Eijk Y., Oostendorp J., Roelofs H., Taube C.. et al. (2016) TNF-alpha and IL-1beta-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir. Res. 17, 3 10.1186/s12931-015-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akram K.M., Samad S., Spiteri M.A. and Forsyth N.R. (2013) Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir. Res. 14, 9 10.1186/1465-9921-14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yew T.L., Hung Y.T., Li H.Y., Chen H.W., Chen L.L., Tsai K.S.. et al. (2011) Enhancement of wound healing by human multipotent stromal cell conditioned medium: the paracrine factors and p38 MAPK activation. Cell Transplant. 20, 693–706 10.3727/096368910X550198 [DOI] [PubMed] [Google Scholar]
- 65.Zhen G., Xue Z., Zhao J., Gu N., Tang Z., Xu Y.. et al. (2010) Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 12, 605–614 10.3109/14653241003745888 [DOI] [PubMed] [Google Scholar]
- 66.Guan X.-J., Song L., Han F.-F., Cui Z.-L., Chen X., Guo X.-J.. et al. (2013) Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF–VEGF receptors. J. Cell. Biochem. 114, 323–335 10.1002/jcb.24377 [DOI] [PubMed] [Google Scholar]
- 67.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K.. et al. (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765 10.1038/nm.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C.L., Xu Z.B., Fan X.L., Chen H.X., Yu Q.N., Fang S.B.. et al. (2018) microRNA-21 mediates the protective effects of mesenchymal stem cells derived from iPSCs to human bronchial epithelial cell injury under hypoxia. Cell Transplant. 27, 571–583 10.1177/0963689718767159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X., Zhang Y., Yeung S.C., Liang Y., Liang X., Ding Y.. et al. (2014) Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Respir. Cell Mol. Biol. 51, 455–465 10.1165/rcmb.2013-0529OC [DOI] [PubMed] [Google Scholar]
- 70.Mueller M. and Kramer B.W. (2017) Stem cells and bronchopulmonary dysplasia - the five questions: Which cells, when, in which dose, to which patients via which route? Paediatr. Respir. Rev. 24, 54–59 [DOI] [PubMed] [Google Scholar]
- 71.Ee M.T. and Thebaud B. (2018) Therapeutic potential of stem cells for bronchopulmonary dysplasia: “It’s about time” or “Not so fast”? Curr. Pediatr. Rev. 14, 227–238 10.2174/1573396314666180911100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aslam M., Baveja R., Liang O.D., Fernandez-Gonzalez A., Lee C., Mitsialis S.A.. et al. (2009) Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 180, 1122–1130 10.1164/rccm.200902-0242OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X., Wang H., Shi Y., Peng W., Zhang S., Zhang W.. et al. (2012) Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol. Int. 36, 589–594 10.1042/CBI20110447 [DOI] [PubMed] [Google Scholar]
- 74.Mobius M.A. and Thebaud B. (2016) Cell therapy for bronchopulmonary dysplasia: promises and perils. Paediatr. Respir. Rev. 20, 33–41 [DOI] [PubMed] [Google Scholar]
- 75.Chang Y.S., Ahn S.Y., Yoo H.S., Sung S.I., Choi S.J., Oh W.I.. et al. (2014) Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 164, 966e6–972e6 10.1016/j.jpeds.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 76.Popova A.P., Bozyk P.D., Bentley J.K., Linn M.J., Goldsmith A.M., Schumacher R.E.. et al. (2010) Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics 126, e1127–e1133 10.1542/peds.2009-3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonfield T.L. and Caplan A.I. (2010) Adult mesenchymal stem cells: an innovative therapeutic for lung diseases. Discov. Med. 9, 337–345 [PubMed] [Google Scholar]
- 78.Sutton M.T. and Bonfield T.L. (2014) Stem cells: innovations in clinical applications. Stem Cells Int. 2014, 516278 10.1155/2014/516278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang G.S., Bunnell B.A., Painter R.G., Quiniones B.C., Tom S., Lanson N.A.. et al. (2005) Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: Potential therapy for cystic fibrosis. Proc. Natl. Acad. Sci. U.S.A. 102, 186–191 10.1073/pnas.0406266102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loi R., Beckett T., Goncz K.K., Suratt B.T. and Weiss D.J. (2006) Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am. J. Respir. Crit. Care Med. 173, 171–179 10.1164/rccm.200502-309OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farrow N., Cmielewski P., Donnelley M., Rout-Pitt N., Moodley Y., Bertoncello I.. et al. (2018) Epithelial disruption: a new paradigm enabling human airway stem cell transplantation. Stem Cell Res. Ther. 9, 153 10.1186/s13287-018-0911-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conese M., Beccia E., Castellani S., Di Gioia S., Colombo C., Angiolillo A.. et al. (2018) The long and winding road: stem cells for cystic fibrosis. Expert Opin. Biol. Ther. 18, 281–292 10.1080/14712598.2018.1413087 [DOI] [PubMed] [Google Scholar]
- 83.Piro D., Rejman J. and Conese M. (2008) Stem cell therapy for cystic fibrosis: current status and future prospects. Expert Rev. Respir Med. 2, 365–380 10.1586/17476348.2.3.365 [DOI] [PubMed] [Google Scholar]
- 84.Sun Z., Li F., Zhou X., Chung K.F., Wang W. and Wang J. (2018) Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J. Thorac Dis. 10, 1084–1098 10.21037/jtd.2018.01.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee M., Jeong S.Y., Ha J., Kim M., Jin H.J., Kwon S.J.. et al. (2014) Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 446, 983–989 10.1016/j.bbrc.2014.03.051 [DOI] [PubMed] [Google Scholar]
- 86.Schu S., Nosov M., O’Flynn L., Shaw G., Treacy O., Barry F.. et al. (2012) Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 16, 2094–2103 10.1111/j.1582-4934.2011.01509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang K., Kang X.W., Wang X.Y., Wu S.J., Xiao J.L., Li Z.G.. et al. (2015) Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol. Med. Rep. 11, 1685–1692 10.3892/mmr.2014.2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai S.X., Liu A.R., Chen S., He H.L., Chen Q.H., Xu J.Y.. et al. (2015) Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res. Ther. 6, 65 10.1186/s13287-015-0060-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Gu W., Song L., Li X.M., Wang D., Guo X.J. and Xu W.G. (2015) Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci. Rep. 5, 8733 10.1038/srep08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong Y., Kim Y.S., Hong S.H. and Oh Y.M. (2016) Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. Exp. Mol. Med. 48, e266 10.1038/emm.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim Y.S., Kim J.Y., Shin D.M., Huh J.W., Lee S.W. and Oh Y.M. (2014) Tracking intravenous adipose-derived mesenchymal stem cells in a model of elastase-induced emphysema. Tuberc Respir Dis. 77, 116–123 10.4046/trd.2014.77.3.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haddad R. and Saldanha-Araujo F. (2014) Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed. Res. Int. 2014, 216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tse W.T., Pendleton J.D., Beyer W.M., Egalka M.C. and Guinan E.C. (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75, 389–397 10.1097/01.TP.0000045055.63901.A9 [DOI] [PubMed] [Google Scholar]
- 94.Klyushnenkova E., Mosca J.D., Zernetkina V., Majumdar M.K., Beggs K.J., Simonetti D.W.. et al. (2005) T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J. Biomed. Sci. 12, 47–57 10.1007/s11373-004-8183-7 [DOI] [PubMed] [Google Scholar]
- 95.Najar M., Raicevic G., Fayyad-Kazan H., De Bruyn C., Bron D., Toungouz M.. et al. (2012) Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev. 8, 1188–1198 10.1007/s12015-012-9408-1 [DOI] [PubMed] [Google Scholar]
- 96.Briones J., Novelli S. and Sierra J. (2011) T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone Marrow Res. 2011, 976793 10.1155/2011/976793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khattar E., Kumar P., Liu C.Y., Akincilar S.C., Raju A., Lakshmanan M.. et al. (2016) Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J. Clin. Invest. 126, 4045–4060 10.1172/JCI86042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akincilar S.C., Khattar E., Boon P.L., Unal B., Fullwood M.J. and Tergaonkar V. (2016) Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov 6, 1276–1291 10.1158/2159-8290.CD-16-0177 [DOI] [PubMed] [Google Scholar]
- 99.Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P.. et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–43 10.1182/blood.V99.10.3838 [DOI] [PubMed] [Google Scholar]
- 100.Xu G., Zhang Y., Zhang L., Ren G. and Shi Y. (2007) The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem. Biophys. Res. Commun. 361, 745–750 10.1016/j.bbrc.2007.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benvenuto F., Ferrari S., Gerdoni E., Gualandi F., Frassoni F., Pistoia V.. et al. (2007) Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 25, 1753–1760 10.1634/stemcells.2007-0068 [DOI] [PubMed] [Google Scholar]
- 102.Groh M.E., Maitra B., Szekely E. and Koc O.N. (2005) Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 33, 928–934 10.1016/j.exphem.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 103.Le Blanc K., Rasmusson I., Gotherstrom C., Seidel C., Sundberg B., Sundin M.. et al. (2004) Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand. J. Immunol. 60, 307–315 10.1111/j.0300-9475.2004.01483.x [DOI] [PubMed] [Google Scholar]
- 104.Glennie S., Soeiro I., Dyson P.J., Lam E.W. and Dazzi F. (2005) Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105, 2821–2827 10.1182/blood-2004-09-3696 [DOI] [PubMed] [Google Scholar]
- 105.Ramasamy R., Tong C.K., Seow H.F., Vidyadaran S. and Dazzi F. (2008) The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell. Immunol. 251, 131–136 10.1016/j.cellimm.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 106.Su J., Chen X., Huang Y., Li W., Li J., Cao K.. et al. (2014) Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 21, 388–396 10.1038/cdd.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren G., Su J., Zhang L., Zhao X., Ling W., L’Huillie A.. et al. (2009) Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27, 1954–1962 10.1002/stem.118 [DOI] [PubMed] [Google Scholar]
- 108.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I.. et al. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 10.1016/j.stem.2007.11.014 [DOI] [PubMed] [Google Scholar]
- 109.Kovach T.K., Dighe A.S., Lobo P.I. and Cui Q. (2015) Interactions between MSCs and immune cells: implications for bone healing. J. Immunol. Res. 2015, 752510 10.1155/2015/752510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luz-Crawford P., Kurte M., Bravo-Alegria J., Contreras R., Nova-Lamperti E., Tejedor G.. et al. (2013) Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 4, 65 10.1186/scrt216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duffy M.M., Ritter T., Ceredig R. and Griffin M.D. (2011) Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2, 34 10.1186/scrt75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sioud M., Mobergslien A., Boudabous A. and Floisand Y. (2011) Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int. J. Oncol. 38, 385–390 10.3892/ijo.2010.869 [DOI] [PubMed] [Google Scholar]
- 113.Lepelletier Y., Lecourt S., Renand A., Arnulf B., Vanneaux V., Fermand J.P.. et al. (2010) Galectin-1 and semaphorin-3A are two soluble factors conferring T-cell immunosuppression to bone marrow mesenchymal stem cell. Stem Cells Dev. 19, 1075–1079 10.1089/scd.2009.0212 [DOI] [PubMed] [Google Scholar]
- 114.Gieseke F., Kruchen A., Tzaribachev N., Bentzien F., Dominici M. and Muller I. (2013) Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur. J. Immunol. 43, 2741–2749 10.1002/eji.201343335 [DOI] [PubMed] [Google Scholar]
- 115.Augello A., Tasso R., Negrini S.M., Amateis A., Indiveri F., Cancedda R.. et al. (2005) Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 35, 1482–1490 10.1002/eji.200425405 [DOI] [PubMed] [Google Scholar]
- 116.Djouad F., Plence P., Bony C., Tropel P., Apparailly F., Sany J.. et al. (2003) Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102, 3837–3844 10.1182/blood-2003-04-1193 [DOI] [PubMed] [Google Scholar]
- 117.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T.. et al. (2007) Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234 10.1182/blood-2006-02-002246 [DOI] [PubMed] [Google Scholar]
- 118.Aggarwal S. and Pittenger M.F. (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- 119.Meisel R., Zibert A., Laryea M., Gobel U., Daubener W. and Dilloo D. (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103, 4619–4621 10.1182/blood-2003-11-3909 [DOI] [PubMed] [Google Scholar]
- 120.Najar M., Rouas R., Raicevic G., Boufker H.I., Lewalle P., Meuleman N.. et al. (2009) Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy 11, 570–583 10.1080/14653240903079377 [DOI] [PubMed] [Google Scholar]
- 121.Yang S.H., Park M.J., Yoon I.H., Kim S.Y., Hong S.H., Shin J.Y.. et al. (2009) Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp. Mol. Med. 41, 315–324 10.3858/emm.2009.41.5.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghosh A.S. and Tergaonkar V. (2010) Telomeres and Inflammation: Rap1 Joins the Ends?, Taylor & Francis; [DOI] [PubMed] [Google Scholar]
- 123.Teo H., Ghosh S., Luesch H., Ghosh A., Wong E.T., Malik N.. et al. (2010) Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nat. Cell Biol. 12, 758 10.1038/ncb2080 [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y., Zhang Z., Gao F., Tse H.F., Tergaonkar V. and Lian Q. (2015) Paracrine regulation in mesenchymal stem cells: the role of Rap1. Cell Death Disease 6, e1932 10.1038/cddis.2015.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qiu B., Han W. and Tergaonkar V. (2015) NUCKS: a potential biomarker in cancer and metabolic disease. Clin. Sci. 128, 715–721 10.1042/CS20140656 [DOI] [PubMed] [Google Scholar]
- 126.Qiu B., Shi X., Wong Ee T., Lim J., Bezzi M., Low D.. et al. (2014) NUCKS Is a Positive Transcriptional Regulator of Insulin Signaling. Cell Rep. 7, 1876–1886 10.1016/j.celrep.2014.05.030 [DOI] [PubMed] [Google Scholar]
- 127.Pomerantz J.L. and Baltimore D. (1999) NF‐κB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK‐related kinase. EMBO J. 18, 6694–6704 10.1093/emboj/18.23.6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y., Chiu S., Liang X., Chai Y.-H., Qin Y., Wang J.. et al. (2017) Absence of NUCKS augments paracrine effects of mesenchymal stem cells-mediated cardiac protection. Exp. Cell Res. 356, 74–84 [DOI] [PubMed] [Google Scholar]
- 129.Cuerquis J., Romieu-Mourez R., Francois M., Routy J.P., Young Y.K., Zhao J.. et al. (2014) Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy 16, 191–202 10.1016/j.jcyt.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 130.Tang G.N., Li C.L., Yao Y., Xu Z.B., Deng M.X., Wang S.Y.. et al. (2016) MicroRNAs involved in asthma after mesenchymal stem cells treatment. Stem Cells Dev. 25, 883–896 10.1089/scd.2015.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang S.Y., Fan X.L., Yu Q.N., Deng M.X., Sun Y.Q., Gao W.X.. et al. (2017) The lncRNAs involved in mouse airway allergic inflammation following induced pluripotent stem cell-mesenchymal stem cell treatment. Stem Cell Res. Ther. 8, 2 10.1186/s13287-016-0456-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bustos M.L., Huleihel L., Meyer E.M., Donnenberg A.D., Donnenberg V.S., Sciurba J.D.. et al. (2013) Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl. Med. 2, 884–895 10.5966/sctm.2013-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Silva L.H.A., Antunes M.A., Dos Santos C.C., Weiss D.J., Cruz F.F. and Rocco P.R.M. (2018) Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 9, 45 10.1186/s13287-018-0802-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y., Chiu S., Liang X., Gao F., Zhang Z., Liao S.. et al. (2015) Rap1-mediated nuclear factor-kappaB (NF-κB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discovery 1, 15007 10.1038/cddiscovery.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pistoia V. and Raffaghello L. (2014) Unveiling the role of TNF-alpha in mesenchymal stromal cell-mediated immunosuppression. Eur. J. Immunol. 44, 352–356 10.1002/eji.201344372 [DOI] [PubMed] [Google Scholar]
- 136.Dorronsoro A., Ferrin I., Salcedo J.M., Jakobsson E., Fernandez-Rueda J., Lang V.. et al. (2014) Human mesenchymal stromal cells modulate T-cell responses through TNF-alpha-mediated activation of NF-kappaB. Eur. J. Immunol. 44, 480–488 10.1002/eji.201343668 [DOI] [PubMed] [Google Scholar]
- 137.Tong L. and Tergaonkar V. (2014) Rho protein GTPases and their interactions with NFkappaB: crossroads of inflammation and matrix biology. Biosci. Rep. 34, e00115 10.1042/BSR20140021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Y., Tergaonkar V., Krishna S. and Androphy E.J. (1999) Human papillomavirus type 16 E6-enhanced susceptibility of L929 cells to tumor necrosis factor alpha correlates with increased accumulation of reactive oxygen species. J. Biol. Chem. 274, 24819–24827 10.1074/jbc.274.35.24819 [DOI] [PubMed] [Google Scholar]
- 139.Xue Q., Luan X.Y., Gu Y.Z., Wu H.Y., Zhang G.B., Yu G.H.. et al. (2010) The negative co-signaling molecule b7-h4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity. Stem Cells Dev. 19, 27–38 10.1089/scd.2009.0076 [DOI] [PubMed] [Google Scholar]
- 140.Saldanha-Araujo F., Haddad R., Farias K.C., Souza Ade P., Palma P.V., Araujo A.G.. et al. (2012) Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: roles of canonical and non-canonical NF-kappaB signalling. J. Cell. Mol. Med. 16, 1232–1244 10.1111/j.1582-4934.2011.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Francois M., Romieu-Mourez R., Li M. and Galipeau J. (2012) Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195 10.1038/mt.2011.189 [DOI] [PubMed] [Google Scholar]
- 142.Gao S., Mao F., Zhang B., Zhang L., Zhang X., Wang M.. et al. (2014) Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappaB and signal transducer and activator of transcription 3 pathways. Exp. Biol. Med. 239, 366–375 10.1177/1535370213518169 [DOI] [PubMed] [Google Scholar]
- 143.Cho D.I., Kim M.R., Jeong H.Y., Jeong H.C., Jeong M.H., Yoon S.H.. et al. (2014) Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 46, e70 10.1038/emm.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ciccocioppo R., Bernardo M.E., Sgarella A., Maccario R., Avanzini M.A., Ubezio C.. et al. (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60, 788–798 10.1136/gut.2010.214841 [DOI] [PubMed] [Google Scholar]
- 145.Paliwal S., Chaudhuri R., Agrawal A. and Mohanty S. (2018) Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mahrouf-Yorgov M., Augeul L., Da Silva C.C., Jourdan M., Rigolet M., Manin S.. et al. (2017) Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 24, 1224–1238 10.1038/cdd.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M.. et al. (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Y., Yu Z., Jiang D., Liang X., Liao S., Zhang Z.. et al. (2016) iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-alpha yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7, 749–763 10.1016/j.stemcr.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen J., Zhang J.H., Xiao H., Wu J.M., He K.M., Lv Z.Z.. et al. (2018) Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis. 9, 81 10.1038/s41419-017-0145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Squillaro T., Peluso G. and Galderisi U. (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25, 829–848 10.3727/096368915X689622 [DOI] [PubMed] [Google Scholar]
- 151.Trounson A. and McDonald C. (2015) Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 152.Cruz F.F. and Rocco P.R.M. (2017) Stem-cell extracellular vesicles and lung repair. Stem Cell Investig. 4, 78 10.21037/sci.2017.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cruz F.F., Borg Z.D., Goodwin M., Sokocevic D., Wagner D.E., Coffey A.. et al. (2015) Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl. Med. 4, 1302–1316 10.5966/sctm.2014-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.de Castro L.L., Xisto D.G., Kitoko J.Z., Cruz F.F., Olsen P.C., Redondo P.A.G.. et al. (2017) Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 8, 151 10.1186/s13287-017-0600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abreu S.C., Weiss D.J. and Rocco P.R. (2016) Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases? Stem Cell Res. Ther. 7, 53 10.1186/s13287-016-0317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]