Abstract
Various miRNAs have been reported to regulate the chondrogenic differentiation of bone marrow mesenchymal stem cells (BMSCs); however, whether miR-134 plays a role in this biological process remains undetermined. In the present study, we first evaluated the chondrogenic differentiation of BMSCs by Alcian blue staining, and examined the miR-134 expression by quantitative real-time PCR (qRT-PCR) during this process. And miR-134 inhibitor was used to investigate the functions of miR-134 in chondrogenic differentiation of BMSCs by Alcian blue staining, qRT-PCR, and Western blot. Subsequently, the correlation between miR-134 and SMAD6 was assessed via bioinformatics analysis and dual-luciferase reporter assay. Finally, the role of SMAD6 in chondrogenic differentiation of BMSCs was also determined through Alcian blue staining, qRT-PCR, and Western blot. As results showed that miR-134 expression was significantly down-regulated during chondrogenic differentiation, and inhibition of miR-134 obviously promoted chondrogenic differentiation. Dual-luciferase reporter assay indicated that miR-134 could directly target the 3′-UTRs of SMAD6, inhibit miR-134 expression in BMSCs, and up-regulate SMAD6 expression. Moreover, we found that overexpression of SMAD6 significantly promoted chondrogenic differentiation, and that SMAD6-induced promotion of chondrogenic differentiation could be reversed by miR-134 mimics. In conclusion, our findings suggest that miR-134 may act as a negative regulator during chondrogenic differentiation of BMSCs by interacting with SMAD6.
Keywords: Bone marrow mesenchymal stem cells, Chondrogenic differentiation, miR-134, SMAD6
Introduction
The damage to articular cartilage, primarily caused by trauma and excessive mechanical conditions, is the most common clinical disease [1,2]. Articular cartilage damage in severe cases probably leads to osteoarthritis (OA), which subsequently causes disability and chronic pain, producing a significant social and economic burden in the world [3–5]. It was reported that the economic costs of OA in the United States were estimated at 15 billion dollars in 2011 alone, making OA the second most expensive disease [6]. The conservative interventions of OA mainly include injection of intra-articular hyaluronic acid, and oral non-steroidal anti-inflammatory drugs; however, these treatments could only alleviate chronic pain [7–9]. Although surgical arthroplasty is applied to treat severe degenerative joint diseases, it may cause several severe postoperative complications [10,11]. Therefore, it is essential to explore some new effective therapeutic measures for articular cartilage damage.
Bone marrow mesenchymal stem cells (BMSCs) are one of the mesenchymal stem cells that can be isolated and differentiated into multiple cell types in vitro, such as osteoblasts, adipocytes, and chondrocytes [12,13]. Due to chondrocytes’ participation in the formation of cartilage tissues, chondrogenic differentiation of BMSCs may play an important role in the regeneration of cartilage [14–16]. Therefore, BMSC transplantation is currently considered to be the most promising measure for OA therapy [17,18]. A variety of growth factors have shown the ability to induce chondrogenic differentiation; however, the application of growth factors may contribute to calcification, limiting their clinical use [19,20]. MiRNAs are endogenous and evolutionary conserved non-coding RNA molecules with 20–24 nucleotides [21,22]. Evidences have demonstrated that miRNAs can regulate the expressions of target genes by directly binding to 3′-UTRs of their mRNA [23,24]. Since the first description of miRNAs in 1993, numerous miRNAs have been identified, and functional assays showed that miRNAs may be involved in almost whole biological processes, including cell proliferation, differentiation, and tumorigenesis [25,26]. Recently, increasing studies have revealed that miRNAs may participate in the chondrogenic differentiation of BMSCs, implying that regulation of miRNAs may be a new strategy for chondrogenic differentiation inducement [27–29].
In the present study, we aimed to investigate the miR-134 roles during chondrogenic differentiation of BMSCs, and attempted to reveal the underlying mechanisms. Due to the bioinformatics analysis results showing that SMAD6 is a targetted gene of miR-134, we hypothesized that the effects of miR-134 on chondrogenic differentiation of BMSCs may be by targetting SMAD6. Therefore, we tested this hypothesis in the present study and demonstrated that miR-134 may act as a negative regulator during chondrogenic differentiation of BMSCs by targetting SMAD6.
Materials and methods
Cell culture and chondrogenic differentiation
BMSCs of Sprague–Dawley rat (catalog number RASMX-01001) were provided by Cyagen Biosciences, Inc. (Guangzhou, China) and cultured at 37°C with the Dulbecco’s modified Eagle’s medium (DMEM; Gibco, MD, U.S.A.), supplemented with 10% FBS, 10 mg/ml of streptomycin, and 10 U/ml of penicillin, in a 5% CO2 humidified incubator. For the chondrogenic differentiation of BMSCs, a Chondrogenic Differentiation Kit (Cyagen Bioscience, Inc., Santa Clara, CA, U.S.A.) was applied under the instruction provided by manufacturers. Briefly, BMSCs were cultured in modified DMEM medium, which supplemented with dexamethasone, ascorbate, sodium pyruvate, and transforming growth factor-β 3 (TGF-β), and the chondrogenic differentiation medium was refreshed every 2 days.
Alcian blue staining
After being cultured in normal DMEM or chondrogenic medium for 0, 14, and 21 days, BMSCs were fixed using 4% formalin for 30 min at 4°C. After being washed with PBS for 5 min, three times, BMSCs were then incubated with 1% Alcian blue 8GX (Sigma) at room temperature for 1 h. Subsequently, stained BMSCs were washed with deionized water for three times, and signals were detected by a flatbed scanner (HP, Palo Alto, CA, U.S.A.). Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD) was applied to quantitate the staining intensity of cells.
RNA extraction and quantitative real-time PCR assay
Total RNAs of culture BMSCs at 0, 14, and 21 days were extracted by an RNAiso Plus reagent (Takara Biotechnology, Japan) according to the protocols obtained from the manufacturer and the RNA concentration was determined using a spectrophotometer at 260 nm, and 2 μg of total RNAs were reverse transcribed into cDNA using BestarTM qPCR RT kit (#2220, DBI Bioscience, China). The qPCR reaction was performed using a BestarTM qPCR MasterMix kit (DBI Bioscience, China) with the Applied Biosystems 7500 PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The sequence of primers used in the present study is shown in Table 1. All primers were synthesized by Sangon, Shanghai, China. The miR-134 expression was normalized to U6. Collage II, SOX9, aggrecan, and SMAD6 expressions were normalized to GAPDH.
Table 1. Primer sequences for quantitative real-time PCR analysis.
| ID | Sequence (5′- 3′) |
|---|---|
| GAPDH F | CCTCGTCTCATAGACAAGATGGT |
| GAPDH R | GGGTAGAGTCATACTGGAACATG |
| Sox9 F | CCTAACGCCATCTTCAAGGC |
| Sox9 R | TTGCACGTCTGTTTTGGGAG |
| Aggrecan F | CTGTTATCGCCACTTTCCCG |
| Aggrecan R | CCCCTCTCATGCCAGATCAT |
| Col II F | GCCAGGATGCCCGAAAATTA |
| Col II R | CGTCAAATCCTCCAGCCATC |
| SMAD6 F | TTGCAACCCCTACCACTTCA |
| SMAD6 R | TTGGTGGCATCTGGAGACAT |
| U6 F | CTCGCTTCGGCAGCACA |
| U6 R | AACGCTTCACGAATTTGCGT |
| All R | CTCAACTGGTGTCGTGGA |
| rno-miR -134-5p | TGTGACTGGTTGACCAGAGGGG |
| rno-miR-134-5p F | ACACTCCAGCTGGGTGTGACTGGTTGACCA |
Cell transfection
Both miR-134 mimics and inhibitors were purchased from GenePharma (Shanghai, China). Before transfection, cells were seeded in 24-well plates and cultured at 37°C for 24 h, then 50 nM miR-134 mimics or inhibitors were added into the medium with lipofectamine 2000 (Invitrogen) and cultured for another 24 h. Nonsense control (NC) of miR-134 was used as control and transfected under the same condition.
Western blot assay
For protein extraction, the cultured BMSCs were lysed with RIPA buffer containing PMSF, and then cell lysis was centrifuged at 10000 g for 15 min at a low temperature. After collecting the total protein extracts, a BCA Protein Assay Kit (Beyotime, China) was used to quantitate the protein concentration. Each sample (40 μg) of protein was loaded and separated by 10% SDS-PAGE, and then transferred onto PVDF membranes (Millipore, U.S.A.). Subsequently, membranes were incubated with 5% non-fat milk in TBST at room temperature for 2 h to block non-specific sites. The membranes were then incubated with corresponding primary antibodies against collage II (1:5000, Rabbit, ab34712, Abcam), SOX9 (1:3000, Rabbit, ab185230, Abcam), aggrecan (1:1000, Rabbit, ab36861, Abcam), and SMAD6 (1:500, Rabbit, ab80049, Abcam) overnight at 4°C. After being washed with TBST for three times, the membranes were subjected to incubation with HRP-conjugated donkey-anti-rabbit IgG (1:10000, ab6802, Abcam) for 2 h. Finally, the target blots were visualized by an enhanced chemiluminescence reagent, and protein expressions of collage II, SOX9, aggrecan, and SMAD6 were normalized to GAPDH (1:10000, Rabbit, ab8245, Abcam).
Plasmid construction and dual-luciferase activity assay
The 3′-UTRs of SMAD6 including the target binding sites for miR-134 were chemically synthesized and cloned into pcDNA3.0 vector (Invitrogen) to form pcDNA3-SMAD6-WT plasmid, and the mutant SMAD6 binding sites were also cloned into pcDNA3.0 to produce pcDNA3-SMAD6-Mut plasmid. For the luciferase reporter assay, BMSCs (2 × 105 cells/well) were cultured in 24-well plates for 24 h, then 100 ng of pcDNA3-SMAD6-WT or pcDNA3-SMAD6-Mut plasmids were co-transfected into BMSCs cells with 100 pmol of miR-134 mimics or NC using Lipofectamine 2000 (Invitrogen) following the protocols obtained from manufacturers. The luciferase activity was determined by a Dual Luciferase Reporter Assay System (Promega), and firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Data of the present study were all expressed as mean ± SEM, and statistical analysis was performed by the Graphpad software (Ver. 7.0, U.S.A.). One-way analysis of variance was applied to assess the difference between means, and the difference between means was considered significant if P<0.05.
Results
miR-134 expression was down-regulated during chondrogenic differentiation of BMSCs
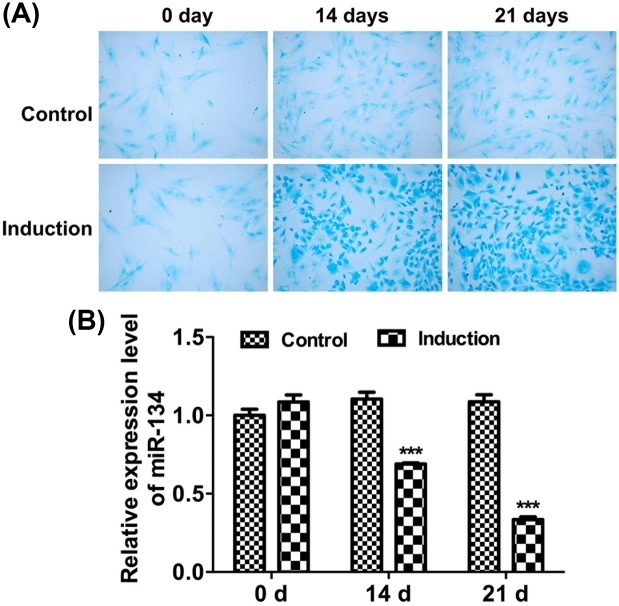
Since glycosaminoglycan deposition is a critical indicator of cartilage extracellular matrix accumulation, Alcian blue staining was performed to assess chondrogenic differentiation of BMSCs by examining glycosaminoglycan accumulation at 0, 14, and 21 days. As results indicated that staining intensity of Alcian blue was significantly increased at 14 and 21 days under chondrogenic medium (induction) (Figure 1A). To determine whether miR-134 plays a role in the chondrogenic differentiation of BMSCs, quantitative real-time PCR (qRT-PCR) assay was applied at 0, 14, and 21 days of chondrogenic differentiation to examine miR-134 expression. Results showed that the relative expression level of miR-134 was remarkably down-regulated during chondrogenic differentiation of BMSCs (Figure 1B).
Figure 1. miR-134 expression in BMSCs during chondrogenic differentiation.
(A) Alcian blue staining of BMSCs cultured in control or chondrogenic medium at 0, 14, and 21 days (10× magnification). (B) The relative mRNA expression was determined by qRT-PCR in BMSCs cultured in control or chondrogenic medium at 0, 14, and 21 days (***P<0.001).
Inhibition of miR-134 promoted chondrogenic differentiation of BMSCs
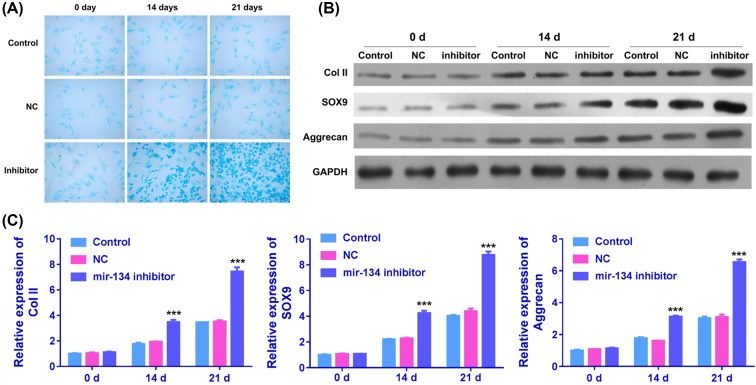
Inhibitor of miR-134 was transfected into BMSCs to evaluate whether miR-134 exhibits effects on chondrogenic differentiation. After treatment, BMSCs at 0, 14, and 21 days were stained with Alcian blue, and expressions of three chondrogenic differentiation markers: collage II, SOX9, and aggrecan were measured by qRT-PCR and Western blot assay. Results from Alcian blue staining indicated that staining intensity of BMSCs with miR-134 inhibitor transfection was obviously enhanced compared with control group and NC-treated group at 14 and 21 days (Figure 2A). Additionally, qRT-PCR and Western blot results suggested that BMSCs with miR-134 transfection significantly up-regulated the mRNA and protein expressions of collage II, SOX9, and aggrecan at 21 days (Figure 2B,C).
Figure 2. Effects of miR-134 inhibitor on chondrogenic differentiation of BMSCs.
(A) Chondrogenic differentiation of BMSCs treated with nothing, NC of miR-134, and miR-134 inhibitor was detected by Alcian blue staining. (B) The protein expressions of collage II, SOX9, and aggrecan were assessed with Western blot assay in control, NC, and inhibitor groups at 0, 14, and 21 days. (C) The relative mRNA expression levels of collage II, SOX9, and aggrecan were measured by qRT-PCR in miR-134 NC or inhibitor treated BMSCs at 0, 14, and 21 days (***P<0.001).
miR-134 directly targetted the 3′-UTRs of SMAD6
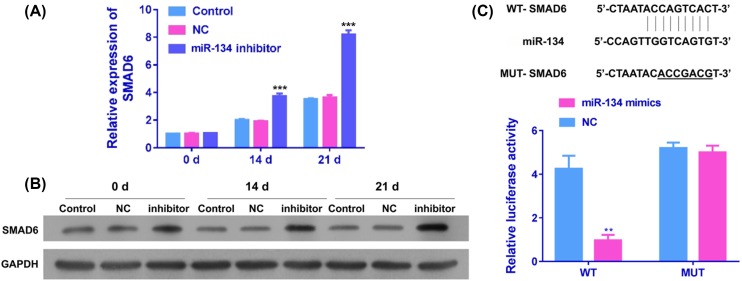
To explore the underlying mechanisms of miR-134 involved in the chondrogenic differentiation of BMSCs, bioinformatics analysis was applied to screen the targets of miR-134. According to the prediction results from Targetscan software, miR-134 was shown to have binding sites in the 3′-UTRs of SMAD6 (Figure 3C, upper panel). To further identify whether miR-134 directly targets SMAD6, luciferase reporter vectors were established with wild-type SMAD6 3′-UTRs (pcDNA3-SMAD6-WT) and mutated SMAD6 3′-UTRs (pcDNA3-SMAD6-Mut). In the luciferase report assay, when pcDNA3-SMAD6-WT and miR-134 mimics were co-transfected into BMSCs, the luciferase activity was significantly attenuated compared with miR-134 NC (Figure 3C, lower panel). Nevertheless, co-transfection of pcDNA3-SMAD6-Mut and miR-134 mimics or NC had no obvious influence on the luciferase activity (Figure 3C, lower panel). In addition, we evaluated the effects of miR-134 inhibition on mRNA and protein expression of SMAD6 in BMSCs by qRT-PCR and Western blot, respectively. As results indicated that SMAD6 mRNA and protein expression in BMSCs transfected with miR-134 inhibitor were significantly increased at day 21 (Figure 3A,B).
Figure 3. SMAD6 expression and correlation with miR-134.
The relative mRNA (A) and protein (B) expressions of SMAD6 were measured by qRT-PCR and Western blot, respectively, in miR-134 NC or inhibitor-treated BMSCs at 0, 14, and 21 days (***P<0.001). (C) Upper panel, the complementary site sequence of miR-134, SMAD6, and mutant SMAD6. Lower panel, luciferase activity was detected in BMSCs co-transfected with pcDNA3-SMAD6-WT or pcDNA3-SMAD6-Mut and miR-134 mimics or NC.
miR-134 inhibited chondrogenic differentiation of BMSCs via SMAD6
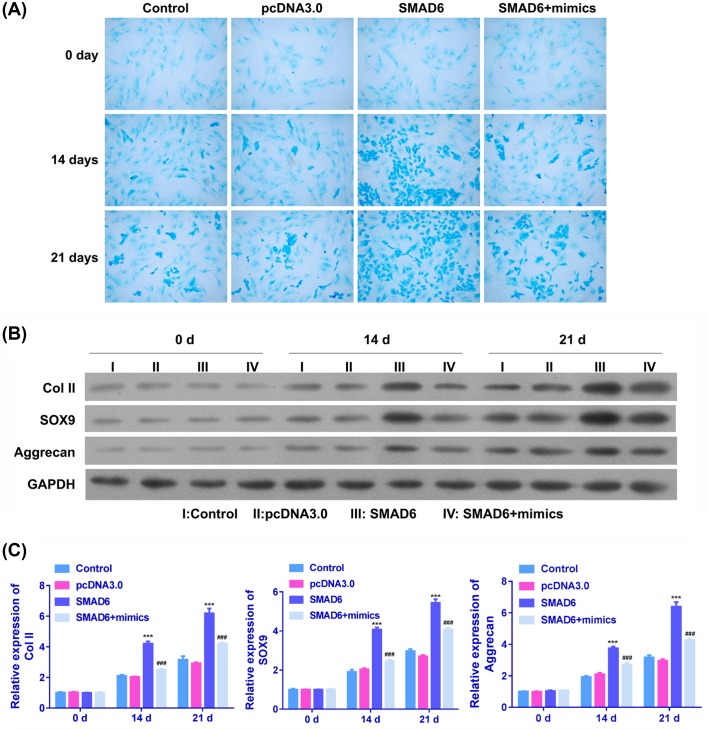
In order to further investigate the underlying mechanisms of interaction between miR-134 and SMAD6 during chondrogenic differentiation, BMSCs transfected with pcDNA 3.0 vector, SMAD6, and SMAD6 plus miR-134 mimics were subjected to Alcian blue staining analysis of chondrogenic differentiation, and qRT-PCR/Western blot analysis of collage II, SOX9, and aggrecan expressions. Alcian staining of BMSCs indicated that SMAD6 transfection remarkably increased the staining intensity compared with pcDNA3.0-transfected cells and blank control cells, and the application of miR-134 mimics could reverse the SMAD6-induced enhancement of intensity (Figure 4A). Moreover, results from qRT-PCR and Western blot indicated that collage II, SOX9, and aggrecan mRNA and protein expressions were significantly up-regulated in SMAD6-treated BMSCs compared with blank control cells and pcDNA3.0 vector-treated cells; however, the SMAD6-induced up-regulation of collage II, SOX9, and aggrecan could be abolished by miR-134 mimics (Figure 4B,C).
Figure 4. Effects of SMAD6 on chondrogenic differentiation of BMSCs.
(A) Chondrogenic differentiation of BMSCs treated with nothing, pcDNA3.0 vector, SMAD6, and SMAD6 plus miR-134 mimics was imaged at 0, 14, and 21 days. (B) Western blot analysis of collage II, SOX9, and aggrecan protein expressions in BMSCs treated with nothing, pcDNA3.0 vector, SMAD6, and SMAD6 plus miR-134 mimics. (C) The qRT-PCR analysis of collage II, SOX9, and aggrecan mRNA expressions in BMSCs treated with nothing, pcDNA3.0 vector, SMAD6, and SMAD6 plus miR-134 mimics (***P<0.001 compared with control, ###P<0.001 compared with SMAD6).
Discussion
Cartilage injuries may be caused by excessive loading on joint, inflammation, and foreign body intrusion [30,31]. Virtually, cartilage could not regenerate or self-heal upon damages or degeneration, because of its low cell density and poor proliferative ability [32,33]. Currently, cartilage tissue regeneration and engineering are two primary treatment methods for cartilage injuries, the former includes all in vivo measures that aim to produce new tissues in situ, while the latter is mainly based on in vitro methods that attempt to engineer neo-cartilage tissues, which can be subsequently implanted into the patients [34–36]. Due to the requirement of donor material and its invasive character, the application of cartilage tissue regeneration is extremely limited, and cartilage tissue engineering obtained quick development during the past decades [37,38]. BMSCs are multipotent cells that could differentiate into musculoskeletal lineages, including chondrocytes, osteoblasts, and myocytes, making BMSCs the most appropriate cell source for cartilage tissue engineering [39,40].
The chondrogenesis process of BMSCs in vitro can be directed by multiple stimulus, such as growth factors, cell–matrix interaction, and mechanical loads [41]. Recently, miRNAs were also demonstrated to be involved in the process of chondrogenesis by interacting with several targetted genes [42,43]. An investigation about miRNA expression profiles was performed by Yang et al. [44] in MSCs during chondrogenic differentiation, and results indicated that eight increased miRNAs and five decreased miRNAs. Moreover, miR-30a was revealed to be up-regulated during chondrogenic differentiation of BMSCs, and miR-30a can promote this process by inhibiting delta-like 4 expression [45]. In addition, miR-145 and miR-495 were reported to be down-regulated, and they can both inhibit chondrogenesis by interacting with the critical chondrogenic transcription factor, SOX9 [46,47]. In the present study, we found that miR-134 expression was significantly down-regulated during chondrogenesis, and this process can be promoted in BMSCs treated with miR-134 inhibitor.
SMADs, the critical mediator of canonical TGF-β signaling pathway, are proteins that are responsible for transducing the TGF-β signal into nucleus. Previous studies revealed that SMADs could regulate the corresponding gene expressions by interacting with transcription factors in the nucleus when TGF-β signaling is activated [48–50]. Dysfunctions of SMAD proteins were presented in numerous diseases, such as kidney, renal, and heart diseases [51–53]. Recently, miR-140 and miR-199 were reported to modulate the process of chondrogenesis by regulating the expressions of SMAD3 and SMAD1, respectively [54,55]. Based on the results from bioinformatics analysis and dual-luciferase reporter assay, we found that SMAD6 was a targetted gene for miR-134, and inhibition of miR-134 in BMSCs could up-regulate the SMAD6 expression. Additionally, transfection with SMAD6 obviously promoted chondrogenesis of BMSCs, and this promotion could be abolished by miR-134 mimics. To our best knowledge, this is the first evidence of miR-134/SMAD6 axis participating in the chondrogenesis of BMSCs.
In conclusion, we revealed that miR-134 may serve as an important negative regulator during chondrogenic differentiation of BMSCs by targetting SMAD6. Further studies are also needed to validate and explore the deeper mechanisms and functions of miR-134 during chondrogenic differentiation of BMSCs by SMAD6. For example, inhibition of miR-134 followed by knockdown of SMAD6 will be performed to explore whether miR-134 can inhibit chondrogenic differentiation of BMSCs via SMAD6.
Abbreviations
- BMSC
bone marrow mesenchymal stem cell
- Col
collage
- DMEM
Dulbecco’s modified Eagle’s medium
- GAPDH
glyceraldehyde phosphate dehydrogenase
- HRP
horseradish peroxidase
- MSC
Mesenchymal stem cells
- OA
osteoarthritis
- qRT-PCR
quantitative real-time PCR
- RIPA
Radio Immunoprecipitation Assay
- TBST
Tris-Buffered Saline Tween
- TGF-β
transforming growth factor-β
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by Zheng Zhou Orthopaedics Hospital.
Author contribution
S.G.X. and X.J.W. designed the experiment, interpreted the data, and wrote the manuscript.
References
- 1.Johnstone B., Alini M., Cucchiarini M.. et al. (2013) Tissue engineering for articular cartilage repair–the state of the art. Eur. Cell Mater. 25, 248–267 10.22203/eCM.v025a18 [DOI] [PubMed] [Google Scholar]
- 2.Vega J., Golano P. and Pena F. (2016) Iatrogenic articular cartilage injuries during ankle arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 24, 1304–1310 10.1007/s00167-014-3237-5 [DOI] [PubMed] [Google Scholar]
- 3.Houard X., Goldring M.B. and Berenbaum F. (2013) Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 15, 375 10.1007/s11926-013-0375-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okubo M. and Okada Y. (2013) Destruction of the articular cartilage in osteoarthritis. Clin. Calcium 23, 1705–1713 [PubMed] [Google Scholar]
- 5.Goldring M.B. and Goldring S.R. (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 1192, 230–237 10.1111/j.1749-6632.2009.05240.x [DOI] [PubMed] [Google Scholar]
- 6.Sen R. and Hurley J.A. (2018) Osteoarthritis, StatPearls, Treasure Island (FL), U.S.A. [Google Scholar]
- 7.Wang Z., Li K., Sun H., Wang J., Fu Z. and Liu M. (2018) Icariin promotes stable chondrogenic differentiation of bone marrow mesenchymal stem cells in selfassembling peptide nanofiber hydrogel scaffolds. Mol. Med. Rep. 17, 8237–8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taruc-Uy R.L. and Lynch S.A. (2013) Diagnosis and treatment of osteoarthritis. Prim. Care 40, 821–836, vii 10.1016/j.pop.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Lluch Girbes E., Nijs J., Torres-Cueco R. and Lopez Cubas C. (2013) Pain treatment for patients with osteoarthritis and central sensitization. Phys. Ther. 93, 842–851 10.2522/ptj.20120253 [DOI] [PubMed] [Google Scholar]
- 10.Hart L. (2012) Prevalence of osteoarthritis and arthroplasty in the hip and knee of former elite athletes. Clin. J. Sport Med. 22, 524–526 10.1097/JSM.0b013e318275d58c [DOI] [PubMed] [Google Scholar]
- 11.Proffen B., Vavken P. and Dorotka R. (2013) Surgical management of osteoarthritis. Wien. Med. Wochenschr. 163, 243–250 10.1007/s10354-013-0199-z [DOI] [PubMed] [Google Scholar]
- 12.Charbord P. (2010) Bone marrow mesenchymal stem cells: historical overview and concepts. Hum. Gene Ther. 21, 1045–1056 10.1089/hum.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Wang Q. and Zhang X.M. (2014) Progress on bone marrow mesenchymal stem cells transplantation for spinal cord injury. Zhongguo Gu Shang 27, 437–440 [PubMed] [Google Scholar]
- 14.Kassem M., Kristiansen M. and Abdallah B.M. (2004) Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin. Pharmacol. Toxicol. 95, 209–214 10.1111/j.1742-7843.2004.pto950502.x [DOI] [PubMed] [Google Scholar]
- 15.Veronesi F., Giavaresi G., Tschon M., Borsari V., Nicoli Aldini N. and Fini M. (2013) Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 22, 181–192 10.1089/scd.2012.0373 [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Wang Y., Gou W., Lu Q., Peng J. and Lu S. (2013) Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int. Orthop. 37, 2491–2498 10.1007/s00264-013-2059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn J.D. and Whartenby K.A. (2014) Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 6, 526–539 10.4252/wjsc.v6.i5.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song F., Tang J., Geng R.. et al. (2014) Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int. J. Clin. Exp. Pathol. 7, 1415–1426 [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller M.B., Fischer M., Zellner J.. et al. (2013) Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int. Orthop. 37, 945–951 10.1007/s00264-013-1800-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelttari K., Winter A., Steck E.. et al. (2006) Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 54, 3254–3266 10.1002/art.22136 [DOI] [PubMed] [Google Scholar]
- 21.Greenberg D.S. and Soreq H. (2014) MicroRNA therapeutics in neurological disease. Curr. Pharm. Des. 20, 6022–6027 10.2174/1381612820666140314151924 [DOI] [PubMed] [Google Scholar]
- 22.Erson-Bensan A.E. (2014) Introduction to microRNAs in biological systems. Methods Mol. Biol. 1107, 1–14 10.1007/978-1-62703-748-8_1 [DOI] [PubMed] [Google Scholar]
- 23.Li Z. and Rana T.M. (2014) Therapeutic targeting of microRNAs: current status and future challenges. Nat. Rev. Drug Discov. 13, 622–638 10.1038/nrd4359 [DOI] [PubMed] [Google Scholar]
- 24.Schmiedel J.M., Klemm S.L., Zheng Y.. et al. (2015) Gene expression. MicroRNA control of protein expression noise. Science 348, 128–132 10.1126/science.aaa1738 [DOI] [PubMed] [Google Scholar]
- 25.Su Q.L., Li S.Q., Wang D.N., Liu F. and Yuan B. (2014) Effects of microRNA-10b on lung cancer cell proliferation and invasive metastasis and the underlying mechanism. Asian Pac. J. Trop. Med. 7, 364–367 10.1016/S1995-7645(14)60056-0 [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Lai N., Liao K., Sun J. and Lin Y. (2015) MicroRNA-210 regulates cell proliferation and apoptosis by targeting regulator of differentiation 1 in glioblastoma cells. Folia Neuropathol. 53, 236–244 10.5114/fn.2015.54424 [DOI] [PubMed] [Google Scholar]
- 27.Mirzamohammadi F., Papaioannou G. and Kobayashi T. (2014) MicroRNAs in cartilage development, homeostasis, and disease. Curr. Osteoporos Rep. 12, 410–419 10.1007/s11914-014-0229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellavia D., Veronesi F., Carina V.. et al. (2018) Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol. Life Sci. 75, 649–667 10.1007/s00018-017-2637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellavia D., Raimondi L., Costa V.. et al. (2018) Engineered exosomes: a new promise for the management of musculoskeletal diseases. Biochim. Biophys. Acta 1862, 1893–1901 10.1016/j.bbagen.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 30.Hayes D.W. Jr, Brower R.L. and John K.J. (2001) Articular cartilage. Anatomy, injury, and repair. Clin. Podiatr. Med. Surg. 18, 35–53 [PubMed] [Google Scholar]
- 31.Brittberg M. and Winalski C.S. (2003) Evaluation of cartilage injuries and repair. J. Bone. Joint Surg. Am., 85–A, 58–69 [DOI] [PubMed] [Google Scholar]
- 32.Meyer U., Wiesmann H.P., Libera J., Depprich R., Naujoks C. and Handschel J. (2012) Cartilage defect regeneration by ex vivo engineered autologous microtissue–preliminary results. In Vivo 26, 251–257 [PubMed] [Google Scholar]
- 33.Pathria M.N., Chung C.B. and Resnick D.L. (2016) Acute and stress-related injuries of bone and cartilage: pertinent anatomy, basic biomechanics, and imaging perspective. Radiology 280, 21–38 10.1148/radiol.16142305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camarero-Espinosa S., Rothen-Rutishauser B., Foster E.J. and Weder C. (2016) Articular cartilage: from formation to tissue engineering. Biomater. Sci. 4, 734–767 10.1039/C6BM00068A [DOI] [PubMed] [Google Scholar]
- 35.Doran P.M. (2015) Cartilage tissue engineering: what have we learned in practice? Methods Mol. Biol. 1340, 3–21 10.1007/978-1-4939-2938-2_1 [DOI] [PubMed] [Google Scholar]
- 36.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C. and Athanasiou K.A. (2015) Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 11, 21–34 10.1038/nrrheum.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seol Y.J., Park J.Y., Jeong W., Kim T.H., Kim S.Y. and Cho D.W. (2015) Development of hybrid scaffolds using ceramic and hydrogel for articular cartilage tissue regeneration. J. Biomed. Mater. Res. A 103, 1404–1413 10.1002/jbm.a.35276 [DOI] [PubMed] [Google Scholar]
- 38.Tuan R.S., Chen A.F. and Klatt B.A. (2013) Cartilage regeneration. J. Am. Acad. Orthop. Surg. 21, 303–311 10.5435/JAAOS-21-05-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S. and Zhu L. (2008) Progress in the study of articular cartilage tissue engineering seeding cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 22, 1505–1507 [PubMed] [Google Scholar]
- 40.Xue J.X., Gong Y.Y., Zhou G.D., Liu W., Cao Y. and Zhang W.J. (2012) Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials 33, 5832–5840 10.1016/j.biomaterials.2012.04.054 [DOI] [PubMed] [Google Scholar]
- 41.Wang H., Li Y., Chen J., Wang X., Zhao F. and Cao S. (2014) Chondrogenesis of bone marrow mesenchymal stem cells induced by transforming growth factor beta3 gene in Diannan small-ear pigs. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 28, 149–154 [PubMed] [Google Scholar]
- 42.Wu C., Tian B., Qu X.. et al. (2014) MicroRNAs play a role in chondrogenesis and osteoarthritis (review). Int. J. Mol. Med. 34, 13–23 10.3892/ijmm.2014.1743 [DOI] [PubMed] [Google Scholar]
- 43.Dong S., Yang B., Guo H. and Kang F. (2012) MicroRNAs regulate osteogenesis and chondrogenesis. Biochem. Biophys. Res. Commun. 418, 587–591 10.1016/j.bbrc.2012.01.075 [DOI] [PubMed] [Google Scholar]
- 44.Yang B., Guo H., Zhang Y., Dong S. and Ying D. (2011) The microRNA expression profiles of mouse mesenchymal stem cell during chondrogenic differentiation. BMB Rep. 44, 28–33 10.5483/BMBRep.2011.44.1.28 [DOI] [PubMed] [Google Scholar]
- 45.Tian Y., Guo R., Shi B., Chen L., Yang L. and Fu Q. (2016) MicroRNA-30a promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting Delta-like 4 expression. Life Sci. 148, 220–228 10.1016/j.lfs.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 46.Yang B., Guo H., Zhang Y., Chen L., Ying D. and Dong S. (2011) MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 6, e21679 10.1371/journal.pone.0021679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S., Yoon D.S., Paik S., Lee K.M., Jang Y. and Lee J.W. (2014) microRNA-495 inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9. Stem Cells Dev. 23, 1798–1808 10.1089/scd.2013.0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hata A. and Chen Y.G. (2016) TGF-β Signaling from Receptors to Smads. Cold Spring Harb Perspect Biol 8, 10.1101/cshperspect.a022061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazawa K. and Miyazono K. (2017) Regulation of TGF-β Family Signaling by Inhibitory Smads. Cold Spring Harb Perspect Biol 9, 10.1101/cshperspect.a022095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan X., Liao H., Cheng M., Shi X., Lin X., Feng X.H. and Chen Y.G. (2016) Smad7 Protein Interacts with Receptor-regulated Smads (R-Smads) to Inhibit Transforming Growth Factor-β (TGF-β)/Smad Signaling. J. Biol. Chem. 291, 382–92 10.1074/jbc.M115.694281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Wang S., Liu S., Li C. and Wang J. (2015) Role of Smad signaling in kidney disease. Int. Urol. Nephrol. 47, 1965–1975 10.1007/s11255-015-1115-9 [DOI] [PubMed] [Google Scholar]
- 52.Meng X.M., Chung A.C. and Lan H.Y. (2013) Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin. Sci. 124, 243–254 10.1042/CS20120252 [DOI] [PubMed] [Google Scholar]
- 53.Lu X.L., Yao X.L., Yan C.Y., Wan Q.L. and Li Y.M. (2016) Functional role of NKX2-5 and Smad6 expression in developing rheumatic heart disease. Eur. Rev. Med. Pharmacol. Sci. 20, 715–720 [PubMed] [Google Scholar]
- 54.Lin E.A., Kong L., Bai X.H., Luan Y. and Liu C.J. (2009) miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J. Biol. Chem. 284, 11326–11335 10.1074/jbc.M807709200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pais H., Nicolas F.E., Soond S.M.. et al. (2010) Analyzing mRNA expression identifies Smad3 as a microRNA-140 target regulated only at protein level. RNA 16, 489–494 10.1261/rna.1701210 [DOI] [PMC free article] [PubMed] [Google Scholar]