Abstract
Objective
Glucagon-like-peptide-1 (GLP-1) receptor analogues have been shown to reduce cardiovascular events in patients with type 2 diabetes. However, the mechanism behind is still unknown. The aim of the study was to investigate the effect of intact GLP-1 (7–36) on coronary microcirculation in overweight adults.
Design and methods
A double-blinded randomized cross-over study was performed, with 12 overweight participants. Effects of intact GLP-1 (7-36) infusion were compared with a saline infusion on separate days. A DPP-4 inhibitor was administered to block degradation of intact GLP-1 (7–36) to the GLP-1 metabolite (9–36). Coronary microcirculation was assessed by Doppler coronary flow velocity reserve (CFVR) before and after 2 h of infusion. Peripheral endothelial function was assessed by flow mediated dilation (FMD) before and after one hour of infusion.
Results
CFVR was 3.77 ± 1.25 during GLP-1 infusion and 3.85 ± 1.32 during saline infusion, endothelial function was 16.3 ± 15.5 % during GLP-1 infusion and 7.85 ± 7.76 % during saline infusion. When adjusting for baseline values no significant differences in CFVR (ΔCFVR 0.38 ± 0.92 vs. ΔCFVR 0.71 ± 1.03, p = 0.43) and no difference in peripheral endothelial function (ΔFMD 7.34 ± 11.5 % vs. ΔFMD –1.25 ± 9.23%, p = 0.14) was found.
Conclusions
We found no effect of intact GLP-1 (7–36), protected from DPP4 mediated degradation on coronary microcirculation in overweight adults.
Keywords: Coronary microcirculation; Coronary flow velocity reserve; Glucagon-like peptide-1 (7–36); GLP-1, flow mediated dilation; Endothelial function
Abbreviations: GLP-1, glucagon-like peptide-1; MACE, major adverse cardiac event; CFVR, coronary flow velocity reserve; CMD, coronary microvascular dysfunction; FMD, flow mediated dilation; DPP-4, dipeptidyl peptidase-4; TTDE, trans-thoracic Doppler echocardiography; LAD, left anterior descending artery; RPP, rate pressure product; QC, quality control; NMD, nitroglycerine-mediated dilation
1. Introduction
Glucagon-like peptide-1 (GLP-1), an incretin hormone produced by intestinal L-cells in response to meal intake, acts to reduce postprandial glucose levels [1,2]. However GLP-1 receptors have been localized in most organs of the human body including the heart [3,4] and in addition to its well-characterized glycaemic actions, studies in both animals and humans have repeatedly demonstrated a beneficial action of GLP-1 on the cardiovascular system [[5], [6], [7]]. However, the physiological effects of GLP-1 on the heart still raise many questions. In patients with type 2 diabetes, semaglutide, liraglutide and albiglutide (GLP-1 analogues) have been shown to reduce the risk of cardiovascular events and mortality in large prospective outcome trials [[8], [9], [10]], but the mechanism behind is still unknown.
Coronary microvascular circulation is the ability to increase vasodilator reserve of the small coronary arteries in response to increase oxygen demand and thus coronary microvascular dysfunction (CMD) is defined as reduced vasodilator reserve of the small coronary arteries in response to increased oxygen demand. CMD may precede development of macrovascular atherosclerosis [11,12] and is associated with increased cardiovascular mortality [13]. Type 2 diabetes and obesity are associated with CMD [13,14]. A former study performed by our group indicated that long-term treatment with liraglutide, a GLP-1 receptor agonist, may improve CMD in patients with type 2 diabetes [15]. However, hyperglycaemia per se seems to impair coronary microvascular function [16] and several studies have shown that weight loss improves CMD. Therefore, the effect of long-term treatment with GLP-1 analogues on CMD may be indirect caused by weight loss and improvements in HbA1c.
Both endothelial dependent and independent pathways may lead to coronary microvascular dysfunction [17,18]. As CMD, endothelial dysfunction may be observed prior to development of macrovascular atherosclerosis and is associated with obesity and type 2 diabetes [19,20].
The aim of the present study was to investigate the effect of intact GLP-1 on coronary microvascular function and peripheral endothelial function in obese individuals without diabetes.
2. Method
2.1. Study protocol
The study was designed and performed in accordance to the Helsinki Declaration of Good Clinical Practise and approved by the ethical committee of Region Hovedstaden, Denmark, and the Danish Health and Medicines Authority. All participants gave oral and written consent before participation.
The study was registered at www.clinicaltrials.gov (NCT02333591).
2.2. Study population
Thirteen overweight adults were included in the study, but one was excluded due to side-effects. Twelve overweight adults (7 male/5 female) completed, all were healthy, non-smokers with no history of cardiovascular disease. Participants who met inclusion criteria underwent a treadmill exercise test with two-dimensional echocardiography, performed by experienced cardiologists, to exclude coronary macrovascular disease.
2.3. Study design
A randomised, double-blinded, crossover study was conducted, consisting of two infusion treatments: (1) 2.5 h intravenous infusion of intact GLP-1 (7–36) in combination with oral administration of DPP-4 inhibitor (100 mg Sitagliptin taken the night before infusion and 100 mg Sitagliptin taken immediately before infusion) (2) 2.5 h intravenous infusion of saline, with a washout period of at least 24 h between infusion 1 and 2. DPP-4 inhibitor is added to prevent degradation of GLP-1 (7–36) to the GLP-1 metabolite, which would otherwise occur rapidly by the enzyme depetidyl-peptidase-4 (DPP-4).
All participants received both treatments in a randomised order after an over-night fast. They refrained from caffeine and food containing significant amounts of methylxanthine (coffee, tea, chocolate, cola, and bananas) for 24 h prior to the treatments.
Coronary flow velocity reserve (CFVR) was measured by trans-thoracic Doppler flow echocardiography (TTDE) of the left anterior descending artery (LAD) before every infusion, and after 2 h of infusion. Peripheral endothelial function was measured by flow mediated dilation (FMD) before every infusion and after 1 h of each infusion. Heart rate and blood pressure were measured using an electric sphygmomanometer according to standardized procedures every 30 min during infusion. Venous blood samples were drawn for 7-point measurements for every infusion period; the first two were drawn at baseline with 15 minute interval, and thereafter every half hour during the infusion. The last sample was drawn just before infusion was discontinued. Blood samples were analysed for glucose, insulin, C-peptide, glucagon, total GLP-1 (i.e., GLP-1 (7–36) + GLP-1 (9–36)) and GLP-1 (7–36). A DPP-4 inhibitor (valine pyrrolidide; 0.01 mmol/L) was added to chilled EDTA tubes for GLP-1 (7–36) measurement to prevent post-sampling degradation of GLP-1.
2.4. Peptide
Purified GLP-1 (7–36) ready for human use was purchased as a Clinalfa product from Bachem AG (Bubendorf, Switzerland), and was stored frozen at −20° until use. On the examination day, GLP-1 was diluted in saline and human serum albumin was added to the solution to a final albumin concentration of 2% to avoid binding of the compound to the infusion material.
A gradually declining bolus infusion was given during the first 10 min (5.91 pmol/kg/min until time 2 min, 2.53 until time 4 min, 2.34 until time 6 min, 2.2 until time 8 min, 2.02 until time 10 min). From time 10 min, the infusion rate was 1.5 pmol/kg/min until the end of examination, expected to result in a “therapeutic”, slightly supraphysiological plasma concentration of GLP-1 of approximately 100 pmol/l [21].
2.5. Analyses
Plasma glucose was measured by the glucose oxidase method, using a glucose analyser (Yellow Springs Instrument, YSI Inc., Yellow Springs, OH). Serum insulin and C-peptide levels were determined using the IMMULITE 2000 immunoassay system (Siemens Healthcare, Erlangen, Germany). Glucagon and GLP-1 concentrations in plasma were measured after extraction of plasma with 70% ethanol. The glucagon radioimmunoassay was directed against the C-terminus of the glucagon molecule (antibody code no. 4305) and therefore mainly measures glucagon of pancreatic origin [22,23]. Plasma concentrations of GLP-1 were measured [24] against standards of synthetic GLP-1 (7–36) amide using antiserum code no. 89390, which is specific for the amidated C-terminus of GLP-1 and therefore mainly reacts with GLP-1 of intestinal origin. The assay reacts equally with intact GLP-1 and with GLP-1 (9–36) amide, the primary metabolite. For both assays, sensitivity was below 1 pmol/l, intraassay coefficient of variation below 6% at 20 pmol/l, and recovery of standard, added to plasma before extraction, about 100% when corrected for losses inherent in the plasma extraction procedure.
Intact GLP-1 (7–36) was measured using a 2-site sandwich assay involving 2 monoclonal antibodies; C-terminal GLP-1F5 and N-terminal Mab26.1 [25]. Standards are GLP-1 (7–36) amide prepared in human plasma depleted of endogenous intact GLP-1 immunoreactivity. Detection limit <0.5 pmol/l. Intra- and inter-assay coefficient of variations are 2 and 5%, respectively. Quality control (QC) samples (“low” and “high”) are included in each assay, alongside the samples. HOMA-IR was obtained using the HOMA2 calculator (available at www.dtu.ox.ac.uk/homacalculator/), using fasting concentrations of glucose and insulin from the first day of examination.
2.6. Echocardiography
Echocardiographic examinations with measurement of coronary flow velocities were performed using a GE Healthcare Vivid E9 cardiovascular ultrasound system (GE Healthcare, Horten, Norway) with a 2.7–8 MHz transducer (GE Vivid 6S probe) for TTDE. Two experienced echocardiographers performed all examinations in the same setting. All four examinations of each participant were performed by the same echocardiographer. The echocardiographer was blinded to the infusion product.
As previously described [26], coronary flow velocities were measured in the LAD by pulsed-wave Doppler as a laminar flow towards the transducer during rest and high-dose adenosine infusion over 6 min (0.14 mg/kg/min). CFVR was calculated as the ratio between peak velocities during stress and during rest. Every examination was analysed independently by two experienced echocardiographers blinded to subject data. The second reading was used except in case of discrepancies (estimates differing by >0.2) in which case the examination was re-evaluated by the two analysers and an agreement was reached.
In the absence of major coronary artery stenosis, CFVR measures coronary microcirculation. The regarded reference standard for measuring coronary microcirculation is by positron emission tomography (PET). However, previous validation studies have shown high inter- and intra-observer reproducibility of CFVR measured by TTDE [[27], [28], [29]].
Rate pressure product (RPP), a measurement of myocardial demand, was calculated as heart rate multiplied by systolic blood pressure. CFVR was corrected for rate pressure product by dividing coronary flow velocities at rest by the rate pressure product and multiplying with 10,000 [27].
2.7. Peripheral vascular function by flow-mediated dilation
Endothelial function was measured by FMD according to guidelines [30]. Examinations were done before and after 60 min of infusion. To minimize potentially negative effects of environmental and physiological influences, participants rested for at least 20 min in a room with monitored temperature (22–25 °C), prior to examination. The ultrasound transducer was placed parallel to the brachial artery 3–5 cm proximally of the antecubital fossa. When high quality image of the brachial artery was acquired, baseline arterial diameter was determined continuously for 1 min, followed by rapid cuff inflation to occlusion at 300 mm Hg for 5 min. Flow and diameter of the brachial artery were measured continuously from 30 s before rapid cuff deflation and during the following 2.5 min. After 10 minute resting period, the endothelium-independent response (nitroglycerine-mediated dilation (NMD)) was performed. Ultrasound images were continuously recorded and obtained using the same GE Healthcare Vivid E9 cardiovascular ultrasound system with a 10 MHz linear array transducer (GE Vivid 9L probe) in duplex mode.
Image analyses were performed offline using state-of-the-art automatic edge-detection software (Vascular Tools 6, MIA, LLC, IA, USA). Peak dilation within 2.5 min from cuff deflation, FMD, was determined as the maximum 5-second averaged diameter in percent of the baseline diameter.
The unscaled FMD, the NMD response, the resting and peak arterial diameter as well as time to peak, were reported.
2.8. Calculations and statistical analysis
Sample size was estimated prior to study commencement: 10 subjects should complete the study based on the ability to detect a 0.3 change in CFVR with a power of 80% and a two-sided significance level of 0.05. A change of 0.3 in CFVR was considered of clinical importance.
CFVR (with and without correction for RPP), FMD, blood pressure, pulse and blood sample levels from the two infusion days were compared. Results are shown as mean or as delta values with standard deviation on each examination day. Linear mixed modelling was used for the analysis of repeated measures and followed by the post hoc Bonferroni's test. The effect of the intervention on CFVR and FMD was analysed using linear mixed modelling with subject-specific and subject-within-visit random effects. The model included a time-treatment-visit-interaction. A potential carry-over effect was adjusted for. Differences resulting in values of p < 0.05 were considered significant.
The graphical illustrations were performed using GraphPad Prism (version 7.02; GraphPad software) and statistical analyses were performed by R (version 3.3.2.).
3. Results
Twelve subjects completed the study, mean age was 54 ± 9.7 years and mean BMI was 30.9 ± 2.9 kg/m2. All had central obesity, measured by waist circumference, of 104 ± 9 cm. HOMA2 IR was 0.81 ± 0.50 and HbA1c was 36.8 ± 2.3 mmol/mol (Supplemental Table I).
3.1. Glucose
A time × intervention interaction was found in the repeated measure of plasma glucose (PG) (p ≤ 0.0001) (Supplemental Fig. I). Baseline PG did not differ between infusion days, but PG concentration decreased during infusion with GLP-1. The mean difference in PG between the two infusions measured just before the CFVR examination was 0.5 mmol/l (4.9 vs 5.4 mmol/l, p = 0.02). However, PG stayed within normal range throughout infusions with a nadir of 4.0 mmol/l during GLP-1 infusion.
3.2. Vital signs
Throughout the GLP-1 infusion heart rate increased from 57 ± 10 beats/min to 64 ± 11 beats/min (p = 0.0004) (Table 1). Just before CFVR measurement heart rate was increased by 6 beats/min (p = 0.009) compared to baseline, and by 7 beats/min compared to saline infusion (p = 0.0002) (Supplemental Fig. IIA). We found a significant increase in average systolic blood pressure measured throughout the GLP-1 infusion compared to baseline (from 125 ± 11 to 138 ± 13 mm Hg, p = 0.007) (Supplemental Fig. IIB). However, no significant difference was found when comparing delta values of systolic blood pressure between the two infusion days (Table 1).
Table 1.
Vital signs.
| Saline |
GLP-1 |
Intervention effect |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | During infusion | p | T = 120 | p | Baseline | During infusion | p | T = 120 | p | Saline Δ | GLP-1 Δ | Estimate | p⁎ | |
| Heart rate (beats/min) | 53 ± 10 | 56 ± 10 | 0.02 | 56 ± 10 | 0.08 | 57 ± 10 | 64 ± 11 | 0.0004 | 63 ± 11 | 0.009 | 2.6 ± 4.7 | 6.7 ± 7.4 | 4.08 | 0.15 |
| sBP (mm Hg) | 129 ± 14 | 129 ± 13 | 0.35 | 131 ± 14 | 0.58 | 125 ± 11 | 136 ± 12 | 0.007 | 138 ± 13 | 0.02 | 1.9 ± 11.8 | 13.2 ± 16.5 | 11.3 | 0.07 |
| dBT (mm Hg) | 74 ± 10 | 74 ± 10 | 0.37 | 74 ± 8 | 0.86 | 75 ± 8 | 78 ± 9 | 0.18 | 75 ± 10 | 0.98 | −0.5 ± 9.9 | −0.2 ± 10.6 | 0.33 | 0.94 |
Data are means ± SD. Vital sign data in obese adults at baseline, on average throughout infusions and after 120 min of infusion of saline or glucagon-like peptide-1 (7–36). sBP, systolic blood pressure; dBP, diastolic blood pressure. Δ is change between baseline and timepoint 120 min.
Is comparison of change from baseline to time point 120 min, between saline and GLP-1 infusion.
No overall difference was found for diastolic blood pressure.
3.3. GLP-1
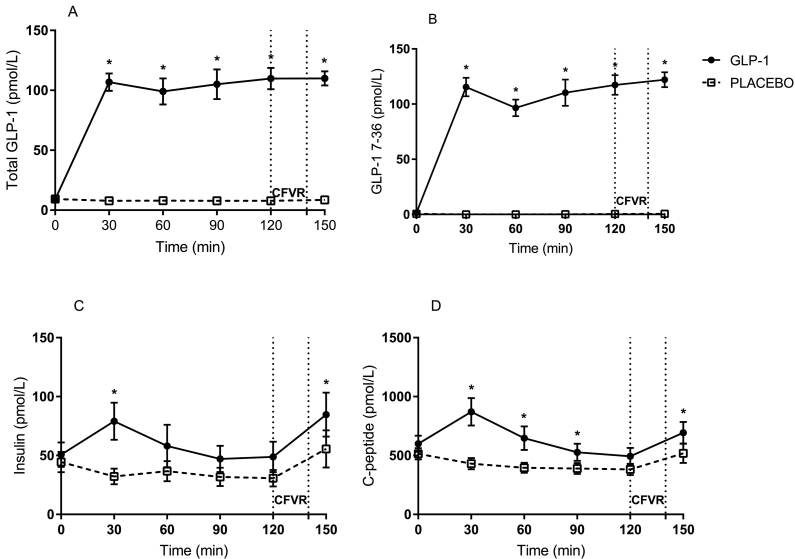
Total plasma GLP-1 (Fig. 1A) and plasma GLP-1 (7–36) (Fig. 1B) increased significantly within the first 30 min of infusion compared to saline and were maintained at supraphysiological levels throughout GLP-1 infusion. The mean level of GLP-1 (7–36) just before CFVR measurement was 117.3 pmol/l versus 0.4 pmol on saline (p ≤ 0.0001).
Fig. 1.
Concentrations (means ± SEM) of total GLP-1 (A), GLP-1 (7–36) (B), insulin (C) and C-peptide (D). CFVR: coronary flow velocity reserve.
3.4. Insulin, C-peptide and glucagon
Insulin (Fig. 1C) and C-peptide (Fig. 1D) showed similar curves, with GLP-1 infusion causing a rise in both, peaking at 30 min and thereafter returning to basal levels. Before the CFVR measurement, there were no difference between GLP-1 and saline infusion.
No significant changes in plasma glucagon were seen between GLP-1 and saline infusion.
3.5. Coronary flow velocity reserve
Median baseline CFVR was 3.26 ± 0.81 and none of the participants had impaired coronary microvascular function (defined as CFVR < 2). CFVR increased during both infusions with no difference between groups (CFVR was 3.77 ± 1.25 during GLP-1 infusion vs. 3.85 ± 1.32 during saline infusion). When comparing delta values, there were neither any significant differences between GLP-1 and saline infusion (ΔCVFR 0.38 ± 1.03 vs. Δ0.71 ± 0.0.92, p = 0.43). Coronary flow velocities corrected for rate pressure product (e.g. heart rate and systolic blood pressure) did not alter the p value (Table 2).
Table 2.
Coronary flow velocities.
| Saline |
GLP-1 |
Intervention effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | T = 120 | Δ | p | Baseline | T = 120 | Δ | p | Estimate | CI | p⁎ | |
| CFVR | 3.13 ± 0.85 | 3.85 ± 1.32 | 0.71 ± 1.03 | 0.02 | 3.39 ± 0.79 | 3.77 ± 1.25 | 0.38 ± 0.92 | 0.15 | −0.33 | −1.16;0.50 | 0.43 |
| CFV at rest | 0.24 ± 0.06 | 0.19 ± 0.06 | −0.05 ± 0.03 | 0.06 | 0.21 ± 0.05 | 0.23 ± 0.08 | 0.02 ± 0.09 | 0.50 | |||
| CFV at hyperaemia | 0.72 ± 0.16 | 0.69 ± 0.19 | −0.04 ± 0.14 | 0.39 | 0.69 ± 0.13 | 0.79 ± 0.13 | 0.10 ± 0.17 | 0.0003 | |||
| CFVR RPP corrected | 2.15 ± 0.77 | 2.80 ± 1.20 | 0.65 ± 0.98 | 0.04 | 2.35 ± 0.54 | 3.27 ± 1.28 | 0.92 ± 1.02 | 0.01 | 0.27 | −0.57;1.11 | 0.53 |
Data are means ± SD. Coronary flow velocities in obese adults at baseline and after 120 min infusion of saline or glucagon-like peptide-1 (7–36). CFVR, coronary flow velocity reserve; CFV, coronary flow velocity; RPP, rate pressure product. Δ is change between baseline and timepoint 120 min.
Is comparison of change from baseline to time point 120 min, between saline and GLP-1 infusion.
3.6. Peripheral vascular function
Seven participants had valid FMD measurements from both examination days. We found no effect of intact GLP-1 infusion on endothelial dependent microvascular function assessed by FMD compared to saline infusion (ΔFMD 7.34 ± 11.5 vs. ΔFMD −1.25 ± 0.9.23, p = 0.14) (Table 3).
Table 3.
Flow mediated dilation.
| Saline |
GLP-1 |
Intervention effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | T = 60 | Δ | p | Baseline | T = 60 | Δ | p | Estimate | CI | p⁎ | |
| FMD (%) | 9.10 ± 5.08 | 7.85 ± 7.76 | −1.25 ± 9.23 | 0.73 | 8.94 ± 7.31 | 16.3 ± 15.5 | 7.34 ± 11.5 | 0.14 | 8.97 | −2.99;20.9 | 0.14 |
| Baseline diameter (mm) | 3.57 ± 0.65 | 3.70 ± 0.53 | 0.13 ± 0.19 | 0.12 | 3.61 ± 0.61 | 3.64 ± 0.69 | 0.026 ± 0.15 | 0.66 | |||
| Peak diameter (mm) | 3.90 ± 0.82 | 3.99 ± 0.65 | 0.082 ± 0.49 | 0.68 | 3.92 ± 0.58 | 4.17 ± 0.59 | 0.26 ± 0.31 | 0.07 | |||
| Time to peak (s) | 43 ± 20 | 49 ± 46 | 6 ± 63 | 0.27 | 98 ± 54 | 76 ± 47 | −21 ± 76 | 0.49 | |||
| NMD (%) | 24.4 ± 7.5 | 27.9 ± 6.9 | 3.44 ± 4.0 | 0.06 | 23.2 ± 6.9 | 35.5 ± 14.1 | 12.3 ± 16.1 | 0.09 | 8.12 | −5.00;21.2 | 0.23 |
Data are means ± SD. Flow mediated dilation in obese adults at baseline and after 60 min infusion of saline or glucagon-like peptide-1 (7–36). FMD, flow mediated dilation; NMD, nitroglycerine mediated dilation. Δ is change during placebo or active infusion.
Is comparison of change from baseline to time point 60 min, between saline and GLP-1 infusion.
3.7. Adverse effects
Five of 13 included participants experienced a transient mild nausea during infusion of GLP-1, three had more severe nausea and were vomiting during GLP-1 infusion, one of the three were excluded for this reason. Vomiting is a well-known side effect to acute administration of GLP-1. No side effects were observed during saline infusion.
4. Discussion
We found no effect of infusion of intact GLP-1 on coronary flow velocity reserve and no effect on peripheral endothelial function and thus no indication of a direct effect of intact GLP-1 on coronary microvascular function in overweight adults without diabetes.
Several studies have indicated beneficial effects of GLP-1 on the cardiovascular system [[8], [9], [10]]. Treatment with the GLP-1 analogue, Liraglutide, significantly reduced the risk of MACE and mortality from cardiovascular disease in patients with type 2 diabetes [8]. The once weekly GLP-1 analogues semaglutide and albiglutide also reduced the risk of MACE in patients with type 2 diabetes though no significant reduction in death from cardiovascular disease were observed [9,10]. The mechanism of risk reduction by GLP-1 treatment is unknown though antiatherogenic effects may be an explanation [31].
Coronary microvascular dysfunction and peripheral endothelial function is associated with obesity, diabetes, hypertension and dyslipidaemia. CMD may precede macrovascular atherosclerosis [11,12] and is an independent predictor of cardiovascular disease [13,14]. Obesity and type 2 diabetes are characterized by a chronic low-grade inflammation associated with increased oxidative stress and high plasma levels of various atherogenic lipids leading to increased risk of endothelial dysfunction and cardiovascular disease [32]. Studies have indicated a direct and indirect anti-inflammatory effect of GLP-1 [33,34].
However, only few studies have examined the effect of GLP-1 on coronary microvascular function. In obese patients with type 2 diabetes the GLP-1 receptor agonist exenatide, administered for 12 weeks, improved coronary microvascular function with concomitant improvement in HbA1c and weight loss [35]. In a randomised cross-over study 10 weeks treatment with the GLP-1 analogue liraglutide non-significantly improved coronary microvascular function concomitantly with significant weight loss and improvement in HbA1c [15]. Extensive weight loss following gastric bypass [36] as well as a more moderate weight loss of 10% obtained by lifestyle intervention in overweight women [37] improved coronary microvascular function. Furthermore, hyperglycaemia per se seems to impair coronary microvascular function. Thus, the effect of long-term treatment with GLP-1 on coronary microcirculation may be indirect due to weight loss and improvement in HbA1c.
To our knowledge only one other study has examined the acute effect of GLP-1 on coronary microcirculation. In contrast to our findings, Subaran el al. found significant increase in myocardial microvascular blood flow, assessed by microbubble void imaging, after acute infusion of intact GLP-1 [38]. Their patient population was young healthy individuals (18–35 years of age) with normal body weight (BMI 21.8 ± 0.4 kg/m2). In contrast we find no increase in coronary microcirculation in obese older subject after acute infusion of intact GLP-1. Thus, there may be differences in the physiological action of intact GLP-1 in young healthy individuals compared to older overweight people. Differences in GLP-1 secretion and action between healthy subjects, obese subjects and patients with type 2 diabetes have formerly been documented [39,40]. A newly published study with prolonged treatment with the GLP-1 analogue liraglutide in overweight patients with heart failure showed no improvement in CFVR [41] supporting our findings of no direct effect of GLP-1 on the microcirculation in obese subjects.
By infusion of intact GLP-1, a high concentration of metabolite will be present in the circulation [42] as intact GLP-1 is metabolized by the ubiquitous enzyme DPP-4 [2]. In contrast to the study by Subaran et al., we administered an oral DPP-4 inhibitor prior to our examinations to rule out an effect of the metabolite, focusing only on the effect of intact GLP-1, which exert its effect through the GLP-1 receptor. Thus, discrepancies between our results and the study by Subaran et al. [38] may be explained by the level of GLP-1 metabolite and intact GLP-1 or influence of other hormone and metabolite changes caused by the DPP-4 inhibition [7].
We found that intact GLP-1 infusion increased heart rate by 7 beats per minutes and systolic blood pressure by 13 mm Hg, though blood pressure was only borderline significantly increased when compared to saline infusion. GLP-1 analogues are known to increase heart rate and furthermore, GLP-1 receptors have been identified in the sinoatrial node [43] thus increased heart rate may be direct receptor mediated.
We found no effect of intact GLP-1 infusion on peripheral endothelial function in overweight adults, and it is unclear whether the GLP-1 receptor is expressed in endothelial cells. Previous studies have examined the effect of GLP-1 on endothelial function, however the results have been contradicting [6,[44], [45], [46], [47]]. Devin et al. demonstrated that infusion of GLP-1 into the brachial artery had no effect on forearm blood flow [44]. Like our study, the investigators added a DPP-4 inhibitor, thereby avoiding potential influence of degrading metabolites. Furthermore, other studies found no effect on endothelial function during long-term treatment with liraglutide in patients with type 2 diabetes [45,46].
In the present study, obese participants with normal glucose tolerance were studied, to avoid the confounding effect of changes in plasma glucose during the experimental days. Another strength of our study is that the plasma levels of GLP-1 obtained during infusion correspond to the plasma levels reported during treatment with GLP-1 receptor analogues [48]. A limitation of our study is our small sample size. However, we performed a power calculation prior to the study, based on a former study by our group in which we needed 10 participants to detect a change of 0.3 which is considered of clinical importance. Though, in our study we had a considerable inter-measurement variability we see no tendency of improved CFVR and thus we have no reason to believe in a type 2 error.
5. Conclusion
We found no effect of DPP-4-protected intact GLP-1 on coronary microcirculation nor on peripheral vascular function in obese glucose tolerant adults.
The following are the supplementary data related to this article.
Plasma glucose concentrations (means ± SEM) during GLP-1 (7–36) and saline infusion. CFVR: coronary flow velocity reserve.
Vital signs. Effect of GLP-1 (7–36) and saline infusion on heart rate (A) and systolic blood pressure (B). Values are means ± SEM. CFVR: coronary flow velocity reserve.
Baseline characteristics of participants.
Conflicts of interest
JH has been consulting with Novo Nordisk. The other authors declare no relationships that could be construed as a conflict of interest.
Funding
This study was funded in part by donations from the private Danish foundations: “Toyota-Fonden”, Denmark “Snedkermester Sophus Jacobsens Fond”, Denmark and “Murermester Lauritz Peter Christensen og hustru Kirsten Sigrid Christensens Fond”, Denmark. The foundations had no role in the study design, data collection, analysis, and result interpretation of this study.
Authors' contributions
MN: study design, subject recruitment, data collection and analyses, writing the manuscript. ES and KB: data collection and analyses, critical revision of the manuscript. TH, JH, SM and EP: study design, data interpretation and critical revision of the manuscript. MZ: study design, data interpretation and writing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Malin Nilsson, Email: malin.sofia.desiree.nilsson@regionh.dk.
Thomas Hermann, Email: tshermann@dadlnet.dk.
Sten Madsbad, Email: sten.madsbad@regionh.dk.
Jens Juul Holst, Email: jjholst@sund.ku.dk.
Eva Prescott, Email: eva.irene.bossano.prescott@regionh.dk.
Mette Zander, Email: m.zander@dadlnet.dk.
References
- 1.Holst J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Baggio L.L., Yusta B., Mulvihill E.E., Cao X., Streutker C.J., Butany J. GLP-1 receptor expression within the human heart. Endocrinology. 2018;159:1570–1584. doi: 10.1210/en.2018-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Drucker D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Nystrom T., Gutniak M.K., Zhang Q., Zhang F., Holst J.J., Ahren B. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 7.Ban K., Noyan-Ashraf M.H., Hoefer J., Bolz S.S., Drucker D.J., Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 8.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez A.F., Green J.B., Janmohamed S., D'Agostino R.B., Sr., Granger C.B., Jones N.P. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 11.Marzilli M., Merz C.N., Boden W.E., Bonow R.O., Capozza P.G., Chilian W.M. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J. Am. Coll. Cardiol. 2012;60:951–956. doi: 10.1016/j.jacc.2012.02.082. [DOI] [PubMed] [Google Scholar]
- 12.Lanza G.A., Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 13.Murthy V.L., Naya M., Foster C.R., Gaber M., Hainer J., Klein J. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawata T., Daimon M., Hasegawa R., Toyoda T., Sekine T., Himi T. Prognostic value of coronary flow reserve assessed by transthoracic Doppler echocardiography on long-term outcome in asymptomatic patients with type 2 diabetes without overt coronary artery disease. Cardiovasc. Diabetol. 2013;12:121. doi: 10.1186/1475-2840-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faber R., Zander M., Pena A., Michelsen M.M., Mygind N.D., Prescott E. Effect of the glucagon-like peptide-1 analogue liraglutide on coronary microvascular function in patients with type 2 diabetes - a randomized, single-blinded, cross-over pilot study. Cardiovasc. Diabetol. 2015;14:41. doi: 10.1186/s12933-015-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi M.S., Williams D., Horlock D., Samarasinghe T., Andrews K.L., Jefferis A.M. Role of mitochondrial dysfunction in hyperglycaemia-induced coronary microvascular dysfunction: protective role of resveratrol. Diab. Vasc. Dis. Res. 2015;12:208–216. doi: 10.1177/1479164114565629. [DOI] [PubMed] [Google Scholar]
- 17.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: an update. Eur. Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flintholm Raft K., Frestad D., Michelsen M.M., Suhrs H.E., Rask A.B., Nilsson M. Peripheral endothelial function and coronary flow velocity reserve are not associated in women with angina and no obstructive coronary artery disease: the iPOWER study. J. Vasc. Res. 2017;54:309–319. doi: 10.1159/000479374. [DOI] [PubMed] [Google Scholar]
- 19.Deanfield J., Donald A., Ferri C., Giannattasio C., Halcox J., Halligan S. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Brunner H., Cockcroft J.R., Deanfield J., Donald A., Ferrannini E., Halcox J. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ryan A.S., Egan J.M., Habener J.F., Elahi D. Insulinotropic hormone glucagon-like peptide-1-(7-37) appears not to augment insulin-mediated glucose uptake in young men during euglycemia. J. Clin. Endocrinol. Metab. 1998;83:2399–2404. doi: 10.1210/jcem.83.7.4988. [DOI] [PubMed] [Google Scholar]
- 22.Holst J.J. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33–69) of glicentin. Biochem. J. 1982;207:381–388. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wewer Albrechtsen N.J., Veedfald S., Plamboeck A., Deacon C.F., Hartmann B., Knop F.K. Inability of some commercial assays to measure suppression of glucagon secretion. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/8352957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orskov C., Rabenhoj L., Wettergren A., Kofod H., Holst J.J. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 25.Vilsboll T., Krarup T., Sonne J., Madsbad S., Volund A., Juul A.G. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 26.Michelsen M.M., Pena A., Mygind N.D., Frestad D., Gustafsson I., Hansen H.S. Coronary flow velocity reserve assessed by transthoracic Doppler: the iPOWER study: factors influencing feasibility and quality. J. Am. Soc. Echocardiogr. 2016;29:709–716. doi: 10.1016/j.echo.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Michelsen M.M., Mygind N.D., Pena A., Olsen R.H., Christensen T.E., Ghotbi A.A. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction: the iPOWER study. Int. J. Cardiol. 2017;228:435–443. doi: 10.1016/j.ijcard.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Olsen R.H., Pedersen L.R., Snoer M., Christensen T.E., Ghotbi A.A., Hasbak P. Coronary flow velocity reserve by echocardiography: feasibility, reproducibility and agreement with PET in overweight and obese patients with stable and revascularized coronary artery disease. Cardiovasc. Ultrasound. 2016;14:22. doi: 10.1186/s12947-016-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraste M., Koskenvuo J., Knuuti J., Toikka J., Laine H., Niemi P. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin. Physiol. 2001;21:114–122. doi: 10.1046/j.1365-2281.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 30.Thijssen D.H., Black M.A., Pyke K.E., Padilla J., Atkinson G., Harris R.A. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron-Vendrig A., Reheman A., Siraj M.A., Xu X.R., Wang Y., Lei X. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes. 2016;65:1714–1723. doi: 10.2337/db15-1141. [DOI] [PubMed] [Google Scholar]
- 32.Hajjar D.P., Gotto A.M., Jr. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013;182:1474–1481. doi: 10.1016/j.ajpath.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasner N.M., Ido Y., Ruderman N.B., Cacicedo J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan A.E., Gaoatswe G., Lynch L., Corrigan M.A., Woods C., O'Connell J. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57:781–784. doi: 10.1007/s00125-013-3145-0. [DOI] [PubMed] [Google Scholar]
- 35.Wei R., Ma S., Wang C., Ke J., Yang J., Li W. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2016;310:E947–E957. doi: 10.1152/ajpendo.00400.2015. [DOI] [PubMed] [Google Scholar]
- 36.Nerla R., Tarzia P., Sestito A., Di Monaco A., Infusino F., Matera D. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr. Metab. Cardiovasc. Dis. 2012;22:626–634. doi: 10.1016/j.numecd.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Coppola A., Marfella R., Coppola L., Tagliamonte E., Fontana D., Liguori E. Effect of weight loss on coronary circulation and adiponectin levels in obese women. Int. J. Cardiol. 2009;134:414–416. doi: 10.1016/j.ijcard.2007.12.087. [DOI] [PubMed] [Google Scholar]
- 38.Subaran S.C., Sauder M.A., Chai W., Jahn L.A., Fowler D.E., Aylor K.W. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin. Sci. (Lond.) 2014;127:163–170. doi: 10.1042/CS20130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aulinger B.A., Vahl T.P., Wilson-Perez H.E., Prigeon R.L., D'Alessio D.A. Beta-cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. J. Clin. Endocrinol. Metab. 2015;100:2489–2496. doi: 10.1210/jc.2014-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nauck M., Stockmann F., Ebert R., Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen R., Jorsal A., Iversen P., Tolbod L.P., Bouchelouche K., Sorensen J. Effect of liraglutide on myocardial glucose uptake and blood flow in stable chronic heart failure patients: a double-blind, randomized, placebo-controlled LIVE sub-study. J. Nucl. Cardiol. 2017 doi: 10.1007/s12350-017-1000-2. [DOI] [PubMed] [Google Scholar]
- 42.Zander M., Madsbad S., Madsen J.L., Holst J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 43.Pyke C., Heller R.S., Kirk R.K., Orskov C., Reedtz-Runge S., Kaastrup P. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 44.Devin J.K., Pretorius M., Nian H., Yu C., FTt Billings, Brown N.J. Dipeptidyl-peptidase 4 inhibition and the vascular effects of glucagon-like peptide-1 and brain natriuretic peptide in the human forearm. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nandy D., Johnson C., Basu R., Joyner M., Brett J., Svendsen C.B. The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diab. Vasc. Dis. Res. 2014;11:419–430. doi: 10.1177/1479164114547358. [DOI] [PubMed] [Google Scholar]
- 46.Nomoto H., Miyoshi H., Furumoto T., Oba K., Tsutsui H., Miyoshi A. A comparison of the effects of the GLP-1 analogue Liraglutide and insulin glargine on endothelial function and metabolic parameters: a randomized, controlled trial Sapporo athero-incretin study 2 (SAIS2) PLoS One. 2015;10 doi: 10.1371/journal.pone.0135854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu A., Charkoudian N., Schrage W., Rizza R.A., Basu R., Joyner M.J. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 48.DeFronzo R.A., Okerson T., Viswanathan P., Guan X., Holcombe J.H., MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr. Med. Res. Opin. 2008;24:2943–2952. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma glucose concentrations (means ± SEM) during GLP-1 (7–36) and saline infusion. CFVR: coronary flow velocity reserve.
Vital signs. Effect of GLP-1 (7–36) and saline infusion on heart rate (A) and systolic blood pressure (B). Values are means ± SEM. CFVR: coronary flow velocity reserve.
Baseline characteristics of participants.