Abstract
Introduction
Although an undoubted association between epicardial fat tissue (EFT) and atrial fibrillation (AF) has been recently approved, the association between EFT and post-ablation AF recurrence is not evident yet. This study aimed to assess the association between EFT and AF recurrence after ablation.
Methods
The present study was a systematic review and meta-analysis using related literature available in electronic databases until July 2018 via “atrial fibrillation” and “epicardial fat” as the main keywords. Considering the different methods of EFT measurement, three different pooled meta-analyses were conducted in this study including: 1) comparison of total EFT volume, 2) left atrium (LA)-EFT volume, and 3) EFT thickness between two groups with and without AF recurrence estimating standardized mean difference (SMD) through a random and non-random effect meta-analysis. Statistical analysis was also performed using Comprehensive Meta-analysis (CMA) Software.
Results
Following a search into a total number of 518 articles, the findings of 12 studies published in 10 articles were enrolled in this meta-analysis. Accordingly, the results of meta-analysis showed that LA-EFT and total EFT volumes were higher in recurrent subjects (LA-EFT: SMD = 0.862 ml; I2 = 0.00, 95% confidence interval (CI) = 0.567–1.156; total EFT: SMD = 1.017 ml, I2 = 0.00, 95% CI = 0.748–1.286). Besides, a significant higher EFT thickness in patients with AF recurrence compared to those with no AF recurrence was observed (SMD = 0.808 mm, I2 = 91.07, 95% CI = 0.215–1401).
Conclusion
The total EFT and LA-EFT volumes, as well as EFT thickness, seemed to be associated with AF recurrence in patients undergoing AF ablation.
Keywords: Epicardial fat, Adipose tissue, Atrial fibrillation, Recurrence, Risk assessment, Prognosis
1. Introduction
Atrial fibrillation (AF) is known as the most common sustained supraventricular arrhythmia with an increasing global incidence and prevalence [1]. Rhythm control strategies, mainly with catheter ablation (CA) or cryoballon ablation (CBA), have also become optimal and well-established treatment options in the domain of symptomatic AF [2]. However, ablation therapy as an interventional procedure has still brought about various issues regarding procedural complications, high expenditure, as well as arrhythmia recurrence [3]. Moreover, the success rate of AF ablation has been only reported by 70% in patients with paroxysmal AF (PAF) and 50% in persistent AF (PeAF), which means that the recurrence rate of a single ablation procedure ranges from 30 to 50% [4]. In fact, the high acute success rates for CA procedure are deemed to be achievable and its durable efficacy for AF has remained the main challenge [4,5]. Thus, appropriate patient selection considering the prediction factors of AF recurrence after an initially successful ablation has also become a significant issue for obtaining better outcomes [6]. In this regard, different clinical and para-clinical factors including older age, female gender, classical cardiovascular risk factors, non-paroxysmal AF, left ventricular (LV) dysfunction, myocardial fibrosis, and atrial enlargement have been further identified as possible predictors of post-ablation AF recurrence [7,8].
Epicardial fat tissue (EFT), as a specialized visceral adipose tissue, has been also considered as an endocrine organ producing various pro-inflammatory and pro-atherogenic factors such as leptin, IL-6, adipocytokines, and TNF-alpha that promote the initiation and development of coronary artery atherosclerosis [9]. The EFT has been also suggested to play a significant role in promoting arrhythmogenesis due to its pro-inflammatory properties and anatomical proximity to the myocardium [10,11].Correspondingly, some believe that EFT has an additional role in the modulation of different triggers, including metabolic and biochemical triggers, leading to AF [12]. The relationship between increase in EFT amount and AF occurrence and severity has been similarly shown in several recent studies [13]. Moreover, the association between EFT and AF recurrence after ablation is not evident yet although newly conducted meta-analysis comparing EFT volume in healthy and AF subjects has revealed an undoubted association between EFT and AF [14].
The abundance of EFT and post-ablation AF recurrence was firstly described by Tsao et al.; while more than ten different studies have investigated this association up to now [15]. However, these studies have been conducted in different populations, various disease stages (paroxysmal or persistent AF), a variety of EFT measurement methods (computed tomography (CT) or echocardiography), numerous methods of rhythm control strategy (CA or CBA), and diverse intervals of follow-up for AF recurrence [[16], [17], [18], [19], [20], [21], [22], [23], [24]]. The present study was the first attempt to verify the association between AF recurrence and EFT, conducting a meta-analysis of investigations comparing patients with and without AF recurrence after ablation.
2. Methods
2.1. Protocol
This systematic review and meta-analysis conducted by the principles set in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement [25].
2.2. Eligibility criteria and study selection
Studies were included if they were: 1) reported EFT thickness or volume with any statistical indexes, 2) reported EFT parameters in two groups of patients with and without AF recurrence after ablation, and 3) measured EFT by CT, magnetic resonance imaging (MRI), or echocardiography. Studies published as conference abstracts were considered eligible for inclusion; however, case reports and review articles were excluded. These articles were independently reviewed by two assessors to ensure full compliance with the study inclusion and exclusion criteria and were discussed and revised in the cases of disagreements.
2.3. Information sources and search
Databases including MEDLINE/PubMed, EBSCO, Thomson Reuters' Web of Science, the Cochrane Library, and Google Scholar, were searched since inception until July 2018 using the following keywords: “Atrial fibrillation; Arrhythmias, Cardiac; Adipose Tissue, Epicardial adipose, Epicardial fat, Epicardial adipose tissue.” Titles and abstracts were screened for the exclusion of unrelated articles. Also, the references of all finally included articles were reviewed in case of finding any related article. No language restrictions were assumed for the included articles.
2.4. Data collection process
Using a data extraction table, the required information from each article including first authors name, publication year, study design, sample size, method of AF ablation (CA or CBA), imaging method of EFT measurement (CT, MRI, or Echocardiography), type of reported EFT (thickness or volume), the location of measured EFT, and follow-up duration for AF recurrence were extracted. The table was completed by the first author and checked by another author for verifying.
2.5. Quality assessment
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was used to assess the quality of the included studies with full-text [26]. The STROBE contained 22 items for quality assessment of the information reported in different sections of a study including introduction, study design and setting, statistical evaluation, results, discussion, and findings. A summary of the results of STROBE judgment for all finally included studies was prepared based on a rating scale ranging from 0 to 22. Quality assessment was performed by two different assessors and a third assessor in case of discrepancies. In the case of conference abstracts, studies without full-text, another type of STROBE checklist, contained 12 items to be included when reporting observational studies in a conference abstract, was used.
2.6. Summary measures and synthesis of results
The PRISMA declaration was followed for statistical analysis. Q statistic of Chi-square value test and I2 index (inconsistency index) were used to evaluate the heterogeneity of individual studies contributing to the pooled estimate. I2 > 50% suggested heterogeneity [27]. P < 0.05 was considered statistically significant, suggesting the presence of heterogeneity. In the case of heterogeneity, a random effects model was used. Probable sources of heterogeneity through the studies were explored using meta-regression and subgroup analysis. The probability of publication bias was evaluated through the Egger's regression test and Funnel plots [28]. The funnel plots exhibit a graphical representation of possible publication bias so that any asymmetry in the plot can represent publication bias, and Egger's regression is also the statistical counterpart of this asymmetry. Considering the different methods of EFT measurement, three different pooled meta-analyses were done in this systematic review including 1) comparison of total EFT volume, 2) LA-EFT volume, and 3) EFT fat thickness between two groups of with and without recurrence AF. All statistical tests were two-tailed and the type I error rate was set at 5%. Statistical analysis was performed using Comprehensive Meta-analysis (CMA) version 2.
3. Results
3.1. Study selection
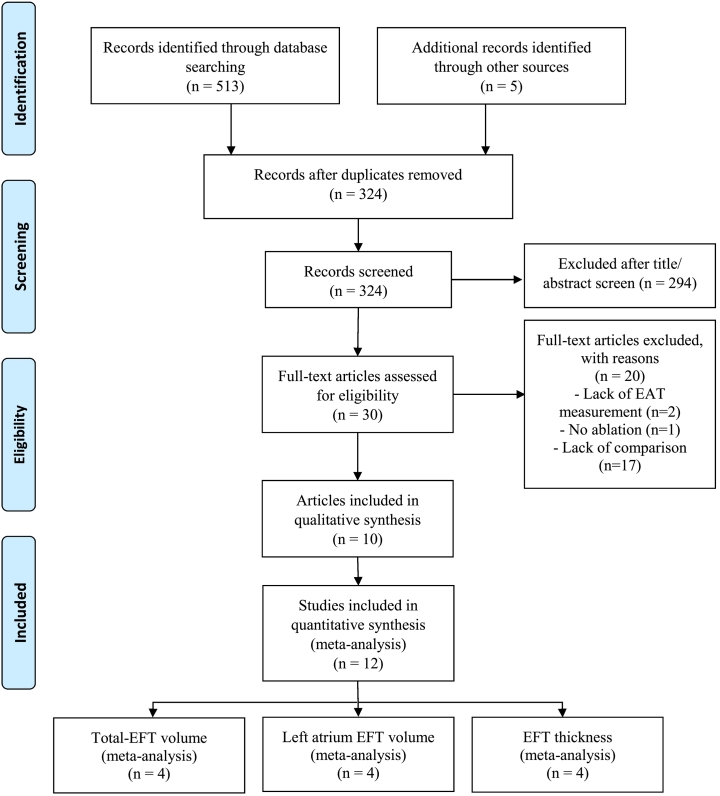
The literature search resulted in 518 records. After exclusion of 194 duplicated records, of the 324 remaining records, 294 were not related to the topic. After assessing the full texts of 30 remaining studies, 20 of the articles excluded. Finally, the findings of the 12 studies published in 10 articles were enrolled in this study (Fig. 1). Finally, three different pooled meta-analyses were done, assessing the association of total EFT volume (n = 4), LA-EFT volume (n = 4), and EFT fat thickness (n = 4) between two groups of with and without recurrence AF.
Fig. 1.
PRISMA flow chart of included studies.
EFT: epicardial fat tissue, LA: left atrium.
3.2. Excluded studies
Twenty studies were excluded after reviewing the original text for the following reasons: 1) lack of reporting EFT, and considering pericardial adipose tissue as the main outcome (n = 2); 2) using electrical cardioversion, not ablation for AF (n = 1); and 3) no comparison between two groups of with and without AF recurrence was available (n = 17).
3.3. Study characteristics
The analysed studies had included 1208 individuals including patients with AF recurrence (n = 816) and without AF recurrence (n = 392) after ablation. Among the AF patient, 889 patients had PAF, and 333 had PeAF. Most of the articles (7/10) were in the full-text format, while three articles were in the conference abstract [17,19,20]. CT [[15], [16], [17],[19], [20], [21], [22], [23]] and echocardiography [18,24] were the used imaging modalities for EFT measurements. The least sample size was 38 [17], and the largest ones were related to Chao et al., [18] with 283 patients. The first article was published in 2011 [15], and the latest article in 2016 [24] (Table 1). Other characteristics of the included studies including study location, type of ablation, follow-up duration, age, body mass index (BMI), and gender distribution are shown in Table 2.
Table 1.
Characteristics of the included studies.
| Reference | Year | Sample size (PAF/PeAF) | AF recurrence |
No AF recurrence |
Type of EAT | Imaging | Article type | Quality score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SDa | n | Mean ± SDa | |||||||
| Tsao [15] | 2011 | 68 (43/25) | 24 | 35.2 ± 12.5 | 44 | 26.8 ± 11.1 | LA-EFT volume | CT | OA | 18/22 |
| Nagashima [16] | 2011 | 40 (24/16) | 15 | 239 ± 90.2 | 25 | 153.5 ± 42.7 | Total EFT volume | CT | OA | 18/22 |
| 15 | 69.6 ± 35.5 | 25 | 40.7 ± 13.9 | LA-EFT volume | CT | |||||
| Murakami [17] | 2012 | 38 (32/6) | 12 | 200 ± 62 | 26 | 145 ± 37 | Total EFT volume | CT | CA | 5/12 |
| Chao [18] | 2013 | 227 (all PAF) | 64 | 6.3 ± 0.6 | 149 | 5.7 ± 0.6 | RV wall EFT thickness | Echo | OA | 19/22 |
| 56 (all PeAF) | 31 | 7.3 ± 0.6 | 25 | 6.7 ± 0.7 | RV wall EFT thickness | Echo | ||||
| Kawakami [19] | 2013 | 95 (74/21) | 34 | 147.3 ± 35.8 | 61 | 109.5 ± 34.9 | Total EFT volume | CT | CA | 4/12 |
| Soucek [20] | 2014 | 102 (74/28) | 27 | 115.8 ± 38.2 | 75 | 86.6 ± 38.2 | Total EFT volume | CT | CA | 5/12 |
| Nakahara [21] | 2014 | 60 (0/60) | 47 | 33.2 ± 10.7 | 13 | 25.1 ± 11 | LA-EFT volume | CT | OA | 18/22 |
| Masuda [22] | 2015 | 53 (22/31) | 24 | 35.1 ± 13.1 | 29 | 25.0 ± 9.5 | LA-EFT volume | CT | OA | 22/22 |
| Kocyigit [23] | 2015 | 249 (203/46) | 60 | 4.4 ± 2.1 | 189 | 4.3 ± 1.6 | RV wall EFT thickness | CT | OA | 19/22 |
| Canpolat [24] | 2016 | 234 (190/44) | 45 | 7.79 ± 2.2 | 189 | 5.79 ± 1.38 | RV wall EFT thickness | Echo | OA | 19/22 |
PAF/PeAF: paroxysmal/persistent atrial fibrillation; LA: left atrium; EAT: epicardial adipose tissue, CT: computed tomography; OA: original article; CA: conference abstract; RV: right ventricle; Echo: echocardiography.
Notice: Unit of the volume and thickness variables are milliliter (ml) and millimeter (mm), respectively.
Table 2.
Settings and baseline characteristics of the included studies.
| Reference | Location | Type of ablation | Age, years |
Gender, male (%) |
BMI, kg/m2 |
Follow/up duration | |||
|---|---|---|---|---|---|---|---|---|---|
| AF Rec (−) | AF Rec (+) | AF Rec (−) | AF Rec (+) | AF Rec (−) | AF Rec (+) | ||||
| Tsao [15] | Taiwan | RFCA | 53.3 ± 9.0 | 57.1 ± 7.1 | 34 (77%) | 18 (75%) | 25.3 ± 3.0 | 26.2 ± 3.9 | 3 months |
| Nagashima [16] | Japan | RFCA | 56.4 ± 11.0 | 60.7 ± 8.5 | 18 (72.0%) | 13 (86.7%) | 22.4 ± 2.4 | 24.1 ± 2.6 | 3–6 month |
| Murakami [17] | Japan | RFCA | N/A | N/A | N/A | 3 months | |||
| Chao [18] | Taiwan | RFCA | Total: 54.6 ± 10.4 | Total: 197 (69.6%) | Total: 25.3 ± 3.6 | 16 ± 9 months | |||
| Kawakami [19] | Japan | RFCA | Total: 60.5 ± 11.8 | Total: 73 (76.8) | N/A | One year | |||
| Soucek [20] | USA | RFCA | N/A | Total: 85 (83.3) | N/A | 3 months | |||
| Nakahara [21] | Japan | RFCA | Total: 63.1 ± 10.4 | Total: 50 (83%) | NA | 16.0 (12–16) months | |||
| Masuda [22] | Japan | RFCA | 61 ± 13 | 62 ± 9 | 22 (76) | 14 (58) | 24.3 ± 3.2 | 24.0 ± 3.2 | 16 ± 4 months |
| Kocyigit [23] | Turkey | Cryo | 55.1 ± 10.7 | 56.9 ± 10.4 | 86 (45.5) | 34 (56.7) | 24.2 ± 1.6 | 24.4 ± 1.6 | 29 (8–48) months |
| Canpolat [24] | Turkey | Cryo | 53.1 ± 10.6 | 57.8 ± 10.8 | 94 (49.7%) | 26 (57.8%) | 25.3 ± 5.2 | 26.0 ± 3.3 | 20 (13–24) months |
Rec: recurrence; BMI: body mass index; N/A: not available; RFCA: radio frequency catheter ablation; Cryo: cryoballon ablation.
3.4. Total epicardial fat volume
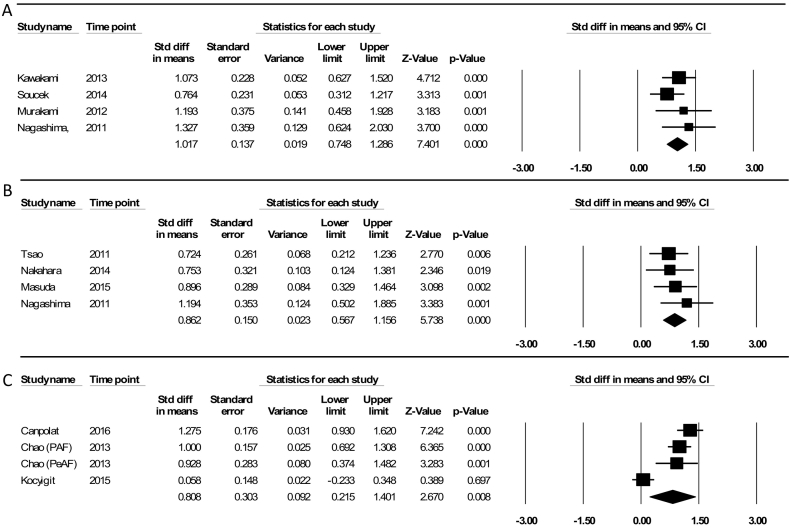
The comparison of total EFT volume between two groups of patients with and without AF recurrence using a random effect model showed an SDM of 1.02 ml (95% CI = 0.74–1.28), indicating that EFT volume was higher in AF recurrence patients. No significant heterogeneity was observed among the studies' results (P = 0.526, Q-value = 2.29, df (Q) = 3, I2 = 0.0%) (Fig. 2-A).
Fig. 2.
(A) Fixed effect meta-analysis comparing total EFT volume of patients with AF recurrence and without AF recurrence; (B) fixed effect meta-analysis comparing left atrium (LA)-EFT volume of patients with AF recurrence and without AF recurrence; (C) random effect meta-analysis comparing epicardial fat tissue (EFT) thickness of patients with AF recurrence and without AF recurrence.
3.5. Left atrium epicardial fat volume
About the association between the LA-EFT volume and the AF recurrence, there was a significant difference in the SDM between patients with and without AF recurrence (0.86 ml, 95% CI = 0.56–1.15), showing a higher LA-EFT volume in AF recurrence patients. The heterogeneity was not found among the studied studies (P = 0.731, Q-value = 1.29, df (Q) = 3, I2 = 0.0%,) using the fixed effect model (Fig. 2-B).
3.6. Epicardial fat thickness
The patients with AF recurrence had a significant higher echocardiographic EFT thickness than those without AF recurrence (SDM = 0.80, 95% CI = 0.21–1.40). Considering the founded heterogeneity among the studied studies (P = 0.000, Q-value = 33.60, df (Q) = 33.60, I2 = 91.07%), the random effect model was used (Fig. 2-C).
3.7. Quality assessment and publication bias
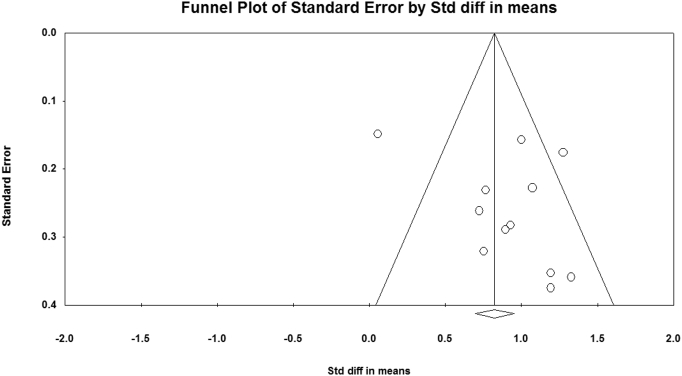
All studies in the current meta-analysis were not of the same quality; the lowest STROBE score was 18, and the highest was 22 for the articles with full-text (Table 1). Description of any efforts to address potential sources of bias was not clear in most of the articles with full-text (6/7). Moreover, the eligibility criteria and the sources and methods of selection of participants were not also clear in 90.0% of the articles. Regarding the Egger's regression test (P = 0.187), there was no published bias in the studies (Fig. 3).
Fig. 3.
Illustration of funnel plot asymmetry due to heterogeneity including all studies.
4. Discussion
4.1. Summary of main results
The main findings of the present study showed an association between increased EFT and AF recurrence. In this respect, three different meta-analyses were conducted to investigate the association between EFT volume, both total and LA specified EFT, as well as EFT thickness on right ventricle free-wall and post-ablation AF recurrence. The findings were all in a similar line, showing a positive association between increased EFT and AF recurrence. Surprisingly, the present meta-analysis demonstrated a significant difference in EFT thickness between two groups with and without AF recurrence; similar to what was reported by CT about EFT volumes. These findings put emphasis on the importance of further evaluations on EFT reports about echocardiography, as a safe and non-invasive imaging modality [29]. Furthermore, the present study, as the first attempt to clarify the association between EFT and AF recurrence revealed a new possible imaging marker for the prediction of AF recurrence, which required further carefully-designed studies for more accurate evaluations.
4.2. Why was this meta-analysis important?
While it is still unclear how EFT affects electrical and structural AF substrates, there are some mechanisms including electrical remodeling, inflammation, oxidative stress, genetic factors, neural mechanism, and aromatase estrogenic capacity that can be taken into account [[30], [31], [32]]. The findings of this study suggested that an increased amount of EFT was much more popular in cases of post-ablation AF recurrence, implying the importance of EFT reduction as a part of strategic planning for AF recurrence prevention. However, the effect of EFT reduction on the outcomes of AF ablation required different clinical trials, but the evidence from observational studies reviewed in this study supported the probable beneficial effects of EFT decline on post-ablation AF recurrence reduction. The study argument was also put on the grounds that: 1) EFT had a strategic location, which was mostly accumulated between the myocardium and the visceral pericardium, without any separating structure from the myocardium and the epicardial vessels [10,33,34]; 2) aside from the approved mechanisms of EFT effects on arrhythmia, EFT might have an effect on triggers from different sites, mainly pulmonary veins, that could interact with a remodelled atrial substrate to sustain AF [12]; 3) association between EFT and AF incidence had been proven in a recent meta-analysis [14], and 4) the present meta-analysis showed the evidence of EFT effects on AF recurrence. In fact, it was assumed that EFT reduction would have more beneficial effects on the outcomes of ablation rather than whole body weight loss through a targeted therapy strategy; however, the impact of whole body weight loss on the EFT reduction was certainly not negligible [35,36]. Likewise, it should be noted that weight loss and statins are so far the only recommended solutions for EFT reduction, and other therapeutic agents such as Eicosapentaenoic acid and Botulinum toxin are even now on their initial phases of clinical trials [[37], [38], [39]].
4.3. Comparison with other meta-analyses
Although the association between the amount of EFT and AF has been recently approved in another meta-analysis [14], the present study was the first meta-analysis on the association between EFT and post-ablation AF recurrence. Therefore, the findings of this meta-analysis were incomparable to another review study in this domain. However, the present meta-analysis, similar to other recent meta-analyses, helped to find another piece of the puzzle of EFT and AF association. In Gaeta et al., the piece of the association between EFT and AF incidence or prevalence was merely found; while in the present study, the piece of association between EFT and AF recurrence was completed.
4.4. Causes of heterogeneity
One of the most interesting points about this meta-analysis was the method of classification of studies in three categories of LA-EFT volume, total EFT volume, and EFT thickness. Using this approach, the evident source of heterogeneity among studies was reduced; wherein no heterogeneity was reported in two meta-analyses of comparing EFT volumes in two groups with and without AF recurrence. Although, the I2 index was found to be 0.0% for meta-analysis of EFT volumes, including LA and total-EFT; it was reported to be >90% for EFT thickness, indicating the presence of heterogeneity sources among the studies. Given the obtained heterogeneity, the random effect model was used in EFT thickness meta-analysis. Considering the number of studies, a subgroup analysis could not be performed to find the possible sources of heterogeneity among various probable factors such as female gender, ablation type, AF type (PAF or PeAF), and criteria used to define AF recurrence, as well as quality of studies based on STROBE Checklist. Moreover, BMI and age, as well as other classical coronary artery disease (CAD) risk factors were additional possible sources of heterogeneity whose role was impossible to be assessed as the sources of heterogeneity with meta-regression due to no reference to them in the majority of studies. However, in another meta-analysis, it had been reported that EFT was not only associated with AF independently from the presence of classical CAD risk factors, but also the EFT amount was regarded as a better risk marker associated with arrhythmia.
4.5. Overall completeness, applicability, and quality of evidence
The number of high-quality studies investigating the association between EFT and AF incidence and prevalence seemed not to be enough to reach a definite conclusion. Although a strong association was found in this study showing that people with AF recurrence had an increased volume and thickness of EFT, the included evidence in this meta-analysis was not sufficient considering its quantity and quality. So, it is believed that there is still a long way to go for an undoubted approval of the association between increased EFT and a higher risk of AF recurrence. Considering the low number of studies in this field, there were attempts to include as much study as possible in this meta-analysis, taking no notice of the quality of the reported evidence, which reduced the strength of the results of this study for generalization. In this regard, three studies without any full-texts were included, which had been only published as abstracts in conference abstract books, as well as three investigations with a quality score equal to 18 (of 22). A total number of 1222 patients, from 10 articles, were included in this systematic review, and the number of patients reached around 250–750 individuals in each meta-analysis, which seemed little for the generalization of the results. Besides, the prospective design for studies was evident in only two cases, and most of the investigations did not bring up their retrospective or prospective approaches in the process of data collection.
4.6. Study limitations
All the included studies in this meta-analysis had an observational design, which by itself had no high position among the Evidence-Based Medicine Pyramid (EBMP). Besides, all the included studies were single-center trials with small sample size. On the other hand, the findings of some studies were limited only to a three-month follow-up. Another limitation in the given studies might be the presence of reporting bias; therefore, AF recurrence as a complication of AF ablation might have been underestimated in some studies. Moreover, we could not adjust our results based on age, gender, and major CAD risk factors, since not all the studies have reported any adjustment for these possible confounders.
5. Conclusions
According to the findings of the present meta-analysis, the EFT measurement, both volume and thickness measurements, seemed to be acceptable strategies for risk stratification of AF recurrence. The present meta-analysis showed that total and LA-EFT volumes, as well as EFT thickness, were higher in patients with AF recurrence in comparison to those without AF recurrence after ablation. However, the amount of evidence in this regard is still inadequate, requiring further cohort studies.
5.1. Implications for further research
Future studies would be required to indicate which type and what protocol of imaging as well as which location of EFT measurement can be the best choices for risk stratification. Moreover, further studies with longer follow-up periods as well as larger sample sizes, are still needed to assess the EFT between two groups with and without AF recurrence. Furthermore, achieving a certain cut-off point from the echocardiographic thickness of EFT and total or LA-EFT are expected to be a challenging issue for future prediction of post-ablation AF recurrence, and this could be also a good idea for future studies.
5.2. Implications for practice
Based on this meta-analysis, it seemed that clinicians should evaluate the EFT, whether as EFT thickness by echocardiography or as EFT-volume via CT, as a possible imaging marker for a better assessment of AF ablation clinical outcomes. Thus; lowering the amount of EFT after ablation, using the available strategies such as weight loss or statin therapy, might be useful for moderating the risk of post-ablation AF recurrence.
Author contributions
Alireza Sepehri Shamloo, Nikolaos Dagres, Arash Arya: Concept/design, Data analysis/interpretation, Drafting article, Approval of article.
Boris Dinov, Philipp Sommer, Daniella Husser-Bollmann, Andreas Bollmann, Gerhard Hindricks: Concept/design, Critical revision of article, Approval of article.
Conflict of interests
N.D. and G.H. report research grants from Abbott, Biotronik, Boston Scientific and Medtronic to the institution (Heart Center Leipzig) without personal financial benefits.
Acknowledgments
Acknowledgements
None.
Funding source
None.
References
- 1.Gaita F., Scaglione M., Battaglia A., Matta M., Gallo C., Galatà M. Very long-term outcome following transcatheter ablation of atrial fibrillation. Are results maintained after 10 years of follow up? EP Europace. 2018;20:443–450. doi: 10.1093/europace/eux008. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y.H., Lu Z.Y., Xiang Y., Hou J.W., Wang Q., Lin H. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784–794. doi: 10.1093/europace/euw330. [DOI] [PubMed] [Google Scholar]
- 3.Ebert M., Stegmann C., Kosiuk J., Dinov B., Richter S., Arya A. Predictors, management, and outcome of cardioversion failure early after atrial fibrillation ablation. Europace. 2018;20:1428–1434. doi: 10.1093/europace/eux327. [DOI] [PubMed] [Google Scholar]
- 4.Deng H., Bai Y., Shantsila A., Fauchier L., Potpara T.S., Lip G.Y. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clin. Res. Cardiol. 2017;106:813–823. doi: 10.1007/s00392-017-1123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 6.Kornej J., Hindricks G., Arya A., Sommer P., Husser D., Bollmann A. The APPLE score - a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degiovanni A., Boggio E., Prenna E., Sartori C., De Vecchi F., Marino P.N. Association between left atrial phasic conduit function and early atrial fibrillation recurrence in patients undergoing electrical cardioversion. Clin. Res. Cardiol. 2018;107:329–337. doi: 10.1007/s00392-017-1188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mujović N., Marinković M., Lip G.Y., Potpara T.S. Predicting recurrent atrial fibrillation after catheter ablation. Europace. 2018;20:f460–f461. doi: 10.1093/europace/euy022. [DOI] [PubMed] [Google Scholar]
- 9.Chistiakov D.A., Grechko A.V., Myasoedova V.A., Melnichenko A.A., Orekhov A.N. Impact of the cardiovascular system-associated adipose tissue on atherosclerotic pathology. Atherosclerosis. 2017;263:361–368. doi: 10.1016/j.atherosclerosis.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Wong C.X., Ganesan A.N., Selvanayagam J.B. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2017;38:1294–1302. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 11.Goudis C.A., Vasileiadis I.E., Liu T. Epicardial adipose tissue and atrial fibrillation: pathophysiological mechanisms, clinical implications and potential therapies. Curr. Med. Res. Opin. 2018:1–11. doi: 10.1080/03007995.2018.1462786. [DOI] [PubMed] [Google Scholar]
- 12.Okumura Y. Cardiac arrhythmia due to epicardial fat: is it a modifiable risk? Curr. Cardiovasc. Risk Rep. 2017;11:23. [Google Scholar]
- 13.Betancur L., Vásquez E.M., Duque L., Díaz J.C., Velásquez J.E., Aristizábal J. Association of epicardial adipose tissue with incidence, severity and recurrences of atrial fibrillation: results of a systematic review. Rev. Mex. Cardiol. 2018;29:55–66. [Google Scholar]
- 14.Gaeta M., Bandera F., Tassinari F., Lorenzo C., Cargnelutti M., Pelissero G. Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. Europace. 2017;19:747–752. doi: 10.1093/europace/euw398. [DOI] [PubMed] [Google Scholar]
- 15.Tsao H.-M., Hu W.-C., Wu M.-H., Tai C.-T., Lin Y.-J., Chang S.-L. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am. J. Cardiol. 2011;107:1498–1503. doi: 10.1016/j.amjcard.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Nagashima K., Okumura Y., Watanabe I., Nakai T., Ohkubo K., Kofune T. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ. J. 2011;75:2559–2565. doi: 10.1253/circj.cj-11-0554. [DOI] [PubMed] [Google Scholar]
- 17.Murakami C., Nagai T., Akira F., Kido T., Nishimura K., Inoue K. Total epicardial fat volume is associated with early recurrence of atrial fibrillation after catheter ablation. J. Am. Coll. Cardiol. 2012;59 [Google Scholar]
- 18.Chao T.-F., Hung C.-L., Tsao H.-M., Lin Y.-J., Yun C.-H., Lai Y.-H. Epicardial adipose tissue thickness and ablation outcome of atrial fibrillation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami H., Satomi K., Nakajima I., Miyamoto K., Yamada Y., Okamura H. Total epicardial adipose tissue volume is associated with outcome of pulmonary vein isolation for atrial fibrillation. Europace. 2013;15:ii218. [Google Scholar]
- 20.Soucek F., Covassin N., Singh P., Ruzek L., Kara T., Suleiman M. Epicardial adipose tissue volume predicts atrial fibrillation recurrence after pulmonary vein isolation. Eur. Heart J. 2014:431. (OXFORD UNIV PRESS GREAT CLARENDON ST, OXFORD OX2 6DP, ENGLAND) [Google Scholar]
- 21.Nakahara S., Hori Y., Kobayashi S., Sakai Y., Taguchi I., Takayanagi K. Epicardial adipose tissue-based defragmentation approach to persistent atrial fibrillation: its impact on complex fractionated electrograms and ablation outcome. Heart Rhythm. 2014;11:1343–1351. doi: 10.1016/j.hrthm.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Masuda M., Mizuno H., Enchi Y., Minamiguchi H., Konishi S., Ohtani T. Abundant epicardial adipose tissue surrounding the left atrium predicts early rather than late recurrence of atrial fibrillation after catheter ablation. J. Interv. Card. Electrophysiol. 2015;44:31–37. doi: 10.1007/s10840-015-0031-3. [DOI] [PubMed] [Google Scholar]
- 23.Kocyigit D., Gurses K.M., Yalcin M.U., Turk G., Evranos B., Yorgun H. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. J. Cardiovasc. Comput. Tomogr. 2015;9:295–302. doi: 10.1016/j.jcct.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Canpolat U., Aytemir K., Yorgun H., Asil S., Dural M., Özer N. The impact of echocardiographic epicardial fat thickness on outcomes of cryoballoon-based atrial fibrillation ablation. Echocardiography. 2016;33:821–829. doi: 10.1111/echo.13193. [DOI] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaderi F., Eshraghi A., Shamloo A.S., Mousavi S. Assosiation of epicardial and pericardial fat thickness with coronary artery disease. Electron Physician. 2016;8:2982–2989. doi: 10.19082/2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong C.X., Mahajan R., Pathak R., D J.T., Sanders P. The role of pericardial and epicardial fat in atrial fibrillation pathophysiology and ablation outcomes. J. Atr. Fibrillation. 2013;5:790. doi: 10.4022/jafib.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatem S.N., Redheuil A., Gandjbakhch E. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc. Res. 2016;109:502–509. doi: 10.1093/cvr/cvw001. [DOI] [PubMed] [Google Scholar]
- 32.Viviano A., Yin X., Zampetaki A., Fava M., Gallagher M., Mayr M. Proteomics of the epicardial fat secretome and its role in post-operative atrial fibrillation. Europace. 2018;20:1201–1208. doi: 10.1093/europace/eux113. [DOI] [PubMed] [Google Scholar]
- 33.Douglass E., Greif S., Frishman W.H. Epicardial fat: pathophysiology and clinical significance. Cardiol. Rev. 2017;25:230–235. doi: 10.1097/CRD.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 34.Ansari M.A., Mohebati M., Poursadegh F., Foroughian M., Shamloo A.S. Is echocardiographic epicardial fat thickness increased in patients with coronary artery disease? A systematic review and meta-analysis. Electron Physician. 2018;10:7249–7258. doi: 10.19082/7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabkin S., Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes. Rev. 2015;16:406–415. doi: 10.1111/obr.12270. [DOI] [PubMed] [Google Scholar]
- 36.Middeldorp M.E., Pathak R.K., Meredith M., Mehta A.B., Elliott A.D., Mahajan R. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. doi: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]
- 37.Pokushalov E., Kozlov B., Romanov A., Strelnikov A., Bayramova S., Sergeevichev D. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: results of a randomized pilot study. J. Am. Coll. Cardiol. 2014;64:628–629. doi: 10.1016/j.jacc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 38.Alexopoulos N., Melek B.H., Arepalli C.D., Hartlage G.-R., Chen Z., Kim S. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning) J. Am. Coll. Cardiol. 2013;61:1956–1961. doi: 10.1016/j.jacc.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 39.Kitamura K., Shibata R., Tsuji Y., Shimano M., Inden Y., Murohara T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1814–H1821. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]