Abstract
Recurrent miscarriage (RM) is currently defined as two or more losses of a clinically established intrauterine pregnancy. Despite years of research, RM continues to be a clinically frustrating challenge for patients and physicians, and its etiology remains poorly understood. Accumulating evidence has suggested that epigenetic modifications are involved in early embryogenesis, and defects in epigenetic patterning contribute to the development of RM. Here, we studied the role of enhancer of zeste homolog 2 (EZH2) in the pathogenesis of RM and found that the EZH2 expression was significantly decreased in the villi from women with RM compared with that in control villi. EZH2 promoted the invasion of trophoblast cells. Moreover, EZH2 could promote epithelial-mesenchymal transition by epigenetically silencing CDX1. Both chromatin immunoprecipitation (ChIP)-PCR and dual-luciferase report assays demonstrated that EZH2 repressed CDX1 transcription via direct binding to its promoter region and then trimethylating Histone3-Lysine27. Furthermore, we discovered that progesterone, which is used extensively in the treatment of miscarriage and RM, increased the expression of EZH2 via the extracellular signaling-regulated kinase (ERK1/2) pathway. These findings revealed that EZH2 may regulate trophoblast invasion as an epigenetic factor, suggesting that EZH2 might be a potential therapeutic target for RM.
Keywords: EZH2, H3K27me3, trophoblast, recurrent miscarriages, EMT, CDX1
Introduction
Recurrent miscarriage is defined as two or more failed clinical pregnancies, which are verified by ultrasonography or histopathologic examination.1 Recurrent miscarriage (RM) is one of the most common clinical problems in reproduction, and it affects 2%–5% of childbearing couples.2 Common established etiology includes advanced maternal ages, uterine anomalies, immune dysregulations, hormonal and metabolic disorders, environmental factors, and cytogenetic abnormalities. However, less than 50% of couples with RM are diagnosed with an exact etiology.3 Previous studies have revealed that shallow or insufficient extravillous trophoblast (EVT) invasion is implicated in the pathogenesis of RM.4, 5 In recent years, it has been increasingly appreciated that epigenetic factors, such as histone modification and DNA methylation, are involved in pregnancy outcomes through regulating trophoblast functions, which are responsible for the healthy development of the fetus.6, 7, 8, 9
Histone modification is one of the major epigenetic mechanisms.8, 10 Specific histone modifications confer active or repressive transcriptional states.11 For instance, the trimethylation of Histone3-Lysine27 (H3K27me3) is a marker of tightly packaged heterochromatin and is associated with gene repression.12 Together with DNA hypermethylation, transcriptional factors, and other regulatory subunits, H3K27me3 typically forms complexes to control target gene expressions.8 In human placental villi, most of the EVTs are H3K27me3-positive when stained by immunohistochemistry.12
Enhancer of zeste homolog 2 (EZH2), the catalytic subunit of polycomb repressive complex 2, mediates the transcriptional silencing of target genes through H3K27me3. EZH2 overexpression is associated with aggressiveness and advanced diseases in some cancers.13, 14, 15, 16 Additionally, EZH2 has extensive roles in reproduction. It is a driver of epithelial-mesenchymal transition (EMT) in endometriosis, which impairs fertility.17 EZH2 is also essential for the early embryogenesis of mice.18 EZH2-deficient embryos display impaired growth potential and embryonic lethality in mice.19 However, the functions and mechanisms of EZH2 in human trophoblasts remain unknown.
Caudal-related homeobox transcription factor 1 (CDX1) plays essential roles in anteroposterior vertebral patterning and intestinal homeostasis.20 Increasing evidence demonstrates that CDX1 and CDX2 function as tumor suppressors.21, 22 In addition, the CpG islands of the CDX1 promoter showed abnormal DNA methylation levels in pre-eclampsia patients compared with those from healthy cases.7 Moreover, CDX1 restricts the invasion of HTR-8/SVneo trophoblast cells by inhibiting MMP-9 expression.23 However, the relationship between CDX1 and RM, as well as the upstream epigenetic regulation of CDX1, has not been sufficiently addressed. In this study, we demonstrate that the expression of EZH2 was reduced in the villi of patients with RM, and its downregulation affected trophoblast invasion by inhibiting EMT via epigenetically repressing CDX1. We also found that progesterone (P4) leads to the upregulation of EZH2 through the extracellular signaling-regulated kinase (ERK1/2) pathway in trophoblasts.
Results
The Expression of EZH2 Is Decreased in the Chorionic Villous Tissues of RM Patients
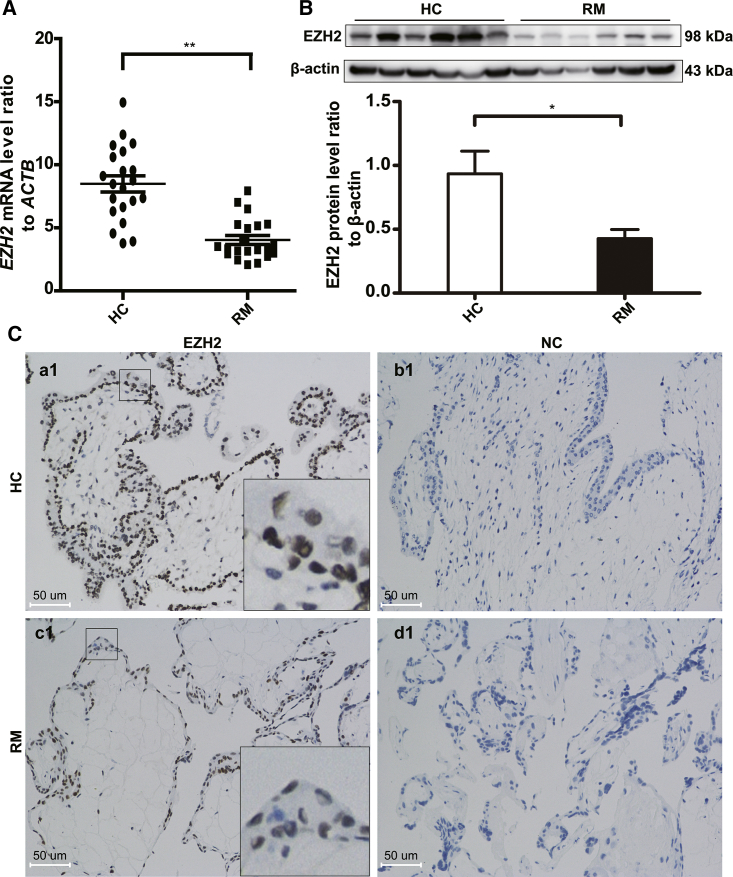
To determine whether EZH2 contributes to the pathogenesis of RM, we first conducted qRT-PCR and western blotting of the villous tissues in first trimester. At both mRNA and protein levels, EZH2 was significantly decreased in the villous tissues of RM patients compared with those of healthy controls (HCs) (Figures 1A and 1B). The immunohistochemical staining of paraffin-embedded villous tissues was performed to further investigate the abundance of EZH2. After staining, the strong expression of EZH2 was observed in the HC group, with the staining mainly locating in the cytotrophoblast (Figure 1Ca1), whereas a much weaker staining was detected in the RM group (Figure 1Cc1).
Figure 1.
EZH2 Expression Was Decreased in Villi of Women with RM
(A) EZH2 mRNA expression levels in the villous tissue of recurrent miscarriage (RM) patients (n = 21) and healthy controls (HCs) (n = 22) were determined by qPCR. The relative RNA amount was calculated with the 2−ΔΔCt method and normalized to the internal control ACTB. (B) Expression of EZH2 protein relative to β-actin (n = 12 for each group). The data are shown as the mean ± SEM. *p < 0.05; **p < 0.01. (C) Representative images of EZH2 protein expression in the villous tissues from HCs and RM patients after immunohistochemistry. Brown staining represents the target protein EZH2. HC, healthy pregnancy control; NC, negative control; RM, recurrent miscarriage. Scale bars, 50 μm.
EZH2 Promotes the Invasion of Trophoblast Cells
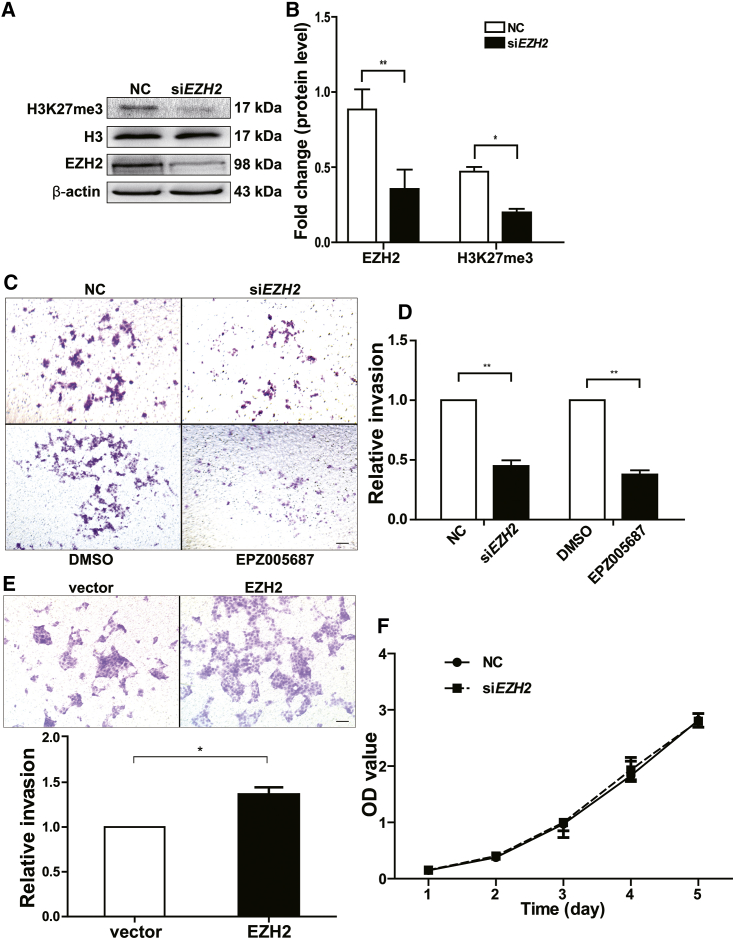
To evaluate the role of EZH2 in the invasion of trophoblast cells, transwell assays were performed using the cell line JAR as a model of EVT, owing to the difficulty of acquiring primary EVT cells. First, the knockdown efficiency of EZH2 was tested by western blotting, and the results showed that both EZH2 and its effector target H3K27me3 were significantly reduced (Figures 2A and 2B). Then, transwell assays revealed that EZH2 knockdown significantly decreased the invasion of trophoblast cells, and treatment with EPZ005687 (an EZH2 inhibitor) also led to a significant reduction in invasiveness (Figures 2C and 2D). Moreover, the overexpression of EZH2 promoted trophoblast invasion, which was in line with the knockdown and inhibition results (Figure 2E). Considering the link between EZH2 overexpression and tumor invasion and proliferation in cancers,15 we also conducted CCK-8 assays and found that the knockdown of EZH2 did not influence the proliferation of trophoblast cells (Figure 2F).
Figure 2.
EZH2 Promoted Trophoblast Invasion In Vitro
(A) Knockdown of EZH2 by siRNA decreased H3K27me3 and western blot analysis of EZH2 and H3K27me3 expressions in trophoblast cells transfected with siEZH2 or NC after 48 h. H3 and β-actin were used as internal controls relative to H3K27me3 and EZH2, respectively. (B) Statistical analysis results of (A). (C) EZH2 knockdown or an EZH2 inhibitor EPZ005687 significantly decreased trophoblast cell invasion compared with that of the controls. (D) Statistical analysis results of (C). (E) EZH2 overexpression significantly increased trophoblast cell invasion. After the transfection of EZH2 and vector plasmids for 48 h, the overexpression of EZH2 enhanced the invasion of trophoblast cells in comparison with that of the control group. (F) Trophoblast cell proliferation was measured after transient transfection of siEZH2 at the indicated times using a Cell Counting Kit-8 (CCK-8) assay kit. The scale bars represent 200 μm. All data are reported as the mean ± SEM. *p < 0.05; **p < 0.01. NC, scrambled siRNA.
EZH2 Regulates the Transcription and Expression of E-Cadherin and CDX1
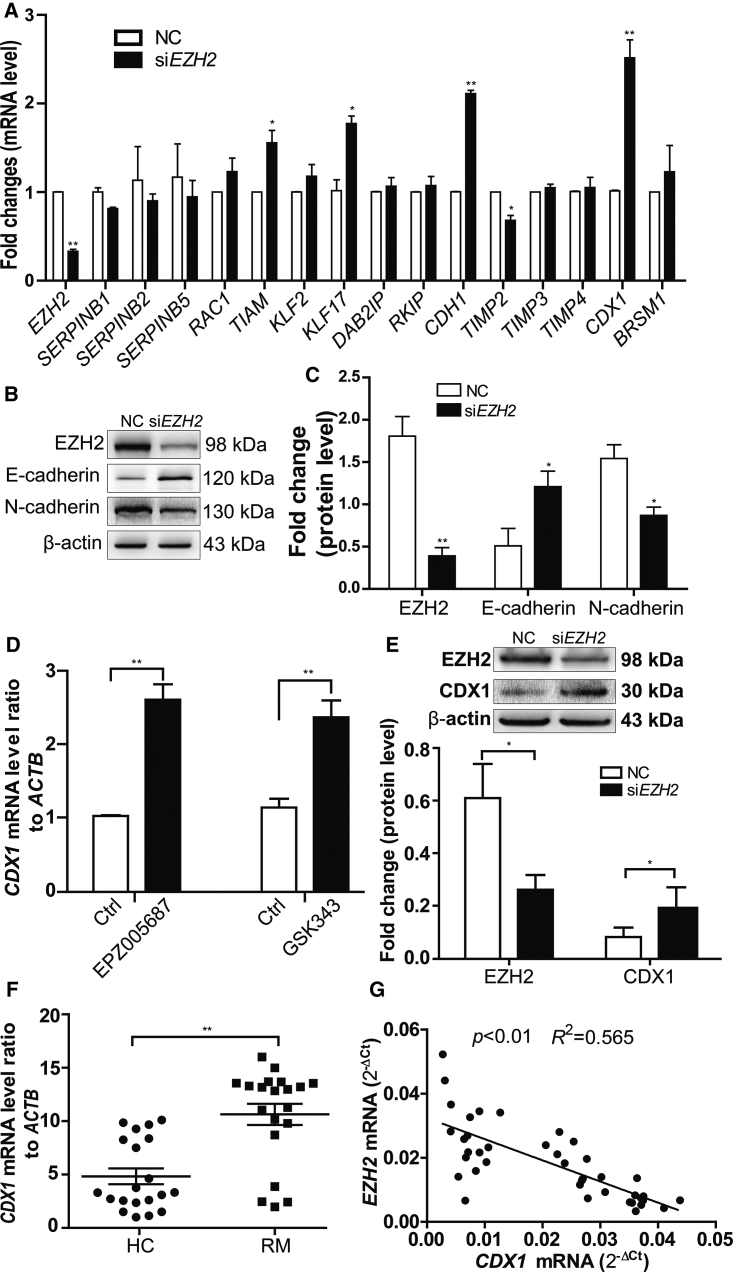
Because EZH2 promoted cell invasion of trophoblast cells, to determine the mechanisms, we screened 15 anti-invasion genes based on the literature regarding trophoblast cells by qRT-PCR, and we found that EZH2 knockdown significantly increased the mRNA levels of TIAM-1, KLF17, CDH1 (the gene encoding E-cadherin), and CDX1 (Figure 3A). Among these genes, the last two changed most significantly; consequently, we focused on the roles of E-cadherin and CDX1. Earlier studies from other groups suggest an inverse relationship between EZH2 and E-cadherin expression, and they demonstrate that E-cadherin is downregulated in EZH2-overexpressing cells through H3K27 trimethylation of the E-box regions in the CDH1 promoter.14, 17, 24 Therefore, we analyzed whether the decrease in EZH2 protein level could restore E-cadherin protein using western blotting. The results showed that, following the knockdown of EZH2 by siRNA transfection, E-cadherin protein in the trophoblast cells was significantly upregulated (Figures 3B and 3C). Additionally, the expression of N-cadherin protein was reduced simultaneously (Figures 3B and 3C).
Figure 3.
Silencing EZH2 Increased E-Cadherin and CDX1 Expressions in the Trophoblasts
(A) The effects of EZH2 knockdown on the expression of 15 invasion suppressor genes in trophoblast cells detected by qPCR. (B) Knockdown of EZH2 increased the expression of E-cadherin and reduced N-cadherin levels by western blot analysis. (C) Statistical analysis of the western blot results in (B). (D) Inhibition of EZH2 enzymatic activity by GSK343 and EPZ005687 increased the expression of CDX1 mRNA, as detected by qPCR. (E) Knockdown of EZH2 increased the expression of CDX1 by western blot analysis. (F) CDX1 mRNA levels in the villous tissue of RM patients (n = 21) and HCs (n = 22) were determined by qPCR. (G) The CDX1 mRNA levels in the villous tissues were measured using qPCR and correlated with the EZH2 mRNA level in the same cohort (n = 40). All data are reported as the mean ± SEM. *p < 0.05; **p < 0.01. Ctrl, DMSO control; NC, scrambled siRNA.
Because CDX1 plays a pivotal role as a tumor suppressor,25 we focused on whether CDX1 is another candidate target gene of EZH2. In accordance with the above result (Figure 3A), EZH2 inhibitors, including GSK343 and EPZ005687, both increased the mRNA level of CDX1 in trophoblast cells (Figure 3D). To further confirm the results, western blotting was performed, and the result showed that silencing EZH2 upregulated the expression of CDX1 at the protein level (Figure 3E). In light of the inverse relationship between EZH2 and CDX1, it is interesting to find that the levels of CDX1 increased significantly in the villous tissues of RM patients compared with those of HC (Figure 3F). Linear correlation analysis also showed that the CDX1 mRNA level was negatively correlated with that of EZH2 in villous tissue (Figure 3G). Taken together, these data suggested that the downregulation of EZH2 promoted CDX1 expression.
EZH2 Inhibits the Transcription of CDX1
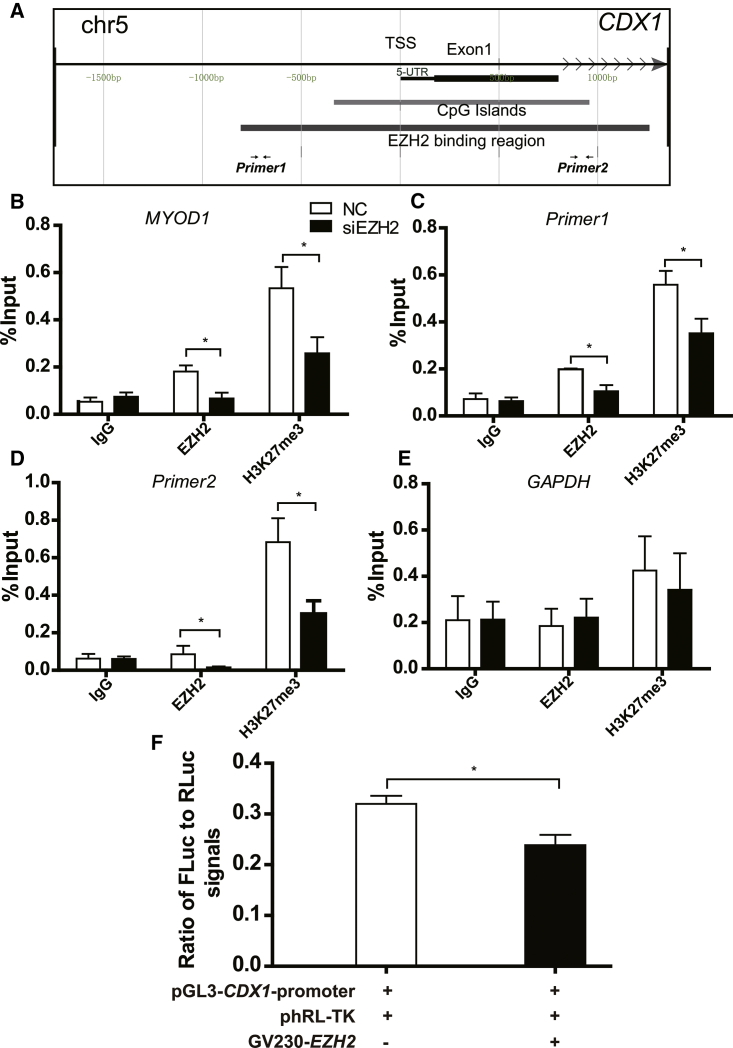
To examine whether EZH2 repressed CDX1 expression by physical DNA binding to the CDX1 promoter, we performed chromatin immunoprecipitation (ChIP) assays. The isolated DNA was subjected to qPCR using primer sets (Table 2) designed to amplify the region of the CDX1 promoter harboring the EZH2 binding sites (Primer1 and Primer2 in the diagrammatic sketch of Figure 4A). Among the primer sets, MYOD1 was used as a positive control (Figure 4B), whereas GAPDH was utilized as a negative control (Figure 4E). We found that EZH2 was directly recruited to the putative EZH2-binding regions of the CDX1 promoter, and this recruitment was accompanied by the detection of high levels of the repressive marker H3K27me3 (Figures 4C and 4D). To further determine the repression of CDX1 by EZH2, we next conducted dual-luciferase report assays in JAR cells using the luciferase reporter plasmid containing a 2-kb CDX1 promoter and EZH2-overexpressing plasmid. The transient enhanced expression of wild-type EZH2 resulted in significant inhibition of CDX1 promoter activity (Figure 4F). Thus, we speculated that EZH2 might regulate CDX1 expression through direct binding at the CDX1 promoter. These results suggested that EZH2 may promote invasion in trophoblast cells through downregulation of CDX1.
Table 2.
ChIP-qPCR Primer Sets
| Gene | Primers for ChIP | Size |
|---|---|---|
| MYOD1 | F: 5′-CCGCCTGAGCAAAGTAAATGAG-3′ | 82 |
| R: 5′-CACCTTGGGCAACCGCTG-3′ | ||
| CDX1-Primer1 | F: 5′-AGTTTCCTGTAATGTCCCCAGAAG-3′ | 80 |
| R: 5′-AAAGTTCTCAAAAGGCAGTGTGTG-3′ | ||
| CDX1-Primer2 | F: 5′-AGCACAGCCTAACCCTCTCTCTG-3′ | 110 |
| R: 5′-GGGAGCCTGCGTTCTAGTTCG-3′ | ||
| GAPDH | F: 5′-TACTAGCGGTTTTACGGGCG-3′ | 166 |
| R: 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′ |
Figure 4.
EZH2 Regulated CDX1 Expression via Direct Binding to Its Promoter
(A) Schematic diagram of the CDX1 promoter region showing the location of CpG islands, the putative EZH2-binding region, and the spanning region of primers used for ChIP assay and PCR analysis. (B–E) Knockdown of EZH2 decreased the binding of both EZH2 and H3K27me3 on the promoter of CDX1. Immuno-precipitated DNA fragments were analyzed by qPCR with specific primer sets. Chromatin obtained from trophoblast cells was immune-precipitated using antibodies against EZH2, histone H3, and IgG. Each ChIP experiment was repeated three times. MYOD1 (B) was positive control; Primer1 (C) and Primer2 (D) were for EZH2; and GAPDH (E) was negative control. (F) Overexpression of EZH2 decreased the transcriptional activity of CDX1 promoter, as detected by dual-luciferase report assays. Approximately 2 kb upstream of the transcriptional start site (TSS) on the promoter of CDX1 was cloned into pGL3 vector. Three plasmids were cotransfected into trophoblast cells for 48 h. Fluorescence measurement was performed using dual-luciferase report assays. RLuc was used for the internal control. All data are reported as the mean ± SEM. *p < 0.05; **p < 0.01. FLuc, firefly luciferase; GAPDH, negative control; MYOD1, positive control; RLuc, Renilla luciferase.
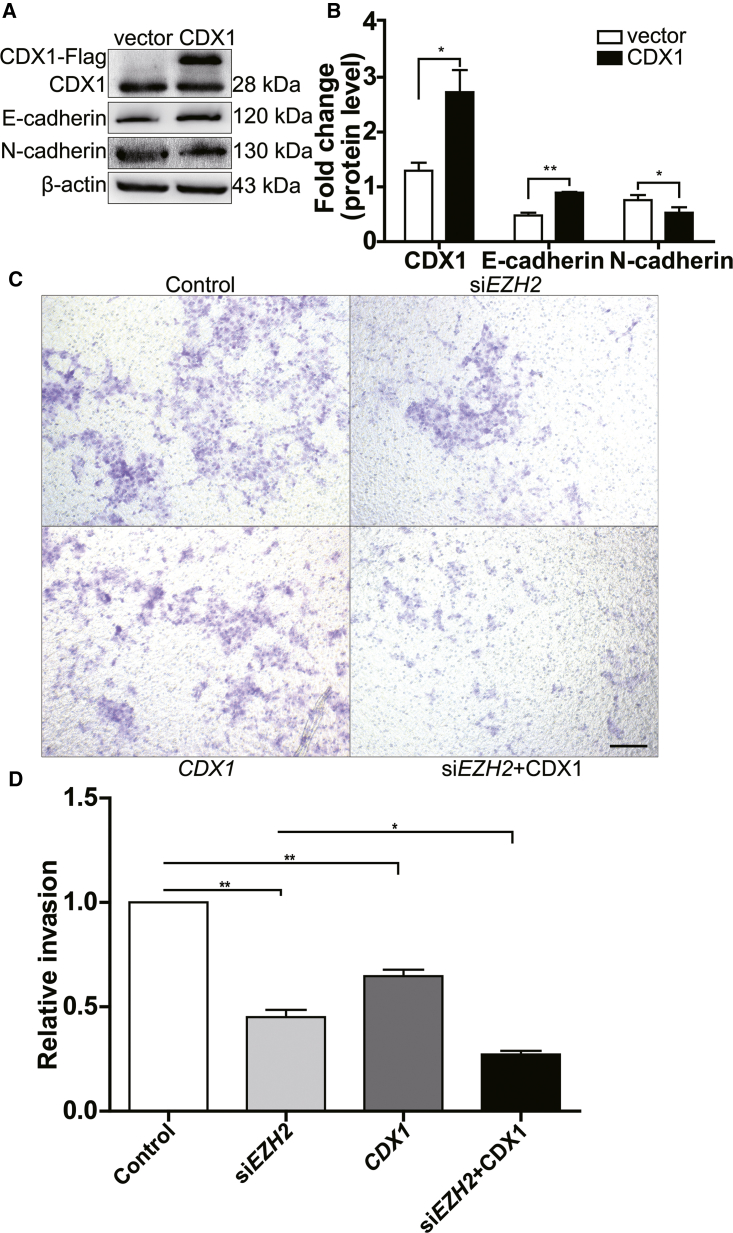
The Overexpression of CDX1 Upregulates E-Cadherin Expression
A previous study demonstrated that CDX1 is one of the EMT-related genes;26 it could induce E-cadherin adhesion activity by reducing beta-catenin and p120 tyrosine phosphorylation.27 In a mouse tumor model, CDX1 knockout tumors lose the expression of the epithelial marker E-cadherin and gain the expression of vimentin, Twist1, Zeb1, and Zeb2, as is typical of EMT.25 Consistent with the above findings, the enhanced expression of CDX1 by the transient transfection of CDX1 plasmids upregulated E-cadherin protein in the trophoblast cells and reduced the expression of N-cadherin (Figures 5A and 5B). The transwell assays further showed that CDX1 overexpression significantly decreased the invasion of trophoblast cells. Additionally, CDX1 overexpression also enhanced EZH2 siRNA-mediated deficient cell invasion (Figures 5C and 5D).
Figure 5.
CDX1 Overexpression Attenuated EMT in Trophoblasts
(A) Overexpression of CDX1 increased the expression of E-cadherin and reduced the N-cadherin level by western blot analysis. (B) Statistical analysis of the western blot results in (A). (C and D) CDX1 overexpression significantly decreased trophoblast cell invasion. After transfection for 48 h, the overexpression of CDX1 attenuated the invasion of trophoblast cells in comparison with that of the control group. CDX1 overexpression also enhanced EZH2 siRNA-mediated deficient cell invasion. (D) is the statistical result of (C). The scale bar represents 200 μm. All data are reported as the mean ± SEM. *p < 0.05; **p < 0.01. EMT, epithelial-mesenchymal transition.
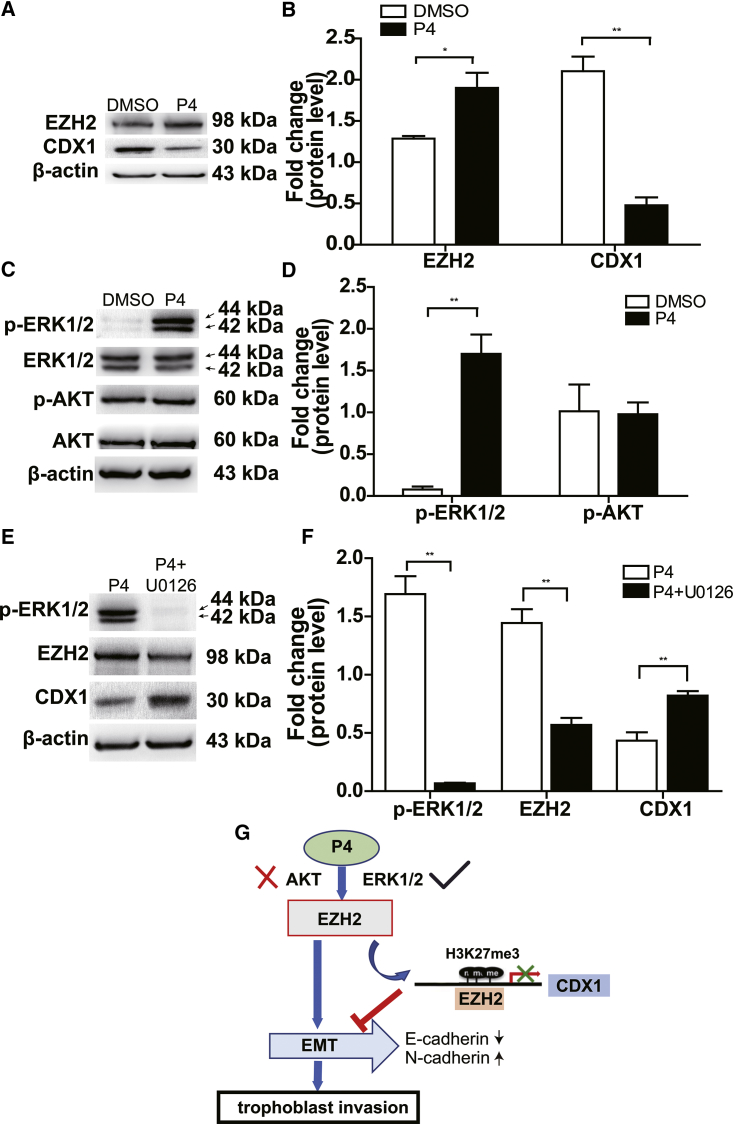
Progesterone Upregulates EZH2 Expression through ERK1/2 Activation
Progesterone has been proposed and used extensively in the treatment of different reproduction-associated pathologies, such as miscarriages, RMs in early pregnancy, and the maintenance of pregnancy.28 A close association has been recently described in mammary physiology during pregnancy between progesterone and EZH2 expression.29 Therefore, we explored whether progesterone augments EZH2 expression in trophoblast cells. A western blot analysis revealed significant increases in the level of EZH2 after treatment with progesterone for 24 h. Meanwhile, a striking decrease in CDX1 expression was detected (Figures 6A and 6B).
Figure 6.
Progesterone Upregulated EZH2 Expression through the ERK1/2 Pathway
(A and B) After treatment with progesterone for 24 h, EZH2 protein was upregulated, whereas CDX1 expression was reduced, as determined by western blotting. (B) is the statistical result of (A). (C and D) p-ERK1/2 protein levels, but not p-AKT levels, increased the following treatment with progesterone for 24 h. (D) is the statistical result of (C). (E and F) Inhibition of the ERK1/2 signaling pathway using U0126 for 24 h attenuated the protein level of EZH2; meanwhile, the CDX1 level was upregulated. (F) is the statistical result of (E). (G) The schematic roles of EZH2 in regulating the invasion behavior of trophoblasts. All data are shown as the mean ± SEM. *p < 0.05; **p < 0.01. P4, progesterone.
It is well-known that both the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway and the mitogen-activated protein kinase (MAPK)/ERK signal pathway are critical for the proliferation and invasion of trophoblast cells. Some researchers have previously highlighted evidence in some cancer cells suggesting that EZH2 may be regulated by the above pathways.30, 31 Promoter analysis shows that three Elk-1 binding motifs are located within the EZH2 promoter, and the MEK-ERK1/2-Elk-1 pathway leads to EZH2 upregulation, as detected by dual-luciferase assays.32 In our study, after treatment with progesterone for 24 h, we found that ERK1/2 signaling was intensely activated, whereas AKT signaling was not (Figures 6C and 6D). To further determine the upstream signaling pathway mediating the ERK1/2-dependent expression of EZH2, we blocked the ERK1/2 signaling pathway using U0126 (20 μM) and found a significant reduction in EZH2 protein, followed by increased expression of CDX1 (Figures 6E and 6F). These data suggest that progesterone upregulated the expression of EZH2 through the ERK1/2 signaling pathway. The schematic diagram of EZH2 mediating the invasion of trophoblast is shown in Figure 6G.
Discussion
The resemblance in the invasive behavior between placental trophoblasts and cancer cells and the role of aberrant epigenetic regulation in cancer development are well-known.33 Delicate control of trophoblast invasion is important for implantation, uterine spiral artery remodeling, and maternal-fetal communication.34 Moreover, numerous studies have established a strong link between epigenetic regulation and placental trophoblast function by demonstrating the critical role of epigenetic regulators in maintaining a healthy pregnancy.8, 35, 36 In this study, we investigated the role of EZH2 in the invasiveness of trophoblast. Our data showed that the expression of EZH2 both at mRNA and protein levels was significantly decreased in the villi of women who suffered from RM compared with those from normal pregnant women. The ChIP and dual-luciferase report assays showed that EZH2 could inhibit CDX1 transcription directly through trimethylation of H3K27 at the promoter of CDX1, which inhibited EMT in trophoblast. Thus, EZH2 could indirectly promote trophoblast EMT via CDX1. Moreover, we found that progesterone, which is used to treat or prevent early miscarriages and RM upregulated EZH2 expression via the ERK1/2 signaling pathway, which means the epigenetic mechanisms in the pathogenesis of RM have long been targeted for its therapy to reverse this disadvantage.
It is now well established that impaired trophoblast invasion is implicated in the etiology of pregnancy complications, including RM.33 In our present study, trophoblast invasion was attenuated by silencing of EZH2 or inhibiting of EZH2 enzyme activity with inhibitors. EZH2 overexpression also promoted invasion of trophoblasts. EZH2 can promote the invasion of malignant cancer cells by silencing target genes that are tumor suppressors.37, 38, 39, 40, 41 Among these genes, CDH1 (the gene that encodes E-cadherin) is putatively one of the targets.14, 24 EZH2 facilitates tumor metastasis by directly repressing CDH1 transcription via methylating Histone3-Lysine 27 at the promoter region mainly through E-box regions.14 E-cadherin is a marker of epithelial cells and is of crucial importance in cancer invasion and metastasis. EMT, featured by reduced E-cadherin and increased N-cadherin expression, has been identified as a characteristic of aggressive tumors; it entitles cells to migratory and invasive capability in carcinogenesis.42, 43 Previous studies have shown that EZH2 physically associates with CDH1 and inhibits E-cadherin transcription via H3K27me3.14, 44 In this study, EZH2 also repressed E-cadherin transcription and protein expression, which was accompanied by the upregulation of N-cadherin and proved that EZH2 directly promoted EMT.
CDX1 is also a tumor suppressor gene; the ablation of CDX1 and CDX2 results in a significant increase in tumor formation in the colon.21 CDX1 is regulated by epigenetic modification; the hypermethylation of the CpG island at the promoter is positively associated with severe clinical phenotypes.26 In addition, it is one of the EMT-related genes. CDX1 or CDX2 expression enhances E-cadherin-mediated cell-cell adhesion in human COLO 205 cells.27, 45 In our study, we found there exists a physical association between EZH2 and H3K27me3 at the CDX1 promoter in trophoblast cells by ChIP-qPCR. Furthermore, CDX1 promoter activity significantly diminished after the overexpression of EZH2, indicating the methylation of H3-K27 by EZH2 at the CDX1 promoter and consequent suppression of CDX1. Moreover, the expression of CDX1 increased in the villous tissues of RM patients, which was opposite to the EZH2 expression. In addition, our study also showed that enhanced CDX1 expression inhibited invasion of trophoblast, upregulated E-cadherin, and downregulated N-cadherin, which means that the CDX1 expression inhibited EMT. Overall, we speculate that EZH2 could promote trophoblast invasion via the indirect promotion of EMT by inhibiting the CDX1 expression.
The PI3K/AKT and ERK1/2 signaling pathways are both verified to be involved in the progress of cellular growth, proliferation, and invasion. Whereas EZH2 expression is regulated by the AKT and ERK1/2 pathways,31 phos (p)-AKT and p-ERK1/2 proteins are less expressed in the RM group compared with the control group.46 In addition, previous research has reported that trophoblast invasion and migration could be promoted by progesterone, which is widely used in clinical practice.28, 47, 48 Furthermore, Pal et al.29 found that the expression and phosphorylation of EZH2 (Thr487) coincide with H3K27me3 modification and peak during pregnancy, which is driven by progesterone. Therefore, we examined p-AKT and p-ERK1/2 expression after progesterone treatment. Our work indicated that progesterone could activate the ERK1/2 pathway and, thus, significantly increase the expression of EZH2 accompanied with the decreased level of CDX1, but not the PI3K/AKT signaling pathway. This is in accordance with the results that report that progesterone is able to induce phosphorylation of ERK1/2 in two choriocarcinoma cell lines (BeWo and JEG-3)49 and is consistent with the reports demonstrating that the phosphorylation of EZH2 at serine 21 suppresses its methyltransferase activity responsible for H3-K27 trimethylation.50, 51 The decreased expression of EZH2 in women with RM provides new insights into the epigenetic mechanisms underlying the pathogenesis of RM and paves new avenues for the development of novel therapeutics.
Materials and Methods
Villous Samples Collection
Twenty-one patients with RM between the ages of 23 and 35 years (mean age 29.0 ± 3.4 years) who had been treated at the Department of Obstetrics and Gynecology of Renji Hospital between July 2016 and September 2016 were recruited for this study. Among these RM patients, 13 patients had two miscarriages, and 8 patients had three miscarriages. Twenty-two women between the ages of 23 and 35 years (mean age 28.1 ± 4.5 years) with healthy early pregnancies that were artificially terminated for nonmedical reasons were included as HCs. The gestational ages when the pregnancy was terminated for the HC and RM groups were 9.2 ± 1.3 and 8.2 ± 2.3 weeks, respectively. Patients with the following features were excluded: (1) genital malformation on pelvic examination and ultrasound, (2) history of genetic diseases, and (3) symptoms of endocrine or metabolic diseases. The Renji Hospital Research and Ethics Committee approved the form and use of the villous tissues (No. 2018033004). The villous tissues were collected immediately after suction and curettage, and blood was removed by scrubbing the villi on cotton gauze. Collected tissues were then quickly frozen in liquid nitrogen and stored at −80°C before use.
Western Blot Analysis
Total protein was isolated from villous tissues and cultured JAR cells using ice-cold radio immunoprecipitation assay (RIPA) (Beyotime Biotechnology, Haimen, China) containing protease inhibitor cocktail tablets and phosphatase inhibitor cocktail tablets (Roche, Basel, Switzerland). Twenty-five micrograms of protein was electrophoresed in SDS-polyacrylamide gels and transferred onto nitrocellulose filter membranes (GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK). Then the membranes were incubated with primary antibodies at 4°C overnight. After extensive washing, the bound primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (1:5,000; Proteintech) with a chemiluminescent detection system. The primary antibodies used in the assays were EZH2 (1:1,000; 5246; Cell Signaling, Danvers, MA, USA), H3K27me3 (1:1,000; 9733; Cell Signaling), H3 (1:1,000; 9728; Cell Signaling), p44/42 (1:1,000, 4695; Cell Signaling), p-p44/42 (1:1,000, 4695; Cell Signaling), AKT (1:1,000; 4691; Cell Signaling), p-AKT (1:2,000, 4060; Cell Signaling), CDX1 (1:1,000; ab126748; Abcam), E-cadherin (1:1,000; sc8426; Santa Cruz), N-cadherin (1:1,000; 22018-1-AP; Proteintech), and β-actin (1:5,000; sc47778; Santa Cruz).
Immunohistochemistry
Immunohistochemical staining was performed on paraffin-embedded sections, as previously described.52, 53, 54, 55 In brief, the slides were deparaffinized, rehydrated in water, and washed in PBS thrice. After inactivation of endogenous peroxidase activity and antigen retrieval, the sections were subjected to incubation with 0.3% Triton X-100 for 20 min. After blocking with immunoglobulin G and incubation with the primary antibody overnight at 4°C, the samples were handled using a two-step plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (PV-9000; ZSGB-BIO, Beijing, China). Finally, the slides were counterstained with hematoxylin, dehydrated with 95% alcohol, and mounted in neutral balsam. Negative controls were treated with nonimmune serum instead of the primary antibody. The antibody against EZH2 used for immunohistochemistry was the same as that used for western blotting.
qPCR Analysis
The total RNA was prepared using an animal total RNA isolation kit (Foregene, Chengdu, China) according to the manufacturers’ instructions. After reverse transcription, the cDNAs were amplified on an ABI 7300 Real-Time PCR System (Applied Biosystems) under the following conditions: initial denaturation for 3 min at 95°C followed by 40 cycles of denaturation at 95°C for 40 s, annealing at 59°C for 40 s and extension at 72°C for 20 s, and a final extension for 5 min at 72°C. Beta-actin was used for normalization. The results were analyzed using the ΔΔCt method. The sequences of the primers are given in Table 1.
Table 1.
Primer Sequences for qRT-PCR
| Gene | Primers | Size (bp) |
|---|---|---|
| EZH2 | F: 5′-TTCATGCAACACCCAACACT-3′ | 96 |
| R: 5′-CTCCCTCCAAATGCTGGTAA-3′ | ||
| CDX1 | F: 5′-CCAAAACCGGCGGGCAAAGGAG-3′ | 102 |
| R: 5′-GCTGGGGTGGCCGTGATGTCGT-3′ | ||
| ACTB | F: 5′-GGGAAATCGTGCGTGACATTAAG-3′ | 275 |
| R: 5′-TGTGTTGGCGTACAGGTCTTTG-3′ | ||
| TIMP2 | F: 5′-AGCATTTGACCCAGAGTGGAA-3′ | 64 |
| R: 5′-CCAAAGGAAAGACCTGAAGGA-3′ | ||
| TIMP3 | F: 5′-TCTGCAACTCCGACATCGT-3′ | 121 |
| R: 5′-TTGGTGAAGCCTCGGTACAT-3′ | ||
| TIMP4 | F: 5′-GCTGGGTGAGGCATGCAGCT-3′ | 119 |
| R: 5′-CAGGGTCTGCACTGGCCGGA-3′ | ||
| TIAM1 | F: 5′-TCATTCTGCTCATTACCTGCG-3′ | 206 |
| R: 5′-ACTTCTCAATGTGGATGCTCTG-3′ | ||
| RAC1 | F: 5′-GCCGCTTCCTATCTCAGC-3′ | 197 |
| R: 5′-ATGCATTGGTTGTGTAACTGATC-3′ | ||
| RKIP | F: 5′-CTACACCTTGGTCCTGACAGA-3′ | 138 |
| R: 5′-GAGCCCACATAATCGGAGAGG-3′ | ||
| SERPINB1 | F: 5′-TCAGCTTGCCCAGGTTCAAACTG-3′ | 300 |
| R: 5′-GGATGCTACCTGAGGAATTATGC-3′ | ||
| SERPINB2 | F: 5′-GCTGGAGATGTTAGCATGTTCTTG-3′ | 300 |
| R: 5′-GGCTTGGTGGAACACTTCAGAAAG-3′ | ||
| SERPINB5 | F: 5′-CATGGAGGCCACGTTCTGTATG-3′ | 417 |
| R: 5′-CCTGGCACCTCTATGGAATCCC-3′ | ||
| KLF2 | F: 5′-AGAGGGTCTCCCTCGATGAC-3′ | 193 |
| R: 5′-TCTCACAAGGCATCACAAGC-3′ | ||
| KLF17 | F: 5′-GCTCTGGAGTGCACACCTCTT-3′ | 74 |
| R: 5′-CAGCATCTCTGCGCTGTGA-3′ | ||
| BRMS1 | F: 5′-AAGGCACCTCTGGTTTCTGG-3′ | 146 |
| R: 5′-TGTGAACAGCAGGGTCAAGGT-3′ | ||
| CDH1 | F: 5′-CGAGAGCTACACGTTCACGG-3′ | 119 |
| R: 5′-GGGTGTCGAGGGAAAAATAGG-3′ |
Transwell Assay
Cell invasion was measured using Matrigel precoated transwell inserts (24-well plates; Corning Life Sciences, Tewksbury, MA, USA), as previously described.56 In brief, the inserts (8-mm pore size; Corning) were precoated with 25 μL of 1:8 diluted Matrigel. After transfection with NC or siEZH2, EZH2 or vector plasmids (GV230-EZH2 or GV230, kindly provided by Rujuan Zuo, Shanghai Jiaotong University, China),57 CDX1 or vector plasmids (pIRES2-CDX1 or pIRES2) for 48 h, or treatment with EPZ005687 (20 μM; Selleckchem, Houston, TX, USA) for 24 h, the cells (1 × 105 in 200 μL of DMEM/F12 containing 1% FBS) were cultured in 1% FBS media and seeded in the inserts. Then the inserts were placed into 24-well plates containing 600 μL of 20% FBS media in each well, and the cells were incubated for 24 h. The inserts were then removed and washed in PBS, and the noninvading cells, together with the Matrigel, were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10 min at room temperature and stained with crystalline iodine. The cells were observed under a fluorescence microscope (Zeiss, Germany). The cells that migrated to the lower surfaces were counted in five predetermined fields at a magnification of 100×. The ratio of the number of cells in the treated group to the number of cells in the control group was calculated as the “relative invasion.” Each experiment was carried out in triplicate and repeated at least three times. The sequences of EZH2 siRNA and CDX1 overexpression plasmids are in Tables S1 and S2.
ChIP Assay
In brief, the cells were harvested at 80% confluence in a 10-cm dish after 0.25% trypsin-EDTA digestion, washed twice with ice-cold PBS containing protease inhibitors, and centrifuged at 2,500 rpm for 3 min. The cell pellet was swirled to mix after sequential addition of 225 μL of buffer I (15 mM Tris-HCl [pH 7.5], 60 mM KCl, 5 mM MgCl2, 0.1 mM EGTA, and 0.3 M sucrose) and 250 μL of lysis buffer (15 mM Tris-HCl [pH 7.5], 60 mM KCl, 5 mM MgCl2, 0.1 mM EGTA, 0.3 M sucrose, 0.5% Nonidet P-40 [NP-40], and 1% sodium deoxycholate). Then, the mixture was lysed on ice for 10 min. After addition of 500 μL of MNase buffer (85 mM Tris-HCl [pH 7.5], 3 mM MgCl2, 2 mM CaCl2, and 0.3 M sucrose) containing 2 μg/mL BSA and 4000 U/mL Micrococcal Nuclease (M0247; NEB) per immunoprecipitation (IP) prep, the nuclei were then subjected to digestion for 10 min at 37°C, with frequent inversion every 3–5 min. The digestion was stopped by the addition of EDTA to a final concentration of 20 mM. The fragmented chromatin DNA was isolated by centrifugation at 12,000 rpm for 10 min at 4°C, then was immunoprecipitated with the antibodies of interest at 4°C overnight. The immune complexes were collected with protein A/G agarose beads (Santa Cruz) by incubating for 2 h at 4°C. The beads were first washed with washing buffer and were then eluted with elution buffer at 65°C for 10 min twice. The DNA was then subjected to RNase A treatment at 37°C for 30 min and was purified by Proteinase K digestion at 65°C for 2 h, followed by phenol-chloroform (Sigma) extraction and ethanol precipitation. The purified DNA was dissolved in sterile double-distilled water for further qPCR analysis. Similar amounts of fragmented DNA without precipitation were used as inputs. The primers designed for ChIP-qPCR are listed in Table 2. The primers for the GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) promoter were used as a negative control, and MYPD1 (Myoblast determination protein 1) was used as a positive control. qPCR results were analyzed using the following equation to express the abundance of the proteins enriched at the respective promoters: Percent input = 100% × 2(C [T] Input Sample–C [T] IP Sample).
Transient Transfection Assay and Dual-Luciferase Assay
The human placental choriocarcinoma cell line (JAR) was obtained from Shanghai Cellular Library of Chinese Academy of Sciences; the verification of the JAR cell line is supplied in the Supplemental Information. JAR cells were seeded in a 24-well culture plate in triplicate and cotransfected with EZH2 or vector plasmids (GV230-EZH2 or GV230)57 and CDX1-promoter plasmids (pGL3-CDX1-promoter; Transheep, Shanghai, China), along with phRL-TK plasmids (Transheep), which were used as internal controls. The ratio of the plasmid DNA (EZH2 [or Vector]:CDX1-promoter:phRL-TK) was 4:5:1. After 48 h of transfection by Lipofectamine 3000 Reagent (1.8 μL/well; Invitrogen), the cells were lysed for the measurement of luciferase activity using a Dual-Luciferase Reporter Assay System (E1910; Promega). CDX1-promoter-driven firefly luciferase activity was normalized for transfection efficiency by comparing with Renilla luciferase activity (indicated as “FLuc ratio to RLuc”). The sequences of CDX1-Luc plasmids are in Table S3.
Cell Culture and Treatment
JAR cells were maintained in six-well plates and cultured in phenol red-free DMEM/F-12 containing glutamine (GIBCO, Grand Island, NY, USA), 1% Insulin-Transferrin-Selenium (Invitrogen, Carlsbad, CA, USA), 5 × 10−2 g/L antibiotics (GIBCO), and 10% charcoal-stripped FBS (Biological Industries, Beit Haemek, Israel) at 37°C and in 5% CO2. After treatment with 10−7 M progesterone (Sigma Chemical, St. Louis, MO, USA) for 24 h, the cells were lysed with ice-cold RIPA for further analysis. For transient transfection, when cells reached 60%–70% confluence, synthetic siRNAs (GenePharma, Shanghai, China) or constructed plasmids were transfected using the Lipofectamine 3000 transfection kit (Invitrogen, Carlsbad, CA, USA) for 48 h according to the manufacturer’s instructions.
Statistical Analysis
The statistical analysis was conducted using SPSS statistical software (version 22.0; IBM, Chicago, IL, USA). The differences were analyzed using an independent sample t test between two groups. Comparison among multiple groups was carried out by a one-way ANOVA followed by Tukey’s post hoc test. The data are reported as the mean ± SEM. A value of p < 0.05 was considered statistically significant.
Author Contributions
S.L. and C.Z. designed the experiments, which were conducted by S.L., N.W., H.L., J.Y., J.L., and C.Z. S.L., Z.-J.C., and C.Z. wrote the manuscript. S.L., W.-P.L., and C.Z. edited the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The authors would like to thank all of the RM patients for their participation during this study. This work was supported by the Major Program of the National Natural Science Foundation of China (grant 81490743 to Z.-J.C.), National Key R&D Program of China (grant 2017YFC1001403), National Natural Science Foundation of China (NSFC; grants 31671199 and 31871512 to C.Z.), and Shanghai Commission of Science and Technology (grant 17DZ2271100).
Footnotes
Supplemental Information includes three tables and one STR profile report and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.12.011.
Contributor Information
Wei-Ping Li, Email: liweiping@renji.com.
Cong Zhang, Email: zhangxinyunlife@163.com.
Zi-Jiang Chen, Email: chenzijiang@hotmail.com.
Supplemental Information
References
- 1.Practice Committee of American Society for Reproductive Medicine Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil. Steril. 2013;99:63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 2.El Hachem H., Crepaux V., May-Panloup P., Descamps P., Legendre G., Bouet P.E. Recurrent pregnancy loss: current perspectives. Int. J. Womens Health. 2017;9:331–345. doi: 10.2147/IJWH.S100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McQueen D.B., Perfetto C.O., Hazard F.K., Lathi R.B. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil. Steril. 2015;104:927–931. doi: 10.1016/j.fertnstert.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Rugg-Gunn P.J. Epigenetic features of the mouse trophoblast. Reprod. Biomed. Online. 2012;25:21–30. doi: 10.1016/j.rbmo.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 5.DaSilva-Arnold S., James J.L., Al-Khan A., Zamudio S., Illsley N.P. Differentiation of first trimester cytotrophoblast to extravillous trophoblast involves an epithelial-mesenchymal transition. Placenta. 2015;36:1412–1418. doi: 10.1016/j.placenta.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Novakovic B., Gordon L., Wong N.C., Moffett A., Manuelpillai U., Craig J.M., Sharkey A., Saffery R. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: implications and opportunities for understanding trophoblast function. Mol. Hum. Reprod. 2011;17:344–353. doi: 10.1093/molehr/gar005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia R.Z., Zhang X., Hu P., Liu X.M., Hua X.D., Wang X., Ding H.J. Screening for differential methylation status in human placenta in preeclampsia using a CpG island plus promoter microarray. Int. J. Mol. Med. 2012;30:133–141. doi: 10.3892/ijmm.2012.983. [DOI] [PubMed] [Google Scholar]
- 8.Kohan-Ghadr H.R., Kadam L., Jain C., Armant D.R., Drewlo S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016;10:126–135. doi: 10.1080/19336918.2015.1098800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Z., Wang Y., Lin J., Xu J., Ding G., Huang H. Genetic and epigenetic risks of assisted reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017;44:90–104. doi: 10.1016/j.bpobgyn.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Berger S.L. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 2002;12:142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 11.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 12.Fogarty N.M.E., Burton G.J., Ferguson-Smith A.C. Different epigenetic states define syncytiotrophoblast and cytotrophoblast nuclei in the trophoblast of the human placenta. Placenta. 2015;36:796–802. doi: 10.1016/j.placenta.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Shin Y.J., Kim J.H. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS ONE. 2012;7:e30393. doi: 10.1371/journal.pone.0030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Q., Yu J., Dhanasekaran S.M., Kim J.H., Mani R.S., Tomlins S.A., Mehra R., Laxman B., Cao X., Yu J. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–7284. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren G., Baritaki S., Marathe H., Feng J., Park S., Beach S., Bazeley P.S., Beshir A.B., Fenteany G., Mehra R. Polycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancer. Cancer Res. 2012;72:3091–3104. doi: 10.1158/0008-5472.CAN-11-3546. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J., Roh J.W., Bandyopadhyay S., Chen Z., Munkarah A.R., Hussein Y., Alosh B., Jazaerly T., Hayek K., Semaan A. Overexpression of enhancer of zeste homolog 2 (EZH2) and focal adhesion kinase (FAK) in high grade endometrial carcinoma. Gynecol. Oncol. 2013;128:344–348. doi: 10.1016/j.ygyno.2012.07.128. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Dong P., Liu X., Sakuragi N., Guo S.W. Enhancer of Zeste homolog 2 (EZH2) induces epithelial-mesenchymal transition in endometriosis. Sci. Rep. 2017;7:6804. doi: 10.1038/s41598-017-06920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge S.Q., Lin S.L., Zhao Z.H., Sun Q.Y. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: implications for male fertility and offspring health. Oncotarget. 2017;8:53804–53818. doi: 10.18632/oncotarget.17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X.J., Wang X., Ma X., Sun S.C., Zhou X., Zhu C., Liu H. EZH2 is essential for development of mouse preimplantation embryos. Reprod. Fertil. Dev. 2014;26:1166–1175. doi: 10.1071/RD13169. [DOI] [PubMed] [Google Scholar]
- 20.Savory J.G.A., Bouchard N., Pierre V., Rijli F.M., De Repentigny Y., Kothary R., Lohnes D. Cdx2 regulation of posterior development through non-Hox targets. Development. 2009;136:4099–4110. doi: 10.1242/dev.041582. [DOI] [PubMed] [Google Scholar]
- 21.Hryniuk A., Grainger S., Savory J.G., Lohnes D. Cdx1 and Cdx2 function as tumor suppressors. J. Biol. Chem. 2014;289:33343–33354. doi: 10.1074/jbc.M114.583823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat. Rev. Cancer. 2003;3:592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- 23.Jia R.Z., Rui C., Li J.Y., Cui X.W., Wang X. CDX1 restricts the invasion of HTR-8/SVneo trophoblast cells by inhibiting MMP-9 expression. Placenta. 2014;35:450–454. doi: 10.1016/j.placenta.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Fujii S., Ochiai A. Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells. Cancer Sci. 2008;99:738–746. doi: 10.1111/j.1349-7006.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo R.J., Suh E.R., Lynch J.P. The role of Cdx proteins in intestinal development and cancer. Cancer Biol. Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 26.Tahara T., Shibata T., Okubo M., Ishizuka T., Nakamura M., Nagasaka M., Nakagawa Y., Ohmiya N., Arisawa T., Hirata I. DNA methylation status of epithelial-mesenchymal transition (EMT)–related genes is associated with severe clinical phenotypes in ulcerative colitis (UC) PLoS ONE. 2014;9:e107947. doi: 10.1371/journal.pone.0107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezaki T., Guo R.J., Li H., Reynolds A.B., Lynch J.P. The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing beta- and p120-catenin tyrosine phosphorylation. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G54–G65. doi: 10.1152/ajpgi.00533.2006. [DOI] [PubMed] [Google Scholar]
- 28.Di Renzo G.C., Giardina I., Clerici G., Brillo E., Gerli S. Progesterone in normal and pathological pregnancy. Horm. Mol. Biol. Clin. Investig. 2016;27:35–48. doi: 10.1515/hmbci-2016-0038. [DOI] [PubMed] [Google Scholar]
- 29.Pal B., Bouras T., Shi W., Vaillant F., Sheridan J.M., Fu N., Breslin K., Jiang K., Ritchie M.E., Young M. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Rep. 2013;3:411–426. doi: 10.1016/j.celrep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Riquelme E., Behrens C., Lin H.Y., Simon G., Papadimitrakopoulou V., Izzo J., Moran C., Kalhor N., Lee J.J., Minna J.D., Wistuba I.I. Modulation of EZH2 Expression by MEK-ERK or PI3K-AKT signaling in lung cancer is dictated by different KRAS oncogene mutations. Cancer Res. 2016;76:675–685. doi: 10.1158/0008-5472.CAN-15-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraro A., Mourtzoukou D., Kosmidou V., Avlonitis S., Kontogeorgos G., Zografos G., Pintzas A. EZH2 is regulated by ERK/AKT and targets integrin alpha2 gene to control Epithelial-Mesenchymal Transition and anoikis in colon cancer cells. Int. J. Biochem. Cell Biol. 2013;45:243–254. doi: 10.1016/j.biocel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Fujii S., Tokita K., Wada N., Ito K., Yamauchi C., Ito Y., Ochiai A. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30:4118–4128. doi: 10.1038/onc.2011.118. [DOI] [PubMed] [Google Scholar]
- 33.Rahat B., Thakur S., Hamid A., Bagga R., Kaur J. Association of aberrant methylation at promoter regions of tumor suppressor genes with placental pathologies. Epigenomics. 2016;8:767–787. doi: 10.2217/epi.16.7. [DOI] [PubMed] [Google Scholar]
- 34.Wu F., Tian F., Zeng W., Liu X., Fan J., Lin Y., Zhang Y. Role of peroxiredoxin2 downregulation in recurrent miscarriage through regulation of trophoblast proliferation and apoptosis. Cell Death Dis. 2017;8:e2908. doi: 10.1038/cddis.2017.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemberger M. Epigenetic landscape required for placental development. Cell. Mol. Life Sci. 2007;64:2422–2436. doi: 10.1007/s00018-007-7113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sailasree S.P., Srivastava S., Mishra R.K. The placental gateway of maternal transgenerational epigenetic inheritance. J. Genet. 2017;96:465–482. doi: 10.1007/s12041-017-0788-5. [DOI] [PubMed] [Google Scholar]
- 37.Jia J., Li F., Tang X.S., Xu S., Gao Y., Shi Q., Guo W., Wang X., He D., Guo P. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7:37868–37881. doi: 10.18632/oncotarget.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deb G., Thakur V.S., Limaye A.M., Gupta S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2015;54:485–499. doi: 10.1002/mc.22121. [DOI] [PubMed] [Google Scholar]
- 39.Chen H., Tu S.W., Hsieh J.T. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J. Biol. Chem. 2005;280:22437–22444. doi: 10.1074/jbc.M501379200. [DOI] [PubMed] [Google Scholar]
- 40.Xu C., Hou Z., Zhan P., Zhao W., Chang C., Zou J., Hu H., Zhang Y., Yao X., Yu L., Yan J. EZH2 regulates cancer cell migration through repressing TIMP-3 in non-small cell lung cancer. Med. Oncol. 2013;30:713. doi: 10.1007/s12032-013-0713-6. [DOI] [PubMed] [Google Scholar]
- 41.Zingg D., Debbache J., Schaefer S.M., Tuncer E., Frommel S.C., Cheng P., Arenas-Ramirez N., Haeusel J., Zhang Y., Bonalli M. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat. Commun. 2015;6:6051. doi: 10.1038/ncomms7051. [DOI] [PubMed] [Google Scholar]
- 42.Appolloni I., Barilari M., Caviglia S., Gambini E., Reisoli E., Malatesta P. A cadherin switch underlies malignancy in high-grade gliomas. Oncogene. 2015;34:1991–2002. doi: 10.1038/onc.2014.122. [DOI] [PubMed] [Google Scholar]
- 43.Schäfer G., Narasimha M., Vogelsang E., Leptin M. Cadherin switching during the formation and differentiation of the Drosophila mesoderm—implications for epithelial-to-mesenchymal transitions. J. Cell Sci. 2014;127:1511–1522. doi: 10.1242/jcs.139485. [DOI] [PubMed] [Google Scholar]
- 44.Herranz N., Pasini D., Díaz V.M., Francí C., Gutierrez A., Dave N., Escrivà M., Hernandez-Muñoz I., Di Croce L., Helin K. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol. Cell. Biol. 2008;28:4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller M.S., Ezaki T., Guo R.J., Lynch J.P. Cdx1 or Cdx2 expression activates E-cadherin-mediated cell-cell adhesion and compaction in human COLO 205 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G104–G114. doi: 10.1152/ajpgi.00484.2003. [DOI] [PubMed] [Google Scholar]
- 46.Ismail A.M., Abbas A.M., Bakry A.K., Abu-Elhassan A.M., Mohamed A.O., Badr G., Youssef M.A. Expression of ERK and Akt proteins in women with unexplained first-trimester recurrent miscarriage. Middle East Fertil. Soc. J. 2017;22:33–38. [Google Scholar]
- 47.Liu J., Matsuo H., Laoag-Fernandez J.B., Xu Q., Maruo T. The effects of progesterone on apoptosis in the human trophoblast-derived HTR-8/SV neo cells. Mol. Hum. Reprod. 2007;13:869–874. doi: 10.1093/molehr/gam078. [DOI] [PubMed] [Google Scholar]
- 48.Halasz M., Polgar B., Berta G., Czimbalek L., Szekeres-Bartho J. Progesterone-induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity. Cell. Mol. Life Sci. 2013;70:4617–4630. doi: 10.1007/s00018-013-1404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zachariades E., Mparmpakas D., Thomas P., Rand-Weaver M., Karteris E. Crucial cross-talk of interleukin-1β and progesterone in human choriocarcinoma. Int. J. Oncol. 2012;40:1358–1364. doi: 10.3892/ijo.2012.1349. [DOI] [PubMed] [Google Scholar]
- 50.Cha T.L., Zhou B.P., Xia W., Wu Y., Yang C.C., Chen C.T., Ping B., Otte A.P., Hung M.C. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 51.Chen B., Liu J., Chang Q., Beezhold K., Lu Y., Chen F. JNK and STAT3 signaling pathways converge on Akt-mediated phosphorylation of EZH2 in bronchial epithelial cells induced by arsenic. Cell Cycle. 2013;12:112–121. doi: 10.4161/cc.23030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo T., Zhang L., Cheng D., Liu T., An L., Li W.-P., Zhang C. Low-density lipoprotein receptor affects the fertility of female mice. Reprod. Fertil. Dev. 2015;27:1222–1232. doi: 10.1071/RD13436. [DOI] [PubMed] [Google Scholar]
- 53.Lv H., Tong J., Yang J., Lv S., Li W.P., Zhang C., Chen Z.J. Dysregulated pseudogene HK2P1 may contribute to preeclampsia as a competing endogenous RNA for hexokinase 2 by impairing decidualization. Hypertension. 2018;71:648–658. doi: 10.1161/HYPERTENSIONAHA.117.10084. [DOI] [PubMed] [Google Scholar]
- 54.Guo S., Yan X., Shi F., Ma K., Chen Z.J., Zhang C. Expression and distribution of the zinc finger protein, SNAI3, in mouse ovaries and pre-implantation embryos. J. Reprod. Dev. 2018;64:179–186. doi: 10.1262/jrd.2017-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui L.L., Yang G., Pan J., Zhang C. Tumor necrosis factor α knockout increases fertility of mice. Theriogenology. 2011;75:867–876. doi: 10.1016/j.theriogenology.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Li M.Q., Hou X.F., Shao J., Tang C.L., Li D.J. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinbeta1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol. Reprod. 2010;82:968–979. doi: 10.1095/biolreprod.109.080739. [DOI] [PubMed] [Google Scholar]
- 57.Zuo R., Liu X., Wang W., Li W., Ying H., Sun K. A repressive role of enhancer of zeste homolog 2 in 11β-hydroxysteroid dehydrogenase type 2 expression in the human placenta. J. Biol. Chem. 2017;292:7578–7587. doi: 10.1074/jbc.M116.765800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.