Abstract
The four components present in the trypanocidal treatment Samorin, the commercially available formulation of isometamidium, were separated and purified by column chromatography. These compounds as well as the Samorin mixture and the other phenanthridine trypanocide, homidium, were tested on Trypanosoma congolense and wild type, diamidine- and isometamidium-resistant Trypanosoma brucei brucei strains using an Alamar blue drug sensitivity assay. EC50 values obtained suggest that M&B4180A (2) was the most active of the components, followed by M&B38897 (1) in all the strains tested, whereas M&B4596 (4) was inactive. Samorin was found to be significantly more active than any of the individual components alone, against T. congolense and all three T. b, brucei strains. Samorin and all its active constituents displayed reduced activity against the previously characterised isometamidium-resistant strain ISMR1.
Keywords: Isometamidium, Trypanosoma congolense, Trypanosoma brucei brucei, Samorin, Drug resistance
Graphical abstract
1. Introduction
Trypanosoma brucei brucei, T. congolense and T. vivax are responsible for African animal trypanosomiasis (AAT) or nagana in cattle (Giordani et al., 2016). Control of these tsetse-fly transmitted animal trypanosomes in sub-Saharan Africa, in the absence of a vector control programme or an effective vaccine, depends solely on chemotherapy using isometamidium (ISM), diminazene and ethidium bromide. If ethidium bromide is withdrawn from use due to its established mutagenic properties (Sayas et al., 2015), ISM and diminazene would be the only trypanocides left for prophylaxis and treatment of veterinary African trypanosomiasis respectively (Delespaux and De Koning, 2007). Resistance to ISM in this situation would therefore threaten the control of animal trypanosomiasis. Resistance to ISM in the field has been confirmed by a number of reports (Jamal et al., 2005; Mongube et al., 2012; Moti et al., 2012; Tihon et al., 2017). Hence, the management of AAT requires both the introduction of new drugs and optimization of available drugs to increase treatment options and reduce the risk of resistance. Optimization of available drugs would involve the determination of their mechanism of action and resistance to understand how best to use the drugs and reduce the incidence of resistance. Resistance to ISM involves a reduction in uptake and/or loss of mitochondrial potential in T. congolense (Sutherland et al., 1992; Wilkes et al., 1997), the latter of which was linked, in T. b. brucei, to mutation of the F1Fo ATPase complex (Eze et al., 2016).
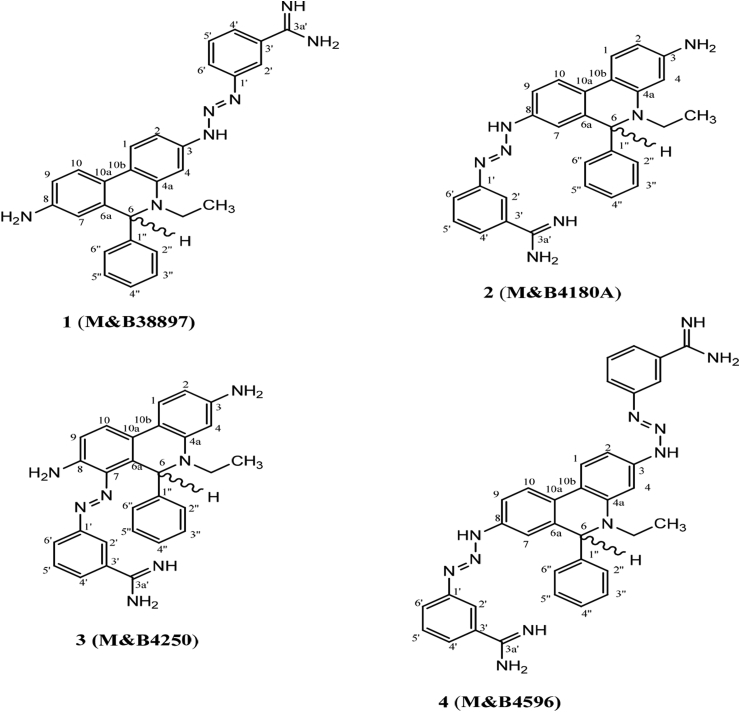
ISM is available commercially as the impure mixture Samorin. The four components which the drug is suspected to contain have been purified to homogeneity, and their structures have been rigorously determined by a combination of NMR and mass-spectrometric techniques (Igoli et al., 2014). For the first time, this analysis showed that the Samorin components exist as reversible di-cationic chloride salts or a dihydro form, with a hydrogen addition to C-6 as depicted in Fig. 1, depending on solvent and pH. The compound 8-(3-m-amidinophenyl-2-triazeno)-3-amino-5-ethyl-6-phenylphenanthridinium chloride hydrochloride (2, M&B4180A) is believed to be the principal active component (Schad et al., 2008), and this is usually the structure for isometamidium given in the literature. The other components isolated, usually dismissed as impurities, were 3-(3-m-amidinophenyl-2-triazeno)-8-amino-5-ethyl-6-phenylphenanthridinium chloride hydrochloride (1, M&B38897, ‘red isomer’), 7-(m-amidinophenyldiazo)-3,8-diamino-5-ethyl-6-phenylphenanthridinium chloride hydrochloride (3, M&B4250, ‘blue isomer’) and 3,8-di(3-m-amidinophenyltriazeno)-5-ethyl-6-phenylphenanthridinium chloride dihydrochloride (4, M&B4596); see Fig. 1. However, the dihydro forms were not observed using NMR with D2O instead of DMSO, suggesting that in aqueous solution the same compounds may be phenanthridinium ions without a C-6 hydrogen, consistent with the belief that this is the biologically active form.
Fig. 1.
Chemical structures of fractions obtained from the fractionation of Samorin. Compounds 1–4 were isolated by Igoli et al. (2014) and matched against their M&B codes. Compounds 1A and 1B are identical (compound 1) but compound 1B contains a significant percentage of ethidium contamination.
In this study, the compounds (Fig. 1) obtained from the purification of Samorin were tested on wild type Trypanosoma brucei brucei 427 and Trypanosoma congolense IL3000, alongside several T. b. brucei strains, including the diamidine-resistant B48 strain (Bridges et al., 2007) and ISM-resistant (ISMR1) strain (Eze et al., 2016). While a report by Sahin et al. (2014) similarly described the effect of individual compounds M&B4180A, M&B4250, B4596 and M&B38897 on trypanosomes, these were separately synthesised rather than purified from commercial Samorin, and the NMR and mass spectra were not made available so their identity and purity could not be verified; the structures were given as phenanthridinium mono-cations. Here we investigate whether purification of Samorin might improve activity and whether the purified compounds all displayed similar activity against T. b. brucei strains resistant to diamidines and phenanthridines, respectively. Absence of cross-resistance for some components would not only point to different mechanisms of action, but highlight a way to bypass isometamidium resistance.
2. Material and methods
2.1. Drugs
Samorin was donated by Mérial, France, while Alamar blue dye was obtained as Resazurin sodium salt from Sigma UK. Compounds 1–4 were fractions from column chromatography purification from Samorin, provided by Professor Graham G. Skellern at the Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow.
2.2. Trypanosome strains and culture
Bloodstream-form of T. b. brucei, strain Lister 427 (s427; MiTat 1.2/BS221), and its derivatives, B48 (diamidine/arsenical resistant) and ISMR1 (phenanthridine resistant) were maintained in full HMI-9 medium with 10% Fetal Bovine Serum at 37 °C as previously described (Bridges et al., 2007). T. congolense IL3000 was grown in Minimum Essential Medium Eagle (MEM; Sigma; supplemented with 20% goat serum). Culture was grown in 5% CO2 at 34 °C and was sub-cultured every 48 h (or whenever cell density reaches about 2 × 106 cells/ml).
Basal MEM medium was prepared by adding the following components to a litre of Hipersolv Chromanorm water (BDH): 1 bottle of MEM powder (Sigma), 5.56 g of HEPES (Sigma), 2.2 g of NaHCO3 (Sigma), 1 g D-glucose (Fisher Scientific), 110 mg sodium pyruvate (Sigma), 10.68 mg adenosine (Sigma), 14 mg hypoxanthine (Sigma), 4 mg thymidine (Sigma), 14.1 mg bathocuproine sulphate acid (Sigma). This was filter-sterilized, after adjusting pH to 7.3, and stored at 4 °C.
One litre of complete Tc—BSF3 medium for T. congolense was prepared with the following components: 750 ml base media, 200 ml goat serum (Gibco), 50 ml serum plus medium supplement (Sigma), 14 μl β-mercaptoethanol (Sigma), 8 ml of 200 mM glutamine (Sigma) and 10 ml penicillin/streptomycin solution (Sigma); storage at 4 °C after filter-sterilization.
2.3. Drug susceptibility assays
Drug susceptibilities were determined for bloodstream-form T. b. brucei, B48 and ISMR1 using the Alamar blue (resazurin) assay as described previously, using 23 doubling dilutions from an initial test compound concentration of 20 μM, and 105 trypanosomes/well; plates were incubated at 37 °C/5% CO2 for 48 h and 24 h before and afterthe addition of Alamar blue, respectively (Rodenko et al., 2007; Gould et al., 2008). EC50 values were calculated by non-linear regression using an equation for a sigmoidal dose–response curve with variable slope (Prism 5.0; GraphPad Software Inc). For Trypanosoma congolense, the same Alamar blue assay was used, but at a final cell density of 2.5 × 105 cells/ml (2.5 × 104 cells/well).
3. Results and discussion
It is widely acknowledged that there is an urgent need for new drugs against animal trypanosomiasis; above anything else, this need is driven by the increasing failure of the long-standing, cheap and widely available drugs isometamidium (ISM, Samorin®/Trypamidium®, Mérial; Veridium®, Sanofi), diminazene aceturate (Berenil®, Hoechst; Veriben®, Sanofi) and homidium bromide (Ethidium®, Laprovet) or homidium chloride (Novidium®, Mérial). AAT drug resistance has now been documented in at least 21 African countries (Assefa and Shibeshi, 2018), a rapid increase from the reported 17 countries in 2013 (Melaku and Birasa, 2013), and the emerging reality is that animal trypanosomiasis can no longer be effectively treated in many parts of Africa, with dire consequences for livelihoods, economies and food security (Giordani et al., 2016; Assefa and Shibeshi, 2018). As new drugs are at the very least years away from making a meaningful contribution in the field, it is essential that we make the most efficient use of the few licenced drugs available. Here we ask the question whether the purification of (any of) the Samorin components would result in a significantly more potent drug able to overcome current levels of resistance.
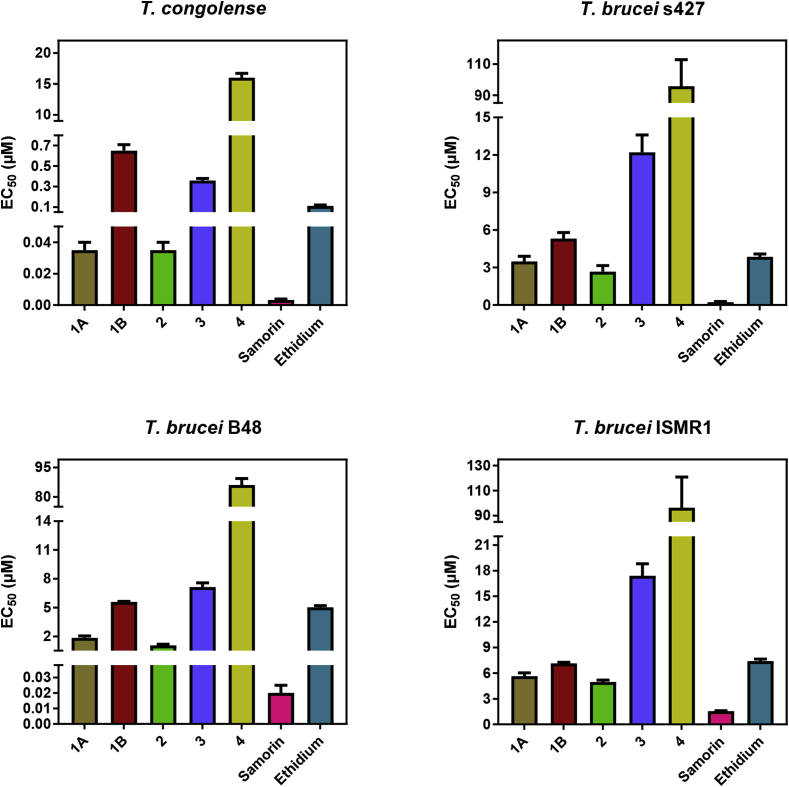
The activity of compounds 1–4 against T. b. brucei s427, B48, ISMR1 and T. congolense are presented in Table 1 and Fig. 2. The main observation was that Samorin was found to be consistently and significantly (P < 0.0001) more active than any of the fractions. Compound 2 was found to be consistently the most active of all the Samorin components, followed by the pure compound 1A, while compound 4 was consistently the least active. Samorin was 10.4-fold, 9-fold, 54-fold and 3.2-fold more active than compound 2 against T. congolense, Tb427, B48 and ISMR1, respectively.
Table 1.
Overview of Samorin fractions and control drugs against T. congolense and T. b. brucei.
| compound |
T. congolense IL3000 |
T. brucei s427 |
T. b. brucei B48 |
T. b. brucei ISMR1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | n | EC50 (μM) | n | EC50 (μM) | n | RF (s427) | P value (s427) | EC50 (μM) | n | RF (s427) | P value (s427) | |
| 1A | 0.035 ± 0.005 | 5 | 3.48 ± 0.42 | 4 | 1.85 ± 0.19 | 4 | 0.53 | 0.012 | 5.64 ± 0.40 | 4 | 1.62 | 0.0093 |
| 1B | 0.65 ± 0.06 | 5 | 5.31 ± 0.48 | 4 | 5.58 ± 0.07 | 4 | 1.05 | 0.59 | 7.14 ± 0.14 | 4 | 1.34 | 0.010 |
| 2 | 0.035 ± 0.005 | 5 | 2.67 ± 0.48 | 4 | 1.06 ± 0.11 | 4 | 0.40 | 0.017 | 4.97 ± 0.22 | 4 | 1.86 | 0.0047 |
| 3 | 0.36 ± 0.02 | 5 | 12.2 ± 1.4 | 6 | 7.13 ± 0.43 | 6 | 0.59 | 0.005 | 17.4 ± 1.4 | 6 | 1.43 | 0.025 |
| 4 | 16.0 ± 0.7 | 6 | 95.7 ± 17.0 | 4 | 86.1 ± 3.3 | 4 | 0.90 | 0.60 | 96.4 ± 24.6 | 4 | 1.01 | 0.98 |
| Samorin | 0.0033 ± 0.0006 | 17 | 0.29 ± 0.04 | 12 | 0.020 ± 0.005 | 10 | 0.068 | 0.00001 | 1.55 ± 0.05 | 14 | 5.33 | >0.00001 |
| Ethidium | 0.11 ± 0.01 | 5 | 3.85 ± 0.22 | 4 | 5.03 ± 0.17 | 4 | 1.31 | 0.0051 | 7.41 0.23 | 4 | 1.92 | 0.00003 |
| Pentamidine | 0.82 ± 0.02 | 11 | 0.0051 ± 0.0004 | 6 | 0.63 ± 0.03 | 6 | 124 | >0.00001 | 0.016 ± 0.002 | 6 | 3.24 | >0.00001 |
The 50% Effective Concentration (EC50) is given as average and SEM, and the number of replicates (n) for each determination is included in a separate column. RF is the Resistance Factor, the ratio of EC50 (s427) and EC50 (B48 or ISMR1). The P value is the outcome of an unpaired, two-tailed Student's t-test of the B48 or ISMR1 EC50 with the corresponding value of s427.
Fig. 2.
Bar graphs showing the EC50 values obtained for Trypanosoma congolense IL3000, and wildtype s427, diamidine-resistant (B48) and ISM-resistant (ISMR1) T. b. brucei strains. EC50 values were determined from alamar blue assays using 105 cells/ml of each cell line. Each bar represents the average of at least three independent assays as indicated in Table 1; error bars are SEM. Statistical significance relative to wild-type T. b. brucei is also included in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
As expected, neither Samorin nor any of the fractions displayed cross-resistance with pentamidine in the B48 strain. Indeed, B48, a multi-drug resistant strain for all diamidines and melaminophenyl arsenicals, was 14.6-fold more sensitive to Samorin (P = 0.00001) than the wild-type s427 cells. In addition, compounds 1, 2, and 3 all were significantly more active against this cell line, which was 124-fold resistant to pentamidine in this series of experiments (P < 0.00001). Interestingly, ethidium bromide was less active against B48 (P < 0.01), albeit by only 1.3-fold, which seems to indicate that at least some aspect of its mechanism of action or cellular entry is different from isometamidium. All the phenanthridines except 4 displayed significantly reduced activity for the isometamidium-resistant strain ISMR1. Indeed, the resistance factors of all the Samorin fractions and ethidium were highly similar (1.3–1.9-fold; Table 1), although the resistance to Samorin exceeded 5-fold with the present assay protocol.
The overall conclusion is that the impure Samorin is significantly more efficacious than each individual fraction in its pure form, suggesting a form of synergistic action by more than any one individual component. Moreover, all of the active compounds displayed reduced activity against the ISM resistant strain ISMR1, showing clearly that the production of any one of the pure compounds out of the Samorin mix is not a viable answer to the drug resistance crisis. Given these observations, and the fact that a pure isometamidium would be much more expensive, we would advocate an increased investment in new AAT drug discovery instead.
Acknowledgements
A.A.E. was supported by a Commonwealth Scholarship (grant reference NGCS-2009-246).
References
- Assefa A., Shibeshi W. Drug resistance in African animal trypanosomiasis: a review. Afr. J. Microbiol. Res. 2018;12:380–386. [Google Scholar]
- Bridges D.J., Gould M.K., Nerima B., Mӓser P., Burchmore R.J., De Koning H.P. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 2007;71:1098–1108. doi: 10.1124/mol.106.031351. [DOI] [PubMed] [Google Scholar]
- Delespaux V., De Koning H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updates. 2007;10:30–50. doi: 10.1016/j.drup.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Eze A.A., Gould M.K., Munday J.C., Tagoe D.N.A., Stelmanis V., Schnaufer A.C., De Koning H.P. Reduced Mitochondrial Membrane Potential is a late adaptation of Trypanosoma brucei brucei to isometamidium preceded by mutations in the γ subunit of the F1F0-ATPase. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani F., Morrison L.J., Rowan T.G., De Koning H.P., Barrett M.P. The animal trypanosomiases and their chemotherapy: a review. Parasitology. 2016;143:1862–1889. doi: 10.1017/S0031182016001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould M.K., Vu X.L., Seebeck T., De Koning H.P. Propidium iodide-based methods for monitoring drug action in the Kinetoplastidae: comparison with the Alamar blue assay. Anal. Biochem. 2008;382:87–93. doi: 10.1016/j.ab.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Igoli J.O., Blackburn G., Gray A.I., Sutcliffe O.B., Watson D.G., Euerby M.R., Skellern G.G. Chromatographic and spectroscopic analysis of the components present in the phenanthridinium trypanocidal agent isometamidium. Anal. Bioanal. Chem. 2014;407:1171–1180. doi: 10.1007/s00216-014-8337-z. [DOI] [PubMed] [Google Scholar]
- Jamal S., Sigauque I., Macuamule C., Neves L., Penzhorn B.L., Marcotty T., Van Den Bossche P. The susceptibility of Trypanosoma congolense isolated in Zambézia province, Mozambique, to isometamidium chloride, diminazene aceturate and homidium chloride. Onderstepoort J. Vet. Res. 2005;72:333–338. doi: 10.4102/ojvr.v72i4.190. [DOI] [PubMed] [Google Scholar]
- Melaku A., Birasa B. Drugs and drug resistance in African animal trypanosomiasis: a review. Eur. J. Appl. Sci. 2013;5:84–91. [Google Scholar]
- Mongube E.O., Vitouley H.S., Allegye-Cudjoe E., Diall O., Boucoum Z., Diarra B., Sanogo Y., Randolph T., Bauer B., Zessin K., Clausen P. Detection of multiple drug-resistant Trypanosoma congolense populations in village cattle of south-east Mali. Parasites Vectors. 2012;5:155–163. doi: 10.1186/1756-3305-5-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moti Y., Fikru R., Van Den Abbeele J., Büscher P., Van den Bossche P., Duchateau L., Delespaux V. Ghibe river basin in Ethiopia: present situation of trypanocidal drug resistance in Trypanosoma congolense using tests in mice and PCR-RFLP. Vet. Parasitol. 2012;189:197–203. doi: 10.1016/j.vetpar.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Rodenko B., Van der Burg A.M., Wanner M.J., Kaiser M., Brun R., Gould M.K., De Koning H.P., Koomen G.J. 2,N6-Disubstituted adenosine analogues with antitrypanosomal and antimalarial activity. Synthesis, uptake studies and in vivo evaluation. Antimicrob. Agents Chemother. 2007;51:3796–3802. doi: 10.1128/AAC.00425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin A., Asencio C., Izotte J., Pillay D., Coustou V., Karembe H., Baltz T. The susceptibility of Trypanosoma congolense and Trypanosoma brucei to isometamidium chloride and its synthetic impurities. Vet. Parasitol. 2014;203:270–275. doi: 10.1016/j.vetpar.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Sayas E., García-López F., Serrano R. Toxicity, mutagenicity and transport in Saccharomyces cerevisiae of three popular DNA intercalating fluorescent dyes. Yeast. 2015;32:595–606. doi: 10.1002/yea.3081. [DOI] [PubMed] [Google Scholar]
- Schad G.J., Allanson A., Mackay S.P., Cannavan A., Tettey J.N.A. Development and validation of an improved HPLC method for the control of potentially counterfeit isometamidium products. J. Pharmaceut. Biomed. Anal. 2008;46:45–51. doi: 10.1016/j.jpba.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Mounsey A., Holmes P.H. Transport of isometamidium (Samorin) by drug-resistant and drug-sensitive Trypanosoma congolense. Parasitology. 1992;104:461–467. doi: 10.1017/s0031182000063721. [DOI] [PubMed] [Google Scholar]
- Tihon E., Imamura H., Van den Broeck F., Vermeiren L., Dujardin J.C., Van Den Abbeele J. Genomic analysis of isometamidium chloride resistance in Trypanosoma congolense. Int. J. Parasitol. Drugs Drug Resist. 2017;7:350–361. doi: 10.1016/j.ijpddr.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes J.M., Mulugeta W., Peregrine A.S. Modulation of mitochondrial electrical potential: a candidate mechanism for drug resistance in African trypanosomes. Biochem. J. 1997;326:755–761. doi: 10.1042/bj3260755. [DOI] [PMC free article] [PubMed] [Google Scholar]