Abstract
Caralluma dalzielii N. E. Brown (Asclepiadaceae) is a cactus-like shaped shrub widely used in traditional medicine for the treatment of rheumatoid arthritis, diabetes, infertility and impotence. The present study evaluated the potential toxicity of aqueous extract of aerial parts of Caralluma dalzielii (AECD) through acute and sub-acute oral administration in mice and rats. During acute toxicity study, female mice and rats were orally administered with AECD at single doses of 175, 500 and 2000 mg/kg according to OECD Guidelines 425. Sub-acute toxicity of AECD (150, 300 and 600 mg/kg p.o) was studied by daily dosing of Wistar rats of both sexes for 28 days. The acute toxicity study revealed no lethal effects and behavioural signs of toxicity at the tested doses indicating that LD50 is greater than 2000 mg/kg. In sub-acute study, a significant reduction in the body weight (p < 0.05), feed and water (p < 0.001) intake of the rats were observed. A significant (p < 0.05) increase in lymphocytes, mean platelet volume counts and alanine aminotransferase were also observed. Histopathological analysis showed mild liver cell distortion in female rats treated at 600 mg/kg of AECD. These results show low toxicity of AECD on short-term use and liver toxicity on long-term use.
Keyword: Toxicology
1. Introduction
Medicinal plants have been used for health and disease management globally. The use of these plants in health care delivery especially in resource poor settings is increasing. About eighty percent of the world's population depend on traditional medicine for primary health care (Ugwah et al., 2013; Ekor, 2014). Even though the use of these plants has shown promising potential with high global demand, there are still concerns about not only their use but also their safety (Obidike and Salawu, 2013).
Herbal products are usually regarded as safe or of low toxicity based on their long history of use by humans (Yuet Ping et al., 2013; Ibrahim et al., 2016). Nevertheless, the latest surveys have indicated that many of these products used in traditional medicine showed adverse effects (Koduru et al., 2006). Since safety continues to be a major issue with the use of medicinal plants, it is important to conduct toxicity studies on them to ascertain their safety profile. Therefore, evaluating the toxicological effects of any medicinal plant extract intended to be used in animals or humans is an important aspect of its assessment for potential toxic effects.
Caralluma dalzielii N.E. Brown commonly known as the mosque stalk is a cactus-like shape plant with 5-merous flowers. It belongs to the family Asclepiadaceae. In Africa, the species is distributed across the Sahel (Plowes, 2008), but grows better in West Africa and Sudan (de Kock and Meve, 2007) and can be up to 1 m high.
In traditional medicine, the aerial parts and sometimes the whole plant is used to treat male and female infertility, rheumatoid arthritis, diabetes, leprosy, snake and scorpion bites and severe pains in the epigastrium. The aerial parts are usually crushed using mortar and pestle and the juice swallowed, directly instilled into the ear or used topically to treat inflammation (Dalziel, 1937; Burkill, 1985; Ugwah-Oguejiofor et al., 2013). A decoction or maceration of the aerial parts is sometimes made and taken as tea once or twice daily for the treatment of infertility (Ugwah-Oguejiofor et al., 2017). Other reported ethnomedicinal uses for this species are for faintness due to fasting, convulsion, emesis, paralysis (Burkill, 1985), otitis (Inngjerdingen et al., 2004) and as aphrodisiac (Oyama et al., 2007; Ibrahim et al., 2010).
Previous scientific evaluations on antiulcer, anti-inflammatory and anti-proliferative activities have been reported (De Leo et al., 2005; Ugwah-Oguejiofor et al., 2013, 2017). Some chemical constituents have been investigated and five new pregnane glycosides, caradalzielosides A–E (1–5) isolated from the aerial parts of the plant (Oyama et al., 2007). Also, cytotoxic activities of the various pregnane glycosides fractions from C. dalzielii have been documented (De Leo et al., 2005).
The use of this plant has gained high popularity globally due to its wide application without adequate information on its toxicity profile. The aim of the present study was therefore to investigate the acute and sub-acute toxicity effects of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats.
2. Materials and methods
2.1. Plant material and preparation of plant extract
The aerial parts of Caralluma dalzielii N.E. Brown was collected from Sokoto North local government area in Sokoto State, North-west Nigeria, in December (2016) after the flowering season. Taxonomic identification and authentication were carried out by Dr. Mshelia, a taxonomist in the Department of Pharmacognosy and Ethnopharmacy, Usmanu Danfodiyo University, Sokoto. A voucher specimen (Pcg/UDUS/Asdy/001) of the plant was deposited in the herbarium of the same department. The plant material was air dried at room temperature to a constant weight and pulverised with pestle and mortar. Five hundred grams of the dried leaf material was macerated in 5 L of distilled water for 48 h. The aqueous extract was filtered through a Whatman filter paper and evaporated to dryness over a regulated hot water bath maintained at 60–70 °C to obtain a yield of 9.12 %(w/w). The dried extract was stored in the refrigerator at 4 °C until further use. The extract was resuspended in distilled water on daily basis during administration to experimental animals.
2.2. Experimental animals
Male and female Wistar rats (Rattus norvergicus) weighing about 150–165 g were obtained from Mike Ugwah animal house in Usmanu Danfodiyo University Teaching Hospital (UDUTH), Sokoto. The mice (20–27 g) were obtained from the animal facility centre of Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria, Nigeria. The animals were acclimatised for 2 weeks before the commencement of the study. Standard commercial chow and water were provided ad libitium for the animals. Housing conditions were maintained at 25 ± 2 °C at 12 h day/night cycles. The study was approved by the Animal Research Ethical Committee, Usmanu Danfodiyo University, Sokoto (PTAC/Cd/MT/002-17). The care and handling of the animals were according to the established public health guidelines in Guide for Care and Use of Laboratory Animals (2011).
2.3. Preliminary phytochemical tests
The plant extract was subjected to qualitative analyses using the methods described by Trease and Evans (1983), El-Olemmy et al. (1994), and Harbone (1993). The presence of alkaloids, glycosides, tannins, saponins, terpenoids, flavonoids, saponins glycosides, cardiac glycosides, anthraquinones, volatile oil, and steroids were tested for.
2.4. Acute and sub-acute toxicity studies
2.4.1. Acute toxicity test
The oral acute toxicity study of the aqueous extract of Caralluma dalzielii was carried out using the ‘Up-and- Down’ method of testing in mice and rats at single doses of 175, 500 and 2000 mg/kg in accordance with the Organization for Economic Development (OECD) guideline no. 425 (Organization for Economic Development, 2001). Five female rats and mice were used for each dose level in the study. An animal was picked at a time, weighed and dosed with the equivalent volume of extract dissolved in distilled water. The extract was administered orally using gastric feeding tube. Each animal was observed after dosing for the first 5 min for signs of regurgitation and kept in a metallic cage. Each was then observed every 15 min in the first 4 h after dosing, every 30 min for 6 h and daily for 48 h for behavioural signs of toxicity (changes in skin, hair, eyes, mucous membranes, and respiratory, circulatory, autonomic and central nervous systems, motor activity, convulsion, tremors, salivation, diarrhoea, lethargy, or sleep) according to the specifications of the OECD (2001). The animals were monitored for a total of 14 days for the long-term possible lethal outcome. The body weights of the animals were measured on days 1, 7, and 14.
2.4.2. Sub-acute toxicity test
Wistar rats of both sexes were assigned randomly to four groups (n = 12/group: six males and six females). Groups I-III received 150, 300 and 600 mg/kg of the extract respectively while group IV received distilled water (5 ml/kg) only. The rats were dosed by oral gavage, using a curved, ball tipped stainless steel feeding needle for 28 days. Daily feed and water intake were recorded for the female rats. The weights of all animals were measured weekly.
On day 29, the rats were weighed and sacrificed. Blood samples were collected via cardiac puncture for biochemical and haematological analyses. The heart, liver, lungs, kidneys, spleens, ovaries and testes were excised, weighed, trimmed and then fixed in Bouin's solution for histological analysis.
2.5. Measurement of body and organ weights
The animals were weighed weekly and the level of weight gain (%) was calculated using the relation;
where Wf = final weight; Wi = initial weight.
After blood collection, the heart, liver, lungs, kidneys, spleen, ovary and testis were excised, weighed and assessed macroscopically. Relative organ weight of each harvested organ was determined using the relation;
2.6. Assessment of haematological parameters
The samples for haematology were put in bottles containing anticoagulant, ethylene diamine tetra-acetic acid (EDTA). Haematological analyses were performed using an automated haematology analyser (Mytaic 22, Germany). Parameters analysed include total and differential leukocyte (WBC), erythrocyte (RBC), Haemoglobin (Hgb), Haematocrit (HCT), platelet count (PLT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), red distribution width (RDW), mean platelet volume (MPV) and platelet distribution width (PDW).
2.7. Assessment of serum biochemical parameters
Blood for biochemical analysis were gently placed in plain bottles to avoid haemolysis of the blood cells. Blood serum was obtained by centrifugation of the blood sample. Biochemical studies were carried out using standard methods for aspartate aminotransferase (AST), alanine aminotransferase (ALT), total and conjugated bilirubin, total protein, albumin, urea and creatinine. The serum electrolytes (Sodium, potassium, chloride and bicarbonate ions) were estimated using an automated ion selective electrode machine (Audicom electrolyte analyser).
2.8. Histopathological examination
The fixed tissues were dehydrated in an ascending series of alcohol, cleared in xylene, and embedded in paraffin wax melting at 60 °C. Serial sections (5-μm thick) obtained by cutting the embedded tissue with microtome, were mounted on 3- aminopropyl triethsilane – coated slides and dried for 24 h at 37 °C (Baravalle et al., 2006). The sections on the slides were deparaffinised with xylene and hydrated in a descending series of alcohol. They were thereafter stained with Mayer's haematoxylin and eosin dyes, dried and mounted on a light microscope (X40, 100 and 200) for histopathological examination.
2.9. Statistical analysis
The results of the study are expressed as the mean ± S. E. M. The results were analysed using Graph Pad Prism version 6 software. Comparison in all the groups was made using one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test. Differences were considered significant at p ≤ 0.05.
3. Results
3.1. Preliminary phytochemical test
Preliminary phytochemical analysis of the extract revealed the presence of alkaloids, flavonoids, saponins, steroids, glycosides, terpenoids, cardiac glycosides and saponins glycosides.
3.2. Effect of plant extract on acute toxicity studies
The acute toxicity test using the Up and Down method at an oral limit doses of 175, 500 and 2000 mg/kg of the aqueous extract of C. dalzielii caused no death in the mice and rats. No lethal effects were noted throughout the short and long-term observation period. No toxicity signs were observed in the animals throughout the 14 days study period. Therefore, the extract may be safe at these doses and the oral LD50 considered greater than 2000 mg/kg in rats and mice.
3.3. Effect of plant extract on sub-acute toxicity studies in rats
All the treated rats of both sexes at the doses of 150, 300 and 600 mg/kg survived throughout the 28 days of treatment. However, the furs of the treated rats at all doses appeared smoother than that of the control. No observable toxicity signs were noticed in the extract treated rats compared to the control.
3.3.1. Effect of the extract on feed and water intake in female rats
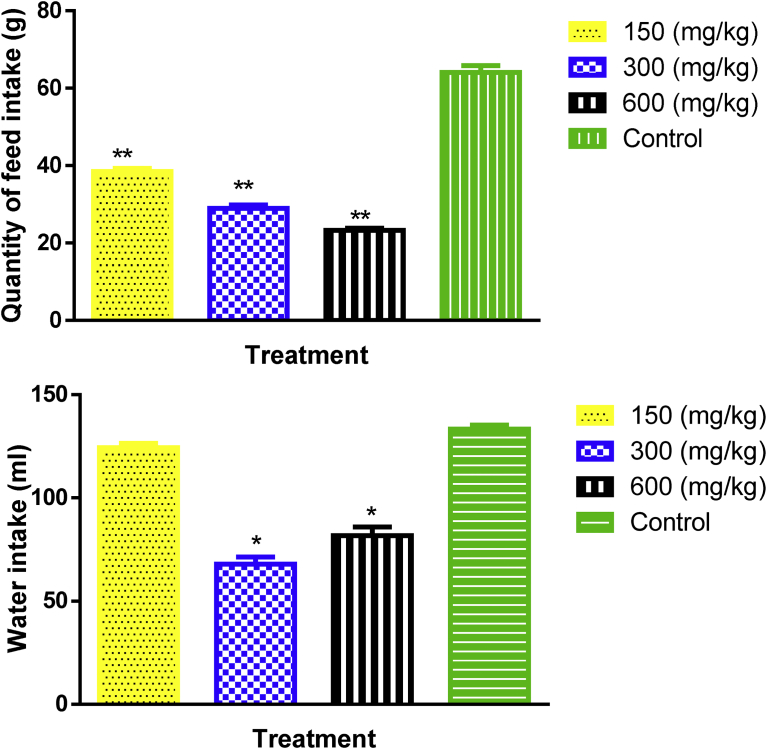
The extract caused a significant (p < 0.001) reduction in the quantity of feed at all doses, and water (p < 0.05) at 300 and 600 mg/kg, consumed by the female rats when compared with the control (Fig. 1).
Fig. 1.
Feed (g/100g body weight) and water (ml/100g body weight) consumption of female animals of control group and treated with aqueous extract of Caralluma dalzielii at the doses of 150, 300 and 600 mg/kg in the sub-acute toxicity study. Values are presented as Mean ± S.E.M, n = 6; Significant in relation to control at *p < 0.05, **p < 0.001; two-way ANOVA followed by Dunnett's post hoc test).
3.3.2. Effect of the extract on body weight gain of rats
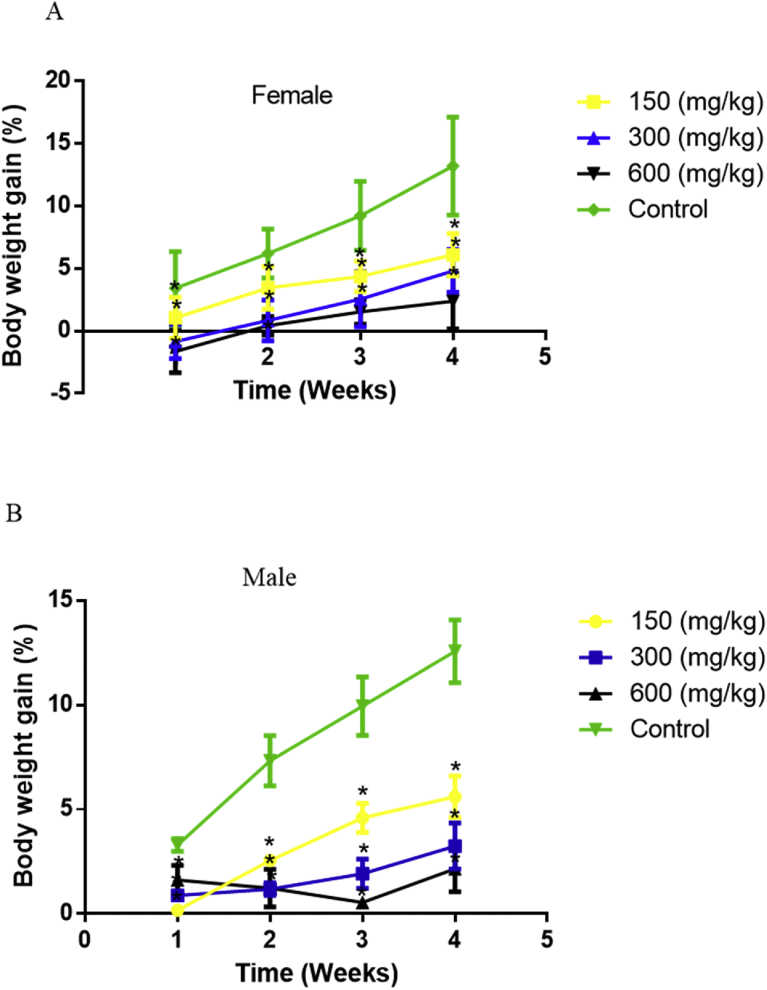
Administration of the extract to the male and female rats caused a dose dependent reduction in the percentage weight gain of the animals compared to the control (Fig. 2).
Fig. 2.
Body weight gain by week of rats, female (A) and male (B), of control group and treated with aqueous extract of Caralluma dalzielii at doses 150, 300 and 600 mg/kg in the sub-acute toxicity study. Values are presented as percentage of mean ± SD, n = 6; Significant in relation to control at *p < 0.05, two-way ANOVA followed by Dunnett's post hoc test).
3.3.3. Effect of the extract on relative organ weight
In the female rats, there was a significant (p < 0.05) reduction in the relative weight of the liver at 300 and 600 mg/kg of the extract when compared to the control (Table 1). The relative weight of the ovaries was significantly (p < 0.05) higher than that of the control at all dose levels. However, this increase was not dose dependent.
Table 1.
Effect of aqueous extract of Caralluma dalzielii on relative organ weight in female and male Wistar rats treated for 28 consecutive days.
| Animal | Organ | Relative organ weight (%) |
|||
|---|---|---|---|---|---|
| 150 mg/kg | 300 mg/kg | 600 mg/kg | Control | ||
| Heart | 0.48 ± 0.04 | 0.44 ± 0.04 | 0.43 ± 0.03 | 0.45 ± 0.04 | |
| Spleen | 0.32 ± 0.04 | 0.28 ± 0.09 | 0.28 ± 0.04 | 0.32 ± 0.04 | |
| Lungs | 0.67 ± 0.09 | 0.69 ± 0.14 | 0.78 ± 0.12 | 0.73 ± 0.14 | |
| Female | Liver | 3.90 ± 0.57 | 2.93 ± 0.7* | 2.88 ± 0.23* | 3.30 ± 0.30 |
| Kidney | 0.31 ± 0.03 | 0.30 ± 0.04 | 0.33 ± 0.03 | 0.29 ± 0.05 | |
| Ovary | 18.02 ± 6.8* | 15.70 ± 3.0* | 16.37 ± 2.5* | 9.83 ± 2.5 | |
| Testes | - | - | - | - | |
| Heart | 0.34 ± 0.04 | 0.29 ± 0.05 | 0.37 ± 0.02* | 0.23 ± 0.04 | |
| Spleen | 0.39 ± 0.07* | 0.35 ± 0.05* | 0.31 ± 0.04 | 0.21 ± 0.03 | |
| Lungs | 0.79 ± 0.1 | 0.67 ± 0.06 | 1.27 ± 0.15** | 0.56 ± 0.10 | |
| Male | Liver | 2.64 ± 0.22 | 2.70 ± 0.39 | 3.24 ± 0.46 | 2.58 ± 0.18 |
| Kidney | 0.61 ± 0.04 | 0.48 ± 0.05 | 0.67 ± 0.07 | 0.50 ± 0.06 | |
| Ovary | - | - | - | - | |
| Testes | 1.85 ± 0.15 | 1.58 ± 0.30 | 2.03 ± 0.12 | 1.79 ± 0.17 | |
Values are presented as Mean ± S.E.M, n = 6; Significant in relation to control at *p < 0.05, **p < 0.01; One-way ANOVA followed by Dunnett's post hoc test.
The male rats showed a significant increase in the relative weight of the heart (p < 0.05) and lungs (p < 0.01) at 600 mg/kg of the extract. There was also a significant increase in the weight of spleen at 150 and 300 mg/kg of the extract when compared to the control (Table 1). All the other organ weight did not differ significantly from the control.
3.3.4. Effect of the extract on haematological parameters
The haematological parameters of the female and male rats revealed significant (p < 0.05) increase in lymphocytes at 300 and 600 mg/kg and mean platelet volume at 600 mg/kg doses of the extract when compared to the control (Table 2). Other haematological parameters were not significantly different from the control (Table 2).
Table 2.
Effect of aqueous extract of Caralluma dalzielii on haematological parameters.
| Animal | Parameter | Dose (mg/kg) |
|||
|---|---|---|---|---|---|
| 150 | 300 | 600 | Control | ||
| WBC (x103/ul) | 8.30 ± 1.60 | 9.42 ± 0.95 | 10.24 ± 2.67 | 8.16 ± 1.92 | |
| LY (x103/ul) | 3.48 ± 1.00 | 4.48 ± 1.07* | 4.38 ± 0.68* | 2.16 ± 0.39 | |
| MO (x103/ul) | 1.20 ± 0.35 | 1.14 ± 0.14 | 1.00 ± 0.36 | 1.06 ± 0.37 | |
| GR (x103/ul) | 3.62 ± 1.43 | 3.88 ± 0.45 | 4.3 ± 2.23 | 4.94 ± 1.77 | |
| LY (%) | 45.9 ± 11.68 | 44.98 ± 7.54 | 54.14 ± 7.90 | 39.06 ± 13.82 | |
| Female | MO (%) | 13.14 ± 1.84 | 12.14 ± 1.66 | 8.88 ± 0.87 | 11.1 ± 2.30 |
| GR (%) | 40.96 ± 11.01 | 42.30 ± 6.29 | 36.62 ± 7.2 | 49.8 ± 11.98 | |
| RBC (x106/ul) | 6.26 ± 0.38 | 6.09 ± 0.25 | 5.93 ± 0.24 | 6.66 ± 0.62 | |
| Hgb (g/dl) | 13.8 ± 0.90 | 13.08 ± 0.63 | 12.94 ± 0.56 | 14.86 ± 1.40 | |
| HCT (%) | 43.36 ± 2.61 | 41.20 ± 1.56 | 40.9 ± 2.08 | 45.54 ± 3.49 | |
| MCV (fl) | 69.24 ± 0.48 | 68.22 ± 0.83 | 68.6 ± 1.16 | 68.92 ± 1.72 | |
| MCH (pg) | 21.98 ± 0.25 | 22.00 ± 0.18 | 21.6 ± 1.17 | 22.32 ± 0.47 | |
| MCHC (g/dl) | 31.74 ± 0.23 | 31.82 ± 0.35 | 31.46 ± 0.46 | 32.42 ± 0.64 | |
| RDW (%) | 14.18 ± 0.28 | 16.38 ± 0.62 | 15.62 ± 0.91 | 15.38 ± 0.56 | |
| PLT (x103/ul) | 531.00 ± 47.07 | 502.00 ± 83.20 | 512.2 ± 36.57 | 467.6 ± 43.13 | |
| PCT (%) | 0.48 ± 0.04 | 0.44 ± 0.07 | 0.47 ± 0.03 | 0.41 ± 0.04 | |
| MPV(fl) | 9.00 ± 0.15 | 8.86 ± 0.17 | 9.20 ± 0.10* | 8.74 ± 0.17 | |
| PDW (fl) | 14.46 ± 0.68 | 13.46 ± 0.65 | 14.16 ± 0.54 | 13.46 ± 0.46 | |
| WBC (x103/ul) | 8.60 ± 2.4 | 12.80 ± 3.29 | 12.10 ± 3.29 | 6.76 ± 0.87 | |
| LY (x103/ul) | 7.38 ± 1.96 | 9.10 ± 2.90* | 9.23 ± 1.87* | 5.14 ± 0.78 | |
| MO (x103/ul) | 0.18 ± 0.11 | 0.58 ± 0.17 | 0.40 ± 0.11 | 0.10 ± 0.03 | |
| GR (x103/ul) | 3.55 ± 0.52 | 3.17 ± 0.90 | 2.98 ± 0.32 | 3.10 ± 0.21 | |
| LY (%) | 86.36 ± 2.11 | 74.00 ± 5.16 | 71.40 ± 3.80 | 75.96 ± 4.84 | |
| Male | MO (%) | 2.24 ± 0.05 | 5.30 ± 1.72 | 4.63 ± 1.71 | 1.50 ± 0.32 |
| GR (%) | 41.23 ± 7.2 | 60.00 ± 3.33 | 58.13 ± 4.15 | 38.50 ± 4.66 | |
| RBC (x106/ul) | 7.7 ± 0.11 | 7.23 ± 0.38 | 7.15 ± 0.36 | 7.71 ± 0.21 | |
| Hgb (g/dl) | 14.58 ± 0.35 | 13.40 ± 0.57 | 13.28 ± 0.49 | 14.50 ± 0.13 | |
| HCT (%) | 42.38 ± 1.57 | 38.64 ± 2.91 | 38.10 ± 1.48 | 40.64 ± 0.51 | |
| MCV (fl) | 53.74 ± 1.52 | 51.78 ± 1.24 | 53.63 ± 1.31 | 52.88 ± 1.04 | |
| MCH (pg) | 19.04 ± 0.5 | 18.62 ± 0.72 | 18.63 ± 0.53 | 18.76 ± 0.30 | |
| MCHC (g/dl) | 35.44 ± 0.11 | 34.98 ± 0.14 | 34.70 ± 0.10 | 35.62 ± 0.35 | |
| RDW (%) | 17.36 ± 0.18 | 18.48 ± 0.29 | 17.70 ± 0.45 | 16.86 ± 0.68 | |
| PLT (x103/ul) | 598 ± 54.72 | 674.80 ± 41.29 | 691.50 ± 71.71 | 737.20 ± 58.02 | |
| PCT (%) | 0.44 ± 0.04 | 0.48 ± 0.07 | 0.41 ± 0.13 | 0.50 ± 0.04 | |
| MPV(fl) | 7.16 ± 0.07 | 4.78 ± 1.79 | 7.85 ± 0.69* | 3.04 ± 1.64 | |
| PDW (fl) | 17.52 ± 1.89 | 20.80 ± 4.50 | 22.53 ± 7.22 | 16.18 ± 0.90 | |
Values are presented as Mean ± S.E.M, n = 6; Significant in relation to control at *p < 0.05, One-way ANOVA followed by Dunnett's post hoc test.
3.3.5. Effect of the extract on biochemical parameters
In the female rats, three parameters were statistically significant during the 28 days treatment period. A significant (p < 0.05) increase in urea at 150 mg/kg, chloride ion at 300 mg/kg and direct bilirubin levels at 600 mg/kg respectively were observed in the female rats when compared to the control. Serum ALT at 600 mg/kg was significantly (p < 0.05) higher than that of the control (Table 3). All other parameters were similar to the control.
Table 3.
Effect of aqueous extract of Caralluma dalzielii on biochemical parameters.
| Animal | Parameter | Dose (mg/kg) |
|||
|---|---|---|---|---|---|
| 150 | 300 | 600 | control | ||
| U (mmol/l) | 7.30 ± 0.86* | 4.66 ± 0.35 | 5.73 ± 0.71 | 4.96 ± 0.27 | |
| Cr (mg/dl) | 0.7 ± 0.00 | 0.7 ± 0.00 | 0.7 ± 0.00 | 0.7 ± 0.00 | |
| Na+ (mmol) | 138 ± 2.8 | 134.8 ± 0.73 | 133 ± 3.01 | 138.4 ± 2.34 | |
| K+ (mmol) | 4.5 ± 0.32 | 3.96 ± 0.21 | 4.03 ± 0.31 | 3.8 ± 0.14 | |
| Female | Cl− (mmol) | 95.3 ± 2.07 | 89.8 ± 1.56* | 95.00 ± 1.91 | 97.2 ± 1.36 |
| HCO3- (mmol) | 21.67 ± 0.52 | 23.6 ± 1.51 | 24.00 ± 1.08 | 23.2 ± 0.66 | |
| Total Protein (g/dl) | 6.33 ± 0.07 | 6.68 ± 0.18 | 6.43 ± 0.17 | 6.12 ± 0.44 | |
| Albumin (g/dl) | 3.00 ± 0.04 | 2.84 ± 0.11 | 2.80 ± 0.09 | 2.66 ± 0.19 | |
| AST (U/L) | 177.33 ± 5.47 | 169.6 ± 17.46 | 188.25 ± 3.90 | 194.2 ± 5.44 | |
| ALT (U/L) | 54.0 ± 10.11 | 47.4 ± 1.94 | 67 ± 3.61* | 49.4 ± 1.78 | |
| T. Bilirubin (mg/dl) | 0.45 ± 0.11 | 1.156 ± 0.51 | 0.73 ± 0.05 | 0.582 ± 1.55 | |
| D. Bilirubin (mg/dl) | 0.10 ± 0.02 | 0.184 ± 0.06 | 0.20 ± 0.02* | 0.112 ± 0.03 | |
| U (mmol/l) | 3.44 ± 0.54 | 4.90 ± 0.06* | 3.53 ± 0.75 | 2.80 ± 0.26 | |
| Cr (mg/dl) | 0.82 ± 0.05 | 0.78 ± 0.05 | 0.85 ± 0.10 | 0.66 ± 0.06 | |
| Na+ (mmol) | 142.60 ± 0.87 | 142.00 ± 0.55 | 143.75 ± 0.85 | 144.40 ± 1.07 | |
| K+ (mmol) | 5.00 ± 0.03 | 5.00 ± 0.10 | 4.55 ± 0.26 | 5.00 ± 0.05 | |
| Male | Cl− (mmol) | 101.80 ± 0.92 | 102.50 ± 1.17 | 102.50 ± 1.44 | 105.80 ± 1.53 |
| HCO3- (mmol) | 25.00 ± 1.52 | 24.20 ± 0.86* | 26.00 ± 1.47 | 27.00 ± 0.84 | |
| Total Protein (g/dl) | 5.80 ± 0.11 | 5.78 ± 0.17 | 5.63 ± 0.23 | 6.06 ± 0.24 | |
| Albumin (g/dl) | 3.12 ± 0.19 | 3.22 ± 0.07 | 3.00 ± 0.17 | 3.20 ± 0.12 | |
| AST (U/L) | 235.20 ± 6.66* | 238.40 ± 18.43 | 257.75 ± 12.48* | 203.00 ± 10.41 | |
| ALT (U/L) | 65.40 ± 19.26 | 68.40 ± 3.23 | 117.56 ± 11.26* | 79.60 ± 5.49 | |
| T. Bilirubin (mg/dl) | 0.33 ± 0.08 | 0.76 ± 0.22 | 0.78 ± 0.20 | 0.50 ± 0.12 | |
| D. Bilirubin (mg/dl) | 0.09 ± 0.03 | 0.11 ± 0.02 | 0.08 ± 0.02 | 0.10 ± 0.02 | |
Data presented as Mean ± S.E.M. *p < 0.05; U= Urea, Cr= Creatinine, aspartate aminotransferase (AST), alanine amino transferase (ALT), T. = Total, C. = Conjugated, D. = Direct.
In the male rats, a significant (p < 0.05) increase in urea and decrease in bicarbonate levels were observed at 300 mg/kg when compared with the control. The serum level of AST was dose dependently higher than that of the control. It increased significantly (p < 0.05) at 150 and 600 mg/kg when compared with the control. Serum ALT at 600 mg/kg was significantly higher than the control (Table 3). All other parameters were not significantly different when compared to the control.
3.3.6. Effect of the extract on histological assessment
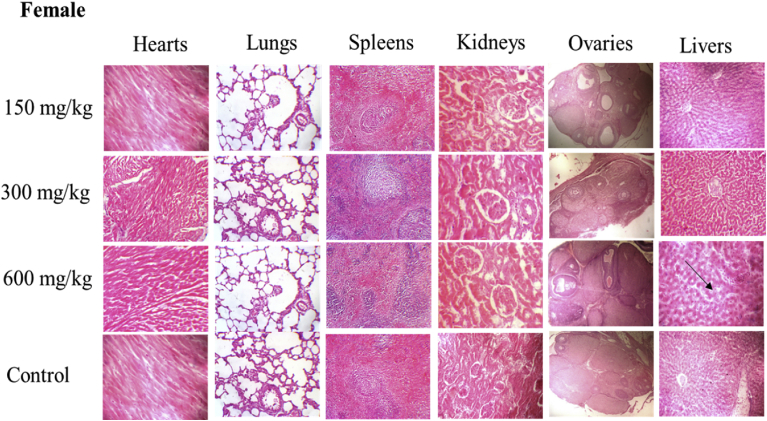
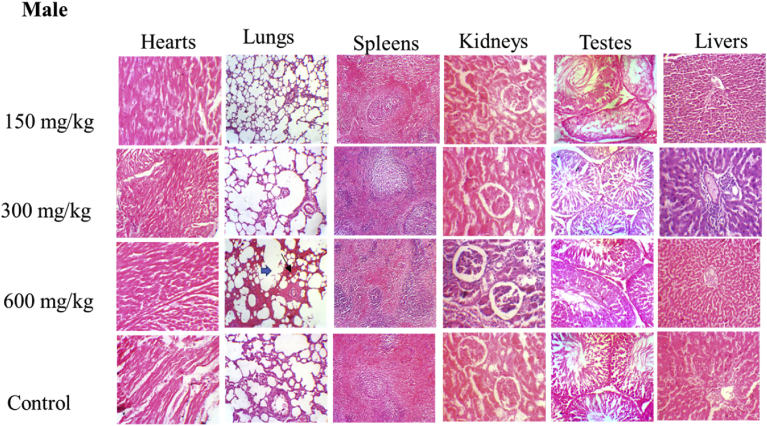
Histological evaluations of the hearts, spleens, kidneys, ovaries and testes in both female and male rats showed no changes when compared to the control. However, in the female rats, a mild inflammatory infiltration within the lobules of the liver was observed (Fig. 3) while in the male rats, the histology of the lungs showed dilated alveoli spaces, expanded septae and regular bronchioles (Fig. 4).
Fig. 3.
Photomicrograph of sections of male hearts, lungs, spleens, kidneys, ovaries and livers in control and treated groups. The liver section at 600 mg/kg of Caralluma dalzielii aqueous extract showed mild inflammatory infiltration within the lobules of the liver. Other organ sections were similar to the control.
Fig. 4.
Photomicrograph of sections of female hearts, lungs, spleens, kidneys, ovaries and livers in control and treated groups. The lungs section at 600 mg/kg of Caralluma dalzielii aqueous extract showed dilated alveoli spaces, expanded septae and regular bronchioles. Other organ sections were similar to the control.
4. Discussion
The principal aim of evaluating the safety of any medicinal plant is to identify the nature and significance of adverse effect and to establish the exposure level at which this effect is observed (Ibrahim et al., 2016). The results of the acute toxicity study indicate that the aqueous extract of the aerial parts of Caralluma dalzielii administered through oral route to rats and mice at 175, 500 and 2000 mg/kg using the up and down method of acute toxicity testing did not produce any sign of toxicity and death in the animals. According to OECD criteria under its Globally Harmonised Classification System (GHS) for chemical substances and mixtures, substances with LD50 > 2000–5000 mg/kg are categorised as unclassified or category 5 (Organization for Economic Development, 2001). This suggests that the oral LD50 of the plant being greater than 2000 mg/kg may be safe. The LD50 of C. dalzielii aerial parts appears to vary depending on the solvent of extraction for example the oral LD50 of ethanol extract of the aerial parts of this plant has been shown to be 2154 mg/kg in rats (Tanko et al., 2013) whereas that of hydroalcoholic extract is 288.53 mg/kg (Umar et al., 2013). This may be due to the altered chemical composition of the resulting extract because of different availability of extractable components (Sultana et al., 2009). Acute toxicity data are usually of limited clinical application. Therefore, sub-acute toxicity study was carried out.
Substances administered in chronic disease conditions may need repeated dosing toxicological evaluation (sub-acute toxicity study) since daily use may result in accumulation in the body with gradual effects on tissues and organs (Abotsi et al., 2011; Bariweni et al., 2018). Sub-acute toxicity testing is useful in assessing target organ and haematological or biochemical effects of extracts since these effects are usually not observable in acute toxicity testing. It is also essential in establishing human safety especially in the development of pharmaceuticals. Hence in this study, the sub-acute toxicity profile of the aerial parts of Caralluma dalzielii extract was evaluated in rats using measurement of feed and water intake, body weight, haematological, biochemical and histological parameters.
Changes in feed and water intake and body weight gain have been used as an indicator of general health status of experimental animals (El-Hilaly et al., 2004). Feed consumption is regulated though several complex biological mechanisms which ensures relatively constant body weight over long periods (Kuriyan et al., 2007). Appetite regulates the body's desire for food and plays important role in weight regulation. Oral administration of the extract of C. dalzielii daily over the period of 28 days exposure test showed no mortality but a significant decrease in the feed and water intake and body weights of both female and male rats. The decrease in body weight could be attributed to the reduction in feed and water intake observed in the rats. Obese individuals have been shown to have increased appetite and reduction of appetite has helped in prevention of further weight gain (Kuriyan et al., 2007). Our result showed that the plant contains glycosides of which have been identified as pregnane glycosides by other researchers (Oyama et al., 2007). Pregnane glycosides possess appetite suppressing properties (Lakshmi et al., 2014). This report agrees with previous studies that showed that some species of Caralluma reduces feed and water intake, body weight and possess anti-obesogenic properties (Kuriyan et al., 2007 and Ambadasu et al., 2013). Hence, our extract may possess anti-obesogenic properties can be used in the development of natural anti-obesity drugs.
Again, in toxicity studies, organ weight changes are sensitive indicators of toxicity, effects on enzymes, physiologic disturbances and target organ injury (Michael et al., 2007). An increase in organ weight suggests the occurrence of hypertrophy while a decrease suggests necrosis in the target organ (Teo et al., 2002). While organ weights provide useful signals indicating test article-related effects, organ weight data must be interpreted in an integrated fashion with gross pathology, clinical pathology, and histopathology findings (Sellers et al., 2007). The decrease in liver weight observed in the female rats could be an indication of toxicity. This is confirmed from the histopathological examination showing mild inflammatory infiltration within the lobules of the female liver tissue. This suggests that the extract on continuous use may be harmful to the liver. However, the changes in the liver was not evident in the male rats. This may be because of hormonal differences in the animals. Again, the weights of the ovaries were significantly increased while there was no change in the testes weight of the male rats. This suggests that the extract may possess compounds that act on the female sex cells more than the males. Therefore, it is important that oestrogenic activity of this plant be studied. Changes were observed in the weights of spleen and heart but there were no changes in their tissue histology demonstrating that this event was irrelevant to the them. There was a significant increase in lungs weight in the male rats. Histopathological examination showed dilated alveoli spaces and expanded septae on the lungs tissue of male animals, therefore, the extract may be slightly toxic to the lungs. According to the Society for Toxicological Pathology, lung weight is an important parameter to evaluate inhalation medicine. An assessment of lung weight in an oral administration study adds little value to a microscopic evaluation and should be optional (Sellers et al., 2007). All the organs and tissue histology were not different from the control therefore it could be claimed that the extract may be safe but should be taken with caution in females.
Analysis of haematological parameters are used to study the extent of toxicity of drug substances including plant extracts (Ibrahim et al., 2016). Haematopoiesis is the process of blood cell formation. Changes in the haematopoietic system have a higher predictive value for human toxicity when data are translated from animal studies (Olson et al., 2000). All blood cells are believed to be derived from the pluripotential stem cell, an immature cell with the capability of becoming an erythrocyte (RBC), a leukocyte (WBC), or a thrombocyte (platelet) (Cavanaugh, 2003). In this study, administration of aqueous extract of C. dalzielii in both female and male rats for a period of 28 days produced no significant change in all blood parameters except an increase in lymphocytes and mean platelets volume. Lymphocytes are dynamic cells and mediate immune response to foreign substances (Pearce et al., 2013). They also produce antibodies enabling the destruction of intracellular microbes and cancer cells (Ganong, 2001). The increase in lymphocytes suggest possible immuno-stimulatory effect of the plant. An abnormally high platelet count may point to haemostatic disorders and eventually lead to thromboembolic diseases (Osei-Bimpong et al., 2012). Oestrogen is usually implicated in the increase in mean platelet volume (Tripathi, 2004). Therefore, the extract may possess oestrogenic activities. However, further studies are needed to ascertain this.
The role of liver and kidney functions are important for survival of animals. Their functionality can be measured by serum biochemical analysis, which are crucial in the toxicological evaluation of xenobiotics (Bariweni et al., 2018). Serum liver function tests provide information about the status of the liver. The liver enzymes (aminotransferases; ALT and AST) describe its cellular integrity, while albumin and total protein levels describe its functionality (Adeoye and Oyedapo, 2004). AST and ALT are principally produced by the liver cells and any assault to the liver may lead to an increase in the serum level of these enzymes (Adedapo et al., 2004). High levels of liver enzymes are signs of hepatocellular toxicity (Brautbar and Williams, 2002), whereas a decrease may indicate enzyme inhibition (Akanji et al., 2013). However, ALT is the most sensitive marker of liver damage or toxicity since AST is also found in abundance in kidneys, testes, cardiac and skeletal muscles (Friedman et al., 1996; Bariweni et al., 2018). In this study, there was a significant increase in the ALT of both the male and female rats at 600 mg/kg. Histopathological examination of the livers showed mild toxic effect in the female liver and none in the male. Therefore, it may be inferred that extract is slightly toxic since it causes elevation of liver enzymes with mild structural change in the liver of the female animals.
The functionality of the liver was assessed by the serum total protein, bilirubin and albumin. A reduction in serum levels of total proteins, bilirubin and albumin depicts reduced synthetic function, which is evident in liver damage or diseases. An increase in these parameters is usually seen in cancerous conditions, or following high protein diet (Tietz et al., 1994). Our study showed a significant increase in direct bilirubin in the female rats confirming the toxic effect of the extract on the liver of the animals. The total protein and albumin serum levels did not differ significantly from the control group. This shows that the effect on the liver could be a mild toxic effect affecting only the female rats. Generally, it appeared that the extract affected the female at a lower dose as compared to the males.
The usual blood test which checks the functionality of the kidneys measures the levels of urea, creatinine and certain dissolved salts (serum electrolyte). In this study, the urea level at a lower dose was significantly elevated. A high serum level of urea indicates that the kidneys may not be working properly, or that the animal is dehydrated whereas, low urea levels are seen in acute liver failure or overhydration (Lum and Leal-Khouri, 1989). Creatinine clearance, an indicator of glomerular filtration rate is used for assessing kidney function. Our extract did not cause any significant change in the creatinine levels when compared with the control suggesting that the extract may not be toxic to the kidney. Histology of the kidneys in the male and female rats did not produce any toxic changes confirming the safety of the extract in the kidney.
5. Conclusion
The oral LD50 of aqueous extract of Caralluma dalzielii has been shown to be greater than 2000 mg/kg and is generally considered safe. C. dalzielii has also been shown to cause appetite suppression and reduction of body weight hence can be used as an anti-obesity agent. Prolonged administration revealed that it may cause elevation of liver enzymes and mild toxicity to the liver. These observations suggest that Caralluma dalzielii may be safe, however liver toxicity on long-term use may be a concern.
Declarations
Author contribution statement
Chinenye Jane Ugwah-Oguejiofor: Conceived and designed the experiments; Wrote the paper.
Charles Ogbonna Okoli: Conceived and designed the experiments.
Michael Oguejiofor Ugwah, Millicent Ladi Umaru: Performed the experiments.
Halilu Emmanuel Mshelia: Contributed reagents, materials, analysis tools or data.
Chiedozie Smart Ogbulie, Mohammed Umar, Anoka Ayembe Njan: Analyzed and interpreted the data.
Funding statement
This work was supported by Management of Usmanu Danfodiyo University Sokoto, Nigeria.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abotsi W.K., Ainooson G.K., Gyasi E.B., Abotsi W.K.M. Acute and sub-acute toxicity studies of the ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae) in rodents. West Afr. J. Pharm. 2011;22:27–35. [Google Scholar]
- Adedapo A.A., Abatan M.O., Olorunsogo O.O. Toxic effects of some plants in the genus Euphorbiaceae on haematological and biochemical parameters of rats. Vet. Arh. 2004;74(1):53–62. [Google Scholar]
- Adeoye B., Oyedapo O.O. Toxicity of Erythophleum guineense stem-bark: role of alkaloid fraction. Afr. J. Trad. Compl. Alt. Med. 2004;1:45–54. [Google Scholar]
- Akanji M.A., Yakubu M.T., Kazeem M.I. Hypolipidemic and toxicological potential of aqueous extract of Rauwolfia vomitoria Afzel root in Wistar rats. J. Med. Sci. 2013;13:253–260. [Google Scholar]
- Ambadasu B., Dange S.V., Walli R.S., Worlikar P.S. Effect of Caralluma fimbriata extract on appetite and lipid profile in rats fed with hypercalorie/cafeteria diet. Int. J. Pharm. Bio. Sci. 2013;4(2):788–793. [Google Scholar]
- Baravalle C., Salvetti N.R., Mira G.A., Pezzone N., Orteaga H.H. Microscopic characterization of follicular structures in Leotrozole-induced Polycystic ovarian syndrome in the rat. Arch. Med. Res. 2006;37:830–839. doi: 10.1016/j.arcmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Bariweni M.W., Yibala O.I., Ozolua R.I. Toxicological studies on the aqueous leaf extract of Pavetta crassipes (K. Schum) in rodents. J. Pharm. Phcog. Res. 2018;6(1):1–16. [Google Scholar]
- Brautbar N., Williams I.J. Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int. J. Hyg. Environ. Health. 2002;25(6):479–491. doi: 10.1078/1438-4639-00175. [DOI] [PubMed] [Google Scholar]
- Burkill H.M. Vol 1. Royal Botanical Gardens; Kew: 1985. (The Useful Plants of West Tropical Africa). [Google Scholar]
- Cavanaugh B.M. FA Davis Company; Philadelphia: 2003. Nurse's Manual of Laboratory and Diagnostic Tests. [Google Scholar]
- Dalziel J.M. Crown Agent for Oversea Governments and Administrations; London: 1937. Useful Plants of West Tropical Africa; pp. 316–317. [Google Scholar]
- de Kock D., Meve U. International Asclepiad Society; UK: 2007. A Checklist of Brachystelma, Ceropegia and the Genera of the Stapeliad; p. 125. [Google Scholar]
- De Leo M., De Tommasi N., Sanogo R., Autore G., Marzocco S., Pizza C., Morelli I., Braca A. New pregnane glycosides from Caralluma dalzielii. Steroids. 2005;70:573–585. doi: 10.1016/j.steroids.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hilaly J., Israili Z.H., Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 2004;91(1):43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- El-Olemmy M.M., Muhtadi F.A., Affifi A.A. King Saudi University; Riyadh: 1994. Experimental Phytochemical: A Laboratory Manual Department of Pharmacognosy; p. 1415. King Saudi University Press. [Google Scholar]
- Friedman L., Martin P., Muooz S.J. A Textbook of Liver Disease. WB Saunders Co.; Philadelphia: 1996. Liver function tests and the objective evaluation of the patient with liver disease; pp. 791–833. [Google Scholar]
- Ganong W.F. Twentieth ed. Lange Medical Books, McGraw Hill Co.; New York: 2001. Review of Medical Physiology. [Google Scholar]
- eighth ed. The National academies press; 2011. Guide for the Care and Use of Laboratory Animals. [PubMed] [Google Scholar]
- Harbone J.B. Chapman and Hall; London: 1993. Phytochemical Methods; p. 68. [Google Scholar]
- Ibrahim J.A., Muazzam I., Jegede I.A., Kunle O.F. Medicinal plants and animals sold by the Yan-Shimfidas of Sabo Wuse in Niger state, Nigeria. Afr. J. Pharm. Pharmacol. 2010;4(6):386–394. [Google Scholar]
- Ibrahim M.B., Sowemimo A.A., Sofidiya M.O., Badmos K.B., Fageyinbo M.S., Abdulkareem F.B., Odukoya O.A. Sub-acute and chronic toxicity profiles of Markhamia tomentosa ethanolic leaf extract in rats. J. Ethnopharmacol. 2016;193:68–75. doi: 10.1016/j.jep.2016.07.036. [DOI] [PubMed] [Google Scholar]
- Inngjerdingen K., Nergård C.S., Diallo D., Mounkoro P.P., Paulsen B.S. An ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa. J. Ethnopharmacol. 2004;92:233–244. doi: 10.1016/j.jep.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Koduru S., Grierson D.S., Afolayan A.J. Antimicrobial activity of Solanum aculeastrum. Pharm. Biol. 2006;44(4):283–286. [Google Scholar]
- Kuriyan R., Raj T., Srinivas S.K., Vaz M., Rajendran R., Kurpad A.V. Effect of Caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite. 2007;48(3):338–344. doi: 10.1016/j.appet.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Lakshmi T., Rajendran R., Raghavan V., Silvester A. HPTLC fingerprinting method to distinguish total extract of Caralluma fimbriata from the modified extracts of Caralluma fimbriata. Biosci. Biotechnol. Res. Asia. 2014;11:785–789. [Google Scholar]
- Lum G., Leal-Khouri S. Significance of low serum urea nitrogen concentrations. J. Clin. Chem. 1989;35(4):639–640. [PubMed] [Google Scholar]
- Michael B., Yano B., Sellers R.S., Perry R., Morton D., Roome N., Johnson J.K., Schafer K. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007;35(5):742–750. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- Obidike I., Salawu O. Pub. InTech; 2013. Screening of Herbal Medicines for Potential Toxicities: Pharmacology, Toxicology and Pharmaceutical Science; New Insight in Toxicity and Drug Testing; pp. 63–67. Chapter 4. [Google Scholar]
- Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., Dorato M. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Development . 2001. Guideline for Testing of Chemicals. Guideance, no. 425. [Google Scholar]
- Osei-Bimpong A., McLean R., Bhonda E., Lewis S.M. The use of the white cell count and haemoglobin in combination as an effective screen to predict the normality of the full blood count. Int. J. Lab. Hematol. 2012;34(1):91–97. doi: 10.1111/j.1751-553X.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama M., Iliya I., Tanaka T., Iinuma M. Five new steroidal glycosides from Caralluma dalzielii. Helv. Chim. Acta. 2007;90:63–71. [Google Scholar]
- Pearce E.L., Poffenberger M.C., Chang C.H., Jones R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowes D.C.H. The stapeliads of Senegal. Cactus World. 2008;26:151–158. [Google Scholar]
- Sellers R.S., Mortan D., Michael B., Roome N., Johnson J.K., Yano B.L., Perry R., Schafer K. Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007;35(5):751–755. doi: 10.1080/01926230701595300. [DOI] [PubMed] [Google Scholar]
- Sultana B., Anwar F., Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14(6):2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanko Y., Daniel P.A., Mohammed K.A., Jimoh A., Yerima M., Mohammed A. Effect of ethanolic extract of Caralluma dalzielii on serum lipid profiles on fructose induced diabetes in wistar rats. Scholar Res. 2013;4:162–166. [Google Scholar]
- Teo S., Stirling D., Thomas S., Hoberman A., Kiorpes A., Khetani V. A 90-day oral gavage toxicity study of d-methylphenidate and d, l-methylphenidate in Sprague–Dawley rats. Toxicol. 2002;179(3):183–196. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- Tietz W.N., Prude E.L., Sirgard-Anderson O. WB Saunders London; UK: 1994. Tietz Textbook of Clinical Chemistry. [Google Scholar]
- Trease G.E., Evans W.C. Bailliere Tindall Press; London: 1983. Pharmacognosy; pp. 309–706. [Google Scholar]
- Tripathi K.D. fifth ed. Jaypee Brothers Medical Publishers limited; New Delhi, India: 2004. Essentials of Medical Pharmacology; pp. 276–282. [Google Scholar]
- Ugwah M.O., Etuk E.U., Bello S.O., Aliero A.A., Ugwah-Oguejiofor C.J. Comparative studies of anti-ulcerogenic activities of three Nigerian medicinal plants: a preliminary evaluation. J. Med. Plants Res. 2013;7(9):490–495. [Google Scholar]
- Ugwah-Oguejiofor C.J., Abubakar K., Ugwah M.O., Njan A.A. Evaluation of the antinociceptive and anti-inflammatory effect of Caralluma dalzielii. J. Ethnopharmacol. 2013;150(3):967–972. doi: 10.1016/j.jep.2013.09.049. [DOI] [PubMed] [Google Scholar]
- Ugwah-Oguejiofor C.J., Inuwa F.S., Abubakar K., Ugwah O.M., Mshelia E.H., Umar M. Evaluation of antiulcer activity of the aerial parts of Caralluma dalzielii N. E. Brown in Wistar rats. Trop. J. Nat. Prod. Res. 2017;1(4):168–172. [Google Scholar]
- Umar A.H., Mabrouk M., Danjuma N.M., Yaro A. Studies on the analgesic and anti-inflammatory properties of hydro-alcohol extract of Caralluma dalzielii NE Br (Asclepiadaceae) in rats and mice. Br. J. Pharmacol. Toxicol. 2013;4(5):169–175. [Google Scholar]
- Yuet Ping K., Darah I., Chen Y., Sreeramanan S., Sasidharan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/182064. [DOI] [PMC free article] [PubMed] [Google Scholar]