Introduction
Pseudohypoparathyroidism type Ia (PHP-Ia) is characterized by end-organ resistance to PTH and Albright’s hereditary osteodystrophy (AHO), involving short stature, obesity, a round face, brachydactyly, osteoma cutis/calcinosis cutis, and mental retardation. PHP-Ia results from reduced activity of the Gsα protein due to heterozygous mutations within the coding region of GNAS gene, resulting in protein inactivation. Furthermore, conditions arising due to resistance to multiple hormones, such as hypothyroidism and hypogonadism, often occur in PHP-Ia. Herein, we report the case of a boy who was diagnosed with PHP-Ia, and in whom, osteoma cutis was detected early by skin biopsy. In addition, genetic analysis showed a novel mutation (c.862dupA) in the GNAS gene in this case.
Case Report
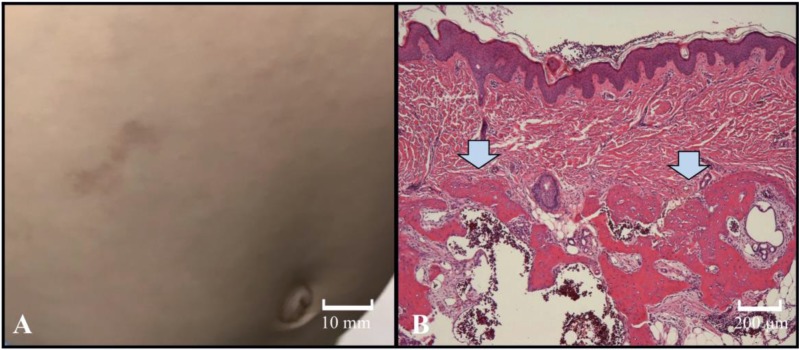
An 11-mo-old boy was referred to our hospital with a delayed psychomotor/developmental milestone. He was born at 40 wk gestation and had no specific symptoms at birth. High blood levels of thyroid stimulating hormone (TSH) were not detected by the newborn mass-screening test. His mother had a resection of subcutaneous ossification of the plantar in her infancy. She had short stature, but no brachydactyly. His 4-yr-old elder sister had a round face, short stature, obesity, brachydactyly, and subcutaneous lesions. On physical examination, his height was 72.6 cm (–0.52 SD) and his weight was 11.3 kg (+2.3 SD). His Kaup index was 21.4 kg/m2 (normal range: 15–18 kg/m2) and he had a round face and short and stubby fingers. The patient could sit and crawl at 11 mo old. He had also recently begun to stand as well while holding on to objects. Subcutaneous lesions were distributed over both of his arms and legs and his abdominal wall. Laboratory data for various parameters which were within normal ranges included: serum calcium: 10 mg/dL (normal range: 8.8–10.6 mg/dL), serum phosphorus: 5.0 mg/dL (normal range: 3.9–6.2 mg/dL), alkaline phosphatase activity: 512 U/L (normal range: 395–1339 U/L), and intact serum PTH: 42 pg/mL (normal range: 10–65 pg/mL). The serum 25-hydroxy vitamin D level (12.9 ng/mL, normal range: 20–100 ng/mL) was significantly low. Serum levels of thyroid hormone and TSH were normal (free T3: 3.31 pg/mL, normal range: 2.2–4.3 pg/mL; free T4: 0.87 ng/dL, normal range: 0.8–1.6 ng/dL; serum TSH: 3.01 μIU/mL, normal range: 0.2–4.5 μIU/mL). Serum insulin-like growth factor-1 (IGF-1) was evaluated to be 32 ng/mL (normal range: 11–149 ng/mL). A skin biopsy of a subcutaneous nodule was done for pathological investigation. Ossification in the dermis and subcutaneous tissue were revealed by histopathological examination, and consequently, osteoma cutis was diagnosed (Fig. 1).
Fig. 1.
(A) Representative image of the subcutaneous lesions that were distributed on his abdominal wall. (B) The arrows indicate histopathological examination of a representative skin biopsy revealing ossification in the dermis and subcutaneous tissue (hematoxylin and eosin staining).
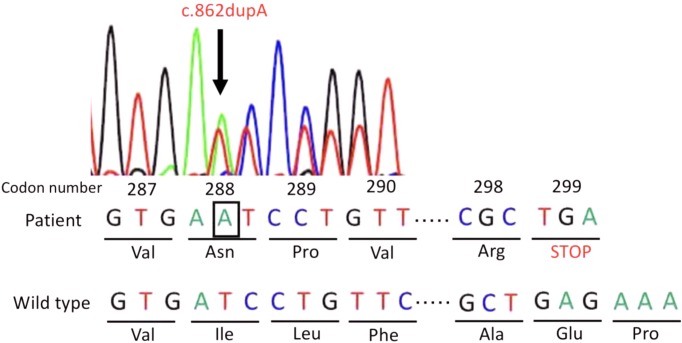
After 1 yr (aged 2 yr), the subcutaneous ossification was slightly increased. At this time, serum intact PTH level was elevated at 81 pg/mL. Serum calcium and serum phosphorus levels were within normal ranges (9.8 mg/dL and 5.3 mg/dL, respectively). Serum 25-hydroxy vitamin D (16.3 ng/mL) was insufficient. Serum TSH level was elevated at 12.201 μIU/mL, with normal serum free T3 and serum free T4 levels (3.46 pg/mL and 1.02 ng/dL, respectively). Based on these clinical and biochemical findings, in addition to family history, we hypothesized that pseudohypoparathyroidism type Ia (PHP-Ia) was associated with Albright hereditary osteodystrophy (AHO). After obtaining informed consent from his parents, we conducted genetic analysis of the GNAS gene. PCR-direct sequencing was used to analyze exons 1-13 of the whole GNAS gene, including intron/exon boundaries. We identified a novel heterozygous mutation, with an adenosine insertion at codon 288 of exon 11 in the GNAS gene (c.862dupA) (according to the sequence NM000516.5 published in GenBank), giving rise to a stop codon (p.Ile288Asnfs*12) (Fig. 2). Genetic analysis could not be done for his mother and elder sister, as no informed consent was obtained. Although serum calcium level was maintained within normal range, preventive treatment with 1, 25 hydroxyvitamin D3 (0.04 μg/kg/d) was initiated. We continued to monitor the serum levels of calcium, thyroid function, and pituitary hormones. Subsequently, we found no episodes of seizure, hypocalcemia, or hypothyroidism.
Fig. 2.
GNAS mutation. The patient’s sequence analysis showed a heterozygous mutation (c.862dupA) due to the insertion of adenosine (indicated in the black box) at codon 288 in exon 11, resulting in a frameshift mutation.
Discussion
We presented a case of PHP-Ia and osteoma cutis, with a novel mutation in the GNAS gene. PHP-Ia is associated with PTH resistance and is characterized by AHO in PHP. The underlying cause for the reduction in the Gsα protein levels is protein inactivation due to a heterozygous mutation within the coding region of the GNAS gene. Since the discovery of a GNAS gene mutation in a PHP patient in 1990, several GNAS mutations have been identified in many families with PHP (1). Associations between PHP and GNAS gene mutations have been well documented. Frameshift mutations caused by insertion or deletion have been detected in nearly half of the GNAS gene mutations. Furthermore, frameshift mutations in the GNAS gene are expected to lead to a truncated protein, which indicates loss of function (2). In our case, because of a single base insertion of adenine at position 862 of the coding DNA region of the GNAS gene, we speculated that this mutation could lead to loss of protein function. This variant has not been previously reported in the Human Gene Mutation Database (HGMD) and Clinvar database. Previous research has shown that a genetic abnormality does not necessarily correlate with a clinical phenotype in PHP, due to GNAS gene variation. The effects of these mutations are tissue-specific, depending on whether there is a biallelic expression of the Gsα protein or it is expressed from only the maternal allele due to imprinting of the paternal allele. The predominant sources of Gsα protein from the maternal allele are the proximal renal tubules, thyroid, gonads, and the pituitary. However, most cells maintain a normal signaling activity as they have half of the normal Gsα protein activity because no paternal imprinting occurs in them (2). There is no association between the position of the GNAS mutation and clinical phenotype. Hence, there are several AHO phenotypes.
Osteoma cutis/calcinosis cutis development is a typical feature of AHO. The osteoma cutis/calcinosis cutis seen in AHO is not associated with hypocalcemia or hyperphosphatemia, as ossification/calcification are not detected in patients with primary hypoparathyroidism (3).
There are no systematic reviews with respect to the frequency of osteoma cutis/calcinosis cutis in AHO. Osteoma cutis/calcinosis cutis is estimated to occur in 25–50% of patients with AHO and PHP-Ia (4). The time taken for the development of osteoma cutis/calcinosis cutis seen in patients with AHO/PHP-Ia within a larger cohort has not been reported; however, it appears to be an earlier symptom of AHO (3). Early detection of osteoma cutis/calcinosis cutis can lead to early diagnosis of PHP-Ia, similar to our case.
Finally, we speculated on the reason why there is a time interval until the onset of PTH resistance. PTH resistance in patients with PHP-Ia rarely appears soon after birth. Moreover, most patients with PHP-Ia exhibit low levels of serum calcium for first few years after birth (3). A previous study in a PHP-Ia murine model reported that GSα protein is expressed in the renal proximal tubules for a certain period of time, despite expression of only maternal allele. For this reason, the suppression of paternal Gsα expression is silenced for certain period of time after birth (2). However, the detailed mechanisms of the onset of resistance to other hormones, except PTH, remain unclear. In current case, subclinical TSH resistance was beginning to appear. Long-term follow-up is required to continuously monitor hypocalcemia and the appearance of resistance to other hormones.
In conclusion, the combination of osteoma cutis, elevated PTH levels, and resistance to TSH levels allowed us to diagnose PHP-Ia at an early stage of life. Osteoma cutis in infants is an important finding leading to the possibility of occurrence of AHO and PHP-Ia at early age.
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
Acknowledgments
We would like to offer special thanks to Dr. Takeshi Usui from the Department of Medical Genetics, Shizuoka General Hospital, for aiding in the genetic diagnosis with Sanger DNA sequence analysis.
References
- 1.Patten JL, Johns DR, Valle D, Eil C, Gruppuso PA, Steele G, et al. Mutation in the gene encoding the stimulatory G protein of adenylate cyclase in Albright’s hereditary osteodystrophy. N Engl J Med 1990;322: 1412–9. doi: 10.1056/NEJM199005173222002 [DOI] [PubMed] [Google Scholar]
- 2.Lemos MC, Thakker RV. GNAS mutations in pseudohypoparathyroidism type 1a and related disorders. Hum Mutat 2015;36: 11–9. doi: 10.1002/humu.22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poomthavorn P, Zacharin M. Early manifestation of obesity and calcinosis cutis in infantile pseudohypoparathyroidism. J Paediatr Child Health 2006;42: 821–3. doi: 10.1111/j.1440-1754.2006.00985.x [DOI] [PubMed] [Google Scholar]
- 4.Riepe FG, Ahrens W, Krone N, Fölster-Holst R, Brasch J, Sippell WG, et al. Early manifestation of calcinosis cutis in pseudohypoparathyroidism type Ia associated with a novel mutation in the GNAS gene. Eur J Endocrinol 2005;152: 515–9. doi: 10.1530/eje.1.01879 [DOI] [PubMed] [Google Scholar]