Introduction
Familial hypercholesterolemia (FH, OMIM number #143890) is an autosomal dominant disorder, resulting in low-density lipoprotein (LDL) receptor dysfunction. The prevalence of FH is estimated to be approximately, 1 in 200 and 1 in 170,000 among heterozygotes and homozygotes, respectively (1). Since FH patients develop severe coronary-artery disease (CAD) due to hypercholesterolemia in early adult life, lipid-lowering treatments must be started at childhood (2).
Although pediatric FH is clinically diagnosed based on the serum LDL cholesterol (LDL-C) levels and a family history of hypercholesterolemia, genetic analysis is useful for a definitive diagnosis, as it can predict the risk stratification of CAD (3, 4) and assist in early diagnosis and intervention, leading to improved prognosis. Here, we present a novel mutation in the LDLR gene, in a patient with heterozygous FH (HeFH).
Patient Report
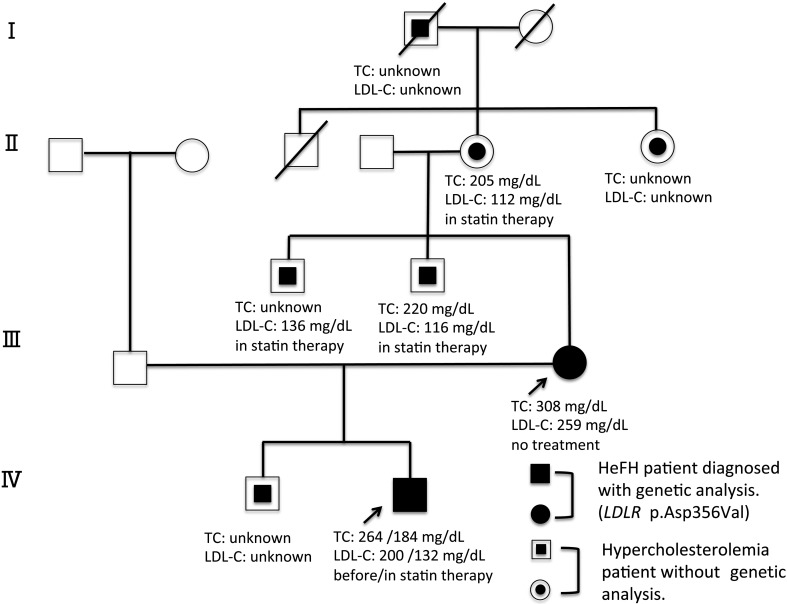
The study subject was an 11-yr-old Japanese boy with Down’s syndrome. He was delivered at 36 wk and 4 d of gestation by cesarean section, due to a history of cesarean birth. His birth weight was 2,160 g (mean –2.1 standard deviation, SD, in Japan), and his body length was 41.0 cm (–3.8 SD). Down’s syndrome was diagnosed based on his facial appearance and genetic analysis. His old brother, mother, maternal uncles, maternal grandmother, maternal grandaunt, and maternal great-grandfather had been diagnosed with hypercholesterolemia. His mother was hypercholesterolemic (serum total cholesterol; TC level 308 mg/dL, LDL-C level 259 mg/dL) at the age of 48 yr. His maternal uncles and maternal grandmother were receiving HMG-CoA reductase inhibitors (statins) for their hypercholesterolemia, but they were hypercholesterolemic despite statin therapy. His older maternal uncle’s serum TC level was unknown, and LDL-C level was 136 mg/dL, at 55 yr of age. His younger maternal uncle’s serum TC level was 220 mg/dL, and LDL-C level was 116 mg/dL, at 52 yr of age. His maternal grandmother’s serum TC level 205 mg/dL, and LDL-C level was 112 mg/dL, at 80 yr of age. The serum lipid values of the other patients in the family were not available (Fig. 1). Further, he had no family history of CAD associated death. The patient was referred to our hospital at 7 yr of age due to hypercholesterolemia (serum total cholesterol; TC level 264 mg/dL, LDL-C level 200 mg/dL). His height was 120.4 cm (approximately 0 SD for the Down’s syndrome patients) and his weight was 21.4 kg (approximately –2.0 SD for the Down’s syndrome patients) or –6.6% of the Japanese standard weight for his height. He did not have xanthoma or Achilles tendon thickening. His serum concentration of thyroid hormones was normal. Based on these findings, we diagnosed him as FH. With the help of his mother, we carried out a lifestyle treatment, which included a proper lipid diet and exercise, for several months. However, his hypercholesterolemia did not improve. Statin therapy (Rosuvastatin Calcium®︎ 2.5 mg daily) was started at 10 yr of age, which improved his hypercholesterolemia (serum TC level from 232 to 184 mg/dL, and LDL-C level from 168 to 132 mg/dL in one month). In Japan, only pitavastatin had been approved as a treatment for children with HeFH. However, he had asymptomatic cholelithiasis without biliary atresia. We started him on rosuvastatin instead of pitavastatin, because pitavastatin was contraindicated for biliary atresia.
Fig. 1.
Pedigree of a Japanese family with familial hypercholesterolemia. Squares and circles indicate males and females, respectively. Black symbols represent individuals with the hypercholesterolemia phenotype and open symbols represent unaffected individuals. The filled symbols indicate patients who were genetically analyzed. The double fill symbols indicate hypercholesterolemia without genetic diagnosis. The proband and his mother are marked by arrows. Roman numerals to the left of the pedigree indicate the generation. The columns under each symbol indicate, from top to bottom, TC and LDL-C concentration (mg/dL).
Mutational Analysis
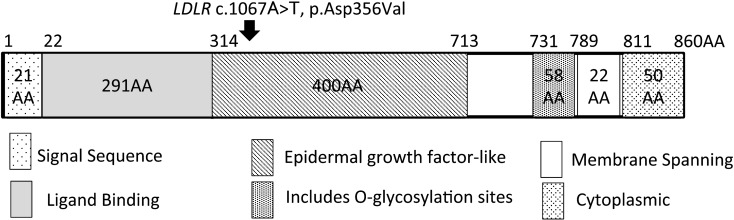
This study was approved by the Institutional Review Board of the Tokyo Metropolitan Children’s Medical Center (H28b-179), and an informed consent was obtained from the patient’s parents. Genomic DNA was extracted from the peripheral blood leukocytes of the patient and his mother. We performed targeted-exome sequencing of seven genes (LDLR, APOB, PCSK9, LDLRAP1, APOE, ABCG5, ABCG8), known to cause hypercholesterolemia, using a next-generation sequencer (5). We used PCR-direct sequencing to make the final determination of the mutation. We identified a novel c.1067A>T (p.Asp356Val) missense mutation in the LDLR gene of the patient and his mother. This mutation was not detected in any of the 150 healthy controls and was absent in the databases, including dbSNP, the 1,000 Genomes Project, Exome Variant Server, NHLBI Exome Sequencing Project, and the Human Genetic Variation Database (HGVD) in Japanese. The mutation was located at the epidermal growth factor-like domain in the extracellular region of LDLR (Fig. 2). Asp356 is evolutionarily highly conserved in humans, rats, and mice. We found no pathological mutations in other genes for which the targeted-exome sequencing was performed.
Fig. 2.
Identification of the sequence variation in LDLR. Schematic diagrams of the LDLR protein. The numbers represents those of the amino acids. The missense mutation, p.Asp356Val in LDLR is located at the epidermal growth factor-like domain in the extracellular region of LDLR. Arrows indicate the mutated amino acids.
Discussion
In the present report, we described a case of HeFH with a missense mutation, p.Asp356Val in LDLR. This mutation is novel and has not been registered with the Human Genome Mutation Database (http://www.hgmd.cf.ac.uk/ac). This variation was segregated from the affected mother. Although the functional consequence of this mutation remains to be determined in vitro, we believe this mutation is pathological because (i) Asp356 is a highly conserved residue as described above, (ii) substitutions of Asp356 to Ala (6), Asp356 to His (7), and Asp356 to Tyr (8), have been reported in FH patients. In silico analyses using SIFT (http:// sift.jcvi.org/), PolyPhen2 (http://genetics.bwh.harvard.edu/pph2/), and M-CAP (http://bejerano.stanford.edu/mcap/) predicted that the mutation would cause functional damage (SIFT score 0.000, PP2 score 0.994, and M-CAP score 0.891).
There were many hypercholesterolemia patients in the maternal family of the study subject. Although their serum lipid values before treatment were unknown, they were likely to be FH patients, and although genetic analysis was not performed, this mutation might have been present in them.
In childhood FH patients, genetic analysis is pivotal for a definite diagnosis, since physical signs such as tendon xanthoma, which can be seen in adult patients, are rarely observed. Furthermore, early diagnosis and intervention are important because intima-media thickness (IMT) starts increasing significantly from 10 yr of age (9). Recent Japanese guidelines for the treatment of pediatric HeFH recommends statins as the first-line therapy at the age of 10 yr (2). FH with a gene mutation has higher risk of CAD than FH without a gene mutation (3,4). Therefore, genetic analysis is useful for selecting the appropriate intervention and improving prognosis, and should be performed on childhood patients with clinical FH.
Conflict of Interests: The authors have nothing to declare.
Acknowledgements
We thank the patient and his family for participation in this study. We are very grateful to James R Valera for his assistance in editing this manuscript.
References
- 1.Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, et al. Hokuriku FH Study Group. Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis 2011;214: 404–7. doi: 10.1016/j.atherosclerosis.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Harada-Shiba M, Ohta T, Ohtake A, Dobashi K, Nohara A, Yamashita S, et al. Japan Pediatric Society and Japan Atherosclerosis Society (eds.). 2017 practice guide for the treatment of pediatric familial hypercholesterolemia in Japan, Article in Japanese, Japan Atherosclerosis Society, 2017. [Google Scholar]
- 3.Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol 2016;67: 2578–89. doi: 10.1016/j.jacc.2016.03.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J 2017;38: 1573–9. doi: 10.1093/eurheartj/ehx004 [DOI] [PubMed] [Google Scholar]
- 5.Tada H, Nomura A, Yamagishi M, Kawashiri MA. First case of sitosterolemia caused by double heterozygous mutations in ABCG5 and ABCG8 genes. J Clin Lipidol 2018;12: 1164–1168.e4. doi: 10.1016/j.jacl.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Marduel M, Carrié A, Sassolas A, Devillers M, Carreau V, Di Filippo M, et al. French ADH Research Network. Molecular spectrum of autosomal dominant hypercholesterolemia in France. Hum Mutat 2010;31: E1811–24. doi: 10.1002/humu.21348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varret M, Rabés JP, Thiart R, Kotze MJ, Baron H, Cenarro A, et al. LDLR Database (second edition): new additions to the database and the software, and results of the first molecular analysis. Nucleic Acids Res. 1998;26:248-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leren TP, Tonstad S, Gundersen KE, Bakken KS, Rødningen OK, Sundvold H, et al. Molecular genetics of familial hypercholesterolaemia in Norway. J Intern Med 1997;241: 185–94. doi: 10.1046/j.1365-2796.1997.78119000.x [DOI] [PubMed] [Google Scholar]
- 9.Wiegman A, de Groot E, Hutten BA, Rodenburg J, Gort J, Bakker HD, et al. Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet 2004;363: 369–70. doi: 10.1016/S0140-6736(04)15467-6 [DOI] [PubMed] [Google Scholar]