Abstract.
Congenital generalized lipodystrophy type 4 (CGL4) is a rare disease caused by mutations in the gene polymerase I and transcript release factor (PTRF), the main symptoms of which are systemic reductions in adipose tissue and muscular dystrophy. The strategy of treating CGL4 is to improve the insulin resistance and hypertriglyceridemia that result from systemic reductions in adipose tissue. Metreleptin, a synthetic analog of human leptin, is effective against generalized lipodystrophies; however, there are no reports of the use of metreleptin in the treatment of CGL4. Herein, we discuss the treatment of a six-year-old boy diagnosed with CGL4 due to a homozygous mutation in PTRF with metreleptin. His serum triglyceride level and homeostasis model assessment of insulin resistance (HOMA-IR) value decreased after two months of metreleptin treatment. However, the efficacy of metreleptin gradually decreased, and the treatment was suspended because anaphylaxis occurred after the dosage administered was increased. Subsequently, his serum triglyceride level and HOMA-IR value significantly increased. Anti-metreleptin-neutralizing antibodies were detected in his serum, which suggested that these antibodies reduced the efficacy of metreleptin and caused increased hypersensitivity. Thus, metreleptin appeared to be efficacious in the treatment of CGL4 in the short term, although an adverse immune response resulted in treatment suspension. Further studies are needed to evaluate metreleptin treatments for CGL4.
Keywords: congenital generalized lipodystrophy, metreleptin, leptin, PTRF
Introduction
Lipodystrophies are characterized by partial or systemic reductions in adipose tissue, and are classified into acquired and inherited types. Inherited lipodystrophies are rare diseases, which are further classified as either autosomal recessive congenital generalized lipodystrophy (CGL) or autosomal dominant familial partial lipodystrophy. Two cases of CGL were initially reported in 1954 (1), and CGL was subsequently classified into four subtypes according to the causal genes (2). CGL1, which occurs with a mutation in the AGPAT2 gene, and CGL2, which occurs with a mutation in the BSCL2 gene, account for the majority of CGL cases and are clinically severe because with these diseases the metabolic activity of adipose tissue is already reduced at birth. The causal genes of CGL3 and CGL4 are caveolin 1 (CAV1) and polymerase I and transcript release factor (PTRF), respectively. CGL3 and CGL4 both cause defects in caveolae, which are specific structures in the plasma membrane that are enriched in cholesterol and sphingolipids. The loss of adipose tissue in CGL4 is commonly recognized later in childhood, and the clinical features that characterize CGL4 include muscular dystrophy resulting from activated myostatin (3). PTRF mutations in CGL4 were initially described in Japan in 2009 (3), and 26 cases have since been reported (3,4,5,6,7,8,9).
In CGL4, as with other generalized lipodystrophies, serum levels of leptin and adiponectin, both of which are appetite controllers and metabolic mediators secreted from adipose tissue, are low because of the reduced amount of adipose tissue. Reductions in serum leptin and adiponectin cause clinical insulin resistance and hypertriglyceridemia. Untreated lipodystrophies increase the risk of diabetes mellitus, acute pancreatitis, hepatic steatosis, and atherosclerosis. Therefore, the strategy of treating lipodystrophies is to improve insulin resistance and hypertriglyceridemia. Metreleptin, a synthetic analog of human leptin, is an effective treatment for lipodystrophies because it improves serum levels of glucose and triglycerides and decreases levels of glycosylated hemoglobin (HbA1C) (10). However, there are no reports of the use of metreleptin treatments for CGL4 because CGL4 is a rare disease. This is the first case report of CGL4 being treated with metreleptin.
Case Report
The clinical manifestations and genetic diagnosis of this case have been reported previously (7). Briefly, a boy exhibited motor developmental delay and poor weight gain at 4 mo of age, and his serum level of creatine kinase was significantly elevated. His parents were not in a consanguineous marriage, and he had no siblings. There were no issues during his perinatal period. At 1 yr of age, he was diagnosed with muscular dystrophy by skeletal muscle biopsy. He subsequently showed a reduction in generalized subcutaneous adipose tissue amounts. Due to the abnormalities in his muscles and adipose tissue, CGL4 was suspected. Therefore, the PTRF gene was examined when he was 1 yr old, and a homozygous mutation of c.696_697insC in this gene was identified. This mutation causes the mislocalization of the PTRF protein and blocks its binding to caveolins (3). He was admitted to our hospital for long-term observation at 5 yr of age.
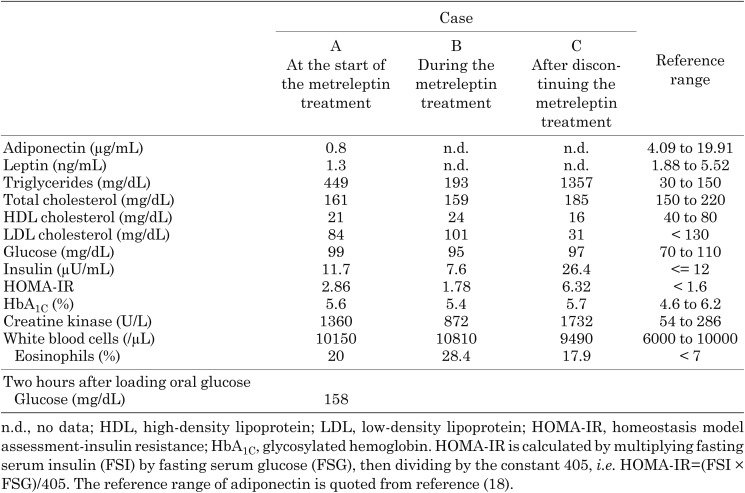
He presented with high serum triglyceride levels, and a high homeostasis model assessment of insulin resistance (HOMA-IR) value revealed that he had high insulin resistance during our hospital examination. We initially attempted diet therapy; however, his serum triglyceride level and HOMA-IR value did not improve after one year and remained at approximately 500 mg/dL and 3.0, respectively. His clinical characteristics at 6 yr of age were as follows: his height and weight were 117.8 cm (–0.17 SD score) and 21.5 kg (–0.30 SD score), respectively. He had abdominal distension, pseudohypertrophy of the appendicular muscle, and a systemic decrease in subcutaneous adipose tissue (Fig. 1). His laboratory data at fasting revealed hypertriglyceridemia, a low high-density lipoprotein cholesterol level, and a high serum immunoreactive insulin level (Table 1A). His serum levels of adiponectin and leptin were low, with values of 0.8 μg/mL and 1.3 ng/mL, respectively. His serum creatine kinase level was high and his white blood cell fraction indicated hyper-eosinophilia. An oral glucose tolerance test revealed impaired glucose tolerance because his glucose level was higher than 140 mg/dL after 2 h, and his HOMA-IR value was 2.86 (Table 1A). His body fat percentage was low, at 7.7% of the whole-body mass excluding the head, as assessed by dual-energy X-ray absorptiometry. Magnetic resonance images revealed a significant decrease in his amounts of subcutaneous and intra-abdominal adipose tissue (Fig. 2). Fatty liver was not present on an abdominal ultrasonogram, and his cardiac output was normal on an echocardiogram.
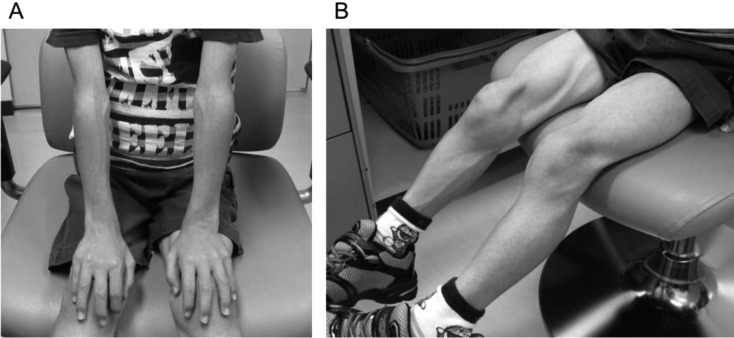
Fig. 1.
Pictures showing the patient’s upper limbs (A) and lower limbs (B) at 6 yr of age. Note the very limited amount of subcutaneous adipose tissue at the extremities and pseudohypertrophy of the appendicular muscles.
Table 1. Laboratory data obtained in the fasting state.
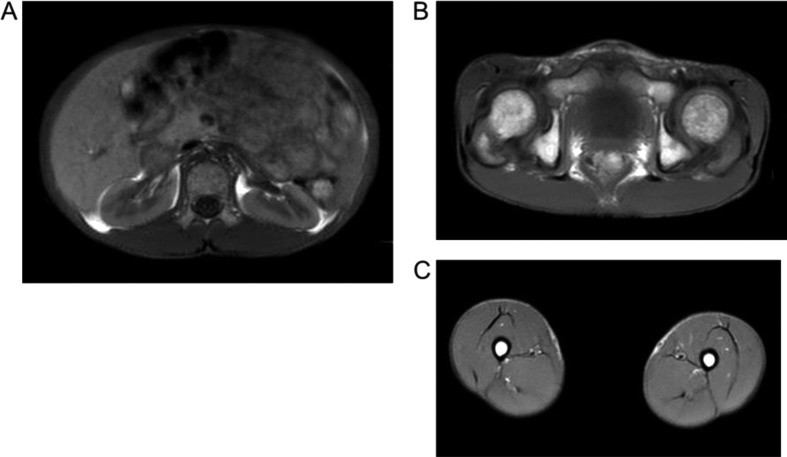
Fig. 2.
Axial T1-weighted magnetic resonance images taken at the umbilical level (A), hip joint level (B), and thigh level (C). Fat typically appears at a high intensity in images; however, the incidence of high intensity areas was reduced, particularly in the subcutaneous area, suggesting that the amount of systemic subcutaneous adipose tissue was markedly decreased.
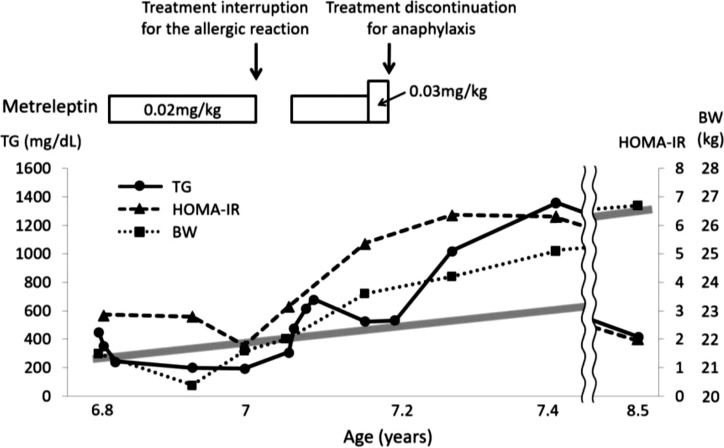
At 6 yr of age, we began a treatment with metreleptin at 0.02 mg/kg/day (Fig. 3). Although the normal initial dose of metreleptin is 0.04 mg/kg/day, we selected a lower dose because he was young and there were no previous reports of CGL4 patients being treated with metreleptin. Metreleptin was administered as an at-home injection under parental guidance. His serum triglyceride level and HOMA-IR value at fasting decreased from 449 to 193 mg/dL and from 2.86 to 1.78, respectively (Table 1B), after 2 mo of the treatment. His appetite and body weight did not increase during the treatment, and his muscle status and amounts of subcutaneous adipose tissue did not change in medical examinations. However, he sometimes developed a cough, eyelid edema, and a rash immediately after the injection, and his blood eosinophil count increased to 6800/µL. Metreleptin was temporarily discontinued because these symptoms indicated an allergic reaction to metreleptin. After discontinuation, his serum triglyceride level began to increase. We restarted the metreleptin treatment along with oral anti-allergic drugs that included a second-generation H1-antihistamine and leukotriene receptor antagonist under hospital supervision, and an allergic reaction was not observed. Since the efficacy of metreleptin gradually decreased during therapy, the dosage was increased to 0.03 mg/kg/day, and dyspnea occurred immediately after an injection on one occasion. Anaphylaxis to metreleptin was suspected, and the treatment was suspended immediately after this episode. We detected anti-metreleptin antibodies in his serum, which exhibited neutralizing activity in vitro. The duration of the metreleptin treatment was approximately 4 mo.
Fig. 3.
The clinical course of this case. TG, triglycerides; HOMA-IR, homeostasis model assessment-insulin resistance; BW, body weight. The gray bold line indicates the normal body weight increase in Japanese boys.
Two months after discontinuing the metreleptin treatment, the patient’s appetite increased, and his body weight increased by 3 kg to 25.1 kg (+0.26 SD score). His serum triglyceride level and HOMA-IR value at fasting significantly increased to 1357 mg/dL and 6.32, respectively (Table 1C). He attempted additional diet therapy with strict nutritional support from family members, and his serum triglyceride level, HOMA-IR value, and SD score of body weight finally decreased to the same level as those before starting the metreleptin treatment (Fig. 3). Furthermore, eosinophilic gastroenteritis with protein loss developed during the observation period without treatment, and so an anti-allergy drug was consequently administered.
Discussion
Reductions in adipose tissue in lipodystrophies result in the insufficient secretion of leptin. Endogenous leptin plays an important role in regulating lipid and glucose metabolism. Leptin inhibits lipogenesis and stimulates lipolysis to promote fatty acid oxidation in white adipocytes, the liver, and skeletal muscle through the autonomic nervous system (11). Glucose metabolism is also partially regulated by leptin through the autonomic nervous system. Leptin suppresses glucose production in the liver and glucagon secretion from pancreatic α-cells and increases glucose uptake in peripheral cells (11).
Metreleptin, a synthetic analog of human leptin, improves lipid and glucose metabolism to levels similar to those achieved by endogenous leptin. The therapeutic effects of metreleptin treatments for lipodystrophies were initially reported in 2002. In that study, nine females with lipodystrophy of various hereditary origins or phenotypes were treated with metreleptin, and serum triglyceride levels and HbA1C decreased (12). Treatment with metreleptin also improved fasting serum triglyceride and glucose levels and insulin resistance in seven patients with generalized lipodystrophy (13). Similarly, the treatment with metreleptin in the present case appeared to improve serum triglyceride levels and insulin resistance. Metreleptin may have suppressed the patient’s appetite in this case because leptin suppresses appetite through acting on the central nervous system (11). His body weight did not increase during the metreleptin treatment, but began to increase after the metreleptin treatment was discontinued, suggesting that his serum triglyceride level and HOMA-IR value increased with body weight gain.
In our patient, the metreleptin treatment was discontinued because of a systemic hypersensitive event, and the treatment’s effect appeared to be reduced by this event. Since anti-metreleptin antibodies were detected in his serum, this systemic hypersensitivity and decreased efficacy may be related to the effects of antibodies to the drug. Generalized lipodystrophies develop due to the absence of leptin, and antibodies to metreleptin, an exogenous leptin, may be produced because it is easily recognized as a foreign protein in vivo or may have high antigenicity. Approximately 15–32% of patients with lipodystrophy develop a hypersensitive reaction to metreleptin (14), and up to 95% of patients develop anti-metreleptin antibodies (10). However, anti-metreleptin antibodies generally appear within 4–6 mo and then their production decreases with continued therapy, and clinical deterioration has only been observed in 4 out of 134 lipodystrophies (15).
Hyper-eosinophilia before the metreleptin treatment may have resulted in the greater severity of hypersensitivity in our case than in previous reports. It remains unclear whether lipodystrophies are correlated with hyper-eosinophilia; however, at least two reports have shown that eosinophilic pneumonitis or pancreatitis is involved in partial or generalized lipodystrophy (16, 17). Moreover, eosinophilic gastroenteritis with protein loss occurred after treatment suspension in the present case. Lipodystrophies with hyper-eosinophilia have also been suggested to exhibit more severe hypersensitivity to metreleptin. Although the underlying immunological mechanisms have not yet been elucidated, it is important to clarify whether hyper-eosinophilia is present in order to use metreleptin safely.
This is the first report of CGL4 being treated by metreleptin. Although the metreleptin treatment was suspended after a short period because of anaphylaxis, it temporarily appeared to be efficacious for CGL4 therapy, similarly to its use with other lipodystrophies; however, its efficacy may have been reduced by the production of anti-metreleptin antibodies. CGL4 is not clinically more severe than CGL1 and CGL2, but still includes the characteristic muscular dystrophy. Therefore, fibrate, a hypolipidemic agent, was not used because one of its side-effects is myopathy, and early detection of this side-effect is difficult in patients with CGL4. Moreover, CGL4 patients are unable to do therapeutic exercise, which limits treatment choices to diet alone. CGL4 is a rare disease and further studies are needed to evaluate potential treatments for CGL4.
Acknowledgments
We would like to thank Dr. Nobuyuki Murakami, who previously treated this patient in early childhood, for referring him to our hospital. We also would like to thank Covance Laboratories, Inc., Charles River Laboratories, Inc., and Aegerion Pharmaceuticals, Inc., for assessing anti-metreleptin antibodies.
References
- 1.Berardinelli W. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 1954;14: 193–204. doi: 10.1210/jcem-14-2-193 [DOI] [PubMed] [Google Scholar]
- 2.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab 2011;96: 3313–25. doi: 10.1210/jc.2011-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest 2009;119: 2623–33. doi: 10.1172/JCI38660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK, Garg A. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet A 2010;152A: 2245–53. doi: 10.1002/ajmg.a.33578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet 2010;6: e1000874. doi: 10.1371/journal.pgen.1000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwianingsih EK, Takeshima Y, Itoh K, Yamauchi Y, Awano H, Malueka RG, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010;101: 233–7. doi: 10.1016/j.ymgme.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 7.Murakami N, Hayashi YK, Oto Y, Shiraishi M, Itabashi H, Kudo K, et al. Congenital generalized lipodystrophy type 4 with muscular dystrophy: clinical and pathological manifestations in early childhood. Neuromuscul Disord 2013;23: 441–4. doi: 10.1016/j.nmd.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 8.Ardissone A, Bragato C, Caffi L, Blasevich F, Maestrini S, Bianchi ML, et al. Novel PTRF mutation in a child with mild myopathy and very mild congenital lipodystrophy. BMC Med Genet 2013;14: 89. doi: 10.1186/1471-2350-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelani M, Ahmed S, Almramhi MM, Mohamoud HS, Bakur K, Anshasi W, et al. Novel nonsense mutation in the PTRF gene underlies congenital generalized lipodystrophy in a consanguineous Saudi family. Eur J Med Genet 2015;58: 216–21. doi: 10.1016/j.ejmg.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez AJ, Mastronardi CA, Paz-Filho GJ. New advances in the treatment of generalized lipodystrophy: role of metreleptin. Ther Clin Risk Manag 2015;11: 1391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 2015;64: 24–34. doi: 10.1016/j.metabol.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346: 570–8. doi: 10.1056/NEJMoa012437 [DOI] [PubMed] [Google Scholar]
- 13.Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007;92: 532–41. doi: 10.1210/jc.2006-1546 [DOI] [PubMed] [Google Scholar]
- 14.Tchang BG, Shukla AP, Aronne LJ. Metreleptin and generalized lipodystrophy and evolving therapeutic perspectives. Expert Opin Biol Ther 2015;15: 1061–75. doi: 10.1517/14712598.2015.1052789 [DOI] [PubMed] [Google Scholar]
- 15.Chan JL, Koda J, Heilig JS, Cochran EK, Gorden P, Oral EA, et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf) 2016;85: 137–49. doi: 10.1111/cen.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith PM, Morgans ME, Clark CG, Lennard-Jones JE, Gunnlaugsson O, Jonasson TA. Lipodystrophy, pancreatitis, and eosinophilia. Gut 1975;16: 230–4. doi: 10.1136/gut.16.3.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn R, Barragry JM. Lipodystrophy and pulmonary eosinophilia. Ir J Med Sci 1981;150: 84–5. doi: 10.1007/BF02938206 [DOI] [PubMed] [Google Scholar]
- 18.Erhardt E, Foraita R, Pigeot I, Barba G, Veidebaum T, Tornaritis M, et al. IDEFICS consortium. Reference values for leptin and adiponectin in children below the age of 10 based on the IDEFICS cohort. Int J Obes 2014;38(Suppl 2): S32–8. doi: 10.1038/ijo.2014.133 [DOI] [PubMed] [Google Scholar]