Abstract
Toxoplasma gondii is an ubiquitous intracellular protozoan parasite. Mammals and birds are intermediate hosts and felid species are definitive hosts. In most human altered habitats the domestic cat is the predominant definitive host. Current knowledge of T. gondii infection in African ecosystems is limited. This study aimed to assess exposure to T. gondii in wild carnivores in the Serengeti ecosystem in East Africa. Carnivores can be infected by the consumption of tissue cysts when feeding on infected animals and by incidental ingestion of oocysts from environmental contamination. Incidental ingestion should occur regardless of a species’ diet whereas the consumption of cysts should increase the chance of infection in carnivorous species. This predicts higher seropositivity in carnivorous than in insectivorous carnivores and lower seropositivity in juvenile carnivores with a long dependency on milk than in adults. We found high seropositivity in carnivorous species: 100% (15 of 15 samples) in adult African lions, 93% (38 of 41 samples) in adult spotted hyenas and one striped hyena sample was positive, whereas all four samples from the insectivorous bat-eared fox were negative. Juvenile hyenas (11 of 19 sera) had significantly lower seropositivity than adults (38 of 41 sera). Long-term monitoring of spotted hyenas revealed no significant difference in seropositivity between two periods (1988–1992 and 2000 to 2016). Identical results were produced in lion and hyena samples by a commercial multi-species ELISA (at serum dilution 1:10) and an in-house ELISA based on a recombinant T. gondii protein (at serum dilution 1:100), making the latter a useful alternative for small amounts of serum. We suggest that diet, age and lifetime range are factors determining seropositivity in carnivores in the Serengeti ecosystem and suggest that the role of small wild felids in the spread of T. gondii in the African ecosystem warrants investigation.
Keywords: African lion, Bat-eared fox, Toxoplasma gondii, Parasite, Serengeti ecosystem, Spotted hyena
Graphical abstract
Highlights
-
•
Most Serengeti lions and spotted hyenas had anti-T. gondii antibodies.
-
•
Spotted hyenas' seropositivity remains similar in two time periods across 28 years.
-
•
The proportion of seropositive juvenile spotted hyenas was lower than in adults.
-
•
No evidence of infection in 4 wild bat-eared foxes, which are insectivorous canids.
-
•
An in-house ELISA permits the use of small amounts of serum.
1. Introduction
Toxoplasma gondii is an intracellular apicomplexan parasite with a worldwide distribution. All mammal and bird species are thought to be potential intermediate hosts and all known definitive host species belong to the family Felidae (Tenter et al., 2000). Humans can also be infected and although the outcome is typically asymptomatic, infection can cause serious health problems, and therefore T. gondii is considered to be an important zoonotic pathogen (Dubey, 2010; Robert-Gangneux and Darde, 2012).
Infection with T. gondii occurs orally either via ingestion of oocysts shed by infected felids or via the consumption of raw meat containing viable tissue cysts (Dubey, 2010). In the majority of urban landscapes worldwide, the domestic cat, Felis catus, is the most abundant definitive host species and primarily responsible for the contamination of urban areas with T. gondii oocysts (Dabritz et al., 2007; Torrey and Yolken, 2013). In landscapes where domestic cats are scarce or absent, environmental contamination by T. gondii oocysts stems mostly from wild felid species (Afonso et al., 2013; Bevins et al., 2012; Lewis et al., 2017). After an initial acute infection, domestic cats shed oocysts for a limited period of days or weeks (Afonso et al., 2006; Zulpo et al., 2018). Antibodies induced by infection are presumed to persist throughout life (Dubey et al., 1995; Afonso et al., 2006; Zarnke et al., 2001) but may decline without further antigenic restimulation, as reported in humans (Rougier et al., 2017).
Toxoplasma gondii oocysts are resilient, particularly in moist environments, thus inadvertent ingestion of oocysts in water or on food may frequently occur in highly contaminated environments (Tenter et al., 2000; VanWormer et al., 2016). This transmission route most likely explains the variable levels of T. gondii seropositivity reported in several wild herbivore species worldwide (Bartova et al., 2007; Gauss et al., 2006; Hove and Mukaratirwa, 2005; Jardine and Dubey, 1996; Kutz et al., 2001; Riemann et al., 1975). The consumption of T. gondii-infected carcasses is also an important transmission route to carnivorous mammalian and avian species, as is the consumption by humans of insufficiently cooked infected meat (Jones and Dubey, 2012). Vertical transmission of T. gondii (in utero) has also been reported from several mammals, including cats and humans (Calero-Bernal et al., 2013; Parameswaran et al., 2009; Powell and Lappin, 2001; Robert-Gangneux and Darde, 2012; Sato et al., 1993; Vargas-Villavicencio et al., 2016; Verma et al., 2016).
Currently little is known about T. gondii infection in large carnivores in protected landscapes in Africa, where domestic cats are absent or scarce. One of the largest protected landscapes in East Africa is the 25,000 km2 Serengeti-Mara ecosystem and adjacent Ngorongoro Conservation Area (Sinclair and Arcese, 1995), hereafter termed the Serengeti ecosystem. This landscape holds 26 wild carnivore species, including six felid species. The largest felid is the African lion, Panthera leo (hereafter lion), which mostly preys on wildebeest (Connochaetes taurinus), plains zebra (Equus quagga), Thomson's gazelle (Eudorcas thomsoni), African buffalo (Syncerus caffer), topi (Damaliscus korrigum), and kongoni (Alcelaphus buselaphus) and warthog (Phacochoerus aethiopicus) (Scheel and Packer, 1995). The spotted hyena (Crocuta crocuta, family Hyaenidae), is the most numerous large carnivore in the Serengeti ecosystem (Hofer and East, 1995). It is an efficient predator and scavenger that consumes a wide range of species (East and Hofer, 2013). In the Serengeti ecosystem it predominantly consumes wildebeest, plains zebra and Thomson's gazelle (Hofer and East, 1993a), and in comparison to other large carnivores, maternal input in terms of lactation is high and the period of lactation long (Hofer et al., 2016). The striped hyena (Hyaena hyaena, family Hyaenidae), is predominantly a scavenger that occurs at low densities in the Serengeti ecosystem (Kruuk, 1976). The bat-eared fox (Otocyon megalotis, family Canidae), is a small insectivorous species, that occasionally consumes small mammals and birds (Lamprecht, 1978). Currently little is known about T. gondii infections in these four species in the Serengeti ecosystem, beyond reports that none of 112 faeces from lions contained T. gondii-like oocysts (Müller-Graf, 1995) or that 12% of 33 faeces from lions contained T. gondii-like oocysts (Bjork et al., 2000), and that a single serum sample from a lion was positive for T. gondii neutralizing antibodies (Riemann et al., 1975). Measures of seropositivity in carnivores are considered a useful general index of the combined infection caused by both the presence of T. gondii in the environment (oocysts) and the consumption of tissue cysts by carnivorous species (Burrells et al., 2013; Millán et al., 2013; Zarnke et al., 2001). Inadvertent infection as a result of the ingestion of oocysts from the environment is expected in all four carnivore species, but infection from the consumption of infected prey is less likely in the insectivorous bat-eared fox than the carnivorous species and in juvenile carnivores than in adults.
Our study aims to (1) determine the proportion of wild carnivores in the Serengeti ecosystem with anti-T. gondii antibodies (2) determine whether the occurrence of anti-T. gondii antibodies was lower in juvenile spotted hyenas than adults; (3) contribute new but limited data on the number of African carnivores in European zoological gardens (zoos) with anti-T. gondii antibodies, and (4) compare results from a commercial multispecies ELISA for detecting anti-T. gondii antibodies with those from an in-house ELISA with a recombinant antigen from T. gondii, using commercially available anti-cat antibodies, with the aim of assessing whether or not the in-house ELISA with defined properties might require less serum, and hence be an attractive proposition when only limited serum is available.
2. Material and methods
2.1. Sample collection
In the Serengeti ecosystem, serum and plasma samples were mostly collected by veterinarians from animals that were anaesthetized for other purposes, such as the removal of wire snares set by bushmeat hunters (Hofer et al., 1993) between 1988 and 2016. Serum and plasma samples were also obtained opportunistically from fresh carcasses of animals killed by predators or vehicles between 1988 and 2016. All samples from species other than the spotted hyena were obtained from adult animals. In spotted hyenas, juveniles were aged at first sighting to an accuracy of ±7 days and were categorized as juvenile when less than 24 months old and as adult when 24 months of age or older (Golla et al., 1999). Juvenile spotted hyenas are dependent on milk for at least the first six months of life (Hofer et al., 2016). Infection with T. gondii via milk has been reported (but see Costa and Langoni, 2010; Powell et al., 2001) but we do not know if this occurs hyenas.
Samples were stored in liquid nitrogen (at −196 °C) or a freezer (−15 °C) in the Serengeti until transported frozen to Germany where they were stored (at −80 °C) until analysed. Sera from 7 captive carnivores from six zoos from Germany and the Netherlands (including Tierpark Berlin, Hodenhagen Zoo, Schwerin Zoo, Opel Zoo and Leipzig Zoo in Germany and Amersfoort Zoo in the Netherlands) were collected by zoo veterinarians primarily during health examinations of animals, including 3 spotted hyenas, 3 lions and one brown hyena, Hyaena brunnea.
2.2. Commercial immunological assays
A total of 35 sera (27 adults, 8 juveniles) collected from spotted hyenas in the Serengeti ecosystem between 1988 and 1992 were tested for anti-T. gondii antibodies in 1992 at the Animal Health Diagnostic Centre at Cornell University (Ithaca, New York), using protocols established by J.P. Dubey and the application of a commercial indirect haemagglutination (IHA ELISA) test (Toxoplasmosis TPM-Test indirect haemagglutination kit, Wampole Laboratories, Princeton, NJ, USA.) Subsequent comparison of this indirect haemagglutination test with a modified commercial multi-species IgG ELISA (Toxo IgG II ELISA kit, Wampole Laboratories, Princeton, NJ, USA) showed very good agreement between the results produced by the two assays using sera from 6 domestic species, including cats and dogs (Schaefer et al., 2011).
Furthermore, we screened 45 samples of serum or plasma collected from wild carnivores in the Tanzanian section of the Serengeti ecosystem between 1997 and 2016, including 25 samples (14 adults and 11 juveniles) from spotted hyenas, 15 samples from adult lions, 4 samples from adult bat-eared foxes, and one from an adult striped hyena. To these samples and to the samples from European zoos we applied the ID Screen® Toxoplasmosis Indirect Multi-species ELISA (IDVet, Grabels, France). The ID Screen® Toxoplasmosis Indirect Multi-species ELISA was used per supplier's instructions. Serum and plasma samples were diluted 1:10. Samples were considered positive if the Sample/Positive control ratio (S/P %) was higher than 50%, doubtful if the S/P % was between 40 and 50% and negative if the S/P % was below 40%. Controls were provided in the kit. We conducted blind screening with regard to species and age.
2.3. In-house ELISA
We applied an in-house ELISA to all lion, spotted hyena and stripped hyena samples, including the samples from European zoos. Expression and purification of recombinant SAG1 (rSAG1-6H) was done as follows. Briefly, C-terminally 6His-tagged SAG1 (amino acids 31–289) from pSAG1-GPI (Seeber et al., 1998) was cloned into plasmid pASG-IBA33 (IBA, Göttingen, Germany) according to the manufacturer's instructions. For expression the resulting plasmid pASG33-SAG1 was transformed into Escherichia coli SHuffle® T7 Express cells (New England Biolabs, Frankfurt am Main, Germany) together with plasmid pMJS9 (Nguyen et al., 2011) to aid in proper disulphide bonding of rSAG1-6H. After induction of expression with 0.5% arabinose and 200 ng/ml anhydrotetracycline for 18 h at 30 °C, rSAG1-6H protein was purified using a HisTrap FF 1 ml column on an ÄktaPurifier FPLC system essentially as described by the manufacturer (GE Healthcare, Chicago, USA). Finally, buffer was exchanged to PBS on a PD10 column (GE Healthcare) before the protein was stored at −20 °C until further use. Protein concentration was determined using the BCA assay (Thermo Fisher, Darmstadt, Germany). Protein purity was assessed by SDS-polyacrylamide gel electrophoresis, silver staining and immunoblot using anti-His tag antibodies and found to be ∼95% pure.
For the ELISA MaxiSorp® plates (Thermo Fisher) were coated overnight at 4 °C with 100 ng of rSAG1-6H per well or PBS as control. All further steps were performed at room temperature. Unspecific binding of serum to the plate was blocked by incubation with 5% soluble milk powder in PBS for 1 h. Then sera from lions and hyenas were serially diluted 1:100, 1:200 and 1:400, added in duplicates to wells and incubated for 90 min. As positive and negative controls we used seropositive plasma and seronegative serum from domestic cats (kindly provided by G. Schares; Friedrich-Loeffler-Institut, Riems, Germany). Controls were used at a dilution of 1:2000. As secondary antibody we used a peroxidase-conjugated goat anti-cat IgG (H + L) at a dilution 1:4000 (KPL, Gaithersburg, MD, USA). SureBlue® TMB Peroxidase substrate (KPL, Gaithersburg, MD, USA) was added and the reaction stopped after 10 min by adding sulphuric acid. The resulting colour signal measured at 450 nm (650 nm reference) at a Tecan Infinite M200 PRO reader. Samples were considered positive when the value was higher than the mean from two independent experiments +3 standard deviations of negative cat or hyena sera of the same dilution.
2.4. Data analysis
Statistical analyses were performed in R, version 3.3.0 (R core team, 2016). Proportions were calculated with the Clopper-Pearson confident intervals (CI) at confidence level of 0.95 using the package DescTools version 0.99.24 (Signorell et al., 2018). We tested for differences between groups using the Pearson's chi-squared test (R core team, 2018). In all statistical tests, the significance threshold was fixed at 5% and all tests were two-tailed.
3. Results
3.1. Anti-T. gondii antibodies in carnivores in the Serengeti ecosystem
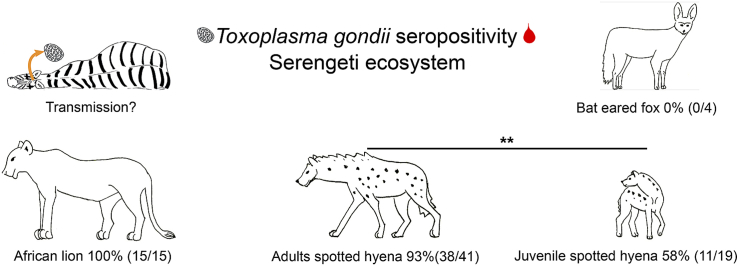
Results from a commercial IHA ELISA revealed that most serum/plasma samples from spotted hyenas between 1988 and 1992 had anti-T. gondii antibodies, including 6 of 8 samples (75%, CI: 35–97%) from juveniles and 24 of 27 samples (89%, CI: 71–98%) from adults. Results from a commercial ID Screen® indirect multi-species ELISA for samples from spotted hyenas obtained between 2000 and 2016 revealed that 5 of 11 samples (45%, CI: 17–77%) from juveniles and 14 of 14 samples (100%, CI: 77–100%) from adults had anti-T. gondii antibodies. As we found no significant difference in the results from adults sampled in these two periods (Chi-squared test, χ2 = 0.44, df = 1, p = 0.51) or for those from juveniles in these two periods (χ2 = 0.67, df = 1, p = 0.41) we combined the results for adults from the two periods, and the results from juveniles in the two periods. Overall, these combined results revealed that 11 of 19 juvenile (58%, CI: 33–80% %) and 38 of 41 adult (93%, CI: 80–98%) spotted hyenas had anti-T. gondii antibodies. Juveniles were less likely to have anti-T. gondii antibodies than adults (χ2 = 8.30, df = 1, p = 0.004).
In total we investigated 20 serum samples from adults in three additional carnivore species in the Serengeti ecosystem between 1997 and 2005, using the ID Screen® indirect multi-species ELISA. The results revealed that all 15 sera (100%, CI: 78–100%) from adult lions and one sample from a striped hyena had anti-T. gondii antibodies whereas sera from four bat-eared foxes were all negative (0% CI: 0–60%). There was no difference in the occurrence of anti-T. gondii antibodies in samples from adult spotted hyenas and adult lions (χ2 = 0.17, df = 1, p = 0.68).
Results from the ID Screen® indirect multi-species ELISA for seven samples from captive carnivores (3 spotted hyenas, 3 lions and one brown hyena) in European zoos were all positive (Table 1, Table 2).
Table 1.
Anti-Toxoplasma gondii antibodies in African lions in zoos and in natural populations worldwide. N, number of samples tested. IFAT, indirect fluorescent antibody test; LAT, latex agglutination test; MTA, modified agglutination test; ELISA, Enzyme-Linked ImmunoSorbent Assay; * ID-Screen T. gondii ELISA also used in this study; N, number of samples tested.
| Location | Positive/N (%) | Test used | Reference | Captive/Wild |
|---|---|---|---|---|
| Germany | 3/3 (100) | ELISA* | Current study | Captive |
| Brazil | 5/9 (56) | IFAT | André et al. (2010) | Captive |
| Brazil | 14/27 (52) | MAT | Silva et al. (2001) | Captive |
| China | 6/6 (100) | MAT | Yang et al. (2017) | Captive |
| Czech Republic | 2/2 (100) | IFAT | Sedlak and Bártová (2006) | Captive |
| Italy | 13/14 (93) | IFAT | Marková et al. (2018) | Captive |
| Mexico | 7/7 (100) | MAT | Alvarado-Esquivel et al. (2013) | Captive |
| Romania | 3/3 (100) | ELISA* | Dărăbuş et al. (2014) | Captive |
| Senegal | 3/7 (43) | ELISA* | Kamga-Waladjo et al. (2009) | Captive |
| South Africa | 10/14 (71) | IFAT | Cheadle et al. (1999) | Captive |
| Thailand | 1/7 (14) | LAT | Thiangtum et al. (2006) | Captive |
| USA | 12/22 (55) | MAT | de Camps et al. (2008) | Captive |
| USA | 8/10 (80) | IFAT | Spencer et al. (2003) | Captive |
| Tanzania (Serengeti NP) | 15/15 (100) | ELISA* | Current study | Wild |
| South Africa (Kruger NP) | 12/12 (100) | IFAT | Penzhorn et al. (2002) | Wild |
| Zimbabwe | 21/21 (100) | IFAT | Penzhorn et al. (2002) | Wild |
| Botswana | 49/53 (92) | IFAT | Penzhorn et al. (2002) | Wild |
| South Africa (Hluhluwe-Umfolozi NP) | 30/30 (100) | IFAT | Penzhorn et al. (2002) | Wild |
| Namibia | 65/66 (98) | ELISA | Spencer and Morkel (1993) | Wild |
Table 2.
Anti-Toxoplasma gondii antibodies in Hyaenidae previously reported in the literature. N, number of samples tested; SFDT, Sabin-Feldman dye test; IFA, immunofluorescence assay; MAT, modified agglutination test; ELISA, Enzyme-Linked ImmunoSorbent Assay; * ID-Screen T. gondii ELISA. Spotted hyena (Crocuta crocuta), Brown hyena (Parahyaena brunnea), Striped hyena (Hyaena hyaena).
| Hyena species | Location | Positive/N (%) | Test | Reference |
|---|---|---|---|---|
| Spotted hyena | Zoos in Germany and Netherlands | 3/3 (100) | ELISA* | Current study |
| Brown hyena | Zoo in Germany | 1/1 (100) | ELISA* | Current study |
| Spotted hyena (juveniles) | free ranging, Serengeti NP (Tanzania) | 11/19 (58) | ELISA* | Current study |
| Spotted hyena (adults) | free ranging, Serengeti NP (Tanzania) | 34/41 (93) | ELISA* | Current study |
| Spotted hyena | free ranging, Kenya | 6/6 (100) | SFDT | Bakal et al. (1980) |
| Brown hyena | Zoos in Czech Republic | 3/3 (100) | IFA | Sedlak and Bártová (2006) |
| Striped hyena | Zoo in France | 1/2 (50) | MAT | Alerte (2008) |
| Spotted hyena | Zoo in France | 1/1 (100) | MAT | Alerte (2008) |
| Striped hyena | Breeding Centre for Endangered Arabian Wildlife, UAE | 3/6 (50) | MAT | Dubey et al. (2010) |
| Spotted hyena | Zoos in Australia | 5/10 (50) | IFA | Wait et al. (2015) |
3.2. Comparison of an in-house ELISA assay with a commercial multi-species ELISA
We assessed a total of 48 samples obtained from lions, spotted hyenas and a striped hyena between 2000 and 2016 in the Serengeti ecosystem and from captive lions, spotted hyenas and a brown hyena, for the presence or absence of anti-T. gondii antibodies using the commercial ID Screen® indirect multi-species ELISA kit and our in-house ELISA. The results in terms of the antibody being detected or not for each of these 48 samples were identical, even though for the commercial ID Screen® indirect multi-species ELISA we applied a serum dilution of 1:10 whereas for the in-house ELISA we used a serum dilution of 1:100.
4. Discussion
Our study revealed that a high proportion of large carnivores in the Serengeti ecosystem had anti-T. gondii antibodies but none of the four samples from the insectivorous bat-eared fox had positive titres. Juvenile spotted hyenas had positive titres less often than adults. The proportion of adult spotted hyenas with anti-T. gondii antibodies in the study population was similar during two sampling periods: from 1988 to 1992 and from 2000 to 2016. In line with previous studies (Table 1, Table 2) we found high T. gondii seropositivity in large African carnivores in zoos.
Our long-term survey of anti-T. gondii antibodies in spotted hyenas in the Serengeti ecosystem revealed that 93% of 41 sera from adults were positive. The only previous report we are aware of found that all of six sera from wild spotted hyenas in Kenya were positive (Bakal et al., 1980). We found that juvenile spotted hyenas in the Serengeti ecosystem were less often seropositive (58% of 19 sera from juveniles) than adults. This in part may be because juveniles in our study population are dependent on milk for the first six months of life and are weaned between 12 and 18 months of age (Hofer et al., 2016; Hofer and East, 1995). As a result, juveniles are less likely to consume T. gondii infected tissue than adults. Also, the likelihood of exposure to a pathogen can increase with age and life-time range, as illustrated by the increase with age in seropositivity to rabies specific virus-neutralizing antibodies in our study population (East et al., 2001). Juveniles remain at communal dens until approximately 12 months of age (Hofer and East, 1993b) and hence their life-time range during their first year of life is small. Incidental contamination of communal den areas with oocysts is probably lower than in other areas in the ecosystem where felids more often deposit faeces, thus chance encounters with contaminated areas should increase with life-time range. In other mammalian (humans, sheep) intermediate hosts and definitive hosts (domestic cats and wild felids), age-dependent increases in seropositivity has been reported (Afonso et al., 2010; Dubey, 2009; Wilking et al., 2016; Zarnke et al., 2001). An age-dependent increase in seropositivity has also been reported in European avian species (Cabezón et al., 2011). We speculate that the consumption of infected tissue probably induces anti-T. gondii antibodies in spotted hyenas more often than the incidental ingestion of oocysts.
Toxoplasma gondii seropositivity in wild populations of the two other carnivorous species in the family Hyaenidae, the striped hyena and brown hyena, is unknown. We found that serum from one adult striped hyena in the Serengeti ecosystem was positive and that most spotted, striped and brown hyenas held in zoos (Table 2) have anti-T. gondii antibodies. Sera from all four bat-eared foxes, an insectivorous canid, in the Serengeti ecosystem were seronegative. To our knowledge, T. gondii seropositivity in the insectivorous member of the family Hyaenidae, the aardwolf (Proteles cristatus) is unknown, but it may be that seropositivity in aardwolves is lower than in carnivorous hyena species. Consistent with the idea of the importance of diet, carnivorous birds have higher T. gondii seropositivity than other bird species (Cabezón et al., 2011), and carnivorous mammals in zoos and circuses in Italy have higher seropositivity than herbivorous mammals (Marková et al., 2018).
In line with studies on lion sera from other locations in Africa (Table 1) we found that all of 15 sera from adult lions in the Serengeti ecosystem contained anti-T. gondii antibodies. Whether lions shed oocysts during a relatively short period after an initial acute infection, as in the domestic cat (Afonso et al., 2006; Zulpo et al., 2018) is not known. Standard coprological examination of samples from lions in the Serengeti ecosystem has revealed either the presence of T. gondii-like oocysts in 12% of fecal samples (Bjork et al., 2000) or the absence of oocysts in faeces (Müller-Graf, 1995). Studies on both the Eurasian lynx (Lynx lynx) and Canadian lynx (Lynx canadensis), also report an absence of T. gondii oocysts in faeces (Ryser-Degiorgis et al., 2006; Simon et al., 2013). Little if anything is known on T. gondii infection and oocyst shedding by the three smaller felid species in the Serengeti ecosystem: the serval (Felis serval), the caracal (Felis caracal) and the African wild cat (Felis sylvestris) or in the rodent and bird prey species of these three species (Hunter and Bowland, 2013; Stuart and Stuart, 2013; Stuart et al., 2013).
Information on T. gondii seropositivity in African wild ungulates provides information on environmental contamination, as herbivores are probably infected by the incidental consumption of oocysts in the environment while grazing or drinking water. In the Serengeti ecosystem, plains zebras are consumed by large predators (Grange et al., 2004) and 3.6% of 29 sera from plains zebra have anti-T. gondii antibodies (Riemann et al., 1975). In Zimbabwe, a wide range of seropositivity (between 10% and 90%) has been reported in wild ungulates (Hove and Dubey, 1999; Hove and Mukaratirwa, 2005). Research is required to investigate the factors determining the occurrence of both anti-T. gondii antibodies and tissue cysts in wild ungulate species, to better understand the relative importance of environmental contamination and the consumption of tissue cysts as transmission routes for T. gondii to definitive felid hosts and intermediate carnivorous mammals and bird hosts. As none of four sera from the insectivorous bat-eared fox were seropositive we cautiously interpret the results from this small samples to suggest that environmental contamination with T. gondii oocysts in the habitats occupied by these bat-eared foxes was low and the chance of infection through the consumption of prey (mostly insects and occasional small birds and mammals) was also low.
Our current knowledge of T. gondii epidemiology in ecosystems, such as the Serengeti, that contain many wild felid species and few domestic cats is limited. If we assume that all six wild felid species in the Serengeti ecosystem are infected with T. gondii and that oocyst shedding is mostly restricted to a limited period following a primary infection, as in the domestic cat, then it could be argued that because of their shorter lifespans, smaller ranges and generally higher densities (Hunter and Bowland, 2013; Stuart and Stuart, 2013; Stuart et al., 2013), small felid species (the African wild cat, serval and caracal) might be expected to contribute more to environmental contamination than the larger felid species (lion, leopard and cheetah) (Caro, 2013; Hunter et al., 2013; West and Packer, 2013).
The current worldwide genetic diversity of T. gondii has been classified into three clonal lineages (named type I, II, or III), that are three of 16 described haplogroups belonging to six major clades (Su et al., 2012). Currently little is known about the genetic diversity of T. gondii strains in Africa, particularly from East Africa (Galal et al., 2017). This lack of information on T. gondii in this region of Africa is also apparent in the Global Mammal Parasite Database (Stephens et al., 2017). Of 568 entries specifying T. gondii as a parasite species, only 28 are from Africa, and none are from East Africa.
Even though experimental infection of laboratory mice with two T. gondii strains of different genotypes has been reported (Brandão et al., 2009; Burrells et al., 2013), it is currently unclear whether this occurs in wild mammalian hosts. Infection with more than one T. gondii genotype challenges the view that T. gondii induces life-long immunity (Zulpo et al., 2018). Analysing tissues known to harbour T. gondii cysts (brain, heart, muscles) from lions and hyena carcasses that potentially sampled a collection of genotypes from infected prey could give insights into the genetic diversity of T. gondii in the Serengeti ecosystem.
A commercial multi-species Toxoplasmosis ELISA kit that is based on the major T. gondii antigen SAG1 was previously shown to work with sera from domestic cats and dogs (Roqueplo et al., 2011) as well as lions (Dărăbuş et al., 2014; Kamga-Waladjo et al., 2009). We showed that this kit and our in-house ELISA, using recombinant SAG1 and commercially available anti-cat antibodies, provided identical results with respect to seropositivity. The in-house ELISA has the advantage of demonstrating seropositivity at dilutions of one or more orders of magnitude higher than the commercial kit. Our ELISA is both cost-effective since it only requires cheap reagents, i.e. antigen and antibody, and is resource-saving as much less serum is required to obtain identical qualitative results. The latter is of importance when serum from wild animals (which can be time consuming and costly to obtain) need to be screened for evidence of exposure to multiple pathogens. Given the recent advances in the use of smartphones as colorimetric readers for ELISA-based assays (Vashist et al., 2015; Vashist and Luong, 2018) together with smartphone apps that can subsequently analyse the data we envisage that cheap in-house ELISAs like ours could be used to provide useful results on the relationship between T. gondii and wildlife in the field.
5. Permits
The research was approved by The Ethics Committee of Leibnitz Institute for Zoo and Wildlife Research (permit number 1997-02-01). Samples from lions in Tanzania were transported with CITES permits issued by the relevant Tanzanian and German Authorities. Research in Tanzania was conducted under clearance granted by the Tanzania Commission for Science and Technology (COSTECH) to HH, SCMF and MLE.
Acknowledgements
We thank the Tanzania Wildlife Research Institute, Tanzanian National Park Authority and COSTECH for their support of our research. SCMF and FT are supported by and SCMF, FT, HH, FS and MLE are members of the Research Training Group 2046 “Parasite Infections: From Experimental Models to Natural Systems” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 251133687/GRK 2046. We thank the DFG (grant EA 5/3-1), the Leibniz-Institute for Zoo and Wildlife Research, the Robert Koch-Institute, the Georg und Agnes Blumenthal-Stiftung and the Max-Planck-Institute for Behavioral Physiology for financial assistance. We thank Klaus Eulenberger, Frank Göritz, Robert Hermes, Thomas Hildebrandt, Gisela von Hegel, Richard Hoare, Mark Jago, Titus Mlengeya, Henning Wiesner, Raymond van der Meer and Harald Wiik for veterinary advice, Mauridi Mdaki and Justine Shamanche at the Serengeti Wildlife Research Centre, and also Annie Francis, Stephan Karl, Sonja Metzger, Thomas Shabani and Dagmar Thierer for assistance. We are grateful to Gereon Schares for cat sera, Lloyd W. Ruddock for plasmids and Timo Schippers for his valuable comments on the manuscript. We thank the editor and two anonymous reviewers for their helpful and constructive comments.
References
- Afonso E., Germain E., Poulle M.L., Ruette S., Devillard S., Say L., Villena I., Aubert D., Gilot-Fromont E. Environmental determinants of spatial and temporal variations in the transmission of Toxoplasma gondii in its definitive hosts. Int J Parasitol Parasites Wildl. 2013;2:278–285. doi: 10.1016/j.ijppaw.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso E., Thulliez P., Gilot-Fromont E. Local meteorological conditions, dynamics of seroconversion to Toxoplasma gondii in cats (Felis catus) and oocyst burden in a rural environment. Epidemiol. Infect. 2010;138:1105–1113. doi: 10.1017/S0950268809991270. [DOI] [PubMed] [Google Scholar]
- Afonso E., Thulliez P., Gilot-Fromont E. Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus) Int. J. Parasitol. 2006;36:1373–1382. doi: 10.1016/j.ijpara.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Alerte V.C.N. Ecole Nationale Vétérinaire de Toulouse; Toulouse: 2008. Prévalence de Toxoplasma gondii sur les animaux d’un parc zoologique (Amneville) : séroprévalence et isolement du parasite. Doctoral dissertation. [Google Scholar]
- Alvarado-Esquivel C., Gayosso-Dominguez E.A., Villena I., Dubey J.P. Seroprevalence of Toxoplasma gondii infection in captive mammals in three zoos in Mexico City, Mexico. J. Zoo Wildl. Med. 2013;44:803–806. doi: 10.1638/2013-0032.1. [DOI] [PubMed] [Google Scholar]
- André M.R., Adania C.H., Teixeira R.H.F., Silva K.F., Jusi M.M.G., Machado S.T.Z., de Bortolli C.P., Falcade M., Sousa L., Alegretti S.M., Felippe P.A.N., Machado R.Z. Antibodies to Toxoplasma gondii and Neospora caninum in captive neotropical and exotic wild canids and felids. J. Parasitol. 2010;96:1007–1009. doi: 10.1645/GE-2502.1. [DOI] [PubMed] [Google Scholar]
- Bakal P.M., Karstad L., In ’T Veld N. Serologic evidence of toxoplasmosis in captive and free-living wild mammals in Kenya. J. Wildl. Dis. 1980;16:559–564. doi: 10.7589/0090-3558-16.4.559. [DOI] [PubMed] [Google Scholar]
- Bartova E., Sedlak K., Pavlik I., Literak I. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in wild ruminants from the countryside or captivity in the Czech Republic. J. Parasitol. 2007;93:1216–1218. doi: 10.1645/GE-1126R.1. [DOI] [PubMed] [Google Scholar]
- Bevins S.N., Carver S., Boydston E.E., Lyren L.M., Alldredge M., Logan K.A., Riley S.P., Fisher R.N., Vickers T.W., Boyce W., Salman M., Lappin M.R., Crooks K.R., VandeWoude S. Three pathogens in sympatric populations of pumas, bobcats, and domestic cats: implications for infectious disease transmission. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork K.E., Averbeck G.A., Stromberg B.E. Parasites and parasite stages of free-ranging wild lions (Panthera leo) of northern Tanzania. J. Zoo Wildl. Med. 2000;31:56–61. doi: 10.1638/1042-7260(2000)031[0056:PAPSOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Brandão G.P., Melo M.N., Gazzinelli R.T., Caetano B.C., Ferreira A.M., Silva L.A., Vitor R.W.A. Experimental reinfection of BALB/c mice with different recombinant type I/III strains of Toxoplasma gondii: involvement of IFN-γ and IL-10. Mem. Inst. Oswaldo Cruz. 2009;104(2):241–245. doi: 10.1590/S0074-02762009000200017. [DOI] [PubMed] [Google Scholar]
- Burrells A., Bartley P.M., Zimmer I.A., Roy S., Kitchener A.C., Meredith A., Wright S.E., Innes E.A., Katzer F. Evidence of the three main clonal Toxoplasma gondii lineages from wild mammalian carnivores in the UK. Parasitology. 2013;140:1768–1776. doi: 10.1017/S0031182013001169. [DOI] [PubMed] [Google Scholar]
- Cabezón O., García-Bocanegra I., Molina-López R., Marco I., Blanco J.M., Höfle U., Margalida A., Bach-Raich E., Darwich L., Echeverría I., Obón E., Hernández M., Lavín S., Dubey J.P., Almería S. Seropositivity and risk factors associated with Toxoplasma gondii infection in wild birds from Spain. PLoS One. 2011;6:1–7. doi: 10.1371/journal.pone.0029549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero-Bernal R., Gómez-Gordo L., Saugar J.M., Frontera E., Pérez-Martín J.E., Reina D., Serrano F.J., Fuentes I. Congenital toxoplasmosis in wild boar (Sus scrofa) and identification of the Toxoplasma gondii types involved. J. Wildl. Dis. 2013;49:1019–1023. doi: 10.7589/2013-01-024. [DOI] [PubMed] [Google Scholar]
- Caro T. Acinonyx jubatus cheetah. In: Kingdon J., Hoffmann M., editors. Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses. Bloomsbury Publishing Plc; London, United Kingdom: 2013. pp. 187–196. [Google Scholar]
- Cheadle M.A., Spencer J.A., Blagburn B.L. Seroprevalences of Neospora caninum and Toxoplasma gondii in nondomestic felids from southern Africa. J. Zoo Wildl. Med. 1999;30:248–251. [PubMed] [Google Scholar]
- Costa V.M., Langoni H. Detection of Toxoplasma gondii in the milk of experimentally infected Wistar female rats. J. Venom. Anim. Toxins incl. Trop. Di. 2010;16(2):368–374. doi: 10.1590/S1678-91992010000200016. [DOI] [Google Scholar]
- Dabritz H.A., Miller M.A., Atwill E.R., Gardner I.A., Leutenegger C.M., Melli A.C., Conrad P.A. Detection of Toxoplasma gondii-like oocysts in cat feces and estimates of the environmental oocyst burden. J. Am. Vet. Med. Assoc. 2007;231:1676–1684. doi: 10.2460/javma.231.11.1676. [DOI] [PubMed] [Google Scholar]
- Dărăbuş G., Afrenie M., Hotea I., Imre M., Morariu S. Endoparasites in mammals from seven zoological gardens in Romania. J. Zoo Wildl. Med. 2014;45:239–246. doi: 10.1638/2012-0170.1. [DOI] [PubMed] [Google Scholar]
- de Camps S., Dubey J.P., Saville W.J.A. Seroepidemiology of Toxoplasma gondii in zoo animals in selected zoos in the midwestern United States. J. Parasitol. 2008;94:648–653. doi: 10.1645/GE-1453.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. second ed. CRC Press; Boca Raton, FL: 2010. Toxoplasmosis of Humans and Animals. [Google Scholar]
- Dubey J.P. Toxoplasmosis in sheep - the last 20 years. Vet. Parasitol. 2009;163:1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Dubey J., Lappin M., Thulliez P. Long-term antibody responses of cats fed Toxoplasma gondii tissue cysts. J. Parasitol. 1995;81(6):887–893. doi: 10.2307/3284035. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Pas A., Rajendran C., Kwok O.C.H., Ferreira L.R., Martins J., Hebel C., Hammer S., Su C. Toxoplasmosis in sand cats (Felis margarita) and other animals in the breeding Centre for endangered arabian wildlife in the United Arab Emirates and Al Wabra wildlife preservation, the state of Qatar. Vet. Parasitol. 2010;172:195–203. doi: 10.1016/j.vetpar.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M.L., Hofer H. Crocuta crocuta spotted hyena. In: Kingdon J., Hoffmann M., editors. Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses. Bloomsbury Pub.; 2013. pp. 273–281. [Google Scholar]
- East M.L., Hofer H., Cox J.H., Wulle U., Wiik H., Pitra C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15026–15031. doi: 10.1073/pnas.261411898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal L., Ajzenberg D., Hamidović A., Durieux M.-F., Dardé M.-L., Mercier A. Toxoplasma and Africa: one parasite, two opposite population structures. Trends Parasitol. 2017;34(2):140–154. doi: 10.1016/j.pt.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Gauss C.B.L., Dubey J.P., Vidal D., Cabezón O., Ruiz-Fons F., Vicente J., Marco I., Lavin S., Gortazar C., Almería S. Prevalence of Toxoplasma gondii antibodies in red deer (Cervus elaphus) and other wild ruminants from Spain. Vet. Parasitol. 2006;136:193–200. doi: 10.1016/j.vetpar.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Golla W., Hofer H., East M.L. Within-litter sibling aggression in spotted hyaenas: effect of maternal nursing, sex and age. Anim. Behav. 1999;58:715–726. doi: 10.1006/anbe.1999.1189. [DOI] [PubMed] [Google Scholar]
- Grange S., Duncan P., Gaillard J.M., Sinclair A.R.E., Gogan P.J.P., Packer C., Hofer H., East M. What limits the Serengeti zebra population? Oecologia. 2004;140:523–532. doi: 10.1007/s00442-004-1567-6. [DOI] [PubMed] [Google Scholar]
- Hofer H., Benhaiem S., Golla W., East M.L. Trade-offs in lactation and milk intake by competing siblings in a fluctuating environment. Behav. Ecol. 2016;27:1567–1578. doi: 10.1093/beheco/arw078. [DOI] [Google Scholar]
- Hofer H., East M. Population dynamics, population size, and the commuting system of Serengeti spotted hyenas. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press; 1995. pp. 332–363. [Google Scholar]
- Hofer H., East M.L. The commuting sytem of Serengeti spotted hyenas: how a predator copes with migratory prey. I. Social organization. Anim. Behav. 1993;46:547–557. [Google Scholar]
- Hofer H., East M.L. The commuting system of Serengeti spotted hyaenas how a predator copes with migratory prey. III. Attendance and maternal care. Anim. Behav. 1993;46:575–589. [Google Scholar]
- Hofer H., East M.L., Campbell K.L.I. Symposia of the Zoological Society of London. 1993. Snares, commuting hyaenas and migratory herbivores: humans as predators in the Serengeti; pp. 347–366. [Google Scholar]
- Hove T., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in sera of domestic pigs and some wild game species from Zimbabwe. J. Parasitol. 1999;85:372–373. [PubMed] [Google Scholar]
- Hove T., Mukaratirwa S. Seroprevalence of Toxoplasma gondii in farm-reared ostriches and wild game species from Zimbabwe. Acta Trop. 2005;94:49–53. doi: 10.1016/j.actatropica.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hunter L., Bowland J. Serval leptailurus serval. In: Kingdon J.S., Hoffman M., editors. vol. 5. Bloomsbury Publishing Plc; London, United Kingdom: 2013. pp. 180–185. (Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses). [Google Scholar]
- Hunter L., Henschel P., Ray J.C. Panthrea pardus leopard. In: Kingdon J., Hoffmann M., editors. Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses. Bloomsbury Publishing Plc; London, United Kingdom: 2013. pp. 159–168. [Google Scholar]
- Jardine J.E., Dubey J.P. Systemic infection with an unidentified Toxoplasma-like Protozoan in a neonatal Lichtenstein's hartebeest (Sigmoceros lichtensteinii) J. Parasitol. 1996;82:515–517. doi: 10.2307/3284099. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Dubey J.P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012;55:845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- Kamga-Waladjo A.R., Gbati O.B., Kone P., Lapo R.A., Dombou E., Chatagnon G., Bakou S.N., Diop P.E.H., Pangui L.J., Tainturier D., Akakpo J.A. Neospora caninum and Toxoplasma gondii in lion (Panthera leo) from Senegal, west Africa. Asian J. Anim. Vet. Adv. 2009;4:346–349. doi: 10.3923/ajava.2009.346.349. [DOI] [Google Scholar]
- Kruuk H. Feeding and social behaviour of the striped hyaena (Hyaena vulgaris Desmarest) Afr. J. Ecol. 1976;14:91–111. [Google Scholar]
- Kutz S.J., Elkin B.T., Panayi D., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in Barren-Ground caribou (Rangifer tarandus groenlandicus) from the Canadian Arctic. J. Parasitol. 2001;87:439–442. doi: 10.1645/0022-3395(2001)087[0439:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lamprecht J. Diet, foraging behavior and interspecific food competition of jackals in Serengeti-National-Park, East-Africa. Zeitschrift für Säugetierkd. 1978;43(4):210–223. [Google Scholar]
- Lewis J.S., Logan K.A., Alldredge M.W., Carver S., Bevins S.N., Lappin M., VandeWoude S., Crooks K.R. The effects of demographic, social, and environmental characteristics on pathogen prevalence in wild felids across a gradient of urbanization. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187035. e0187035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marková J., Machačová T., Bártová E., Sedlák K., Budíková M., Silvestre P., Laricchiuta P., Russo M., Veneziano V. Toxoplasma gondii, Neospora caninum and Encephalitozoon cuniculi in animals from captivity (zoo and circus animals) J. Eukaryot. Microbiol. 2018 doi: 10.1111/jeu.12688. [DOI] [PubMed] [Google Scholar]
- Millán J., Chirife A.D., Kalema-Zikusoka G., Cabezón O., Muro J., Marco I., Cliquet F., León-Vizcaíno L., Wasniewski M., Almería S., Mugisha L. Serosurvey of dogs for human, livestock, and wildlife pathogens, Uganda. Emerg. Infect. Dis. 2013;19:680–682. doi: 10.3201/eid1904.121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Graf C.D. A coprological survey of intestinal parasites of wild lions (Panthera leo) in the Serengeti and the Ngorongoro Crater, Tanzania, east Africa. J. Parasitol. 1995;81:812–814. [PubMed] [Google Scholar]
- Nguyen V.D., Hatahet F., Salo K.E.H., Enlund E., Zhang C., Ruddock L.W. Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E.coli. Microb. Cell Factories. 2011;10:1. doi: 10.1186/1475-2859-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., O'Handley R.M., Grigg M.E., Wayne A., Thompson R.C.A. Vertical transmission of Toxoplasma gondii in Australian marsupials. Parasitology. 2009;136:939–944. doi: 10.1017/S0031182009006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzhorn B.L., Stylianides E., van Vuuren M., Alexander K., Meltzer D.G.A., Mukarati N. Seroprevalence of Toxoplasma gondii in free-ranging lion and leopard populations in southern Africa. S. Afr. J. Wildl. Res. 2002;32:163–165. doi: 10.2307/4356078?ref=no-x-route:178e9888bd4160cfc88999e87f69c73a. [DOI] [Google Scholar]
- Powell C.C., Brewer M., Lappin M.R. Detection of Toxoplasma gondii in the milk of experimentally infected lactating cats. Vet. Parasitol. 2001;102:29–33. doi: 10.1016/S0304-4017(01)00521-0. [DOI] [PubMed] [Google Scholar]
- Powell C.C., Lappin M.R. Clinical ocular toxoplasmosis in neonatal kittens. Vet. Ophthalmol. 2001;4:87–92. doi: 10.1046/j.1463-5224.2001.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann G.P., Burridge M.J., Behymer D.E., Franti C.E. Toxoplasma gondii antibodies in free-living African mammals. J. Wildl. Dis. 1975;11:529–533. doi: 10.7589/0090-3558-11.4.529. [DOI] [PubMed] [Google Scholar]
- Robert-Gangneux F., Darde M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roqueplo C., Halos L., Cabre O., Davoust B. Toxoplasma gondii in wild and domestic animals from New Caledonia. Parasite. 2011;18:345–348. doi: 10.1051/parasite/2011184345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier S., Montoya J.G., Peyron F. Lifelong persistence of Toxoplasma cysts: a questionable dogma? Trends Parasitol. 2017;33:93–101. doi: 10.1016/j.pt.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Ryser-Degiorgis M.-P., Jakubek E.-B., Hård af Segerstad C., Bröjer C., Mörner T., Jansson D.S., Lundén A., Uggla A. Serological survey of Toxoplasma gondii infection in free-ranging Eurasian lynx (Lynx lynx) from Sweden. J. Wildl. Dis. 2006;42:182–187. doi: 10.7589/0090-3558-42.1.182. [DOI] [PubMed] [Google Scholar]
- Sato K., Iwamoto I., Yoshiki K. Experimental toxoplasmosis in pregnant cats. J. Vet. Med. Sci. 1993;55:1005–1009. doi: 10.1292/jvms.55.1005. [DOI] [PubMed] [Google Scholar]
- Schaefer J.J., White H.A., Schaaf S.L., Mohammed H.O., Wade S.E. Modification of a commercial Toxoplasma gondii Immunoglobulin G Enzyme-Linked Immunosorbent Assay for use in multiple animal species. J. Vet. Diagn. Invest. 2011;23:297–301. doi: 10.1177/104063871102300215. [DOI] [PubMed] [Google Scholar]
- Scheel D., Packer C. Variation in predation by bions: tracking a movable feast. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press; 1995. pp. 299–314. [Google Scholar]
- Sedlak K., Bártová E. Seroprevalences of antibodies to Neospora caninum and Toxoplasma gondii in zoo animals. Vet. Parasitol. 2006;136:223–231. doi: 10.1016/j.vetpar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Seeber F., Dubremetz J.F., Boothroyd J.C. Analysis of Toxoplasma gondii stably transfected with a transmembrane variant of its major surface protein. SAG1. J Cell Sci. 1998;111:23–29. doi: 10.1242/jcs.111.1.23. [DOI] [PubMed] [Google Scholar]
- Signorell A. DescTools: tools for descriptive statistics. R package version 0. 2018;99:24. [Google Scholar]
- Silva J.C., Ogassawara S., Marvulo M.F., Ferreira-Neto J.S., Dubey J.P. Toxoplasma gondii antibodies in exotic wild felids from Brazilian zoos. J. Zoo Wildl. Med. 2001;32:349–351. doi: 10.1638/1042-7260(2001)032[0349:TGAIEW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Simon A., Bigras Poulin M., Rousseau A.N., Dubey J.P., Ogden N.H. Spatiotemporal dynamics of Toxoplasma gondii infection in Canadian lynx (Lynx canadensis) in western Québec, Canada. J. Wildl. Dis. 2013;49:39–48. doi: 10.7589/2012-02-048. [DOI] [PubMed] [Google Scholar]
- Sinclair A.R.E., Arcese P. Serengeti in the context of worldwide conservation efforts. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press; 1995. pp. 31–46. [Google Scholar]
- Spencer J.A., Higginbotham M.J., Blagburn B.L. Seroprevalence of Neospora caninum and Toxoplasma gondii in captive and free-ranging nondomestic felids in the United States. J. Zoo Wildl. Med. 2003;34:246–249. doi: 10.1638/02-046. [DOI] [PubMed] [Google Scholar]
- Spencer J.A., Morkel P. Serological survey of sera from lions in etosha national Park. S. Afr. J. Wildl. Res. 1993;23:60–61. [Google Scholar]
- Stephens P.R., Pappalardo P., Huang S., Byers J.E., Farrell M.J., Gehman A., Ghai R.R., Haas S.E., Han B., Park A.W., Schmidt J.P., Altizer S., Ezenwa V.O., Nunn C.L. Global mammal parasite Database version 2.0. Ecology. 2017;98:1476. doi: 10.1002/ecy.1799. [DOI] [PubMed] [Google Scholar]
- Stuart C., Stuart T. Caracal caracal caracal. In: Kingdon J., Hoffmann M., editors. vol. 5. Bloomsbury Publishing Plc; London, United Kingdom: 2013. pp. 174–179. (Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses). [Google Scholar]
- Stuart C., Stuart T., De Smet K.L. Felis silvestris Wildcat. In: Kingdon J., Hoffmann M., editors. vol. 5. Bloomsbury Publishing Plc; London, United Kingdom: 2013. pp. 206–210. (Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses). [Google Scholar]
- Su C., Khan A., Zhou P., Majumdar D., Ajzenberg D., Darde M.L., Zhu X.Q., Ajioka J.W., Rosenthal B.M., Dubey J.P., Sibley L.D. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5844–5849. doi: 10.1073/pnas.1203190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiangtum K., Nimsuphun B., Pinyopanuwat N., Chimnoi W., Tunwattana W., Tongthainan D., Jittapalapong S., Rukkwamsuk T., Maruyama S. Seroprevalence of Toxoplasma gondii in captive felids in Thailand. Vet. Parasitol. 2006;136:351–355. doi: 10.1016/j.vetpar.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Torrey E.F., Yolken R.H. Toxoplasma oocysts as a public health problem. Trends Parasitol. 2013;29:380–384. doi: 10.1016/j.pt.2013.06.001. [DOI] [PubMed] [Google Scholar]
- VanWormer E., Carpenter T.E., Sing P., Shapiro K., Wallender W.W., Conrad P.A., Largier J.L., Maneta M.P., Mazet J.A.K. Coastal development and precipitation drives pathogen flow from land to sea: evidence from a Toxoplasma gondii and felid host system. Sci. Rep. 2016;6:29252. doi: 10.1038/srep29252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Villavicencio J.A., Besne-Merida A., Correa D. Vertical transmission and fetal damage in animal models of congenital toxoplasmosis: a systematic review. Vet. Parasitol. 2016;223:195–204. doi: 10.1016/j.vetpar.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Vashist S.K., Luong J.H.T. Smartphone-nased immunoassays. In: Vashist S.K., Luong J.H.T., editors. Handbook of Immunoassay Technologies. Academic Press; 2018. pp. 433–453. [Google Scholar]
- Vashist S.K., van Oordt T., Schneider E.M., Zengerle R., von Stetten F., Luong J.H.T. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens. Bioelectron. 2015;67:248–255. doi: 10.1016/j.bios.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Verma S.K., Carstensen M., Calero-Bernal R., Moore S.A., Jiang T., Su C., Dubey J.P. Seroprevalence, isolation, first genetic characterization of Toxoplasma gondii, and possible congenital transmission in wild moose from Minnesota, USA. Parasitol. Res. 2016;115:687–690. doi: 10.1007/s00436-015-4789-0. [DOI] [PubMed] [Google Scholar]
- Wait L.F., Srour A., Smith I.G., Cassey P., Sims S.K., McAllister M.M. A comparison of antiserum and protein A as secondary reagents to assess Toxoplasma gondii antibody titers in cats and spotted hyenas. J. Parasitol. 2015;101:390–392. doi: 10.1645/14-705.1. [DOI] [PubMed] [Google Scholar]
- West P.M., Packer C. Panthera leo lion. In: Kingdon J., Hoffmann M., editors. Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses. Bloomsbury Publishing Plc; London, United Kingdom: 2013. pp. 149–159. [Google Scholar]
- Wilking H., Thamm M., Stark K., Aebischer T., Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci. Rep. 2016;6:22551. doi: 10.1038/srep22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-R., Feng Y.-J., Lu Y.-Y., Dong H., Li T.-Y., Jiang Y.-B., Zhu X.-Q., Zhang L.-X. Antibody detection, isolation, genotyping, and virulence of Toxoplasma gondii in captive felids from China. Front. Microbiol. 2017;8:1414. doi: 10.3389/fmicb.2017.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnke R.L., Dubey J.P., Ver Hoef J.M., McNay M.E., Kwok O.C.H. Serologic survey for Toxoplasma gondii in Lynx from interior Alaska. J. Wildl. Dis. 2001;37:36–38. doi: 10.7589/0090-3558-37.1.36. [DOI] [PubMed] [Google Scholar]
- Zulpo D.L., Sammi A.S., Dos Santos J.R., Sasse J.P., Martins T.A., Minutti A.F., Cardim S.T., de Barros L.D., Navarro I.T., Garcia J.L. Toxoplasma gondii: a study of oocyst re-shedding in domestic cats. Vet. Parasitol. 2018;249:17–20. doi: 10.1016/j.vetpar.2017.10.021. [DOI] [PubMed] [Google Scholar]