Abstract
Gene variants of CYP24A1, which encodes the enzyme 24-hydroxylase, are a most unusual cause of maternal hypercalcemia. Loss-of-function mutations in CYP24A1 result in impaired dehydroxylation of active vitamin D (calcitriol). Secondary to this hypercalcemia, hypercalciuria and suppressed parathyroid hormone (P-PTH) can develop. These gene-variants are most often detected in children exposed to vitamin D prophylaxis. These children develop failure to thrive, hypercalciuria, hypercalcemia, and low PTH levels. CYP24A1 variants have also been reported in adults with hypercalcemia and recurrent urolithiasis. This report describes gestational hypercalcemia in two of three sisters with combined CYP24A1 heterozygous variants.
Methods
We retrospectively investigated medical files, clinical information, and calcium levels during and after pregnancy in three sisters giving birth to nine children. All three sisters were also tested genetically.
Results
Two sisters developed hypercalcemia during all seven pregnancies and late-onset hypertension during pregnancy. These sisters had two heterozygote variants in the enzyme CYP24A1: c1186C>T and c443T>C. A third sister had the c1186C>T variant and was normocalcemic. Of the seven children born to the two sisters with combined variants, four had hypercalcemia and five had hypoglycemia as neonates. In these mothers, calcium levels slowly normalized postpartum. In the affected neonates, calcium and blood glucose levels became normal within weeks.
Conclusion
Combined variants of CYP24A1 caused long-standing gestational hypercalcemia and late-onset hypertension. In neonates, elevated serum calcium and hypoglycemia can be consequences necessitating prompt measures. CYP24A1 mutations should be considered in unexplained gestational hypercalcemia. Their combined effects during pregnancy have not been observed previously.
Keywords: maternal, gestational, hypercalcemia, CYP24A1, pregnancy, hypoglycemia
Gestational hypercalcemia is caused mostly by hyperparathyroidism. Other uncommon causes are malignancies and milk alkali syndrome [1]. In rare cases, hypercalcemia is secondary to an inactivating variant in the gene encoding the P450 CYP24A1 24-hydroxylase enzyme, which is inherited recessively. Inactivation of this enzyme reduces the conversion of 1α,25-dihydroxycholecalciferol (S-1,25 OHD) (calcitriol) to inactive 1,24,25(OH)D3 and also decreases the conversion of 25-hydroxycholecalciferol (S-25 OHD) (calcidiol) to 24,25(OH)D3. This causes increased calcitriol and calcidiol concentrations, hypercalcemia, and hypercalciuria and suppresses parathyroid hormones (P-PTHs) [2]. This was first reported in children with “idiopathic infantile hypercalcemia” (online mendelian inheritance in man 143880). Homozygous loss-of-function variants cause the most common and severe forms [3].
We retrospectively investigated three sisters, two of whom developed gestational hypercalcemia, mainly during the last trimester and postpartum. It became obvious that variants in CYP24A1 were a common denominator only after the last of nine pregnancies.
Our aim is to describe the clinical picture, symptoms, treatment, pregnancy outcomes, and postparity effects on calcium levels and blood pressure level in patients with compound mutations in this enzyme and in their children.
1. Methods
All medical files were reviewed manually for clinical information during 1997 to 2016. Laboratory data, symptoms, and treatments were registered. All three sisters were tested genetically.
Urinary calcium/24 hour, parathyroid hormone (P-PTH), S- and P-calcium, S-ionized calcium, and S-25 OHD and 1,25 OHD vitamins were analyzed according to clinical routines. Different assays were applied during this 20-year-period. These data are given in Table 1.
Table 1.
Changes in Laboratory References 1997 to 2016
| Years | References | |
|---|---|---|
| S-calcium in adults | −2005 | 2.20–2.60 mmol/L |
| P-calcium in adults | 2005− | 2.15–2.50 mmol/L |
| S-calcium in children | −2003 | 1.80–2.80 mmol/L <7 d; 2.20–2.70 mmol/L 7 d to 7 y |
| 2003–2005 | 2.20–2.60 mmol/L | |
| 2005− | 2.00–3.00 mmol/L first day; 2.10–2.60 mmol/L d 2 to 12 mo | |
| S-ionized calcium in adults | −2005 | 1.17–1.29 mmol/L |
| 2005− | 1.15–1.33 mmol/L | |
| P-PTH | −1999 | 12–72 ng/L |
| 1999–2015 | 10–65 ng/L = 1.1–6.9 pmol/L | |
| 2015− | 1.1–6.0 pmol/L | |
| S-25 OHD vitamin | −2006 | 10–50 μg/L |
| 2009− | 75–250 nmol/L | |
| S-1,25 OHD vitamin | 1995–2015 | 10–60 ng/L |
| 2015− | 60–208 pmol/L | |
| Urinary calcium/24 h | 1995− | 0.7–7.0 mmol/24 h |
Minus sign before a year indicates ongoing from previous years. Minus sign after a year indicates still ongoing.
For this type of retrospective study, formal ethical approval is not required. Informed and written consent was obtained from the included participants.
A. Genetic analysis
Exons 2 and 9 of CYP24A1 were analyzed by PCR sequencing of both DNA strands of the entire coding region and exon-intron splice junctions by Centogene AG, Rostock, Germany.
2. Results
Two of the sisters developed hypercalcemia in all of their seven gestations. Hypercalcemia was more pronounced during the third trimester and postpartum. Sister A was normocalcemic and had the heterozygous CYP24A1 variant c1186C>T (p.Arg396Trp) (class 1). Her P-PTH and vitamin D levels were normal. Her two children were also unaffected. Sisters B and C had the same gene variant and were also heterozygous carriers for another variant, c443T>C (p.Leu148Pro) (class 3). No deletions or duplications were found. Both sisters B and C developed hypercalcemia during pregnancy, which normalized within 9 months (range, 5 to 9 months). They also had hypertension at the time of parturition. Of these two sisters, four of six infants tested had hypercalcemia, and five of seven also had hypoglycemia at birth. Calcium and glucose levels normalized over time. The clinical data for these pregnancies are described below.
3. Case reports
A. Sister A
The eldest sister had no history of nephrocalcinosis or hypercalcemia. She gave birth to two healthy children, and her two pregnancies were uneventful. She is a heterozygous carrier of the CYP24A1 variant c1186C>T. During follow-up, her albumin-corrected calcium level was 2.40 mmol/L (reference interval, 2.15 to 2.50 mmol/L), S-25 OHD level was 44 nmol/L (75 to 250 nmol/L), S-1,25 OHD level was 123 pmol/L (60 to 208 pmol/L), and P-PTH level was 3.6 pmol/L (1.6 to 6.0 pmol/L). Urinary calcium/24 hour was not tested. All total calcium levels will subsequently be presented as corrected for albumin. No data on S-calcium levels in her offspring are known.
B. Sister B
Sister B presented at 23 years of age with a history of urinary stones, nephrocalcinosis, and ongoing treatment with an antidepressant drug (citalopram). No vitamin D substitution was used during any of her pregnancies. All calcium levels during her pregnancies are presented in Table 2, and her children’s calcium levels are presented in Table 3.
Table 2.
Sister B: Results of the Biochemical Test Performed During and After Her Five Pregnancies
| GW 15 | GW 35 | 3 Days |
10 Days |
20 Days |
2–3 Months |
5–6 Months |
9 Months |
References | |
|---|---|---|---|---|---|---|---|---|---|
| PostP | PostP | PostP | PostP | PostP | PostP | ||||
| Pregnancy 1 | |||||||||
| S-calcium | 4.29 | 2.89 | 2.63 | 2.66 | 2.54 | 2.20–2.60 mmol/L | |||
| S-ion cal | 2.15 | 1.44 | 1.17–1.29 mmol/L | ||||||
| P-PTH | 5 | 12–72 ng/L | |||||||
| S-25 OHD | 39 | 64 | 10–50 μg/L | ||||||
| S-1,25 OHD | 78 | 19 | 10–60 ng/L | ||||||
| Urinary calcium | 2.7 | 0.7–7.0 mmol/24 h | |||||||
| Pregnancy 2 | |||||||||
| S-calcium | 2.89 | 2.88 | 3.51 | 2.82 | 2.52 | 2.20–2.60 mmol/L | |||
| S-ion cal | 1.33 | 1.45 | 1.72 | 1.47 | 1.32 | 1.17–1.29 mmol/L | |||
| P-PTH | <7 | <7 | <7 | 10 | 10–65 ng/L | ||||
| Pregnancy 3 | |||||||||
| S-calcium | 3.31 | 3.23 | 3.22 | 2.84 | 2.64 | 2.20–2.60 mmol/L | |||
| S-ion cal | 1.63 | 1.50 | 1.39 | 1.36 | 1.17–1.29 mmol/L | ||||
| P-PTH | <7 | <7 | <10 | 10–65 ng/L | |||||
| Pregnancy 4 | |||||||||
| S-calcium | 2.88 | 2.63 | 3.49 | 2.20–2.60 mmol/L | |||||
| S-ion cal | 1.79 | 1.63 | 1.74 | 1.74 | 1.48 | 1.38 | 1.14–1.27 mmol/L | ||
| P-PTH | <6 | 10–65 ng/L | |||||||
| Pregnancy 5 | |||||||||
| P-calcium | 2.80 | 2.65 | 2.92 | 3.21 | 3.27 | 2.78 | 2.77 | 1.30 | 2.15–2.50 mmol/L |
| S-ion cal | 1.48 | 1.38 | 1.61 | 1.67 | 1.84 | 1.51 | 1.42 | 1.15–1.33 nmol/L | |
| P-PTH | 0.74 | 0.83 | 0.69 | 0.76 | 0.79 | 1.2 | 1.6–6.0 pmol/L | ||
| S-25 OHD | 128 | 86 | 67 | 77 | 83 | 129 | 81 | 75–250 nmol/L | |
| Urinary calcium/24 h | 15.5 | 0.7–7.0 mmol/24 h | |||||||
Abbreviations: GW, gestational wk; PostP, postpartum; S-ion cal, S-ionized calcium.
Table 3.
Sister B: Calcium and Postpartum Glucose Levels in Her Five Children
| At Birth | 1 Day PostP | 4–5 Days PostP | 10 Days PostP | 1 Month PostP | References | |
|---|---|---|---|---|---|---|
| S-calcium | ||||||
| Baby 1 | ||||||
| Baby 2 | 3.09 | 2.98 | 3.02 | 3.07 | 1.80–2.80 mmol/L | |
| Baby 3 | 3.15 | 2.68 | 2.77 | <7 d | ||
| Baby 4 | 2.28 | 2.28 | 2.20–2.70 mmol/L >7 d | |||
| Baby 5 | 2.56 | 2.00–3.00 mmol/L | ||||
| P-glucose | ||||||
| Baby 1 | 0.2 | 0.1 | 2.0 | 4.0 | ||
| Baby 2 | 1.6 | Normal | ||||
| Baby 3 | Normal | 3.1–5.5 mmol/L | ||||
| Baby 4 | <2.2a | 3.5 | 4.9 | Capillary: >2.6 mmol/La | ||
| Baby 5 | 2.8 | 3.4 |
No calcium tests were performed in sister B’s first child. References for children vary by age.
Abbreviation: PostP, postpartum.
Capillary blood.
B-1. First pregnancy
Three days after her first delivery in gestational week 38, sister B developed severe epigastric pain and hypertension, and her blood pressure level was 160/110 mm Hg. Late-onset preeclampsia was suspected.
Ten days after delivery, as an in-patient, sister B’s blood pressure level had normalized with labetalol 600 mg per day. Her S-creatinine level had increased from 96 (<110) to 143 µmol/L. Her condition did not improve; her calcium level at that time was 4.29 mmol/L (2.20 to 2.60 mmol/L) (Table 2).
Because hypertension and hypercalcemic crisis were present, she was transferred to the intensive care unit. She was treated with hydration, oral furosemide 40 mg × 1, and calcitonin 1 IE/kg in 500 mL NaCl. On the eleventh day after delivery, her S-calcium level was 4.0 mmol/L, P-PTH was 2 ng/L (10 to 65 ng/L), and S‒angiotensin-converting enzyme was 13.2 kE/L (8.0 to 32 kE/L). Pamidronate infusion 60 mg IV was used for 2 consecutive days. Her calcium level decreased to 3.2 mmol/L. S-electrophoresis and a bone marrow biopsy were normal. After 21 days, she was discharged from the hospital in good condition, with normal blood pressure levels without treatment. Her calcium level was 2.89 mmol/L (Table 2).
B-2. Baby B1
A girl weighing 3760 g and with Apgar scores of 9, 10, and 10 was initially tired and irritable. She had severe hypoglycemia, 0.2 mmol/L (3.1 to 5.5 mmol/L), and was admitted to the neonatal intensive care unit (NICU) (Table 3). She had convulsions, and her electroencephalography showed epileptiform spikes. No serum calcium samples were collected. Results of ultrasonography of the brain, echocardiography, and lumbar puncture were normal. Her severe hypoglycemia was difficult to treat. S-insulin was highly elevated, 780 pmol/L (<120 pmol/L). She was given a 20% glucose infusion and the KATP-channel activator diazoxide.
At 3 weeks of age, she developed necrotizing enterocolitis and received total parenteral nutrition. At discharge after 30 days, the colitis was cured, and she had normal S-insulin (25 mmol/L) and blood glucose (4.0 mmol/L) levels without treatment. She is now a healthy woman.
B-3. Second pregnancy
At pregnancy week 32, sister B’s S-calcium level was elevated (2.88 mmol/L), and her P-PTH level was immeasurably low. At gestational week 38 + 3, her hypercalcemia had increased to 3.33 mmol/L and her blood pressure level had increased to 140/90 mm Hg. She delivered vaginally without complications.
After birth, her S-calcium level was 3.51 mmol/L, and her ionized calcium level was 1.72 mmol/L. She recovered slowly. Her blood pressure level was 140/85 mm Hg.
B-4. Baby B2
A girl born in week 38 + 4 and weighing 3290 g with Apgar scores of 9, 10, and 10 had a low blood glucose level of 1.6 mmol/L, and her hypercalcemia value was 3.09 mmol/L (1.80 to 2.80 mmol/L). She was treated with 10% glucose infusions in the NICU for 4 days. Her blood glucose value recovered within days. During these days, her calcium levels were still elevated (Table 3). After 4 days, she was discharged because of her mother’s unwillingness to stay.
B-5. Third pregnancy
Two years later, sister B again developed hypercalcemia during pregnancy. Already in gestational week 18, her S-calcium level was 3.44 mmol/L with an immeasurably low P-PTH level. Nausea was present. Her condition improved after treatment with oral furosemide 40 mg, and in gestational week 23 + 3, her S-calcium level was 2.67 mmol/L. The fetus showed normal growth on ultrasonographic investigation. After gestational week 31, all medication was stopped, her calcium level was 2.97 mmol/L, and her blood pressure was normal. At gestational week 39 + 2, she delivered vaginally. Her postpartum calcium level was 3.37 mmol/L; the following day, it was 3.49 mmol/L. Pamidronate 60 mg IV was given along with oral furosemide. She was feeling well and was discharged from the hospital because of unwillingness to stay. Her blood pressure level was 115/85 mm Hg without medication.
B-6. Baby B3
A boy was born weighing 3740 g and with Apgar scores of 9, 9, and 10. Two days after delivery, his S-calcium level was 3.15 mmol/L (1.80 to 2.80 mmol/L), and it decreased after 10 days (Table 3). Observation at the NICU for 3 days was uneventful. He was doing well and had no hypoglycemia.
B-7. Fourth pregnancy
Sister B’s S-calcium level was elevated in week 15: 2.88 mmol/L. In week 36, her S-calcium level was 2.63 mmol/L with immeasurable P-PTH value; her 25 OHD vitamin level was 113 nmol/L. Her calcium level increased postpartum to 3.24 mmol/L (Table 2). Her blood pressure level during pregnancy was normal, 130/70 mm Hg, but it increased postpartum to 145/100 mm Hg. She was treated with IV fluids. Nine days postpartum, she was transferred to a medical ward because of muscle weakness, fatigue, and elevated calcium level of 3.69 mmol/L (not corrected for albumin). She received 5 L of 0.9% saline infusion, after which her calcium level decreased to 3.32 mmol/L with clinical improvement. Over the next 3 months, saline infusions were administered over a couple of 24-hour periods.
B-8. Baby B4
A boy is born weighing 3310 g and with Apgar scores of 9, 10, and 10. His S-calcium level was normal, but his blood glucose level was low (<2.2 mmol/L), which normalized within days.
B-9. Fifth pregnancy
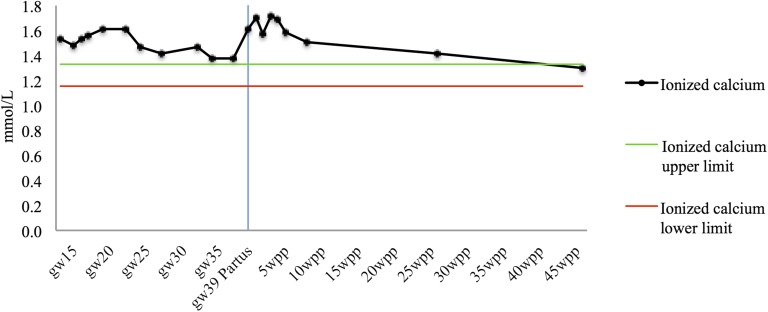
Sister B’s calcium level increased in week 15. She had nausea and muscle weakness. At week 35, labetalol 100 mg × 2 was commenced when her blood pressure level was 140/90 mm Hg. After parturition, her blood pressure level increased to 150/95 mm Hg. Five days later, her S-calcium level was 2.92 mmol/L. Fluids were given. Three weeks after delivery, she was treated at a medical ward because her ionized calcium level was 1.81 mmol/L. Four liters of NaCl was given together with labetalol 100 mg × 3, and her ionized calcium level decreased to 1.68 mmol/L. A week later, her ionized calcium level was 1.59 mmol/L, her total calcium level was 2.90 mmol/L, and she was feeling excellent (Fig. 1; Table 2). One month later, her blood pressure had normalized to 130/80 mm Hg, and labetalol had by then been withdrawn.
Figure 1.
All measured serum ionized calcium levels during sister B’s fifth pregnancy, from gestational week 15 until 45 weeks postpartum, are shown. Gray lines represent the lower and upper limits of the reference range (1.15 to 1.33 mmol/L), and the blue line marks partus. Gw, gestational week; wpp, weeks postpartum.
B-10. Baby B5
A boy was born after induction due to nausea and muscle weakness at gestational week 38 + 5. His weight was 3535 g, and his Apgar scores were 4, 5, and 9, with a quick recovery. He had no hypoglycemia (4.0), and his calcium level at age 1 day was 2.56 mmol/L (2.00 to 3.00 mmol/L). Reference value 3.1-5.5 mmol/L.
C. Sister C
At the age of 21 years and pregnant for the first time, sister C already had nephrocalcinosis. Her S-calcium level was elevated (2.87 mmol/L) in week 15. After week 35, her calcium level was moderately elevated at 2.73 mmol/L, and her P-PTH level was undetectable at <10 ng/L (10 to 65 ng/L) (Table 4). Her blood pressure level was 140/90 mm Hg.
Table 4.
Sister C: Results of the Biochemical Test Performed During and After Her Two Pregnancies and in Her Two Newborns
| 5 Months PreP | 1 Month PreP | Partus | 1 Day PostP | 5 Days PostP | 10–20 Days PostP | 3 Months PostP | 5 Months PostP | References | |
|---|---|---|---|---|---|---|---|---|---|
| S-calcium | |||||||||
| Pregnancy 1 | 2.87 | 2.73 | 3.04 | 2.90 | 3.08 | 3.25 | 2.20–2.60 mmol/L | ||
| Pregnancy 2 | 2.83 | 2.86 | 3.13 | 3.16 | |||||
| S-ionized calcium | 1.17–1.29 mmol/L | ||||||||
| Pregnancy 1 | 1.44 | 1.42 | 1.61 | 1.75 | 1.59 | 1.25 | |||
| Pregnancy 2 | 1.46 | 1.52 | 1.47 | 1.53 | 1.87 | 1.70 | 1.45 | ||
| Urinary calcium | 5.7 | 7.2 | 0.7–7.0 mmol/24 h | ||||||
| P-PTH | <10 | <10 | <10 | 10–65 ng/L | |||||
| Pregnancy 1: 1,25 OHD | 107 | 55 | 10–60 ng/L | ||||||
| Pregnancy 1: | |||||||||
| S-25 OHD | 161 | 40 | 10–50 μg/L | ||||||
| Children | 2.80 | 2.20–2.60 mmol/L | |||||||
| S-calcium | |||||||||
| Baby C1 | |||||||||
| Baby C2 | 2.87 | 2.53 | |||||||
| P-glucose | |||||||||
| Baby C1 | Normal | 3.1–5.5 mmol/L | |||||||
| Baby C2 | 4.0 | 2.9 |
Ionized calcium and calcium have the same reference, unless stated with another reference.
Abbreviations: PreP, preparturition; PostP, postpartum.
She delivered spontaneously at term. Over the following days, her S-calcium level was elevated, with an ionized calcium level of 1.61 mmol/L, and her P-PTH level was <10 ng/L. Five days postpartum, she was admitted with headache, nausea, and severe hypertension (190/110 mm Hg). Treatment with saline infusions and labetalol 100 mg × 2 was initiated. The symptoms disappeared, and all medications were withdrawn after 2 days (Table 4).
C-1. Baby C1
A girl was delivered at week 39 + 5 weighing 3895 g and with Apgar scores of 9, 10, and 10. At 2 days, her calcium level was 2.80 mmol/L (2.20 to 2.60 mmol/L), and she was treated in the NICU for 3 days. She had no hypoglycemia.
C-2. Second pregnancy
Sister C’s calcium level was elevated in weeks 15 and 35 (Table 4). Birth was induced because of rising calcium level and hypertension. Her blood pressure level was elevated to 145/100 mm Hg, which was treated with labetalol 100 mg × 2. Two days postpartum, her blood pressure was normal without medication, but her calcium level had risen to 3.45 mmol/L. She received 5 L of saline infusion, and her calcium value declined to 2.98 mmol/L. The calcium elevation continued until day 10 postpartum (Table 4).
C-3. Baby C2
A girl born at week 39 weighed 3220 g and had Apgar scores of 9, 10, and 10. She stayed in the NICU for 2 days. This child was also affected by hypercalcemia [2.87 (2.20 to 2.60) mmol/L], but no further tests were performed. Her glucose level was slightly low at day five: 2.9 mmol/L.
4. Discussion
All three sisters carried a heterozygote variant in the CYP24A1 c1186C>T gene, but only sisters B and C developed gestational hypercalcemia, and both had an additional heterozygote gene variant, c443T>C. The frequency of the CYP24A1 c1186C>T variant is 80 per 100,000 persons, and for c443T>C with unknown properties, it is 50 per 100,000 [4]. The degree of hypercalcemia in sisters B and C varied from severe to slight and was most pronounced in the third trimester and postpartum; they also developed temporary hypertension. Calcium was analyzed in four of five children of sister B, of whom two had hypercalcemia in the neonatal period, which later normalized. Although sister C had less severe gestational hypercalcemia, her two offspring had elevated S-calcium values. Five of seven infants had hypoglycemia, which was severe in two.
It was only with the combination of the heterozygote variants that hypercalcemia and hypertension developed in sisters B and C. This is a de novo observation for the heterozygote gene variant c443T>C. With these combined variants, patients can develop hypercalcemia in situations with increased levels of D vitamins, as during pregnancy.
Maternal hypercalcemia with CYP24A1 variants was described in a woman who delivered twins in week 32. She was a homozygote carrier of the p.E143del variant [5]. Another woman with hypertension and a CYP24A1 homozygous frameshift mutation developed aggravated hypertension and acute pancreatitis due to hypercalcemia around the time of delivery in her two pregnancies [6]. In a study by Shah et al. [7], a woman was investigated in her fourth pregnancy and had compound heterozygote variants with E143del and R396W mutations in CYP24A1. The latter patient is clinically similar to our patients but had different gene variants. These previously cited cases reported worsening of hypertension during pregnancy in one patient and new-onset hypertension in another. None of them describes hypercalcemia or hypoglycemia in the newborn.
Calcium homeostasis is altered in normal pregnancy, and albumin-adjusted calcium is often slightly elevated. S-1,25 OHD production is increased, predominantly because of parathyroid hormone‒related protein and upregulation of estradiol, prolactin, and placental lactogen [8].
The half-life of S-1,25 OHD is short, estimated to be approximately 15 hours, in comparison with the much longer half-life of weeks for S-25 OHD [9, 10]. Elimination of S-1,25 OHD has been markedly impaired in CYP24A1 null mice [11]. This finding indicates an explanation for the long postpartum hypercalcemic state.
The reason for the hypertension in sisters B and C but normotension in sister A with only one gene variant is unknown. Abnormal placental development, immunologic factors, increased sensitivity to angiotensin-2, systemic endothelial dysfunction, inflammation, infection, and low levels of vitamin D may all play a role in the development of gestational hypertension and preeclampsia [12]. Because none of these factors was driving hypertension in the two sisters, future research may clarify the underlying mechanisms.
The severe hypoglycemia in sister B’s first two children is intriguing and worrisome. In the first infant, this was most probably secondary to hyperinsulinemia. However, insulin was not measured in the other four children with low glucose levels. A mild decrease in blood glucose is normal in newborns, but severe hypoglycemia in infancy is most often seen in infants born small for gestational age, when the mother has type 1 diabetes mellitus or because of rare channelopathies, metabolopathies or nesidioblastosis [13]. We cannot entirely rule out a self-limiting nesidioblastosis as an explanation for temporary hypoglycemia in affected neonates. Hypercalcemia as such may have a hypoglycemic effect. Injection of calcium into the main pancreatic arteries to detect an insulinoma can cause local hyperinsulinemia. Hyperosmolar concentrations of calcium may cause degranulation of pancreatic tumor cells [14]. Further investigations point toward Ca2+ influx into β-cells through voltage-gated calcium channels as the most likely mechanism [15]. Effects on calcium-sensing receptors on human β-cells may also contribute to hyperinsulinemia [16]. With the reservation that hypercalcemia in baby B1 was not established but highly likely because her mother had severe hypercalcemia even at day 10 postpartum, it is probable that the neonatal hypoglycemia was secondary to the elevated calcium level stimulating insulin release. Hypoglycemia is not associated with hypercalcemia in adults. However, infants have lower glycogen depots and may be more vulnerable to hypercalcemia-induced hyperinsulinemia
There is no specific therapy for hypercalcemia due to CYP24A1 variants. Current treatment strategies currently include rehydration and abstaining from dietary intake of calcium, vitamin D, multivitamins, and sunlight exposure [17]. Other advocated treatments are imidazole derivates, which are inhibitors of CYP3A4 hence reducing the formation of 1,25 OHD or rifampin that induces CYP3A4, which degrades 1,25 OHD to inactive metabolites, with normalization of calcium levels [18–20].
Sister B was treated with citalopram during her pregnancies. This antidepressant agent is a known substrate for CYP2C19, CYP2D6, and CYP3A4. Citalopram is not known to affect CYP genes involved in vitamin D metabolism [21]. Hence, it is unlikely that citalopram had effects on calcium levels.
A limitation in all retrospective studies is the risk of ascertainment bias, which was present in this study as well. However, the data from the medical files were extracted as objectively as possible. The lack of important biochemical tests is another limitation.
5. Conclusions
Compound heterozygous variants in CYP24A1 can give rise to very high maternal calcium levels and can affect the fetus. Hypertension can also develop, as well as hyperinsulinemia and hypoglycemia in the newborn, necessitating prompt measures. These combined findings have not been reported previously. Clinical follow-up during pregnancy and genetic counseling should be advised in carriers of homozygous and combined heterozygous variants of CYP24A1.
Acknowledgments
We acknowledge Dr. Inga Bartuviciene and Thomas Gustafsson at the Department of Laboratory Medicine, Karolinska University Hospital, Stockholm, for assistance in chasing shifting laboratory methods and references during these 20 years.
Disclosure statement: The authors have nothing to disclose.
Glossary
Abbreviation:
- NICU
neonatal intensive care unit
- P-PTH
parathyroid hormone
- S-1,25 OHD, 1α
25-dihydroxycholecalciferol
- S-25 OHD
25-hydroxycholecalciferol
References and Notes
- 1. Sato K. Hypercalcemia during pregnancy, puerperium, and lactation: review and a case report of hypercalcemic crisis after delivery due to excessive production of PTH-related protein (PTHrP) without malignancy (humoral hypercalcemia of pregnancy). Endocr J. 2008;55(6):959–966. [DOI] [PubMed] [Google Scholar]
- 2. Nesterova G, Malicdan MC, Yasuda K, Sakaki T, Vilboux T, Ciccone C, Horst R, Huang Y, Golas G, Introne W, Huizing M, Adams D, Boerkoel CF, Collins MT, Gahl WA. 1,25-(OH)2D-24 Hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol. 2013;8(4):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lightwood R, Stapleton T. Idiopathic hypercalcaemia in infants. Lancet. 1953;262(6779):255–256. [DOI] [PubMed] [Google Scholar]
- 4. NHLBI Exome Sequencing Project (ESP). Exome variant server. Available at: http://evs.gs.washington.edu/EVS/ . Accessed 19 June 2018.
- 5. Dinour D, Davidovits M, Aviner S, Ganon L, Michael L, Modan-Moses D, Vered I, Bibi H, Frishberg Y, Holtzman EJ. Maternal and infantile hypercalcemia caused by vitamin-D-hydroxylase mutations and vitamin D intake. Pediatr Nephrol. 2015;30(1):145–152. [DOI] [PubMed] [Google Scholar]
- 6. Woods GN, Saitman A, Gao H, Clarke NJ, Fitzgerald RL, Chi N-W. A young woman with recurrent gestational hypercalcemia and acute pancreatitis caused by CYP24A1 deficiency. J Bone Miner Res. 2016;31(10):1841–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah AD, Hsiao EC, O’Donnell B, Salmeen K, Nussbaum R, Krebs M, Baumgartner-Parzer S, Kaufmann M, Jones G, Bikle DD, Wang Y, Mathew AS, Shoback D, Block-Kurbisch I. Maternal hypercalcemia due to failure of 1,25-dihydroxyvitamin-D3 catabolism in a patient with CYP24A1 mutations. J Clin Endocrinol Metab. 2015;100(8):2832–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):795–826. [DOI] [PubMed] [Google Scholar]
- 9. Jones KS, Schoenmakers I, Bluck LJC, Ding S, Prentice A. Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br J Nutr. 2012;107(8):1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zingone F, Ciacci C. The value and significance of 25(OH) and 1,25(OH) vitamin D serum levels in adult coeliac patients: a review of the literature. Dig Liver Dis. 2018;50(8):757–760. [DOI] [PubMed] [Google Scholar]
- 11. Masuda S, Byford V, Arabian A, Sakai Y, Demay MB, St-Arnaud R, Jones G. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146(2):825–834. [DOI] [PubMed] [Google Scholar]
- 12. Solomon CG, Seely EW. Hypertension in pregnancy. Endocrinol Metab Clin North Am. 2006;35(1):157–171, vii. [DOI] [PubMed] [Google Scholar]
- 13. Dekelbab BH, Sperling MA. Recent advances in hyperinsulinemic hypoglycemia of infancy. Acta Paediatr. 2006;95(10):1157–1164. [DOI] [PubMed]
- 14. Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013;19(6):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henquin J-C, Pattou F, Nenquin M. Insulin secretion in response to high extracellular calcium is not a pathognomonic feature of insulinoma cells [published online ahead of print 30 October 2017]. Diabetes Metab. doi: 10.1016/j.diabet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 16. Gray E, Muller D, Squires PE, Asare-Anane H, Huang G-C, Amiel S, Persaud SJ, Jones PM. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J Endocrinol. 2006;190(3):703–710. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen M, Boutignon H, Mallet E, Linglart A, Guillozo H, Jehan F, Garabedian M. Infantile hypercalcemia and hypercalciuria: new insights into a vitamin D-dependent mechanism and response to ketoconazole treatment. J Pediatr. 2010;157(2):296–302. [DOI] [PubMed] [Google Scholar]
- 18. Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. 2012;97(3):E423–E427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sayers J, Hynes AM, Srivastava S, Dowen F, Quinton R, Datta HK, Sayer JA. Successful treatment of hypercalcaemia associated with a CYP24A1 mutation with fluconazole. Clin Kidney J. 2015;8(4):453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawkes CP, Li D, Hakonarson H, Meyers KE, Thummel KE, Levine MA. CYP3A4 induction by rifampin: an alternative pathway for vitamin D inactivation in patients with CYP24A1 mutations. J Clin Endocrinol Metab. 2017;102(5):1440–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flockhart DA. Drug interaction: cytochrome P450 drug interactions table. Indiana University School of Medicine. Available at:http://medicine.iupui.edu/clinpharm/ddis/main-table/. Accessed June 15, 2018.