Abstract
Natural compounds derived from marine organisms exhibit a wide variety of biological activities. Over the last decades, a great interest has been focused on the anti-tumour role of sponges and algae that constitute the major source of these bioactive metabolites. A substantial number of chemically different structures from different species have demonstrated inhibition of tumour growth and progression by inducing apoptosis in several types of human cancer. The molecular mechanisms by which marine natural products activate apoptosis mainly include (1) a dysregulation of the mitochondrial pathway; (2) the activation of caspases; and/or (3) increase of death signals through transmembrane death receptors. This great variety of mechanisms of action may help to overcome the multitude of resistances exhibited by different tumour specimens. Therefore, products from marine organisms and their synthetic derivates might represent promising sources for new anticancer drugs, both as single agents or as co-adjuvants with other chemotherapeutics. This review will focus on some selected bioactive molecules from sponges and algae with pro-apoptotic potential in tumour cells.
Keywords: apoptosis, alga, sponge, cancer, marine compound
1. Introduction
About 71% of the Earth’s surface is water-covered making the marine environment an immense source of new bioactive compounds with uncommon and unique chemical features. Marine organisms, which include about 2.2 million among algae, corals, sponges, and other invertebrates, are highly competitive and complex species, obliged to share a limited extent habitat [1]. The forced coexistence induced the development of exceptional defensive mechanisms to repel or destroy predators, through the production of potent compounds, often referred to as “secondary metabolites”. Those compounds, which are chemically classified as terpenoids, alkaloids, polyketides, peptides, shikimic acid derivatives, sugars, steroids, and a multitude of mixed biogenesis metabolites have been found to exhibit many biological activities (antimicrobial, antitumor, antidiabetic, anticoagulant, antioxidant, anti-inflammatory, antiviral, antimalarial, antitubercular, anti-aging, antifouling, and antiprotozoal) [2]. Among all of these mechanisms, the antitumor activity is certainly the most promising. The first successful marine-derived anticancer drugs was the nucleoside spongothymidine, obtained from the Caribbean sponge Cryptotethyacrypta (previously known as Tethyacrypta) [3]. In 1969, a derivative of this nucleoside, Ara-C (also known as 1-beta-d-Arabinofuranosylcytosine or cytarabine) was approved by the United States Food and Drug Administration (FDA) under the name of Cytosar-U for the treatment of leukemia and is still in use today for the treatment of acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML), non-Hodgkins lymphoma, and myelodysplastic syndrome (MDS). Nowadays, around 30,000 marine natural products are known, representing a huge industrial and therapeutic potential in virtue of their diversified chemical structures used as privileged scaffolds in drug discovery and development [4]. Overall, more than 3000 new substances identified from marine organisms during the past three decades have shown to have good antitumor activity, and most of them are still in preclinical or early clinical trials, with only a limited number already on the market [5]. To date, five of these compounds have been approved by the FDA for use as pharmaceutical drugs in cancer treatment: Brentuximab Vedotin (Adcetris®, for the treatment of patients with Hodgkin lymphoma), Eribulin Mesylate (Halaven®, for the treatment of patients with metastatic breast cancer), Trabectedin (Yondelis®, for the treatment of patients with unresectable or metastatic liposarcoma or leiomyosarcoma), Fludarabine Phosphate (Fludara®, for the treatment of adult patients with B-cell chronic 95 lymphocytic leukemia), and Nelarabine (Arranon®, for the treatment of patients with T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma) (https://www.fda.gov/Drugs/default.htm).
The potential anticancer activity of marine-derived compounds relies on different cellular and molecular mechanisms, including DNA protection, cell-cycle modulation, induction of apoptosis and autophagy, inhibition of angiogenesis, migration, invasion and metastasis formation [6]. Cancer can be considered an extremely heterogeneous disease. Besides, all types of cancer share some characteristics, defined as hallmarks, responsible for their malignant properties [7]. Most of the traditional anti-cancer drugs have been developed to deliberately target one of these hallmarks. However, malignant capabilities of cancer cells are regulated by partially redundant signalling pathways. Consequently, a therapeutic agent inhibiting only one key pathway in a tumour may allow some cancer cells to survive permitting renewed tumour growth and clinical relapse. Thus, there is actually great attention in exploiting strategies able to simultaneously target these supporting pathways, thus preventing the development of adaptive resistance [8]. Natural compounds usually inhibit cancer formation and development through the interaction with multiple cellular signalling pathways. In addition, they are often characterized by a better safety profile than traditional chemotherapy agents, and they are reasonably priced and easily available [9].
Impairment or resistance to apoptosis has been considered probably the most important challenge in oncology. The molecular mechanisms by which tumour cells gain resistance to apoptosis mainly include (1) a dysregulation of the mitochondrial pathway; (2) the inactivation or the loss of caspases; and/or (3) a deficiency of death signals through the transmembrane death receptors of the tumour necrosis factor (TNF) superfamily [10]. Compounds directly inducing apoptosis, reduce the appearance of drug resistance, decrease mutagenesis and reduce toxicity. In the last decade, numerous apoptosis-inducer compounds have been isolated from marine sponges and algae. This review will focus on some selected agents of marine source, which have been reported to affect apoptotic pathways in tumour cells. Some of them with the relative IC50 and the chemical structure are shown in Table 1.
Table 1.
Marine compounds isolated from sponges and algae with IC50 value.
| Compound | Chemical Structure | IC50 |
|---|---|---|
| Fascaplysin |
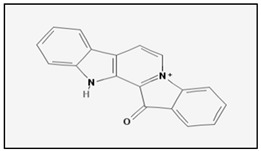
|
SCLC cell lines 0.96 µM NSCLC cell lines 1.15 µM Breast and ovarian cancer cell lines 0.89 µM |
| Monanchocidin A |
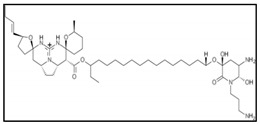
|
HCT 116 4.5 µM DLD-1 and HT-29 >10 µM |
| 10-Acetylirciformonin B |

|
HL-60 1.7 g/mL |
| Heteronemin |
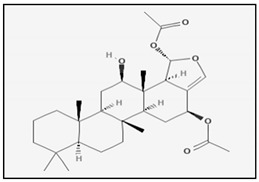
|
ACHN 3.54 µM A498 1.57 µM |
| Laulimalide |

|
MDA-MB-435 5.74 nM SK-OV-3 11.53 SKVLB 1210 |
| C-phycocyanin |
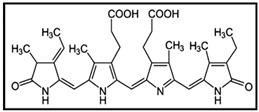
|
S-HepG2 40 µM R-HepG2 50 µM K562 50 µM MDA-MB-231 194.4 µg/mL |
| Elatol |

|
COLO-205 2.5 µg/mL L929 1.1 µM B16F10 10.1 µM |
| Fucoidan |

|
WiDr 0.244 mg/mL MCF-7 0.173 mg/mL |
| BDDE |

|
K562 13.9 µg/mL |
2. Apoptosis: A Target for Anticancer Therapy
Resistance to apoptosis is one of the hallmarks of human cancers and has been reported to be involved in cancer cells evasion to immune-mediated and cytotoxic drug treatment [11].
The expression “apoptosis” was minted by Kerr et al. [12] in 1970 to describe a specific morphological aspect of cell death. It is a highly regulated process that occurs normally during development and aging and acts as a homeostatic mechanism to remove any needless or undesired cells. Apoptosis also takes place as a defence mechanism such as in immune reactions or when cells are flawed by diseases or toxic compounds [13].
There are two main apoptotic pathways: the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway that are activated by both intracellular and extracellular signals. The intracellular signals include ultraviolet radiations, DNA damage and growth factor and cytokine deprivation, whereas the extracellular signals involve death receptors (DR) that are members of the TNF receptor gene superfamily and play a critical role in transmitting the death signal from the cell surface to the intracellular signalling pathways [14]. The best-characterized ligands and corresponding death receptors include FasL/FasR, TNF-α/TNFR1, Apo3L/DR3, Apo2L/DR4 and Apo2L/DR5 [15]. However, both extrinsic and intrinsic pathways are linked and converge at the final phase of apoptosis with the activation of the executive cysteine aspartyl-specific proteases (caspases). Caspases are a class of cysteine proteases that cleave target proteins which coordinate the apoptotic process. Caspases are broadly expressed in their inactivated form (named pro-caspases) in most cells and once activated can in turn activate other pro-caspases, amplifying the apoptotic signalling pathway and leading to rapid cell death. There are more than 13 known caspases that are classified into: initiators, effectors, inflammatory and other caspases. In particular, the most important caspases identified and widely studied are caspase-2, -8, -9, -10 as initiators and caspase-3, -6, -7 as executioners [16].
2.1. Extrinsic or Death Receptor Pathway
The extrinsic pathway is best represented by the FasL/FasR and TNF-α/TNFR1 models. After the binding of the ligand (FasL or TNF-α) to the respective receptor (FasR or TNFR1) an adaptor protein binds to the complex ligand/receptor. These proteins are namely Fas-associated death domain (FADD) and TNF receptor-associated death domain (TRADD) [17]. Adaptor proteins allow the binding and consequent activation of procaspase-8 and -10 in order to form the death-inducing signalling complex (DISC) [18]. Once caspases-8 and -10 are activated the execution phase of apoptosis starts by involving the effector caspases-3, -6 and -7. The extrinsic pathway can be inhibited by cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein (c-FLIP) through the interaction with DR5, FADD and caspase-8 which results in an apoptotic inhibitory complex (AIC). In fact, increased expression of c-FLIP has been reported in different types of cancer [19]. Another extrinsic pathway inhibitor protein is toso, a member of the immunoglobulin gene superfamily, that inhibits TNF-α and Fas signalling pathways and has been identified in several human cancer cell lines [20].
2.2. Intrinsic or Mitochondrial Pathway
The intrinsic signalling pathway is initiated by different stimuli such as toxins, hypoxia and infections. These stimuli change the mitochondrial permeability inducing the release of different pro-apoptotic proteins into the citosol. The most important mitochondrial proteins are: cytochrome c, apoptosis-inducing factor (AIF) and caspase-activated DNase (CAD) [21]. Upon the release, cytochrome c binds to the apoptotic protease activating factor-1(APAF-1) and procaspase-9, forming a complex named apoptosome in which pro-caspase-9 is converted into caspase-9. Once activated, caspase-9 triggers the effector caspases-3 and -7 promptly leading to cell death [22]. Apoptosis-inducing factor and CAD migrate into the nucleus during the late phase of apoptosis causing DNA fragmentation. Apoptosis-inducing factor works in a caspase independent manner, whereas CAD requires caspase-3 binding for inducing DNA fragmentation [23]. All the intrinsic pathway events are regulated by B-cell lymphoma-2 (BCL-2) protein family that can exert both pro- or anti-apoptotic effects. Some of the anti-apoptotic proteins comprise Bcl-2, Bcl-x and BAG, and some of the pro-apoptotic proteins include Bcl-10, Bax, Bak, Bad and Blk. Overexpression or loss of pro- and anti-apoptotic BCL-2 proteins, respectively, is typical in a wide range of human cancer and has frequently been linked with increased chemoresistance and radioresistance [24].
2.3. Other Atypical Forms of Cell Death
Although the use of the term “programmed cell death” is specifically ascribed to apoptosis, there are other different types of cell death which have been evaluated in cancer treatment.
Mitotic catastrophe is a cell death mode that occurs during the metaphase in case of dysregulated mitosis and is characterized by the activation of caspase-2 [25]. Mitotic catastrophe was suggested to be an onco-suppressive mechanism. Thus, faltered mitotic catastrophe can promote the uncontrollable growth of cancer cells [26].
Anoikis is a particular type of apoptosis induced by cell detachment from extracellular matrix having an important role in preventing anchorage-independent growth and epithelial–mesenchymal transition which are typical features of metastasis development. Cancer cells develop anoikis resistance due to several mechanisms, including alteration of integrin, β-catenin/TCF and other pathways involved in cancer development and progression [27].
Pyroptosis is an inflammation related cell death activated by a wide range of stimuli [28]. Pyroptosis is triggered by caspase-1 which is activated by inflammasome, and it is responsible for the production of interleukin 1-beta (IL1-β) and IL-18. Recently, it has been hypothesized that pyroptosis could be exploited as a new and prominent target to mediate anti-cancer treatments [29]. In addition, there are other different types of atypical forms of cell death including: excitotoxicity, Wallerian degeneration, paraptosis, pyronecrosis and entosis [30].
3. Marine-Derived Compounds: Inductors of Apoptosis
3.1. Marine Sponges
Marine sponges are multicellular aquatic animals that filter water through their porous eating bacteria and other particles. There are more than 5000 various species of marine sponges rich in different essential components such as fatty acids, proteins, alkaloids, peroxides and terpenes showing antibacterial, antiviral, antifungal, anti-malarial, anti-helminthic, immune-suppressive and anti-inflammatory effects [31]. In addition, at least 60 different species of sponges possess also chemopreventive and anticancer effects [32]. Unfortunately, their possible use as anticancer drugs is difficult because of the limited supply of the compounds, which are present only in very few amounts in the sponges. However, in the last few years, the synthetic and/or semi-synthetic chemistry applications for natural products have been reported to be a great starting points for generating such compounds [33]. A list of marine spongean compounds with pro-apoptotic effects is reported in Table 2.
Table 2.
Pro-apoptotic compounds isolated from marine sponges.
| Compound | Mitochondrial Pathway | Death Receptor Pathway | Caspases Activation | Other Pathways |
|---|---|---|---|---|
| Alkaloids | ||||
| Aaptamine | NFκB/AP-1 | |||
| Isoaptamine | 3 and 7 | |||
| Crambescidin 800 | 3 | |||
| Fascaplysin | 3 | AKT/AMPK | ||
| Lamellarin D | ✔ | |||
| Makulavamines | 3, 8 and 9 | |||
| Manzamine A | ✔ | 9 | ||
| Monanchocidin A | 3 and 7 | |||
| Renieramycin M | 3 and 7 | |||
| Nortopsentins A-C | ✔ | |||
| Terpenoids | ||||
| 10-Acetylirciformonin B | 3, 8 and 9 | |||
| Heteronemin | ✔ | ✔ | 3, 8, 9 and 10 | |
| Stellettins A and B | ✔ | 8 | ||
| Smenospongine | p38/AMPK | |||
| Macrolides | ||||
| Peloruside A | 3 and 7 | |||
| Salarins | ✔ | 3 and 9 | ||
| Tedanolides | 3 and 12 | |||
| Others | ||||
| Agelasine B | ✔ | 8 | Bcl-2 | |
| Dideoxypetrosynol A | ✔ | 3 and 9 | BAX/Bcl-2 | |
| Jasplakinolide | 3 | BAX/Bcl-2 | ||
| Psammaplysene A | ✔ | FOXO1 |
3.1.1. Alkaloids Isolated from Marine Sponges
Aaptaminoids are alkaloids isolated from different species of Aaptos sponge such as aaptamine, demethylaaptamine, isoaaptamine, aaptosamine, aaptosine and demethyloxy-aaptamine [34]. These alkaloids have been reported to possess different biological activity including anti-neoplastic effect, as demonstrated on murine lymphocytic leukemia P-338 cells, human mouth epidermoid carcinoma KB16 cells, human lung adenocarcinoma A549 and human colon adenocarcinoma HT-29 cells [35]. In particular, aaptamine exhibits DNA intercalating activity and induces G2/M cell cycle arrest of human chronic myeloid leukemia K562 cells [36]. In 2014, Dyshlovoy et al. [37] reported that aaptamine at high but non-toxic concentration induces AP-1 and NF-κB- dependent transcriptional activity which are involved in the induction of apoptosis and in cell transformation. Isoaaptamine was also demonstrated to exert cell growth suppression and apoptosis induction in human breast cancer T-47D cells confirmed by the activation of caspase-3 and -7 and the cleavage of their substrate Poly (ADP-ribose) polymerase (PARP) [38].
Crambescidin 800 is a guanidine alkaloid extracted from sponges of genus Monanchora. It induces differentiation of K562 cells into erythroblasts and accumulation of cells in the S-phase [39]. However, its anti-proliferative and pro-apoptotic effect has been recently demonstrated in triple negative breast cancer cells T11 and SUM159PT and further confirmed by the cleavage of caspase-3 [40].
Fascaplysin is an alkaloid isolated from the Fijian sponge Fascaplysinopsis that exhibits a wide range of pharmacological activities such as antibacterial, antiviral, antifungal and antimalarial. In 2015 it was reported for the first time that fascaplysin induces caspases-mediated apoptosis in HL-60 human pro-myelocytic leukemia cells. In fact, treatment of HL-60 with fascaplysin in the range of nanomolar concentration is able to induce apoptosis in HL-60 cells. In addition fascaplysin also induces autophagy in HL-60 cells and attenuates PI3K/AKT/mTOR signalling [41]. More recently, Rath et al. [42] demonstrated that fascaplysin exerts an anti-proliferative and pro-apoptotic effect in lung cancer and small-cell lung cancer circulating tumour cell lines by involving AKT/PKB and adenosine monophosphate-activated protein kinase (AMPK) pathways.
Lamellarins are a class of alkaloids extracted from different organisms, including molluscs, ascidians and sponges. Lamellarin D represents the lead compound and is the most widely studied in cancer contexts. It showed a potent cytotoxic and pro-apoptotic effect in both human and mouse leukemia cells (Jurkat and P388, respectively). The pro-apoptotic effect of lamellar D is strictly correlated to a direct effect on mitochondria. In fact, lamellarin D induces mitochondrial depolarization which is correlated with nuclear apoptosis [43]. This data has been further confirmed by the addition of cyclosporin A, a potent inhibitor of mitochondrial permeability transition, that totally reverted the pro-apoptotic effect of lamellarin D [44].
Makaluvamines are a class of alkaloids isolated from the marine sponge of genus Zyzzya. They showed promising cytotoxic activity in colon cancer cells HCT 116 [45]. Given their interesting activity in cancer, Wang et al. [46] synthesized different novel makaluvamine analogues and evaluated their activities in breast cancer. In particular FBA-TPQ analogue induced apoptosis in different breast cancer cell lines and inhibited MCF-7 xenograft tumour growth in vivo.
Manzamine A is an alkaloid isolated from sponges of the genus Haliclona sp., Xestospongia sp. and Pellina sp., which demonstrated different pharmacological properties such as anti-inflammatory, antimicrobial and anticancer effects [47]. It showed pro-apoptotic effect in human pancreatic adenocarcinoma cells and human colorectal cancer cells [48]. In particular, tetraethylbenzimi-dazolylcarbocyanine iodide (JC-1) staining, which is used for the evaluation of the membrane integrity, showed that manzamine A induces depolarization of mitochondrial membrane potential and consequent release of cytochrome c which in turn triggers the downstream apoptotic cascades [49].
Monanchocidin A is an alkaloid extracted from the marine sponge Monanchorapulchra [50] which has promising anti-cancer properties. In particular, its cytotoxic activity has been demonstrated in both leukemic and ovarian human cancer cells (THP-1 and Hela respectively) [50]. Moreover, monanchocidin A also exhibits anti-migratory activity in different bladder cancer cell lines and induces autophagy and lysosomal membrane permeabilization [51].
Renieramycin M is a tetrahydroisoquinolinequinone alkaloid isolated from the blue sponge Xestospongia that revealed an encouraging cytotoxic activity in colon, lung and breast cancer cell lines in the range of nanomolar concentrations [52]. Renieramycin M cytotoxic effect is mainly related to apoptosis as determined by flow citometry analysis. Interestingly, renieramycin M is also able to stimulate anoikis in H460 lung cancer cells as confirmed by the XTT assay. Moreover, it also induces inhibition of anchorage-independent growth, invasiveness and migration. Thus, these data suggest that renieramycin M also possesses potential anti-metastasis properties [53].
Nortopsentins A–C are alkaloids isolated from the sponge Spongosoritesruetzleri [54]. These molecules showed in vitro cytotoxicity against P388 leukemia cells. However, due to the very small amount of this substances isolated from the sponge, different nortopsentins synthetic approaches have been proposed [55]. These semi-syntethic compounds showed antiproliferative effect on HCT-116 colorectal cancer cells at low micromolar concentrations. In addition, they induced apoptosis and cell cycle arrest also by inducing mitochondrial dysfunction [56].
3.1.2. Terpenoids Isolated from Marine Sponges
10-Acetylirciformonin B is a furanoterpenoid that was extracted and purified from marine sponge Ircinia sp. This molecule showed cytotoxic activity against HL-60 human leukemia cells in a dose- and time-dependent manner [57]. Its cytotoxic effect was associated to apoptosis as demonstrated by flow cytometry analysis using Annexin V and propidium iodide double staining. The pro-apoptotic effect involves induction of DNA strand breakages and phosphorylation of H2A.X and p-CHK2 that are markers evaluated during nuclear DNA damage [58]. In addition, treatment of HL 60 cells with 10-acetylirciformonin B for 24 h increased the activation of caspases-3, -8 and -9 as well as the cleavage of PARP.
Heteronemin is a sesterterpene extracted from the sponge Hyrtios, that showed different biological activity and cytotoxic effect on several cancer cell lines including leukemia, colon, breast, cervical and renal cancer in the range of nanomolar concentrations [59]. Heteronemin regulates both extrinsic and intrinsic apoptotic pathways in chronic myeloid leukemia K562 and human renal cell carcinoma A498 cells by inhibiting MAPK and AKT signallings which are involved in inflammation and apoptosis [59,60]. More recently Lee et al. [61] reported that heteronemin exerts a pro-apoptotic effect in LNcap prostate cancer cells. In addition, they also reported that heteronemin induced also autophagy in LNcap cells as confirmed by the expression of LC3-II, a soluble protein involved in autophagic activity [62].
Stellettins A and B are triterpenoids isolated from the sponge Stellettatmuis collected from China. Stellettin A was significantly toxic to P388 leukemic cells at micromolar range [63], whereas stellettin B showed an antiproliferative and pro-apoptotic effect in SF295 gliobastoma cell lines [64]. Furthermore, Liu et al. [65] showed that both stellettin A and stellettin B induced oxidative stress and apoptosis in HL-60 human leukemia and LNCaP prostate cancer cell lines confirmed by the activation of caspase-8, the reduction of Bcl-2 protein expression and the increase of FasL.
Smenospongine is a sesquiterpene extracted from the Indonesian sponge Dactylospongiaelegans that has been reported to induce apoptosis and G1 arrest in HL60 and U937 leukemia cells in a dose-dependent manner [66]. Moreover Tang et al. [67] also reported a pro-apoptotic effect of smenospongine in MCF-7 human breast cancer by increasing the phosphorylation level of p38 and AMPKα that have been reported to inhibit cell growth and induce apoptosis.
3.1.3. Macrolides Isolated from Marine Sponges
Peloruside A is a macrolide isolated from the marine sponge Mycale hentscheli that demonstrated cytotoxic and apoptotic effect on human (HL-60) and mouse (32D-ras) myeloid leukemic cells, as well as non-transformed 32D cells [68]. Another similar compound is laulimalide isolated from the sponge Cacospongia. It also showed a pro-apoptotic effect in MDA-MB-435 human breast cancer cell lines by inducing mitotic arrest and activation of the caspase pathways [69]. Moreover, it has been reported that both peruloside and laulimalide show a mechanism of action similar to that of paclitaxel and other taxol drugs binding to β-tubulin and inducing polymerization and stabilization [70].
Salarins are a class of nitrogenous macrolides isolated from the marine sponge Fascaplysinopsis in Madagascar. Among the different salarins, salarin C is the most potent inhibitor of cell proliferation as demonstrated in vitro on K562 leukemia cells. Salarin C induced apoptosis and cell cycle arrest in a dose- and time-dependent manner. This effect was also confirmed by the activation of caspase-3 and -9 and the consequent PARP cleavage. In addition, it has also been demonstrated that salarin C induces mitochondrial permeability alteration supporting cythocrome c release [71].
Tedanolides are a small family of macrolides which includes tedanolide, 13-deoxytedanolide and candidaspongiolide. The latter is isolated from the marine sponge Candidaspongia and has been reported to promote apoptosis in human U251 glioma and HCT116 colon carcinoma cells by triggering caspase-3 and -12 activation [72]. In addition, it has been also demonstrated that candidaspongiolide inhibits proliferation of human melanoma cells in a selective manner compared to breast and lung cancer cell lines [73]. However, the underlying reasons for the selectivity of candidaspongiolide in melanoma cell lines have not been determined and are currently being investigated.
3.1.4. Other Molecules Isolated from Marine Sponges
Agelasine B is a toxin isolated from the marine sponge Agelasclathrodes. This toxin demonstrated cytotoxic effect on two different human breast cancer cell lines (MCF-7 and SKBr3) and on PC3 human prostate cancer cells. In particular, agelasine B releases Ca2+ from the endoplasmic reticulum that is correlated to induced DNA fragmentation and apoptosis [74]. This data has been also further confirmed by the activation of caspase-8 and the reduction of the anti-apoptotic protein Bcl-2 [75].
Dideoxypetrosynol A is a polyacetilene extracted from the Petrosia sponge. It has been widely studied for its pro-apoptotic effect on SK-MEL-2 human melanoma cancer cells and U937 human leukemia cells. In particular, dideoxypetrosynol A-induced chromatin condensation and formation of apoptotic bodies in SK-MEL-2 melanoma cells as demonstrated by morphological analysis with DAPI staining. Moreover, the addition of dideoxypetrosynol A to SK-MEL-2 cells increased accumulation of the sub-G1 phase in a dose-dependent manner quantified by flow cytometry of fixed nuclei [76]. In U937 leukemia cells, dideoxypetrosynol A also showed cell cycle arrest demonstrated by evaluating the expression levels of the cell cycle regulating factors such as cyclin D1, cyclin E, Cdk2, Cdk4 and Cdk6 [77].
Jasplakinolide, also called jaspamide, is a macrocyclic peptide isolated from the marine sponges Jaspis johnstoni and splendens [78]. Jasplakinolide induces apoptosis in Jurkat T cells through the activation of the executioner caspase-3 and the increase of BCL-2 pro-apoptotic proteins family such as BAX [79]. Moreover, studies focused on the murine IL-2-dependent T cell line CTLL-20 showed that the pro-apoptotic role of jasplakinolide is related to the stabilization of polymerized actin which has been reported to be involved in the transduction of apoptotic signal [80].
Psammaplysene A is a bromotyrosine derivative isolated from the sponge Psammaplysilla. It showed an interesting pro-apoptotic activity against two different type of human endometrial cancer cell lines Ishikawa and ECC-1. In particular, Psammaplysene A pro-apoptotic effect is linked to the nuclear export inhibition of FOXO1 transcription factor, which in turn triggers apoptosis by activating the pro-apoptotic protein Bim or inducing the tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis pathway [81]. This result has been also confirmed by using silenced FOXO1 ECC-1 cells and overexpressing FOXO1 Ikasawa cells in which have been evaluated the cleavage of PARP substrate and its correlation with FOXO1 [82]. Moreover, psammaplysene A also showed a cooperative effect with cisplatin in reduction of cell viability and induction of apoptosis in HT29 colon cancer cells [83] and in lung cancer cells [84].
3.2. Marine Algae
Among marine organisms, marine algae, which include microalgae and macroalgae (or seaweeds), constitute a major source, after sponges, of natural compounds with diverse biological activities. Microalgae constitute more than 90% of the oceanic biomass and they are single-cell photosynthetic organisms distinguished in prokaryote (cyanobacteria) and eukaryote [85]. Actually, more than 10,000 species are known, and many of them are edible and rich in essential nutrients such as protein, fibre, vitamins, minerals and polyunsaturated fatty acids. In addition, they have also revealed to be a prolific source of secondary metabolites (polysaccharides, phycobilins, polysaccharides, sterols, tocopherols, terpenes, polyphenols and phycocyanins) with numerous bioactive properties such as antioxidants, anticancer, anti-inflammatory, antihypertensive, anti-hyperlipidaemia, anticoagulant, immunomodulatory, neuroprotective, antiviral and antimicrobial [86]. Traditionally, seaweeds have been utilized in cuisine and medicine in East Asian countries, where there has been observed a lower incidence of chronic diseases, such as hyperlipidaemia, coronary heart disease, diabetes and cancer, compared to Western countries [87]. Finally, marine algae have also the advantages of a faster cultivation, processing, and harvesting cycle, as well as the ability to be cultured on waste materials, which enhance drug cost effectiveness and biopotential. A list of marine algae compounds with pro-apoptotic effects is reported in Table 3.
Table 3.
Pro-apoptotic compounds isolated from marine algae.
| Compound | Mitochondrial Pathway | Death Receptor Pathway | Caspases | Other Pathways |
|---|---|---|---|---|
| Cyanobacteria | ||||
| Apotoxin A | 3 and 7 | |||
| Lagunamide A-C | ✔ | |||
| Dolastatin 10 | ✔ | |||
| Symplostatin 1 | 3 | Bcl-2 | ||
| Kanamienamide | 3 and 7 | |||
| C-phycocyanin | ✔ | ✔ | 3 | NF-κB/MAPK/AKT |
| Cryptophycin 52 | 3 and 1 | p53/Bcl-2 | ||
| Cryptophycin 1 | 3 | |||
| NoA | 3 and 7 | |||
| Coibamide A | 3 | |||
| Chlorophytes | ||||
| C. vulgaris extracts | ✔ | 3, 8 and 9 | p53/Bcl-2/BAX | |
| C. sorokiniana extracts | ✔ | Bcl-2 | ||
| D. salina extracts | ✔ | 8 | p53/BAX | |
| Astaxanthin | NF-κB | |||
| Siphonaxanthin | 3 | Bcl-2 | ||
| Clerosterol | ✔ | 3 and 9 | Bcl-2/BAX | |
| Ulvans | ✔ | 3 and 9 | p53/Bcl-2/BAX | |
| GLP | ✔ | 3 and 9 | ||
| Rhodophyta | ||||
| Obtusol | 6 | |||
| Elatol | 2, 4, 6, 8 and 9 | p53/Bcl-XL/eIF4A1 | ||
| Laurinterol | 3 | p53 | ||
| Mertensene | 3 | MAPK/AKT/NF-κB | ||
| λ and ϊ-carrageenans | ✔ | 3 and 9 | Bcl-2/BAX | |
| ECS | 3, 8 and 9 | Bcl-2/BAX | ||
| TDB | 3 | Bcl-2/p53 | ||
| ESA | 3 | |||
| Phaeophyta | ||||
| F. vesciculous Fucoidans | ✔ | 3, 8 and 9 | Bcl-2/Bcl-XL/MAPK | |
| U. Pinnatifida Fucoidans | ✔ | ✔ | 3, 8 and 9 | p38/AKT/ROS Bax/Bcl-2/β-cat |
| Laminarans | ✔ | ✔ | 3, 6, 7, 8 and 9 | IGF-IR |
| Fucoxanthin | ✔ | 3 and 9 | NF-κB/AP-1/ROS/Bcl-XL | |
| BDDE | ✔ | 3 and 9 | Bax/Bcl-2/ROS | |
| LJGP | ✔ | 3, 8 and 9 | p27/Bcl-2 | |
| Diphlorethohydroxycarmalol | ✔ | Bcl-2 |
3.2.1. Cyanobacteria
Cyanobacterium (marine blue-green algae or Cyanophyceae) are slow-growing photosynthetic prokaryotes, which are responsible for producing atmospheric oxygen as well as numerous bioactive secondary metabolites with several pharmacological properties [88]. Most of these metabolites have a structure type common to a subset of natural products (i.e., rifamycin, lovastatin, vancomycin, bleomycin) utilized as therapeutic agents in some human diseases, including inflammation, bacterial infections and cancer. In fact, they are produced by a biosynthetic pathway that involve either the non-ribosomal polypeptide (NRP) synthase and the mixed polyketide-NRP synthase, resulting in chemically different structures such as linear peptides, cyclic peptides, linear lipopeptides, depsipeptides, cyclicdepsipeptides, fatty acid amides, swinholides, glicomacrolides or macrolactones [89]. The cytotoxic effects of marine cyanobacteria compounds on human tumour cell lines are the most studied, and several compounds have emerged as templates for the development of new anticancer drugs. Among the mechanisms implicated in their cytotoxicity, one of the most studied is the activation of the apoptotic process. Some compounds were found to induce the activation of caspase-3, while others were found to induce cell cycle arrest or mitochondrial dysfunctions or alterations in BCL-2 protein family, important regulators of apoptosis in cancer [90]. Secondary marine cyanobacterial metabolites mainly derived from Oscillatoriales orders followed by Nostocales and Chroococcales. In particular, more than 300 nitrogen-containing metabolites have been isolated from two marine filamentous cyanobacterial genera: Lyngbya and Symploca [91].
Apratoxins, a class of potent cytotoxic cyclic depsipeptides originally isolated from various strains of Lyngbya sp. from Guam, Palau, and Papua New Guinea, exhibited high potency against various cancer cells. Apratoxin A, the parental compound isolated from Lyngbya majuscule, was shown to induce pronounced G1-phase cell cycle arrest and apoptosis in osteosarcoma U2OS cells by activation of caspase-3 and -7 [92]. Apratoxin E, isolated from Lyngbya bouilloni, also displayed strong cytotoxicity against several cancer cell lines derived from colon, cervix and bone [93].
New cyclic depsipeptides, lagunamide A, lagunamide B and lagunamide C were recently also isolated from Lyngbya majuscula, from the western lagoon of Pulau. Lagunamides metabolites displayed potent cytotoxic activity when tested against a panel of cancer cell lines such as P388, A549, PC3, HCT8 and SK-OV3It, which might occur via induction of mitochondrial-mediated apoptosis [94,95].
Hectochlorin and lyngbyabellins are structurally-related lipopeptide and cyclic depsipeptides isolated from the genus Lyngbya, which are described to inhibit cell cycle proliferation in a human Burkitt lymphoma cell line by inducing an arrest in G2/M phase [96,97]. Dolastatins are cytotoxic peptides that were initially isolated from the sea hare Dolabella auricularia and later found to be produced by marine cyanobacterial strains Symploca [98]. Dolastatin 10, the cyanobacterial metabolite, was found to induce G2/M arrest and the activation of the apoptotic mitochondrial “intrinsic” pathway in human lung carcinoma cells [99]. Dolastatin 10 and its synthetic derivative Soblidotin have been used in trials for the treatment of sarcoma, leukemia, lymphoma, liver cancer and kidney cancer (https://clinicaltrials.gov/ct2). Similar to dolastatin 10, symplostatin 1, another metabolite isolated from the genus Symploca, exhibited a great efficacy in inhibiting a variety of cancer cell proliferation. Symplostatin 1 caused the formation of abnormal mitotic spindles and accumulation of cells in metaphase resulting in a G2/M cell cycle arrest. Moreover, symplostatin 1 initiated the phosphorylation of Bcl-2, formation of micronuclei and activation of caspase-3, indicating induction of apoptosis [100].
Kanamienamide, an enamide with an enol ether 11-membered lipopeptide, isolated from Moorea bouillonii, in Kagoshima, exhibited growth-inhibitory activity in HeLa cells and caused apoptosis-like cell death in HeLa cells trough caspases cascade activation [101].
Spirulinales are filamentous cyanobacteria with trichomes regularly spirally coiled, with parietal thylakoids, lacking heterocytes. Previously belonging to the Synechococco phycidae family, now they are considered a new order following whole genome sequencing. However, “Spirulina platensis”, commercially important for its phylogenetic and cytological criteria has been classified in the genus Arthrospira (Oscillatoriales) [102]. Spirulina platensis is widely used as a dietary supplement because of its high nutritional value; it is rich in proteins, essential fatty acid, vitamins and mineral but is also potent in antioxidants including spirulans (sulphated polysaccharides) and phycobiliproteins (C-phycocyanin and allophycocianin). Spirulina has been demonstrated to have hypolipidemic, hypoglycemic and antihypertensive properties. Recently, S. platensis-derived bioactive substances have also been investigated for their anti-cancer functions. In particular, c-phycocyanin, a light-harvesting biliprotein, seems to be implicated in most of the biological effects of spirulina. Indeed, several studies have shown that phycocyanin plays anti-proliferative and pro-apoptotic effects on different cancer cell types such as lung cancer [103], liver cancer [104], colon cancer [105], breast cancer [106,107] and leukemia [108] both in vitro and in vivo, without side effects on normal tissue. C-phycocyanin can affect cancer cell cycle proliferation by inducing cell cycle arrest in G0/G1 phase and by activating the apoptotic pathway in several ways. For example, in MDA-MB-231 breast cancer cells, the antitumor effect of c-phycocyanin was mediated by induction of apoptosis trough upregulation of Fas protein levels of and cleavage of caspase-3, while downregulation of Bcl-2 protein was observed [107]. In K562 leukemia cells, the c-phycocyanin pro-apoptotic effect was mediated by release of cytochrome c from mitochondria into the cytosol instead [108]. In addition, MAPK, PI3K/Akt/mTOR and NF-κB pathways are also involved in phycocyanin-induced apoptosis [103,107]. Interestingly, when phycocyanin was combined with chemotherapeutic drugs, their efficacy was improved resulting in reduction of the dose, and consequently, minimizing the adverse effects. It is reported that diverse drugs may synergize with phycocyanin to kill human cancer. The combination of piroxicam (the traditional non-steroidal anti-inflammatory drug) and phycocyanin was more than 70% effective to inhibit colon cancer development in rat than single-use drugs [109]. Moreover, Bing Li [110] found that an all-trans-retinoic acid (ATRA) and c-phycocyanin combination treatment of HeLa cells could significantly upregulate the expression of caspase-3 while downregulating the anti-apoptotic protein Bcl-2. In addition, c-phycocyanin and betaine combination decreased A549 cell viability up to 60%, and might effectively inhibit tumour growth in rats [111]. C-phycocyanin could be also combined with He-Ne laser for the treatment of tumours, acting as photosensitizer. Thus, when tumour cells (HepG2, MCF-7, MDA-MB-231) were irradiated, the pre-treatment with phycocyanin induced mitochondrial membrane potential loss, increased reactive oxygen species (ROS), released cytochrome c and activated caspase-3, all effects leading cells to apoptosis [106,112,113]. These studies demonstrated the great therapeutic potential of phycocyanin in cancer therapy.
Two potent bioactive metabolites belonging to the Nostocales order are represented by calothrixin A and cryptophycin 52. The calothrixins are quinone-based natural products isolated from Calothrix cyanobacteria that have shown potent antiproliferative effects against several cancer cell lines. Interestingly, calothrixin A has been found to display potent antiproliferative effects against leukemia cells. Beyond a cell cycle block, calothrixin A is described to induce an oxidative stress in Jurkat human T cells that as a consequence foments DNA fragmentation leading to apoptosis [114]. Calothrixin B has shown antiproliferative activity against the HCT-116 colon cancer cell line instead [115]. Cryptophycin 52 is a naturally macrocyclic depsipeptide isolated from the marine cyanobacteria Nostoc spp. [116]. Cryptophycin 52 is an antimicrotubules agent with potent antiproliferative and cytotoxic activity against a broad spectrum of cultured human tumour cell lines, including several multidrug-resistant lines as well as murine tumours model [117]. The cytotoxic effects of cryptophycin 52 appear to be mediated, at least in part via apoptosis. In particular, in several prostate cancer cell lines including PC-3, LNCaP and DU-145, cryptophycin 52 induced apoptosis via proteolytic processing and activation of caspase-3 and caspase-1 and cleavage of the caspase substrate PARP. In addition, cell death was also associated with phosphorylation of Bcl-2 and Bcl-x(L) proteins and upregulation of p53 protein and the p53-regulated gene p21 [118]. Also cryptophycin 1 is described to induce apoptosis in a human ovarian carcinoma cell line, initiating the caspases cascade through caspase-3 activation [119]. Finally, both cryptophycins were found to induce DNA fragmentation [118,119].
Natural oxadiazinenocuolin A (NoA) with a unique 1,2,3-oxadiazine heterocycle (N–N–O) structure was identified in three independent cyanobacterial strains of genera Nostoc, Nodularia, and Anabaena. It has been reported that NoA-induced cell death is clearly consistent with apoptosis as demonstrated by blebbing, caspase activation and PARP-1 cleavage in HeLa cells [120].
To this end, among the members with anti-proliferative activity belonging to Chroococcales order, there is coibamide A, a depsipeptide founded in a Panamanian Leptolyngbya sp. This compound was evaluated in the National Cancer Institute’s NCI’s panel of 60 cancer cell lines, and it exhibited significant activities against human breast cancer (MDA-MB-231), human melanoma (LOX IMVI), human leukemia (HL-60) and human glioblastoma (SNB-75)cells at nanomolar concentrations [121]. Coibamide A was also described as capable to cause the arrest of cell cycle progression in G1 phase in melanoma-like MDA-MB-435 cells and in U87-MG and SF-295 glioblastoma cells [121,122]. A deeper analysis of the mechanisms of action of coibamide A showed caspase-3 activation in dying cells [123].
3.2.2. Chlorophytes
Chlorophyta is a group of green photosynthetic microalgae with biological and pharmacological properties important for human health. They have a long history of use as food source for human consumption, aquaculture and animal feed, as well as for other commercial purposes such as colouring agents, cosmetics and others [124]. Many species of green algae are able to produce several secondary metabolites when they are exposed to stress or sub-optimal conditions linked to nutrient deprivation, light intensity, temperature, salinity and pH which are health-promoting agents. Some of them have also shown promising anticancer activity. Out of 174 strains from 24 genera of green microalgae screened, 10 strains from the species Desmococcus olivaeceus, Chlorella and Scenedesmus showed anticancer activity [125]. The best-known example is Chlorella vulgaris which has been widely used for centuries as a food supplement for its health benefits and its high nutritive value due to the adequate contents in protein, omega-3 polyunsaturated fatty acids, polysaccharides, vitamins and minerals. Clinical trials have suggested that supplementation with C. vulgaris can ameliorate hyperlipidemia and hyperglycemia, and protect against oxidative stress, cancer and chronic obstructive pulmonary disease [126]. Concerning the anticancer properties several studies have been demonstrated that Chlorella extracts exhibit cytotoxic effects in various human cancer cell lines. The anti-cancer effects of C. Vulgaris derivatives have been related to their ability to modulate apoptosis signalling pathways. In fact, C. vulgaris has been shown to inhibit cell proliferation and to induce apoptosis cascades in the hepatoma cell line HepG2 [127], in human lung carcinoma A549 and NCI-H460 cells [128] and in rat models of liver cancer [129]. The mitochondrial signalling pathway was involved in C. vulgaris-induced apoptosis of HepG2 cells with increased expression of pro-apoptotic proteins p53, Bax and caspase-9/3 and decreased expression of Bcl-2 protein [127]. In the same way the chemopreventive role of C. vulgaris in hepatocarcinogenesis-induced rats was mediated by the mitochondria-dependent apoptotic pathway through caspase-8 activation. Other species of green algae belonging to the genus Chlorella have also demonstrated anticancer effects: C. sorokiniana has shown to induce apoptosis in non-small cell lung cancer (NSCLC) cells through the activation of the mitochondrial-mediated apoptotic pathway associated to downregulation of BCL-2 anti-apoptotic family, which resulted in a marked reduction of tumour growth in mice [130]; C. pyrenoidosa induced antineoplastic effects in experimental breast cancer model, instead [131]. Green algae contain also many active pigments such as carotenoids, which are normally used as colouring agents. Generally, carotenoids in plants play important roles in the photosynthetic process, such as light-absorption and quenching of excess energy [132]. Interestingly, several carotenoid extracts from marine Chlorella have demonstrated antioxidant and protective activity on human cells. In particular, carotenoid extracts from C. ellipsoidea (violaxanthin, xanthophylls, antheraxanthin and zeaxanthin) and from C. vulgaris (lutein) have been shown to inhibit human colon cancer HCT116 cell proliferation. Between the two species, pro-apoptotic effect of C. ellipsoidea extract was almost 2.5 times stronger than that of the C. vulgaris extract [133]. Among carotenoids, algal lycopene isolated from C. Marina also showed very significant anti-proliferative and pro-apoptotic effect in human prostate cancer cell lines [134]. Dunaliella salina is a unicellular halophilic green microalga with potent antioxidant activity and plays an important role in reducing the risk of several ROS-associated diseases, such as cancer. Dunaliella salina is known to be a rich source of β-carotene. In particular, under stress conditions, its production increases and as a consequence, the antioxidant and cytotoxic activities of D. Salina results enhanced [135,136]. The extracts from D. salina are widely used as nutrition supplement especially in China, Japan and Taiwan, particularly as natural antimicrobials and antioxidants. The cytotoxic effect of D. salina has been studied in different cancer cell lines [137,138]. An ethanol extract of D. salina, that is higher in β-carotene content, was able to significantly inhibit carcinogenesis in a murine model of squamous cell cancer by enhancing cell immunity [139]. Further, carotenes enriched extracts of D. salina showed significant antioxidant and cytotoxic effects in breast cancer cell lines (MCF-7) [140]. Sheu et al. reported that the anticancer effect of D. Salina was apoptotis-mediated. They demonstrated that ethanol extract of D. salina induced the activation of the extrinsic apoptotic FasL/FasR pathway in A549 lung cancer cells via upregulation of p53. Consequently, the apoptosis-inducing effect of p53 resulted in caspase-8 activation and increased expression of the pro-apoptotic protein Bax [141]. These finding were also confirmed in an in vivo model of mammary carcinogenesis. In this study, female tumour bearing-rats treated whit D. salina lyophilized powder showed up to 83.4% reduction of tumour volume. This chemopreventive effect was obtained through the suppression of cell proliferation and the induction of apoptosis [142]. Haematococcuspluvialis is also a rich source of carotenoid. Astaxanthin (3,3′-dihydroxy-β, β′-carotene-4,4′-dione) a xanthophyll carotenoid has demonstrated antioxidants properties and efficient promotion of apoptosis in prostate cancer cell lines by inhibiting NF-kappa B [143]. Siphonaxanthin is a specific keto-carotenoid of siphonaceous green algae. It represents the major carotenoid in the peripheral light-harvesting complexes (LHCs) of photosystem II and helps to absorb green and blue-green light under water [144]. In edible green algae such as Codium fragile, Cauler palentillifera and Umbraulva japonica, siphonaxanthin content is approximately 0.03–0.1% of the dry weight. Interestingly, siphonaxanthin has been reported to induce apoptosis in human leukemia cells (HL-60). The induction of apoptosis was associated with the decreased expression of Bcl-2, and the subsequently increased activation of caspase-3. In addition, siphonaxanthin upregulated the expression of GADD45α and of the DR5 [145].
C. fragile is also an important source of clerosterol, which is a cholesterol derivative [146]. The cytotoxic effects and mechanism of action of clerosterol were investigated in A2058 human melanoma cells. Cells treated with clerosterol showed chromatin condensation and DNA fragmentation, which are typical morphologic changes associated with apoptosis. Indeed, clerosterol induced apoptosis in A2058 cell via the mitochondria-mediated pathway, which was associated with the modulation of Bcl-2 and Bax expression, and an increase in the levels of cleaved caspases-9 and -3 [147].
Recently, polysaccharides from marine organisms have also garnered attention because of their potential anti-tumour, immune-modulatory and antioxidant effects [148]. The greatest antitumor properties have been reported for ulvans, which are polysaccharides from green microalga from the genus Ulva. Polysaccharides isolated from Ulva lactuca, a marine alga widely distributed at Egyptian sea shores were reported to have anti-proliferative effects on different types of cancer in vitro and in vivo. Kaeffer et al., were the first to show that ulvan sulphated polysaccharide inhibits proliferation of tumoural colon carcinoma cell lines (HCT-29, HCT-116, and Caco-2) [149]. Later, other studies revealed the potential anticancer effects of those ulvans also on tumoural hepatocellular carcinoma cell lines (HepG2) and in Ehrlich ascites carcinoma (EAC)-bearing mice [150]. Finally, ulvan polysaccharides isolated from U. lactuca have shown potent antiproliferative and cytotoxic effect against breast carcinoma cell line in vitro and potential chemopreventive effects against breast carcinogenesis in vivo [151]. The anticarcinogenic and ameliorative effects of these polysaccharides have been demonstrated to be mediated through the enhancement of an antioxidant defence system and induction of apoptosis, which might be associated with the increased expression of p53 and decreased expression of Bcl-2 proteins [150,151]. Sulphated polysaccharides isolated from Ulva pertusa showed little cytotoxicity against gastric adenocarcinoma (AGS) cells while demonstrating strong immunomodulatory activity by stimulating Raw 264.7 cells to produce high amounts of nitric oxide and various cytokines [152]. Polysaccharides obtained from Ulva fasciata, also known as sea lettuce, are reported to be naturally high in Se. Selenium plays an important role in many physiological processes and it has been reported to have potent chemopreventive activities [153]. Selenium-polysaccharide, which contain about 44.4 μg/g of Se, has been demonstrated to gain greater anti-tumour effect than native polysaccharides. It has shown potent anti-proliferative activity against human lung cancer A549 cells, leading to apoptotic mitochondrial-mediated pathway by activating caspase-9 and down-regulating the expression of BCL-2 family proteins [154]. A sulphated polysaccharide, extracted from Ulva intestinalis, is found to have anticancer activity on human hepatoma HepG2 cell line. It induces apoptosis on HepG2 cells involving a caspases-mediated mitochondrial signalling pathway, which is associated with changes in mitochondrial membrane potential, release of cytochrome c to the cytosol, activation of caspase-9 and -3, as well as decrease of Bcl-2 expression and increase of Bax expression [155]. Capsosiphon fulvescens was shown to inhibit the AGS cell proliferation and induced apoptosis by inhibiting insulin-like growth factor-I receptor (IGF-IR) signalling and the PI3K/Akt pathway, which resulted in caspase-3 activation and decrease of Bcl-2 expression levels [156].
A glycoprotein (GLP) with a molecular mass of 48 kDa, extracted and purified from Codium decorticatum, was able to inhibit cell viability by inducing apoptosis in human MDA-MB-231 breast cancer cells. Apoptosis was associated with alterations in the mitochondrial membrane potential, which led to mitochondrial membrane permeabilization, cytochrome c release, and activation of caspases-3 and -9 [157].
3.2.3. Rhodophyta
Rhodophyta, also known as red algae, have been documented as a source of natural nutraceuticals and pharmaceuticals for many years. Recent studies showed that sulphated polysaccharides isolated from red seaweed possess various therapeutic and biological feature such as anti-oxidants, anti-proliferative, anti-tumour, anti-viral and anti-coagulants [158,159].
Genus Laurencia are one of the most studied marine organisms among the red algae. They are found on the inter-tidal rocks of the warm sea waters throughout the world. There are almost 421 species actually known. It has been provend that the genus Laurencia is a rich source of structurally unique secondary metabolites, characterized by a relatively high degree of halogenation and core terpene structures. The halogenated metabolites from Laurencia possess several biological activities, such as antifeedant, anthelmintic, antimalarial, antifouling, antimicrobial and cytotoxicity [160,161]. Two halogenated sesquiterpenes (obtusol and elatol) isolated from L. dendroidea showed a great antiproliferative activity on colorectal adenocarcinoma cells (Colo-205) obtained through the induction of apoptosis. Elatol activity proceeded trough the activation of caspases-2, -4, -6 and -8, on the other hand obtusol activity involved only caspase-6 activation [162]. Similar effects were induced also by the analogue elatol isolated from algae L. microcladia, which inhibited tumour growth in B16F10 melanoma-bearing mice. The anti-tumour properties of elatol were, at least in part, related to cell cycle delay in the G(1)/S transition coupled with the simultaneous induction of apoptosis. In particular, the apoptotic pathway was associated with an increase of p53 expression and caspase-9 cleavage and to a decreased of Bcl-xl protein [163]. Recently, the inhibition of the DEAD-box RNA helicase eIF4A1 has been identified as a new possible mechanism of action for the anticancer activity of elatol [164]. Laurinterol is a marine sesquiterpene extracted from L. Okamurai. Beside its well-known antimicrobial activity, laurinterol has demonstrated to be able to induce apoptosis through a p53-dependent pathway in melanoma cells (B16F1). Anticancer activity of laurinterol against melanoma cells was determined by DNA fragmentation and caspase activation [165]. The marine polyether triterpenoid dehydrothyrsiferol isolated from L. viridis induced apoptosis on in hormone insensitive breast cancer cells by estrogen-dependent and independent pathways [166]. Mertensene, a halogenated monoterpene from the red alga Pterocladiella capillacea, inhibited human colorectal adenocarcinoma cell (HT29 and LS174) proliferation. In HT29 cancer cells, mertensene triggered a caspase-dependent apoptosis characterized by caspase-3 activation and PARP cleavage. In addition it induced G2/M cell cycle arrest associated with a reduction of the cellular level of cyclin-dependent kinases (CDK) 2 and 4. Moreover, the antiproliferative effects seems correlated to the modulation of MAPK ERK1/2, Akt and NF-κB pathways, the major regulators of cell survival, proliferation and metabolism [167]. A new class of diterpene-benzoate macrolides, namely bromophycolides, were isolated from Callophycus serratus. The first identified, bromophycolide A, demonstrated moderate in vitro cytotoxic potency on A2780 human ovarian cells, consequent to the arrest at the G1 phase of the cell cycle and to a simultaneous induction of apoptosis [168]. Later, other bromophycolides (C-I) were obtained from C. serratus with cytotoxic activity against a wide range of cancer cells. Among them, the antiproliferative effect of Bromophycolide D was the most significant [169].
Sulphated polysaccharides extracted from red seaweeds have gained enormous attention over the last decades for their multiple chemical structures and biological activities, particularly for their potential anti-cancer properties [170]. Carrageenans are a small chemical group of linear sulphated polysaccharides, which represent the main composite constituent compounds of cell walls in a large group of edible red algae. Carrageenans are divided into three categories (κ, I or λ) according to their degree of sulfation and water solubility. λ-carrageenan, which had the highest degree of sulfation, exhibited an efficient anti-proliferative activity in the human breast cancer cells MDA-MB-231 via the promotion of caspases-dependent apoptosis and with the contribution of the pro-apoptotic protein Bax. The altered Bax/Bcl-2 ratio led to the disruption of mitochondrial transmembrane potential with the consequent release of cytochrome c triggering the formation of the APAF-1/caspase-9 complex and subsequently inducing the active form of caspase-3 [171]. Similar results were obtained also with degraded ι-carrageenan, which inhibited cell proliferation in Caco-2 and HepG2 cancer cell lines via apoptosis induction [172]. An extracted sulphated carrageenan (ECS), predominantly consisting of ι-carrageenan obtained from the red alga Laurencia papillosa, was characterized for its potential cytotoxic activity against the MDA-MB-231 cancer cell line. This study demonstrated that the algal ESC has anti-proliferative properties that lead to apoptosis. Indeed, ECS induced the extrinsic pathway of apoptosis in MDA-MB-231 cells via the recruitment of caspase-3, caspase-8 and caspase-9, the re-modulation of the Bax/Bcl-2 ratio and DNA damage [173]. Further, λ- and ι-carrageenan isolated from L. papillosa was shown to significantly inhibit MCF-7 breast cancer cell viability by inducing apoptosis-mediated cell death [174].
The next good candidate chemotherapeutic agent, acting through the induction of apoptosis is 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether (TDB), isolated from Symphyocladia latiuscula, which has been shown to induce apoptosis in the MCF-7 cells via caspase-3 activation with PARP cleavage and Bcl-2 downregulation. Treatment with TBD also caused a marked increase in the level of p21WAF1/CIP1 protein in a p53-dependent manner and DNA fragmentation [175].
Eucheuma serra agglutinin (ESA) is a lectin derived from a marine red alga Eucheuma serra inducing tumour cell death by apoptosis. It has been proposed that the antitumour activity of ESA might be related directly to specific intermolecular interactions with unique sugar chains rich in mannose localized on the tumour cell surface. Such specific binding between ESA and cancer cells led to apoptosis activation. Indeed, several studies reported that ESA induced cell death against several cancer cell lines, such as colon cancer (Colo201 and Colo26), cervix cancer (HeLa) and osteosarcoma (OST and LM8). DNA ladder detection and the induction of caspase-3 activity confirmed apoptosis induction by ESA in cancer cells [176,177]. Moreover, an intravenous injection of ESA in the tail of Colon26 tumour-bearing mice significantly inhibited tumour growth by inducing apoptotic cell death, without any serious side-effects for the animals [178]. Furthermore, the administration of PEGylated Span 80 vesicles with ESA encapsulated, is expected to express more effective antitumor activity against cancer cells, increasing the potential of ESA as new effective anti-tumour drug [179].
3.2.4. Phaeophyta
Phaeophyta (brown algae) represent a large group of photoautotrophic marine macro-algae, which include about 265 genera with more than 1500 species. They produce a plenty of secondary metabolites, which have been demonstrated to be potent antibiotic, antifungal, antiviral, or anticancer agents. Among them, brown-algal polysaccharides and carotenoids received considerable attention because of their proven health effects such as antibacterial, antioxidant, anti-inflammatory, anticoagulant and antitumor effects. Utilization of these compounds as an ingredient in some dietary supplement products has been very common in the East Asian countries, particularly in Japan, China and Korea. In addition brown-algal polysaccharides are present in large amounts in algal biomass and are easy to isolate permitting their use as functional food supplements or nutraceuticals [180].
Fucoidans and laminarans represent the most abundant polysaccharides in the cell wall of various species of brown algae, and the principle carbohydrate reserve. Fucoidans are a complex series of sulphated polysaccharide that exists mainly in the cell wall matrix of brown seaweeds such as mozuku, kombu, limumoui, bladderwrack and wakame and are the main components of the sticky substance. The basic structure of fucoidan consists of fucose monosaccharide units with attached sulphate groups. More complex molecular structures of fucoidans strongly depend on the species and other factors such as harvesting time and habitat conditions [181]. Recently, a number of studies have obtained interesting results regarding the anticancer properties of fucoidans both in vitro and in vivo, in different types of cancers. Clinical trials of patients with breast, cervical, renal, and hepatic carcinomas showed a significant improvement in tumour regression among patients who received an alternative medicine treatment regimen based on fucoidan administration [182]. Fucoidan isolated from Fucus vesiculosus and Undaria pinnatifida are the most studied for the comprehension of their chemical and biological properties. Various mechanisms have been postulated for the anticancer activity of fucoidans, such as induction of cell cycle arrest, apoptosis and immune system activation [183]. In particular, it has been demonstrated that in a wide variety of cancers, such as hematopoietic, lung, breast, prostate and colon cancers, fucoidans mediated cell death through induction of apoptosis [184,185,186,187,188,189,190]. Numerous studies have investigated the underlying pathway of fucoidan-induced apoptosis. The extrinsic pathway, which is initiated by activation of death receptors leading to cleavage of caspase-8, was induced by fucoidan in the MCF-7 breast cancer cell line [191]. In contrast, Zhang et al. found that fucoidan from Cladosiphono kamuranus induced apoptosis in MCF-7 through a caspase-independent pathway involving mitochondrial permeabilization, activation of Bcl-2 family proteins, and release of cytochrome c. Thus, they concluded that fucoidan performed its activity through alteration of mitochondrial function. Fucoidan from F. vesiculosus activated caspases via both the death receptor-mediated and mitochondria-mediated apoptotic pathways in human colon cancer cells [185]. Similarly, in HL-60 cells fucoidan treatment induced activation of caspases-8, -9 and -3, the cleavage of Bid, and changed the mitochondrial membrane permeability. In addition MEKK1, MEK1, ERK1/2, and JNK, resulted in important mediators in fucoidan-induced apoptosis of human leukemic cells [190]. Treatment of MDA-MB231 breast cancer cells with low molecular weight fucoidan resulted in a significant decrease in anti-apoptotic proteins Bcl-2, Bcl-xl and Mcl-1 associated with the activation of caspases, mitochondrial dysfunction and alteration of Ca2+ homeostasis [192]. Compared to other sulphated polysaccharides, the fucoidans extracted from the sporophylls of the brown seaweed Undaria pinnatifida showed higher sulphate and L-fucose content, and exhibited a broader range of bioactivities [193]. On human hepatocellular carcinoma (SMMC-7721) cells, Yang et al. demonstrate that the mechanism responsible for fucoidan-induced apoptosis was mediated by ROS. They showed that the increasing ROS production in fucoidan-exposed cells induced mitochondrial oxidative damage, mitochondrial membrane potential depolarization and a release of cytochrome c, with final activation of caspase-9 and caspase-3 [194]. Recently, the same ROS-dependent apoptotic pathway was also reported in human bladder cancer cells (5637HBCC) after treatment with fucoidan. According to the authors, the anticancer effect of fucoidan was related to: (i) increased Bax/Bcl-2 expression ratio; (ii) dissipation of the mitochondrial membrane potential; and (iii) production of intracellular ROS [195]. The pro-apoptotic effects of fucoidan from U. Pinnatifida were also examined on A549 human lung carcinoma cells. In this case, fucoidan was shown to induce caspase-9 activation following upstream downregulation of p38 MAPK and PI3K/Akt pathway [184]. Boo et al. (2013) found that fucoidan isolated from U. Pinnatifida induced apoptotic cell death in human prostate cancer cells (PC-3) through the activation of both intrinsic and extrinsic molecular signalling pathways. Thus, fucoidan treatment led to upregulation of DR5 and cleavage of caspase-8, which are critical in the extrinsic pathway; fucoidan also led to the downregulation of Bcl-2, upregulation of Bax, and activation of caspase-9, which are essential in the intrinsic pathway. Moreover, their results demonstrated that induction of apoptosis following fucoidan addition to in PC-3 cells was associated with downregulation of PI3K/Akt and Wnt/β-catenin signalling pathways [187]. It is interesting to note that some studies have shown that fucoidan and laminaran may enhance the effects of anticancer drugs. For example, the combination of fucoidan with arsenic trioxide (ATO) and ATRA, two well-known synthetic anticancer drugs, significantly reduced the development of the tumour mass in mice bearing NB4 cells (acute promyelocytic leukemia) [196]. Combining fucoidan with standard chemotherapeutic agents resulted in increased apoptosis in cancer cell while reducing chemotherapy-related side effects [192,197]. A recent study showed that fucoidan in combined therapy with cisplatin exerts a greater inhibition of tumour volume in a mouse model of lung cancer. Interesting, an increasing number of clinical results suggested that by combining cisplatin and fucoidan important anticancer effects were obtained in lung cancer patients [198]. Actually, the effect of fucoidan plus platinum-based chemotherapy on quality of life in subjects with NSCLC (stage III and stage IV) (NCT03130829) is under examination by the FDA (https://clinicaltrials.gov/ct2). Innovative fucoidan-coated copper sulphide nanoparticles (F-CuS) have been designed for chemo-photothermal therapy. Fucoidan-coated copper sulphide nanoparticles may potentiate cancer treatment by combining the fucoidan-mediated apoptosis and therapeutic effect of laser irradiation. In addition, using near-infrared light-responsive nanoparticles, the intracellular delivery of fucoidan in cancer cells results also improved. Indeed, this combination effectively eliminated multiple tumours in live mouse models [199].
Laminarans are brown algal polysaccharides mainly composed of β-glucan (β1–3, β1–6-glucan). Beside the structural characteristics, laminarans also exhibit some biological activities of other glucans such as immune-stimulating and anticancer effects, as well as antibacterial activity. Generally, laminarans are present in the cell wall of brown algae (mainly in Laminaria/Saccharina, and to a lesser extent in Ascophyllum and Fucus species) in an amount of about 35% of seaweed dry weight, which can vary according to seaweed species, harvesting season, habitat and extraction method [180]. The antitumor effect of laminarans has been described in different cancer types. Park et al. reported that laminaran obtained from Laminaria japonica inhibited human colon cancer HT-29 cell growth by decreasing cell proliferation and inducing apoptosis. The proposed mechanism of cell death involved Fas-mediated apoptosis because laminaran was found to increase the expression of Fas and FADD protein levels, which in turn induced the activation of caspases cascade. Further results demonstrated that laminaran downregulated IGF-IR, whose overexpression plays a part in cancer cell proliferation and protects cancer cells against apoptosis [200]. In a successive study, laminaran was also shown to induce a dose-dependent sub-G1 and G2-M phase cell cycle arrest, followed by apoptosis in highly proliferative colon cancer cells. The authors found that laminaran antiproliferative effect was dependent on the inhibition of ErbB signalling pathway-related proteins [201]. Similar finding were also confirmed by Ji and Ji, who investigated the underlying mechanisms of laminaran-induced apoptosis in human colon cancer (LoVo) cells. Their results revealed that laminaran induces apoptosis through both extrinsic and intrinsic pathways. Indeed, they observed upregulation of DR-4, DR-5, TRAIL and FADD, which activated caspases-8, -3, -6, and -7 as well as the activation of the mitochondrial pathway, which led to the release of cytocrom c and the activation of caspase-9 and -3 [202,203].
Fucoxanthin is one of the most abundant carotenoids of some brown algae, such as wakame (Undaria pinnatifida) and kombu (Laminaria japonica), which have a long history of use in the diets of Asian cultures and in traditional Chinese medicine. In humans, dietary fucoxanthin is mainly metabolized in the gastrointestinal tract by digestive enzymes to fucoxanthinol, the deacetylated form of fucoxanthin, which is absorbed into intestinal cells. Then, circulating fucoxanthinol is further converted to amarouciaxanthin A in the liver. Fucoxanthinol is considered to be the active form of fucoxanthin and it had a stronger inhibitory effect on the viability of human cancer cells [204]. Fucoxanthins are known to have many health benefits such as anti-inflammatory, anti-obesity, anti-diabetes, hepato-protective and cardiovascular-protective activities [205]. Moreover, accumulating evidence has shown that fucoxanthins and its metabolite fucoxanthinol exert their anti-proliferative and cancer preventive action in several cancer cells and mouse models. The mechanisms of action underlying this anticancer effect mainly relay on the induction of cell-cycle arrest and apoptosis in several tumour cell lines [206,207]. In many cases fucoxanthin was shown to induce cell-cycle arrest during the G0/G1 phase in a dose- and time-dependent manner. The induction of cell-cycle arrest was accompanied to a reduction of the kinase activity of the cyclin D/CDK4 complex. Concomitantly, a caspase-dependent apoptosis was induced, together with downregulation in some apoptosis-related proteins such as Bcl2, XIAP and cellular inhibitor of apoptosis protein 2 (CIAP2). Additionally, the effects of fucoxanthin and fucoxanthinol might be mediated through the inactivation of transcription factor NF-κB or AP-1 [208,209,210,211]. Several studies report that fucoxanthin induced DNA fragmentation in human prostate cancer cells PC-3, DU145 and in human colon cancer cells Caco-2 [212,213] or induced mitochondrial-dependent apoptosis in human promyelocytic leukemia HL-60 [214]. Moreover, it has been reported that fucoxanthin induced caspase-dependent apoptosis following ROS generation and Bcl-XL reduction in HL-60 cells [215]. The same pro-apoptotic effect caspase-mediated was also observed in human bladder cancer cells, in human cervical cancer cells HeLa [216]; in human breast cancer cells MCF-7 and in estrogen-resistant cells MDA-MB-231 [204]. In gastric cancer SGC-7901 cells, fucoxanthin induced autophagy, which in turn promoted apoptosis with caspase-3 cleavage and Bcl-2 downregulation [217]. There is some evidence showing that fucoxanthin could be used to potentiate the action of conventional anticancer drugs. Liu et al. observed that fucoxanthin improved chemotherapeutic efficacy of cisplatin by enhancing cisplatin-induced apoptosis in HepG2 cells.
Fucoxanthin combined with cisplatin also attenuated the expression of DNA repair genes, such as ERCC (excision repair 1), and thymidine phosphorylase, which are responsible of platinum resistance in cancer cells [218]. Temozolomide is an alkylating agent widely used in the therapy of malignant gliomas. However, the main disadvantage related to this drug is the development of resistance. There is evidence that the combination of fucoxanthin with temozolomide could be used to potentiate the action of anticancer drugs in glioblastoma treatment and to overcome the resistance issue. Promising data have been raised also for the treatment of colon cancer through the combination of 5-fluorouracil (5-FU) and fucoxanthin. In fact, it has been demonstrated that the marine compound enhances the cytotoxic effect of 5-FU in colon cancer cells, whereas no marked cytotoxicity was observed in normal colon cell lines (CCD-18Co) [219]. Recently, the effects of TRAIL and fucoxanthin combination treatment has been investigated. TRAIL, a member of the TNF superfamily, can selectively trigger apoptosis in tumour cells and has the ability to circumvent the chemoresistance of conventional therapeutics. Jin et al. [220] found that fucoxanthin enhanced TRAIL sensitivity in TRAIL-resistant human cervical cancer SiHa cells, which resulted in increased cell apoptosis. Other metabolites from Phaeophyta algae have also been described for their anticancer activity.
Bis (2,3-dibromo-4,5-dihydroxybenzyl) ether (BDDE) is a marine bromophenol compound isolated from the marine algae Leathesia nana exhibiting broad and potent cytotoxicity against several cancer cell lines (HeLa, HCT-116, HCT-8, SMMC-7721, A549, and K562 cells). BDDE arrests cell cycle in S phase and apoptosis on K562 cells by a mitochondrial mediated pathway [221]. HFGP from Hizikia fusiformis [86] and LJGP from Laminaria japonica [222] induced apoptosis on HepG2 and HT-29 cells, which was mediated by the Fas signalling pathway. Diphlorethohydroxycarmalol, isolated from Eiseniabicyclis, induced apoptosis on HL60 cells through the accumulation of sub-G1 cell population along with nuclear condensation, the reduction of Bcl-2 expression and the depletion of mitochondrial potential [223].
4. Conclusions
The inhibition of apoptosis is known as one of the hallmarks of cancer. The ability of cancer cells to escape from programmed cell death contributes to tumour growth, promotes tumour cell spread to distal organs and confers resistance to cytotoxic anticancer agents. Therefore, bioactive compounds with the ability to inhibit cancer cell proliferation by activating apoptotic pathways can be employed as potential chemotherapeutic drugs. Marine environments offer an abundant source of new bioactive molecules with unique structural features and a wide range of biological activities, such as antiviral, anti-bacterial, anti-fungal, antiparasitic, anti-oxidant, anti-inflammatory and anti-tumour. Among all marine organisms, sponges and algae represent the richest source of natural marine compounds which have received considerable attention because of their anticancer activity. Several studies have shown that both sponge- and algae-derived compounds inhibit tumour growth and progression through different mechanisms, such as cell-cycle arrest, anti-inflammatory activity, apoptosis, induction of ER stress and inhibition of angiogenesis. In particular, here we reviewed the underlying cellular mechanisms by which these different compounds induce apoptosis of tumour cells. Apoptosis occurs via two principal pathways: the extrinsic (cytoplasmic) pathway whereby death receptors trigger the apoptosis, or the intrinsic (mitochondrial) pathway in which changes in mitochondrial membrane potential lead to cytochrome C release and death signal activation. The two apoptotic pathways converge on caspase-3 activation which is the main effector of apoptosis. The evidence reported in this review highlights the great value of many marine derivatives to promote cell death by activating one or both apoptotic pathways in cancer cells. Moreover, some studies have shown that some of the compounds isolated from sponges or algae may enhance the chemotherapeutic efficacy of commercial anticancer drugs. Therefore, these marine natural products might represent promising sources for new anticancer drug development; however, their utilization as co-adjuvants in therapeutics should also be evaluated.
Author Contributions
G.E. and P.D.C. did the bibliographic research and wrote the manuscript. A.I. critically revised the article for intellectual content.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Simmons T.L., Andrianasolo E., McPhail K., Flatt P., Gerwick W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- 2.Mayer A.M.S., Rodriguez A.D., Taglialatela-Scafati O., Fusetani N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs. 2017;15:273. doi: 10.3390/md15090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann W., Feeney R.J. The Isolation of a New Thymine Pentoside from Sponges1. J. Am. Chem. Soc. 1950;72:2809–2810. doi: 10.1021/ja01162a543. [DOI] [Google Scholar]
- 4.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 5.Altmann K.H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chimia. 2017;71:646–652. doi: 10.2533/chimia.2017.646. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Torres V., Encinar J.A., Herranz-Lopez M., Perez-Sanchez A., Galiano V., Barrajon-Catalan E., Micol V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules. 2017;22:1037. doi: 10.3390/molecules22071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Kumar B., Singh S., Skvortsova I., Kumar V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr. Med. Chem. 2017;24:4729–4752. doi: 10.2174/0929867324666170331123648. [DOI] [PubMed] [Google Scholar]
- 9.Amin A.R., Kucuk O., Khuri F.R., Shin D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer C.M., Singh A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannun Y.A. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997;89:1845–1853. [PubMed] [Google Scholar]
- 12.Galluzzi L., Vicencio J.M., Kepp O., Tasdemir E., Maiuri M.C., Kroemer G. To die or not to die: That is the autophagic question. Curr. Mol. Med. 2008;8:78–91. doi: 10.2174/156652408783769616. [DOI] [PubMed] [Google Scholar]
- 13.Norbury C.J., Hickson I.D. Cellular responses to DNA damage. Ann. Rev. Pharmacol. Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- 14.Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 15.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igney F.H., Krammer P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 17.Sun B.H., Zhao X.P., Wang B.J., Yang D.L., Hao L.J. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J. Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.W., Choi E.J., Joe C.O. Activation of death-inducing signaling complex (DISC) by pro-apoptotic C-terminal fragment of RIP. Oncogene. 2000;19:4491–4499. doi: 10.1038/sj.onc.1203796. [DOI] [PubMed] [Google Scholar]
- 19.Shirley S., Micheau O. Targeting c-FLIP in cancer. Cancer Lett. 2013;332:141–150. doi: 10.1016/j.canlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Hitoshi Y., Lorens J., Kitada S.I., Fisher J., LaBarge M., Ring H.Z., Francke U., Reed J.C., Kinoshita S., Nolan G.P. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/S1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang C., Youle R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.E., Du F., Fang M., Wang X. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc. Natl. Acad. Sci. USA. 2005;102:17545–17550. doi: 10.1073/pnas.0507900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susin S.A., Daugas E., Ravagnan L., Samejima K., Zamzami N., Loeffler M., Costantini P., Ferri K.F., Irinopoulou T., Prevost M.C., et al. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 2000;192:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenzel A., Grespi F., Chmelewskij W., Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis Int. J. Program. Cell Death. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castedo M., Perfettini J.L., Roumier T., Andreau K., Medema R., Kroemer G. Cell death by mitotic catastrophe: A molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 26.Mc Gee M.M. Targeting the Mitotic Catastrophe Signaling Pathway in Cancer. Mediat. Inflamm. 2015;2015:146282. doi: 10.1155/2015/146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoli P., Giannoni E., Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Wree A., Eguchi A., McGeough M.D., Pena C.A., Johnson C.D., Canbay A., Hoffman H.M., Feldstein A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizato N., Luzete B.C., Kiffer L., Correa L.H., de Oliveira Santos I., Assumpcao J.A.F., Ito M.K., Magalhaes K.G. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci. Rep. 2018;8:1952. doi: 10.1038/s41598-018-20422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anjum K., Abbas S.Q., Shah S.A., Akhter N., Batool S., Hassan S.S. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016;24:347–362. doi: 10.4062/biomolther.2016.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essack M., Bajic V.B., Archer J.A. Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment. Mar. Drugs. 2011;9:1580–1606. doi: 10.3390/md9091580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Z. The modification of natural products for medical use. Acta Pharm. Sin. B. 2017;7:119–136. doi: 10.1016/j.apsb.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larghi E.L., Bohn M.L., Kaufman T.S. Aaptamine and related products. Their isolation, chemical syntheses, and biological activity. Tetrahedron. 2009;65:4257–4282. doi: 10.1016/j.tet.2009.03.027. [DOI] [Google Scholar]
- 35.Gul W., Hammond N.L., Yousaf M., Bowling J.J., Schinazi R.F., Wirtz S.S., de Castro Andrews G., Cuevas C., Hamann M.T. Modification at the C9 position of the marine natural product isoaaptamine and the impact on HIV-1, mycobacterial, and tumor cell activity. Bioorgan. Med. Chem. 2006;14:8495–8505. doi: 10.1016/j.bmc.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin M., Zhao W., Zhang Y., Kobayashi M., Duan H., Kong D. Antiproliferative effect of aaptamine on human chronic myeloid leukemia K562 cells. Int. J. Mol. Sci. 2011;12:7352–7359. doi: 10.3390/ijms12117352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyshlovoy S.A., Fedorov S.N., Shubina L.K., Kuzmich A.S., Bokemeyer C., Keller-von Amsberg G., Honecker F. Aaptamines from the marine sponge Aaptos sp. display anticancer activities in human cancer cell lines and modulate AP-1-, NF-kappaB-, and p53-dependent transcriptional activity in mouse JB6 Cl41 cells. BioMed Res. Int. 2014;2014:469309. doi: 10.1155/2014/469309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C.F., Lee M.G., El-Shazly M., Lai K.H., Ke S.C., Su C.W., Shih S.P., Sung P.J., Hong M.C., Wen Z.H., et al. Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress. Mar. Drugs. 2018;16:18. doi: 10.3390/md16010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki S., Kong D., Matsui K., Kobayashi M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res. 2004;24:2325–2330. [PubMed] [Google Scholar]
- 40.Shrestha S., Sorolla A., Fromont J., Blancafort P., Flematti G.R. Crambescidin 800, Isolated from the Marine Sponge Monanchora viridis, Induces Cell Cycle Arrest and Apoptosis in Triple-Negative Breast Cancer Cells. Mar. Drugs. 2018;16:53. doi: 10.3390/md16020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S., Guru S.K., Pathania A.S., Manda S., Kumar A., Bharate S.B., Vishwakarma R.A., Malik F., Bhushan S. Fascaplysin induces caspase mediated crosstalk between apoptosis and autophagy through the inhibition of PI3K/AKT/mTOR signaling cascade in human leukemia HL-60 cells. J. Cell. Biochem. 2015;116:985–997. doi: 10.1002/jcb.25053. [DOI] [PubMed] [Google Scholar]
- 42.Rath B., Hochmair M., Plangger A., Hamilton G. Anticancer Activity of Fascaplysin against Lung Cancer Cell and Small Cell Lung Cancer Circulating Tumor Cell Lines. Mar. Drugs. 2018;16:383. doi: 10.3390/md16100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heiskanen K.M., Bhat M.B., Wang H.W., Ma J., Nieminen A.L. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J. Biol. Chem. 1999;274:5654–5658. doi: 10.1074/jbc.274.9.5654. [DOI] [PubMed] [Google Scholar]
- 44.Ballot C., Kluza J., Lancel S., Martoriati A., Hassoun S.M., Mortier L., Vienne J.C., Briand G., Formstecher P., Bailly C., et al. Inhibition of mitochondrial respiration mediates apoptosis induced by the anti-tumoral alkaloid lamellarin D. Apoptosis Int. J. Program. Cell Death. 2010;15:769–781. doi: 10.1007/s10495-010-0471-2. [DOI] [PubMed] [Google Scholar]
- 45.Barrows L.R., Radisky D.C., Copp B.R., Swaffar D.S., Kramer R.A., Warters R.L., Ireland C.M. Makaluvamines, marine natural products, are active anti-cancer agents and DNA topo II inhibitors. Anti-Cancer Drug Des. 1993;8:333–347. [PubMed] [Google Scholar]
- 46.Wang W., Rayburn E.R., Velu S.E., Nadkarni D.H., Murugesan S., Zhang R. In vitro and in vivo anticancer activity of novel synthetic makaluvamine analogues. Clin. Cancer Res. 2009;15:3511–3518. doi: 10.1158/1078-0432.CCR-08-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radwan M., Hanora A., Khalifa S., Abou-El-Ela S.H. Manzamines: A potential for novel cures. Cell Cycle. 2012;11:1765–1772. doi: 10.4161/cc.20135. [DOI] [PubMed] [Google Scholar]
- 48.Guzman E.A., Johnson J.D., Linley P.A., Gunasekera S.E., Wright A.E. A novel activity from an old compound: Manzamine A reduces the metastatic potential of AsPC-1 pancreatic cancer cells and sensitizes them to TRAIL-induced apoptosis. Investig. New Drugs. 2011;29:777–785. doi: 10.1007/s10637-010-9422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin L.C., Kuo T.T., Chang H.Y., Liu W.S., Hsia S.M., Huang T.C. Manzamine A Exerts Anticancer Activity against Human Colorectal Cancer Cells. Mar. Drugs. 2018;16:252. doi: 10.3390/md16080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzii A.G., Makarieva T.N., Denisenko V.A., Dmitrenok P.S., Kuzmich A.S., Dyshlovoy S.A., Krasokhin V.B., Stonik V.A. Monanchocidin: A new apoptosis-inducing polycyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Organ. Lett. 2010;12:4292–4295. doi: 10.1021/ol101716x. [DOI] [PubMed] [Google Scholar]
- 51.Dyshlovoy S.A., Hauschild J., Amann K., Tabakmakher K.M., Venz S., Walther R., Guzii A.G., Makarieva T.N., Shubina L.K., Fedorov S.N., et al. Marine alkaloid Monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget. 2015;6:17328–17341. doi: 10.18632/oncotarget.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charupant K., Daikuhara N., Saito E., Amnuoypol S., Suwanborirux K., Owa T., Saito N. Chemistry of renieramycins. Part 8: Synthesis and cytotoxicity evaluation of renieramycin M-jorunnamycin A analogues. Bioorgan. Med. Chem. 2009;17:4548–4558. doi: 10.1016/j.bmc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Halim H., Chunhacha P., Suwanborirux K., Chanvorachote P. Anticancer and antimetastatic activities of Renieramycin M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anticancer Res. 2011;31:193–201. [PubMed] [Google Scholar]
- 54.Sakemi S., Sun H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Organ. Chem. 1991;56:4304–4307. doi: 10.1021/jo00013a044. [DOI] [Google Scholar]
- 55.Carbone A., Parrino B., Barraja P., Spano V., Cirrincione G., Diana P., Maier A., Kelter G., Fiebig H.H. Synthesis and antiproliferative activity of 2,5-bis(3′-indolyl)pyrroles, analogues of the marine alkaloid nortopsentin. Mar. Drugs. 2013;11:643–654. doi: 10.3390/md11030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carbone A., Parrino B., Di Vita G., Attanzio A., Spano V., Montalbano A., Barraja P., Tesoriere L., Livrea M.A., Diana P., et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo[2,3-b]pyridines and indolyl-thiazolyl-pyrrolo[2,3-c]pyridines, nortopsentin analogues. Mar. Drugs. 2015;13:460–492. doi: 10.3390/md13010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su J.H., Chang W.B., Chen H.M., El-Shazly M., Du Y.C., Kung T.H., Chen Y.C., Sung P.J., Ho Y.S., Kuo F.W., et al. 10-acetylirciformonin B, a sponge furanoterpenoid, induces DNA damage and apoptosis in leukemia cells. Molecules. 2012;17:11839–11848. doi: 10.3390/molecules171011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma A., Singh K., Almasan A. Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol. Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 59.Wu S.Y., Sung P.J., Chang Y.L., Pan S.L., Teng C.M. Heteronemin, a Spongean Sesterterpene, Induces Cell Apoptosis and Autophagy in Human Renal Carcinoma Cells. BioMed Res. Int. 2015;2015:738241. doi: 10.1155/2015/738241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schumacher M., Cerella C., Eifes S., Chateauvieux S., Morceau F., Jaspars M., Dicato M., Diederich M. Heteronemin, a spongean sesterterpene, inhibits TNF alpha-induced NF-kappa B activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol. 2010;79:610–622. doi: 10.1016/j.bcp.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 61.Lee M.G., Liu Y.C., Lee Y.L., El-Shazly M., Lai K.H., Shih S.P., Ke S.C., Hong M.C., Du Y.C., Yang J.C., et al. Heteronemin, a Marine Sesterterpenoid-Type Metabolite, Induces Apoptosis in Prostate LNcap Cells via Oxidative and ER Stress Combined with the Inhibition of Topoisomerase II and Hsp90. Mar. Drugs. 2018;16:204. doi: 10.3390/md16060204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanida I., Ueno T., Kominami E. LC3 and Autophagy. Methods Mol. Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 63.Su J.Y., Meng Y.H., Zeng L.M., Fu X., Schmitz F.J. Stellettin A, a new triterpenoid pigment from the marine sponge Stelletta tenuis. J. Nat. Prod. 1994;57:1450–1451. doi: 10.1021/np50112a017. [DOI] [PubMed] [Google Scholar]
- 64.Tang S.A., Zhou Q., Guo W.Z., Qiu Y., Wang R., Jin M., Zhang W., Li K., Yamori T., Dan S., et al. In vitro antitumor activity of stellettin B, a triterpene from marine sponge Jaspis stellifera, on human glioblastoma cancer SF295 cells. Mar. Drugs. 2014;12:4200–4213. doi: 10.3390/md12074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W.K., Cheung F.W., Che C.T. Stellettin A induces oxidative stress and apoptosis in HL-60 human leukemia and LNCaP prostate cancer cell lines. J. Nat. Prod. 2006;69:934–937. doi: 10.1021/np058122y. [DOI] [PubMed] [Google Scholar]
- 66.Kong D., Aoki S., Sowa Y., Sakai T., Kobayashi M. Smenospongine, a sesquiterpene aminoquinone from a marine sponge, induces G1 arrest or apoptosis in different leukemia cells. Mar. Drugs. 2008;6:480–488. doi: 10.3390/md20080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang J., Wu W., Yang F., Liu L., Yang Z., Liu L., Tang W., Sun F., Lin H. Marine sponge-derived smenospongine preferentially eliminates breast cancer stem-like cells via p38/AMPKalpha pathways. Cancer Med. 2018;7:3965–3976. doi: 10.1002/cam4.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller J.H., Rouwe B., Gaitanos T.N., Hood K.A., Crume K.P., Backstrom B.T., La Flamme A.C., Berridge M.V., Northcote P.T. Peloruside A enhances apoptosis in H-ras-transformed cells and is cytotoxic to proliferating T cells. Apoptosis Int. J. Program. Cell Death. 2004;9:785–796. doi: 10.1023/B:APPT.0000045789.54694.cf. [DOI] [PubMed] [Google Scholar]
- 69.Mooberry S.L., Tien G., Hernandez A.H., Plubrukarn A., Davidson B.S. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999;59:653–660. [PubMed] [Google Scholar]
- 70.Hamel E., Day B.W., Miller J.H., Jung M.K., Northcote P.T., Ghosh A.K., Curran D.P., Cushman M., Nicolaou K.C., Paterson I., et al. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol. Pharmacol. 2006;70:1555–1564. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Califa N., Bishara A., Kashman Y., Neumann D. Salarin C, a member of the salarin superfamily of marine compounds, is a potent inducer of apoptosis. Investig. New Drugs. 2012;30:98–104. doi: 10.1007/s10637-010-9521-4. [DOI] [PubMed] [Google Scholar]
- 72.Trisciuoglio D., Uranchimeg B., Cardellina J.H., Meragelman T.L., Matsunaga S., Fusetani N., Del Bufalo D., Shoemaker R.H., Melillo G. Induction of apoptosis in human cancer cells by candidaspongiolide, a novel sponge polyketide. J. Natl. Cancer Inst. 2008;100:1233–1246. doi: 10.1093/jnci/djn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitson E.L., Pluchino K.M., Hall M.D., McMahon J.B., McKee T.C. New candidaspongiolides, tedanolide analogues that selectively inhibit melanoma cell growth. Organ. Lett. 2011;13:3518–3521. doi: 10.1021/ol201329p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pimentel A.A., Felibertt P., Sojo F., Colman L., Mayora A., Silva M.L., Rojas H., Dipolo R., Suarez A.I., Compagnone R.S., et al. The marine sponge toxin agelasine B increases the intracellular Ca(2+) concentration and induces apoptosis in human breast cancer cells (MCF-7) Cancer Chemother. Pharmacol. 2012;69:71–83. doi: 10.1007/s00280-011-1677-x. [DOI] [PubMed] [Google Scholar]
- 76.Choi H.J., Bae S.J., Kim N.D., Jung J.H., Choi Y.H. Induction of apoptosis by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., in human skin melanoma cells. Int. J. Mol. Med. 2004;14:1091–1096. doi: 10.3892/ijmm.14.6.1091. [DOI] [PubMed] [Google Scholar]
- 77.Park C., Kim G.Y., Kim G.D., Lee W.H., Cheong J.H., Kim N.D., Bae S.J., Jung J.H., Choi Y.H. Suppression of U937 human monocytic leukemia cell growth by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., via induction of Cdk inhibitor p16 and down-regulation of pRB phosphorylation. Oncol. Rep. 2006;16:171–176. doi: 10.3892/or.16.1.171. [DOI] [PubMed] [Google Scholar]
- 78.Ebada S.S., Wray V., de Voogd N.J., Deng Z., Lin W., Proksch P. Two new jaspamide derivatives from the marine sponge Jaspis splendens. Mar. Drugs. 2009;7:434–444. doi: 10.3390/md7030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Odaka C., Sanders M.L., Crews P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin. Diagn. Lab. Immunol. 2000;7:947–952. doi: 10.1128/CDLI.7.6.947-952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Posey S.C., Bierer B.E. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J. Biol. Chem. 1999;274:4259–4265. doi: 10.1074/jbc.274.7.4259. [DOI] [PubMed] [Google Scholar]
- 81.Lu H., Huang H. FOXO1: A potential target for human diseases. Curr. Drug Targets. 2011;12:1235–1244. doi: 10.2174/138945011796150280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berry E., Hardt J.L., Clardy J., Lurain J.R., Kim J.J. Induction of apoptosis in endometrial cancer cells by psammaplysene A involves FOXO1. Gynecol. Oncol. 2009;112:331–336. doi: 10.1016/j.ygyno.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez de Mattos S., Villalonga P., Clardy J., Lam E.W. FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol. Cancer Ther. 2008;7:3237–3246. doi: 10.1158/1535-7163.MCT-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H., Yin J., Wang C., Gu Y., Deng M., He Z. FOXO3a mediates the cytotoxic effects of cisplatin in lung cancer cells. Anti-Cancer Drugs. 2014;25:898–907. doi: 10.1097/CAD.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 85.Tandon P., Jin Q., Huang L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Fact. 2017;16:219. doi: 10.1186/s12934-017-0834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alves C., Silva J., Pinteus S., Gaspar H., Alpoim M.C., Botana L.M., Pedrosa R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018;9:777. doi: 10.3389/fphar.2018.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown E.S., Allsopp P.J., Magee P.J., Gill C.I., Nitecki S., Strain C.R., McSorley E.M. Seaweed and human health. Nutr. Rev. 2014;72:205–216. doi: 10.1111/nure.12091. [DOI] [PubMed] [Google Scholar]
- 88.Singh R.K., Tiwari S.P., Rai A.K., Mohapatra T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011;64:401–412. doi: 10.1038/ja.2011.21. [DOI] [PubMed] [Google Scholar]
- 89.Shah S.A.A., Akhter N., Auckloo B.N., Khan I., Lu Y., Wang K., Wu B., Guo Y.W. Structural Diversity, Biological Properties and Applications of Natural Products from Cyanobacteria. A Review. Mar. Drugs. 2017;15:354. doi: 10.3390/md15110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costa M., Costa-Rodrigues J., Fernandes M.H., Barros P., Vasconcelos V., Martins R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs. 2012;10:2181–2207. doi: 10.3390/md10102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan L.T. Filamentous tropical marine cyanobacteria: A rich source of natural products for anticancer drug discovery. J. Appl. Phycol. 2010;22:659–676. doi: 10.1007/s10811-010-9506-x. [DOI] [Google Scholar]
- 92.Luesch H., Chanda S.K., Raya R.M., DeJesus P.D., Orth A.P., Walker J.R., Izpisua Belmonte J.C., Schultz P.G. A functional genomics approach to the mode of action of apratoxin A. Nat. Chem. Biol. 2006;2:158–167. doi: 10.1038/nchembio769. [DOI] [PubMed] [Google Scholar]
- 93.Matthew S., Schupp P.J., Luesch H. Apratoxin E, a Cytotoxic Peptolide from a Guamanian Collection of the Marine Cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008;71:1113–1116. doi: 10.1021/np700717s. [DOI] [PubMed] [Google Scholar]
- 94.Tripathi A., Puddick J., Prinsep M.R., Rottmann M., Chan K.P., Chen D.Y., Tan L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry. 2011;72:2369–2375. doi: 10.1016/j.phytochem.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Tripathi A., Fang W., Leong D.T., Tan L.T. Biochemical studies of the lagunamides, potent cytotoxic cyclic depsipeptides from the marine cyanobacterium Lyngbya majuscula. Mar. Drugs. 2012;10:1126–1137. doi: 10.3390/md10051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marquez B.L., Watts K.S., Yokochi A., Roberts M.A., Verdier-Pinard P., Jimenez J.I., Hamel E., Scheuer P.J., Gerwick W.H. Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J. Nat. Prod. 2002;65:866–871. doi: 10.1021/np0106283. [DOI] [PubMed] [Google Scholar]
- 97.Han B.N., McPhail K.L., Gross H., Goeger D.E., Mooberry S.L., Gerwick W.H. Isolation and structure of five lyngbyabellin derivatives from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron. 2005;61:11723–11729. doi: 10.1016/j.tet.2005.09.036. [DOI] [Google Scholar]
- 98.Luesch H., Moore R.E., Paul V.J., Mooberry S.L., Corbett T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001;64:907–910. doi: 10.1021/np010049y. [DOI] [PubMed] [Google Scholar]
- 99.Catassi A., Cesario A., Arzani D., Menichini P., Alama A., Bruzzo C., Imperatori A., Rotolo N., Granone P., Russo P. Characterization of apoptosis induced by marine natural products in non small cell lung cancer A549 cells. Cell. Mol. Life Sci. CMLS. 2006;63:2377–2386. doi: 10.1007/s00018-006-6264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mooberry S.L., Leal R.M., Tinley T.L., Luesch H., Moore R.E., Corbett T.H. The molecular pharmacology of symplostatin 1: A new antimitotic dolastatin 10 analog. Int. J. Cancer. 2003;104:512–521. doi: 10.1002/ijc.10982. [DOI] [PubMed] [Google Scholar]
- 101.Sumimoto S., Iwasaki A., Ohno O., Sueyosh K., Teruya T., Suenaga K. Kanamienamide, an Enamide with an Enol Ether from the Marine Cyanobacterium Moorea bouillonii. Organ. Lett. 2016;18:4884–4887. doi: 10.1021/acs.orglett.6b02364. [DOI] [PubMed] [Google Scholar]
- 102.Walter J.M., Coutinho F.H., Dutilh B.E., Swings J., Thompson F.L., Thompson C.C. Ecogenomics and Taxonomy of Cyanobacteria Phylum. Front. Microbiol. 2017;8:2132. doi: 10.3389/fmicb.2017.02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hao S., Yan Y., Li S., Zhao L., Zhang C., Liu L., Wang C. The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells. Mar. Drugs. 2018;16:178. doi: 10.3390/md16060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy K.R., Arunasree K.M., Reddy N.P., Dheeraj B., Reddy G.V., Reddanna P. Alteration of mitochondrial membrane potential by Spirulina platensis C-phycocyanin induces apoptosis in the doxorubicinresistant human hepatocellular-carcinoma cell line HepG2. Biotechnol. Appl. Biochem. 2007;47:159–167. doi: 10.1042/BA20060206. [DOI] [PubMed] [Google Scholar]
- 105.Wang H., Liu Y., Gao X., Carter C.L., Liu Z.R. The recombinant beta subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Lett. 2007;247:150–158. doi: 10.1016/j.canlet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Li B., Chu X., Gao M., Li W. Apoptotic mechanism of MCF-7 breast cells in vivo and in vitro induced by photodynamic therapy with C-phycocyanin. Acta Biochim. Biophys. Sin. 2010;42:80–89. doi: 10.1093/abbs/gmp104. [DOI] [PubMed] [Google Scholar]
- 107.Jiang L., Wang Y., Liu G., Liu H., Zhu F., Ji H., Li B. C-Phycocyanin exerts anti-cancer effects via the MAPK signaling pathway in MDA-MB-231 cells. Cancer Cell Int. 2018;18:12. doi: 10.1186/s12935-018-0511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subhashini J., Mahipal S.V., Reddy M.C., Mallikarjuna Reddy M., Rachamallu A., Reddanna P. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem. Pharmacol. 2004;68:453–462. doi: 10.1016/j.bcp.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 109.Saini M.K., Vaiphei K., Sanyal S.N. Chemoprevention of DMH-induced rat colon carcinoma initiation by combination administration of piroxicam and C-phycocyanin. Mol. Cell. Biochem. 2012;361:217–228. doi: 10.1007/s11010-011-1106-9. [DOI] [PubMed] [Google Scholar]
- 110.Yang F., Li B., Chu X.M., Lv C.Y., Xu Y.J., Yang P. Molecular mechanism of inhibitory effects of C-phycocyanin combined with all-trans-retinoic acid on the growth of HeLa cells in vitro. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014;35:5619–5628. doi: 10.1007/s13277-014-1744-0. [DOI] [PubMed] [Google Scholar]
- 111.Bingula R., Dupuis C., Pichon C., Berthon J.Y., Filaire M., Pigeon L., Filaire E. Study of the Effects of Betaine and/or C-Phycocyanin on the Growth of Lung Cancer A549 Cells In Vitro and In Vivo. J. Oncol. 2016;2016:8162952. doi: 10.1155/2016/8162952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bharathiraja S., Seo H., Manivasagan P., Santha Moorthy M., Park S., Oh J. In Vitro Photodynamic Effect of Phycocyanin against Breast Cancer Cells. Molecules. 2016;21:1470. doi: 10.3390/molecules21111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang C.Y., Wang X., Wang Y., Zhou T., Bai Y., Li Y.C., Huang B. Photosensitization of phycocyanin extracted from Microcystis in human hepatocellular carcinoma cells: Implication of mitochondria-dependent apoptosis. J. photochem. photobiol. B Biol. 2012;117:70–79. doi: 10.1016/j.jphotobiol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Chen X.X., Smith G.D., Waring P. Human cancer cell (Jurkat) killing by the cyanobacterial metabolite calothrixin A. J. Appl. Phycol. 2003;15:269–277. doi: 10.1023/A:1025134106985. [DOI] [Google Scholar]
- 115.Hatae N., Satoh R., Chiba H., Osaki T., Nishiyama T., Ishikura M., Abe T., Hibino S., Choshi T., Okada C., et al. N-Substituted calothrixin B derivatives inhibited the proliferation of HL-60 promyelocytic leukemia cells. Med. Chem. Res. 2014;23:4956–4961. doi: 10.1007/s00044-014-1061-6. [DOI] [Google Scholar]
- 116.Trimurtulu G., Ohtani I., Patterson G.M.L., Moore R.E., Corbett T.H., Valeriote F.A., Demchik L. Total Structures of Cryptophycins, Potent Antitumor Depsipeptides from the Blue-Green-Alga Nostoc Sp Strain Gsv-224. J. Am. Chem. Soc. 1994;116:4729–4737. doi: 10.1021/ja00090a020. [DOI] [Google Scholar]
- 117.Wagner M.M., Paul D.C., Shih C., Jordan M.A., Wilson L., Williams D.C. In vitro pharmacology of cryptophycin 52 (LY355703) in human tumor cell lines. Cancer Chemother. Pharmacol. 1999;43:115–125. doi: 10.1007/s002800050871. [DOI] [PubMed] [Google Scholar]
- 118.Drew L., Fine R.L., Do T.N., Douglas G.P., Petrylak D.P. The novel antimicrotubule agent cryptophycin 52 (LY355703) induces apoptosis via multiple pathways in human prostate cancer cells. Clin. Cancer Res. 2002;8:3922–3932. [PubMed] [Google Scholar]
- 119.Mooberry S.L., Busquets L., Tien G. Induction of apoptosis by cryptophycin 1, a new antimicrotubule agent. Int. J. Cancer. 1997;73:440–448. doi: 10.1002/(SICI)1097-0215(19971104)73:3<440::AID-IJC20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 120.Voracova K., Hajek J., Mares J., Urajova P., Kuzma M., Cheel J., Villunger A., Kapuscik A., Bally M., Novak P., et al. The cyanobacterial metabolite nocuolin a is a natural oxadiazine that triggers apoptosis in human cancer cells. PLoS ONE. 2017;12:e0172850. doi: 10.1371/journal.pone.0172850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Medina R.A., Goeger D.E., Hills P., Mooberry S.L., Huang N., Romero L.I., Ortega-Barria E., Gerwick W.H., McPhail K.L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008;130:6324–6325. doi: 10.1021/ja801383f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serrill J.D., Wan X., Hau A.M., Jang H.S., Coleman D.J., Indra A.K., Alani A.W., McPhail K.L., Ishmael J.E. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Investig. New Drugs. 2016;34:24–40. doi: 10.1007/s10637-015-0303-x. [DOI] [PubMed] [Google Scholar]
- 123.Hau A.M., Greenwood J.A., Lohr C.V., Serrill J.D., Proteau P.J., Ganley I.G., McPhail K.L., Ishmael J.E. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS ONE. 2013;8:e65250. doi: 10.1371/journal.pone.0065250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plaza M., Herrero M., Cifuentes A., Ibanez E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009;57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- 125.Skjanes K., Rebours C., Lindblad P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit. Rev. Biotechnol. 2013;33:172–215. doi: 10.3109/07388551.2012.681625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Panahi Y., Darvishi B., Jowzi N., Beiraghdar F., Sahebkar A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016;22:164–173. doi: 10.2174/1381612822666151112145226. [DOI] [PubMed] [Google Scholar]
- 127.Yusof Y.A., Saad S.M., Makpol S., Shamaan N.A., Ngah W.Z. Hot water extract of Chlorella vulgaris induced DNA damage and apoptosis. Clinics. 2010;65:1371–1377. doi: 10.1590/S1807-59322010001200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Z.D., Liang K., Li K., Wang G.Q., Zhang K.W., Cai L., Zhai S.T., Chou K.C. Chlorella vulgaris Induces Apoptosis of Human Non-Small Cell Lung Carcinoma (NSCLC) Cells. Med. Chem. 2017;13:560–568. doi: 10.2174/1573406413666170510102024. [DOI] [PubMed] [Google Scholar]
- 129.Mohd Azamai E.S., Sulaiman S., Mohd Habib S.H., Looi M.L., Das S., Abdul Hamid N.A., Wan Ngah W.Z., Mohd Yusof Y.A. Chlorella vulgaris triggers apoptosis in hepatocarcinogenesis-induced rats. J. Zhejiang Univ. Sci. B. 2009;10:14–21. doi: 10.1631/jzus.B0820168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin P.Y., Tsai C.T., Chuang W.L., Chao Y.H., Pan I.H., Chen Y.K., Lin C.C., Wang B.Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017;17:88. doi: 10.1186/s12906-017-1611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kubatka P., Kapinova A., Kruzliak P., Kello M., Vybohova D., Kajo K., Novak M., Chripkova M., Adamkov M., Pec M., et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition. 2015;31:560–569. doi: 10.1016/j.nut.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto H., Uragami C., Cogdell R.J. Carotenoids and Photosynthesis. Sub-Cell. Biochem. 2016;79:111–139. doi: 10.1007/978-3-319-39126-7_4. [DOI] [PubMed] [Google Scholar]
- 133.Cha K.H., Koo S.Y., Lee D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008;56:10521–10526. doi: 10.1021/jf802111x. [DOI] [PubMed] [Google Scholar]
- 134.Renju G.L., Muraleedhara Kurup G., Bandugula V.R. Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014;35:10747–10758. doi: 10.1007/s13277-014-2339-5. [DOI] [PubMed] [Google Scholar]
- 135.Murthy K.N.C., Vanitha A., Rajesha J., Swamy M.M., Sowmya P.R., Ravishankar G.A. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005;76:1381–1390. doi: 10.1016/j.lfs.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 136.Hu C.C., Lin J.T., Lu F.J., Chou F.P., Yang D.J. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008;109:439–446. doi: 10.1016/j.foodchem.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 137.Emtyazjoo M., Moghadasi Z., Rabbani M., Emtyazjoo M., Samadi S., Mossaffa N. Anticancer effect of Dunaliella salina under stress and normal conditions against skin carcinoma cell line A431 in vitro. Iran. J. Fish. Sci. 2012;11:283–293. [Google Scholar]
- 138.Atasever-Arslan B., Yilancioglu K., Bekaroglu M.G., Taskin E., Altinoz E., Cetiner S. Cytotoxic effect of extract from Dunaliella salina against SH-SY5Y neuroblastoma cells. Gen. Physiol. Biophys. 2015;34:201–207. doi: 10.4149/gpb_2014034. [DOI] [PubMed] [Google Scholar]
- 139.Xue L.X. [Experimental study on extract of Dunaliella salina in preventing NSAR-induced cancer of proventriculus in mice] Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prevent. Med.] 1993;27:350–353. [PubMed] [Google Scholar]
- 140.Singh P., Baranwal M., Reddy S.M. Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharm. Biol. 2016;54:2269–2275. doi: 10.3109/13880209.2016.1153660. [DOI] [PubMed] [Google Scholar]
- 141.Sheu M.J., Huang G.J., Wu C.H., Chen J.S., Chang H.Y., Chang S.J., Chung J.G. Ethanol extract of Dunaliella salina induces cell cycle arrest and apoptosis in A549 human non-small cell lung cancer cells. In Vivo. 2008;22:369–378. [PubMed] [Google Scholar]
- 142.Srinivasan R., Chaitanyakumar A., Mageswari A., Gomathi A., Pavan Kumar J.G.S., Jayasindu M., Bharath G., Shravan J.S., Gothandam K.M. Oral administration of lyophilized Dunaliella salina, a carotenoid-rich marine alga, reduces tumor progression in mammary cancer induced rats. Food Funct. 2017;8:4517–4527. doi: 10.1039/C7FO01328K. [DOI] [PubMed] [Google Scholar]
- 143.Fan M., Nath A.K., Tang Y., Choi Y.J., Debnath T., Choi E.J., Kim E.K. Investigation of the Anti-Prostate Cancer Properties of Marine-Derived Compounds. Mar. Drugs. 2018;16:160. doi: 10.3390/md16050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen G., Niu X., Chen X., Li L., Kuang T., Li S. Characterization of chlorophyll-protein complexes isolated from a Siphonous green alga, Bryopsis corticulans. Photosynth. Res. 2008;96:75–81. doi: 10.1007/s11120-007-9286-6. [DOI] [PubMed] [Google Scholar]
- 145.Ganesan P., Noda K., Manabe Y., Ohkubo T., Tanaka Y., Maoka T., Sugawara T., Hirata T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta. 2011;1810:497–503. doi: 10.1016/j.bbagen.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 146.Rubinstein I., John Goad L. Sterols of the siphonous marine alga Codium fragile. Phytochemistry. 1974;13:481–484. doi: 10.1016/S0031-9422(00)91238-X. [DOI] [Google Scholar]
- 147.Kim A.D., Lee Y., Kang S.H., Kim G.Y., Kim H.S., Hyun J.W. Cytotoxic effect of clerosterol isolated from Codium fragile on A2058 human melanoma cells. Mar. Drugs. 2013;11:418–430. doi: 10.3390/md11020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fedorov S.N., Ermakova S.P., Zvyagintseva T.N., Stonik V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs. 2013;11:4876–4901. doi: 10.3390/md11124876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaeffer B., Benard C., Lahaye M., Blottiere H.M., Cherbut C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med. 1999;65:527–531. doi: 10.1055/s-1999-14009. [DOI] [PubMed] [Google Scholar]
- 150.Ahmed O.M., Rasha R.A. Anti-Proliferative and Apoptotic Efficacies of Ulvan Polysaccharides against Different Types of Carcinoma Cells In Vitro and In Vivo. J. Cancer Sci. Ther. 2014;6:202–208. doi: 10.4172/1948-5956.1000272. [DOI] [Google Scholar]
- 151.Abd-Ellatef G.F., Ahmed O.M., Abdel-Reheim E.S., Abdel-Hamid A.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer. 2017;9:67–83. doi: 10.2147/BCTT.S125165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tabarsa M., Han J.H., Kim C.Y., You S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food. 2012;15:135–144. doi: 10.1089/jmf.2011.1716. [DOI] [PubMed] [Google Scholar]
- 153.El-Bayoumy K., Sinha R. Mechanisms of mammary cancer chemoprevention by organoselenium compounds. Mutat. Res. 2004;551:181–197. doi: 10.1016/j.mrfmmm.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 154.Sun X., Zhong Y., Luo H., Yang Y. Selenium-Containing Polysaccharide-Protein Complex in Se-Enriched Ulva fasciata Induces Mitochondria-Mediated Apoptosis in A549 Human Lung Cancer Cells. Mar. Drugs. 2017;15:215. doi: 10.3390/md15070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X., Chen Y., Wang J., Liu Z., Zhao S. Antitumor activity of a sulfated polysaccharide from Enteromorpha intestinalis targeted against hepatoma through mitochondrial pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014;35:1641–1647. doi: 10.1007/s13277-013-1226-9. [DOI] [PubMed] [Google Scholar]
- 156.Kwon M.J., Nam T.J. A polysaccharide of the marine alga Capsosiphon fulvescens induces apoptosis in AGS gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway. Cell Biol. Int. 2007;31:768–775. doi: 10.1016/j.cellbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 157.Thangam R., Senthilkumar D., Suresh V., Sathuvan M., Sivasubramanian S., Pazhanichamy K., Gorlagunta P.K., Kannan S., Gunasekaran P., Rengasamy R., et al. Induction of ROS-dependent mitochondria-mediated intrinsic apoptosis in MDA-MB-231 cells by glycoprotein from Codium decorticatum. J. Agric. Food Chem. 2014;62:3410–3421. doi: 10.1021/jf405329e. [DOI] [PubMed] [Google Scholar]
- 158.Lee J.C., Hou M.F., Huang H.W., Chang F.R., Yeh C.C., Tang J.Y., Chang H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013;13:55. doi: 10.1186/1475-2867-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Magalhaes K.D., Costa L.S., Fidelis G.P., Oliveira R.M., Nobre L.T., Dantas-Santos N., Camara R.B., Albuquerque I.R., Cordeiro S.L., Sabry D.A., et al. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011;12:3352–3365. doi: 10.3390/ijms12053352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suzuki M., Takahashi Y., Nakano S., Abe T., Masuda M., Ohnishi T., Noya Y., Seki K. An experimental approach to study the biosynthesis of brominated metabolites by the red algal genus Laurencia. Phytochemistry. 2009;70:1410–1415. doi: 10.1016/j.phytochem.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 161.Dias D.A., Urban S. Phytochemical studies of the southern Australian marine alga, Laurencia elata. Phytochemistry. 2011;72:2081–2089. doi: 10.1016/j.phytochem.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 162.Barcellos Marini M., Rodrigues de Freitas W., Lacerda da Silva Machado F., Correa Ramos Leal I., Ribeiro Soares A., Masahiko Kanashiro M., Frazao Muzitano M. Cytotoxic activity of halogenated sesquiterpenes from Laurencia dendroidea. Phytother. Res. PTR. 2018;32:1119–1125. doi: 10.1002/ptr.6052. [DOI] [PubMed] [Google Scholar]
- 163.Campos A., Souza C.B., Lhullier C., Falkenberg M., Schenkel E.P., Ribeiro-do-Valle R.M., Siqueira J.M. Anti-tumour effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J. Pharm. Pharmacol. 2012;64:1146–1154. doi: 10.1111/j.2042-7158.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- 164.Peters T.L., Tillotson J., Yeomans A.M., Wilmore S., Lemm E., Jimenez-Romero C., Amador L.A., Li L., Amin A.D., Pongtornpipat P., et al. Target-Based Screening against eIF4A1 Reveals the Marine Natural Product Elatol as a Novel Inhibitor of Translation Initiation with In Vivo Antitumor Activity. Clin. Cancer Res. 2018;24:4256–4270. doi: 10.1158/1078-0432.CCR-17-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim M.M., Mendis E., Kim S.K. Laurencia okamurai extract containing laurinterol induces apoptosis in melanoma cells. J. Med. Food. 2008;11:260–266. doi: 10.1089/jmf.2007.575. [DOI] [PubMed] [Google Scholar]
- 166.Pec M.K., Aguirre A., Moser-Thier K., Fernandez J.J., Souto M.L., Dorta J., Diaz-Gonzalez F., Villar J. Induction of apoptosis in estrogen dependent and independent breast cancer cells by the marine terpenoid dehydrothyrsiferol. Biochem. Pharmacol. 2003;65:1451–1461. doi: 10.1016/S0006-2952(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 167.Tarhouni-Jabberi S., Zakraoui O., Ioannou E., Riahi-Chebbi I., Haoues M., Roussis V., Kharrat R., Essafi-Benkhadir K. Mertensene, a Halogenated Monoterpene, Induces G2/M Cell Cycle Arrest and Caspase Dependent Apoptosis of Human Colon Adenocarcinoma HT29 Cell Line through the Modulation of ERK-1/-2, AKT and NF-kappaB Signaling. Mar. Drugs. 2017;15:221. doi: 10.3390/md15070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kubanek J., Prusak A.C., Snell T.W., Giese R.A., Hardcastle K.I., Fairchild C.R., Aalbersberg W., Raventos-Suarez C., Hay M.E. Antineoplastic diterpene-benzoate macrolides from the Fijian red alga Callophycus serratus. Organ. Lett. 2005;7:5261–5264. doi: 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kubanek J., Prusak A.C., Snell T.W., Giese R.A., Fairchild C.R., Aalbersberg W., Hay M.E. Bromophycolides C-I from the Fijian red alga Callophycus serratus. J. Nat. Prod. 2006;69:731–735. doi: 10.1021/np050463o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Patel S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech. 2012;2:171–185. doi: 10.1007/s13205-012-0061-9. [DOI] [Google Scholar]
- 171.Jazzara M., Ghannam A., Soukkarieh C., Murad H. Anti-Proliferative Activity of lambda-Carrageenan Through the Induction of Apoptosis in Human Breast Cancer Cells. Iran. J. Cancer Prevent. 2016;9:e3836. doi: 10.17795/ijcp-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ariffin S.H., Yeen W.W., Abidin I.Z., Abdul Wahab R.M., Ariffin Z.Z., Senafi S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complement. Altern. Med. 2014;14:508. doi: 10.1186/1472-6882-14-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murad H., Ghannam A., Al-Ktaifani M., Abbas A., Hawat M. Algal sulfated carrageenan inhibits proliferation of MDA-MB-231 cells via apoptosis regulatory genes. Mol. Med. Rep. 2015;11:2153–2158. doi: 10.3892/mmr.2014.2915. [DOI] [PubMed] [Google Scholar]
- 174.Ghannam A., Murad H., Jazzara M., Odeh A., Allaf A.W. Isolation, Structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. Int. J. Biol. Macromol. 2018;108:916–926. doi: 10.1016/j.ijbiomac.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 175.Lee J.H., Park S.E., Hossain M.A., Kim M.Y., Kim M.N., Chung H.Y., Choi J.S., Yoo Y.H., Kim N.D. 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether induces growth inhibition and apoptosis in MCF-7 human breast cancer cells. Arch. Pharm. Res. 2007;30:1132–1137. doi: 10.1007/BF02980248. [DOI] [PubMed] [Google Scholar]
- 176.Fukuda Y., Sugahara T., Ueno M., Fukuta Y., Ochi Y., Akiyama K., Miyazaki T., Masuda S., Kawakubo A., Kato K. The anti-tumor effect of Euchema serra agglutinin on colon cancer cells in vitro and in vivo. Anti-Cancer Drugs. 2006;17:943–947. doi: 10.1097/01.cad.0000224458.13651.b4. [DOI] [PubMed] [Google Scholar]
- 177.Sugahara T., Ohama Y., Fukuda A., Hayashi M., Kawakubo A., Kato K. The cytotoxic effect of Eucheuma serra agglutinin (ESA) on cancer cells and its application to molecular probe for drug delivery system using lipid vesicles. Cytotechnology. 2001;36:93–99. doi: 10.1023/A:1014057407251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hayashi K., Walde P., Miyazaki T., Sakayama K., Nakamura A., Kameda K., Masuda S., Umakoshi H., Kato K. Active Targeting to Osteosarcoma Cells and Apoptotic Cell Death Induction by the Novel Lectin Eucheuma serra Agglutinin Isolated from a Marine Red Alga. J. Drug Deliv. 2012;2012:842785. doi: 10.1155/2012/842785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Omokawa Y., Miyazaki T., Walde P., Akiyama K., Sugahara T., Masuda S., Inada A., Ohnishi Y., Saeki T., Kato K. In vitro and in vivo anti-tumor effects of novel Span 80 vesicles containing immobilized Eucheuma serra agglutinin. Int. J. Pharm. 2010;389:157–167. doi: 10.1016/j.ijpharm.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 180.Sanjeewa K.K.A., Lee J.S., Kim W.S., Jeon Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017;177:451–459. doi: 10.1016/j.carbpol.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 181.Imbs T.I., Ermakova S.P., Malyarenko Vishchuk O.S., Isakov V.V., Zvyagintseva T.N. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydr. Polym. 2016;135:162–168. doi: 10.1016/j.carbpol.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 182.Nishimoto S. Clinical Improvement in Cancer Patients through Integrative Medicine Supplemented Mainly with Low-Molecular Fucoidan. The Fourth Report. J. Int. Soc. Life Inf. Sci. 2016;34:153. doi: 10.18936/islis.34.2_153. [DOI] [Google Scholar]
- 183.Atashrazm F., Lowenthal R.M., Woods G.M., Holloway A.F., Dickinson J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs. 2015;13:2327–2346. doi: 10.3390/md13042327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boo H.J., Hyun J.H., Kim S.C., Kang J.I., Kim M.K., Kim S.Y., Cho H., Yoo E.S., Kang H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. PTR. 2011;25:1082–1086. doi: 10.1002/ptr.3489. [DOI] [PubMed] [Google Scholar]
- 185.Kim E.J., Park S.Y., Lee J.Y., Park J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10:96. doi: 10.1186/1471-230X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aisa Y., Miyakawa Y., Nakazato T., Shibata H., Saito K., Ikeda Y., Kizaki M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005;78:7–14. doi: 10.1002/ajh.20182. [DOI] [PubMed] [Google Scholar]
- 187.Boo H.J., Hong J.Y., Kim S.C., Kang J.I., Kim M.K., Kim E.J., Hyun J.W., Koh Y.S., Yoo E.S., Kwon J.M., et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs. 2013;11:2982–2999. doi: 10.3390/md11082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Banafa A.M., Roshan S., Liu Y.Y., Chen H.J., Chen M.J., Yang G.X., He G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013;33:717–724. doi: 10.1007/s11596-013-1186-8. [DOI] [PubMed] [Google Scholar]
- 189.Haneji K., Matsuda T., Tomita M., Kawakami H., Ohshiro K., Uchihara J.N., Masuda M., Takasu N., Tanaka Y., Ohta T., et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer. 2005;52:189–201. doi: 10.1207/s15327914nc5202_9. [DOI] [PubMed] [Google Scholar]
- 190.Jin J.O., Song M.G., Kim Y.N., Park J.I., Kwak J.Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010;49:771–782. doi: 10.1002/mc.20654. [DOI] [PubMed] [Google Scholar]
- 191.Yamasaki-Miyamoto Y., Yamasaki M., Tachibana H., Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009;57:8677–8682. doi: 10.1021/jf9010406. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Z., Teruya K., Eto H., Shirahata S. Induction of apoptosis by low-molecular-weight fucoidan through calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci. Biotechnol. Biochem. 2013;77:235–242. doi: 10.1271/bbb.120631. [DOI] [PubMed] [Google Scholar]
- 193.Kim K.J., Lee O.H., Lee B.Y. Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010;48:1101–1104. doi: 10.1016/j.fct.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 194.Yang L., Wang P., Wang H., Li Q., Teng H., Liu Z., Yang W., Hou L., Zou X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs. 2013;11:1961–1976. doi: 10.3390/md11061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Han M.H., Lee D.S., Jeong J.W., Hong S.H., Choi I.W., Cha H.J., Kim S., Kim H.S., Park C., Kim G.Y., et al. Fucoidan Induces ROS-Dependent Apoptosis in 5637 Human Bladder Cancer Cells by Downregulating Telomerase Activity via Inactivation of the PI3K/Akt Signaling Pathway. Drug Dev. Res. 2017;78:37–48. doi: 10.1002/ddr.21367. [DOI] [PubMed] [Google Scholar]
- 196.Atashrazm F., Lowenthal R.M., Dickinson J.L., Holloway A.F., Woods G.M. Fucoidan enhances the therapeutic potential of arsenic trioxide and all-trans retinoic acid in acute promyelocytic leukemia, in vitro and in vivo. Oncotarget. 2016;7:46028–46041. doi: 10.18632/oncotarget.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zuo T., Li X., Chang Y., Duan G., Yu L., Zheng R., Xue C., Tang Q. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015;6:415–422. doi: 10.1039/C4FO00567H. [DOI] [PubMed] [Google Scholar]
- 198.Hsu H.Y., Lin T.Y., Hu C.H., Shu D.T.F., Lu M.K. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018;432:112–120. doi: 10.1016/j.canlet.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 199.Jang B., Moorthy M.S., Manivasagan P., Xu L., Song K., Lee K.D., Kwak M., Oh J., Jin J.O. Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget. 2018;9:12649–12661. doi: 10.18632/oncotarget.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Park H.K., Kim I.H., Kim J., Nam T.J. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. Int. J. Mol. Med. 2012;30:734–738. doi: 10.3892/ijmm.2012.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Park H.K., Kim I.H., Kim J., Nam T.J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013;32:291–295. doi: 10.3892/ijmm.2013.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ji C.F., Ji Y.B. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol. Lett. 2014;7:1728–1732. doi: 10.3892/ol.2014.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ji Y.B., Ji C.F., Zhang H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules. 2012;17:9947–9960. doi: 10.3390/molecules17089947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rwigemera A., Mamelona J., Martin L.J. Comparative effects between fucoxanthinol and its precursor fucoxanthin on viability and apoptosis of breast cancer cell lines MCF-7 and MDA-MB-231. Anticancer Res. 2015;35:207–219. [PubMed] [Google Scholar]
- 205.Peng J., Yuan J.P., Wu C.F., Wang J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs. 2011;9:1806–1828. doi: 10.3390/md9101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Satomi Y. Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017;37:1557–1562. doi: 10.21873/anticanres.11484. [DOI] [PubMed] [Google Scholar]
- 207.Martin L.J. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs. 2015;13:4784–4798. doi: 10.3390/md13084784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Das S.K., Hashimoto T., Shimizu K., Yoshida T., Sakai T., Sowa Y., Komoto A., Kanazawa K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta. 2005;1726:328–335. doi: 10.1016/j.bbagen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 209.Das S.K., Hashimoto T., Kanazawa K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim. Biophys. Acta. 2008;1780:743–749. doi: 10.1016/j.bbagen.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 210.Ishikawa C., Tafuku S., Kadekaru T., Sawada S., Tomita M., Okudaira T., Nakazato T., Toda T., Uchihara J.N., Taira N., et al. Anti-adult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer. 2008;123:2702–2712. doi: 10.1002/ijc.23860. [DOI] [PubMed] [Google Scholar]
- 211.Tafuku S., Ishikawa C., Yasumoto T., Mori N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol. Rep. 2012;28:1512–1518. doi: 10.3892/or.2012.1947. [DOI] [PubMed] [Google Scholar]
- 212.Kotake-Nara E., Kushiro M., Zhang H., Sugawara T., Miyashita K., Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001;131:3303–3306. doi: 10.1093/jn/131.12.3303. [DOI] [PubMed] [Google Scholar]
- 213.Hosokawa M., Kudo M., Maeda H., Kohno H., Tanaka T., Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta. 2004;1675:113–119. doi: 10.1016/j.bbagen.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 214.Kotake-Nara E., Terasaki M., Nagao A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem. 2005;69:224–227. doi: 10.1271/bbb.69.224. [DOI] [PubMed] [Google Scholar]
- 215.Kim K.N., Heo S.J., Kang S.M., Ahn G., Jeon Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA. 2010;24:1648–1654. doi: 10.1016/j.tiv.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 216.Ye G., Lu Q., Zhao W., Du D., Jin L., Liu Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014;35:11261–11267. doi: 10.1007/s13277-014-2337-7. [DOI] [PubMed] [Google Scholar]
- 217.Zhu Y., Cheng J., Min Z., Yin T., Zhang R., Zhang W., Hu L., Cui Z., Gao C., Xu S., et al. Effects of fucoxanthin on autophagy and apoptosis in SGC-7901cells and the mechanism. J. Cell. Biochem. 2018;119:7274–7284. doi: 10.1002/jcb.27022. [DOI] [PubMed] [Google Scholar]
- 218.Liu C.L., Lim Y.P., Hu M.L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFkappaB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs. 2013;11:50–66. doi: 10.3390/md11010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lopes-Costa E., Abreu M., Gargiulo D., Rocha E., Ramos A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Health Part A. 2017;80:776–787. doi: 10.1080/15287394.2017.1357297. [DOI] [PubMed] [Google Scholar]
- 220.Jin Y., Qiu S., Shao N., Zheng J. Fucoxanthin and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Synergistically Promotes Apoptosis of Human Cervical Cancer Cells by Targeting PI3K/Akt/NF-kappaB Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:11–18. doi: 10.12659/MSM.905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu M., Zhang W., Wei J., Qiu L., Lin X. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012;211:126–134. doi: 10.1016/j.toxlet.2012.03.771. [DOI] [PubMed] [Google Scholar]
- 222.Go H., Hwang H.J., Nam T.J. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. Toxicology In Vitro. 2010;24:1546–1553. doi: 10.1016/j.tiv.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 223.Zou Y., Qian Z.J., Li Y., Kim M.M., Lee S.H., Kim S.K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008;56:7001–7009. doi: 10.1021/jf801133h. [DOI] [PubMed] [Google Scholar]


