Abstract
Acute myeloid leukemia (AML) is an aggressive malignancy, which is highly heterogeneous with regard to chemosensitivity and biological features. The AML cell population is organized in a hierarchy that is reflected in the in vitro growth characteristics, with only a minority of cells being able to proliferate for more than two weeks. In this study, we investigated the ability of AML stem cells to survive and proliferate in suspension cultures in the presence of exogenous mediators but without supporting non-leukemic cells. We saw that a high number of maintained stem cells (i.e., a large number of clonogenic cells after five weeks of culture) was associated with decreased overall survival for patients receiving intensive chemotherapy; this prognostic impact was also detected in the multivariate/adjusted analysis. Furthermore, the patients with many clonogenic cells presented more frequently with mutations in transcription-related genes, and also showed a higher abundance of proteins involved in transcription at the time of diagnosis. In conclusion, the growth characteristics of the long-term proliferating leukemic stem cells seem to have an independent prognostic impact in human AML, and these characteristics appear to be reflected by the mutational landscape and the proteome of the patients at the time of diagnosis.
Keywords: acute myeloid leukemia, clonogenic cells, colony formation, cytokines, differentiation, transcription
1. Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy, which mainly affects the elderly. It is characterized by an accumulation of immature myeloblasts in the bone marrow [1]. The backbone of treatment is intensive chemotherapy, potentially combined with stem cell transplantation. Still, overall survival is low due to a high relapse rate, and the high number of elderly or unfit patients, who cannot undergo the intensive and potentially curative treatment [2].
Acute myeloid leukemia is a highly heterogeneous malignancy and prognosis, and thus overall survival is correlated with both cytogenetics and specific gene mutations, such as FMS-related tyrosine kinase 3 internal tandem repeats (Flt3-ITD; unfavorable) and nucleophosmin (NPM1; favorable in absence of a coinciding Flt3-ITD) mutations [3]. Morphologically, AML is classified into eight distinct categories according to the French-American-British system (FAB M0-7), which defines at which stage in hematopoiesis maturation arrest occurs [4,5]. Cell differentiation is further reflected by downregulation of the CD34 stem cell marker upon maturation [6].
AML cell populations have a hierarchical organization, and a major part of the cells will undergo spontaneous (i.e., stress-induced) apoptosis during the first 3–5 days of in vitro culture [7,8]. However, for some patients, a subset of cells will still proliferate after 2–8 weeks despite the initial apoptosis [9], and our present long-term cultures correspond to these previous in vitro assays for AML stem cells [7]. Furthermore, AML stem cells can also be assayed by xenografts, which is achievable mainly for cells from patients with high-risk leukemia [10]. Finally, total AML cell populations seem to reflect at least certain characteristics of the leukemic stem cells that are thought to be responsible for chemoresistance and leukemia relapse because gene expression and epigenetic profiles of the overall population are associated with prognosis [11,12]. Based on these observations, our hypothesis is that the ability of long-term in vitro growth in the absence of AML-supporting non-leukemic cells differs between patients. This capacity of long-term proliferation reflects a clinically relevant difference in the cellular ability to survive and proliferate under suboptimal conditions and is probably caused by differences in regulation of proliferation and/or balance between pro- and anti-apoptotic signaling. To identify these mechanisms, we characterized a large group of patients with regard to long-term proliferation. Furthermore, to identify possible molecular mechanisms contributing to stress-resistance (i.e., long-term in vitro survival and proliferation) we also performed transcriptomic and proteomic comparisons of primary AML cells with or without long-term in vitro proliferation.
2. Results
2.1. Long-Term In Vitro Proliferation of AML Cells in the Absence of Supportive Non-Leukemic Cells Is Detected Only for a Subset of Patients
Primary AML cells derived from 68 unselected patients (Table 1) were cultured in suspension (six-well culture plates); the culture medium was only supplemented with thrombopoietin (TPO), Flt3 ligand (Flt3L), stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin 3 (IL-3). After 35 days, the non-adherent cells were harvested and thereafter seeded in methylcellulose medium (MethoCultTM H4434 containing erythropoietin, EPO) and incubated for an additional 14 days before the number of colonies was estimated. Thus, the cells were cultured for a total of 7 weeks, and clonogenic cells could then be detected for 38 of the 68 patients. A minority of colonies of erythroid origin (i.e., presenting with morphological signs of hemoglobinization) were detected for 16 of the 38 patients, whereas two exceptional patients showed only erythroid colonies. Thus, non-erythroid colonies dominated for the large majority of patients. The number of colonies obtained from the parallels of samples containing 25,000 and/or 50,000 seeded viable cells per well were back calculated to the initial 2 × 106 cells, taking into consideration that cells from 20 cultures had been removed during the course of suspension culture in order to prevent overgrowth with cell density exceeding 2 × 106 cells/mL. The number of adjusted colonies showed a considerable variation among patients and ranged from 1.5 up to 35,050 colonies per 2 × 106 initial cells (median number: 324 colonies).
Table 1.
Biological and clinical characteristics of the 68 acute myeloid leukemia (AML) patients included in the study.
| Patient Characteristics, Cell Morphology | Disease Etiology and Cell Morphology | Cell Genetics | |||
|---|---|---|---|---|---|
| Age | De novo AML | 45 | Cytogenetics | ||
| Median (yrs) | 64 | Favorable | 7 | ||
| Range (yrs) | 18–92 | Secondary AML | Intermediate | 5 | |
| MDS | 10 | Normal | 35 | ||
| Gender | CMML | 2 | Adverse | 15 | |
| Females | 33 | CML | 1 | n.d. | 6 |
| Males | 35 | CLL | 1 | ||
| MF | 3 | Flt3 mutations | |||
| FAB classification | PV | 1 | ITD 2 | 16 | |
| M0 | 4 | Chemotherapy | 2 | Wild-type 2 | 39 |
| M1 | 17 | n.d. | 13 | ||
| M2 | 13 | AML relapse 1 | 7 | ||
| M4 | 15 | NPM1 mutations | |||
| M5 | 14 | CD34 receptor | Mutated | 20 | |
| n.d. | 5 | Negative (≤20%) | 21 | Wild-type | 33 |
| Positive (>20%) | 41 | n.d. | 15 | ||
| n.d. | 6 | ||||
| Flt3 plus NPM1 mutation | 9 | ||||
1 Three of the patients had a relapse of secondary AML. 2 One patient in each group has a point mutation at D835. n.d.: not determined. Abbreviations: MDS: myelodysplastic syndrome; CMML: chronic myelomonocytic leukemia; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia; MF: myelofibrosis; PV: polycytemia vera; ITD: internal tandem repeat.
We estimated the number of viable cells after the initial five weeks of suspension cultures, and this number showed a significant correlation with the capacity of colony formation (Kendall’s tau-b: r = 0.431; p < 0.0001). We further observed borderline correlation with peripheral blood blast count (Kruskal-Wallis test, p = 0.055; data from relapse patients were censored) as the median count increased from 40.8 × 109 blasts/L for cultures with less than 0.5 × 106 viable cells to 105 × 109 blasts/L for cultures containing more than 2.0 × 106 viable cells. We defined a threshold of 200 colonies, corresponding to 0.01% long-term proliferating cells, to divide the patients into groups with few and many colonies, respectively. We did this in order not to overestimate the significance of a few observed colonies, which in case of a high cell number can lead to a rather high adjusted colony number. The group with few colonies then comprised 16 patients with a median of 19 colonies whereas the group with many colonies contained 22 patients with a median of 1367 colonies per 2.0 × 106 seeded cells. The number of viable cells after five weeks in suspension culture varied considerably between the groups with no or few detectable colonies on one side and the group of cultures with >200 colonies on the other side (Table 2). Thus, it appears as if cultures with few colonies have more in common with the cultures without detectable colonies, as compared to the group with more than 0.01% long-term proliferating cells. Using this definition, only 1/30 cultures with less than 0.5 × 106 viable cells showed colony formation as opposed to only 2/14 cultures with more than 2.0 × 106 viable cells that did not form at least 200 colonies (Fisher’s exact test: p < 0.0001).
Table 2.
Overview of median and range values for colony number and cell population for the groups without detectable colonies, with few colonies and with many colonies.
| Number of Colonies | |||
|---|---|---|---|
| Not Observed | 1–200 | >200 | |
| Colony number | 0 | 19 (1.5–180) | 1367 (285–35,050) |
| Viable cell number (106) | 0.26 (0–3.06) | 0.18 (0.01–2.25) | 2.46 (0.30–53.5) |
2.2. The Capacity of Cytokine-Supported Long-Term In Vitro Proliferation Is Not Associated with Patient Age, Morphological Differentiation, CD34 Expression, Karyotype, or Molecular Genetic Abnormalities
In agreement with previous results [13], the capacity of long-term AML cell proliferation in the absence of supportive cells was not associated with the differentiation status of the primary AML cells at the time of diagnosis, i.e., cultures with and without this capacity did not differ with regard to morphological signs of differentiation (FAB classification) or expression of the CD34 stem cell marker. Furthermore, apart from cell number colony formation after long-term culture appeared to be independent of common prognostic factors such as patient age, hemoglobin levels, peripheral blood blast count, cytogenetics, NPM1 mutations, and secondary or relapsed versus de novo AML (an overview of patient details is provided in Supplementary Table S1). Only Flt3-ITD positive cells showed a non-significant trend towards a higher percentage of clonogenic cells because 20% of cultures with <200 colonies presented with Flt3-ITD, as opposed to 45% of cultures with higher colony numbers. Thus, at least in this patient cohort, the capacity of long-term in vitro proliferation in the absence of non-leukemic supportive cells seems to be essentially independent of the genetic abnormalities with an established prognostic impact.
2.3. A high Frequency of AML Cells Showing Long-Term Cytokine-Supported Proliferation Is Associated with Reduced Survival for Patients Receiving Intensive Anti-Leukemic Therapy
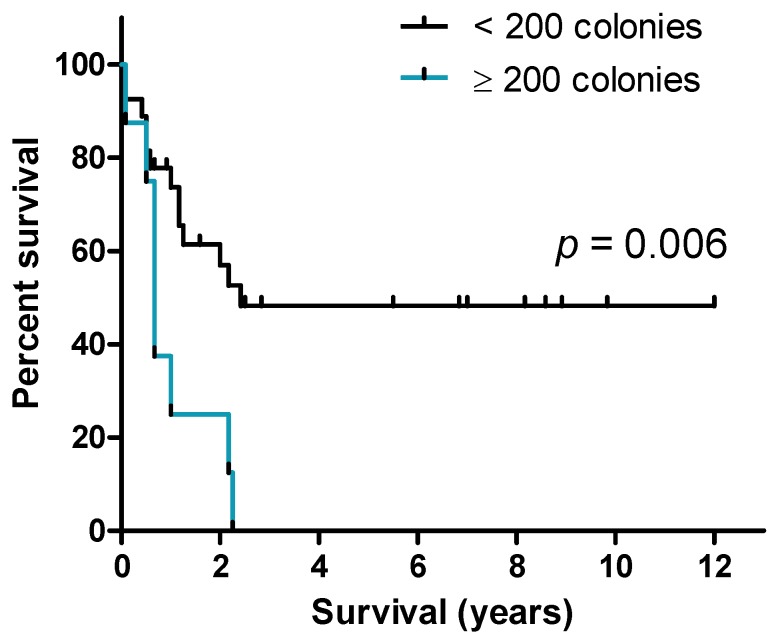
We compared the overall survival for patients whose blasts did or did not show long-term cytokine-supported proliferation; this comparison included only relatively young and fit consecutive patients with newly diagnosed AML who had completed their intensive and thus potentially curative induction therapy followed by the planned consolidation treatment. The 18 patients showing long-term proliferation were compared with the 17 patients where long-term proliferation supported by cytokines alone was not detectable. Overall survival did not differ between these two groups of patients and the 2-years survival was 40%. However, when we analyzed the quantitative instead of the qualitative differences in long-term proliferation (i.e., the number of colonies/clonogenic cells after 5 weeks of suspension cultures), we detected that the subset of patients with a high number of clonogenic cells (>200 colonies) showed decreased overall survival (median overall survival for patients with no/few detectable colonies being 2.4 years as compared to 8 months for patients with many colonies; log-rank test: 0.006; Figure 1).
Figure 1.
Patient survival dependent on colony number. Patients with blasts giving rise to more than 200 colonies after long-term culture (blue line) showed significantly (log-rank test) worse outcome than the patients with fewer colony forming cells.
We also performed a multivariate analysis including the known prognostic parameters age (younger or older than 60 years), Flt3-ITD, NPM1 insertions, favorable and adverse/intermediate cytogenetics and disease etiology (secondary versus de novo AML) in addition to the number of colonies (below or above 200 colonies) (Table 3). In the Cox regression analysis, two parameters emerged as independent risk factors for reduced survival: Patient age (hazard ratio, HR = 5.67; p = 0.011) and colony number (HR = 5.82; p = 0.005). Because the mutation status and/or cytogenetics for four patients were missing (three patients without detected colonies, one with >200 colonies; no long-term survivors), the latter analysis only contained 31 out of the 35 patients with survival data. The lack in associations between prognosis and Flt3/NPM1 mutations is most likely due to the relatively small number of heterogeneous patients in our cohort and a rather large overlap of patients in the groups with NPM1-insertions and Flt3-ITDs, as 7/18 patients with acquired mutations present with alterations in both genes.
Table 3.
Multivariate (adjusted) hazard ratio analysis for parameters associated with prognosis in AML. Significant p-values are highlighted.
| Variable | Adj. HR | 95% CI | p-Value |
|---|---|---|---|
| Age (≥60 years) 1 | 5.67 | 1.49–21.56 | 0.011 |
| Colony number (≥200) | 5.82 | 1.72–19.66 | 0.005 |
| Flt3-ITD | 1.83 | 0.48–6.92 | 0.377 |
| NPM1-insertion | 2.33 | 0.65–8.39 | 0.194 |
| Etiology (de novo) | 0.44 | 0.05–3.89 | 0.458 |
| Favorable cytogenetics | 0.78 | 0.11–5.61 | 0.802 |
| Unfavorable/intermediate cytogenetics | 0.58 | 0.11–3.00 | 0.513 |
1 Reference value, i.e., age ≥ 60 years is a risk factor. HR: hazard ratio; CI: confidence interval.
2.4. Increased Levels of HGF and IL-1RA during Long-Term Suspension Culture Are Associated with Maintenance of Clonogenic Cells during Culture
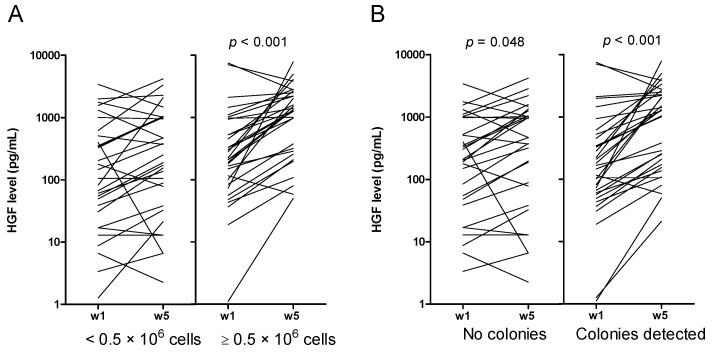
We investigated long-term AML cell proliferation in the presence of exogenous cytokines. However, primary AML cells themselves show constitutive release of a wide range of soluble mediators [14,15]. To characterize the mediator release by the leukemic cells during long-term suspension culture we measured the supernatant levels of 19 constitutively released mediators after 7 and 35 days of culture. We first investigated whether the levels of these mediators varied during the five weeks of suspension cultures (Wilcoxon signed-rank test for paired samples; week 5 vs. week 1 values), and whether these variations showed any associations with proliferation/viability during short-term culture, specific AML subgroups (i.e., FAB classification, cytogenetics, gene mutations, disease etiology) or colony formation. First, five of the 19 mediators showed no significant variation (due to a large number of comparisons, the significance level was set to 0.01) during the culture period: the chemokines (CCL5 and CXCL5), the interleukins (IL-1β and IL-6), and granulocyte colony-stimulating factor (G-CSF). Second, the levels of the eight mediators CCL2-4, CXCL8, IL-1 receptor antagonist (IL-1RA), matrix metalloproteases (MMP) 1 and 2, and cystatin C significantly increased during the suspension culture for all patients, except for those without detectable cytokine-dependent proliferation in the one-week 3H-thymidine assay and/or extensive spontaneous in vitro apoptosis characterized by less than 30% viable cells after two days of suspension culture. A gradual increase during long-term suspension culture of these eight cytokines thus seems to reflect that viable cells are still present during culture. Third, an increase during culture for the five mediators CXCL1/10, MMP-9, serpin E1 and tumor necrosis factor α (TNFα) was especially seen for patient cells without monocytic differentiation (i.e., FAB M0-2; Supplementary Figure S1). Finally, an increase in hepatocyte growth factor (HGF) was associated with cultures containing >0.5 × 106 viable cells after five weeks of suspension culture, and with colony forming cultures (Figure 2).
Figure 2.
Differences in hepatocyte growth factor (HGF) secretion dependent on cell population and colony number. (A) HGF concentrations do not significantly increase between week 1 and week 5 for cultures containing less than 0.5 × 106 viable cells after 5 weeks of culturing. (B) Increase in HGF levels is more pronounced in cultures with detectable colonies (right). Note the large overlap between the patients with higher cell numbers and colony formation.
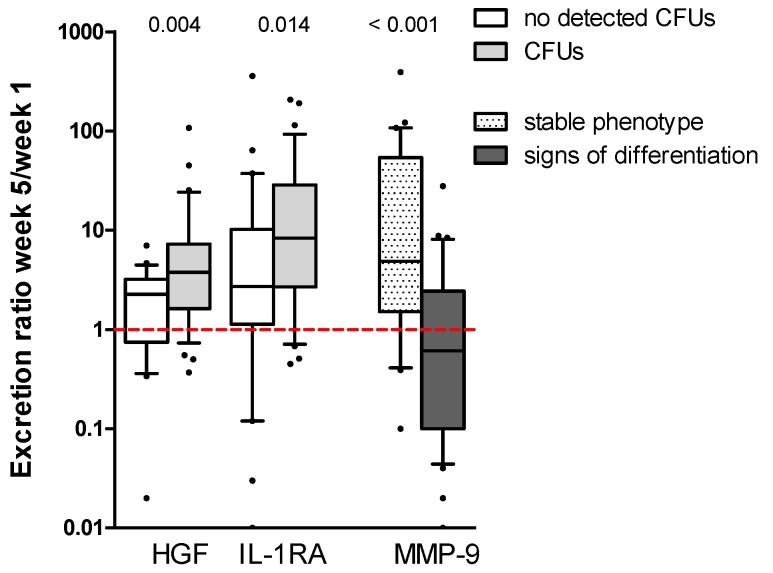
On the other hand, when we compared the cytokine secretion ratios between different patient subgroups (Mann-Whitney U-test; week 5/week 1 ratios; p ≤ 0.01), different patterns were observed for seven mediators: CCL2, CCL3, HGF, IL-1RA, MMP-9, cystatin C, and TNFα. Higher ratios of CCL2, CCL3, and cystatin C were observed for cells without NPM1 insertions and for CD34+ cells (Supplementary Figure S2). Furthermore, higher secretion ratios of IL-1RA, MMP-9 and TNFα were associated with FAB M0-2 (Supplementary Figure S3). On the other hand, the MMP-9 decrease over time was linked with cells showing morphological changes (i.e., plastic adherence, increased cellular size and/or presence of pseudopodia) over time (p < 0.001). Finally, higher ratios of HGF (p = 0.004) and borderline of IL-1RA (p = 0.014) were observed for cultures with colony forming cells (Figure 3; Supplementary Tables S2 and S3). However, the increase in HGF was most pronounced for the patient group with few colonies. The release ratios for the latter cytokines showed also a weakly positive correlation with the number of colonies: IL-1RA (Kendall’s tau-b: r = 0.203; p = 0.029) and HGF (r = 0.251; p = 0.007).
Figure 3.
Cytokine secretion week 5/week 1 ratios for the two cytokines that were significantly higher expressed by cultures containing clonogenic cells. Median ratios HGF: 2.26 vs. 3.77; IL-1RA: 2.71 vs. 8.31. The right part of the figure shows that matrix metalloprotease (MMP)-9 is depleted from the medium over time for cultures, where cells present with a change in their phenotype: 4.86 vs. 0.61. CFU: colony forming unit.
2.5. High Constitutive Cytokine Release Is Associated with an Altered AML Cell Phenotype during the Initial Five Weeks of Suspension Culture
We reproduced the results from a previous study of primary AML cells [14], which showed that high constitutive release of a wide range of soluble mediators during short-term in vitro culture is associated with signs of monocytic differentiation (FAB M4/5; Supplementary Table S4) and the favorable inv(16) abnormality with especially high secretion levels at week 1 and 5, respectively. Also in concurrence with the former study, NPM1-insertions and/or absence of the stem cell marker CD34 were associated with lower mediator levels. However, according to the current study, the AML cell population showing morphological alterations after 2–3 weeks of suspension culture had the highest absolute mediator levels of all samples (Mann-Whitney U-test; p ≤ 0.01): initial (week 1) high levels for CCL4, CXCL8, IL-1RA, HGF, MMP-1/9, and cystatin C; and persistent (week 1 and week 5) high levels for CCL2, CXCL5/10, IL-1β, G-CSF, and TNFα (Supplementary Table S4). The presence of morphological changes was associated with monocytic differentiation of the primary cells (FAB M4/5; Fisher’s exact test; p = 0.011). All cells with the inv(16)/t(16;16) abnormalities also showed a change in their phenotype, high cytokine levels and in general absence of colony formation, with exception of the single t(16;16) patient (396 colonies). Interestingly, there was, in general, no distinct correlation between constitutive cytokine secretion levels and the number of viable cells after five weeks in suspension culture or colony number after seven weeks of culture (Supplementary Table S5). Long-term maintenance seems rather to be associated with a higher relative increase in HGF and probably also in IL-1RA during culture (Section 2.4).
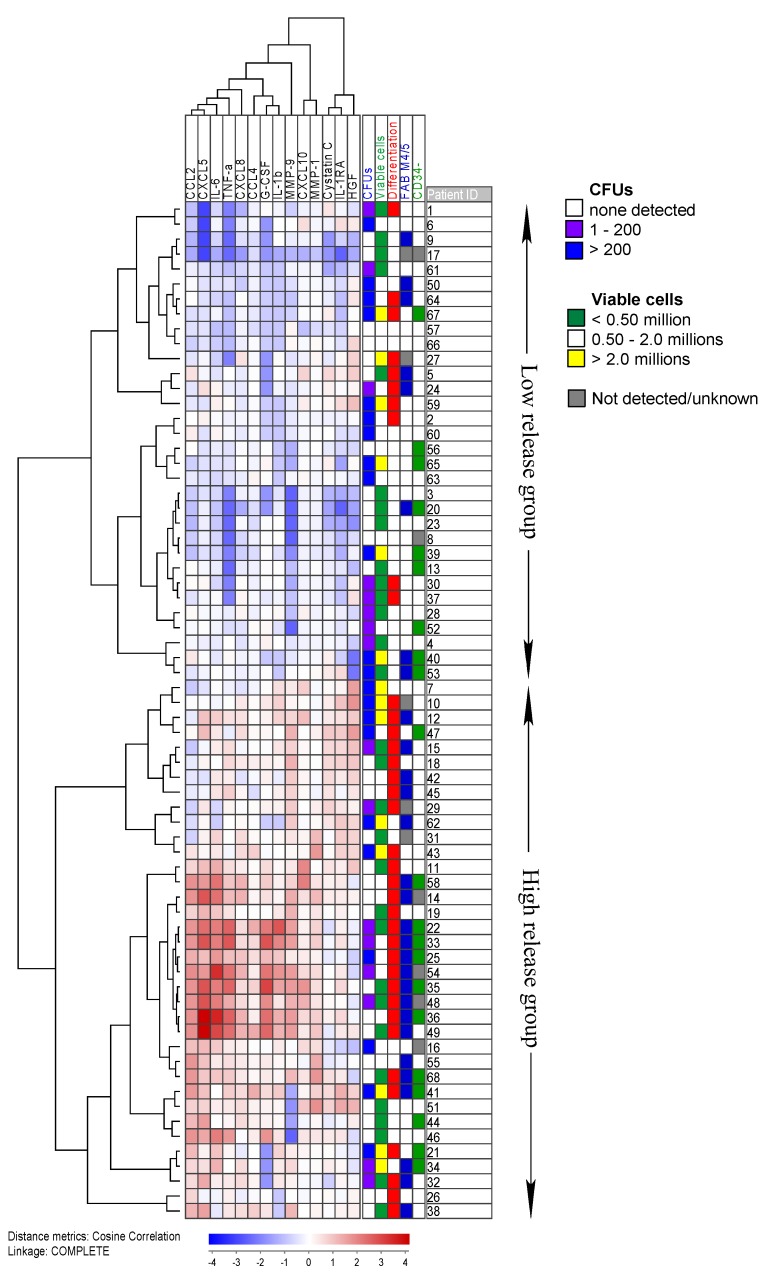
We further performed an unsupervised clustering (Figure 4) including the 14 mediators, which were secreted at significantly higher levels in those cell cultures that showed morphological alterations during long-term culture. In the cluster, the patients divided into two groups: Low and high initial cytokine release. As expected from previous results [14], all five patients with inv(16)/t(16;16) and a majority of 72% of FAB M4/5 patients (Fisher’s exact test; p = 0.005) clustered in the high release group. Furthermore, 73% of cultures showing morphological signs of differentiation (increased cellular size and/or presence of pseudopodia) after 5 weeks in suspension clustered in the high release group (Fisher’s exact test; p = 0.001). Regarding colony formation, there was no significant difference between the two groups. Intriguingly, morphological alterations during suspension culture appeared to correlate with increased patient survival, independent of colony number (log-rank test p = 0.006; Supplementary Figure S4).
Figure 4.
A cluster of the 14 cytokines that were differentially expressed for cells that showed morphological changes during suspension culture. The cluster shows the week 1 concentrations for all 68 patients. All values were median normalized and log transformed, thus the blue color indicates values below the median. Information about the number of detected colonies, the viable cell population, the presence of morphological changes over time and the blast morphology, according to the French-American-British (FAB) system and CD34 expression, is provided on the right. Both blasts with a change in their phenotype during culture and with signs of monocytic differentiation (FAB M4/5) are overrepresented in the high release group. On the other hand, colony number correlates with cell number but not with cytokine expression.
2.6. Gene Expression and Mutation Profiling Identifies Differences between Patient Groups with High and Low Numbers of Clonogenic Cells
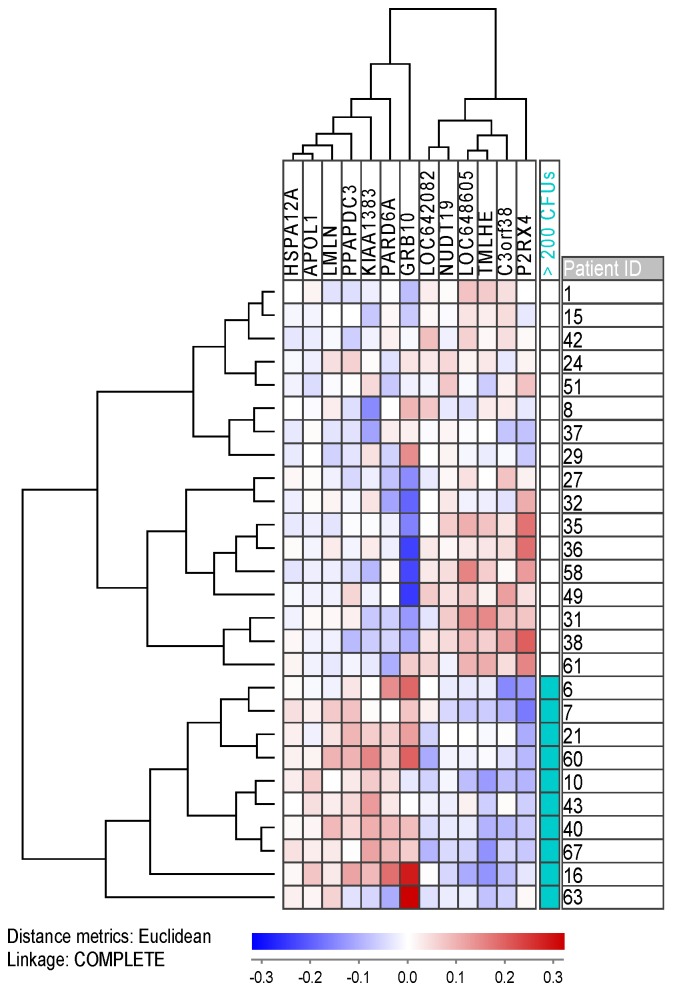
Using JExpress, we performed a feature subset selection (FSS)/Anova microarray analysis comparing 10 unselected patients with many (>200 colonies) colony forming units (CFUs) with 17 patients with fewer colonies. We set the cut-off to an absolute t-score of 4.00, corresponding to p < 5.0 × 10−4. We found 14 genes (including two probes for C3orf38) differentially expressed, thereof 12 mapped genes. Five of these genes were overexpressed in cultures with <200 colonies (Figure 5; Table 4; Supplementary Table S6). Most of the genes are associated with metabolism and energy pathways (NUDT19, P2RX4, and TMLHE), whereas C3orf38 is possibly linked with apoptosis. The seven upregulated genes for cultures with many colonies, on the other hand, include genes that are linked with mitosis (KIAA1383 and PARD6A), invasion and metastasis (LMLN and PARD6A), and protein folding (HSPA12A). The most interesting gene, however, might be the proto-oncogene GRB10 (coding for growth factor receptor-bound protein 10) which has been investigated in AML, where it was linked to leukemogenesis [16] and relapse due to resistant disease [17].
Figure 5.
A cluster of the 13 differentially expressed genes between cultures with many (>200) and few colony forming cells. Probes of differentially expressed mRNA with an absolute t-score > 4.00 were median normalized and log transformed prior to clustering. C3orf38 represents the mean value for the two gene probes. Red and blue color represent up- and downregulated genes, respectively. RPS4Y1, a gene encoded on the Y-chromosome, was excluded from the cluster as it divides the patients by gender rather than colony number; the patient subgroups presented by the microarray coincidentally contained 70% female patients in the high colony subgroup, and 82% male patients in the low colony subgroup.
Table 4.
Classification of the genes that were found to be differentially expressed for patient populations with >0.01% clonogenic cells. The upregulated genes are associated with cell cycle and signaling, whereas the downregulated genes mostly are linked with metabolism. For details on the encoded proteins, see Supplementary Table S6.
| Classification Based on Protein Function | Gene Expression in AML Population with Colony Formation | |
|---|---|---|
| Upregulated | Downregulated | |
| Cell cycle, mitosis | KIAA1383/MTR120; LMLN; PARD6A; GRB10 | |
| Transcription | (RPS4Y1) | |
| Apoptosis | C3orf38? | |
| Chaperone | HSPA12A | |
| Signaling, transport | APOL1; GRB10; PPADDC3/PLPP7 | P2XR4; TMLHE |
| Metabolism | NUDT19; TMLHE | |
| Unknown | LOC642082; LOC648605 | |
Furthermore, for 35 patients—thereof eight with >200 colonies—we have more in-depth information about the mutational landscape including 35 additional mutations to Flt3-ITD and NPM1-insertions, which all are associated with AML (Supplementary Table S7). It appears as if mutations in genes, where the products are involved in chromatid separation (i.e., the chromatid modifier ASXL1 and subunits of the cohesin complex, namely RAD21, SMC1A and STAG2), are more frequent among patients with >0.01% clonogenic cells (0.75 as compared to 0.15 mutations/patient; Fisher’s exact test p = 0.010).
2.7. Gene Expression Profiling Identifies Differentially Expressed Genes for Both Cultures Which Present with an Altered AML Phenotype and Cultures with Erythroid Colonies
We performed FSS analysis as described in Section 2.6 for cultures that differed in their AML blast phenotype, and for cultures with and without colonies of erythroid origin.
First, FSS identified 61 probes coding for 35 annotated genes which were differentially expressed between the patient groups with (10 patients) and without (17 patients) stable AML cell phenotype during long-term suspension culture (Section 2.5; Supplementary Figure S5; Supplementary Table S8). The encoded proteins fulfil a wide range of functions with signaling (7 genes), transcription (8 genes) and metabolism (10 genes) being the most prominent (Supplementary Table S9). Five of these genes have been studied in AML (see [16,18,19,20,21,22]).
Second, FSS identified 36 differentially expressed probes, thereof 23 annotated genes (Section 2.1; Supplementary Figure S6; Supplementary Table S10), which differentiated the cultures with erythroid colonies (7 patients) from those containing only myeloid colonies (9 patients). Most of the genes were upregulated in the subset with erythroid colonies and coded for proteins involved in metabolism (5 proteins) and transcription (4 proteins; Supplementary Table S11). Two of the upregulated genes have been investigated in AML (see [23,24,25]).
2.8. Proteomics Analysis Identifies Proteins Involved in Transcription to Be More Abundant for Patients with a High Number of Clonogenic Cells
We performed both proteomic and phosphoproteomic analyses on cells from 15 consecutive AML patients (8 with no/few and 7 with many colonies) obtained at diagnosis. Thirty-three proteins, thereof 31 with an identified function, showed to be differentially abundant in the proteomic analysis. Transcription factors and proteins involved in mRNA splicing dominated for the patient group >200 colonies, whereas proteins involved in protein synthesis and modification (kinases) were found to be decreased (Table 5; Supplementary Table S12). Two of the proteins had been studied in AML previously: VIM (vimentin) and SMG1 (nonsense-mediated mRNA decay associated PI3K related kinase). Vimentin (increased for the group >200 colonies) is considered a negative prognostic factor in AML [26], whereas SMG1 (decreased for the group >200 colonies) is a potential tumor suppressor in hypoxic tumors and associated with regulation of AML cell proliferation [27].
Table 5.
Classification of the proteins that were found to be differentially abundant (left) or differentially phosphorylated (right) for patient populations with >0.01% clonogenic cells. The proteins with higher abundance are associated with transcription and lipid/ion transport. The differentially phosphorylated proteins, on the other hand, are mainly linked with protein biosynthesis and the cytoskeleton. For details on the proteins, see Supplementary Tables S12 and S13.
| Biological Process | Proteomic Analysis | Phosphoproteomic Analysis | ||
|---|---|---|---|---|
| Increased ≥200 Colonies | Decreased ≥200 Colonies | Increased ≥200 Colonies | Decreased ≥200 Colonies | |
| mRNA splicing | RBMXL1; TXNL4A; ZNF830 | PCBP1 2 | PAPOLA; PRPF31; PRPF40A | |
| Transcription | MED1; POLR3C; ZNF830 | SMG1 2 | DOT1L 2; TCEAL3; ZNF521 2 | CBFB 2; ISL2; LEO1 2; MYSM1 |
| Transcription repressor | SPEN; ZNF521 2 | AEBP2 2; GATAD2B | ||
| Translation | ABCF1; EIF2B2; FARSB; MRPL3 | DNAJC2; EIF5B | ||
| Kinase | CAMK2D; DGKZ; SMG1 2 | NEK1; PRKD2 | ||
| Chaperone | DNAJC2; DNAJC5 | |||
| Metabolism | CPD 1; HEXB; MPDU1 | USP4 | CAST 2; CPD 1; DOT1L 2 | CTPS1; MYO9B; PPP6R3; RASGRP2 |
| Transporter, ion channel | CLIC4; CPD 1; CRIP1; RALGAPA2 | ABCF1; CLCN3; RBP4 | CPD | ATP13A1; OSBP; SCAP |
| Signaling | RALGAPA2; VIM 1,2 | CAP1; MRC2 | VIM 1,2; ZFYVE26 | LAT 2; LSP12; mTOR 2; RNF31; TMPO |
| Cytoskeleton | VIM 1,2 | AAMP; CAP1; MYOF | SP100; VIM 1,2; ZFYVE26 | DBNL; MYO9B; SH3BP1; TMPO |
| Apoptosis | MED1; RMDN3; TMEM173 | GRAMD4; MAP1S 2 | ||
| Cell cycle/mitosis | KIF4A | NEK1 | ||
| Proliferation regulation | FAM53C; SCRIB | |||
| Others | C19orf19/TRIR; MOB4 | BOD1L1; CAD; IRF2BP2; TTC7A | ||
| Unknown | GPALPP1 | MAP7D1 1 | MAP7D1 1; TOR4A | |
1 These proteins are found in both proteomic and phosphoproteomic analysis. 2 These proteins have been studied in AML.
Phosphoproteomic analysis revealed 49 differentially phosphorylated proteins between the two groups, including three proteins that were already identified by the proteomic analysis: carboxypeptidase D (CPD), MAP7 domain containing 1 (MAP7D1) and VIM (Table 5; Supplementary Table S13). Again, proteins involved in transcription, translation, and protein-modifying (19 proteins) dominated, in addition to proteins involved in cytoskeleton structure and signaling (7 proteins). As many as 12 proteins had been studied in AML previously. Expression of three of the proteins is linked with mixed-lineage leukemia (MLL/11q23) rearranged AML: The transcription factors DOT1L and ZNF521, and VIM. Furthermore, expression of four proteins is associated with tumor progression/high risk AML: DOT1L, mTOR, VIM and ZNF521 [26,27,28,29,30], whereas the expression of six proteins is associated with tumor suppression/low risk AML: AEBP2, CAST, CBFB, LSP1, MAP1S and probably PCBP1 [31,32,33,34,35]. Finally, the effect of de/phosphorylation has been studied for LEO homolog 1 (LEO1), and dephosphorylation of this protein was then linked to leukemogenesis [36].
3. Discussion
In AML, the leukemic cells are organized in a hierarchy [10] and can be divided into three different subgroups with decreasing percentage of the total amount of cells: (i) The non-cycling blasts, which usually undergo apoptosis in cell culture during the first week; (ii) a subset of cells with pro-longed self-renewal capacity (clonogenic cells); and (iii) a small subset of cells with a long-term proliferative capability (4–8 weeks) in cell culture [7,8,9]. The latter include those cells that are referred to as the leukemic stem cells (LSCs) that are usually associated with the CD34+CD38− cell subpopulation [37]. Even though maturation is usually associated with loss of CD34 expression and absence of long-term proliferation [8,9], exceptional patients present with CD34− LSCs [9,38,39]. Altogether, these observations fit with our present as well as previous studies [13] where colony formation ability could neither be linked with FAB classification nor CD34 expression. Because the fraction of cells with clonogenic potential is low (median: 1.6 × 10−2; mean: 0.16%) these cells can virtually be present in any AML subtype. Since clonogenic blasts in certain cases even exist within the more mature CD34− cell fraction, the cell marker CD34 itself can be used as a predictor neither for colony formation [39] nor for patient survival [37].
One might debate, whether the observed colonies indeed are derived from long-term proliferating leukemic cells or whether they result from contamination with normal hematopoietic stem cells (HSCs). The latter view might be supported by the presence of erythroid colonies for some patients. However, we regard the CFUs to be of leukemic origin. First, a previous study suggested that contamination with healthy HSCs is common [40], and these cells may then proliferate in response to EPO and IL-3, which are present in the MethoCult medium [41,42,43]. Therefore, one would expect to detect erythroid CFUs for most cultures, but only 26% of patients presented with erythroid colonies after 7 weeks and these colonies dominated only for five patients. Second, microarray analysis identified 36 differentially expressed genes (Supplementary Figure S6; Supplementary Tables S10 and S11) between primary cell populations with versus without erythroid colonies. The different expression patterns between these two patient subgroups suggest that the observed difference lies within the leukemic cell populations, which give rise to different colony types.
We saw an inverse correlation between an increased percentage of clonogenic cells (>0.01%) and patient survival (Figure 1), which appears to be reasonable as these cells are expected to maintain the disease. A larger fraction of blasts with long-term proliferative capacity thus might lead to increased leukemic blast burden in the patients. Long-term replenishing of non-cycling cells could also be observed in the cultures that contained the highest fractions of clonogenic cells, and these cultures frequently (12/22 cultures with >200 colonies as opposed to 2/46 remaining cultures; Fisher’s exact test, p < 0.0001) showed a higher number of viable cells after five weeks in suspension culture than the originally seeded 2.0 × 106.
Previous studies have shown that AML patients differ in their requirements for proliferation during in vitro culture [44,45,46]. Our present assay for long-term in vitro proliferation has two steps that are based on different culture media; first five weeks of suspension culture followed by two weeks of a methylcellulose-based clonogenic assay. The culture medium is thus different but highly standardized in the two assays. Classification of patients as capable of long-term proliferation thus requires survival of sufficient long-term proliferating cells during the first suspension culture, and the ability to proliferate also in the second assay (weeks six and seven of in vitro culture). The two steps in the long-term proliferation assay can explain why certain patients show discrepancies between the numbers of viable cells after five weeks of suspension culture and the number of colonies in the final clonogenic assay, where cultures presenting with few colonies showed a similar amount of viable cells as cultures without colony formation.
We used primary AML cells derived from peripheral blood of AML patients with high percentages and/or levels of circulating leukemia cells, and as discussed in detail in previous methodological articles we thus have a selection of patients [7,47]. However, our patients should be regarded as representative with respect to the most important biomarkers for in vivo chemosensitivity in AML, i.e., the generally accepted prognostic factors of this disease [47]. In addition, our study shows similar frequencies of various mutants as previous studies of a general AML cell population have indicated [48]. Despite this, we want to emphasize that our results should be interpreted with care because we cannot exclude the possibility that the cell populations are representative only for this selected subset of patients. The advantage of our methodological strategy is that we used a simple and standardized method for preparation of highly enriched AML cells without induction of functional alteration due to more complex separation methods, e.g., antibody-based separation with ligation of surface molecules [7,49]. Finally, the culture of similar AML cell population without subset selection has also been used in previous studies of leukemic stem cells, i.e., long-term proliferating AML cells in the presence of leukemia-supporting stromal cells (see [7,49]).
Another question is whether cells derived from the peripheral blood are representative for the leukemic cells in the bone marrow microenvironment, which is regarded as the site of initial leukemogenesis. A previous study compared the cell surface molecular profile for paired cell samples derived from bone marrow and peripheral blood of the same patients; in these studies, the cells showed only relatively small quantitative differences for a limited number of adhesion molecules [50]. Furthermore, in a recent study, we also showed that constitutive activation of the PI3K-Akt-mTOR pathway varies considerably among patients but has large similarities when comparing paired blood and bone marrow samples from the same patients [51]. These observations are not surprising when taking into account that blood and bone marrow cells are hierarchically organized and are expected to have the same AML-associated genetic abnormalities. Taken together, these observations suggest that peripheral blood samples are representative for the AML cell population in general, but again we would emphasize that our results should be interpreted carefully due to our analysis of peripheral blood rather than bone marrow cells.
The constitutive cytokine secretion during long-term culture follows the same pattern for a majority (13/19) of mediators, regardless of AML subclassification or long-term proliferation capacity. Thus, the patient heterogeneity with respect to cytokine levels was to a large degree maintained during culture. The cultures that differed from this pattern showed low proliferation and low viability even during the first 2–8 days, indicating that apoptotic/necrotic cells do not considerably contribute to mediator levels due to leakage through damaged/ruptured cell membranes. An exceptional mediator is MMP-9. Even though the levels at week 5 are still highest for both blasts with monocytic differentiation and with altered morphology, the start median values are divided in half for these groups, whereas the concentrations increase by a 4-fold for FAB M0-2 and cells with a stable phenotype. In addition, cultures with clonogenic cells show a trend towards an increase in MMP-9 concentration over time. Together with HGF and IL-1RA–which significantly increased during long-term culture–these were also the only cytokines where the absolute concentrations after five weeks exceeded those of the cultures that lacked clonogenic cells (Supplementary Table S3).
Increased levels for HGF and IL-1RA for cell cultures containing clonogenic cells have been reported previously [13]. At the detected concentrations (<100 ng/mL), IL-1RA stimulates AML cell proliferation [52]. HGF has been thoroughly studied in AML, and enhanced release has been associated with an adverse prognosis [53]. Inhibition of HGF expression in AML cell lines resulted in increased apoptosis and reduced proliferation [54]. Furthermore, inhibition of the HGF-receptor c-MET resulted in reduced colony formation ability of AML cells [55]. Targeting PI3K and mTOR decreased supernatant levels of both HGF and MMP-9 [56]. MMPs play an important role in tumor invasion and metastasis as they degrade extracellular matrix components [57,58]. Especially MMP-9 is associated with extramedullary infiltration and leukemia invasiveness [57,59]. It seems as if HGF promotes cell cycle progression and blast proliferation, whilst inhibiting apoptosis, and increases the expression of MMP-9 [54]. The increased levels of MMP-9 during pro-longed culturing of cultures containing clonogenic cells might, therefore, be a consequence of the increased HGF levels. However, it appears as if the MMP-9 increase during long-term culture is not a specific feature for cultures containing clonogenic cells. Rather than that, depletion of MMP-9 is correlated with cells that show morphological alterations and may be involved in the change of phenotype.
In our present study, we observed a significant difference in supernatant levels only for a few of the constitutively secreted cytokines when comparing cells with and without the capacity of long-term proliferation. These levels reflect the balance between constitutive release, cytokine receptor binding due to autocrine loops, and possibly degradation due to the release of proteases. However, the role of soluble mediators in leukemogenesis is probably very complex, and we, therefore, emphasize that only experiments based on cytokine knockdown, cytokine neutralization, or specific inhibition of cytokine receptors and their downstream signaling can verify whether a certain cytokine is important for the observed difference in long-term AML cell proliferation. Both receptor-blocking of HGF [55] and the use of IL-1β specific antibodies [60] can reduce colony formation, verifying their involvement in the process. Furthermore, as shown in neutralization experiments, even soluble mediators below detection limit may contribute to cell proliferation [47], although the effects induced by the same mediator may vary vastly among different patients [61,62,63,64]. Finally, autocrine effects of a cytokine can be mediated both on intracellular/endogenous as well as extracellular loops [65]. Taken together, differences in cytokine levels among patients (see Figure 2 and Figure 3) do not necessarily reflect the molecular mechanisms behind differences in long-term in vitro proliferation, whereas even mediators with similar supernatant levels in the two patient groups may contribute to long-term proliferation through differences in responsiveness towards the mediator. However, the observed cytokine differences among patients with and without long-term proliferation (Figure 2 and Figure 3) suggest that these two subsets show additional differences with regard to communication via the local cytokine network (i.e., IL-1/IL-1RA, HGF) with neighboring AML-supporting stromal cells in their common bone marrow microenvironment.
We further observed that the cells which during the course of five weeks showed morphological alterations had the highest constitutive cytokine levels after short-term (1 week) culture for 14/19 mediators (p ≤ 0.01), including IL-1RA, HGF and MMP-9 (Figure 4). The week 1 values were chosen because these early measurements correspond the most to the originally cell cultures as (1) no cells have been removed from the cultures, and (2) morphological alterations are not detectable yet. We also want to stress that the 7-day cytokine profile is very similar to that obtained after only two days of culture: 55/68 of the samples were additionally cultured in StemSpan SFEMTM medium at a concentration of 1.0 × 106 cells/mL for two days. The distribution of the patients into two groups with high and low secretion of the same 14 cytokines showed a large degree of overlap with the cluster presented in Figure 4 (Fisher’s exact test; p < 0.001). Cultures containing cells with an altered phenotype, therefore, seem to present with an altered cytokine profile; this may explain why these cultures show significantly increased cytokine levels for 7 mediators at week 5, even though the number of viable cells is lower than in cultures with many clonogenic cells (median: 0.88 × 106 vs. 2.46 × 106 cells, respectively; p = 0.001). Thus, the week 1 cytokine profile might predict a differentiation potential of the blasts and thereby a favorable prognosis; this would be in agreement with our previous study where prolonged patient survival was associated with a high constitutive cytokine secretion profile after 2 days in culture [14]. This improved survival might then be due to the leukemic cells’ capability to differentiate upon autocrine cytokine stimulation. However, we have to stress that a high number of long-term proliferating cells and an altered phenotype are not mutually exclusive as 50% of cultures with >200 colonies also showed changes in morphology. Still, morphological changes during suspension culture were associated with increased patient survival in our study (Supplementary Figure S4).
There are three reasons for why we chose to follow the change in phenotype by light microscopy/cell morphology. First, tracking changes in morphology by light-microscopy has been characterized in previous studies and should, therefore, be regarded as a reliable way to survey alterations in the phenotype, i.e., a monocyte-macrophage differentiation program [66]. Second, repeated evaluations during culture are allowed. Third, the alternative strategy of studying the expression of a single or a few surface markers may reflect aberrant molecular expression (a well-known phenomenon in acute leukemia) rather than an altered phenotype [67]. Thus, in our opinion, this simple methodological strategy is reliable, even though it does not allow any detailed examination.
We also compared the global gene expression profiles of primary AML cell populations with and without more than 0.01% long-term proliferating cells. Since the fraction of clonogenic cells in the cell population is small, it may not be surprising that few genes were found to be differentially expressed between the two groups. Most of the downregulated genes for cultures >200 colonies are involved in (energy) metabolism, whereas the upregulated genes especially code for proteins, which are involved in cell cycle/mitosis and invasion. Of the latter, increased expression of GRB10 was associated with Flt3-ITD, relapse, increased proliferation, reduced apoptosis and even increased colony formation in short-term cultures (2 + 5 days) [17]. Since long-term culturing of AML blasts is not practical in the clinic, these differentially expressed genes (Figure 5; Table 4) and especially GRB10 might act as biomarkers in order to identify patients with a high fraction of long-term proliferating cells and, thus, worsened prognosis.
We further found that mutations in the cohesin complex (represented by three of the five proteins) and in ASXL1 are more frequent in the patient group with many clonogenic cells (Supplementary Table S7). ASXL1, together with the cohesin complex, regulates gene expression in normal hematopoietic cells. Mutations in ASXL1 lead to loss of function and altered expression of cohesin target genes [68], and is therefore correlated with poor prognosis in AML [69]. On the other hand, mutations in cohesin are also associated with loss of function, secondary AML, and poor prognosis [70]. Interestingly, co-mutations in ASXL1 and cohesin genes also appear to be more frequent in the patient group with more than 0.01% long-term proliferating cells. Thus, the loss of function of several proteins with overlapping function might contribute to increased colony formation ability.
Finally, the proteomic analysis showed that many of the differentially abundant proteins are involved in protein biosynthesis, from mRNA splicing to folding and modification of the nascent protein (Table 5; Supplementary Table S12). Among these proteins were the negative prognostic factor VIM and the tumor suppressor SMG1, which were found to be increased for the patients with many and few colony forming cells, respectively. Regarding phosphoproteomics, we identified 49 differentially phosphorylated proteins, of which 11 have been linked to prognosis in AML. Knockdown of two of the proteins (histone methylating DOT1L and transcription factor ZNF521) has even shown to reduce colony formation ability in short-term cultures [28,71]. It is remarkable that 40% of the differentially phosphorylated proteins are involved in protein biosynthesis. We hypothesize that there might be a link between the frequent gene mutations in AML and the aberrant phosphorylation profile in the proteins with a similar function. Only six of the 35 patients with mutation data do not present with mutations in epigenetic modifiers, transcription factors/repressors, spliceosome and cohesin complex (Supplementary Table S7). Co-mutations in these gene groups are more frequent in the group with many colonies (2.6 as compared to 1.5 mutations/patient; Fisher’s exact test p = 0.035). Therefore, aberrant protein expression in AML cells with clonogenic ability might be reflected at both the gene and the protein level.
Our hypothesis was that the ability of long-term in vitro growth in the absence of AML-supporting non-leukemic cells differed among patients and had clinical relevance. The obtained results strongly suggest that this is true. However, we also hypothesized that differences in the capacity of long-term in vitro proliferation are due to differences in the regulation of AML cell proliferation and/or the balance between pro- and anti-apoptotic signaling; while this may be true, the molecular mechanisms behind these differences still remain to be resolved.
4. Materials and Methods
4.1. Patients and Preparation of AML Cells
The study was approved by the local Ethics Committee (Regional Ethics Committee III, University of Bergen, REK 2017-305, Bergen, Norway) and samples were collected after written informed consent. AML blasts from peripheral blood were derived from 68 unselected patients with a high number and/or percentage of circulating leukemic cells (33 females and 35 males; median age 64 years with range 18–92 years). A majority of 45 patients had de novo AML whereas 7 patients had relapsed AML, and 19 patients had AML secondary to chronic myeloproliferative neoplasia, myelodysplastic syndromes or previous chemotherapy (Table 1). Thirty-five of the patients received potentially curative treatment including induction therapy based on cytarabine plus an anthracycline followed by intensive consolidation treatment.
4.2. Preparation of Enriched AML Cells
AML cells were isolated from peripheral blood by density gradient separation (Lymphoprep; Axis-Shield, Oslo, Norway; specific density 1.077 g/mL) and due to our selection of patients with high absolute/relative levels of circulating AML cells the gradient-separated cell populations contained at least 90% leukemia cells. The cells were cryopreserved and stored in liquid nitrogen [62]. All cell samples were isolated, cryopreserved, and thawed based on the same highly standardized methods.
4.3. Analyses of AML Cell Viability and Short-Term Proliferation
4.3.1. Analysis of AML Cell Viability
AML cells were cultured for 40 h in Stem Span SFEMTM medium (Stem Cell Technologies; Vancouver, BC, Canada) and cell viability analyzed by flow cytometry using propidium iodide-Annexin V (Tau Technologies BV; Kattendijke, The Netherlands) staining as described previously [14].
4.3.2. Proliferation Assay
As described in detail previously [14], AML cells were cultured for one week in serum-free Stem Span in the presence of Flt3L, SCF and GM-CSF, until proliferation was assayed as nuclear 3H-thymidine incorporation after 8 days. Detectable proliferation was defined as nuclear incorporation corresponding to >1000 cpm.
4.4. Long-Term Suspension Cultures
AML cells were cultured in Endothelial Cell Growth Medium (EGM-2 medium, Lonza; Walkersville, MD, USA), which contains, among others, 2% fetal bovine serum and vascular endothelial growth factor (VEGF) to facilitate rapid proliferation. According to the manufacturer’s descriptions, the medium was further supplemented with 20 ng/mL of TPO, Flt3L, SCF, GM-CSF and IL-3 (Peprotech; Rocky Hill, NJ, USA). The EGM-2 medium has shown to support the long-term proliferation of AML blasts [13].
We initially seeded 2 × 106 AML cells in 5 mL EGM-2 medium in 6-well tissue plates (Nunc; Roskilde, Denmark), and the cells were incubated in suspension cultures for five weeks. Once a week, 1–2 mL of medium or cell suspension—for cultures containing high cell density as judged by light microscopy—was removed and replaced by fresh complete EGM-2 medium. Cell supernatants were harvested after week 1 and week 5 and stored at −80 °C prior to analysis.
4.5. Colony Formation Assay
Long-term cultured cells were tested in our colony formation assay after five weeks of suspension culture. The non-adherent AML cells were then collected, centrifuged, and resuspended in 0.5 mL RPMI medium (Sigma Aldrich; St. Louis, MO, USA). The number of viable cells was determined using trypan blue staining (Fluka; St. Louis, MO, USA). Subsequently, duplicates of 2.5 × 104 and, where available, 5.0 × 104 viable AML cells were seeded in 0.5 mL MethoCultTM H4434 classic medium (StemCell Technologies; Vancouver, BC, Canada) containing EPO in flat-bottomed 24-well plates (Nunc). Lower viable cell numbers were seeded for patients with low cell populations after five weeks in culture. After an additional 14 days of in vitro culture in the colony formation assay colonies containing more than 30 cells (i.e., CFUs) were scored using an inverted microscope. Colonies were classified as being of either myeloid or erythroid origin, where the latter showed morphological signs of hemoglobinization.
4.6. Analysis of Soluble Mediator Levels in Cell Culture Supernatants
The sampled cell culture supernatants were analyzed by Luminex (R&D Systems; Minnesota, MN, USA). Excretion levels for following 19 proteins were measured: (i) The chemokines CCL2-5, CXCL1/5/8/10; (ii) the interleukins IL-1β, IL-1RA and IL-6; (iii) the growth factors G-CSF and HGF; (iv) the matrix metalloproteases MMP-1/2/9; (v) the protease inhibitors cystatin C and serpin E1; and (vi) TNFα.
4.7. RNA Preparation and Analysis of Global Gene Expression
RNA preparation, labeling, and microarray hybridization have been described in detail previously [72]. Briefly, the gene expression profiles were analyzed using the Illumia iScan Reader (Illumina Inc., San Diego, CA, USA) for fluorescent detection of biotin-labeled complementary RNA (cRNA). The latter was quality-controlled by both NanoDrop and an Agilent 2100 Bioanalyzer (Agilent Tachnologies, Santa Clara, CA, USA). Finally, 750 ng biotin-labeled cRNA was hybridized to the HumanHT-12 V4 Expression BeadChip (Illumina Inc.), which targets 47,231 probes. The microarray data were quantile normalized prior to analysis in J-Express 2012 (MolMine AS, Bergen, Norway) [73].
4.8. Proteomic and Phosphoproteomic Analyses
The methods for analysis of proteomic and phosphoproteomic profiles in primary human AML cells have been described in detail previously [74,75,76]. Briefly, each patient sample was spiked with a heavy super-SILAC mix [77] and prepared according to the filter-aided sample preparation protocol [78] for proteome and phosphoproteome analysis using nano-liquid chromatography online coupled with a QExactive HF Orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany).
4.9. Statistical and Bioinformatical Analyses
The statistical analyses were performed with the IBM Statistical Package for the Social Sciences (SPSS) version 25 (Chicago, IL, USA) and with GraphPad Prism 5 (San Diego, CA, USA). The Wilcoxon signed-ranked test was used to compare paired samples, whereas Mann-Whitney U-tests and Kruskal-Wallis tests were used to compare different patient subgroups. The χ2 test was used to analyze categorized data and the Kendall’s tau-b test for correlation analyses. Finally, Kaplan Meier analysis and log-rank test in addition to Cox regression for multivariate analyses were used for patient survival statistics. In general, p-values < 0.05 were regarded as statistically significant.
The statistical analyses of proteomic and phosphoproteomic data were performed with a Welch t-test in Perseus (Max Planck Institute of Biochemistry, Martinsried, Germany) [79], and the significance of fold changes was assessed using Z-statistics [80].
For hierarchical clustering of the cytokine release levels, all values were median normalized and log transformed prior to clustering using J-Express (MolMine AS, Bergen, Norway). Complete linkage and cosine correlation were used as the linkage method and distance measurement, respectively.
5. Conclusions
In this study, more than half of the AML patients had cells with long-term (i.e., seven weeks) proliferative capacity and/or a stable phenotype during culture; both these characteristics are associated with an adverse prognosis for patients receiving intensive induction therapy. Strikingly, these two parameters were not associated with other unfavorable prognostic factors. Cultures containing clonogenic cells presented with a higher increase in HGF and IL-1RA concentrations than the others during culture, and had, in general, higher numbers of viable cells after 5 weeks in suspension culture. From our results, it appears as if aberrant transcription and transcription regulation is associated with a high percentage of clonogenic cells, as genes involved in transcription more frequently are mutated for these patients, and proteins which are involved in protein synthesis are overexpressed or differentially phosphorylated for the patient group. Thus, these characteristics may be used to predict the presence of many clonogenic cells at the time of diagnosis, and thus an adverse prognosis.
Acknowledgments
The technical assistance of Kristin P. Rye was highly appreciated.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6694/11/1/73/s1. Figure S1: FAB M0-2 is associated with a relative concentration increase between weeks 1 and 5 for the mediators CXCL1, CXCL10, MMP-9, serpin E1, and TNFα. Figure S2: Increase in CCL2, CCL3, and cystatin C levels over time is associated with the NPM1-wt and CD34+ patient subsets. Figure S3: Increase in IL-1RA, MMP-9, and TNFα levels over time is associated with the FAB M0-2 patient subset. Figure S4: Patient survival dependent on cell phenotype. Figure S5: Cluster of the 58 differentially expressed genes between cultures with and without signs of morphological differentiation. Figure S6: Cluster of the 36 differentially expressed genes between cultures with and without erythroid colonies. Table S1: Detailed information about the 68 AML patients included in the study. Table S2: Week5/week 1 excretion ratios for 19 soluble mediators. Table S3: HGF, IL-1RA and MMP-9 levels from long-time suspension cultures of primary human AML cells. Table S4: Differences in the secretion ratios for 19 mediators depending on morphology according to FAB and morphological changes during suspension culture. Table S5: Correlation between cytokine levels and cell or colony number. Table S6: Single genes identified in the comparison of global gene expression profiles for patients with and without at least 0.01% long-term proliferating cells. Table S7: Overview over the mutational landscape of 35 consecutive patients. Table S8: Single genes identified in the comparison of global gene expression profiles for patients with and without signs of morphological differentiation. Table S9: Classification of the genes that were found to be differentially expressed for patient populations that show signs of differentiation. Table S10: Single genes identified in the comparison of global gene expression profiles for patients with and without signs of erythroid colonies. Table S11: Classification of the genes that were found to be differentially expressed for patient populations, which showed colonies of erythroid origin. Table S12: Comparison of the proteomic profile at the time of diagnosis for primary human AML cells derived from patients with and without colony formation. Table S13: Comparison of the phosphoproteomic profile at the time of diagnosis for primary human AML cells derived from patients with and without colony formation.
Author Contributions
Conceptualization, Ø.B..; methodology, A.K.B., E.A., M.H.-V., F.S., F.B., I.-S.G. and S.B.-B.; formal analysis, A.K.B., E.A., M.H.-V., I.-S.G. and S.B.-B; writing—original draft and review, A.K.B and Ø.B..; visualization, A.K.B.; All authors read and approved the final version of the manuscript.
Funding
This research was funded by Helse-Vest (grant 911788) and the Norwegian Cancer Society (grants 17813, 62370, 145008, 15752 and 145007).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Caceres-Cortes R.J. Blastic leukaemias (AML): A biologist’s view. Cell Biochem. Biophys. 2013;66:13–22. doi: 10.1007/s12013-012-9392-8. [DOI] [PubMed] [Google Scholar]
- 2.Burnett A., Wetzler M., Löwenberg B. Therapeutic advances in acute myeloid leukemia. J. Clin. Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 3.Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett J.M., Catovsky D., Daniel M.T., Flandrin G., Galton D.A., Gralnick H.R., Sultan C. Proposal for the recognition of minimally differentiated acute myeloid leukaemia (AML-MO) Br. J. Haematol. 1991;78:325–329. doi: 10.1111/j.1365-2141.1991.tb04444.x. [DOI] [PubMed] [Google Scholar]
- 5.Neame P.B., Soamboonsrup P., Browman G.P., Meyer R.M., Benger A., Wilson W.E., Walker I.R., Saeed N., McBride J.A. Classifying acute leukemia by immunophenotyping: A combined FAB-immunologic classification of AML. Blood. 1986;68:1355–1362. [PubMed] [Google Scholar]
- 6.Sidney L.E., Branch M.J., Dunphy S.E., Dua H.S., Hopkinson A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruserud Ø., Gjertsen B.T., Foss B., Huang T.S. New strategies in the treatment of acute myelogenous leukemia (AML): In vitro culture of AML cells—The present use in experimental studies and the possible importance for future therapeutic approaches. Stem Cells. 2001;19:1–11. doi: 10.1634/stemcells.19-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Ryningen A., Stapnes C., Bruserud Ø. Clonogenic acute myelogenous leukemia cells are heterogeneous with regard to regulation of differentiation and effect of epigenetic pharmacological targeting. Leuk Res. 2007;31:1303–1313. doi: 10.1016/j.leukres.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland H.J., Blair A., Zapf R.W. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood. 1996;87:4754–4761. [PubMed] [Google Scholar]
- 10.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 11.Eppert K., Takenaka K., Lechman E.R., Waldron L., Nilsson B., van Galen P., Metzeler K.H., Poeppl A., Ling V., Beyene J., et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 12.Prada-Arismendy J., Arroyave J.C., Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31:63–76. doi: 10.1016/j.blre.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Hatfield K.J., Reikvam H., Bruserud Ø. Identification of a subset of patients with acute myeloid leukemia characterized by long-term in vitro proliferation and altered cell cycle regulation of the leukemic cells. Expert Opin. Targets. 2014;18:1237–1251. doi: 10.1517/14728222.2014.957671. [DOI] [PubMed] [Google Scholar]
- 14.Brenner A.K., Tvedt T.H., Nepstad I., Rye K.P., Hagen K.M., Reikvam H., Bruserud Ø. Patients with acute myeloid leukemia can be subclassified based on the constitutive cytokine release of the leukemic cells; the possible clinical relevance and the importance of cellular iron metabolism. Expert Opin. Targets. 2017;21:357–369. doi: 10.1080/14728222.2017.1300255. [DOI] [PubMed] [Google Scholar]
- 15.Bruserud Ø., Ryningen A., Olsnes A.M., Stordrange L., Øyan A.M., Kalland K.H., Gjertsen B.T. Subclassification of patients with acute myelogenous leukemia based on chemokine responsiveness and constitutive chemokine release by their leukemic cells. Haematologica. 2007;92:332–341. doi: 10.3324/haematol.10148. [DOI] [PubMed] [Google Scholar]
- 16.Casas S., Ollila J., Aventin A., Vihinen M., Sierra J., Knuutila S. Changes in apoptosis-related pathways in acute myelocytic leukemia. Cancer Genet. Cytogenet. 2003;146:89–101. doi: 10.1016/S0165-4608(03)00102-X. [DOI] [PubMed] [Google Scholar]
- 17.Kazi J.U., Ronnstrand L. FLT3 signals via the adapter protein Grb10 and overexpression of Grb10 leads to aberrant cell proliferation in acute myeloid leukemia. Mol. Oncol. 2013;7:402–418. doi: 10.1016/j.molonc.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodner S.M., Naeve C.W., Rakestraw K.M., Jones B.G., Valentine V.A., Valentine M.B., Luthardt F.W., Willman C.L., Raimondi S.C., Downing J.R., et al. Cloning and chromosomal localization of the gene encoding human cyclin D-binding Myb-like protein (hDMP1) Gene. 1999;229:223–228. doi: 10.1016/S0378-1119(98)00591-5. [DOI] [PubMed] [Google Scholar]
- 19.Caligiuri M.A., Briesewitz R., Yu J., Wang L., Wei M., Arnoczky K.J., Marburger T.B., Wen J., Perrotti D., Bloomfield C.D., et al. Novel c-CBL and CBL-b ubiquitin ligase mutations in human acute myeloid leukemia. Blood. 2007;110:1022–1024. doi: 10.1182/blood-2006-12-061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui X.Y., Skretting G., Jing Y., Sun H., Sandset P.M., Sun L. Hypoxia influences stem cell-like properties in multidrug resistant K562 leukemic cells. Blood Cells Mol. Dis. 2013;51:177–184. doi: 10.1016/j.bcmd.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Han Q., Lu J., Wang J., Ye J., Jiang X., Chen H., Liu C., Chen L., Lin T., Chen S., et al. H2AFY is a novel fusion partner of MECOM in acute myeloid leukemia. Cancer Genet. 2018;222–223:9–12. doi: 10.1016/j.cancergen.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Yang C., Shao T., Zhang H., Zhang N., Shi X., Liu X., Yao Y., Xu L., Zhu S., Cao J., et al. MiR-425 expression profiling in acute myeloid leukemia might guide the treatment choice between allogeneic transplantation and chemotherapy. J. Transl. Med. 2018;16:267. doi: 10.1186/s12967-018-1647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole A., Wang Z., Coyaud E., Voisin V., Gronda M., Jitkova Y., Mattson R., Hurren R., Babovic S., Maclean N., et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2015;27:864–876. doi: 10.1016/j.ccell.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin K., Byrd J.C. Antagonizing ClpP: A new power play in targeted therapy for AML. Cancer Cell. 2015;27:747–749. doi: 10.1016/j.ccell.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Qu X., Davison J., Du L., Storer B., Stirewalt D.L., Heimfeld S., Estey E., Appelbaum F.R., Fang M. Identification of differentially methylated markers among cytogenetic risk groups of acute myeloid leukemia. Epigenetics. 2015;10:526–535. doi: 10.1080/15592294.2015.1048060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S., Du Y., Beckford J., Alachkar H. Upregulation of the EMT marker vimentin is associated with poor clinical outcome in acute myeloid leukemia. J. Transl. Med. 2018;16:170. doi: 10.1186/s12967-018-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du Y., Lu F., Li P., Ye J., Ji M., Ma D., Ji C. SMG1 acts as a novel potential tumor suppressor with epigenetic inactivation in acute myeloid leukemia. Int. J. Mol. Sci. 2014;15:17065–17076. doi: 10.3390/ijms150917065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germano G., Morello G., Aveic S., Pinazza M., Minuzzo S., Frasson C., Persano L., Bonvini P., Viola G., Bresolin S., et al. ZNF521 sustains the differentiation block in MLL-rearranged acute myeloid leukemia. Oncotarget. 2017;8:26129–26141. doi: 10.18632/oncotarget.15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W., Deng L., Song Y., Redell M. DOT1L inhibition sensitizes MLL-rearranged AML to chemotherapy. PLoS ONE. 2014;9:e98270. doi: 10.1371/journal.pone.0098270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafer D., Grant S. Update on rational targeted therapy in AML. Blood Rev. 2016;30:275–283. doi: 10.1016/j.blre.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haimovici A., Brigger D., Torbett B.E., Fey M.F., Tschan M.P. Induction of the autophagy-associated gene MAP1S via PU.1 supports APL differentiation. Leuk Res. 2014;38:1041–1047. doi: 10.1016/j.leukres.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Morita K., Noura M., Tokushige C., Maeda S., Kiyose H., Kashiwazaki G., Taniguchi J., Bando T., Yoshida K., Ozaki T., et al. Autonomous feedback loop of RUNX1-p53-CBFB in acute myeloid leukemia cells. Sci. Rep. 2017;7:16604. doi: 10.1038/s41598-017-16799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niapour M., Farr C., Minden M., Berger S.A. Elevated calpain activity in acute myelogenous leukemia correlates with decreased calpastatin expression. Blood Cancer J. 2012;2:e51. doi: 10.1038/bcj.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsson L., Zettermark S., Biloglav A., Castor A., Behrendtz M., Forestier E., Paulsson K., Johansson B. The genetic landscape of paediatric de novo acute myeloid leukaemia as defined by single nucleotide polymorphism array and exon sequencing of 100 candidate genes. Br. J. Haematol. 2016;174:292–301. doi: 10.1111/bjh.14056. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X., Li Y., Wu H. A novel scoring system for acute myeloid leukemia risk assessment based on the expression levels of six genes. Int. J. Mol. Med. 2018;42:1495–1507. doi: 10.3892/ijmm.2018.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong P.S.Y., Zhou J., Chooi J.Y., Chan Z.L., Toh S.H.M., Tan T.Z., Wee S., Gunaratne J., Zeng Q., Chng W.J. Non-canonical activation of beta-catenin by PRL-3 phosphatase in acute myeloid leukemia. Oncogene. 2018 doi: 10.1038/s41388-018-0526-3. [DOI] [PubMed] [Google Scholar]
- 37.Van Rhenen A., Feller N., Kelder A., Westra A.H., Rombouts E., Zweegman S., van der Pol M.A., Waisfisz Q., Ossenkoppele G.J., Schuurhuis G.J. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin. Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 38.Blair A., Hogge D.E., Sutherland H.J. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(−)/HLA-DR. Blood. 1998;92:4325–4335. [PubMed] [Google Scholar]
- 39.Terpstra W., Prins A., Ploemacher R.E., Wognum B.W., Wagemaker G., Löwenberg B., Wielenga J.J. Long-term leukemia-initiating capacity of a CD34-subpopulation of acute myeloid leukemia. Blood. 1996;87:2187–2194. [PubMed] [Google Scholar]
- 40.Schuurhuis G.J., Meel M.H., Wouters F., Min L.A., Terwijn M., de Jonge N.A., Kelder A., Snel A.N., Zweegman S., Ossenkoppele G.J., et al. Normal hematopoietic stem cells within the AML bone marrow have a distinct and higher ALDH activity level than co-existing leukemic stem cells. PLoS ONE. 2013;8:e78897. doi: 10.1371/journal.pone.0078897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Migliaccio G., Migliaccio A.R., Visser J.W. Synergism between erythropoietin and interleukin-3 in the induction of hematopoietic stem cell proliferation and erythroid burst colony formation. Blood. 1988;72:944–951. [PubMed] [Google Scholar]
- 42.Öhler L., Geissler K. Semi-solid colony growth in acute myeloid leukemia and its relation to cytogenetic risk groups. Leuk Lymphoma. 2002;43:1743–1747. doi: 10.1080/1042819021000006484. [DOI] [PubMed] [Google Scholar]
- 43.Goodman J.W., Hall E.A., Miller K.L., Shinpock S.G. Interleukin 3 promotes erythroid burst formation in “serum-free” cultures without detectable erythropoietin. Proc. Natl. Acad. Sci. USA. 1985;82:3291–3295. doi: 10.1073/pnas.82.10.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruserud Ø., Frostad S., Foss B. In vitro culture of acute myelogenous leukemia blasts: A comparison of four different culture media. J. Hematother. 1999;8:63–73. doi: 10.1089/106161299320587. [DOI] [PubMed] [Google Scholar]
- 45.Bruserud Ø., Gjertsen B.T., von Volkman H.L. In vitro culture of human acute myelogenous leukemia (AML) cells in serum-free media: Studies of native AML blasts and AML cell lines. J. Hematother. Stem Cell Res. 2000;9:923–932. doi: 10.1089/152581600750062372. [DOI] [PubMed] [Google Scholar]
- 46.Salem M., Delwel R., Touw I., Mahmoud L., Löwenberg B. Human AML colony growth in serum-free culture. Leuk Res. 1988;12:157–165. doi: 10.1016/0145-2126(88)90076-8. [DOI] [PubMed] [Google Scholar]
- 47.Bruserud Ø., Hovland R., Wergeland L., Huang T.S., Gjertsen B.T. Flt3-mediated signaling in human acute myelogenous leukemia (AML) blasts: A functional characterization of Flt3-ligand effects in AML cell populations with and without genetic Flt3 abnormalities. Haematologica. 2003;88:416–428. [PubMed] [Google Scholar]
- 48.Papaemmanuil E., Gerstung M., Bullinger L., Gaidzik V.I., Paschka P., Roberts N.D., Potter N.E., Heuser M., Thol F., Bolli N., et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gjertsen B.T., Øyan A.M., Marzolf B., Hovland R., Gausdal G., Døskeland S.O., Dimitrov K., Golden A., Kalland K.H., Hood L., Bruserud Ø. Analysis of acute myelogenous leukemia: Preparation of samples for genomic and proteomic analyses. J. Hematother. Stem Cell Res. 2002;11:469–481. doi: 10.1089/15258160260090933. [DOI] [PubMed] [Google Scholar]
- 50.Reuss-Borst M.A., Klein G., Waller H.D., Müller C.A. Differential expression of adhesion molecules in acute leukemia. Leukemia. 1995;9:869–874. [PubMed] [Google Scholar]
- 51.Nepstad I., Hatfield K.J., Aasebø E., Hernandez-Valladares M., Brenner A.K., Bartaula-Brevik S., Berven F., Selheim F., Skavland J., Gjertsen B.T., et al. Two acute myeloid leukemia patient subsets are identified based on the constitutive PI3K-Akt-mTOR signaling of their leukemic cells; a functional, proteomic, and transcriptomic comparison. Expert Opin. Targets. 2018;22:639–653. doi: 10.1080/14728222.2018.1487401. [DOI] [PubMed] [Google Scholar]
- 52.Stosić-Grujicić S., Basara N., Milenković P., Dinarello C.A. Modulation of acute myeloblastic leukemia (AML) cell proliferation and blast colony formation by antisense oligomer for IL-1 beta converting enzyme (ICE) and IL-1 receptor antagonist (IL-1ra) J. Chemother. 1995;7:67–70. doi: 10.1179/joc.1995.7.1.67. [DOI] [PubMed] [Google Scholar]
- 53.Kim J.G., Sohn S.K., Kim D.H., Baek J.H., Lee N.Y., Suh J.S., Chae S.C., Lee K.S., Lee K.B. Clinical implications of angiogenic factors in patients with acute or chronic leukemia: Hepatocyte growth factor levels have prognostic impact, especially in patients with acute myeloid leukemia. Leuk Lymphoma. 2005;46:885–891. doi: 10.1080/10428190500054491. [DOI] [PubMed] [Google Scholar]
- 54.Guo J.R., Li W., Wu Y., Wu L.Q., Li X., Guo Y.F., Zheng X.H., Lian X.L., Huang H.F., Chen Y.Z. Hepatocyte growth factor promotes proliferation, invasion, and metastasis of myeloid leukemia cells through PI3K-AKT and MAPK/ERK signaling pathway. Am. J. Transl. Res. 2016;8:3630–3644. [PMC free article] [PubMed] [Google Scholar]
- 55.Kentsis A., Reed C., Rice K.L., Sanda T., Rodig S.J., Tholouli E., Christie A., Valk P.J., Delwel R., Ngo V., et al. Autocrine activation of the MET receptor tyrosine kinase in acute myeloid leukemia. Nat. Med. 2012;18:1118–1122. doi: 10.1038/nm.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reikvam H., Nepstad I., Bruserud Ø., Hatfield K.J. Pharmacological targeting of the PI3K/mTOR pathway alters the release of angioregulatory mediators both from primary human acute myeloid leukemia cells and their neighboring stromal cells. Oncotarget. 2013;4:830–843. doi: 10.18632/oncotarget.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aref S., El-Sherbiny M., Mabed M., Menessy A., El-Refaei M. Urokinase plasminogen activator receptor and soluble matrix metalloproteinase-9 in acute myeloid leukemia patients: A possible relation to disease invasion. Hematology. 2003;8:385–391. doi: 10.1080/10245330310001621323. [DOI] [PubMed] [Google Scholar]
- 58.Xian J., Shao H., Chen X., Zhang S., Quan J., Zou Q., Jin H., Zhang L. Nucleophosmin mutants promote adhesion, migration and invasion of human leukemia THP-1 cells through MMPs op-regulation via Ras/ERK MAPK signaling. Int. J. Biol. Sci. 2016;12:144–155. doi: 10.7150/ijbs.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefanidakis M., Karjalainen K., Jaalouk D.E., Gahmberg C.G., O’Brien S., Pasqualini R., Arap W., Koivunen E. Role of leukemia cell invadosome in extramedullary infiltration. Blood. 2009;114:3008–3017. doi: 10.1182/blood-2008-04-148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey A., Edwards D.K.t., Eide C.A., Newell L., Traer E., Medeiros B.C., Pollyea D.A., Deininger M.W., Collins R.H., Tyner J.W., et al. Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017;18:3204–3218. doi: 10.1016/j.celrep.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruserud Ø. Effects of endogenous interleukin 1 on blast cells derived from acute myelogenous leukemia patients. Leuk Res. 1996;20:65–73. doi: 10.1016/0145-2126(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 62.Bruserud Ø., Ryningen A., Wergeland L., Glenjen N.I., Gjertsen B.T. Osteoblasts increase proliferation and release of pro-angiogenic interleukin 8 by native human acute myelogenous leukemia blasts. Haematologica. 2004;89:391–402. [PubMed] [Google Scholar]
- 63.Hatfield K., Ryningen A., Corbascio M., Bruserud Ø. Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts. Int. J. Cancer. 2006;119:2313–2321. doi: 10.1002/ijc.22180. [DOI] [PubMed] [Google Scholar]
- 64.Ryningen A., Wergeland L., Glenjen N., Gjertsen B.T., Bruserud Ø. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leuk Res. 2005;29:185–196. doi: 10.1016/j.leukres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Santos S.C., Dias S. Internal and external autocrine VEGF/KDR loops regulate survival of subsets of acute leukemia through distinct signaling pathways. Blood. 2004;103:3883–3889. doi: 10.1182/blood-2003-05-1634. [DOI] [PubMed] [Google Scholar]
- 66.Langley G.R., Smith L.J., McCulloch E.A. Adherent cells in cultures of blast progenitors in acute myeloblastic leukemia. Leuk Res. 1986;10:953–959. doi: 10.1016/0145-2126(86)90248-1. [DOI] [PubMed] [Google Scholar]
- 67.Levine J.H., Simonds E.F., Bendall S.C., Davis K.L., Amir el A.D., Tadmor M.D., Litvin O., Fienberg H.G., Jager A., Zunder E.R., et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z., Zhang P., Yan A., Guo Z., Ban Y., Li J., Chen S., Yang H., He Y., Li J., et al. ASXL1 interacts with the cohesin complex to maintain chromatid separation and gene expression for normal hematopoiesis. Sci. Adv. 2017;3:e1601602. doi: 10.1126/sciadv.1601602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bullinger L., Döhner K., Döhner H. Genomics of acute myeloid leukemia diagnosis and pathways. J. Clin. Oncol. 2017;35:934–946. doi: 10.1200/JCO.2016.71.2208. [DOI] [PubMed] [Google Scholar]
- 70.Thota S., Viny A.D., Makishima H., Spitzer B., Radivoyevitch T., Przychodzen B., Sekeres M.A., Levine R.L., Maciejewski J.P. Genetic alterations of the cohesin complex genes in myeloid malignancies. Blood. 2014;124:1790–1798. doi: 10.1182/blood-2014-04-567057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rau R.E., Rodriguez B.A., Luo M., Jeong M., Rosen A., Rogers J.H., Campbell C.T., Daigle S.R., Deng L., Song Y., et al. DOT1L as a therapeutic target for the treatment of DNMT3A-mutant acute myeloid leukemia. Blood. 2016;128:971–981. doi: 10.1182/blood-2015-11-684225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reikvam H., Tamburini J., Skrede S., Holdhus R., Poulain L., Ersvær E., Hatfield K.J., Bruserud Ø. Antileukaemic effect of PI3K-mTOR inhibitors in acute myeloid leukaemia-gene expression profiles reveal CDC25B expression as determinate of pharmacological effect. Br. J. Haematol. 2014;164:200–211. doi: 10.1111/bjh.12611. [DOI] [PubMed] [Google Scholar]
- 73.Dysvik B., Jonassen I. J-Express: Exploring gene expression data using Java. Bioinformatics. 2001;17:369–370. doi: 10.1093/bioinformatics/17.4.369. [DOI] [PubMed] [Google Scholar]
- 74.Aasebø E., Mjaavatten O., Vaudel M., Farag Y., Selheim F., Berven F., Bruserud Ø., Hernandez-Valladares M. Freezing effects on the acute myeloid leukemia cell proteome and phosphoproteome revealed using optimal quantitative workflows. J. Proteom. 2016;145:214–225. doi: 10.1016/j.jprot.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez-Valladares M., Aasebø E., Mjaavatten O., Vaudel M., Bruserud Ø., Berven F., Selheim F. Reliable FASP-based procedures for optimal quantitative proteomic and phosphoproteomic analysis on samples from acute myeloid leukemia patients. Biol. Proced. Online. 2016;18:13. doi: 10.1186/s12575-016-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hernandez-Valladares M., Aasebø E., Selheim F., Berven F.S., Bruserud Ø. Selecting sample preparation workflows for mass spectrometry-based proteomic and phosphoproteomic analysis of patient samples with acute myeloid leukemia. Proteomes. 2016;4:24. doi: 10.3390/proteomes4030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aasebø E., Vaudel M., Mjaavatten O., Gausdal G., Van der Burgh A., Gjertsen B.T., Døskeland S.O., Bruserud Ø., Berven F.S., Selheim F. Performance of super-SILAC based quantitative proteomics for comparison of different acute myeloid leukemia (AML) cell lines. Proteomics. 2014;14:1971–1976. doi: 10.1002/pmic.201300448. [DOI] [PubMed] [Google Scholar]
- 78.Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 79.Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 80.Arntzen M.Ø., Koehler C.J., Barsnes H., Berven F.S., Treumann A., Thiede B. IsobariQ: Software for isobaric quantitative proteomics using IPTL, iTRAQ, and TMT. J. Proteome Res. 2011;10:913–920. doi: 10.1021/pr1009977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.