Abstract
Leaf senescence is a genetically controlled process that involves the perception of extracellular signals and signal transduction. The receptor-like protein kinases (RLKs) are known to act as an important class of cell surface receptors and are involved in multiple biological processes such as development and stress responses. The functions of a number of RLK members have been characterized in Arabidopsis and other plant species, but only a limited number of RLK proteins have been reported to be associated with leaf senescence. In the present study, we have characterized the role of the somatic embryogenesis receptor kinase 4 (SERK4) gene in leaf senescence. The expression of SERK4 was up-regulated during leaf senescence and by several abiotic stress treatments in Arabidopsis. The serk4-1 knockout mutant was found to display a significant early leaf senescence phenotype. Furthermore, the results of overexpression analysis and complementary analysis supported the idea that SERK4 acts as a negative regulator in the process of leaf senescence.
Keywords: SERK4, leaf senescence, LRR-RLK, cell-to-cell communication, cell death
1. Introduction
Leaf senescence in plants is a predetermined cell-death mechanism and a coordinated process that involves the remobilization of nutrients [1]. Prior to death, plants undergo a process of recycling and translocating nutrients from senescing organs to non-senescing parts such as seeds and other storage tissues, ensuring their survival and propagation. The senescence process of leaves thus largely affects the yield and nutritional value of food crops [2,3]. Apparently, leaf senescence is marked by the yellowing of old leaves from tip to base due to the degeneration of chlorophylls. At the biochemical level, macromolecules such as lipids, proteins and carbohydrates are degraded [4], while at the molecular level, a number of signaling pathways operate with the involvement of key regulatory components to activate the expression of a subset of senescence associated genes (SAGs), participating in senescence regulation [5,6].
Among the regulatory components, the cell surface receptors are important structural proteins, serving as the scaffold to perceive signals which coordinate cell-to-cell communication. The receptor-like kinases (RLKs) are the most characterized class of cell-surface receptors, with unique structural features [7,8]. A typical RLK comprises an extracellular binding domain (ECD) to perceive specific ligands, a transmembrane domain (TM) to fix the protein on the plasma membrane and a cytoplasmic kinase domain (KD) to transduce the signals into the cell to activate the downstream regulatory components via phosphorylation [7]. The phylogenetic study of the RLKs indicated that nearly 610 members are encoded by the Arabidopsis genome, representing a large monophyletic gene super family [9]. Among the 44 subclasses of RLKs, leucine-rich repeat receptor-like protein kinase (LRR-RLK) represents the largest subfamily in the Arabidopsis with over 200 members [9]. The ECD of LRR-RLKs are furnished with individual leucine-repeat units, 24 amino acids long, varying in both number and arrangement [7]. To date, a large number of LRR-RLK members have been identified in Arabidopsis, rice, poplar, potato, tomato and other plant species [9,10,11,12]. However, only limited members have been assigned to various biological functions including plant growth and development and stress responses [7].
Being a development and age-dependent process, leaf senescence can be triggered by various intrinsic and extrinsic factors including age and hormones as well as biotic and abiotic stresses [13]. The initiation and execution of leaf senescence are driven by the differential expression of genes [14]. Among them, a number of transcription factors, including members of the NAC, WRKY and MYB families, have been found to play important roles in regulating leaf senescence [15,16,17,18,19]. The ability of LRR-RLKs to sense various signals at the cell surface and regulate the activities of transcription factor through signal transduction makes them potential candidates to be components of the senescence regulatory network. In fact, several members of the LRR-RLK family have been identified as playing a role in the progress of leaf senescence. One LRR-RLK member, senescence-associated receptor like kinase (SARK), was reported to regulate leaf senescence in Phaseolus vulgaris [20]. Further, soybean (Glycine max) senescence-associated receptor-like kinase (GmSARK) and its homologue AtSARK in Arabidopsis were functionally characterized, and their knockout mutant exhibited precocious leaf senescence [21,22]. Another LRR-RLK member RPK1 was up-regulated by age and ABA, while acting as a positive regulator of leaf senescence in Arabidopsis [23].
In an attempt to isolate senescence-regulating LRR-RLKs, Arabidopsis T-DNA insertion lines of selected LRR-RLK family genes were screened for leaf senescence-related phenotypes followed by the systematic analysis of related genes. Here, we describe the characterization of an important LRR-RLK member which has been reported as the somatic embryogenesis receptor-like kinase 4 (SERK4) and grouped into the SERK subfamily in Arabidopsis [24]. In prior studies, SERK4 was found to negatively regulate cell death through elusive mechanisms [25,26,27]. Notably, the SERK subfamily members have been reported in various studies with different functions including brassinosteroid signaling, stomatal patterning, immunity and cell death [25,28,29]. In this study, the SERK4 gene was found to be highly expressed in senescent leaves and to control leaf senescence in Arabidopsis.
2. Materials and Methods
2.1. Plant Materials
The Arabidopsis wild type Col-0 was kept in our laboratory and the T-DNA insertion line SALK_057955, which has been reported previously [29], was obtained from the Arabidopsis Biological Resource Center (ABRC). Homozygous mutant plants were identified with the PCR based method using three primers including the T-DNA left border primer (5′-ATTTTGCCGATTTCGGAAC-3′), the gene specific left primer (5′-TGGCTCAGAAGAAAACCACAG-3′), and the right border primer (5′-CTGCTCCACTTCTGTTTCCAC-3′).
2.2. Plant Growth and Stress Treatments
Arabidopsis seeds were surface-sterilized by immersion in 70% ethanol and were well dried before sowing on Petri dishes containing half-strength MS (Murashige and Skoog) solid media. Two-week old seedlings with four leaves from the plates were transplanted to pots containing soil mixture (pindstrup–peat moss and vermiculite, 3:1 v/v). Arabidopsis plants were kept in a growth room at 22 °C with continuous light. Different tissues, including the shoot, root, flower, young leaf, and senescence leaf of four-week old Arabidopsis plants, were harvested for gene expression analysis. Leaves at different developmental stages were used for gene expression analysis. This includes young leaves that are still undergoing expansion, mature leaves that are fully expanded with no senescence phenotype, early senescence leaves with senescence initiated at the tip, and late senescence leaves in which senescence has proceeded to the middle section of a leaf. Environmental stresses such as extreme temperature, salt, and drought can induce senescence. According to previous studies, Arabidopsis seedlings were treated with 150 mM NaCl, 200 µM mannitol, 35 °C and 4 °C for 1, 3 and 6 h, respectively [13,30]. All of these samples were frozen in liquid nitrogen immediately after harvest and stored at −80 °C before analysis.
2.3. RNA Extraction and qRT-PCR
Total RNA was extracted using RNAiso (TaKaRa, Shiga, Japan) based on the manufacturer’s instructions. The first-strand cDNA synthesis was performed with 2 μg RNA using the PrimeScript™ RT reagent Kit (TaKaRa). Further, the qRT-PCR analysis was conducted using an ABI 7500 real-time PCR system (Applied Biosystems, Waltham, MA, USA) with a 20 μL reaction volume including SYBR (TaKaRa) 10 μL, 10 μM forward/reverse primer 0.4 μL, and 100 ng/μL cDNA 0.2 μL. The ACTIN2 (ACT2) gene was used as the internal control for normalization, whereas the SAG12 and RBCS genes were used as senescence markers [15,31]. The relative fold change was calculated based on the 2−ΔΔct method. The qRT-PCR assay results were collected from three independent biological repeats with three technical replications. The t-tests were performed with GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Sequences of primers are listed in Supplementary Table S1.
2.4. Subcellular Localization Analysis
The coding sequence (CDS) of SERK4 was amplified and ligated into the pEasy-Blunt vector (Transgen Biotech, Beijing, China). Then, the CDS of SERK4 excluding the stop codon together with a green fluorescent protein (GFP) fragment were inserted into the SacI site of the pCHF3 vector by Infusion (Clontech, Palo Alto, CA, USA). The 35S::SERK4-GFP construct and a previously reported 35S::GFP control [32] were used for Agrobacterium-mediated transient expression in Nicotiana benthamiana leaves [33]. The GFP fluorescence signals were captured by a confocal microscope (TCS-SP8, Leica, Wetzlar, Germany) three to four days after the injections.
2.5. Overexpression and Complementation Test
For overexpression analysis, the CDS of SERK4 was amplified and inserted into the SacI site of the pCHF3 vector by Infusion (Clontech). For complementation testing, the native promoter (2.2 kb) of SERK4 was PCR-amplified and cloned into the EcoRI and SacI sites of the pPZP211 vector by Infusion (Clontech), resulting in the recombinant construction named pPZP211-pSERK4. Further, the CDS of SERK4 was amplified and inserted into the SalI site of pPZP211-pSERK4 by Infusion (Clontech). After the sequencing of these recombinant constructions, the respective plasmids were transferred into GV3101 Agrobacterium competent cells. The positive colonies of Agrobacterium were selected and used to transform Col-0 or serk4-1 mutant plants through the floral dip method [34]. The transformed Arabidopsis were selected on plates containing 50 mg/L kanamycin.
2.6. Measurements of Chlorophyll Content, Fluorescence and Ion Leakage
The chlorophyll content was quantified as described previously [35]. The fluorescence in leaves was measured with a chlorophyll fluorometer (Opti-Sciences, Tyngsboro, MA, USA) according to the manufacturer’s instructions. For ion leakage measurement, leaves were immersed in deionized distilled water, shaken at 25 °C for 30 min, and the beginning conductivity was measured using a digital conductivity meter (Thermo Fisher Scientific Traceable, Hampton, NH, USA). The samples were then boiled for 15 min and then the second conductivity was measured. The percentage of the first measurement over the second measurement was used as the membrane leakage indicator [35]. These assay results were retrieved from five independent biological repeats. The t-tests were performed with GraphPad Prism 5 (GraphPad Software Inc.).
3. Results
3.1. The SERK4 Gene is Up-Regulated During Leaf Senescence
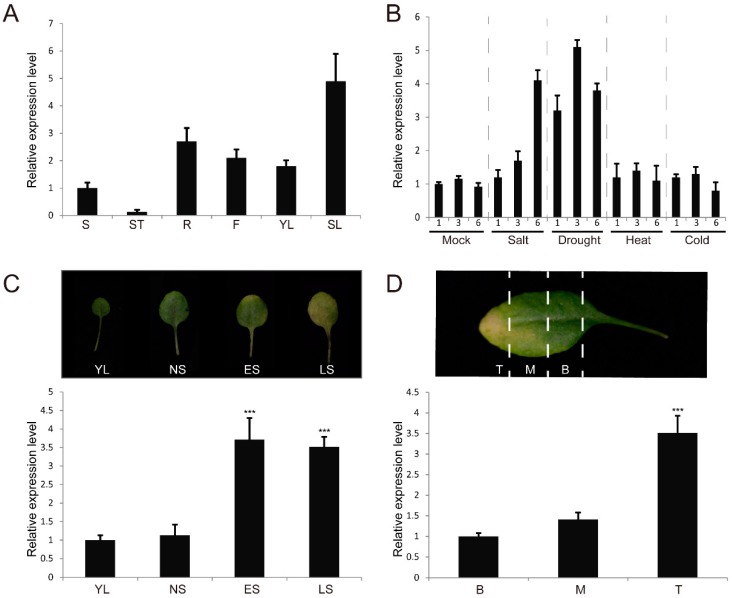
The expression pattern of SERK4 was examined by qRT-PCR. The results showed that SERK4 exhibited low expression in stem, root, flower and young leaf, but high expression in senescence leaf (Figure 1A). Besides this, the expression levels of SERK4 under abiotic stress treatments were also determined by qRT-PCR. It was found that the transcript levels of SERK4 were significantly induced by drought and salt stresses and reached peak levels with five-fold and four-fold increase after 3 h and 6 h treatments, respectively. However, SERK4 expression seemed not to be responsive to heat or chilling stress treatments (Figure 1B).
Figure 1.
Expression analysis of the SERK4 gene: (A) SERK4 expression levels in tested tissues. The ratios of SERK4 gene expression level in different tissues were calculated relative to the shoot. S, shoot; St. shoot tip; R, root; F, flower; YL, young leaf; SL, senescence leaf; (B) SERK4 gene expression changes under abiotic stress treatments. The ratios of SERK4 gene expression level under tested treatments were calculated relative to the untreated seedlings; (C) SERK4 gene expression in leaves at different developmental stages. The ratios of SERK4 gene expression level were calculated relative to the YL stage. YL, young leaf; NS, fully-expanded, non-senescent leaf; ES, early senescent leaf; LS, late senescent leaf; (D) SERK4 gene expression at different sections of a senescing leaf. The ratios of SERK4 gene expression level were calculated relative to the base section. B, base; M, middle; T, tip. In A, B, C and D, the expression data are means ± SD of three biological repeats. *** p < 0.001 (t-tests).
Further, the SERK4 expression pattern in a single leaf was examined under four growth stages, including the expanding young leaf stage, the fully expanded mature leaf stage, the early senescence leaf stage and late senescence leaf stage. The SERK4 transcripts were detected to be highly expressed in leaves at both early and late senescence stages (Figure 1C). Since natural senescence proceeds from the tip section toward the base section of the leaf in Arabidopsis, a representative wildtype Arabidopsis leaf at the early senescence stage was divided into three parts, and the SERK4 gene was found to be highly expressed in the tip part of the leaf (Figure 1D). These results suggested that the expression of SERK4 is up-regulated during the process of leaf senescence.
3.2. The SERK4 Protein is Localized on the Cell Membranes
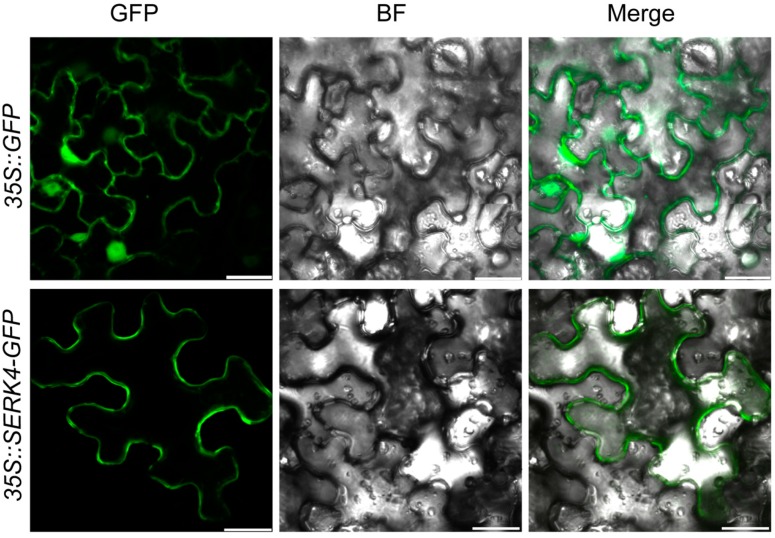
The SERK4 gene is predicted to encode a typical LRR-RLK protein, which can be grouped with other SERK members to form a separated branch in the Arabidopsis LRR-RLK family. To determine the subcellular localization of the SERK4 protein, the CDS of SERK4 gene excluding the stop codon was inserted to be in frame with a GFP reporter gene, under the control of the CaMV-35S promoter. The fluorescence of the fusion protein was specifically localized on the cell membranes, whereas the signals of 35S::GFP were found to be distributed in both membrane and cytosolic fractions (Figure 2).
Figure 2.
Subcellular localization analysis of the SERK4 protein: the green fluorescent protein (GFP) and SERK4-GFP fusion constructs under the control of the CaMV-35S promoter were transiently expressed in tobacco leaves. The scale bar represents 25 μm. GFP, GFP fluorescence; BF, bright field; Merge, the merged image of GFP and BF.
3.3. Loss-of-Function of SERK4 Causes Early Senescence
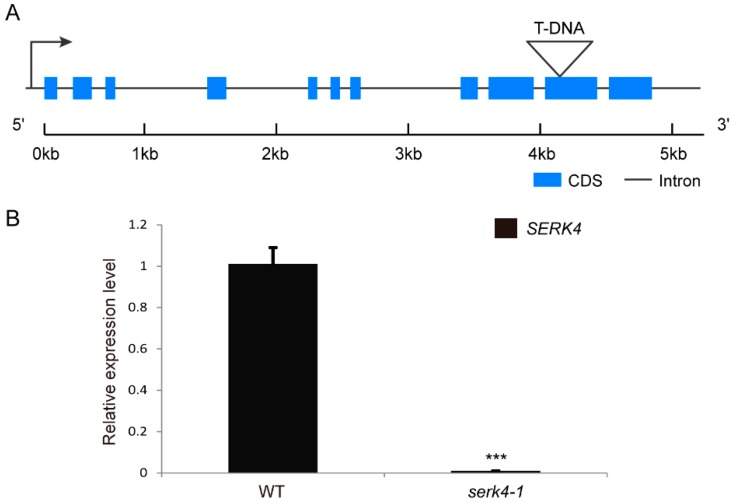
To investigate the role of SERK4 in the regulation of leaf senescence, a mutant line named serk4-1 was used for loss-of-function analysis. serk4-1 (SALK_057955) was obtained from the Arabidopsis Biological Resource Center (ABRC) which harbored a T-DNA insertion in the second last exon of the SERK4 gene. The homozygotes of serk4-1 were confirmed by PCR-based genotyping and no SERK4 transcript was detected in the LS-stage rosette leaves of the serk4-1 mutant plants by qRT-PCR (Figure 3).
Figure 3.
Expression of SERK4 in the T-DNA insertion line: (A) the exon/intron structure of the SERK4 gene and location of the T-DNA insertion; (B) the expression level of SERK4 revealed by qRT-PCR in LS leaves of wildtype and the T-DNA insertion line, the ratios of SERK4 gene expression level were calculated relative to the wildtype leaf. CDS, coding sequence; WT, wildtype. The expression data are means ± SD of three biological repeats. *** p < 0.001 (t-tests).
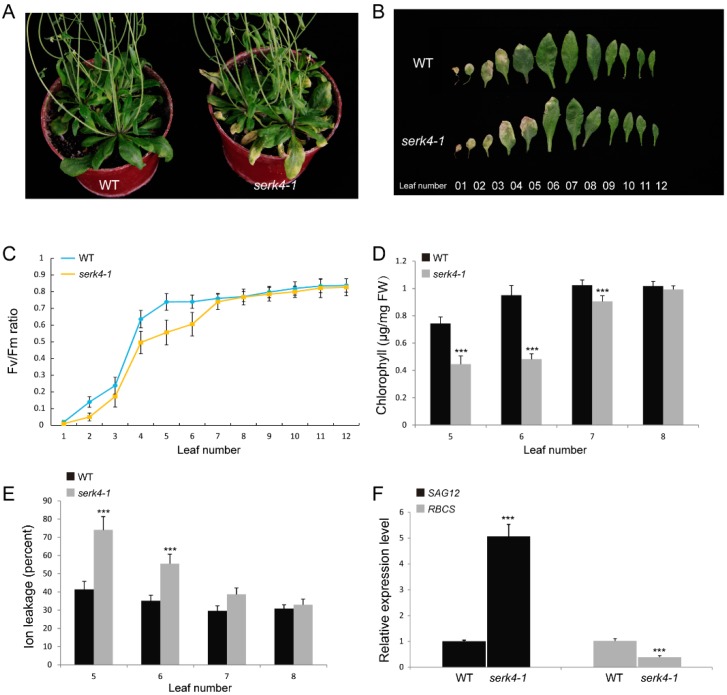
No significant difference of phenotypes between serk4-1 and wild-type plants was observed at the early developmental stages, and bolting time was not affected in the serk4-1 mutant. The five-week-old serk4-1 mutant plants, however, showed an early senescence phenotype compared with the wild-type plants after bolting (Figure 4A). Further, 12 detached rosette leaves from mutant and wild-type plants were prepared as described previously and compared for their senescence phenotypes [15]. The results also showed a clear early senescence phenotype of the serk4-1 mutant (Figure 4B). The Fv/Fm ratio is an indicator of the photosynthesis efficiency. A lower Fv/Fm ratio was found at four leaf positions of the mutant in comparison to the wild-type plants (Figure 4C). Consistent with the early senescence phenotype of the serk4-1 mutant, the chlorophyll content of serk4-1 plants was lower than that of the wildtype at leaf position 5–8 (Figure 4D). Furthermore, ion leakage, which is an important plasma membrane integrity indicator, was found to be lower at the four leaf positions of serk4-1 than the wild-type plant (Figure 4E).
Figure 4.
The early senescence phenotype of the serk4-1 mutant; (A) the leaf senescence phenotype of the five-week-old serk4-1 mutant and the wild-type plants; (B) phenotype of 12 detached rosette leaves from the serk4-1 mutant and the wild type; (C) The Fv/Fm of the serk4-1 mutant and the wild type; (D) the chlorophyll content of the serk4-1 mutant and the wild type; (E) the ion leakage of the serk4-1 mutant and the wild type; (F) the expression levels of SAG12 and RBCS in the number 6 rosette leaf of the serk4-1 mutant and the wild type. The ratios of SAG12 and RBCS gene expression level were calculated relative to the wild type, respectively. WT, wildtype. In D, E and F, the data are means ± SD of three biological repeats. *** p < 0.001 (t-tests).
Molecular makers could help in estimating the senescence process prior to the visible phenotypes. The SAG12 gene was reported to encode a cysteine proteinase and has been well accepted as a molecular marker for age-induced leaf senescence [30]. On the other hand, the RBCS gene was reported to be involved in photosynthesis and the expression level was found to be negatively correlated with the leaf senescence process [31]. As expected, the SAG12 expression level of the serk4-1 mutant was higher than wild-type plants, while the RBCS expression level of serk4-1 plants was lower than for the wild type (Figure 4F).
3.4. Leaf Senescence is Delayed in Plants Overexpressing SERK4
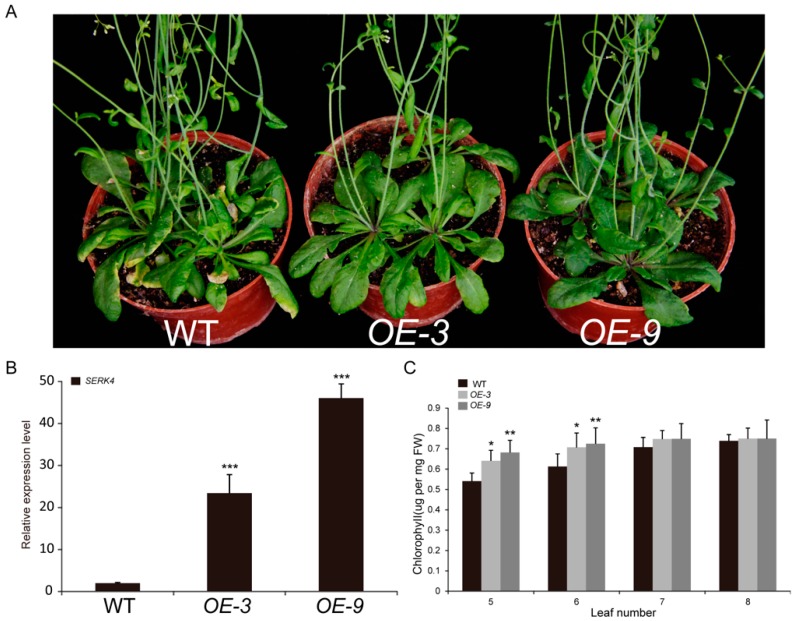
To further investigate the roles of SERK4 in leaf senescence, gain-of-function analysis was carried out. The CDS of SERK4 was fused into the pCHF3 vector after the 35S promoter and transgenic lines were generated to overexpress SERK4 in the wild-type background. Hence, two overexpression lines, named OE-3 and OE-9, were selected for phenotyping. Compared to the six-week-old wildtype plants, the overexpression of SERK4 in transgenic Arabidopsis resulted in a significant delay of leaf senescence (Figure 5A,B). Notably, the bolting time was not affected in these two SERK4 overexpression lines. The overexpression lines showed higher chlorophyll content than the wild-type plants (Figure 5C).
Figure 5.
The delayed senescence phenotype of the SERK4 overexpression lines; (A) the leaf senescence phenotype of the six-week-old wildtype and two SERK4 overexpression lines plants; (B) the expression level of SERK4 gene in wildtype and two SERK4 overexpression lines, the ratios of SERK4 gene expression level were calculated relative to the wildtype; (C) the chlorophyll contents of the wildtype and two SERK4 overexpression lines. WT, wildtype; OE, overexpression line. In B and C, the data are means ± SD of three biological repeats. * p < 0.05, ** p < 0.01, *** p < 0.001 (t-tests).
3.5. The SERK4 Gene Rescues the Serk4 Mutant Phenotypes
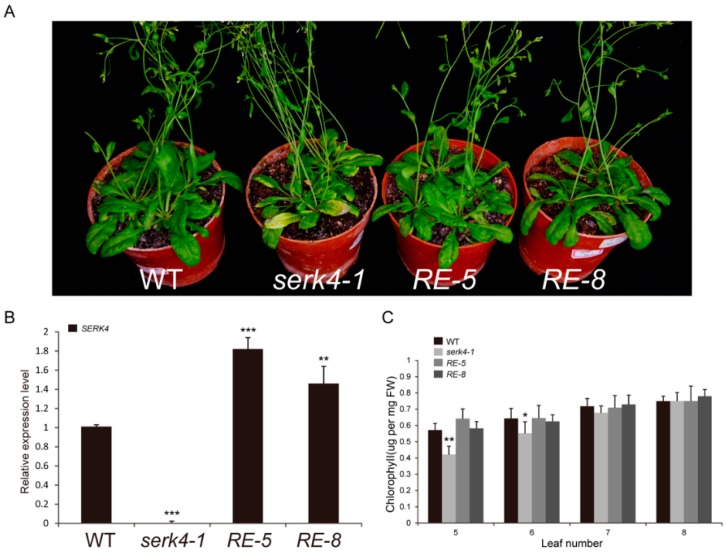
To further confirm these senescence-related phenotypes were conferred by alteration of the SERK4 gene expression, a complementation test was carried out. Driven by the 2.2 kb native SERK4 promoter, the CDS of the SERK4 gene was inserted into the pPZP211 vector, and the resulting complementation construct was used to transform the serk4-1 mutant plants. The early senescence phenotype of serk4-1 was restored to the wild type in two independent transgenic lines (Figure 6A,B). The senescence phenotypes were further supported by the chlorophyll content results (Figure 6C).
Figure 6.
Complementation assay of the serk4-1 mutant with the SERK4 gene; (A) the leaf senescence phenotype of the five-week old wild-type, serk4-1 mutant and two rescue-line plants; (B) the expression level of the SERK4 gene in the wild type, serk4-1 mutant and two rescue lines, the ratios of SERK4 gene expression level were calculated relative to the wild type; (C) the chlorophyll contents of the wild type, serk4-1 mutant and two rescue lines. WT, wildtype; RE, rescue line. In B and C, the data are means ± SD of three biological repeats. * p < 0.05, ** p < 0.01, *** p < 0.001 (t-tests).
4. Discussion
Leaf senescence is a genetically controlled cell-death process. During the past two decades, a large number of SAGs have been identified and a subset of SAGs have been characterized to be involved in the regulation of the initiation and execution of leaf senescence [13]. Here, we characterized one of the Arabidopsis LRR-RLK family members, SERK4, which participated in the regulation network of leaf senescence. The expression of SERK4 was found to be up-regulated in the senescence leaf. Compared to the wild-type plant, the serk4-1 mutant displayed an early senescence phenotype with a higher expression level of the senescence marker gene SAG12, lower chlorophyll content and Fv/Fm ratio, and high ion leakage. Although only one single loss-of-function mutant was analyzed, the fact that the early senescence phenotype of the serk4-1 mutant was successfully rescued to the wild type by the SERK4 gene, and that two transgenic lines overexpressing SERK4 exhibited a delayed leaf senescence phenotype, fully supported the role of SERK4 as a negative regulator of leaf senescence in Arabidopsis.
Comprising five members (SERK1-5) in Arabidopsis, the SERK family has been characterized as co-receptors in a number of signaling pathways [36,37]. In previous studies, SERK1 was reported as a co-receptor and found to interact with BRI1, PSKR and HAESA respectively, resulting in heterodimeric complexes which work in various signaling pathways [37,38,39]. Besides this, the SERK family member SERK3/BAK1 was observed to interact with BRI1 in regulating BR signaling, while BAK1 was also found to interact with FLS2 and contribute to conferring plants with innate immunity [40,41]. Furthermore, SERK4 was reported to interact with SERK3/BAK1, while the silencing of SERK3/BAK1 and SERK4 at the same time caused cell death and H2O2 production in Arabidopsis, suggesting that SERK4 is also a negative regulator of cell death [25,26,27]. Programmed cell death is a genetically controlled process of cell suicide, while leaf senescence as the last stage of leaf development is a type of programmed cell death which is featured by catabolic events such as proteins and nucleic acid degradation [13,25,42]. Based on the present clues, it is suggested that SERK4 acts as a negative regulator in both cell death and leaf senescence, and may coordinate these two important processes in Arabidopsis. Besides this, SERK4 may also act as a co-receptor in negatively regulating leaf senescence, which could be explored in further studies.
Acknowledgments
The authors thank the Guo laboratory members at the Tobacco Research Institute, Chinese Academy of Agricultural Sciences (CAAS) for discussion and critical reading of the manuscript.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4409/8/1/50/s1, Supplementary Table S1: The PCR primers used in this study.
Author Contributions
X.L. and S.A. conducted the research and participated in the drafting of the manuscript. A.A., C.G., H.L., J.Y., Y.Z. and X.G. assisted to the data collection and analysis. Y.G. conceived this research, designed the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (31571494), the Agricultural Science and Technology Innovation Program (ASTIP-TRIC02) and the Fundamental Research Funds for Central Non-profit Scientific Institution (Y2017JC27).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gan S., Amasino R.M. Making sense of senescence (molecular genetic regulation and manipulation of leaf senescence) Plant Physiol. 1997;113:313–319. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hörtensteiner S., Feller U. Nitrogen metabolism and remobilization during senescence. J. Exp. Botany. 2002;53:927–937. doi: 10.1093/jexbot/53.370.927. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y., Gan S.S. Translational researches on leaf senescence for enhancing plant productivity and quality. J. Exp. Botany. 2014;65:3901–3913. doi: 10.1093/jxb/eru248. [DOI] [PubMed] [Google Scholar]
- 4.Humbeck K., Quast S., Krupinska K. Functional and molecular changes in the photosynthetic apparatus during senescence of flag leaves from field-grown barley plants. Plant Cell Environ. 1996;19:337–344. doi: 10.1111/j.1365-3040.1996.tb00256.x. [DOI] [Google Scholar]
- 5.Guo Y., Gan S.S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2012;35:644–655. doi: 10.1111/j.1365-3040.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 7.Gish L.A., Clark S.E. The RLK/Pelle family of kinases. Plant J. 2011;66:117–127. doi: 10.1111/j.1365-313X.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiu S.H., Bleecker A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Nat. Acad. Sci. USA. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiu S.H., Karlowski W.M., Pan R., Tzeng Y.H., Mayer K.F., Li W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Z., Wang J., Yang S., Song Y. Identification and expression analysis of the LRR-RLK gene family in tomato (Solanum lycopersicum) Heinz 1706. Genome. 2015;58:121–134. doi: 10.1139/gen-2015-0035. [DOI] [PubMed] [Google Scholar]
- 11.Zan Y., Ji Y., Zhang Y., Yang S., Song Y., Wang J. Genome-wide identification, characterization and expression analysis of populus leucine-rich repeat receptor-like protein kinase genes. BMC Genomics. 2013;14 doi: 10.1186/1471-2164-14-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Salman A., Guo C., Yu J., Cao S., Gao X., Li W., Li H., Guo Y. Identification and characterization of LRR-RLK family genes in potato reveal their involvement in peptide signaling of cell fate decisions and biotic/abiotic stress responses. Cells. 2018;7:120. doi: 10.3390/cells7090120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y., Gan S. Leaf senescence: Signals, execution, and regulation. Curr. Top. Dev. Biol. 2005;71:83–112. doi: 10.1016/S0070-2153(05)71003-6. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y. Towards systems biological understanding of leaf senescence. Plant Mol. Biol. 2013;82:519–528. doi: 10.1007/s11103-012-9974-2. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Gan S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006;46:601–612. doi: 10.1111/j.1365-313X.2006.02723.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K., Gan S.S. An ABA-AtNAP transcription factor-SAG113 PP2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2011;158:961–969. doi: 10.1104/pp.111.190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balazadeh S., Siddiqui H., Allu A.D., Matallana-Ramirez L.P., Caldana C., Mehrnia M., Zanor M.I., Köhler B., Mueller-Roeber B. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010;62:250–264. doi: 10.1111/j.1365-313X.2010.04151.x. [DOI] [PubMed] [Google Scholar]
- 18.Zentgraf U., Laun T., Miao Y. The complex regulation of WRKY53 during leaf senescence of Arabidopsis thaliana. Eur. J. Cell Biol. 2010;89:133–137. doi: 10.1016/j.ejcb.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y., Gan S.S. AtMYB2 regulates whole plant senescence by inhibiting cytokinin-mediated branching at late stages of development in Arabidopsis. Plant Physiol. 2011;156:1612–1619. doi: 10.1104/pp.111.177022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajouj T., Michelis R., Gepstein S. Cloning and characterization of a receptor-like protein kinase gene associated with senescence. Plant Physiol. 2000;124:1305–1314. doi: 10.1104/pp.124.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X.P., Gan R., Li P.L., Ma Y.Y., Zhang L.W., Zhang R., Wang Y., Wang N.N. Identification and functional characterization of a leucine-rich repeat receptor-like kinase gene that is involved in regulation of soybean leaf senescence. Plant Mol. Biol. 2006;61:829–844. doi: 10.1007/s11103-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 22.Xu F., Meng T., Li P., Yu Y., Cui Y., Wang Y., Gong Q., Wang N.N. A Soybean dual-specificity kinase GmSARK and its Arabidopsis homologue regulate leaf senescence through synergistic actions of auxin and ethylene. Plant Physiol. 2011;157:2131–2153. doi: 10.1104/pp.111.182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee I.C., Hong S.W., Whang S.S., Lim P.O., Nam H.G., Koo J.C. Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol. 2011;52:651–662. doi: 10.1093/pcp/pcr026. [DOI] [PubMed] [Google Scholar]
- 24.Aan den Toorn M., Albrecht C., de Vries S. On the origin of SERKs: Bioinformatics analysis of the somatic embryogenesis receptor kinases. Mol. Plant. 2015;8:762–782. doi: 10.1016/j.molp.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 25.De Oliveira M.V., Xu G., Li B., de Souza Vespoli L., Meng X., Chen X., Yu X., de Souza S.A., Intorne A.C., Manhães A.M., et al. Specific control of Arabidopsis BAK1/SERK4-regulated cell death by protein glycosylation. Nat. Plants. 2016;2 doi: 10.1038/nplants.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He K., Gou X., Yuan T., Lin H., Asami T., Yoshida S., Russell S.D., Li J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr. Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 27.Kemmerling B., Schwedt A., Rodriguez P., Mazzotta S., Frank M., Qamar S.A., Mengiste T., Betsuyaku S., Parker J.E., Müssig C., et al. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr. Biol. 2007;17:1116–1122. doi: 10.1016/j.cub.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Wu W., Wu Y., Gao Y., Li M., Yin H., Lv M., Zhao J., Li J., He K. Somatic embryogenesis receptor-like kinase 5 in the ecotype Landsberg erecta of Arabidopsis is a functional RD LRR-RLK in regulating brassinosteroid signaling and cell death control. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng X., Chen X., Mang H., Liu C., Yu X., Gao X., Torii K.U., He P., Shan L. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Curr. Biol. 2015;25:2361–2372. doi: 10.1016/j.cub.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S.W., Jon J.H., Kwak J.M., Nam H.G. Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt, and cold treatments in Arabidopsis thaliana. Plant Physiol. 1997;113:1203–1212. doi: 10.1104/pp.113.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan X., Wang J., Chua L., Jiang D., Peng W., Xie D. A role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol. 2010;155:751–764. doi: 10.1104/pp.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Hamyat M., Liu C., Salman A., Gao X., Guo C., Wang Y., Guo Y. Identification and characterization of the WOX family genes in five Solanaceae species reveal their conserved roles in peptide signaling. Genes. 2018;9:260. doi: 10.3390/genes9050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheludko Y., Sindarovska Y., Gerasymenko I., Bannikova M., Kuchuk N. Comparison of several Nicotiana species as hosts for high-scale Agrobacterium-mediated transient expression. Biotechnol. Bioeng. 2007;96:608–614. doi: 10.1002/bit.21075. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X., Henriques R., Lin S.S., Niu Q.W., Chua N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006;1:641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- 35.Zhao L., Xia Y., Wu X.Y., Schippers J.H., Jing H.C. Phenotypic Analysis and Molecular Markers of Leaf Senescence. In: Guo Y., editor. Plant Senescence–Methods and Protocols. Humana Press; New York, NY, USA: 2018. pp. 35–48. [DOI] [PubMed] [Google Scholar]
- 36.Brandt B., Hothorn M. SERK co-receptor kinases. Curr. Biol. 2016;26:R225–R226. doi: 10.1016/j.cub.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Santiago J., Henzler C., Hothorn M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science. 2013;341:889–892. doi: 10.1126/science.1242468. [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Li H., Han Z., Zhang H., Wang T., Lin G., Chang J., Yang W., Chai J. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature. 2015;525:265–268. doi: 10.1038/nature14858. [DOI] [PubMed] [Google Scholar]
- 39.Santiago J., Brandt B., Wildhagen M., Hohmann U., Hothorn L.A., Butenko M.A., Hothorn M. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife. 2016:5. doi: 10.7554/eLife.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nam K.H., Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 41.Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D., Felix G., Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 42.Lim P.O., Kim H.J., Gil Nam H. Leaf senescence. Annu. Rev. Plant Biol. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.