Abstract
Summary scores provide an alternative approach to measuring dietary quality. The Growing Up Milk-Lite (GUMLi) Trial was a multi-centre, double-blinded, randomised controlled trial of children randomised to receive a reduced protein GUM (GUMLi) or unfortified cow’s milk (CM). In a secondary analysis of the GUMLi Trial, we used the Probability of Adequate Nutrient Intake (PANDiet) to determine the nutritional adequacy of the diets of participating children living in Auckland. The PANDiet was adapted to the New Zealand Nutrient Reference Values and data from four 24 h Recalls (24HR) collected at months 7, 8, 10, and 11 post-randomisation were used. Differences between randomised groups (GUMLi vs. CM) of the PANDiet and its components were made. Eighty-three Auckland participants were included in the study (GUMLi n = 41 vs. CM n = 42). Total PANDiet scores were significantly higher in the GUMLi group (p < 0.001), indicating better overall nutrient adequacy and diet quality. Dietary intakes of children in both groups met the recommendations for fat, total carbohydrates and most micronutrients; however, protein intakes exceeded recommendations. Consumption of GUMLi was associated with higher nutritional adequacy, with an increased likelihood of meeting nutrient requirements; however, the impact of the family diet and GUMLi on dietary diversity requires further evaluation.
Keywords: diet quality, PANDiet index, early childhood, nutritional adequacy, nutrient intake quality, growing up milk
1. Introduction
Early food habits, practices, and dietary patterns develop rapidly within the first two years of life [1,2]; with evidence that diet quality may decline as children age [3]. Evaluating diet quality in paediatric populations is of increasing interest, however, due to a paucity of evidence-based dietary guidelines for children under two, combining these multidimensional behaviours into a single meaningful measure remains a challenge [4].
Diet quality can be determined using nutrient, food, or food and nutrient-based indices [5]. Index scores are determined ‘a priori’, using dietary guidelines, recommended nutrient intakes, or current nutrition knowledge of optimal dietary patterns [6,7,8,9]. The resulting numeric representation of dietary quality or nutrient adequacy can be used as a nutritional benchmark in identifying relationships between the whole-of-diet and later health [6,7,10,11]. Nutrient-based measures of diet quality reflect adequacy of nutrient intake, however, require detailed dietary assessment, additional analyses and statistical modelling before a final score is calculated [5,6]. In contrast, food-based indices provide an indirect measure of nutrient and non-nutrient interactions, where a score is easily calculated based on awarding points for fulfilling certain criteria [5,6]. The Probability of Adequate Nutrient Intake (PANDiet) score is a complete, nutrient-based diet quality index, employing probabilistic calculations of nutrient adequacy [12]. The index has been evaluated in French [12], US [12] adult populations and a UK [13] paediatric population and has shown to be a useful tool in assessing diet quality at the population level [12].
There is limited research on the contribution of milk to the diets of children under two [14,15,16], specifically, whether Growing Up Milks (GUM) provide a nutritional advantage compared to standard cow’s milk (CM) [17]. Simulation data have shown that replacing CM with GUM resulted in protein intakes more in line with recommendations, reduced saturated fatty acid (SFA) intake and increased likelihood of adequate intakes of vitamin D and iron [17,18]. We aimed to evaluate the dietary quality of the Auckland children participating in the GUMLi Trial aged 18- to 23-months, using an adapted PANDiet index and determine nutritional adequacy according to intervention allocation.
2. Materials and Methods
2.1. Study Design and Participants
This is a secondary analysis of data collected as part of the GUMLi trial. Briefly, the GUMLi trial was a multi-centre, double blinded, randomised controlled trial performed in Auckland, New Zealand (n = 108) and Brisbane, Australia (n = 52) from 2015 to 2017. One hundred and sixty healthy children aged one were randomised 1:1 to receive unfortified cow’s milk (CM) or a reduced protein GUM (GUMLi), fortified with iron, vitamin D, pre- and probiotics (Danone Pty Ltd., Auckland, New Zealand) until the age of two. GUMLi had a reduced energy and protein content compared to commercial GUM on the market, 60 kcal/100 mL vs. 71 kcal/100 mL and 1.7 g/100 mL protein vs. 2.2 g/100 mL. An energy-matched, non-fortified cow’s milk was used as an active control, with a protein content of 3.1 g/100 mL. The primary trial outcome evaluated the effect of consuming GUMLi versus unfortified CM as part of a whole diet for 12-months on body composition at two years of age [19]. Secondary outcomes included dietary intake (food frequency questionnaire or 24 h), micronutrient status, and cognitive development.
The study received ethical approval from the Health and Disability Ethics Committee of the Ministry of Health, New Zealand (14/NTB/152), and the University of Queensland Medical Research Ethics Committee, Brisbane, Australia (2014001318). The GUMLi Trial was registered with the Australian New Zealand Clinical Trials Registry, reference number: ACTRN12614000918628. Written informed consent was obtained from all participants. At month six post-randomisation, primary caregivers were invited to complete four record-assisted twenty-four-hour recalls (24 h). Of the 108 Auckland participants, 83 (77%) completed four 24 h. Four (4%) opted out of the study (but continued with the main trial), eleven (10 %) withdrew from the main trial and nine (8%) took part, but did not complete four 24 h. Only 14 (27%) participants from Brisbane completed four 24 h, therefore, the decision was made not to include them in the analysis.
2.2. Dietary Intakes
A dietitian collected dietary data over the phone using record-assisted 24HRs between months 6 to 11 post-randomisation, according to a standardised procedure [20]. Four 24 h were collected per participant on randomly allocated days (three weekdays and one weekend day). The record-assisted 24HR differed from standard 24HRs, as caregivers recoded their child’s intake over the pre-defined 24-h period preceding the phone call. This methodology was used in a pilot validation study for the New Zealand Children’s Nutrition Survey [21] and the Australian Children’s Nutrition and Physical Activity Survey (CNPAS) [22,23]. A ‘Foods fed by other adults’ form, adapted from the Feeding Infants and Toddlers study (FITS) [24,25] was used to record intake if the child was in the care of another adult i.e., day-care. Use of dietary supplements, homemade recipes, and portion sizes (household measures or gram weight) were recorded. A food model booklet, reproduced with permission from CNPAS was used to assist with describing serving sizes [22,23]. Breastfeeding was recorded as time (minutes) and quantity estimated using a conversion factor of 10 mL/min, max 10 min [26,27]. All 24HR were double-checked to identify mistakes, missing foods, or clarify recipes. A dietetics student entered the data into Foodworks® (version 9, Xyris Software, Pty Ltd., Australia) and checked for completeness. Nutritional data were derived from the FOODfiles 2016 database [28] and nutritional profiles of commercial toddler foods sourced from companies or nutrient information panels.
2.3. Assessment of Nutrient Intakes with Nutrient Reference Values
Nutrient intakes were compared to the Australian and New Zealand Nutrient Reference Values (NRVs) [29]. Prevalence of inadequate intakes were assessed using the cut-off point method for nutrients with an Estimated Average Requirement (EAR) value [30]. This method has previously been shown to produce realistic estimates of the prevalence of inadequate dietary intakes [30]. The EAR, derived by the Institute of Medicine (IoM) was used for vitamin D (10 µg/day) [31].
2.4. Assessment of Diet Quality Using the PANDiet Score
The development and design of the PANDiet score has been reported in detail elsewhere [12,32]. Briefly, the PANDiet provides a measure of diet quality through the probability of having adequate nutrient intake, ranging from 0–100, where the higher the score, the better the diet quality and nutrient adequacy [12,32]. The PANDiet is an average of the Adequacy and Moderation sub-scores, which rely on the calculation of probability of adequacy for 25 nutrients and consider duration of dietary assessment, day-to-day variability, nutrient reference values, inter-variability of intake, and mean nutrient intakes [12,32]. The Adequacy sub-score calculates the probability that usual nutrient intake is above a reference value and the Moderation sub-score calculates the probability that the usual nutrient intake meets requirements and does not exceed a reference value [12,33]. Using the original methods [12], the PANDiet score calculation for protein and micronutrients was adjusted using the Australian and New Zealand NRVs and inter-variability for children one- to three years of age [29]. There are no recommendations for total fat, poly-unsaturated fatty acids (PUFA) and carbohydrate in children under two. Therefore, as seen in Verger et al. [32], we used the reference values set by the European Food Safety Authority (EFSA) [34], Nordic recommendations for SFA and non-milk extrinsic sugars (NMES) [35] and the IoM upper limit for protein [36]. The risk of excessive intakes were assessed using a penalty value system [12], using the upper limit as a reference [29] (Table S1).
Participants were classified according to their randomisation into the trial and allocation to receive GUMLi or CM. The trial analysis was conducted based on the assumption that the PANDiet index was suitable to use as an outcome measure and the difference between randomised groups, if observed, would indicate an effect of the intervention.
2.5. Statistical Analysis
A sample size of 64 participants in each arm is required to have 80% power at 5% significant level (two-sided) to detect a 0.5 SD difference in body fat percent (primary outcome) between the two arms at the end of the 12-month intervention. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Baseline characteristics were summarised by treatment group (GUMLi vs. CM) using descriptive statistics. Continuous variables were reported as mean and standard deviation (SD) and categorical variables described as frequencies and percentages. The characteristics of the Auckland sub-group included in this study (N = 83) were compared to those in the Auckland cohort who did not participate (N = 25). Chi-Square test or Fisher’s Exact test were used for categorical variables, and the Kruskal-Wallis test or two-sample t-test was used for continuous variables. The impact of the intervention on energy and nutrient intakes were evaluated at each 24HR time point (month 7, 8, 10, and 11 post-randomisation), using random effect mixed models with an autoregressive covariance structure on repeated measures. The fixed effects model included participant sex, treatment group, time point and its interaction with the treatment group. Model-adjusted mean differences between nutrient intakes of both groups and 95% confidence intervals (95% CI) were reported at each time point, with associated p-values. The impact of the intervention on the overall PANDiet score, sub-scores and components using all 24 h data were evaluated using linear regression models adjusting for sex. Model-adjusted mean differences between two groups were estimated and tested. All statistical tests were two-sided with a statistical significance of p < 0.05. As a secondary analysis, missing data was not imputed and no adjustment for multiple comparisons were considered.
3. Results
One hundred and eight Auckland children participated in the main GUMLi trial. Of these, 83 (77%) were included in this sub-study, with no significant differences between GUMLi and CM groups for any baseline characteristics (Table 1), therefore, it was assumed that any differences in PANDiet scores would be attributed to the intervention milk. No statistical differences were observed between the Auckland participants included in the analysis (N = 83) and those excluded (N = 25), except for maternal educational attainment (80% vs. 60%; p = 0.047) (Table S2). GUMLi and CM composition are presented in Table S3. Both milks were energy-matched per 100 mL, however compared to CM, GUMLi was lower in SFA and protein, with higher carbohydrate and dietary fibre, and nutritionally significant amounts of iron and vitamin D (cholecalciferol).
Table 1.
Child and maternal characteristics of the Auckland cohort (N = 83) included in the PANDiet cohort.
| Baseline Demographics | Study Group | p-Value * | |
|---|---|---|---|
| Intervention (N = 41) n (%) | Control (N = 42) n (%) | ||
| Child’s sex | 0.062 | ||
| Boy | 19 (46) | 28 (67) | |
| Girl | 22 (54) | 14 (33) | |
| Other children in the family | 0.222 | ||
| No | 16 (39) | 22 (52) | |
| Yes | 25 (61) | 20 (48) | |
| Day care attendance | 0.893 | ||
| No | 25 (61) | 25 (60) | |
| Yes | 16 (39) | 17 (40) | |
| Breastfed at baseline | 0.415 | ||
| No | 27 (66) | 24 (57) | |
| Yes | 14 (34) | 18 (43) | |
| Mother’s Ethnicity | 0.903 | ||
| Māori | 8 (20) | 6 (14) | |
| Pacific | 0 (0) | 1 (2) | |
| Asian | 3 (7) | 2 (5) | |
| European | 23 (56) | 26 (62) | |
| Other | 7 (17) | 7 (17) | |
| Mother’s Age, years (mean ± SD) | 32 ± 5 | 32 ± 4 | 0.874 |
| Mother’s BMI, kgm2 (mean ± SD) | 26 ± 5 | 27 ± 6 | 0.916 |
| Mother’s Highest Level of Education | 0.589 | ||
| No school qualifications | 0(0) | 0(0) | |
| Primary | 2 (5) | 0 (0) | |
| Secondary | 5 (12) | 7 (17) | |
| Tertiary | 33 (80) | 33 (79) | |
| Other | 1 (2) | 2 (5) | |
| Mother’s Employment Status | 0.082 | ||
| Full-time caregiver | 14 (34) | 15 (36) | |
| Full-time paid employment | 5 (12) | 13 (31) | |
| Part-time paid employment | 14 (34) | 13 (31) | |
| Receiving a benefit | 1 (2) | 0 (0) | |
| Unemployed, no benefit | 3 (7) | 0 (0) | |
| Other | 4 (10) | 1 (2) | |
| Smoking | |||
| Current smoking | 1 (2) | 1 (2) | 1.000 |
| Smoking before pregnancy | 5 (12) | 2 (5) | 0.432 |
| Smoking during pregnancy | 1 (2) | 0 (0) | 0.494 |
* Unadjusted p-values, Chi-square test or Fisher’s Exact test is used to test the difference between groups for categorical variables; the Kruskal-Wallis test or two-sample t-test is used to compare the medians/means between groups for continuous variables.
3.1. Evaluation of Nutrient Intakes
Mean (SD) daily nutrient intakes at the four 24HR time points are displayed in Table 2, according to GUMLi or CM group. For the purpose of table length, only nutrients with significant relationships at any time point are displayed. A full table is presented in Table S4. There were no differences between groups at any time point for energy, sodium, PUFA, vitamin A, vitamin B-6, folate, magnesium, and selenium. Children in the GUMLi group had significantly higher intakes of vitamin C and iron across all time points, and children in the CM group had significant higher intakes of riboflavin and potassium.
Table 2.
Nutrient intake among Auckland children (N = 83) from 18 and 23 months of age (month 7–11 post randomisation) 1,2.
| Usual Intake Values | Adjusted Difference (95%CI) | p * | ||
|---|---|---|---|---|
| Nutrients | Intervention (N = 41) Mean (SD) | Control (N = 42) Mean (SD) | ||
| Energy (kcal) | ||||
| Month 07 | 1135.92 (294.19) | 1122.34 (187.51) | 36.61 (−93.18, 166.41) | 0.579 |
| Month 08 | 1114.07 (277.52) | 1246.07 (378.83) | −108.96 (−238.76, 20.84) | 0.100 |
| Month 10 | 1128.31 (383.78) | 1068.61 (291.44) | 82.74 (−47.05, 212.54) | 0.210 |
| Month 11 | 1190.24 (288.14) | 1118.93 (283.21) | 94.34 (−35.45, 224.14) | 0.154 |
| Carbohydrate (g) | ||||
| Month 07 | 142.45 (40.53) | 127.44 (36.36) | 18.26 (−0.25, 36.76) | 0.053 |
| Month 08 | 138.50 (38.12) | 144.65 (56.59) | −2.90 (−21.41, 15.61) | 0.758 |
| Month 10 | 138.25 (45.72) | 123.41 (41.01) | 18.09 (−0.42, 36.59) | 0.055 |
| Month 11 | 145.81 (41.01) | 126.61 (43.06) | 22.45 (3.94, 40.96) | 0.018 * |
| Total fat (g) | ||||
| Month 07 | 38.49 (13.70) | 43.91 (11.34) | −4.69 (−11.04, 1.65) | 0.146 |
| Month 08 | 37.76 (14.79) | 46.21 (16.22) | −7.72 (−14.07, −1.37) | 0.017 * |
| Month 10 | 39.53 (17.61) | 40.34 (13.50) | −0.08 (−6.42, 6.27) | 0.981 |
| Month 11 | 43.99 (15.81) | 43.75 (13.59) | 0.97 (−5.38, 7.32) | 0.764 |
| Saturated fat (g) | ||||
| Month 07 | 18.98 (7.37) | 21.16 (5.98) | −1.96 (−5.27, 1.34) | 0.243 |
| Month 08 | 18.11 (7.41) | 22.16 (8.16) | −3.83 (−7.14, −0.53) | 0.023 * |
| Month 10 | 19.46 (8.88) | 19.73 (7.77) | −0.05 (−3.35, 3.26) | 0.977 |
| Month 11 | 20.93 (8.11) | 21.00 (6.84) | 0.15 (−3.16, 3.45) | 0.930 |
| NMES (g) | ||||
| Month 07 | 45.46 (18.22) | 42.02 (17.88) | 4.33 (−5.27, 13.93) | 0.375 |
| Month 08 | 45.90 (19.00) | 49.01 (30.16) | −2.22 (−11.83, 7.38) | 0.649 |
| Month 10 | 40.13 (23.17) | 39.00 (19.24) | 2.03 (−7.58, 11.63) | 0.678 |
| Month 11 | 48.53 (25.02) | 39.29 (21.47) | 10.14 (0.54, 19.74) | 0.039 * |
| Protein (g) | ||||
| Month 07 | 46.07 (17.15) | 50.13 (10.13) | −3.26 (−9.65, 3.13) | 0.316 |
| Month 08 | 46.09 (14.01) | 56.47 (17.08) | −9.58 (−15.97, −3.19) | 0.004 * |
| Month 10 | 46.45 (18.34) | 44.33 (12.51) | 2.92 (−3.47, 9.31) | 0.369 |
| Month 11 | 44.59 (14.36) | 47.48 (13.13) | −2.09 (−8.48, 4.30) | 0.520 |
| Thiamin (mg) | ||||
| Month 07 | 1.50 (0.63) | 1.19 (0.84) | 0.34 (0.03, 0.64) | 0.030 * |
| Month 08 | 1.54 (0.56) | 1.29 (0.72) | 0.28 (−0.02, 0.59) | 0.069 |
| Month 10 | 1.35 (0.70) | 1.03 (0.82) | 0.36 (0.05, 0.66) | 0.022 * |
| Month 11 | 1.36 (0.68) | 0.99 (0.64) | 0.40 (0.10, 0.71) | 0.010 * |
| Riboflavin (mg) | ||||
| Month 07 | 1.82 (0.64) | 2.12 (0.64) | −0.29 (−0.56, −0.02) | 0.037 * |
| Month 08 | 1.71 (0.54) | 2.30 (0.77) | −0.58 (−0.85, −0.30) | <0.0001 * |
| Month 10 | 1.66 (0.50) | 2.07 (0.67) | −0.39 (−0.66, −0.11) | 0.006 * |
| Month 11 | 1.63 (0.61) | 2.11 (0.57) | −0.47 (−0.74, −0.20) | 0.001 * |
| Niacin (mg) | ||||
| Month 07 | 19.97 (7.25) | 17.79 (4.64) | 2.49 (−0.12, 5.09) | 0.061 |
| Month 08 | 20.63 (5.18) | 20.09 (7.30) | 0.85 (−1.75, 3.45) | 0.521 |
| Month 10 | 19.34 (6.64) | 15.80 (5.87) | 3.84 (1.24, 6.45) | 0.004 * |
| Month 11 | 19.09 (5.31) | 17.28 (5.39) | 2.11 (−0.49, 4.71) | 0.112 |
| Vitamin B12 (µg) | ||||
| Month 07 | 2.36 (1.12) | 2.78 (1.09) | −0.41 (−0.91, 0.09) | 0.108 |
| Month 08 | 2.25 (1.30) | 3.17 (1.55) | −0.91 (−1.41, −0.41) | 0.0004 * |
| Month 10 | 2.14 (0.94) | 2.57 (0.93) | −0.42 (−0.92, 0.07) | 0.095 |
| Month 11 | 2.03 (0.91) | 2.58 (1.15) | −0.55 (−1.05, −0.05) | 0.031 * |
| Vitamin C (mg) | ||||
| Month 07 | 104.00 (44.39) | 45.38 (37.36) | 57.38 (35.76, 79.01) | <0.0001 * |
| Month 08 | 99.95 (39.44) | 50.22 (54.71) | 48.48 (26.86, 70.11) | <0.0001 * |
| Month 10 | 92.84 (34.62) | 50.54 (65.97) | 41.05 (19.43, 62.68) | 0.0002 * |
| Month 11 | 92.51 (48.54) | 58.80 (61.49) | 32.47 (10.85, 54.10) | 0.003 * |
| Vitamin D (µg) | ||||
| Month 07 | 6.02 (6.57) | 3.23 (3.18) | 2.80 (1.07, 4.53) | 0.002 * |
| Month 08 | 4.73 (2.70) | 3.59 (4.00) | 1.16 (−0.57, 2.89) | 0.188 |
| Month 10 | 5.17 (2.76) | 2.92 (2.49) | 2.27 (0.54, 4.00) | 0.011 * |
| Month 11 | 4.86 (3.44) | 3.73 (4.75) | 1.15 (−0.58, 2.88) | 0.194 |
| Calcium (mg) | ||||
| Month 07 | 901.26 (268.15) | 898.06 (287.37) | 8.15 (−113.76, 130.06) | 0.895 |
| Month 08 | 808.31 (257.94) | 943.49 (314.09) | −130.24 (−252.15, −8.33) | 0.036 * |
| Month 10 | 899.34 (284.54) | 836.03 (251.58) | 68.25 (−53.65, 190.16) | 0.271 |
| Month 11 | 830.41 (284.29) | 891.05 (280.79) | −55.70 (−177.60, 66.21) | 0.369 |
| Zinc (mg) | ||||
| Month 07 | 6.75 (2.76) | 6.11 (1.51) | 0.71 (−0.22, 1.64) | 0.133 |
| Month 08 | 6.64 (2.24) | 6.85 (2.65) | −0.13 (−1.07, 0.80) | 0.776 |
| Month 10 | 6.44 (2.45) | 5.37 (1.74) | 1.14 (0.21, 2.07) | 0.017 * |
| Month 11 | 6.42 (1.77) | 5.45 (1.69) | 1.04 (0.11, 1.97) | 0.029 * |
| Phosphorus (mg) | ||||
| Month 07 | 1023.06 (284.32) | 1004.05 (202.02) | 33.99 (−83.88, 151.87) | 0.571 |
| Month 08 | 966.96 (257.85) | 1106.43 (293.65) | −124.49 (−242.36, −6.62) | 0.039 * |
| Month 10 | 984.67 (335.64) | 930.98 (252.54) | 68.68 (−49.20, 186.55) | 0.252 |
| Month 11 | 989.53 (278.71) | 988.18 (262.19) | 16.33 (−101.54, 134.20) | 0.785 |
| Potassium (mg) | ||||
| Month 07 | 1666.69 (703.04) | 1962.08 (433.28) | −283.10 (−528.37, −37.83) | 0.024 * |
| Month 08 | 1537.51 (481.42) | 2232.75 (761.17) | −682.95 (−928.21, −437.68) | <0.0001 * |
| Month 10 | 1406.79 (493.64) | 1861.05 (503.26) | −441.97 (−687.24, −196.70) | 0.001 * |
| Month 11 | 1512.12 (526.22) | 1987.09 (511.40) | −462.67 (−707.94, −217.40) | 0.000 * |
| Iron (mg) | ||||
| Month 07 | 10.62 (3.36) | 6.23 (2.82) | 4.58 (3.31, 5.85) | <0.0001 * |
| Month 08 | 10.80 (2.96) | 6.90 (2.75) | 4.10 (2.83, 5.37) | <0.0001 * |
| Month 10 | 9.83 (2.89) | 5.64 (3.07) | 4.38 (3.11, 5.65) | <0.0001 * |
| Month 11 | 10.26 (3.24) | 5.24 (2.35) | 5.21 (3.93, 6.48) | <0.0001 * |
| Copper (mg) | ||||
| Month 07 | 0.62 (0.32) | 0.6 (0.24) | 0.04 (−0.08, 0.15) | 0.524 |
| Month 08 | 0.6 (0.26) | 0.68 (0.35) | −0.06 (−0.18, 0.05) | 0.255 |
| Month 10 | 0.63 (0.28) | 0.5 (0.21) | 0.15 (0.04, 0.26) | 0.010 * |
| Month 11 | 0.6 (0.18) | 0.53 (0.18) | 0.09 (−0.02, 0.2) | 0.115 |
| Iodine (µg) | ||||
| Month 07 | 64.08 (23.15) | 52.65 (24.78) | 11.80 (0.49, 23.11) | 0.041 * |
| Month 08 | 63.58 (29.84) | 55.72 (21.88) | 8.22 (−3.09, 19.54) | 0.154 |
| Month 10 | 60.53 (27.92) | 53.13 (26.95) | 7.77 (−3.55, 19.08) | 0.178 |
| Month 11 | 65.92 (30.56) | 53.53 (21.06) | 12.76 (1.45, 24.07) | 0.027 |
* p < 0.05. 1 Repeated measures mixed model with an autoregressive covariance structure, adjusting for sex. 2 Only nutrients with significant relationships at any of the four time points are displayed.
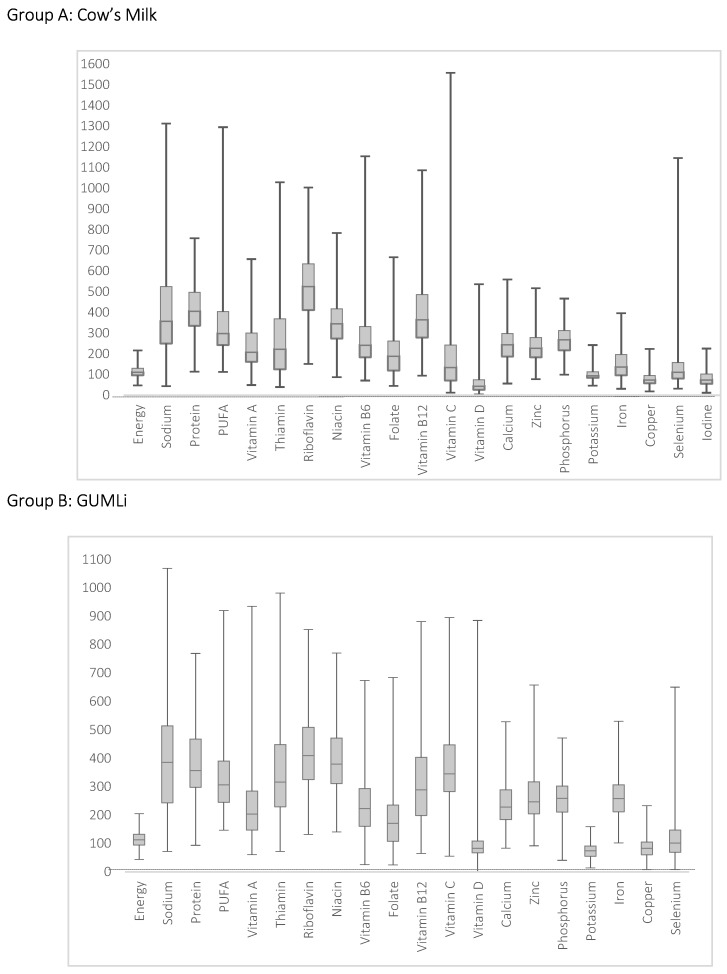
Compared with New Zealand NRVs [29], intakes of most nutrients were adequate, i.e., median intake (average all four 24 h) ≥ nutrient reference value across both groups Figure 1. Nutrients with median intakes below reference values in both groups were vitamin D, potassium, copper, and iodine.
Figure 1.
Intake of energy and nutrients as a percentage of New Zealand reference values (28) in 18- to 23-month-old children from the Auckland cohort participating in the GUMLi trial (median (—), interquartile range (box; 25th and 75th percentiles), minimum and maximum value). GUMLi = Growing Up Milk—Lite.
3.2. PANDiet Scores According to Intake of GUMLi or CM
Mean PANDiet score, sub-scores and individual components are displayed in Table 3. After adjusting for sex, children in the GUMLi group had significantly higher PANDiet scores and Adequacy scores compared to the CM group (adjusted mean difference +3.11 and +4.17, respectively). There was no difference in the Moderation sub score and energy intake between groups. Of note, the Adequacy sub-score was 2.5 and 2.6 times greater than the Moderation sub-score in the GUMLi and CM group, respectively, indicating poor adherence to the recommendations for avoiding excessive nutrient intakes.
Table 3.
PANDiet scores, sub-scores, and individual components, among Auckland children (N = 83) from 18 and 23 months of age (month 7–11 post randomisation) 1,2.
| Score | Intervention (N = 41) | Control (N = 42) | Adjusted Difference (95% CI) | p-Value * |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| PANDiet 3 | 52.9 (3.07) | 50.12 (3.97) | 3.11 (1.56, 4.67) | 0.0001 * |
| Moderation sub-score | 29.82 (6.47) | 27.77 (6.58) | 2.06 (−0.87, 4.99) | 0.1660 |
| Protein | 0.41 (0.50) | 0.33 (0.48) | 0.08 (−0.14, 0.30) | 0.4747 |
| Total Fat | 0.10 (0.30) | 0.48 (0.51) | −0.40 (−0.59, −0.22) | <0001 * |
| Total Carbohydrate | 0.85 (0.36) | 0.55 (0.50) | 0.33 (0.14, 0.53) | 0.0011 * |
| SFA | 0.10 (0.16) | 0.06 (0.12) | 0.05 (−0.01, 0.11) | 0.1231 |
| NMES | 0.16 (0.22) | 0.23 (0.28) | −0.08 (−0.19, 0.03) | 0.1699 |
| Sodium | 0.03 (0.14) | 0.02 (0.04) | 0.01 (−0.03, 0.06) | 0.5832 |
| Adequacy sub-score | 75.98 (4.98) | 72.46 (5.88) | 4.17 (1.82, 6.51) | 0.0007 * |
| Protein | 0.99 (0.03) | 1.00 (0.001) | −0.01 (−0.01, 0.002) | 0.1697 |
| Total Carbohydrate | 0.98 (0.16) | 1.00 (0.00) | −0.02 (−0.07, 0.03) | 0.4293 |
| Total Fat | 1.00 (0.00) | 0.86 (0.35) | 0.14 (0.03, 0.26) | 0.0140 * |
| PUFA | 0.15 (0.19) | 0.18 (0.23) | −0.01 (−0.10, 0.08) | 0.7975 |
| Vitamin A | 0.96 (0.08) | 0.98 (0.02) | −0.02 (−0.05, 0.001) | 0.0560 |
| Thiamin | 1.00 (0.005) | 0.94 (0.09) | 0.06 (0.03, 0.09) | 0.0001 * |
| Riboflavin | 1.00 (0.001) | 1.00 (0.0003) | −0.0003 (−0.001, 0.00) | 0.0853 |
| Niacin | 1.00 (0.00001) | 1.00 (0.0005) | 0.0001 (0.00, 0.0002) | 0.1819 |
| Vitamin B6 | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00,0.00) | 0.9775 |
| Folate | 0.89 (0.18) | 0.91 (0.21) | −0.01 (−0.10, 0.07) | 0.7717 |
| Vitamin B12 | 0.99 (0.02) | 1.00 (0.01) | −0.01 (−0.02, −0.003) | 0.0081 * |
| Vitamin C | 1.00 (0.01) | 0.71 (0.32) | 0.30 (0.20, 0.40) | <0001 * |
| Vitamin D | 0.43 (0.34) | 0.19 (0.31) | 0.25 (0.10, 0.39) | 0.0011 * |
| Calcium | 0.99 (0.02) | 1.00 (0.02) | −0.002 (−0.01, 0.01) | 0.7227 |
| Magnesium | 0.99 (0.02) | 1.00 (0.004) | −0.005 (−0.01, 0.002) | 0.1826 |
| Zinc | 1.00 (0.003) | 0.99 (0.02) | 0.01 (−0.0002, 0.01) | 0.0585 |
| Phosphorus | 1.00 (0.01) | 1.00 (0.002) | −0.001 (−0.003, 0.0005) | 0.1323 |
| Potassium | 1.00 (0.002) | 1.00 (1E−6) | −0.0003 (−0.001, 0.0002) | 0.1998 |
| Iron | 1.00 (0.01) | 0.78 (0.32) | 0.25 (0.16, 0.35) | <0001 * |
| Copper | 0.34 (0.30) | 0.27 (0.27) | 0.10 (−0.03, 0.23) | 0.1203 |
| Selenium | 0.57 (0.37) | 0.70 (0.28) | −0.09 (−0.23, 0.06) | 0.2314 |
| Iodine | 0.46 (0.28) | 0.29 (0.24) | 0.18 (0.06, 0.30) | 0.0035 * |
NMES, Non-milk Extrinsic Sugars; PANDiet, The Probability of Adequate Nutrient Intake score; PUFA, Poly0unsaturated Fatty Acids; SFA, Saturated Fatty Acids. * p < 0.05. 1 Linear regression model, adjusting for sex. 2 All the PANDiet component scores range from 0 to 1, where 1 represents a 100% probability that the intake is adequate according to a reference value. 3 Combined data from all four 24HR were used to calculate the overall PANDiet score and adequacy and moderation sub-scores, which ranged from 0 to 100. The higher the score or sub-scores, the better the nutrient adequacy of the diet.
There were no differences in component of the Moderation sub-score, except for total fat and total carbohydrates, where the CM group had a higher probability of avoiding excessive total fat intake and the GUMLi group had a higher probability of avoiding excessive total carbohydrate intake. The GUMLi group tended to have higher probability of avoiding excessive protein intake (not significant). The mean probabilities for avoiding excessive intakes were low for sodium (≤0.03), SFA (≤0.10) and NMES (≤0.23) in this population. There were no differences between groups in components of the Adequacy sub-score, except for total fat, thiamin, vitamin C, vitamin D, iron, and iodine where the GUMLi group had a higher probability of having adequate intakes for these nutrients, and vitamin B12 where the CM group had a higher probability of having an adequate intake.
4. Discussion
Using the PANDiet index, we have evaluated the diet quality and nutritional adequacy of 18- to−23-month-old Auckland children participating in the GUMLi Trial, according to GUMLi or CM consumption. This is the first study to use data from a randomised controlled trial to measure the impact of a dietary intervention, such as GUM on diet quality using a nutrient-based index, such as the PANDiet score. Total PANDiet scores were significantly higher in the GUMLi group, indicating better overall nutrient adequacy and diet quality. Nutrient intakes of children in both groups met recommendations for fat, total carbohydrates and most micronutrients; however, protein sodium, NMES, and SFA intakes exceeded recommendations. Whilst average total energy intakes were similar, the children consuming GUMLi had higher probabilities of having adequate intakes of vitamin C, vitamin D and iron, and were less likely to have insufficient intakes of vitamin D. Further analysis of food group consumption, adherence to dietary guidelines, or nutrient densities would elucidate whether the GUMLi intervention had an impact on dietary diversity, as an inverse relationship between dietary diversity and formula intake has previously been reported in 12- to 16-month-old Australian children [16].
4.1. Diet Quality and PANDiet Scores According to GUMLi or CM Allocation
GUM has been shown to improve intakes of iron, vitamin C, vitamin D, and PUFA’s during the dietary transition from a milk-based intake to a ‘family diet’ in cross-sectional, observational studies [14,15,16,37]. The PANDiet has previously been evaluated in 12- to 18-month-old-children in the U.K. according to GUM or commercial infant foods (CIF) consumption [32]. Consuming GUM was associated with greater nutritional adequacy with a mean PANDiet score of 74.1 compared to children who did not consume GUM or CIF (difference of +7.2 points) [32]. A much smaller difference of +2.78 was observed in our sample, where consuming GUMLi was associated with greater nutritional adequacy. More recently, the difference in PANDiet scores for ‘at risk children with Diabetic mothers’ and ‘not at risk’ children in the BABYDIET study was similar at +2.4 points (65.9 and 68.3, respectively) [13].
It is important to note the effect of differences in national nutrient recommendations on PANDiet calculations and resultant scores. In the present study, the PANDiet score calculation was adjusted according to Australia and New Zealand NRVs where available [29], and if not, the reference values determined by Verger et al. [32] who used nutrient recommendations for UK children 12- to 36-months-of-age [34,35,38]. The greatest variation in recommendations were for selenium and folate, where the New Zealand NRVs are 1.7–2.4 times higher than the UK, (folate: 120 µg vs. 50 µg and selenium: 20 µg vs. 11.5 µg). The probability of adequate would be higher than the current calculation if we used the UK recommendations in our sub-score calculation. As all probabilities of adequacy are equally weighted, higher component scores will contribute to a higher total Adequacy sub-score and resultant PANDiet score [12].
In this population, the quality of both fat and carbohydrates are of concern. Children had low probabilities for avoiding excessive intakes for SFA (≤0.10) and low probabilities for having adequate PUFA intakes (<0.20). An altered ratio of total and SFA has been described in two other studies, and is an important consideration, given the role of PUFA’s in cognitive and visual development [37,39]. At each time point in the study, NMES exceeded the recommendations (>11% EI). Similar intakes were observed in a nationally representative sample of one- to four-year-old Irish children, where mean NMES intakes exceeded recommendations at 12% energy intake (EI) and increased with age [40].
4.2. Strengths and Limitations
The PANDiet provides an accurate measure of diet quality at an individual and population level through assessing global nutrient adequacy, and is strengthened by the use of a probabilistic calculation of nutrient adequacy, as previously described [12]. The index was designed to be as exhaustive as possible, and describes the role that different foods/food groups have in contributing to diet quality, at the nutrient level [12]. Our analysis is strengthened by the use of New Zealand NRVs to assess nutrient adequacy in the New Zealand context, however, because of this, cross-national comparisons of the PANDiet score are limited. Previous studies have used large, observational cohorts, where each subject has one PANDiet score calculated at a single time point, using multiple measures of dietary assessment [12,32,33]. For the present study, dietary data were collected on one day per month, over four months; therefore, month-to-month variation was considered in the PANDiet calculation. Using a record-assisted 24HR, allowed inclusion of children in the care of other adults (i.e., at day care), however, the reliance on parent and other adult-reported measures may lead to an increase in misreporting or social desirability bias. Mother’s in our sample were older and highly educated, which may have affected total PANDiet scores. The ethnicity distribution in our sample was not considered representative of the Auckland population; therefore, no differences between ethnicities were evaluated. The validity of the PANDiet index was not evaluated for RCT data, therefore, further evaluation of the PANDiet in a larger, more representative cohort of New Zealand children under two is recommended to determine whether the PANDiet has predictive validity with respect to longitudinal health outcomes.
5. Conclusions
The consumption of GUMLi was associated with higher nutritional adequacy of the diets of children 18- to 23-months-of-age defined by PANDiet score, with increased likelihood of meeting nutrient requirements. However, consumption of GUMLi did not guarantee 100% nutrient adequacy. GUMLi consumers still had excessive protein intakes, but were more likely to have carbohydrate and SFA intakes that were in line with recommendations and improved iron and vitamin D intakes. Although GUMLi had a positive effect on index scores, consumption toward the latter half of the second year of life may not have the same impact as during early childhood as previously reported in younger children according to GUM consumption [32]. This may be because in the latter part of the second year of life, children are more likely to be following a family diet of varying quality, with a reduced reliance on fortified milks. Suggesting that other dietary strategies to promote a healthy diet through optimising nutrient intake could also result in more favourable dietary intake profiles, rather than solely concentrating on milk [41], however, further research is required on the consequences of consuming GUMLi on overall dietary diversity.
Acknowledgments
The authors thank E.O. Verger for his assistance in calculating and interpreting the PANDiet index.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/11/1/203/s1, Table S1. PANDiet for Australian and New Zealand children aged from 18 to 23 months: components, reference values and inter-individual variability, Table S2. Nutritional composition of CM and GUMLi per 100 mL of prepared product, Table S3. Characteristics of the Auckland cohort included versus excluded in the PANDiet analysis, Table S4. Nutrient intake among Auckland children (N = 83) from 18 to 23 months of age (month 7–11 post randomisation).
Author Contributions
Conceptualisation: C.R.W. and C.C.G.; methodology: A.L.L. and C.R.W.; formal analysis: Y.J. and R.X.C.; data curation: A.L.L. and T.M.; writing—original draft preparation: A.L.L.; writing—review and editing: A.L.L., C.R.W., and C.C.G.; project administration: A.L.L. and T.M.; funding acquisition: C.R.W. and C.C.G.
Funding
The GUMLi Trial received an investigator-initiated grant from Danone Nutricia Research. The funder had no role in the study design, data collection, analysis, and interpretation of the study. There are no restrictions or delays on the timely publication of the results of the trial.
Conflicts of Interest
C.R.W. has received honoraria for presentations and consultations from Danone, Nutricia, Pfizer, and Fonterra. C.C.G. has received honoraria for consultations from Fonterra. A.L.L. states no conflict of interest. T.M. states no conflict of interest. Y.J. states no conflict of interest. R.X.C. states no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Northstone K., Emmett P.M. Are dietary patterns stable throughout early and mid-childhood? A birth cohort study. Br. J. Nutr. 2008;100:1069–1076. doi: 10.1017/S0007114508968264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan J. Nutrition for toddlers: The foundation for good health—2. Current problems and ways to overcome them. J. Fam. Health Care. 2005;15:85–88. [PubMed] [Google Scholar]
- 3.Golley R.K., Hendrie G.A., McNaughton S.A. Scores on the Dietary Guideline Index for Children and Adolescents Are Associated with Nutrient Intake and Socio-Economic Position but Not Adiposity—3. J. Nutr. 2011;141:1340–1347. doi: 10.3945/jn.110.136879. [DOI] [PubMed] [Google Scholar]
- 4.Ruel M.T., Menon P. Child feeding practices are associated with child nutritional status in Latin America: Innovative uses of the demographic and health surveys. J. Nutr. 2002;132:1180–1187. doi: 10.1093/jn/132.6.1180. [DOI] [PubMed] [Google Scholar]
- 5.Kant A.K. Indexes of overall diet quality: A review. J. Am. Diet. Assoc. 1996;96:785–791. doi: 10.1016/S0002-8223(96)00217-9. [DOI] [PubMed] [Google Scholar]
- 6.Marshall S., Burrows T., Collins C.E. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J. Hum. Nutr. Diet. 2014;27:577–598. doi: 10.1111/jhn.12208. [DOI] [PubMed] [Google Scholar]
- 7.Wirt A., Collins C.E. Diet quality—What is it and does it matter? Public Health Nutr. 2009;12:2473–2492. doi: 10.1017/S136898000900531X. [DOI] [PubMed] [Google Scholar]
- 8.Waijers P.M., Feskens E.J., Ocké M.C. A critical review of predefined diet quality scores. Br. J. Nutr. 2007;97:219–231. doi: 10.1017/S0007114507250421. [DOI] [PubMed] [Google Scholar]
- 9.Hu F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Lazarou C., Newby P.K. Use of dietary indexes among children in developed countries. Adv. Nutr. 2011;2:295–303. doi: 10.3945/an.110.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smithers L.G., Golley R.K., Brazionis L., Emmett P., Northstone K., Lynch J.W. Dietary patterns of infants and toddlers are associated with nutrient intakes. Nutrients. 2012;4:935–948. doi: 10.3390/nu4080935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verger E.O., Mariotti F., Holmes B.A., Paineau D., Huneau J. Evaluation of a diet quality index based on the probability of adequate nutrient intake (PANDiet) using national French and US dietary surveys. PLoS ONE. 2012;7:e42155. doi: 10.1371/journal.pone.0042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoen S., Jergens S., Barbaresko J., Nöthlings U., Kersting M., Remer T., Stelmach-Mardas M., Ziegler A.G., Hummel S. Diet quality during infancy and early childhood in children with and without risk of type 1 diabetes: A DEDIPAC study. Nutrients. 2017;9:48. doi: 10.3390/nu9010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghisolfi J., Fantino M., Turck D., de Courcy G.P., Vidailhet M. Nutrient intakes of children aged 1–2 years as a function of milk consumption, cows’ milk or growing-up milk. Public Health Nutr. 2013;16:524–534. doi: 10.1017/S1368980012002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walton J., Flynn A. Nutritional adequacy of diets containing growing up milks or unfortified cow’s milk in Irish children (aged 12–24 months) Food Nutr. Res. 2013;57:21836. doi: 10.3402/fnr.v57i0.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne R., Magarey A., Daniels L. Food and beverage intake in Australian children aged 12–16 months participating in the NOURISH and SAIDI studies. Aust. N. Z. J. Public Health. 2014;38:326–331. doi: 10.1111/1753-6405.12249. [DOI] [PubMed] [Google Scholar]
- 17.Vandenplas Y., De Ronne N., Van De Sompel A., Huysentruyt K., Robert M., Rigo J., Scheers I., Brasseur D., Goyens P. A Belgian consensus-statement on growing-up milks for children 12–36 months old. Eur. J. Pediatr. 2014;173:1365–1371. doi: 10.1007/s00431-014-2321-7. [DOI] [PubMed] [Google Scholar]
- 18.Eussen S.R., Pean J., Olivier L., Delaere F., Lluch A. Theoretical impact of replacing whole cow’s milk by young-child formula on nutrient intakes of UK young children: Results of a simulation study. Ann. Nutr. Metab. 2015;67:247–256. doi: 10.1159/000440682. [DOI] [PubMed] [Google Scholar]
- 19.Wall C.R., Hill R.J., Lovell A.L., Matsuyama M., Milne T., Grant C.C. A Multi-Centre, Double Blind, Randomised, Placebo Controlled Trial to Evaluate the Effect of Consuming Growing Up Milk. ‘Lite’ on Body Composition in Children Aged 12–23 Months. [(accessed on 29 December 2018)]; doi: 10.1093/ajcn/nqy302. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366785. [DOI] [PubMed]
- 20.Blanton C.A., Moshfegh A.J., Baer D.J., Kretsch M.J. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J. Nutr. 2006;136:2594–2599. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- 21.Watson P. Development & Pretesting of Methodologies for the Children’s Nutrition Survey: Validation Report: A Report to the Ministry of Health. Report Three. Institute of Food, Nutrition and Human Health, Massey University; Palmerston North, NZ, USA: 2003. [Google Scholar]
- 22.Coblac L., Bowen J., Burnett J., Syrette J., Dempsey J., Balle S., Wilson C., Flight I., Good N., Saunders I. Australian National Children’s Nutrition and Physical Activity Survey: Main Findings. Australian Bureau of Statistics; Canberra, Australia: 2008. [Google Scholar]
- 23.User Guide . Australian National Children’s Nutrition and Physical Activity Survey. Commonwealth Scientific Industrial Research Organisation; Canberra, Australia: 2010. [Google Scholar]
- 24.Briefel R.R., Reidy K., Karwe V., Devaney B. Feeding infants and toddlers study: Improvements needed in meeting infant feeding recommendations. J. Am. Diet. Assoc. 2004;104(Suppl. 1):31. doi: 10.1016/j.jada.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Devaney B., Kalb L., Briefel R., Zavitsky-Novak T., Clusen N., Ziegler P. Feeding infants and toddlers study: Overview of the study design. J. Am. Diet. Assoc. 2004;104(Suppl. 1):8. doi: 10.1016/j.jada.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Emmett P., North K., Noble S. Types of drinks consumed by infants at 4 and 8 months of age: A descriptive study. Public Health Nutr. 2000;3:211–217. doi: 10.1017/S1368980000000240. [DOI] [PubMed] [Google Scholar]
- 27.Lennox A., Sommerville J., Ong K., Henderson H., Allen R. Diet and Nutrition Survey of Infants and Young Children. Department of Health and Food Standards Agency; London, UK: 2013. [Google Scholar]
- 28.Institute for Plant & Food Research Limited, Ministry of Health . New Zealand Food composition Database: New Zealand FOODfiles. Institute for Plant & Food Research Limited, Ministry of Health; Wellington, New Zealand: 2016. [Google Scholar]
- 29.National Health and Medical Research Council . Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. National Health and Medical Research Council; Canberra, Australia: 2006. [Google Scholar]
- 30.Carriquiry A.L. Assessing the prevalence of nutrient inadequacy. Public Health Nutr. 1999;2:23–34. doi: 10.1017/S1368980099000038. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine . Dietary Reference Itakes for Calcium and Vitamin, D. Institute of Medicine; Washington, DC, USA: 2011. [Google Scholar]
- 32.Verger E.O., Eussen S., Holmes B.A. Evaluation of a nutrient-based diet quality index in UK young children and investigation into the diet quality of consumers of formula and infant foods. Public Health Nutr. 2016;19:1785–1794. doi: 10.1017/S1368980015003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verger E.O., Eussen S., Holmes B.A. Diet quality and nutritional adequacy of young children in the UK according to their consumption of young child formula and commercial infant food. Proc. Nutr. Soc. 2015;74:E250. doi: 10.1017/S002966511500292X. [DOI] [Google Scholar]
- 34.Agostoni C.V., Berni Canani R., Fairweather Tait S., Heinonen M., Korhonen H., La Vieille S., Marchelli R., Martin A., Naska A., Neuhäuser Berthold M., et al. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union: EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) EFSA J. 2013;11:1–103. [Google Scholar]
- 35.Nordic Council of Ministers . Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity. Nordic Council of Ministers; Copenhagen, Denmark: 2014. [Google Scholar]
- 36.Institute of Medicine (US) Panel on Macronutrients, Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Institute of Medicine; Washington, DC, USA: 2005. [Google Scholar]
- 37.Hilbig A., Drossard C., Kersting M., Alexy U. Nutrient adequacy and associated factors in a nationwide sample of German toddlers. J. Pediatr. Gastroenterol. Nutr. 2015;61:130–137. doi: 10.1097/MPG.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 38.Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy . Dietary Reference Values for Food Energy and Nutrients for the United Kingdom: Report of the Panel on Dietary Reference Values of the committee On Medical Aspects of Food Policy. HM Stationery Office; Richmond, UK: 1991. [PubMed] [Google Scholar]
- 39.Butte N.F., Fox M.K., Briefel R.R., Siega-Riz A.M., Dwyer J.T., Deming D.M., Reidy K.C. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J. Am. Diet. Assoc. 2010;110:S37. doi: 10.1016/j.jada.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Walton J., Kehoe L., McNulty B.A., Nugent A.P., Flynn A. Nutrient intakes and compliance with nutrient recommendations in children aged 1–4 years in Ireland. J. Hum. Nutr. Diet. 2017;30:665–676. doi: 10.1111/jhn.12452. [DOI] [PubMed] [Google Scholar]
- 41.Hojsak I., Bronsky J., Campoy C., Domellöf M., Embleton N., Fidler Mis N., Hulst J., Indrio F., Lapillonne A., Mølgaard C., et al. Young Child Formula: A Position Paper by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018;66:177–185. doi: 10.1097/MPG.0000000000001821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.