Abstract
Aflatoxin B1 (AFB1) is a widely spread mycotoxin contaminates food and feed, causing severe oxidative stress damages and immunotoxicity. Grape seed proanthocyanidin (GSPE), a natural antioxidant with wide range of pharmacological and medicinal properties. The goal of the present study was to investigate the protective effects of GSPE against AFB1-induced immunotoxicity and oxidative stress via NF-κB and Nrf2 signaling pathways in broiler chickens. For the experiment, 240 one-day old Cobb chicks were allocated into four dietary treatment groups of six replicates (10 birds per replicate): 1. Basal diet (control); 2. Basal diet + AFB1 1mg/kg contaminated corn (AFB1); 3. Basal diet + GSPE 250 mg/kg (GSPE); 4. Basal diet + AFB1 1 mg/kg + GSPE 250 mg/kg (AFB1 + GSPE). The results showed that GSPE significantly decreased serum inflammatory cytokines TNF-α, IFN-γ, IL-1β, IL-10, and IL-6 induced by AFB1. Similarly, GSPE + AFB1 treated group revealed a significant decrease in mRNA expressions of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-6) in the splenic tissue compared to the AFB1 treatment group. In addition, western blotting results manifested that GSPE treatment normalized the phosphorylation of nuclear factor kappa B (p65) and the degradation of IκBα protein induced by AFB1. Furthermore, GSPE enhanced the antioxidant defense system through activating the nuclear factor-erythroid-2-related factor (Nrf2) signaling pathway. The mRNA and protein expression level of Nrf2 and its down streaming associated genes were noted up-regulated by the addition of GSPE, and down-regulated in the AFB1 group. Taken together, GSPE alleviates AFB1-induced immunotoxicity and oxidative damage by inhibiting the NF-κB and activating the Nrf2 signaling pathways in broiler chickens. Conclusively, our results suggest that GSPE could be considered as a potential natural agent for the prevention of AFB1-induced immunotoxicity and oxidative damage.
Keywords: Aflatoxin B1, Grape Seed Proanthocyanidin Extract, Immunotoxicity, NF-κB, oxidative stress, Nrf2, Broilers
1. Introduction
Mycotoxins are hazardous to human and animals health worldwide because of their frequent occurrence in food and/or feedstuffs, and high probability to affect human health, and livestock and poultry productivity [1,2]. Aflatoxins are the widely studied mycotoxins, mainly produced by Aspergillus flavus and Aspergillus parasiticus [3]. The most common natural aflatoxins found in food and/or feed materials are B1, B2, G1, and G2 type. Among the major aflatoxins, Aflatoxin B1 (AFB1) is reported as a widespreading and highly toxic type of aflatoxins [4,5,6]. AFB1 can affect several organs at a time, and classified as a Group-1 carcinogen to humans, with no safe dose [7]. Meanwhile, it has hepatotoxic, immunotoxic, carcinogenic, mutagenic, teratogenic, and other adverse health effects on humans and animals [8,9,10]. In poultry, AFB1 can severely affect the immune system, AFB1 immunosuppressive nature is the well-documented area of its toxicity [11]. Simultaneously, AFB1 can alter the size of the immune system organs, hence and severely altering the immunological functions in chickens [12]. The spleen is the largest lymphoid organ in the body, with a large number of T and B lymphocytes, and plays a vital role in the protective immune reactions [13]. Meanwhile, its functional ability in the generation, maturation, and storage of lymphocytes plays an important role in the humoral and cellular immune responses [14].
NF-κB is a transcription factor that has an essential role in cell proliferation, immunity inflammation, and oxidative stress [15,16,17]. Previously it was found that AFB1 combined with ochratoxin A (OTA) could exacerbate the immunotoxicity through the NF-κB signaling pathway [18]. However, antioxidant, anti-inflammatory and immunomodulatory properties of GSPE has been reported [19,20]. Therefore, it indicates that GSPE could play an important role in protecting the spleen immune injury from the damage caused by AFB1.
AFB1 increases the production of free radicals, lipid peroxidation, augments the oxidative damage, resulting in severe cell damage and/or cell death cycle to animals or humans. Oxidative stress has been reported to play a major role in the toxicity mechanism of AFB1 [21,22], whereas, the liver is considered the principal target organ for aflatoxins which is major metabolizing and detoxifying organ in the body [23,24]. Several studies have demonstrated that dysregulation of the Nrf2 signaling pathway is associated with AFB1-induced oxidative damage [25,26].
The Nrf2 signaling pathway has anti-oxidative effects by alleviating toxicant-induced oxidative stress damage [27]. It has been suggested that the anti-oxidative stress system can be activated through the Nrf2 signaling pathway, hence leads to excrete the toxic metabolites through the regulation of many intracellular antioxidant genes expression [28,29]. The Nrf2 is a primarily expressed gene within the metabolically active organs such as the liver. Therefore, the Nrf2 pathway is considered the most important therapeutic target for the prevention and treatment of oxidative stress-induced liver and its associated diseases [30,31,32].
Phytochemicals produced by plants through primary and secondary metabolism that have potential to activate the Nrf2 signaling pathway which may produce beneficial protecting effects against AFB1-induced liver damages [33,34,35]. Proanthocyanidins are one of the natural compounds that can found in fruits, seeds, vegetables, nuts, flowers, cereals and bark. Grape seed proanthocyanidin extract (GSPE) derived from grape seed, enriched with oligomeric proanthocyanidin, polymerized and polyphenolic flavonoids [36,37]. GSPE has potency as a powerful antioxidant for its ability to scavenge free oxygen radicals, with anti-cancer and anti-inflammatory effects [38]. GSPE has proven to have protective effect against zearalenone-induced hepatic injury and oxidative stress in the liver of Kunming mice [19]. Meanwhile, GSPE can protect the functions of major organs by improving the antioxidant system, and play a preventive role against carbon tetrachloride and ischemia/reperfusion [39,40]. Moreover, GSPE improves suppressed immune response induced by AFB1 in the spleen of mice [20]. In addition, GSPE demonstrated to have the ability to ameliorate lead-induced liver oxidative damage via the Nrf2/antioxidant response element (ARE) pathway [41].
In our previous study, we reported that GSPE had the ability to ameliorate oxidative damage induced by AFB1 in broiler chickens [42]. However, the precise molecular mechanism by which GSPE attenuates oxidative damage and immune injury caused by AFB1 in broiler chickens remained unclear. In the present study, we hypothesized that (1) GSPE will protect the spleen from AFB1-induced immune injury by suppressing the inflammatory response and inhibiting the NF-κB expression in broilers. (2) The protective effects of GSPE against AFB1-induced oxidative damage might be through regulating the Nrf2 signaling pathway in the liver of broiler chickens. Presumably, this is the first study to investigate the role of NF-κB and Nrf2 in the protective effects of GSPE against AFB1-induced immune injury and oxidative damage in broiler chickens.
2. Results
2.1. Serum Inflammatory Cytokines
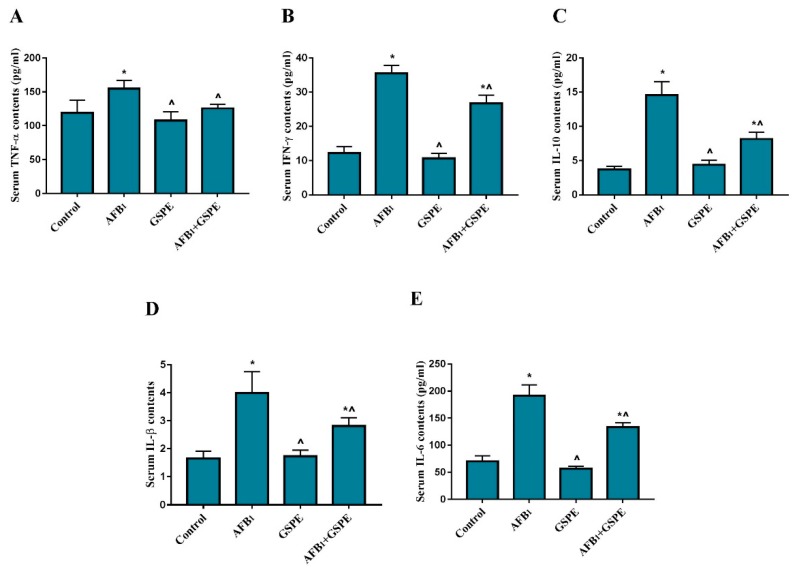
The effect of GSPE supplementation on the levels of inflammatory cytokines in the serum of control and experimental groups of broilers are depicted in Figure 1. Broilers exposed to AFB1 showed (p < 0.05) increase in the serum TNF-α, IFN-γ, IL-1β, IL-10, and IL-6, as compared to the control group. However, AFB1 + GSPE co-treated group showed significant reduction in TNF-α, IFN-γ, IL-1β, IL-10, and IL-6, as compared to the AFB1 treated group. Besides this, single GSPE treatment group did not show any significant difference in the serum inflammatory cytokines as compared to the control group.
Figure 1.
Protective role of grape seed proanthocyanidin extract (GSPE) treatment on Aflatoxin B1 (AFB1)-induced levels of the inflammatory cytokines in the serum of broilers. All data were expressed as mean ± SD (n = 6). Different symbols among groups indicate significant difference by least significant difference test (LSD) test (p < 0.05). * (p < 0.05) significant differences compared to the control group and ^ (p < 0.05) significant differences compared to the AFB1 group. (A) Tumor necrosis factor alpha (TNF-α); (B) interferon gamma (IFN-γ); (C) interleukin-1 beta (IL-1β); (D) interleukin 10 (IL-10); (E) interleukin 6 (IL-6).
2.2. Pro-Inflammatory Cytokines Gene Expression
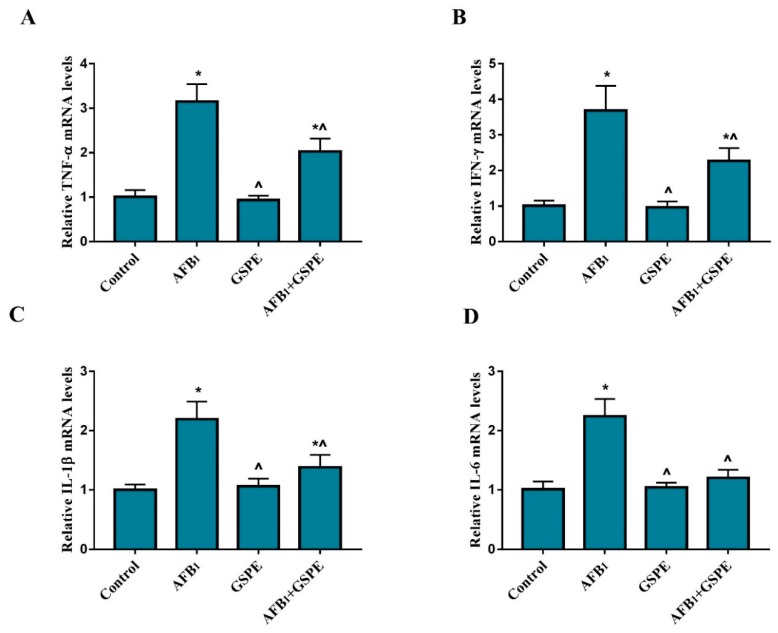
To elucidate the protective role of GSPE on the AFB1-induced immune response, the gene expression of pro-inflammatory cytokines at mRNA levels were quantified by quantitative real-time PCR in the splenic tissue of broilers. As shown in Figure 2, the mRNA expressions of TNF-α, IFN-γ, IL-1β, and IL-6 were significantly up-regulated in the AFB1 group, as compared to the control group. However, the addition of GSPE to AFB1 contaminated diet (p < 0.05) revert the negative effect of AFB1 on the mRNA levels of TNF-α, IFN-γ, IL-1β, and IL-6, as compared to the AFB1 treated group. Accordingly, our results suggest that pro-inflammatory cytokines gene expression inhibited by GSPE and thereby inflammatory response alleviated in the spleen induced by AFB1 in broilers.
Figure 2.
Protective role of GSPE treatment on AFB1-induced mRNA expression levels of the pro-inflammatory genes in the spleen of broilers. The mRNA expression of TNF-α, IFN-γ, IL-1β, and IL-6 were detected by quantitative real-time PCR. All data were expressed as mean ± SD (n = 6). Different symbols among groups indicate significant difference by LSD test (p < 0.05). * (p < 0.05) significant differences compared to the control group and ^ (p < 0.05) significant differences compared to the AFB1 group. (A) Tumor necrosis factor alpha (TNF-α); (B) interferon gamma (IFN-γ); (C) interleukin-1 beta (IL-1β); (D) interleukin 6 (IL-6).
2.3. Effects of GSPE and AFB1 on the Degradation of IκBα and the Phosphorylation of NF-κB (p65)
The NF-κB signaling pathway is considered a key factor to monitor the genes responsible for the immune and inflammatory responses. To confirm our hypothesis that NF-κB signaling mechanism involves in immunotoxicity, that is induced by AFB1 in the spleen of broilers. In the current study, the western blotting approach was used to determine the AFB1 toxicity impact and inhibition by GSPE. We found that AFB1 caused a (p < 0.05) degradation in the IκBα protein as compared to the control group. However, supplementation of GSPE into AFB1 contaminated diet showed prominent (p < 0.05) inhibition of AFB1-induced IκBα protein degradation. Figure 3A. Furthermore, AFB1 significantly elevated the phosphorylation of NF-κB (p65), while the addition of GSPE into AFB1 contaminated diet significantly reduced the phosphorylation of NF-κB (p65) Figure 3B.
Figure 3.
Protective role of GSPE treatment on the AFB1-induced degradation IκBα (A) and phosphorylation of NF-κB (p65) (B). The protein expression of β-actin, p65, pp65, and IκBα protein were detected by western blotting in the spleen of broilers. All data were expressed as mean ± SD. Different symbols among groups indicate significant difference by LSD test (p < 0.05). * (p < 0.05) significant differences compared to the control group and ^ (p < 0.05) significant differences compared to the AFB1 group.
2.4. Nrf2 and Its Downstream Genes (HO-1, GPx1, NQO1, and GCLC) mRNA Expression
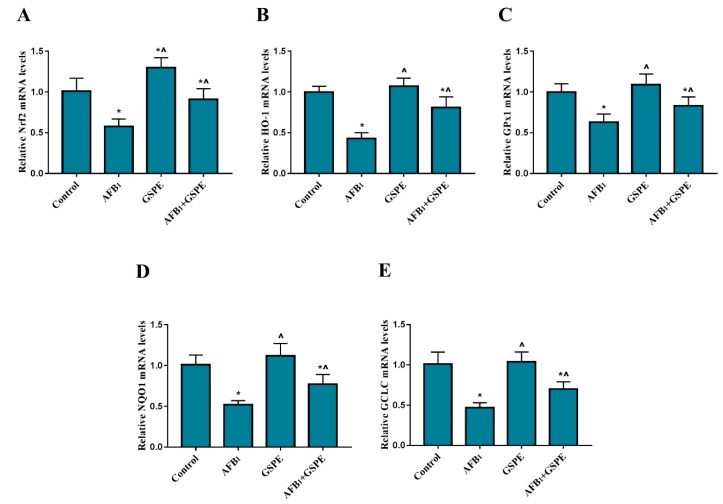
We assessed the protective effects of GSPE against AFB1-induced oxidative damage through regulating the Nrf2 signaling pathway in the liver of broiler chickens. The mRNA levels of HO-1, GPx1, NQO1, GCLC, and Nrf2 were examined by quantitative real-time PCR. The results of AFB1 treatment group showed (Figure 4) a significant decrease in the mRNA level of Nrf2 gene, as compared to the control group. The supplementation of GSPE to dietary treatments showed (p < 0.05) increase in the gene expression of Nrf2 in the liver of broilers by 22% and 56% when compared with the control and AFB1 group, respectively (Figure 4A). Moreover, compared to the control group, the mRNA expression level of HO-1, GPx1, NQO1, and GCLC genes in the AFB1 group were (p < 0.05) down-regulated. In contrast, the addition of GSPE to the AFB1 contaminated diet showed significant improvement in the mRNA expression levels of HO-1, GPx1, NQO1, and GCLC decreased by AFB1 Figure 4B–E.
Figure 4.
Protective role of GSPE treatment on AFB1-induced mRNA expression levels of the Nrf2 signaling pathway in the liver of broilers. The mRNA expression of Nrf2, HO-1, GPx1, NQO1, and GCLC were detected by quantitative real-time PCR. All data were expressed as mean ± SD (n = 6). Different symbols among groups indicate significant difference by LSD test (p < 0.05). * (p < 0.05) significant differences compared to the control group and ^ (p < 0.05) significant differences compared to the AFB1 group. (A) Nuclear erythroid-2-related factor (Nrf2); (B) heme oxygenase-1 (HO-1); (C) glutathione peroxidase (GPx1); (D) quinone oxidoreductase 1 (NQO1); (E) glutamate-cysteine ligase catalytic subunit (GCLC).
2.5. Nrf2 and Its Downstream Genes (HO-1, GPx1, NQO1, and GCLC) Protein Expression
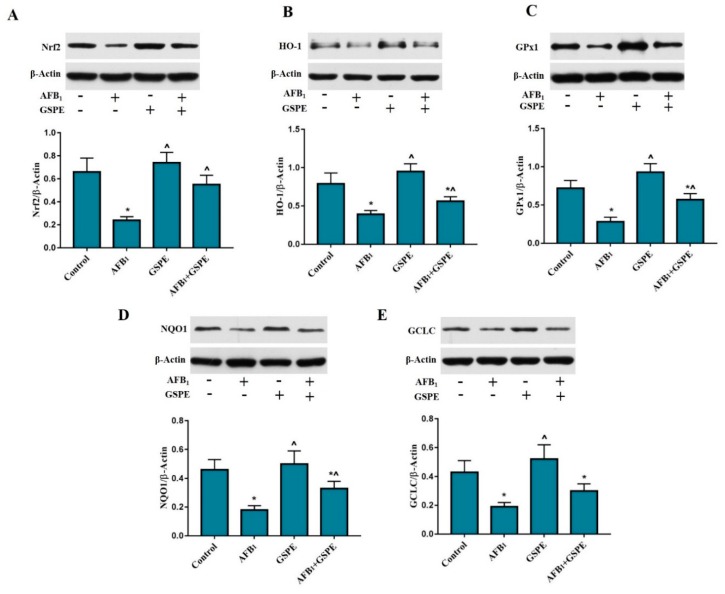
To understand whether the protective effects of GSPE against AFB1-induced oxidative damage is associated with the Nrf2 gene activation, we measured the protein level of Nrf2 and its target proteins, HO-1, GPx1 NQO1, and GCLC in broiler liver by western blotting (Figure 5). Exposure to AFB1 resulted in the down-regulation of the Nrf2 protein expression, and this effect was alleviated by the addition of GSPE into diets contaminated with AFB1, with a significant up-regulation of the Nrf2 protein expression when compared to the AFB1 treated group (Figure 5A). Furthermore, we noted that protein expression of HO-1, GPx1, NQO1, and GCLC showed a (p < 0.05) reduced in the AFB1 fed group, as compared to the control group. While the supplementation of dietary GSPE into AFB1 contaminated diet significantly ameliorated HO-1, GPx1, and NQO1 protein expressions when compared to the AFB1 group Figure 5B–D. However, the numerical difference was found in GCLC protein expression, as compared to the AFB1 group.
Figure 5.
Protective role of GSPE treatment on AFB1-induced protein expression levels of the Nrf2 signaling pathway in the liver of broilers. The protein expression of β-actin, Nrf2, HO-1, GPx1, NQO1, and GCLC were detected by western blotting. All data were expressed as mean ± SD. Different symbols among groups indicate significant difference by LSD test (p < 0.05). * (p < 0.05) significant differences compared to the control group and ^ (p < 0.05) significant differences compared to the AFB1 group. (A) Nuclear erythroid-2-related factor (Nrf2); (B) heme oxygenase-1 (HO-1); (C) glutathione peroxidase 1 (GPx1); (D) quinone oxidoreductase 1 (NQO1); (E) glutamate-cysteine ligase catalytic subunit (GCLC).
3. Discussion
The immunosuppressive nature of AFB1 is a well-supported area of its severe toxicity, and specifically, in poultry the immune system of birds is severely affected by AFB1 [11]. In the present study, a significant increase in inflammatory cytokines TNF-α, IFN-γ IL-1β, IL-10, and IL-6 in AFB1 group was observed in the serum of broilers, as compared to the control group. These results are in consistency with previous reports which indicated that AFB1 could lead to inflammation and alter the immune response [20,43]. However, the level of TNF-α, IFN-γ IL-1β, IL-10, and IL-6 remained lower in the normal physiological state, comparing with extrinsic effects. Meanwhile, cytokines are secreted and released by affected cells and released into the blood, which increases the levels of cytokines in the serum, indicating inflammation of tissue. Previously it was reported that GSPE could significantly improve the serum inflammatory cytokines induced by AFB1 in the mice [20]. The findings were similar to our results, which showed that the addition of GSPE to diets contaminated with AFB1 significantly ameliorated TNF-α, IFN-γ IL-1β, IL-10, and IL-6 induced by AFB1 in the serum of broilers.
In avian species, the spleen is the peripheral lymphoid organ, which plays a major protective role against inflammation and acquired immune response. The pro-inflammatory cytokines such as TNF-α, IFN-γ IL-1β, and IL-6 could trigger immune responses, as well as interrelate cells in order to eliminate the toxic effects and actively release other inflammation mediators [13]. Therefore, we determined the mRNA expression of the pro-inflammatory cytokines within the spleen, which can reflect the immune status of the broiler chickens. The current study showed that mRNA expression of TNF-α, IFN-γ IL-1β, and IL-6 were increased in the spleen of broilers exposed to AFB1. Moreover, our results indicated that the inclusion of AFB1 in the diet leads to an inflammation response in the spleen, similar effects had been observed in previous studies [20,43,44]. However, GSPE inhibited the mRNA expression of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and IFN-γ, of spleens induced by AFB1 in broilers. These results are consistent with those of [45,46,47] who have demonstrated that GSPE has anti-inflammatory effects in various animal models. Our findings indicated that GSPE has anti-inflammatory properties via modulating the secretion of pro-inflammatory cytokines.
Nuclear factor- κB (NF-κB) is a transcriptional factor that regulates immune and inflammatory responses in various condition [48,49]. In the current study, we found that the immunotoxicity induced by AFB1 promoted the phosphorylation of the NF-κB and the degradation of the IκBα protein. However, AFB1 toxicity was significantly ameliorated by the GSPE treatment. NF-κB is isolated from the cytoplasm through direct interaction with one of the inhibitor proteins of the IκB family such as IκBα. The phosphorylation and degradation of the IκBα proteins showed to be essential for the activation of the NF-κB, hence leads to a rapid translocation of NF-κB from the cytoplasm to the nucleus [50]. Our results are in accordance with the previous study [18], revealing that the presence of AFB1 in combination with OTA could aggravate immunotoxicity through the NF-κB signaling pathway. Furthermore, the addition of GSPE into AFB1 contaminated diet blocked the phosphorylation of NF-κB and the degradation of IκBα protein, which was the primary protein to activate NF-κB. The present study results indicated that the activation of NF-κB might be the reason for the spleen immune injury and inflammatory response in broilers. Our current study findings are suggesting that the mitigation of NF-κB activation might be responsible for the protective effects of GSPE against immunotoxicity of broilers induced by AFB1.
The Nrf2 signaling pathway has anti-oxidative effects on alleviating toxicant-induced oxidative stress and hepatotoxicity [27]. Activation of the Nrf2 signaling pathway enables to protect the cells from the oxidative damage. The antioxidative stress system can be activated by the Nrf2 signaling pathway, in parallel with regulating the expression of many intracellular antioxidant genes leads to excrete toxins [28,29]. Nrf2 and its target genes such as HO-1, GSH-Px, NQO1, and GCLC are critical components of the endogenous redox system. These proteins have been known to have the cytoprotective resistant effect to oxidative stress [51,52,53,54]. In our previous work, we found that GSPE could ameliorate AFB1-induced hepatotoxicity and oxidative damage in the liver of broiler chickens [42]. However, the precise mechanism by which GSPE attenuates oxidative damage caused by AFB1 in broiler chickens remained unclarified. Therefore, in the current study, we investigated the protective effects of GSPE on AFB1-induced oxidative damage through regulating the Nrf2 signaling pathway in the liver of broiler chickens. In the present study, the addition of GSPE to AFB1 contaminated diet attenuated the AFB1-induced decrease in Nrf2 in both mRNA and protein expression in the liver of broilers. Moreover, the mRNA and protein expressions of HO-1, GPx1, NQO1, and GCLC were up-regulated by the addition of GSPE to AFB1 diet. Our results indicated that GSPE could activate the Nrf2, resulting in enhancing the expression of HO-1, GPx1, NQO1, and GCLC, leading to improve the oxidative stress resistance, hence maintain the redox balance and enhance the resistance ability to oxidative stress induced by AFB1 in the liver of broilers. The present study results are continuity of previous studies that showed GSPE could ameliorate toxicant-induced oxidative damage via activating the Nrf2 signaling pathway. Long et al. [19], reported that GSPE attenuated the zearalenone-induced oxidative damage in the Kunming mice and the mechanism was related to the activation of the Nrf2 signaling pathway. Moreover, GSPE can induce Nrf2 expression and ARE-mediated transcription, thereby reducing oxidative stress. For instance, GSPE inhibited lead-induced liver oxidative damage and elevated antioxidant capacity via the activation of the Nrf2/ARE signaling pathway [41]. Furthermore, oligomeric proanthocyanidins markedly enhanced the nuclear translocation of Nrf2, promoted the expression of HO-1, NQO1, and thioredoxin reductase 1, and suppressed H2O2-induced oxidative damage in A549 cells [55]. The present study revealed that the protective effect of GSPE against AFB1 induced oxidative damage might be triggering the Nrf2 signaling pathway.
4. Conclusions
In summary, our findings indicated that the exposure to AFB1 could exacerbate inflammatory response, immunotoxicity and oxidative damage in broilers. Conversely, GSPE protects the immunotoxicity and oxidative damage AFB1-induced through the modulation of the NF-κB and Nrf2 signaling pathways. Taken together, our data demonstrated that NF-κB and Nrf2 responses were modulated by GSPE, which indicated the potential application of GSPE against immunotoxicity and oxidative stress in broiler chickens. Moreover, our findings propose a potential explanation for the mechanism of the antioxidant and immunomodulatory activities of GSPE and could be considered as a potential natural agent for the prevention of AFB1-induced immunotoxicity and oxidative damage in humans and animals.
5. Material and Methods
5.1. Fungal Isolation
In the present study Aspergillus flavus (NRRL-3357) strain was used for AFB1 production [56]. The strain was maintained as a glycerol stock preparation at −80 °C. It was grown on Petri dishes containing potato dextrose agar (E. Merck, Darmstadt, Germany) medium at 30 °C for seven days.
5.2. Aflatoxin B1 Production
Aflatoxin B1 was produced in corn by inoculating the Aspergillus flavus (NRRL-3357) according to the technique proposed by [57]. Briefly, twenty grams of ground maize were placed in Erlenmeyer flasks, then autoclaved at 121 °C for 20 min to eliminate their natural micro-flora. Each flask was inoculated by Aspergillus flavus containing 1 × 106 spores/grams. Mature spores were harvested with sterile 0.85% physiological saline. The moisture content was adjusted to the desired level (20%) by adding the necessary calculated volume of sterilized distilled water. The flasks were sealed with plastic film, allowing gaseous and vapor exchange, and then were mixed thoroughly by vigorous shaking to obtain a homogeneous distribution of the inoculum. The flasks were incubated under stationary conditions in a constant temperature and humidity incubator at 30 ± 1 °C and relative humidity (85%) for 15 days. Each flask was shaken once a day. The inoculated corn was incubated for 15 days to obtain the approximate AFB1 content of 64 mg/kg. The AFB1 contaminated corn was stored at 4 °C prior to treatment.
5.3. Aflatoxin B1 Analysis
The concentration of aflatoxin B1 was detected by High-Performance Liquid Chromatography (Waldbronn, Germany) equipped with a fluorescence detector and chromatographic separation was achieved using a C18 column (250 × 4.6 mm, 5 µm, Agilent, Santa Clara, CA, USA) following the methodology proposed by [57].
5.4. Compliance with Ethical Standards
All the experiments procedures were approved on 7 August 2017 (approval No. HZAUCH-2017-007) and conducted under the guidelines provided by the Institutional Animal Care and Ethics Committee of Huazhong Agricultural University, Wuhan, China.
5.5. Bird, Diets, and Management
GSPE was purchased from Zelang Medical Technology Company (Nanjing, China; purity ≥ 98%). For the present experiment, 240 one-day old Cobb broilers were obtained from a commercial hatchery (Jingzhou Kang Poultry Co., Ltd., Jingzhou, China). After three days acclimation, birds with similar body weight were randomly divided into four groups with six replicates of ten birds each (n = 60 per group). Groups were allocated based on the following four dietary treatments; 1. Basal diet without addition of GSPE and AFB1 (Control), 2. Basal diet supplemented with AFB1 1 mg/kg (AFB1), 3. Basal diet supplemented with GSPE 250 mg/kg (GSPE), 4. Basal diet supplemented with AFB1 1 mg/kg + GSPE 250 mg/kg (AFB1 + GSPE). The doses of AFB1 and GSPE were chosen considering our previous study [42]. Birds were fed ad libitum and provided fresh drinking water during the whole experimental period (28 days). The experiment was conducted under standard temperature and hygienic conditions. The composition of the basal diet has been presented in Table 1.
Table 1.
Basal diet formulation and nutritional value.
| Ingredients | Percentage % |
|---|---|
| Corn | 58.3 |
| Soybean meal | 30.2 |
| Fish meal | 5.6 |
| Soybean oil | 2.3 |
| Dicalcium phosphate | 1.2 |
| Lime stone | 1.00 |
| Salt | 0.2 |
| Methionine | 0.2 |
| Premix 1 | 1.00 |
| Total | 100.00 |
| Calculated chemical composition | |
| Crude protein | 21.87 |
| Metabolisable energy (MJ/kg) | 13.45 |
| Lysine | 1.14 |
| Methionine | 0.40 |
| Methionine + Cystine | 0.94 |
| Calcium | 0.95 |
| Available phosphorus | 0.49 |
1 The premix contained (per kg of diet): Fe, 60 mg; Cu, 7.5 mg; Zn, 65 mg; Mn, 110 mg; I, 1.1 mg; Se, 0.4 mg; Biotin, 0.04 mg; choline chloride, 400 mg; vitamin A (from retinyl acetate), 4500 IU; vitamin D3 (from cholecalciferol), 1000 IU; vitamin K (menadione sodium bisulphate), 1.3 mg; vitamin B1, 2.2 mg; vitamin B2, 10 mg; vitamin B3, 10 mg; vitamin B5, 50 mg; vitamin B6, 4 mg; vitamin B11, 1 mg; vitamin B12, 0.013 mg.
5.6. Collection of Samples
At the 28th day of age, one bird close to the average weight was selected from each replicate. After chickens fasted for 12 h, blood samples were collected in tubes by puncture of the wing vein. The blood samples were centrifuged (Eppendorf centrifuge 5804R, Hamburg, Germany) at 3000× g at 4 °C for 10 min, and the serum was separated and stored at −20 °C for cytokines analysis. After taking blood samples, birds were euthanized by cervical dislocation, and spleen and liver were collected. The spleen and liver were snap frozen in liquid nitrogen and later stored at −80 °C for further analysis.
5.7. Determination of Serum Cytokines
The immune response in the serum was determined by measuring the levels of TNF-α (CSB-E11231Ch), IFN-γ (CSB-E08550Ch), IL-1β, IL-10 (CSB-E12835Ch), and IL-6 (CSB-E08549Ch). These cytokines were analyzed using the Enzyme Linked-Immunosorbent Assay (ELISA) method through the specific assay kits. The details of all the determination procedures followed by manufactures protocols for the commercial kits supplied by (Cusabio Biotech Co. Ltd., Wuhan, China). The levels of cytokines were expressed as picogram per milliliter (pg/mL).
5.8. Total RNA Extraction and Quantitative Real-Time PCR
Total mRNA was extracted from spleen and liver tissues with Trizol® (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The purity and concentration of RNA samples were estimated by nucleic acid concentration analyzer NanoDrop 2000 (Thermo Fisher, Waltham, MA, USA) based on the ratio of the absorbance at 260 and 280 nm. The cDNA was synthesized from 1 µg of total RNA by reverse transcription in a 20 µL reaction using a PrimeScriptTM RT reagent Kit (Takara DRR037A, Dalian, China) following the manufacturer’s protocol. The expression levels of pertaining genes (β-actin, TNF-α, IFN-γ, IL-1β, IL-10, IL-6, Nrf2, HO-1, GPx1, NQO1, and GCLC) were analyzed by quantitative real-time PCR (CFX384, Bio-Rad, Hercules, CA, USA) using the SYBER® Green PCR Master Mix (Applied Biosynthesis, Waltham, MA, USA), following the method according to our previous study [58] and the manufacturer’s instructions. The primer sequences used for each gene are presented in Table 2. The 2-∆∆Ct method was used for quantification with the β-actin as a reference gene, and the relative abundance was normalized to the control (as 1) [59,60]. The results were expressed as relative mRNA levels.
Table 2.
Primer used for quantitative real-time PCR.
| Target Gene | Primer | Primer Sequence (5′→3′) | Accession No. |
|---|---|---|---|
| β-Actin | Forward Reverse |
CCCGCAAATGCTCTAAACC CCAATCCTGTCTTGTTTTATGC |
L08165 |
| IL-1β | Forward Reverse |
GGTCAACATCGCCACCTACA CATACGAGATGCAAACCAGCAA |
NM_204524.1 |
| IL-6 | Forward Reverse |
GGTGATAAATCCCGATGAAGT TCTCCATAAACGAAGTAAAGTCTC |
NM_204628 |
| IFNγ | Forward Reverse |
TGAGCCAGATTGTTTCGATG TCCTTTTGAAACTCGGAGGA |
NM_205149 |
| TNFα | Forward Reverse |
TGTGTATGTGCAGCAACCCGTAGT GGCATTGCAATTTGGACAGAAGT |
AY765397.1 |
| Nrf2 | Forward Reverse |
GATGTCACCCTGCCCTTAG CTGCCACCATGTTATTCC |
NM_205117 |
| HO-1 | Forward Reverse |
GGTCCCGAATGAATGCCCTTG ACCGTTCTCCTGGCTCTTGG |
HM237181.1 |
| GPx1 | Forward Reverse |
GACCAACCCGCAGTACATCA GAGGTGCGGGCTTTCCTTTA |
NM_001277853.1 |
| NQO1 | Forward Reverse |
CAGTGGCATGCACCCAGGGAA GCATGCCCCTTTTAGCCTTGGCA |
NM_001277619.1 |
| GCLC | Forward Reverse |
AGTGCTGAGTGGCGAAGAAGT GCAGCCTCTTGCCTCCTCTT |
XM_419910.5 |
5.9. Western Blot Analysis
Protein expressions of Nrf2, HO-1, GPx1, NQO1 and GCLC in the liver and NF-κB, p-NF-κB, and IκBα in the spleen of broilers were determined by western blot according to our previous study [58]. The following antibodies used for the present study were purchased from indicated sources: Anti-NF-κB, Anti-p-NF-κB, Anti-Nrf2, Anti-HO-1, anti-GPx1, (Abcam, Cambridge, MA, USA), Anti-NQO1, GCLC (Abclonal Technology, Woburn, MA, USA), Anti-β-Actin, Anti-IκBα (Cell Signaling Technology, Boston, MA, USA). The HRP-labeled goat anti-rabbit IgG (Servicebio Technology, Wuhan, China) was used as the secondary antibody. Samples were analyzed in triplicate; a representative blot is shown in respective figures. The proteins bands were detected via chemiluminescence WesternBrightTM ECL substrate kit (Advansta, Menlo Park, CA, USA), then visualized and quantified by FluroChem FC2 Imaging System.
5.10. Statistical Analysis
The experimental data were analyzed by one-way ANOVA using IBM SPSS Statistic 22 (IBM Corporation, Armonk, New York, NY, USA). Differences were considered to be significant at p < 0.05, the least significant difference test (LSD) was used to separate the significant differences between means. Results were presented as mean ± SD.
Author Contributions
The authors’ responsibilities were as follows—S.A.R. and D.Q.: designed the research; S.A.R., L.S., N.-Y.Z., M.M.K., Z.L., L.C., S.W., and A.S.: conducted the research; F.A.K., I.R.R., and D.M.B.: guided and performed the statistical analysis; S.A.R.: wrote the manuscript and hold primary responsibility for the final content; and all authors: read and approved the final manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2016YFD0501207) and National Natural Science Foundation of China (NSFC) grant number (31772635).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
GSPE alleviates AFB1-induced immune injury by suppressing the inflammatory response and inhibiting the NF-κB expression. GSPE ameliorates AFB1-induced oxidative stress through activating the Nrf2 pathway in broilers.
References
- 1.Bünger J., Westphal G., Mönnich A., Hinnendahl B., Hallier E., Müller M. Cytotoxicity of occupationally and environmentally relevant mycotoxins. Toxicology. 2004;202:199–211. doi: 10.1016/j.tox.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Méndez A. Decontamination of aflatoxin duckling feed with aqueous citric acid treatment. Anim. Feed Sci. Technol. 2007;135:249–262. doi: 10.1016/j.anifeedsci.2006.07.009. [DOI] [Google Scholar]
- 3.Abrar M., Anjum F.M., Butt M.S., Pasha I., Randhawa M.A., Saeed F., Waqas K. Aflatoxins: Biosynthesis, occurrence, toxicity, and remedies. Crit. Rev. Food Sci. Nutr. 2013;53:862. doi: 10.1080/10408398.2011.563154. [DOI] [PubMed] [Google Scholar]
- 4.Ferenčík M., Ebringer L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol. 2003;48:417–426. doi: 10.1007/BF02931378. [DOI] [PubMed] [Google Scholar]
- 5.Herzallah S.M. Aflatoxin B1 residues in eggs and flesh of laying hens fed aflatoxin B1 contaminated diet. Am. J. Agric. Biol. Sci. 2013;8:156–161. doi: 10.3844/ajabssp.2013.156.161. [DOI] [Google Scholar]
- 6.Alpsoy L., Yalvac M.E., Litwack G. Key roles of vitamins a, c, and e in aflatoxin B1-induced oxidative stress. Vitam. Horm. Adv. Res. Appl. 2011;86:287–305. doi: 10.1016/B978-0-12-386960-9.00012-5. [DOI] [PubMed] [Google Scholar]
- 7.Craig A.W. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: International Agency for Research on Cancer. IARC; Lyon, France: 1986. [Google Scholar]
- 8.Stettler P.M., Sengstag C. Liver carcinogen aflatoxin B1 as an inducer of mitotic recombination in a human cell line. Mol. Carcinog. 2001;31:125. doi: 10.1002/mc.1047. [DOI] [PubMed] [Google Scholar]
- 9.Zhang N.Y., Qi M., Zhao L., Zhu M.K., Guo J., Liu J., Gu C.Q., Rajput S.A., Krumm C.S., Qi D.S. Curcumin prevents aflatoxin b1 hepatoxicity by inhibition of cytochrome p450 isozymes in chick liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissonnier G.M., Pinton P., Laffitte J., Cossalter A.M., Gong Y.Y., Wild C.P., Bertin G., Galtier P., Oswald I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008;231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Yunus A.W., Razzazifazeli E., Bohm J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins. 2011;3:566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence T. The nuclear factor nf-kb pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;10:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui W., Cui H., Peng X., Fang J., Liu X., Wu B. Effect of vanadium on splenocyte apoptosis in broilers. J. Med. Chem. 2012;2:57–60. [Google Scholar]
- 14.Subhashinie K., Johnson T.J., Zhou H., Li X., Nate B., Emma B., Megan O., Sandford E.E., Liu P., Nolan L.K. Spleen transcriptome response to infection with avian pathogenic escherichia coli in broiler chickens. BMC Genomics. 2011;12:469. doi: 10.1186/1471-2164-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden M.S., Ghosh S. Nf-îºb, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diks S.H., van Deventer S.J., Peppelenbosch M.P. Lipopolysaccharide recognition, internalisation, signalling and other cellular effects. J. Endotoxin Res. 2001;7:335–348. doi: 10.1179/096805101101532909. [DOI] [PubMed] [Google Scholar]
- 17.Guzik T.J., Korbut R., Adamekguzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 18.Hou L., Fang G., Xuan Z., Zhou Y., Gang Q., Liu Z., Huang K. Immunotoxicity of ochratoxin a and aflatoxin B1 in combination is associated with the nuclear factor kappa b signaling pathway in 3d4/21 cells. Chemosphere. 2018;199:718. doi: 10.1016/j.chemosphere.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Long M., Yang S.H., Han J.X., Li P., Zhang Y., Dong S., Chen X., Guo J., Wang J., He J.B. The protective effect of grape-seed proanthocyanidin extract on oxidative damage induced by zearalenone in kunming mice liver. Int. J. Mol. Sci. 2016;17:808. doi: 10.3390/ijms17060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long M., Zhang Y., Li P., Yang S.H., Zhang W.K., Han J.X., Wang Y., He J.B. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 2016;17:516. doi: 10.3390/ijms17040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Wahhab M.A., Aly S.E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J. Agric. Food Chem. 2003;51:2409–2414. doi: 10.1021/jf0209185. [DOI] [PubMed] [Google Scholar]
- 22.Shen H.M., Shi C.Y., Lee H.P., Ong C.N. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol. Appl. Pharmacol. 1994;127:145. doi: 10.1006/taap.1994.1148. [DOI] [PubMed] [Google Scholar]
- 23.Sumit R., Ji E.K., Roger C.J. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010;89:325. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Grant D., Mendicino M., Levy G. Xenotransplantation: Just around the corner? Surgery. 2001;129:243. doi: 10.1067/msy.2001.118380. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Muhammad I., Li W., Sun X., Cheng P., Zhang X. Sensitivity of arbor acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-ii enzymes and Nrf2. Environ. Toxicol. Pharmacol. 2018:94–104. doi: 10.1016/j.etap.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Vipin A.V., Rao R., Kurrey N.K., Venkateswaran G. Protective effects of phenolics rich extract of ginger against aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017;91:415. doi: 10.1016/j.biopha.2017.04.107. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y.M., Wang Y., Tan H.S., Yu T., Fan X.M., Chen P., Zeng H., Huang M., Bi H.C. Schisandrol b protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the Nrf2/are signaling pathway. Acta Pharmacol. Sin. 2016;37:382. doi: 10.1038/aps.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang I.G., Min K.C. Recent updates on acetaminophen hepatotoxicity: The role of Nrf2 in hepatoprotection. Toxicol. Res. 2013;29:165–172. doi: 10.5487/TR.2013.29.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sykiotis G.P., Habeos I.G., Samuelson A.V., Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.S., Surh Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Eggler A.L., Gay K.A., Mesecar A.D. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2008;52:S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J., Wang H., Chen F., Fu J., Xu Y., Hou Y., Kou H.H., Zhai C., Nelson M.B., Zhang Q. An overview of chemical inhibitors of the Nrf2—are signaling pathway and their potential applications in cancer therapy. Free Radic. Biol. Med. 2016;99:544–556. doi: 10.1016/j.freeradbiomed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Costa S., Utan A., Speroni E., Cervellati R., Piva G., Prandini A., Guerra M.C. Carnosic acid from rosemary extracts: A potential chemoprotective agent against aflatoxin B1. An in vitro study. J. Appl. Toxicol. 2007;27:152–159. doi: 10.1002/jat.1186. [DOI] [PubMed] [Google Scholar]
- 34.Cavin C., Marin-Kuan M., Langouet S., Bezencon C., Guignard G., Verguet C., Piguet D., Holzhauser D., Cornaz R., Schilter B. Induction of Nrf2-mediated cellular defenses and alteration of phase i activities as mechanisms of chemoprotective effects of coffee in the liver. Food Chem. Toxicol. 2008;46:1239–1248. doi: 10.1016/j.fct.2007.09.099. [DOI] [PubMed] [Google Scholar]
- 35.Hayes J.D., Mcmahon M., Chowdhry S., Dinkovakostova A.T. Cancer chemoprevention mechanisms mediated through the keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 36.Zhen J., Qu Z., Fang H., Fu L., Wu Y., Wang H., Zang H., Wang W. Effects of grape seed proanthocyanidin extract on pentylenetetrazole-induced kindling and associated cognitive impairment in rats. Int. J. Mol. Med. 2014;34:391. doi: 10.3892/ijmm.2014.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Ashmawy I.M., Saleh A., Salama O.M. Effects of marjoram volatile oil and grape seed extract on ethanol toxicity in male rats. Basic Clin. Pharmacol. Toxicol. 2007;101:320–327. doi: 10.1111/j.1742-7835.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 38.Ouédraogo M., Charles C., Ouédraogo M., Guissou I.P., Stévigny C., Duez P. An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr. Cancer. 2011;63:1163. doi: 10.1080/01635581.2011.607549. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z.C., Yin J., Zhou B., Liu Y.T., Yu Y., Li G.Q. Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J. Gastroenterol. 2015;21:7468–7477. doi: 10.3748/wjg.v21.i24.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai N., Zou Y., Zhu L., Wang H.F., Dai M.G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (ccl4)-induced steatosis and liver injury in rats via cyp2e1 regulation. J. Med. Food. 2014;17:663–669. doi: 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long M., Liu Y., Cao Y., Wang N., Dang M., He J. Proanthocyanidins attenuation of chronic lead-induced liver oxidative damage in kunming mice via the Nrf2/are pathway. Nutrients. 2016;8:656. doi: 10.3390/nu8100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajput S.A., Sun L., Zhang N., Khalil M.M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins. 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Ma Q.G., Zhao L.H., Wei H., Duan G.X., Zhang J.Y., Ji C. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int. J. Mol. Sci. 2014;15:5649–5662. doi: 10.3390/ijms15045649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Q., Yan L., Yu F., Zhao L., Hua W., Cheng J., Zhang J. Molecular mechanisms of lipoic acid protection against aflatoxin B1-induced liver oxidative damage and inflammatory responses in broilers. Toxins. 2015;7:5435–5447. doi: 10.3390/toxins7124879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee T., Kwon H.S., Bang B.R., Lee Y.S., Park M.Y., Moon K.A., Kim T.B., Lee K.Y., Moon H.B., Cho Y.S. Grape seed proanthocyanidin extract attenuates allergic inflammation in murine models of asthma. J. Clin. Immunol. 2012;32:1292–1304. doi: 10.1007/s10875-012-9742-8. [DOI] [PubMed] [Google Scholar]
- 46.Subarnas A., Wagner H. Analgesic and anti-inflammatory activity of the proanthocyanidin shellegueain a from polypodium feei mett. Phytomedicine. 2000;7:401–405. doi: 10.1016/S0944-7113(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 47.Ahmad S.F., Zoheir K.M., Abdelhamied H.E., Attia S.M., Bakheet S.A., Ashour A.E., Abdallah A.R. Grape seed proanthocyanidin extract protects against carrageenan-induced lung inflammation in mice through reduction of pro-inflammatory markers and chemokine expressions. Inflammation. 2014;37:500–511. doi: 10.1007/s10753-013-9764-2. [DOI] [PubMed] [Google Scholar]
- 48.Han Y.M., Koh J., Ji W.K., Lee C., Koh S.J., Kim B.G., Lee K.L., Im J.P., Kim J.S. Nf-kappa b activation correlates with disease phenotype in crohn’s disease. PLoS ONE. 2017;12:e0182071. doi: 10.1371/journal.pone.0182071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin Z., Kong C., Yang X., Cui X., Lin X., Zhe Z. Protein kinase c-α (pkcα) modulates cell apoptosis by stimulating nuclear translocation of nf-kappa-b p65 in urothelial cell carcinoma of the bladder. BMC Cancer. 2017;17:432. doi: 10.1186/s12885-017-3401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Didonato J.A., Mercurio F., Karin M. Phosphorylation of i kappa b alpha precedes but is not sufficient for its dissociation from nf-kappa b. Mol. Cell. Biol. 1995;15:1302–1311. doi: 10.1128/MCB.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otterbein L.E., Choi A.M.K. Heme oxygenase: Colors of defense against cellular stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:1029–1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 52.Moffit J.S., Aleksunes L.M., Kardas M.J., Slitt A.L., Klaassen C.D., Manautou J.E. Role of nad(p)h:Quinone oxidoreductase 1 in clofibrate-mediated hepatoprotection from acetaminophen. Toxicology. 2007;230:197–206. doi: 10.1016/j.tox.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haines D.D., Lekli I., Teissier P., Bak I., Tosaki A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: Focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012;204:487–501. doi: 10.1111/j.1748-1716.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 54.Mulcahy R.T., Wartman M.A., Bailey H.H., Gipp J.J. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/tre sequence. J. Biol. Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 55.Sun C., Jin W., Shi H. Oligomeric proanthocyanidins protects a549 cells against H2O2-induced oxidative stress via the Nrf2-are pathway. Int. J. Mol. Med. 2017;39:1548. doi: 10.3892/ijmm.2017.2971. [DOI] [PubMed] [Google Scholar]
- 56.Schmidtheydt M., Abdelhadi A., Magan N., Geisen R. Complex regulation of the aflatoxin biosynthesis gene cluster of aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 2009;135:231–237. doi: 10.1016/j.ijfoodmicro.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 57.Liu J., Sun L., Zhang N., Zhang J., Guo J., Li C., Rajput S.A., Qi D. Effects of nutrients in substrates of different grains on aflatoxin B1 production by aspergillus flavus. BioMed Res. Int. 2016;2016:7232858. doi: 10.1155/2016/7232858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao X., Xiao Z., Li C., Zhang J., Zhu L., Sun L., Zhang N., Khalil M.M., Rajput S.A., Qi D. Prenatal exposure to zearalenone disrupts reproductive potential and development via hormone-related genes in male rats. Food Chem. Toxicol. 2018;16:11–19. doi: 10.1016/j.fct.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Sun L.H., Lei M.Y., Zhang N.Y., Zhao L., Krumm C.S., Qi D.S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014;74:289. doi: 10.1016/j.fct.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 60.Sun L.H., Lei M.Y., Zhang N.Y., Gao X., Li C., Krumm C.S., Qi D.S. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on brl 3a rat liver cells. Toxicon. 2015;95:6–12. doi: 10.1016/j.toxicon.2014.12.010. [DOI] [PubMed] [Google Scholar]