Abstract
A lytic bacteriophage PHB01 specific for Pasteurella multocida type D was isolated from the sewage water collected from a pig farm. This phage had the typical morphology of the family Podoviridae, order Caudovirales, presenting an isometric polyhedral head and a short noncontractile tail. PHB01 was able to infect most of the non-toxigenic P. multocida type D strains tested, but not toxigenic type D strains and those belonging to other capsular types. Phage PHB01, the first lytic phage specific for P. multocida type D sequenced thus far, presents a 37,287-bp double-stranded DNA genome with a 223-bp terminal redundancy. The PHB01 genome showed the highest homology with that of PHB02, a lytic phage specific for P. multocida type A. Phylogenetic analysis showed that PHB01 and PHB02 were composed of a genus that was close to the T7-virus genus. In vivo tests using mouse models showed that the administration of PHB01 was safe to the mice and had a good effect on treating the mice infected with different P. multocida type D strains including virulent strain HN05. These findings suggest that PHB01 has a potential use in therapy against infections caused by P. multocida type D.
Keywords: bacteriophage, lytic, P. multocida type D, isolation, therapeutic application
1. Introduction
Pasteurella multocida isolates are generally classified into five capsular types (A, B, D, E, and F) [1], and are commonly associated with respiratory diseases and hemorrhagic septicemia in a wide range of domestic and wild animals [2]. While it rarely occurs, P. multocida infections in humans have been continuously reported [3,4,5,6,7], and most of these cases are likely to be transmitted from pets such as dogs and cats [4,8]. It has been reported that the infection of P. multocida isolates displays host predilection; different capsular types are associated with specific types of diseases [2,8,9,10].
Known as the natural predators of bacteria, bacteriophages (phages) are probably the most abundant biological entity on the Earth [11]. Regarding their ability to kill pathogens with high specificity, phages are proposed as promising therapeutic tools, and the use of phages/phage-derivatives to combat bacterial infections has been widely studied for bacteria such as Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Clostridium difficile [12,13,14,15]. However, current knowledge on P. multocida phages, especially the lytic phages, is limited.
P. multocida phages were first reported in 1956 [16], however, the lytic P. multocida phages had not been characterized until 2018 [17,18]. To date, there have been only five whole genome sequences of P. multocida phages available in the GenBank database including the three temperate transducing Pasteurella phages F108 (Accession: NC_008193) [19], AFS-2018a (Accession: MH238466), and Pm86 (Accession: MH238467) [20], and two lytic phages PMP-GADVASU-IND (Accession: KY203335) [18], and PHB02 (Accession: MF034659) [17]. While lytic phages specific for P. multocida capsular types A and B have been characterized recently [17,18], there is still a lack of information on lytic phages for other P. multocida capsular types. In this study, a novel lytic T7-like phage designated vB_PmuP_PHB01 (hereafter referred to as phage PHB01) specific for P. multocida capsular type D strains was isolated and characterized. To the best of our knowledge, this was the first report of a T7-like phage specific for P. multocida capsular type D strains.
2. Materials and Methods
2.1. Phage Isolation
PHB01 was isolated from the sewage water collected from a pig farm in Hubei Province in January 2016 using the conventional double-layer agar method described previously [17,21]. Briefly, 20 mL of sewage water was sterilized by filtration through a 0.22 μm pore size membrane. After that, 5 mL of the filtrate was mixed with 10 mL of indicator bacteria (P. multocida capsular type D strain HND065, a nontoxigenic isolate) at mid-log phase and incubated at 37 °C for 4 h. The mixture was then centrifuged at 12,000× g for 10 min, and filtered using a 0.22 μm pore size membrane. A total of 100 μL of the supernatant was then mixed with 300 μL of the indicator bacterium, and was poured into 6 mL of molten soft Tryptic Soy Broth (TSB medium with 0.7% w/v agar) containing 10% v/v of newborn calf serum (NBS). Finally, the mixture was poured onto a prepared Tryptic Soy Agar (TSA medium with 1.5% w/v agar; SA top agar) containing 10% v/v of NBS and incubated overnight at 37 °C to numerate the plaques.
After the plaques were numerated, a single plaque was picked and re-suspended in 6 mL of a modified SM buffer [5.8 g of NaCl, 2.0 g of MgSO4·7H2O, 50 mL of Tris-HCl (pH 7.4), 5.0 mL of 2% gelatin] [22] for 3 h. The phage-containing SM buffer was then centrifuged at 12,000× g for 30 s and the supernatant was filtered through a 0.22 μm pore size membrane. After that, the phage preparations were given serial 10-fold dilutions with sterile SM buffer. Phage isolation by the double-layer agar method was repeated four more times, and the phage suspensions were stored at 4 °C. The phages were purified by CsCl gradient ultra-centrifugation, as described previously [17].
2.2. Electron Microscopy
The morphology of the phages was determined using a 100-kV transmission electron microscope (HITACHI H-7650, Tokyo, Japan) with the same protocol previously described [17]. The phage filtrate was stained negatively with 2% uranyl acetate after the addition of a drop of a phage suspension onto a grid surface, and the excess stain was removed immediately.
2.3. Thermolability and pH Sensitivity
The thermolability and pH sensitivity of PHB01 was tested as previously described [23] with minor modifications. The purified phage particles were given incubation at different temperatures (4 °C, 20 °C, 40 °C, 50 °C, 60 °C, and 70 °C) for 1 h to test the thermolability. Incubations at 37 °C for 1 h under different pH levels (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12) were set to test the pH sensitivity of the phage. Each assay was performed in triplicate. Samples were titered by the double-layer agar plate method [17].
2.4. One-Step Growth Curve
To test the one-step growth curve, PHB01 was mixed with its indicator bacteria (mid-log phase) at multiplicity of infection (MOI) of 0.001 and incubated at 37 °C for 5 min. After incubation, unabsorbed free phages were removed by centrifugation at 12,000× g for 30 s. The pellets were washed using pre-warmed TSB (37 °C) first, and then, the suspension was transferred to 20 mL of TSB followed by incubation at 37 °C [24]. From this moment (t = 0 min), a 0.5-mL sample was collected every 10 min for 90 min. A double-layer agar method [17] was used to determine the titration of the phage particles. The experiment was repeated three times. The latent period was followed by a single burst of phages, where the burst size was the average number of phages released per infected host cell and calculated as the ratio between the number of phages before and after the burst [25].
2.5. Host Range
Spot tests were performed to determine the host specificity of phage PHB01, in accordance with previous study [26]. A total of 48 P. multocida clinical isolates collected from different locations in China including 37 capsular type D strains, 10 capsular type A strains, and 1 capsular type F strain as well as other bacterial species including Escherichia coli, Salmonella enterica serovar typhimurium, Salmonella enterica serovar choleraesuis, and Bordetella bronchiseptica were used. Summarily, bacterial strains were grown to mid-log phase at 37 °C, and 300 μL of each bacterial culture was added into 3 mL of molten SA top agar. After each overlay solidified, 4 μL of the phage lysate (1 × 109 pfu/mL) was spotted onto the bacterial overlays, dried, and then incubated at 37 °C for 8 h. The same volume of sterile phage buffer was also spotted onto the bacterial overlays and incubated under the same conditions as the controls. Lytic specificity was defined based on the formation of bacteriophage plaques. The spot tests were repeated three times to confirm the results. The efficiency of plating (EOP) values were determined by calculating the ratio of pfus of each phage-susceptible strain to the pfus of the indicator strain (P. multocida HND065). This experiment was repeated three times [17].
2.6. DNA Extraction and Analysis of Genome Sequence
The genomic DNA of PHB01 was extracted using the phenol-chloroform method [25]. Briefly, the purified phages were treated by proteinase K (100 mg/mL), SDS (10%, w/v), and EDTA (0.5 mM, pH 8.0) at 56 °C in water for 2 h. After that, the sample was washed three times by using an equal volume of mixture composed of phenol, chloroform, and isoamyl alcohol (25:24:1), followed by centrifugation at 4 °C, 12,000× g for 10 min, to remove the debris. In the next step, the supernatant was mixed with isoamyl alcohol kept at −20 °C overnight. The air-dried precipitate was washed three times with cold 75% ethanol, and the phages’ genomic DNA was finally dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]).
Whole genome sequencing was performed at BGI (Shenzhen, China) on an Illumina HiSeq 2500 sequencer with 2 × 100 bp read length [17]. Libraries with an insert size of 270 bp were constructed using the NEBNext®Ultra™ II DNA Library Prep Kit (NEB, Ipswich, MA, US). Raw reads with low quality were filtered and eliminated by SOAPnuke (version 1.5.0) software (https://github.com/BGI-flexlab/SOAPnuke) [27] according to the following criteria: reads with a certain proportion of low quality (20) bases (40% as the default, parameter setting at 20 bp), and/or with a certain proportion of Ns (10% as the default, parameter setting at 1 bp) were removed. Adapter contamination (15 bp overlap between the adapter and reads as the default, parameter setting at 15 bp) and duplication contamination were also removed. The high-quality reads were then de novo assembled into the genome by means of SOAPdenovo2.04 [28,29]. The terminal sequences of the virus genome were determined by a modified statistical method, as described previously [30]. Glimmer 3.0 [31], Tandem Repeat Finder 4.09 [32], and tRNAscan-SE 2.0 [33] were used to predict protein-encoding putative open reading frames (ORFs), Tandem repeats, and transfer RNAs (tRNAs) encoded by the PHB01 genome, respectively, with the default parameters. The PHB01 whole genome sequence (WGS) and its annotations were finally deposited into GenBank under accession number MF166859. When required, a sequence comparison was performed using Easyfig v.2.0 [34]. Phylogenetic trees were constructed using MEGAX [35] with 1000 Bootstrap replications.
2.7. Animals and Ethic Statement
BALB/c mice (5-week-old) used in this study were purchased from the Huazhong Agricultural University Laboratory Animal Center (Wuhan, China). All animal tests performed in this study followed the Guide for the Care and Use of Laboratory Animals of Hubei Province (approved by the Standing Committee of the People’s Congress of Hubei Province) and were approved by the Ethical Committee for Animal Experiments at Huazhong Agricultural University, Wuhan, China. The approved number is HZAUMO-2018-023.
2.8. Safety Test
To study the toxicity of PHB01, six 5-week-old female BALB/c mice were randomly divided into two groups with three mice in each group. Mice in each group received an intraperitoneal injection of 100 μL PHB01 (109 pfu/mL), and 100 μL PBS buffer, respectively. All mice were housed under the same conditions and were observed for seven days.
The health status of each group of mice was recorded by giving different scores (0: dead; 1: near death; 2: exudative accumulation around partially closed eyes; 3: lethargy and hunched back; 4: decreased physical activity and ruffled fur; 5: normal health, condition unremarkable), as previously described [36]. The total score for each group was recorded at least three times a day. At seven days post-injection, all mice were euthanized, and the livers, spleens, kidneys, and lungs were collected for histological examination. The data describing the health status of each group of mice are presented as the mean ± SD.
2.9. Mouse Infection and Treatment
A non-toxigenic P. multocida capsular type D isolate HND065 and a non-toxigenic P. multocida capsular type D virulent isolate HN05 that we had sequenced previously [9] were used to generate the mouse-infection model in this study. Before study, the minimum lethal dose (MLD) of each isolate on mice was determined as described previously [37]. After that, thirty-six 5-week-old female BALB/c mice were divided into six groups (I–VI) and each group contained six mice. Mice in Groups I~II and groups IV~V were challenged intraperitoneally with HND065 and HN05 at 2 × MLD (2 × 107 CFU for HND065 and 3.2 × 104 CFU for HN05) while mice in Groups III and VI received a challenge of PHB01 (108 PFU) and PBS through the same routine, respectively. In the next step, each of the mice in P. multocida-challenged groups were given a treatment of PHB01 at 108 PFU (Groups II and V) or PBS (Groups I and IV) at 6 h following bacterial inoculation, and then twice a day for five days (Figure 1). All mice were housed under the same conditions. Health statuses were monitored and recorded at least three times a day for 21 days. Scores were given as above-mentioned and the data describing the health status of the mice were expressed as the mean ± SD. Survival was analyzed using the Kaplan–Meier analysis with a log-rank test (statistically significant at p < 0.05).
Figure 1.
Experimental scheme for the evaluation of PHB01 treatment efficacy in mice infected with P. multocida type D. Each of the mice in the P. multocida-challenged groups were given a treatment of PHB01 at 108 PFU (Groups II and V) or PBS (Groups I and IV) at 6 h following bacterial inoculation, and then twice a day for five days.
3. Results
3.1. Morphological Characteristics
Using a P. multocida capsular type D strain HND065 as the indicator, a Pasteurella bacteriophage designated vB_PmuP_PHB01 (PHB01 for short) was isolated from the pig farm sewage water through the double-layer agar method [21]. After incubation and purification, PHB01 formed round transparent plaques with a clear boundary in the double agar. The plaques were approximately 0.5–1.5 mm in diameter with a surrounding halo (2–3 mm in diameter; Figure 2A). Electron microscopy showed that PHB01 had an isometric polyhedral head approximately 55 nm in diameter and a short tail ~13 nm in length (Figure 2B). Based on these morphological characteristics and according to the latest International Committee on Taxonomy of Viruses (ICTV) classification, PHB01 was determined as a member of the subfamily Autographivirinae, family Podoviridae, and the order Caudovirales.
Figure 2.
Morphological characteristics of phage PHB01. (A) Plaques of phage PHB01 on Pasteurella multocida HND065; (B) Transmission electron micrograph of phage PHB01 (marked with white arrows).
3.2. Life Cycle Parameters
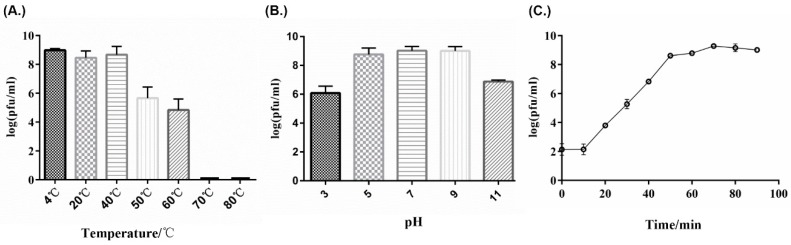
Thermolability tests showed that PHB01 was stable from 4 to 40 °C, but showed a titer reduction from 50 to 60 °C; moreover, the titer dramatically decreased (approximately 4.8 log units) from 60 to 70 °C (Figure 3A). For pH sensitivity, PHB01 was stable from pH 5.0 to 9.0, but the titer dropped approximately 3 and 2 log units at pH 3.0 and 11.0, respectively (Figure 3B). The one-step growth curve determination test showed that the entire life cycle of PHB01 consisted of an approximately 70-min infection process and an approximately 10-min eclipse period; the average burst size was 190 phage particles per infected cell after 70 min at 37 °C (Figure 3C).
Figure 3.
Biological properties of phage PHB01. (A) Sensitivity to temperature variations; (B) Sensitivity to pH variations; (C) One-step growth curve.
3.3. Host Range of PHB01
Host range tests showed that PHB01 was able to lyse most of the P. multocida capsular type D isolates (22 out of 37) tested; all of these sensitive isolates were non-toxigenic. A small number of non-toxigenic capsular type D isolates (4 out of 37) displayed resistance to PHB01 (Table 1). It is quite strange that PHB01 had no effect on the capsular type D isolates which produce toxin (Table 1). In addition, PHB01 also did not show activity on lysing P. multocida capsular type A and F isolates as well as the bacteria in other genus (E. coli, Salmonella spp., and B. bronchiseptica, Table 1).
Table 1.
Host range of phage PHB01 1.
| Strain | Strain (Genotype) | Isolated Locations | Efficiency of Plating (EOP) |
|---|---|---|---|
| 1 | Pasteurella multocida D strain HND065 | Henan, China | 1 |
| 2 | Pasteurella multocida D | Guangdong, China | <0.01 |
| 3 | Pasteurella multocida D | Hubei, China | 1.1 |
| 4 | Pasteurella multocida D | Anhui, China | 0.66 |
| 5 | Pasteurella multocida D | Henan, China | 1.33 |
| 6 | Pasteurella multocida D | Hubei, China | 1 |
| 7 | Pasteurella multocida D | Hubei, China | 0.5 |
| 8 | Pasteurella multocida D | Hubei, China | 0.06 |
| 9 | Pasteurella multocida D | Guangdong, China | <0.01 |
| 10 | Pasteurella multocida D | Hubei, China | <0.01 |
| 11 | Pasteurella multocida D | Hubei, China | 0.84 |
| 12 | Pasteurella multocida D | Anhui, China | 0.83 |
| 13 | Pasteurella multocida D | Fujian, China | <0.001 |
| 14 | Pasteurella multocida D | Guangdong, China | 0.25 |
| 15 | Pasteurella multocida D | Hubei, China | 1.5 |
| 16 | Pasteurella multocida D | Shanxi, China | 0.58 |
| 17 | Pasteurella multocida D | Shanghai, China | 0.5 |
| 18 | Pasteurella multocida D | Shanxi, China | 0.75 |
| 19 | Pasteurella multocida D | Hubei, China | 0.5 |
| 20 | Pasteurella multocida D | Hubei, China | 0.66 |
| 21 | Pasteurella multocida D | Hubei, China | <0.001 |
| 22 | Pasteurella multocida D | Guangdong, China | <0.001 |
| 23 | Pasteurella multocida D | Guangdong, China | - |
| 24 | Pasteurella multocida D | Anhui, China | - |
| 25 | Pasteurella multocida D | Shanxi, China | - |
| 26 | Pasteurella multocida D | Guangdong, China | - |
| 27 | Pasteurella multocida D | Unknown | - |
| 28 | Pasteurella multocida D | Unknown | - |
| 29 | Pasteurella multocida D | Unknown | - |
| 30 | Pasteurella multocida D | Unknown | - |
| 31 | Pasteurella multocida D | Unknown | - |
| 32 | Pasteurella multocida D | Unknown | - |
| 33 | Pasteurella multocida D | Unknown | - |
| 34 | Pasteurella multocida D strain HN06 | Hainan, China | - |
| 35 | Pasteurella multocida D | Hubei, China | - |
| 36 | Pasteurella multocida D | Guangdong, China | - |
| 37 | Pasteurella multocida D | Shanxi, China | - |
| 38 | Pasteurella multocida A | Beijing, China | - |
| 39 | Pasteurella multocida A | Hubei, China | - |
| 40 | Pasteurella multocida A | Hubei, China | - |
| 41 | Pasteurella multocida A | Hunan | - |
| 42 | Pasteurella multocida A | Sichuan | - |
| 43 | Pasteurella multocida A | Sichuan | - |
| 44 | Pasteurella multocida A | Guangdong | - |
| 45 | Pasteurella multocida A | Guangdong | - |
| 46 | Pasteurella multocida A strain HB01 | Hubei | - |
| 47 | Pasteurella multocida A strain HB03 | Hubei | - |
| 48 | Pasteurella multocida F strain HN07 | Henan | - |
| 49 | Escherichia coli DH5α | - | |
| 50 | Salmonella enterica serovar typhimurium ATCC14028 | - | |
| 51 | Salmonella enterica serovar choleraesuis | - | |
| 52 | Bordetella bronchiseptica | - | |
1 The EOP values were determined by calculating the ratio of plaque-forming units (PFUs) of each phage-susceptible strain to the PFUs of indicator strain (P. multocida HND065); toxigenic capsular type D strains were marked in bold; (-) indicates that no plaques were observed.
3.4. Genomic Characteristics of PHB01
PHB01 possessed a linear double-stranded DNA genome with a size of 37,287 bp in length, with a 223-bp terminal redundancy. The average G + C content of the genome sequence was approximately 40.7%. Prediction using Glimmer 3.0 identified 43 putative ORFs; of which 24 ORFs were assigned putative functions and the remaining 19 ORFs were annotated as hypothetical proteins (8 ORFs) and proteins with unknown functions (11 ORFs) (Table S1 in supplemental materials). The proteins with known functions participated in DNA packing and morphogenesis, lysis, replication, and regulation (Table S1 in supplemental materials). There were no transfer RNAs (tRNA) or other small RNA genes detected in the PHB01 genome sequence using tRNAscan-SE 2.0.
Phylogenetic analyses using the sequences of either the major capsid protein (Figure 4A), the DNA polymerases (Figure 4B), and/or the RNA polymerases (Figure 4C) showed that PHB01 belonged to subfamily Autographivirinae, and was closest to phage PHB02.
Figure 4.
Phylogenetic tree analysis based on the alignments of amino acid sequences of the major capsid protein ((A); Protein ID: ASD51044.1), the DNA polymerases ((B); Protein ID: ASD51034.1), and the RNA polymerases ((C); Protein ID: ASD51019.1) of the Autographivirinae subfamily phages from GenBank. Phage PHB01 is indicated by the black circle. The evolutionary trees were constructed using the neighbor-joining method with the Poisson correction model. One thousand bootstrap repetitions were performed. Sequences were aligned by the ClustalW package carried by MEGAX [35].
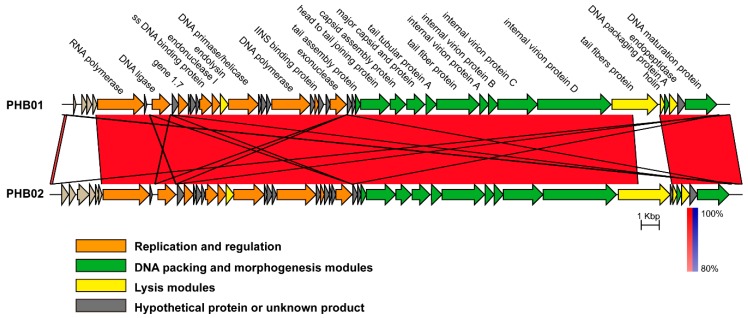
Sequence comparison showed that the average nucleotide identity between the genomes of PHB01 and PHB02 was 96.16% (calculated by ANI, http://enve-omics.ce.gatech.edu/ani/). PHB01 possessed a similar genetic structure as PHB02; most proteins encoded by the PHB02 genome were seen in PHB01 (Figure 5). With the exception of several hypothetical or function-unknown proteins, only a tail fiber protein (gp17) displayed a level of difference between the two phages (Figure 4). Compared to the tail fiber protein encoded by PHB02, the protein encoded by PHB01 had an insertion of 18 amino acids at the N-terminal and multiple amino acids deletion and change at its C-terminal (Figure S1 in supplemental materials).
Figure 5.
A co-linearity comparison diagram of the genomic organization at the nucleotide level between Pasteurella phages PHB01 and PHB02. The figure was generated via Easyfig v.2.0. The color code refers to the BLASTn identity of those regions between genomes. Arrows represent putative CDSs encoded by different genomes.
3.5. Acute Toxicity
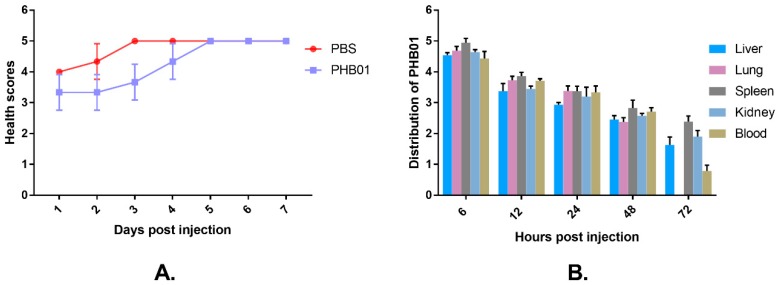
After an injection of PHB01 at a dose of 1.0 × 108 PFU, the mice did not show any abnormal behavior and/or appearance when compared to the control mice (Figure 6A). The amounts of PHB01 in livers (pfu/g), lungs (pfu/g), spleens (pfu/g), kidneys (pfu/g), and blood (pfu/mL) of the injected mice decreased as time passed; no phages were detected in the lung of the injected mice at 72 h post injection (Figure 6B). In addition, none of the mice injected with the phage showed any changes in the eosinophils or basophils or other pathological changes among the main organs when compared with the control mice (Figure 7).
Figure 6.
Safety test of PHB01 on mice. (A) Health scores given to the mice in different groups; (B) Distribution of phage PHB01 on the main organs of the mice. Data are presented as the mean ± SD.
Figure 7.
Histopathological analysis of the main organs collected from the mice received a challenge of phage PHB01 and/or PBS. The liver, spleen, kidney, and lungs were fixed with 4% formalin. Tissue sections were stained with hematoxylin and eosin or toluidine blue.
3.6. Therapeutic Effect of the Phage
To test the therapeutic effect of PHB01, we challenged mice with both the host strain of PHB01 and a non-toxigenic P. multocida capsular type D virulent isolate HN05 and then treated the infected mice with either the phage or PBS (Figure 1). Challenged mice treated with PBS (Groups I and IV) showed severe clinical signs (Figure 8A). Over 80% of the mice in these two groups died within five days post challenge (Figure 8B). In contrast, those challenged-mice treated with PHB01 (Groups II and V) showed much milder signs and a significantly increased survival rate (p ≤ 0.05) (Figure 8A,B). There were no observed clinical signs and no deaths recorded for the PHB01 (Group III) and/or PBS (Groups VI) control groups (Figure 8A,B).
Figure 8.
Protective effects of the phage PHB01 in mice challenged with wild-type P. multocida strain HND065 and HN05. (A) Health scores given to the mice in different groups. The total score for the health status of each group was recorded at least three times per day. The data are expressed as the mean ± SD; (B) Survival curve of the mice in each of the groups during the experiment.
4. Discussion
It has been more than 135 years since P. multocida was first shown to be the causative agent of fowl cholera by Louis Pasteur in 1881, and many studies on P. multocida have been published. However, the history on the study of P. multocida phages is relatively short, and only a few reports regarding P. multocida phages are available. To the best of our knowledge, the first paper involved in the P. multocida phage was published in 1956 [16,37], but it was not until 2006, when the first temperate transducing phage for P. multocida, designated F108, was sequenced and its whole genome sequence was published [19]. Since then, there have been no reports on P. multocida phages for 12 years. In 2018, the first complete genome sequences of lytic bacteriophages for P. multocida capsular types A and B were reported [17,18]. However, there is still a lack of reporting on lytic bacteriophages for P. multocida strains of other capsular types (D, E, and F). In the present study, the lytic bacteriophage specific for P. multocida capsular type D was isolated, characterized, and sequenced for the first time, which helps us understand more about P. multocida phages.
It has been reported that more than 95% of all phages described in the literature belong to the order Caudovirales (tailed ds-DNA phages), which comprises three families: the families Myoviridae (viruses with contractile tails), Siphoviridae (viruses with long, noncontractile tails), and Podoviridae (viruses with short noncontractile tails) [18,38]. Viruses in different families have icosahedral or oblate heads, but differ in the length and contractile abilities of their tails [38]. Electron microscopy showed that the appearance of PHB01 was composed of an isometric polyhedral head approximately 55 nm in diameter and a short tail ~13 nm in length (Figure 2B). These morphological characteristics are quite similar to phage PHB02, a lytic P. multocida phage belonging to the family Podoviridae in the order Caudovirales [17], but differ from the reported Pasteurella phages in family Siphoviridae, which have long noncontractile tails (≥100 nm) [18,39,40] and/or in the family Siphoviridae with long, contractile tails (≥100 nm) [19]. These findings suggest that PHB01 is a member of the family Podoviridae in the order Caudovirales.
PHB01 and PHB02 are also genetically and phylogenetically related. Phylogenetic analysis using the sequences of either the major capsid protein, the DNA polymerases, and/or the RNA polymerases showed that PHB01 was closest to PHB02 (Figure 4A–C). Comparative genomics analysis showed that PHB01 shared a similar genetic structure to PHB02; most of the function-known proteins encoded by PHB01 and PHB02 were the same, with the exception of the tail fiber protein (gp17) (Figure 5). It has been reported that Caudovirales phages use the tail fiber protein to recognize and attach to the bacterial surface at the early stage of infection by specifically digesting the polysaccharides, the primary receptors for phages [41,42,43,44,45]. It is also known that PHB01 and PHB02 are specific for P. multocida capsular type D and A strains, respectively [18], and P. multocida strains of capsular type D and A produce different polysaccharides [46]. Therefore, the different tail fiber proteins encoded by PHB01 and PHB02 might be associated with the host specificity of the two phages. Only when more phage genomes from different P. multocida capsular types become available can we finally confirm this suggestion.
Host range tests showed that PHB01 displayed good specificity for killing P. multocida capsular type D isolates (Table 1). However, some type D isolates, particularly the toxigenic type D isolates, were highly resistant to PHB01 (Table 1). It has been documented that bacteria resist phages by preventing phage adsorption, preventing phage DNA entry, cutting phage nucleic acids, or by a wide range of heterologous proteins that provide resistance through the abortion of phage infection (known as the abortive infection systems) [47]. In the next step, we will undertake further study to find out about such mechanisms in those type D isolates. In vivo tests using mouse models showed that the administration of PHB01 was safe for the mice and had a good effect on treating the mice infected with different P. multocida strains including virulent strain HN05 (Figure 8A,B). These findings suggest that PHB01 has a potential use in therapy against P. multocida infections.
5. Conclusions
A novel lytic bacteriophage PHB01 specific for P. multocida type D was characterized in the present study. Both morphological and genetical analysis indicated that this phage was a member of subfamily Autographivirinae, family Podoviridae, and order Caudovirales. However, PHB01 did not belong to any genera previously identified in subfamily Autographivirinae, and should be assigned into a new genus. In addition, PHB01 displayed good ability on killing most of P. multocida type D strains, and it also had good therapeutic effect on the infections caused by P. multocida type D. This phage has a potential application against P. multocida capsular type D.
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4915/11/1/86/s1, Figure S1: Sequence comparison of the tail fiber protein encoded by PHB01 and PHB02, Table S1: The annotation of the phage PHB01 genome.
Author Contributions
Conceptualization, Y.C., Z.P. and B.W.; Methodology, Y.C., G.G., E.S., J.S., L.Y., L.Z., W.L., L.H., and Z.P.; Validation, Z.P., B.W., and H.C.; Formal analysis, Z.P.; Writing—original draft preparation, Z.P. and Y.C.; Writing—review and editing, Z.P., X.T., B.W., and H.C.; Visualization, W.L. and Z.P.; Supervision, Z.P. and B.W.; Project administration, Z.P. and B.W.; Funding acquisition, H.C.
Funding
This research was funded by the Agricultural Science and Technology Innovation Program of Hubei Province, the Ministry of Science and Technology of the Peoples’ Republic of China (grant numbers 2018YFD0500804 and 2018YFD0500204), and the National Natural Science Foundation of China (NSFC, grant number 31421064).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Carter G.R. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am. J. Vet. Res. 1955;16:481–484. [PubMed] [Google Scholar]
- 2.Wilkie I.W., Harper M., Boyce J.D., Adler B. Pasteurella multocida: Diseases and pathogenesis. Curr. Top. Microbiol. Immunol. 2012;361:1–22. doi: 10.1007/82_2012_216. [DOI] [PubMed] [Google Scholar]
- 3.Katechakis N., Maraki S., Dramitinou I., Marolachaki E., Koutla C., Ioannidou E. An unusual case of Pasteurella multocida bacteremic meningitis. J. Infect. Public Health. 2018 doi: 10.1016/j.jiph.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Abreu F., Rodriguez-Lucas C., Rodicio M.R., Vela A.I., Fernandez-Garayzabal J.F., Leiva P.S., Cuesta F., Cid D., Fernandez J. Human Pasteurella multocida Infection with Likely Zoonotic Transmission from a Pet Dog, Spain. Emerg. Infect. Dis. 2018;24:1145–1146. doi: 10.3201/eid2406.171998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pak S., Valencia D., Decker J., Valencia V., Askaroglu Y. Pasteurella multocida pneumonia in an immunocompetent patient: Case report and systematic review of literature. Lung India. 2018;35:237–240. doi: 10.4103/lungindia.lungindia_482_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maleb A., Elmalki J., Bouayadi O., Ben Lahlou Y., Frikh M., Abdeljaouad N., Lemnouer A., Yacoubi H., Elouennass M. Serious phlegmonous lesion of the hand following an injury by vegetal thorn: Never forget Pasteurella multocida! Trauma Case Rep. 2018;13:18–21. doi: 10.1016/j.tcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang J., Kim S.H., Yoo G., Hwang G.Y., Uh Y., Yoon K.J. First Case of Pasteurella multocida Pneumonic Bacteremia in Korea. Ann. Lab. Med. 2018;38:490–491. doi: 10.3343/alm.2018.38.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson B.A., Ho M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013;26:631–655. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Z., Liang W., Wang F., Xu Z., Xie Z., Lian Z., Hua L., Zhou R., Chen H., Wu B. Genetic and Phylogenetic Characteristics of Pasteurella multocida Isolates From Different Host Species. Front. Microbiol. 2018;9:1408. doi: 10.3389/fmicb.2018.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubatzky K.F. Pasteurella multocida and immune cells. Curr. Top. Microbiol. Immunol. 2012;361:53–72. doi: 10.1007/82_2012_204. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasiah S., Bhavsar J., Thapar K., Liles M., Schoenfeld T., Wommack K.E. Phages across the biosphere: Contrasts of viruses in soil and aquatic environments. Res. Microbiol. 2008;159:349–357. doi: 10.1016/j.resmic.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Can K., Aksu U., Yenen O.S. Investigation of PhiKZ phage therapy against Pseudomonas aeruginosa in mouse pneumonia model. Turk. J. Med. Sci. 2018;48:670–678. doi: 10.3906/sag-1711-22. [DOI] [PubMed] [Google Scholar]
- 13.Akusobi C., Chan B.K., Williams E., Wertz J.E., Turner P.E. Parallel Evolution of Host-Attachment Proteins in Phage PP01 Populations Adapting to Escherichia coli O157:H7. Pharmaceuticals. 2018;11:60. doi: 10.3390/ph11020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Euler C.W., Delaune A., Fischetti V.A. Using a Novel Lysin To Help Control Clostridium difficile Infections. Antimicrob. Agents Chemother. 2015;59:7447–7457. doi: 10.1128/AAC.01357-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao F., Wang X., Wang L., Li Z., Che J., Wang L., Li X., Cao Z., Zhang J., Jin L., et al. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. Biomed. Res. Int. 2015;2015:752930. doi: 10.1155/2015/752930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchner C., Eisenstark A. Lysogeny in Pasteurella multocida. Am. J. Vet. Res. 1956;17:547–548. [PubMed] [Google Scholar]
- 17.Chen Y., Sun E., Song J., Yang L., Wu B. Complete Genome Sequence of a Novel T7-Like Bacteriophage from a Pasteurella multocida Capsular Type A Isolate. Curr. Microbiol. 2018;75:574–579. doi: 10.1007/s00284-017-1419-3. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi S., Saxena H.M., Imam N., Kashoo Z., Sharief Banday M., Alam A., Malik M.Z., Ishrat R., Bhat B. Isolation and genome analysis of a lytic Pasteurella multocida Bacteriophage PMP-GAD-IND. Lett. Appl. Microbiol. 2018;67:244–253. doi: 10.1111/lam.13010. [DOI] [PubMed] [Google Scholar]
- 19.Campoy S., Aranda J., Alvarez G., Barbe J., Llagostera M. Isolation and sequencing of a temperate transducing phage for Pasteurella multocida. Appl. Environ. Microbiol. 2006;72:3154–3160. doi: 10.1128/AEM.72.5.3154-3160.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillol-Salom A., Martinez-Rubio R., Abdulrahman R.F., Chen J., Davies R., Penades J.R. Phage-inducible chromosomal islands are ubiquitous within the bacterial universe. ISME J. 2018 doi: 10.1038/s41396-018-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang H.C., Chen C.R., Lin J.W., Shen G.H., Chang K.M., Tseng Y.H., Weng S.F. Isolation and characterization of novel giant Stenotrophomonas maltophilia phage phiSMA5. Appl. Environ. Microbiol. 2005;71:1387–1393. doi: 10.1128/AEM.71.3.1387-1393.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chibani-Chennoufi S., Sidoti J., Bruttin A., Kutter E., Sarker S., Brussow H. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: Implications for phage therapy. Antimicrob. Agents Chemother. 2004;48:2558–2569. doi: 10.1128/AAC.48.7.2558-2569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M., Xu J., Yao H., Lu C., Zhang W. Isolation, genome sequencing and functional analysis of two T7-like coliphages of avian pathogenic Escherichia coli. Gene. 2016;582:47–58. doi: 10.1016/j.gene.2016.01.049. [DOI] [PubMed] [Google Scholar]
- 24.Ul Haq I., Chaudhry W.N., Andleeb S., Qadri I. Isolation and partial characterization of a virulent bacteriophage IHQ1 specific for Aeromonas punctata from stream water. Microb. Ecol. 2012;63:954–963. doi: 10.1007/s00248-011-9944-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Cai L., Ma R., Xu Y., Tong Y., Huang Y., Jiao N., Zhang R. A novel roseosiphophage isolated from the oligotrophic South China Sea. Viruses. 2017;9:109. doi: 10.3390/v9050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen K.C., Hair B.B., Wienclaw T.M., Murdock M.H., Hatch J.B., Trent A.T., White T.D., Haskell K.J., Berges B.K. Isolation and Host Range of Bacteriophage with Lytic Activity against Methicillin-Resistant Staphylococcus aureus and Potential Use as a Fomite Decontaminant. PLoS ONE. 2015;10:e0131714. doi: 10.1371/journal.pone.0131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Chen Y., Shi C., Huang Z., Zhang Y., Li S., Li Y., Ye J., Yu C., Li Z., et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. 2018;7:1–6. doi: 10.1093/gigascience/gix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R., Zhu H., Ruan J., Qian W., Fang X., Shi Z., Li Y., Li S., Shan G., Kristiansen K., et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20:265–272. doi: 10.1101/gr.097261.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R., Li Y., Kristiansen K., Wang J. SOAP: Short oligonucleotide alignment program. Bioinformatics. 2008;24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 30.Li S., Fan H., An X., Fan H., Jiang H., Chen Y., Tong Y. Scrutinizing virus genome termini by high-through put sequencing. PLoS ONE. 2014;9:e85806. doi: 10.1371/journal.pone.0085806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delcher A.L., Bratke K.A., Powers E.C., Salzberg S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe T.M., Chan P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016;44:W54–W57. doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L., Li D., Li X., Hu L., Cheng M., Xia F., Gong P., Wang B., Ge J., Zhang H., et al. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci. Rep. 2016;6:29344. doi: 10.1038/srep29344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Sun E., Yang L., Song J., Wu B. Therapeutic Application of Bacteriophage PHB02 and its Putative Depolymerase Against Pasteurella multocida Capsular Type A in Mice. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu S., Le S., Tan Y., Zhu J., Li M., Rao X., Zou L., Li S., Wang J., Jin X., et al. Genomic and proteomic analyses of the terminally redundant genome of the Pseudomonas aeruginosa phage PaP1: Establishment of genus PaP1-like phages. PLoS ONE. 2013;8:e62933. doi: 10.1371/journal.pone.0062933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackermann H.W., Karaivanov L. Morphology of Pasteurella multocida bacteriophages. Can. J. Microbiol. 1984;30:1141–1148. doi: 10.1139/m84-179. [DOI] [PubMed] [Google Scholar]
- 40.Kolaskar A.S., Tongaonkar P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–174. doi: 10.1016/0014-5793(90)80535-Q. [DOI] [PubMed] [Google Scholar]
- 41.Yan J., Mao J., Xie J. Bacteriophage polysaccharide depolymerases and biomedical applications. BioDrugs Clin. Immunother. Biopharma. Gene Ther. 2014;28:265–274. doi: 10.1007/s40259-013-0081-y. [DOI] [PubMed] [Google Scholar]
- 42.Leiman P.G., Battisti A.J., Bowman V.D., Stummeyer K., Muhlenhoff M., Gerardy-Schahn R., Scholl D., Molineux I.J. The structures of bacteriophages K1E and K1-5 explain processive degradation of polysaccharide capsules and evolution of new host specificities. J. Mol. Biol. 2007;371:836–849. doi: 10.1016/j.jmb.2007.05.083. [DOI] [PubMed] [Google Scholar]
- 43.Leiman P.G., Molineux I.J. Evolution of a new enzyme activity from the same motif fold. Mol. Microbiol. 2008;69:287–290. doi: 10.1111/j.1365-2958.2008.06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Koc C., Kuhner P., Stierhof Y.D., Krismer B., Enright M.C., Penades J.R., Wolz C., Stehle T., Cambillau C., et al. An essential role for the baseplate protein Gp45 in phage adsorption to Staphylococcus aureus. Sci. Rep. 2016;6:26455. doi: 10.1038/srep26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latka A., Maciejewska B., Majkowska-Skrobek G., Briers Y., Drulis-Kawa Z. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl. Microbiol. Biotechnol. 2017;101:3103–3119. doi: 10.1007/s00253-017-8224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyce J.D., Chung J.Y., Adler B. Pasteurella multocida capsule: Composition, function and genetics. J. Biotechnol. 2000;83:153–160. doi: 10.1016/S0168-1656(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 47.Labrie S.J., Samson J.E., Moineau S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010;8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.