Abstract
Chemical investigation of MeOH extract of a South China Sea sponge Cacospongia sp. yielded 15 terpenoids belonging to three different skeleton-types, including the unusual C17 γ-lactone norditerpenoids (1–3), the rare C21 pyridine meroterpenoid (7), and the notable C25 manoalide-type sesterterpenoids (4–6, 8–10). Compounds 1–5 were initially obtained as enantiomers, and were further separated to be optically pure compounds (1a, 1b, 2a, 2b, 3a-r, 3b-r, 4a, 4b, 5a and 5b) by chiral HPLC, with a LiAlH4 reduction aid for 3. Compounds 3a/3b (a pair of inseparable enantiomers), 4a, 5a, 6, and 7 were identified as new compounds, while 1a/1b and 2a/2b were obtained from a natural source and were determined for their absolute configurations for the first time. This is also the first time to encounter enantiomers of the well-known manoalide-type sesterterpenoids from nature. The structures with absolute configurations of the new compounds were unambiguously determined by comprehensive methods including HR-ESI-MS and NMR data analysis, optical rotation comparison, experimental and calculated electronic circular dichroism (ECD), and Mo2(OAc)4 induced circular dichroism (ICD) methods. The cytotoxicity of the isolates against selected human tumor cell lines was evaluated, however, the tested compounds showed no activity against selected cell lines.
Keywords: marine sponge, Cacospongia, terpenoids
1. Introduction
Linear terpenoids, represented by linear furano- and pyrrolo-terpenoids, are a unique family of natural products with widespread bioactivities [1,2]. Among all the isolated linear terpenoids, C25 linear sesterterpenoids are the major components with more than 200 compounds been isolated, such as nitropyrrolins, heronapyrroles, fukanedones, and manoalide-type sesterterpenoids [3,4,5,6,7]. Inspiringly, the manoalide-type C25 sesterterpenoids, which are frequently obtained from marine sponges, have been studied as topical antipsoriatic lead drug candidates for their potent anti-inflammatory activity [8,9]. Apart from the common C15 sesquiterpenoids, C20 diterpenoids, and C25 sesterterpenoids, a few linear terpenoids take irregular C17 or C21 carbon skeletons [1,2,3,4,5,6,7]. To the best of our knowledge, there are only four C17 terpenoids been isolated from red algae Laurencia viridis [10], marine spong Fasciospongia cavernosa [11], and Chloranthaceae plant Chloranthus sessilifolius [12]. Only one of them takes the linear structure. C21 linear terpenoids are also a rare class of natural products which are mainly isolated from marine sponges of the genera Cacospongia, Carteriospongia, Dysidea, Fasciospongia, Hippospongia, Leiosella, Spirastrella, and Spongia [1,2].
Marine sponges of the genus Cacospongia (order Dictyoceratida, family Thorectidae) draw much attention for its production of diverse terpenoids [13], such as C21-difuran terpenoid cacospongienone A from C. scalaris [14], manoalide-type sesterterpenoid cacospongionolide from C. mollior [15], and tetracyclic scalarane-type sesterterpenoid scalarin from C. scalaris [16]. However, only four out of the total 17 identified Cacospongia species have been studied for their chemical constituents. Besides, the unidentified species of Cacospongia (Cacospongia sp.) with potential morphological challenge are found to be significant resource for novel terpenoids. For example, two new terpenoids, namely cacofuran and (+)-isojaspic acid with novel bridged tricyclic carbon skeletons were isolated from two unidentified Cacospongia species collected from Okinawa and Papua New Guinea, respectively [17,18]. Furthermore, the Cacospongia derived terpenoids usually exhibit excellent pharmacological potentials, such as antibacterial activity, anti-inflammatory, and cytotoxicity [13,14,15,16,17,18].
In the course of our continuing search for new bioactive metabolites from Xisha Island sponges [19,20], an unidentified Cacospongia species was collected and investigated for its chemical components, yielding three pairs of rare C17 γ-lactone norditerpenoid enantiomers (1a, 1b, 2a, 2b and the inseparable 3a/3b), an infrequent C21 pyridine terpenoid (7), and six notable C25 manoalide-type sesterterpenoids (4–6, 8–10) including two pairs of enantiomers (4a, 4b, 5a and 5b) (Figure 1). Among them, (±)-8,13-secoepicavernosine (3a/3b), (+)-hippolide E (4a), (+)-(6E)-neomanoalide (5a), (3R,4R)-14,18-secoluffariolide C (6), and cacospongine A (7) were identified as new compounds, while the enantiomers 1a/1b and 2a/2b were separated by chiral HPLC. This is the first time to obtain manoalide-type enantiomers from nature. The structures with absolute configurations of the new compounds were unambiguously elucidated by a combinatorial methods, including HR-ESI-MS, 1D and 2D NMR spectra analysis, optical rotation comparison, experimental and calculated ECD comparison, and Mo2(OAc)4 induced circular dichroism (ICD) method. Furthermore, cytotoxicity of these compounds was tested against selected tumor cell lines. Herein, we report the isolation, chiral resolution, structure elucidation, and bioactivity of the isolated linear terpenoids.
Figure 1.
Structures of compounds 1–10.
2. Results and Discussion
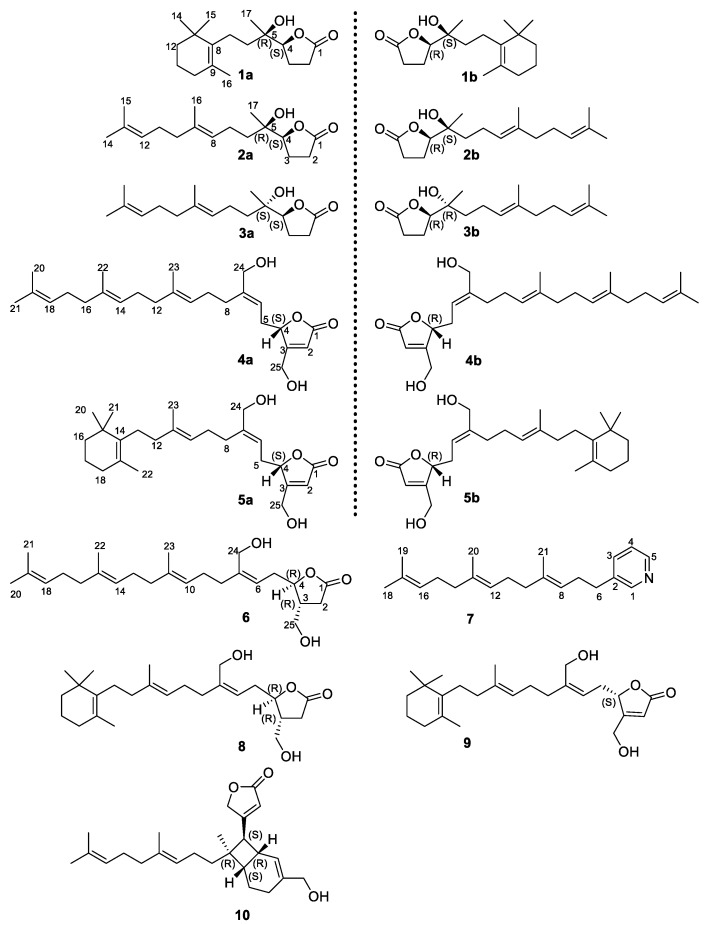
Compound 1 was obtained as colorless oil, and its molecular formula was determined as C17H28O3 by HR-ESI-MS (m/z 303.1936, [M+Na]+, calcd 303.1931, Supplementary Figure S8). A comparison of the 1H and 13C NMR data (Table 1) with literature suggested 1 to be the reported cavernosine definitely [11]. However, compound 1 showed neither optical activity () nor Cotton effect on its CD spectra, just as cavernosine did in the original report () [11], which suggested 1 to be a racemic mixture. Then, chiral HPLC resolution of 1 afforded a pair of stereoisomers 1a () and 1b () with a ratio of approximately 1:1 (Supplementary Figure S3). The relative configuration of the C-4–C-5 fragment in 1a and 1b were both assigned as erythro type (4S*,5R*) by comparing the 1H and 13C NMR data (Table 1, Supplementary Figures S9 and S10) with those of the synthesized erythro (±)-cavernosine and threo (±)-epicavernosine [21]. The absolute configurations of 1a and 1b were established by ECD methods. According to literature, the γ-chiral center of a saturated γ lactone would induce a Cotton effect at 213 nm, which is mainly attributed to the πxπx* electronic transition of the carbonyl group in the γ-lactone ring [22]. Accordingly, 1a and 1b were found to take a negative and positive Cotton effects at 213 nm, respectively. Thus, 1a and 1b were proposed to have the 4S and 4R absolute configurations [22]. The result was further confirmed by a comparison of the experimental ECD spectra with the calculated curves of the four possible candidate stereoisomers of 1. The calculated ECD data suggested that the valuence of the Cotton effect at 213 nm was solely related to the absolute configuration of C-4 (negative-4S, positive-4R) (Figure 2), while the Cotton effect intensity could be affected by the auxochromic hydroxy group at C-5. Finally, the absolute configurations of 1a [(+)-cavernosine] and 1b [(−)-cavernosine] were assigned as 4S,5R and 4R,5S, respectively. This is the first time to separate enantiomers of cavernosine and to determine their absolute configurations.
Table 1.
1H and 13C NMR Data for Compounds 1–3 in CDCl3 (500 MHz for 1H and 125 MHz for 13C).
| No. | 1a/1b | 2a/2b | 3a/3b | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 177.3, C | 177.5, C | 177.2, C | |||
| 2 | 28.9, CH2 | 2.56, m | 28.8, CH2 | 2.51, m | 29.0, CH2 | 2.56, m |
| 3 | 21.8, CH2 | 2.15, m; 2.27, m | 21.7, CH2 | 2.11, m; 2.22, m | 22.2, CH2 | 2.26, m; 2.16, m |
| 4 | 85.6, CH | 4.35, t (7.6) | 85.6, CH | 4.31, t (7.5) | 85.0, CH | 4.37, t (7.7) |
| 5 | 73.0, C | 72.6, C | 73.0, C | |||
| 6 | 36.9, CH2 | 1.41, m | 36.9, CH2 | 1.38, m; 1.48, m | 39.2, CH2 | 1.60, m; 1.68, m |
| 7 | 21.9, CH2 | 1.89, t (6.2) | 21.6, CH2 | 2.02, m; 2.11, m | 22.2, CH2 | 2.10, m; 2.05, m |
| 8 | 136.2, C | 123.5, CH | 5.08, t (7.1) | 123.6, CH | 5.12, t (6.6) | |
| 9 | 127.5, C | 135.6, C | 135.9, C | |||
| 10 | 32.7, CH2 | 2.00, td (13.0, 5.6) 2.15, m | 39.5, CH2 | 1.94, m | 39.6, CH2 | 1.98, m |
| 11 | 19.4, CH2 | 1.55, m; 1.68, m | 26.4, CH2 | 2.02, m | 26.6, CH2 | 2.05, m |
| 12 | 39.7, CH2 | 1.48, m; 1.55, m | 124.0, CH | 5.04, t (6.9) | 124.2, CH | 5.08, t (6.7) |
| 13 | 35.0, C | 131.2, C | 131.5, C | |||
| 14 | 28.6, CH3 | 0.99, s | 25.5, CH3 | 1.63, s | 25.7, CH3 | 1.68, s |
| 15 | 28.5, CH3 | 0.97, s | 17.5, CH3 | 1.56, s | 17.7, CH3 | 1.60, s |
| 16 | 19.7, CH3 | 1.58, s | 15.8, CH3 | 1.58, s | 16.0, CH3 | 1.62, s |
| 17 | 23.24, CH3 | 1.34, s | 23.0, CH3 | 1.26, s | 20.9, CH3 | 1.14, s |
Figure 2.
Experimental CD spectra of 1a and 1b overlaid with calculated spectra for the candidate stereostructures.
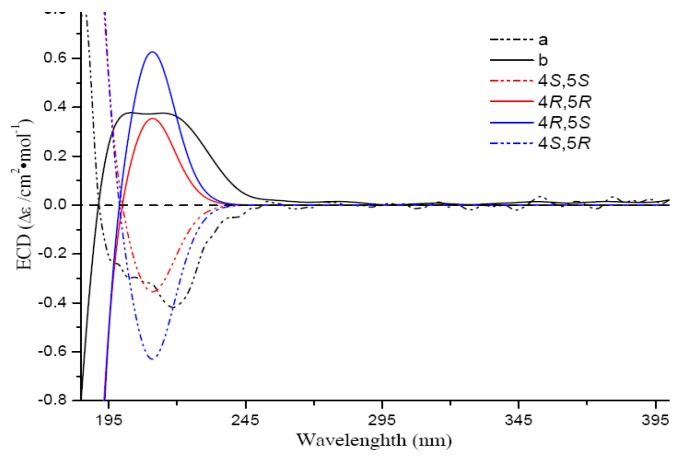
Compounds 2 and 3, having the same molecular formula of C17H28O3 with 1, were isolated as colorless oil. A comparison of the 1D NMR data of 2 with those of 1 (Table 1, Supplementary Figures S13–S15, S21 and S22) suggested that they both take the same γ-lactone ring and C-4-C-7 fragments. However, the rest fragment of 2 is obviously different with that of 1 according to their 1D data, especially the two methine signals (δH 5.08, t, J = 7.1 Hz and δH 5.04, t, J = 6.9 Hz) observed in 2. HMBC correlations from H3-14/H3-15 to two alkenyl carbon signals at δC 124.0 (C-12) and δC 131.2 (C-13) strongly suggested the formation of a double bound of C-12 = C-13 in 2 (Supplementary Figure S18). Thus compound 2 was determined as a 8,13-seco product of 1. Compound 2 has been synthesized as a side product [23]. The mostly identical 1D and 2D NMR data of 3 and 2 indicated that they both take the same planar structure. The slight difference of Me-17 in 2 and 3 (δH 1.14, δC 20.9 in 3 vs. δH 1.26, δC 23.0 in 2) suggested they may be a pair of epimers. Geometrical configurations of double bonds ∆8 in 2 and 3 were both deduced as E geometry, which was deduced from NOE correlations of H3-16/H2-7 and H2-10/H-8, and from the chemical shifts of C-16 (<20 ppm, a methyl resonance appearing at a chemical shift less than 20 ppm is indicative of an ‘E’ configuration, whereas a value larger than 20 ppm indicates a ‘Z’ configuration) [24]. Comparisons of chemical shifts of H3-17 in 2 (δH 1.02; C6D6) and 3 (δH 0.74; C6D6) with that of the synthesized erythro 8,13-secocavernosine (δH 1.08; C6D6) revealed 2 possessing a erythro-type relative configuration for the C-4–C-5 fragment [23]. While chemical shifts of Me-17 in 3 (δH 1.14 vs. δH 1.26 in 2; CDCl3) was in agreement with that of the synthesized threo-type analog [(S)-5-((S)-2-hydroxy-6-methylhept-5-en-2-yl)-dihydrofuran-2(3H)-one] (δH 1.16; CDCl3) [25]. Thus, the relative configurations of 2 and 3 were assigned as erythro (4S*,5R*) and threo (4S*,5S*), respectively. Optical rotation value and ECD experiment disclosed that 2 and 3 were both optical inactive. Further chiral resolution of 2 afforded a pair of stereoisomers 2a () and 2b () (Supplementary Figure S4). The absolute configurations of 2a and 2b were established as 4S,5R and 4R,5S, respectively, by experimental and calculated ECD spectra comparison (Figure 3) Thus, 2a and 2b were finally established as (+)-8,13-secocavernosine and (−)-8,13-secocavernosine, respectively.
Figure 3.
Experimental CD spectra of 2a and 2b overlaid with calculated spectra for the candidate stereostructures.
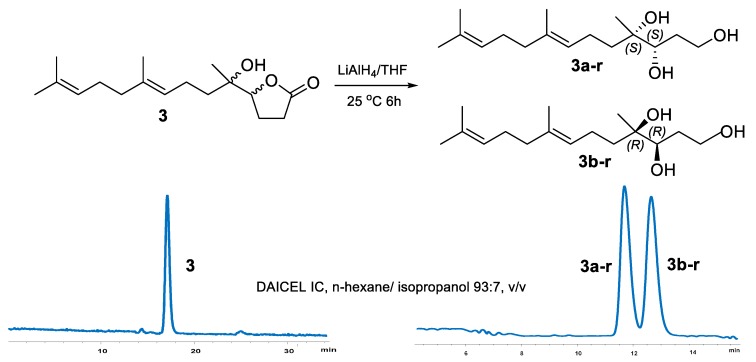
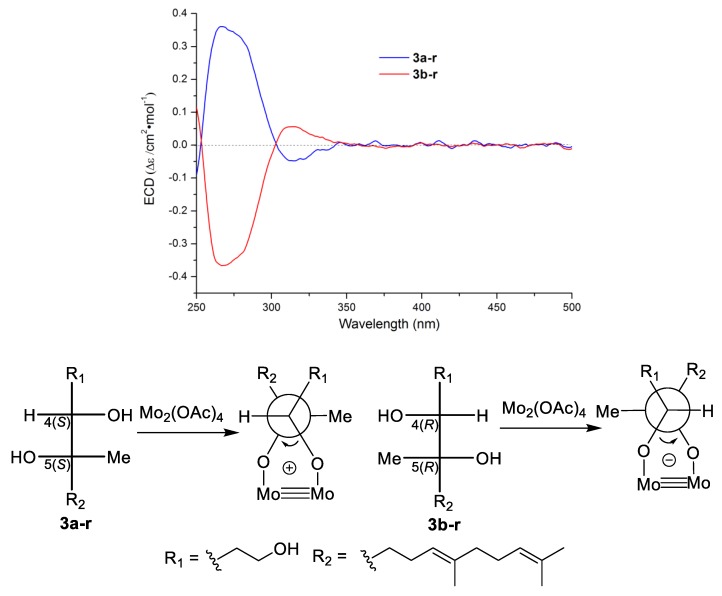
However, the optical inactive compound 3 could not be further separated by chiral HPLC (Supplementary Figure S5). To address the absolute configuration of 3, several chemical tailoring reactions were tried. During the NaOH hydrolysis of 3, the hydrolytic product 3-h was unstable and spontaneously formed the original γ-lactone 3, even using CH2N2 as a protective agent for the carboxyl of 3-h (Supplementary Scheme S1). Finally, compound 3 was successfully reduced to acyclic 1,4,5-triol derivative using LiAlH4 (Scheme 1) [11]. The reduced product was further separated to be a pair of isomers 3a-r () and 3b-r (), with a ratio of 1:1 on chiral HPLC (Scheme 1). On the basis of Snatzke’s theory [26,27], Mo2(OAc)4 induced circular dichroism (ICD) spectra of acyclic adjacent diol substrate (relative configuration determined) could be used to established the absolute configurations of the diol fragment according to the key diagnostic Cotton effect at 310 nm (band IV). Thus, Mo2(OAc)4 ICD experiments was carried out, and a positive and a negative Cotton effect at 310 nm on the ICD spectra of 3a-r and 3b-r were observed, respectively. The absolute configurations of 3a-r and 3b-r were determined to be 4S,5S and 4R,5R (Figure 4), respectively [26,27]. This result confirmed 3 to be a pair of inseparable new enantiomers bearing the 4S,5S and 4R,5R absolute configurations.
Scheme 1.
LiAlH4 reduction and chiral resolution of compound 3.
Figure 4.
Mo2(OAc)4 complexes ICD of 3a-r and 3b-r, and absolute configuration analysis of 3a-r and 3b-r by Snatzke’s theory.
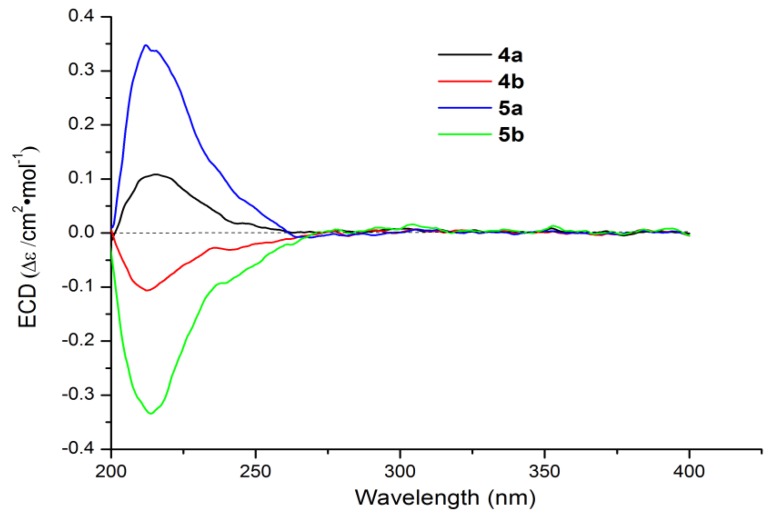
Compounds 4a/4b and 5a/5b, having the same molecular formula of C25H40O4 as deduced by HR-ESI-MS data (Supplementary Figures S27 and S31), were obtained as two pairs of enantiomers both with an approximate ratio of 1:3 (Supplementary Figure S7). Each pair of isomers possess the same NMR data (Table 2), while their optical rotations ( 4a + 4.1 vs. 4b − 7.4; 5a + 3.6 vs. 5b − 7.7) and ECD curves (Figure 5) are almost opposite. Comparisons of 1D NMR data of 4a/4b and 5a/5b (Table 2, Supplementary Figures S28–S30 and S32–S34) with those of literatures revealed their planar structure to be hippolide E and (E)-neomanoalide, respectively [28,29]. The absolute configurations of 4b [(−)-hippolide E] and 5b [(−)-(E)-neomanoalide] were assigned to be the same as hippolide E and (6E)-neomanoalide-24-ol [28,29] by CD spectra comparisons. Their enantiomers 4a [(+)-hippolide E] and 5a [(+)-(6E)-neomanoalide-24-ol] were both deduced to be 4S configuration according to their opposite CD absorptions (Figure 5) and opposite optical rotations comparing with 4b and 5b. Thus, 4a and 5a were identified as two new enantiomers of hippolide E and (E)-neomanoalide-24-ol, respectively. This is the first time to obtain the natural-occurring enantiomers of the well-known manoalide-type sesterterpenoids.
Table 2.
1H and 13C NMR Data for Compounds 4–7 (500 MHz for 1H and 125 MHz for 13C).
| No. | 4a/4b a | 5a/5b a | 6 a | 7 b | ||||
|---|---|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 172.2, C | 172.2, C | 176.0, C | 149.6, CH | 8.40, s | |||
| 2 | 116.3, CH | 6.05, s | 116.3, CH | 6.05 s | 31.7, CH2 | 2.39, dd (8.2, 17.1) 2.62, m | 137.0, C | |
| 3 | 170.8, C | 170.9, C | 41.4, CH | 2.50, m | 135.8, CH | 7.60, d (,7.6) | ||
| 4 | 81.5, CH | 5.07, t (5.6) | 81.5, CH | 5.07, t (5.3) | 82.8, CH | 4.44, dd (4.9, 10.4) | 123.2, CH | 7.27, dd (6.7, 5.6) |
| 5 | 30.5, CH2 | 2.72, m; 2.46, m | 30.5, CH2 | 2.72, m; 2.47, m | 32.5, CH2 | 2.57, m | 147.0, CH | 8.38, d (4.1) |
| 6 | 117.8, CH | 5.39, t (7.0) | 117.7, CH | 5.39 t (7.3) | 121.7, CH | 5.40, t (7.5) | 32.3, CH2 | 2.60, t (7.6) |
| 7 | 143.4, C | 143.4, C | 142.8, C | 29.0, CH2 | 2.25, q (7.3) | |||
| 8 | 28.5, CH2 | 2.12, m | 28.5, CH2 | 2.12, m | 35.8, CH2 | 2.17, m | 122.9, CH | 5.12, t (6.9) |
| 9 | 26.6, CH2 | 2.06, m | 27.8, CH2 | 2.03, m | 26.6, CH2 | 2.06, m | 135.5, C | |
| 10 | 123.3, CH | 5.11, m | 122.7, CH | 5.13, t (6.5) | 123.6, CH | 5.10, m | 39.66, CH2 | 1.92, m |
| 11 | 136.2, C | 137.1, C | 135.8, C | 25.99, CH2 | 2.01, m | |||
| 12 | 39.7, CH2 | 1.98, m | 39.8, CH2 | 2.01, m | 39.7, CH2 | 1.97, m | 123.8, CH | 5.05, m |
| 13 | 26.7, CH2 | 2.06, m | 26.7, CH2 | 2.11, m | 26.7, CH2 | 2.06, m | 134.3, C | |
| 14 | 124.0, CH | 5.10, m | 137.0, C | 124.1, CH | 5.10, m | 39.6, CH2 | 1.92, m | |
| 15 | 135.1, C | 127.0, C | 135.0, C | 26.6, CH2 | 2.01, m | |||
| 16 | 39.7, CH2 | 1.98, m | 32.8, CH2 | 1.90, t (6.1) | 39.7, CH2 | 1.98, m | 124.1, CH | 5.05, m |
| 17 | 26.8, CH2 | 2.06, m | 19.5, CH2 | 1.57, m | 26.7, CH2 | 2.15, m | 130.6, C | |
| 18 | 124.3, CH | 5.09, m | 40.2, CH2 | 1.41, m | 124.3, CH | 5.10, m | 25.5, CH3 | 1.63, s |
| 19 | 131.3, C | 35.0, C | 131.3, C | 17.5, CH3 | 1.54, s | |||
| 20 | 25.7, CH3 | 1.68, s | 28.6, CH3 | 0.99, s | 25.7, CH3 | 1.68, s | 15.7, CH3 | 1.54, s |
| 21 | 17.7, CH3 | 1.60, s | 28.6, CH3 | 0.99, s | 17.7, CH3 | 1.60, s | 15.7, CH3 | 1.47, s |
| 22 | 16.1, CH3 | 1.60, s | 19.8, CH3 | 1.63, s | 16.1, CH3 | 1.60, s | ||
| 23 | 16.0, CH3 | 1.60, s | 16.1, CH3 | 1.60, s | 16.0, CH3 | 1.60, s | ||
| 24 | 66.4, CH2 | 4.06, s | 66.4, CH2 | 4.07, s | 60.2, CH2 | 4.08, d (11.9) 4.17, d (12.1) | ||
| 25 | 58.8, CH2 | 4.54, d (16.7); 4.45, d (16.5) | 58.8, CH2 | 4.55, d (16.8) 4.45, d (16.5) | 63.2, CH2 | 3.65, dd (7.2, 10.1) 3.75, dd (4.5, 10.6) | ||
a Recorded in CDCl3; b Recorded in DMSO-d6.
Figure 5.
Experimental CD spectra for 4a/4b and 5a/5b.
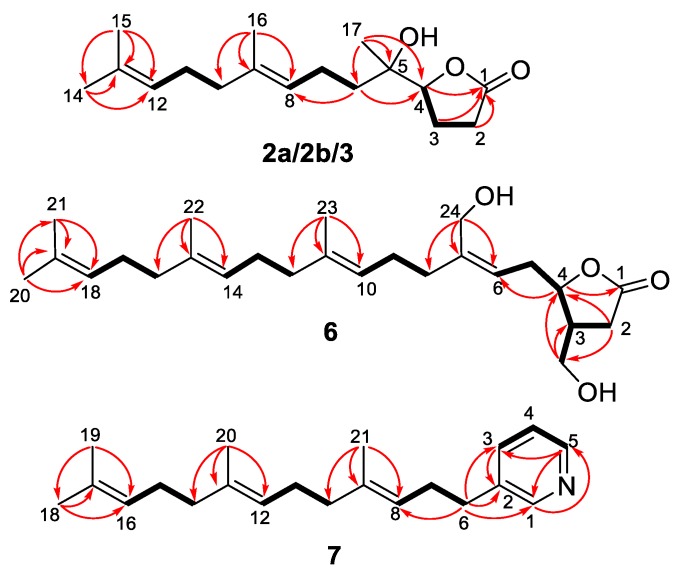
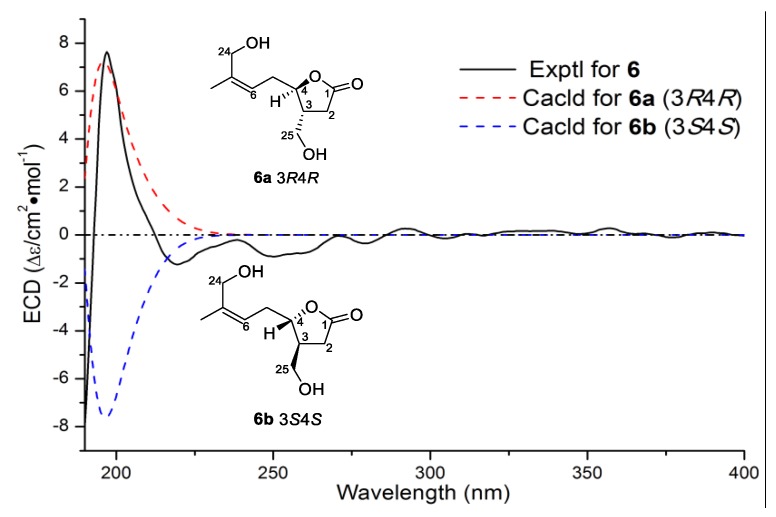
Compound 6, with the molecular formula of C25H40O4, was also considered as a manoalide-type sesterterpenoid according to its similar 1D NMR data with those of 4a/4b and 5a/5b (Table 2, Supplementary Figures S36–S38). Further 1D NMR data comparison of 6 with those of co-isolated luffariolide C (8) revealed their structural similarity, except for a slight difference of Me-20 (δH 1.68 in 6 vs. δH 0.99 in luffariolide C) and Me-21 (1.60 vs. 0.99) [30]. HMBC correlations from H3-20/H3-21 to the olefinic carbons of C-18 (δC 124.3) and C-19 (δC 131.3) (Figure 6, Supplementary Figure S41) suggested that the cyclohexene ring in luffariolide C was cleaved and formed an extra double bound of C-18=C-19 in 6. Further 2D NMR analysis, such as 1H-1H COSY correlations of H2-16/H2-17/H-18 and HMBC correlations from H3-22 to C-14/C-15/C-16, (Figure 6) confirmed the planar structure of 6 to be 14,18-seco luffariolide C. Double bounds of ∆10 and ∆14 were both deduced to be E geometry according to NOE correlations of H3-23/H2-9 and H3-22/H2-13 (Figure 7). While double bound of ∆6 was assigned as Z geometry by NOE correlation of H2-24/H2-5. Additionally, NOE correlations of H-3/H2-5 and H-4/H2-25 (Figure 7), as well as the coupling constant between H-3 and H-4 (J = 0 Hz) in 6, suggested it takes the same 3,4-trans relative configuration with luffariolide C. Moreover, the similar optical rotations of 6 () and luffariolide C () revealed the same 3R,4R absolute configurations. The result was further supported by experimental and theoretical ECD comparisons of the main chiral lactone structure in 6 (Figure 8). Thus, 6 was determined as (3R,4R)-14,18-secoluffariolide C.
Figure 6.
Key COSY (▬) and HMBC () correlations of 2a/2b, 3, 6, and 7.
Figure 7.
Key NOESY correlations of compounds 6.
Figure 8.
Experimental CD spectra of 6 overlaid with calculated spectra for the candidate stereostructures of the main chiral partial structure in 6.
The molecular formula of compound 7 was deduced as C21H31N by HR-ESI-MS at m/z 298.2532 ([M + H]+ calcd 298.2529, Supplementary Figure S43). 1H NMR spectrum of 7 showed four aromatic proton signals (δH 8.40, s; 8.38, d, J = 4.1 Hz; 7.60, d, J = 7.6 Hz; 7.27, dd, J = 6.7, 5.6 Hz). The remaining proton signals in upfield were similar with those of 6, especially the four olefinic methyls of H3-18 (δH 1.63 in 7 vs. 1.68 in 6), H3-19 (1.54 vs. 1.60), H3-20 (1.54 vs. 1.60) and H3-21 (1.47 vs. 1.60). 1H-1H COSY correlations of H2-6/H2-7/H-8, H2-10/H2-11/H-12 and H2-14/H2-15/H-16, together with HMBC correlations from H3-18/ H3-19 to C-16/C-17, from H3-20 to C-12/C-13/C-14, and from H3-21 to C-8/C-9/C-10, indicated a C16 prenyl chain (C-6-C-18). Additionally, a monosubstituted pyridine ring was constructed based on 1H-1H COSY correlations of H-3/H-4/H-5 and HMBC correlations from H-5 to C-1/C-3, from H-3 to C-2 and from H-1 to C-5 (Figure 6, Supplementary Figures S48 and S49). The C16 linear chain was connected with the pyridine ring at C-2 by HMBC correlations from H2-6 (δH 2.60, t, J = 7.6 Hz) to C-1 (δC 149.6), C-2 (δC 137.0) and C-3 (δC 135.8) (Figure 6). The E geometry of double bounds ∆8 and ∆12 were both deduced from the chemical shifts of C-21 and C-20 (δC both at 15.7, < 20 ppm) [24]. Thus compound 7 was determined as a C21 pyridine terpenoid and was named as cacospongine A.
The structures of the known analogs of luffariolide C (8) [30], (Z)-neomanoalide (9) [29] and hippolide J (10) [31] were determined by comparison of their 1D/2D NMR data, ESI-MS data and optical rotations with those of reported values.
The cytotoxicity of the isolates against human tumor cell lines of K562, HCT116, Hep3B, A-549, and Jurkat was evaluated, using MTT method [32] with adriamycin as positive control. However, all the tested compounds were inactive (IC50 > 10 μM).
3. Experimental Section
3.1. General Methods
Optical rotations were measured on a JASCO P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were measured on a Beckman DU640 spectrophotometer. ECD spectra were obtained on a Jasco J-815 CD spectrometer. IR spectra were recorded on a Nicolet Nexus 470 (FT-IR) spectrophotometer (Thermo Electron Co., Madison, WI, USA), KBr pellets. NMR spectra were recorded on a Bruker DRX-500MHz instrument (Bruker BioSpin GmbH Co., Rheinstetten, Germany), 500 MHz for 1H NMR and 125 MHz for 13C NMR in CDCl3; chemical shifts δ in ppm referred to the solvent peaks at δH 7.26 and δC 77.0 for CDCl3, δH 2.50 and C 39.5 for DMSO-d6 and δH 7.16 for C6D6, and coupling constant J in Hz. HR-ESI-MS were obtained from a Micromass Q-Tof Ultima GLOBAL GAA076 LC-mass spectrometer (Waters Corporation, Milford, MA, USA). HPLC separation was performed on an Agilent 1100 series instrument with DAD detector (Agilent technologies, Santa Clara, CA, USA), equipped with a semi-preparative reversed-phased column (YMC-packed C18, 5 μm, 250 × 10 mm, 1.5 mL/min) or an analytic chiral column DAICEL IC-3 (DAICEL chiral technologies, Shanghai, China). Precoated silica gel plates (GF254, Qingdao Marine Chemical Inc., Qingdao, China) were used for TLC analyses. Silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) was used for column chromatography (CC).
3.2. Animal Material
The sponge Cacospongia sp. is in irregular shape, with black-brownish color, and covered with a tight encrusting crust. There are irregularity distributed holes on the surface, wihch is thick and solid, and is in the dimensions of around 4 × 5 cm 6 × 8 cm (see Supplementary I. Photos of sponge specimen). The specimen was collected from the coral-reef regions of Yong Xing Island (16°50′ N, 112°20′ E) in the South China Sea, at a depth ranging from 18 to 25 m, in November 2010. The specimen, with a voucher of no. XS-2009-34, was frozen immediately at −20 °C until it was examined. The voucher was deposited at the School of Medicine and Pharmacy, Ocean University of China, China. The sponge was identified by Dr. N.J.d.V. (Naturalis Biodiversity Centre, Leiden, The Netherlands).
3.3. Extraction and Isolation
The frozen sponge (2.6 kg, wet weight) was minced and extracted with MeOH for three times (each time for one day) at room temperature (5 L × 3). The combined solution was evaporated in vacuum and desalinated for three times to yield a residue (56.0 g). The crude extract was then subjected to a reduced pressure silica gel column eluting with a step-by-step gradient elution of acetone-petroleum ether (from 0:1 to 1:0, v/v), to give six fractions. Fraction 2 was then further separated by silica gel column eluting with petroleum ether/acetone (2:1, 1:1, 0:1, v/v) to give subfractions F2-1–F2-5. F2-2 was further purified by semi-preparative HPLC with a mobile phase of MeOH/H2O (70:30, v/v) to give three pairs of isomer mixtures 1 (6.0 mg; tR 53.0 min), 2 (30.1 mg; tR 59.0 min) and 3 (4.1 mg; tR 56.0 min). These isomers were finally separated by chiral HPLC with a mobile phase of n-hexane/ isopropanol (93:7, v/v), and yielded 1a (2.3 mg; tR 15.0 min), 1b (2.4mg; tR 28.0 min), 2a (12.1 mg; tR 15.0 min), 2b (12.0 mg; tR 24.5 min), 3a/3b (enantiomeric mixture, 4.1 mg; tR 17.5 min). HPLC purification (85:15, MeOH/H2O, v/v) of F2-3 afforded compound 7 (6.1 mg; tR 37.0 min). Fraction 3 was chromatographed on another silica gel column to give two subfractions F3-1 and F3-2, which were found to contain terpenoid components by TLC analyses. Then F3-1 was further separated by HPLC (85:15, MeOH/H2O, v/v) to give 6 (2.4 mg; tR 42.0 min), 8 (1.5 mg; tR 37.0 min), 9 (4.6 mg; tR 56.0 min) and 10 (5.0 mg; tR 61.0 min). The two pairs of isomers of 4a (1.6 mg; tR 47.0 min)/4b (4.2 mg; tR 42.0 min) and 5a (1.5 mg; tR 49.0 min) /and 5b (4.3 mg; tR 44.0 min) were ultimately separated by chiral HPLC with a mobile phase of n-hexane/ethanol (94:6, v/v) from F3-2.
Cavernosine (1): colorless oil; (+)-cavernosine (1a), (c 0.1, MeOH); (−)-cavernosine (1b), (c 0.1, MeOH); IR (KBr) vmax 3479, 2926, 1779, 1450, 1374, 1191 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; HRESIMS m/z 303.1936 [M + Na]+ (calcd for C17H28O3Na, 303.1931).
8,13-secocavernosine (2): colorless oil; (+)-8,13-secocavernosine (2a): (c 0.1, MeOH); (−)-8,13-secocavernosine (2b), (c 0.1, MeOH); IR (KBr) vmax 3469, 2928, 1774, 1453, 1377, 1191 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; 1H NMR in C6D6 (500 MHz) δ 5.22 (t, J = 6.9 Hz, 1H), 5.16 (t, J = 6.9 Hz, 1H), 3.64 (t, J = 7.5 Hz, 1H), 1.68 (s, 3H), 1.57 (s, 3H), 1.57 (s, 3H), 1.02 (s, 3H); HRESIMS m/z 303.1938 [M + Na]+ (calcd for C17H28O3Na, 303.1931).
(±)-8,13-secoepicavernosine (3a/3b, unseparated): colorless oil (MeOH); (c 0.1, MeOH); IR (KBr) vmax 3473, 2919, 1774, 1649, 1456, 1379, 1190, 1004 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; 1H NMR in C6D6 (500 MHz) δ 5.25 (t, J = 6.9 Hz, 1H)), 5.20 (t, J = 7.0 Hz, 1H), 3.62 (t, J = 7.5 Hz, 1H), 1.70 (s, 3H), 1.61 (s, 3H), 1.59 (s, 3H), 0.74 (s, 3H); HRESIMS m/z 303.1936 [M + Na]+ (calcd for C17H28O3Na, 303.1931).
(+)-hippolide E (4a): colorless oil (MeOH); (c 0.1, MeOH); IR (KBr) vmax 3410, 2925, 2360, 2338, 1746, 1700, 1650, 1540, 1455, 1381 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 2; HRESIMS m/z 425.2671 [M + Na]+ (calcd for C25H38O4Na, 425.2662).
(+)-(6E)-neomanoalide (5a): colorless oil (MeOH); (c 0.1, MeOH); IR (KBr) vmax 3415, 2926, 2860, 2359, 2338, 1744, 1647, 1455, 1380, 1143, 1063 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 2; HRESIMS m/z 425.2674 [M + Na]+ (calcd for C25H38O4Na, 425.2662).
(3R,4R)-14,18-secoluffariolide C (6): colorless oil (MeOH); (c 0.1, MeOH); IR (KBr) vmax 3384, 2922, 2856, 2359, 2337, 1750, 1451, 1375, 1265, 1196, 1018 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 2; HRESIMS m/z 405.2995 [M + H]+ (calcd for C25H41O4, 405.2999).
Cacospongine A (7): colorless oil (MeOH); IR (KBr) vmax 2924, 2854, 2361, 2339, 1718, 1453, 1379, 1190 cm−1; 1H NMR (DMSO-d6, 500 MHz) and 13C NMR (DMSO-d6, 125 MHz), see Table 2; HRESIMS m/z 298.2532 [M + H]+ (calcd for C21H32N, 298.2529).
3.4. LiAlH4 Reduction of 3
The solution of 3 (3 mg) in dry THF (2 mL) containing LiAlH4 (3 mg) were stirred for 6 h at room temperature, under the protection of argon atmosphere. The reduction reaction was quenched by addition of 2 mL of 10% KOH aqueous solution, and the mixture was then extracted by EtOAc to yielded crude products (2.7 mg) [11]. The isomer mixtures were further separated by chiral HPLC (n-hexane/ isopropanol, 93:7, v/v) to give 3a-r (1.3 mg; tR 11.7 min) and 3b-r (1.2 mg; tR 12.7 min).
Compounds3a-r and 3b-r: colorless oil from MeOH; for 3a-r and +11.1 for 3b-r (c 0.1, MeOH); 1H NMR (CDCl3, 500 MHz) δ 5.14 (1H, t, J = 6.7 Hz, H-8), 5.08 (1H, t, J = 6.7 Hz, H-12), 3.70 (2H, m, H2-1), 3.46 (1H, d, J = 10.9 Hz, H-4), 2.11 (1H, m, H-7a), 2.06 (1H, m, H-7b), 2.06 (2H, m, H2-11), 1.98 (2H, m, H2-10), 1.75 (2H, m, H2-2), 1.68 (3H, s, H3-14), 1.62 (3H, s, H3-16), 1.60 (3H, s, H3-15), 1.56 (1H, m, H-6a), 1.50 (each 1H, m, H-6b, H-3a), 1.40 (1H, m, H2-3b), 1.12 (3H, s, H3-17); 13C NMR (CDCl3, 125 MHz) δ 135.8 (C, C-9), 131.5 (C, C-13), 124.2 (CH, C-12), 124.1 (CH, C-8), 77.2 (CH, C-4), 75.1 (C, C-5), 62.9 (CH2, C-1), 39.7 (CH2, C-10), 38.8 (CH2, C-6), 30.1 (CH2, C-2), 28.6 (CH2, C-3), 26.6 (CH2, C-11), 25.7 (CH3, C-14), 20.8 (CH3, C-17), 22.0 (CH2, C-7), 17.7 (CH3, C-15), 16.0 (CH3, C-16).
3.5. Determination of the Absolute Configuration of the Diol Moiety in 3a-r and 3b-r by Snatzke’s Method
ICD spectra of Mo-complexes of 3a-r and 3b-r were obtained according to reported procedures [26,27]. The reduced products (each 0.5 mg) and Mo2(OAc)4 (1.0 mg) were dissolved in 1.5 mL of dry DMSO to give a solution, with the ligand to metal molar ratio being around 1:1.2. The electronic transitions of the metal complexes in DMSO were monitored by a Jasco J-815 CD spectrometer in the UV–vis region of 250–500 nm, and stationary ICD spectra were obtained after 50 min at 15 °C. Because there were no inherent absorptions for the reduced products, the observed ICD spectra could be directly used to analyze the absolute configurations of diol fragments in the ligands with the characteristic cotton effects around 310 nm, according to Snatzke’s theory [26,27].
3.6. Calculating Section
The quantum chemical calculations were performed using the density functional theory (DFT) by Gaussian 09 [33]. The initial key chiral structures in compounds 1a, 1b, 2a, 2b and 6 were built with Spartan 10 software, and all trial structures were first minimized based on molecular mechanics calculations. Conformational search was performed by Spartan 10 software using MMFF force filed, and conformers occurring within a 10 kcal/mol energy window from the global minimum were chosen for geometry optimization in the gas phase with the DFT method at the B3LYP/DGDZVP level. The B3LYP/DGDZVP harmonic vibrational frequencies were further calculated to confirm their stability. The spin-allowed excitation energies and rotatory (Rn) and oscillator strengths (fn) of the lowest excited states of stable conformers were calculated for ECD spectra using TD-DFT method with the basis set RB3LYP/DGDZVP. Solvent effects of methanol solution were evaluated at the same DFT level by using the SCRF/PCM method in agreement with the experiment condition. Electronic transitions were expanded as Gaussian curves with a FQHM (full width at half maximum) for each peak of 0.32 eV. The ECD spectra were combined after Boltzmann weighting according to their population contribution.
3.7. Cytotoxicity
In vitro cytotoxicity was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric assay against K562, HCT116, Hep3B, A-549 and Jurkat cell lines by reported procedures [32]. All the cell lines were purchased from Shanghai Institute of Cell Biology (Shanghai, China). Adriamycin (doxorubicin, ADM) was used as a positive control.
4. Conclusions
In summary, the present study of sponge Cacospongia sp. led to the identification of 15 optically pure terpenoids. This is the first time to encounter a series of stereoisomers of the rare linear C17 terpenoid and well-known manoalide-type sesterterpenes from an individual sponge species. Furthermore, there is only one C17 γ-lactone terpenoid named cavernosine and no C21 pyridine terpenoid had been reported before [10]. The structural diversity of manoalides was usually derived from the multi-site cyclization andoxidation of the linear prenyl chain, such as compounds 5, 8–10. However, two pairs of enantiotopic manoalide-type sesterterpenoids (4a/4b and 5a/5b) expanded the chemical diversity of manoalides by a stereoisomer manner. The present results showed a remarkable structural diversity of terpenoids in the sponge Cacospongia sp. The unique C17 and C21 meroterpenoids showed chemotaxonomy significance for the unidentified species of Cacospongia.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/17/1/14/s1. Photos of sponge specimen, Chiral HPLC separation profiles of 1a/1b, 2a/2b, 3, 3a-r/3b-r, 4a/4b and 5a/5b, hydrolysis and reduction scheme of 3, HR-MS and NMR spectra for the new compounds, as well as the computational details (PDF).
Author Contributions
X.Z. performed most of the experiments, analyzed the data and prepared the draft manuscript. P.-L.L. analyzed the data, performed the chemical calculations and revised the manuscript. G.-F.Q. checked the data. S.L. revised the manuscript. N.J.d.V. identified the sponge species. G.-Q.L. and X.-L.T. designed the research and revised the final version.
Fundings
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21572210, 41776136, 41522605 and 81741155), AoShan Talents Program Supported by Qingdao National Laboratory for Marine Science and Technology (No. 2015ASTP), and Shandong Provincial Natural Science Foundation (No. ZR2017BD009). Special thanks are given to J.L. (Key Laboratory of Marine Drugs, Chinese Ministry of Education, Ocean University of China) for the cytotoxicity tests and to N.J.d.V. for the identification of the sponge species.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu Y., Zhang S., Abreu P.J. Heterocyclic terpenes: Linear furano- and pyrroloterpenoids. Nat. Prod. Rep. 2006;23:630–651. doi: 10.1039/b604586c. [DOI] [PubMed] [Google Scholar]
- 2.Wang B., Wang L., Li Y., Liu Y. Heterocyclic terpenes: Linear furano- and pyrroloterpenoids. RSC Adv. 2014;4:12216–12234. doi: 10.1039/C3RA48040B. [DOI] [PubMed] [Google Scholar]
- 3.Hanson J.R. Sesterterpenoids. Nat. Prod. Rep. 1986;3:123–132. doi: 10.1039/np9860300123. [DOI] [Google Scholar]
- 4.Hanson J.R. The sesterterpenoids. Nat. Prod. Rep. 1992;9:481–489. doi: 10.1039/np9920900481. [DOI] [Google Scholar]
- 5.Hanson J.R. The sesterterpenoids. Nat. Prod. Rep. 1996;13:529–535. doi: 10.1039/np9961300529. [DOI] [Google Scholar]
- 6.Liu Y., Wang L., Jung J.H., Zhang S. Sesterterpenoids. Nat. Prod. Rep. 2007;24:1401–1429. doi: 10.1039/b617259h. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Yang B., Lin X.P., Zhou X.F., Liu Y. Sesterterpenoids. Nat. Prod. Rep. 2013;30:455–473. doi: 10.1039/c3np20089b. [DOI] [PubMed] [Google Scholar]
- 8.Ebada S.S., Lin W.H., Proksch P. Bioactive Sesterterpenes and Triterpenes from marine sponges: Occurrence and pharmacological significance. Mar. Drugs. 2010;8:313–346. doi: 10.3390/md8020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross H., König G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006;5:115–141. doi: 10.1007/s11101-005-5464-3. [DOI] [Google Scholar]
- 10.Cen-Pacheco F., Nordström L., Souto M.L., Martín M.N., Fernández J.J., Daranas A.H. Studies on polyethers produced by red algae. Mar. Drugs. 2010;8:1178–1188. doi: 10.3390/md8041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braekman J.C., Daloze D., Bertau R., Macedo de Abreu P. Cavernosine, a novel ichthyotoxic terpenoid lactone from the sponge Fasciospongia cavernosa. Bull. Soc. Chim. Belg. 1982;91:791–796. doi: 10.1002/bscb.19820910907. [DOI] [Google Scholar]
- 12.Wang L.J., Xiong J., Lau C., Pan L.L., Hu J.F. Sesquiterpenoids and further diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius. J. Asian Nat. Prod. Res. 2015;17:1220–1230. doi: 10.1080/10286020.2015.1118622. [DOI] [PubMed] [Google Scholar]
- 13.Mehbub M.F., Perkins M.V., Zhang W., Franco C.M.M. New marine natural products from sponges (Porifera) of the order Dictyoceratida (2001 to 2012); a promising source for drug discovery, exploration and future prospects. Biotechnol. Adv. 2016;34:473–491. doi: 10.1016/j.biotechadv.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Guella G., Amade P., Pietra F. Cacospongione A, cacospongienone A, and cacospongienone B, new C21 difuran terpenoids from the marine sponge Cacospongia scalaris SCHMIDT of the Côte d’Azur. Helv. Chim. Acta. 1986;69:726–733. doi: 10.1002/hlca.19860690325. [DOI] [Google Scholar]
- 15.Derosa S., Destefano S. Cacospongionolide. A new antitumoral sesterterpene, from the marine sponge Cacospongia mollior. J. Org. Chem. 1988;53:5020–5023. doi: 10.1021/jo00256a022. [DOI] [Google Scholar]
- 16.Fattorusso E., Magno S., Santacroce C., Sica D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron. 1972;28:5993–5997. doi: 10.1016/0040-4020(72)88132-8. [DOI] [Google Scholar]
- 17.Tanaka J., Marriott G., Higa T., Higa T. Cacofurans A and B, new furanoditerpenes from a marine sponge. J. Nat. Prod. 2001;64:1468–1470. doi: 10.1021/np010203g. [DOI] [PubMed] [Google Scholar]
- 18.Rubio B.K., van Soest R.W.M., Crews P. Extending the record of meroditerpenes from Cacospongia marine sponges. J. Nat. Prod. 2007;70:628–631. doi: 10.1021/np060633c. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q., Tang X.L., Luo X.C., de Voogd N.J., Li P.L., Li G.Q. (+)-and (−)-Spiroreticulatine, A pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China Sea sponge Fascaplysinopsis reticulate. Org. Lett. 2015;17:3458–3461. doi: 10.1021/acs.orglett.5b01503. [DOI] [PubMed] [Google Scholar]
- 20.Liu C.X., Tang X.L., Li P.L., Li G.Q. Suberitine A–D, four new cytotoxic dimeric aaptamine alkaloids from the marine sponge Aaptos suberitoides. Org. Lett. 2012;14:1994–1997. doi: 10.1021/ol3004589. [DOI] [PubMed] [Google Scholar]
- 21.Jefford C.W., Jaggi D., Bernardinelli G., Boukouvalas J. The synthesis of (±)-cavernosine. Tetrahedron Lett. 1987;28:4041–4044. doi: 10.1016/S0040-4039(01)83856-9. [DOI] [Google Scholar]
- 22.Nishida Y., Konno T., Ohrui H., Meguro H. Circular Dichroism (CD) of marmelo lactones and the effect of the unsaturation at C-5 on the cotton effect of γ-lactone. Agr. Biol. Chem. Tokyo. 1983;47:2683–2684. doi: 10.1080/00021369.1983.10866017. [DOI] [Google Scholar]
- 23.Underwood B.S., Tanuwidjaja J., Ng S.S., Jamison T.F. Total syntheses of the squalene-derived halogenated polyethers ent-dioxepandehydrothyrsiferol and armatol A via bromonium-and Lewis acid-initiated epoxide-opening cascades. Tetrahedron. 2013;69:5205–5220. doi: 10.1016/j.tet.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange G.L., Lee M. 13C NMR determination of the configuration of methyl-substituted double bonds in medium-and large-ring terpenoids. Magn. Reson. Chem. 1986;24:656–658. doi: 10.1002/mrc.1260240804. [DOI] [Google Scholar]
- 25.Mehl F., Bombarda I., Vanthuyne N., Faure R., Gaydou E.M. Hemisynthesis and odour properties of δ-hydroxy-γ-lactones and precursors derived from linalool. Food Chem. 2010;121:98–104. doi: 10.1016/j.foodchem.2009.12.010. [DOI] [Google Scholar]
- 26.Frelek J., Ikekawa N., Takatsuto S., Snatzke G. Application of [Mo2(OAc)4] for determination of absolute configuration of brassinosteroid vic-diols by circular dichroism. Chirality. 1997;9:578–582. doi: 10.1002/(SICI)1520-636X(1997)9:5/6<578::AID-CHIR27>3.0.CO;2-K. [DOI] [Google Scholar]
- 27.Di Bari L., Pescitelli G., Pratelli C., Pini D., Salvadori P. Determination of absolute configuration of acyclic 1, 2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 2001;66:4819–4825. doi: 10.1021/jo010136v. [DOI] [PubMed] [Google Scholar]
- 28.Piao S.J., Zhang H.J., Lu H.Y., Yang F., Jiao W.H., Yi Y.H., Chen W.S., Lin H.W. Hippolides A–H, acyclic manoalide derivatives from the marine sponge Hippospongia lachne. J. Nat. Prod. 2011;74:1248–1254. doi: 10.1021/np200227s. [DOI] [PubMed] [Google Scholar]
- 29.Desilva E.D., Scheuer P.J. Three new sesterterpenoid antibiotics from the marine sponge Luffariella variabilis (Polejaff) Tetrahedron Lett. 1981;22:3147–3150. doi: 10.1016/S0040-4039(01)81849-9. [DOI] [Google Scholar]
- 30.Tsuda M., Shigemori H., Ishibashi M., Sasaki T., Kobayashi J. Luffariolides A–E, new cytotoxic sesterterpenes from the Okinawan marine sponge Luffariella sp. J. Org. Chem. 1992;57:3503–3507. doi: 10.1021/jo00038a051. [DOI] [Google Scholar]
- 31.Jiao W.H., Hong L.L., Sun J.B., Piao S.J., Chen G.D., Deng H., Wang S.P., Yang F., Lin H.W. (±)-Hippolide J–A Pair of unusual antifungal enantiomeric sesterterpenoids from the marine sponge Hippospongia lachne. Euro. J. Org. Chem. 2017;24:3421–3426. doi: 10.1002/ejoc.201700248. [DOI] [Google Scholar]
- 32.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 33.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2009. Revision A.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.