Abstract
Carotenoids are diet-dependent milk components that are important for the visual and cognitive development of an infant. This study determined β-carotene, lycopene and lutein + zeaxanthin in breastmilk and its associations with dietary intake from healthy Polish mothers in the first six months of lactation. Concentrations of carotenoids in breastmilk were measured by HPLC (high-performance liquid chromatography) (first, third, sixth month of lactation) and dietary intake was assessed based on a three-day dietary record (third and sixth month of lactation). The average age of participants (n = 53) was 31.4 ± 3.8 years. The breastmilk concentrations of carotenoids were not changed over the progress of lactation. Lycopene was a carotenoid with the highest content in breastmilk (first month 112.2 (95% CI 106.1–118.3)—sixth month 110.1 (103.9–116.3) nmol/L) and maternal diet (third month 7897.3 (5465.2–10329.5) and sixth month 7255.8 (5037.5–9474.1) µg/day). There was a positive correlation between carotenoids in breastmilk and dietary intake (lycopene r = 0.374, r = 0.338; lutein + zeaxanthin r = 0.711, r = 0.726, 3rd and 6th month, respectively) and an inverse correlation with maternal BMI in the third month of lactation (β-carotene: r = −0.248, lycopene: r = −0.286, lutein + zeaxanthin: r = −0.355). Adjusted multivariate regression models confirmed an association between lutein + zeaxanthin intake and its concentration in breastmilk (third month: β = 0.730 (0.516–0.943); 6th: β = 0.644 (0.448–0.840)). Due to the positive associations between dietary intake and breastmilk concentrations, breastfeeding mothers should have a diet that is abundant in carotenoids.
Keywords: bioactive factors, carotenoids, dietary intake, high-performance liquid chromatography (HPLC), human milk, lactation, maternal diet, prospective study
1. Introduction
Breastfeeding is the best feeding method for newborns, infants and toddlers. International organizations (e.g., WHO, UNICEF, AAP, ESPGHN) recommend exclusive breastfeeding during the first six months of life and further breastfeeding along with complementary feeding up to two years or more, as long as the infant and mother desire [1,2,3]. Exclusive breastfeeding at this time provides sufficient energy and macronutrients as well as most of the micronutrients (except vitamin D and K) to meet the mean requirements of healthy term-born infants [3,4]. During this time, breastmilk provides not only necessary nutrients, however also a variety of bioactive factors, such as immunoglobins, stem cells, cytokines, hormones, growth factors and phytochemicals (e.g., carotenoids and flavonoids) [5,6,7]. These compounds are crucial for optimal development and further health, including decreased risk of infectious diseases during childhood as well as chronic non-communicable diseases throughout the lifespan [2,8]. Previous studies have shown that breastmilk composition is influenced by many determinants, including maternal factors (e.g., age, nutritional status, dietary intake, tobacco use or passive smoking), infant factors (gestational age, age, gender) and physiological factors (lactation stage, nursing stage, diurnal variation) [9,10,11,12,13,14]. The content of some nutrients in breast milk is dependent on maternal dietary intake, including fatty acid profile [15], iodine [16] and vitamins B1, B2, B6 and B12 [17]. However, maternal dietary intake has a limited influence on breastmilk macronutrient composition [14] and some micronutrients, e.g., folic acid [17] and zinc [18]. Some studies have indicated that maternal nutritional status measured as body mass index (BMI) may influence the milk fat concentrations [14] as well as carotenoid concentrations [19].
Carotenoids (β-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, astaxanthin) are plant bioactive compounds that cannot be synthesized by mammals however are supplied with a high dietary intake of vegetables and fruits (especially green, yellow, orange or red) or some animal products (e.g., yolk, salmon or rainbow trout) [20,21,22]. Numerous studies in recent years have shown their positive impact on human health, mainly in adults [23,24,25], however also during pregnancy and early childhood, as noted previously [6,26]. Recent studies focusing on breastmilk carotenoids have shown that their concentration depends on maternal dietary intake [27] and varies among populations [19,28,29,30], especially due to different dietary habits. Other factors may also affect the carotenoid concentration in breast milk, such as education status or anthropometric parameters of mothers [19]. Giordano and Quadro [26], in a recent review, emphasize the importance of monitoring maternal and infant lutein and zeaxanthin status due to its importance during pregnancy and the neonatal period [26]. An analysis of breastmilk carotenoids may be a useful, non-invasive method due to the correlations between maternal carotenoid status, breastmilk carotenoids and infant carotenoid status [27,30,31,32,33]. To date, few studies have considered the impact of dietary intake of carotenoids and maternal factors on breastmilk carotenoid concentration and there is a lack of long-term studies on the subject.
The aims of this study were (1) to investigate the concentrations of selected carotenoids (β-carotene, lycopene and lutein + zeaxanthin) in breastmilk from healthy mothers living in an urban area of Poland in the first six months of lactation (2) to determine the dietary intake of carotenoids in the third and sixth months of lactation and (3) to explore the associations between breastmilk carotenoids and dietary intake and other maternal factors.
2. Materials and Methods
2.1. Ethical Approval
The study was approved by the Ethics Committee of the Medical University of Warsaw in 2015, Resolution No. AKBE/139/15. Written consent was obtained from all participants and the study was conducted in compliance with the Helsinki Declaration.
2.2. Study Design
The study consisted of three study sessions at the first, third and sixth month of lactation. All mothers completed a questionnaire which included detailed questions about lifestyle, sociodemographic, health factors and nutrition (e.g., maternal education, household income, dietary supplement use) during the pre-conceptional, prenatal and postnatal periods. At each study visit, breastmilk samples were also collected and anthropometric measurements were assessed in infants and mothers, maternal psychological status was evaluated, as well as the infant psychomotor development in the sixth month. Detailed information about the study design is shown in Table 1. The data used in this paper are part of the data that were obtained during this study.
Table 1.
Study design characteristics.
| Study Visit | Assessment | ||||
|---|---|---|---|---|---|
| Breastmilk | Nutritional | Anthropometric | Psychological | ||
| 1st (1st month) |
Mother | - macronutrients - carotenoids - fatty acid profile |
- Food Frequency Questionnaire FFQ-6 1 [34] - dietary supplement use |
- pre-pregnancy, 14 Hbd 2, 27 Hbd body mass - current body mass |
- EPDS 3 [35] - PSS-10 4 [36,37] |
| Infant | - | - breastfeeding frequency - dietary supplement use |
- birth parameters - body mass - body length - head circumference |
- | |
| 2nd (3rd month) |
Mother | - macronutrients - carotenoids - fatty acid profile |
- 3-day dietary record - carotenoid intake - dietary supplement use |
- current body mass | - EPDS 3 [35] - PSS-10 4 [36,37] |
| Infant | - | - breastfeeding frequency - dietary supplement use |
- body mass - body length - head circumference |
- | |
| 3rd (6th month) |
Mother | - macronutrients - carotenoids - fatty acid profile |
- 3-day dietary record - carotenoid intake - dietary supplement use |
- current body mass | - EPDS 3 [35] - PSS-10 4 [36,37] |
| Infant | - | - breastfeeding frequency - dietary supplement use - introduced to other foods or drinks |
- body mass - body length - head circumference |
- DSR 5 Scale [38]. | |
1 FFQ-6—Food Frequency Questionnaire; 2 Hbd—hebdomas (weeks of gestation); 3 EPDS—Edinburgh Postpartum Depression Scale; 4 PSS-10—Perceived Stress Scale; 5 DSR—Children Development Scale.
2.3. Study Participants
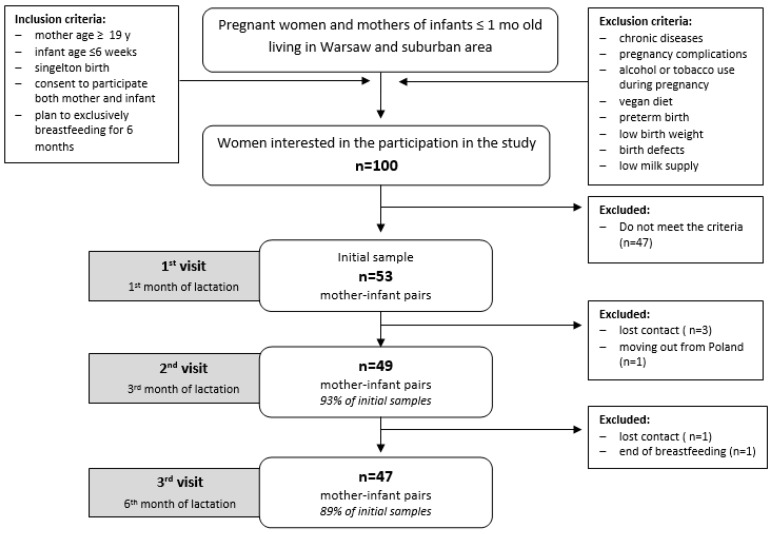
Recruitment was conducted between April 2015 and July 2017 in the Holy Family Hospital in Warsaw among patients of Obstetrics Clinic as well as breastfeeding women from the local community using social media groups. Eligibility criteria included women between 18 and 45 years of age giving birth to a single, healthy infant. The exclusion criteria included maternal chronic disease (kidney, liver, gastrointestinal diseases influencing nutrient absorption, hypertension, diabetes type I or II) and pregnancy complications (including preeclampsia or eclampsia, pregnancy induced hypertension). The study also included 53 mother-infant pairs (Figure 1). The mean age of mothers (n = 53) was 31.4 ± 3.8 years. Most of the mothers had a university education and a high average income. Before pregnancy, most of them had a normal BMI (n = 46; 87%); at the first month of lactation, 78% were classified as having normal body weight (n = 40), 86% (n = 42) at the third month and 85% (n = 40) at the sixth month. Further, 25 participants (47%) were primiparas and 53% of the infants were females (n = 28; detailed study group characteristics are shown in Table S1). Most of the study visits took place during the spring-summer period (no statistically significant differences between the number of visits in particular seasons, p = 0.482).
Figure 1.
Flowchart: study design and study sample collection.
2.4. Breastmilk Collection
Breastmilk samples were collected by participants at home after being given detailed instructions. The same amount (5–10 mL) of pre-feeding and post-feeding breastmilk was collected 24 hours prior to each study visit at four time periods (06:00–12:00; 12:00–18:00; 18:00–24:00; 24:00–06:00) to minimize the daily differences in milk composition due to the 24-hour variation in carotenoid concentrations that is described in literature [39,40]. The samples were obtained by a breast pump or manually from the breast(s) that the infant fed from. Breastmilk samples were transported to the Holy Family Hospital in Warsaw under cooling conditions. Next, the same amount from all four samples was collected and mixed in a Vortex shaker IKA MS2 (IKA Works Inc., Wilmington, North Carolina, USA) for one minute. The pooled sample was distributed in 2 mL clear polypropylene tubes, labelled and stored at −80 °C for later analysis within six months of collection. Precautions were taken throughout the breastmilk sample collection and procedures to minimize the exposure of samples to temperature, light and air.
2.5. Breastmilk Composition Analysis
Breastmilk energy value and fat content per 100 mL of the raw sample of breastmilk were analysed using a MIRIS human milk analyser HMA (Miris, Uppsala, Sweden). Prior to the analysis, each sample (n = 149) was warmed to 40 °C and homogenized (1.5 s/1 mL of sample) using a sonicator (milk homogenizer, Miris, Uppsala, Sweden) according to the method described previously [14]. From each raw pooled sample, macronutrient composition was analysed three times (~2 mL per analysis) and the average of the three measurements was used for further statistical analysis.
2.6. Breastmilk Carotenoid Analysis
Carotenoid concentration (β-carotene, lycopene and lutein + zeaxanthin) in milk samples was assessed using high-performance liquid chromatography (HPLC). Milk samples for analysis were prepared based on the modified method published by Macias and Schweigert [41]. To 2 mL of milk, 500 μL of a 12% solution of pyrogallol, 50 μL, 1% ascorbic acid in 0.1 M HCl, 1.5 mL 30% KOH solution and 2.5 mL ethanol were successively added. The mixture was shaken for 30 seconds with a Vortex shaker and was then incubated in a 50 °C water bath for 40 minutes. Subsequently, 1 mL of saturated NaCl solution and 1 mL of n-hexane were added to the ice-cooled samples, followed by vigorous shaking for three minutes. The sample was then centrifuged for 10 minutes at 4 °C (8000 rpm). Immediately after centrifugation, the upper hexane layer was transferred to a new tube. The extraction was repeated two more times. The combined hexane extracts were evaporated in a vacuum evaporator (30 minutes at 30 °C). The formed precipitate was dissolved in 0.5 mL of hexane and was transferred to dark glass vials.
The content of individual carotenoids in the prepared milk samples was determined using the Shimadzu HPLC system (Japan: 2 LC-20AD pumps, CMB-20A controller system, SIL-20AC autosampler, UV/IS SPD-20AV detector, CTD-20AC controller) using C18 Synergi Fusion-RP 80i columns (250 × 4.60 mm, Phenomenex, CA, USA). The determination of the studied carotenoids was carried out at a wavelength of 471 nm for lycopene, 450 nm for β-carotene and 445 nm for lutein + zeaxanthin. The mobile phase consisted of two phases—phase A: acetonitrile/methanol mixed in proportions of 90:10 (90/10; v/v) and phase B: methanol/ethyl acetate, in a 34:16 ratio (34/16; v/v). The flow rate of the developing mixture was 1.0 cm3/min and the sample injection was 100 μL. Individual carotenoid concentrations in milk were calculated by comparing them with a corresponding standards curve (standards of catalogue numbers: β-carotene C4582, lutein + zeaxanthin X6250, lycopene L9879 from Sigma-Aldrich Inc., Merck KGaA, Darmstadt, Germany). The standard curves for all carotenoids are shown in supplementary materials (Figure S1). The concentrations of the studied carotenoids were expressed in nmol/L. Since lutein and zeaxanthin could not be completely resolved and were summed, all references to milk lutein concentrations refer to lutein + zeaxanthin.
2.7. Dietary Intake Analysis
The maternal diet at the 3rd and 6th month of lactation was assessed using the 3-day dietary record conducted by respondents before the study visits (on three typical days, two weekdays and one weekend day). Respondents received all necessary information and instructions on conducting a dietary record, including the importance of scrupulosity recording all consumed foods and beverages during the period covered by the 3-day dietary records. During the recording, respondents used a kitchen scale to measure the weight of the serving, or if they did not have kitchen scales, they estimated the servings using typical household measures. All sizes of portions were verified using the “Album of Photographs of Food Products and Dishes” created by the National Food and Nutrition Institute [42]. All nutritional data were collected and analysed by the qualified dietician.
Based on the data from the 3-day record of daily energy value and dietary intake of selected macronutrients (protein, carbohydrates, fat) and micronutrients (vitamin A, E, D, B1, B2, B3, B6, B12, C, folic acid; iodine, calcium, potassium, phosphor, magnesium, iron, zinc, copper, manganese), the dietary fibre and fatty acid profile were calculated using Dieta 5.0 Software (National Food and Nutrition Institute, Warsaw, Poland) based on Polish food-composition tables [43]. The total dietary intake of selected carotenoids (lutein + zeaxanthin, lycopene and β-carotene) were estimated using data from the USDA Database [22]. In addition, data on dietary supplement use in the preconceptional prenatal and postnatal periods were collected including the used dose, name and the brand of dietary supplement. The data were used to calculate the average daily nutrient intake with dietary supplements. The intake of nutrients was calculated as a mean from the three recorded days to obtain the mean daily intake from diet, dietary supplements and the sum of both for each participant. For the purpose of this study, we used only energy value, fat intake and intake of antioxidant fat-soluble vitamins (A and E) and carotenoids (lutein + zeaxanthin, lycopene and β-carotene) for analysis.
2.8. Anthropometric Measurements
The body weight (kg) and height (cm) of mothers were measured according to the International Standards for Anthropometric Assessment [44] to the nearest 0.1 kg or 0.1 cm, respectively, using a professional stadiometer (Seca 799, Hamburg, Germany). All measurements were taken in light clothing and without shoes. Body mass index (BMI, kg/m2) was calculated and classified according to the World Health Organization (WHO) [45]. BMI and body mass changes between visits were calculated.
2.9. Statistical Analysis
The parameters that were analysed in this study were presented as: means and standard deviation or 95% coefficient intervals (CI), minimum and maximum values. To assess the normality of distributions, the Shapiro–Wilk test was applied. The differences between groups were analysed using the Wilcoxon matched pairs test (for two repeated measurements) or the Friedman ANOVA test (for three repeated measurements). For qualitative variables, the Cochrane Q test was used to check the differences between three repeated measurements and the Chi2 McNemar test was used for the two repeated measurements.
The correlations between breastmilk carotenoids and the maternal anthropometric and sociodemographic characteristics, as well as dietary intake, were estimated with Spearman’s rank or Kendall’s tau correlations (if one of the variables was measured on an ordinal scale) coefficient. Linear regression was used to investigate the relationship between breastmilk carotenoids and the dietary intake of its carotenoids. The one univariate and final three multivariate models were specifically adjusted for season, maternal age, BMI, education, mode of delivery, fat, vitamin E and vitamin A intake. All of the analyses were performed using Statistica 13.1 software (Dell Inc., Round Rock, TX, USA). For all of the tests, p ≤ 0.05 was considered as significant.
3. Results
3.1. Breastmilk Composition and Carotenoid Concentration
The average energy value, fat content and carotenoid concentrations in breastmilk at the 1st, 3rd and 6th month of lactation are shown in Table 2. The energy value and fat content in breastmilk were, on average, around 68–69 kcal/100mL and 3.8–3.9 g/100mL, respectively, and this level was similar regardless of the lactation month. The highest concentration of determined carotenoids was observed for lycopene (minimum–maximum values 77.2–176.9 nmol/L). The concentrations of β-carotene and lutein + zeaxanthin were at similar levels—mean values around 33 nmol/L (despite the insignificant 12% higher concentration of lutein + zeaxanthin at the sixth month due to the higher dietary intake at this month) and also did not differ between the months of lactation.
Table 2.
Energy value, fat and carotenoid content in breastmilk in the first, third and sixth month of lactation.
| Nutrient (Unit) |
Breastmilk Composition Mean Value (95% CI 1) Min–Max |
p-Value 2 | ||
|---|---|---|---|---|
| First Month (n = 53) |
Third Month (n = 49) |
Sixth Month (n = 47) |
||
| Energy (kcal/100 mL) |
69.5 (67.5–71.6) 57.0–89.7 |
68.2 (65.3–71.0) 49.3–95.5 |
68.4 (66.5–70.4) 53.7–90.3 |
0.777 |
| Total fat (g/100 mL) |
3.8 (3.6–4.1) 2.2–6.0 |
3.8 (3.5–4.1) 1.7–6.9 |
3.9 (3.7–4.1) 2.2–6.4 |
0.595 |
| β-carotene (nmol/L) |
33.2 (33.0–33.5) 31.9–36.3 |
33.1 (32.9–33.3) 31.8–35.9 |
33.3 (33.1–33.6) 32.3–35.4 |
0.436 |
| Lycopene (nmol/L) |
112.2 (106.1–118.3) 77.2–169.0 |
111.2 (105.0–117.3) 75.4–176.9 |
110.1 (103.9–116.3) 78.3–176.7 |
0.457 |
| Lutein + zeaxanthin (nmol/L) |
33.0 (26.3–39.7) 2.7–123.2 |
33.0 (24.1–41.8) 3.2–139.9 |
37.1 (26.5–47.8) 1.8–169.0 |
0.640 |
1 CI—coefficient interval; 2 Friedman ANOVA test.
3.2. Dietary Intake
Table 3 shows the energy value and dietary intake of selected nutrients at the third and sixth month of lactation. There was no statistical difference between the third and sixth month for energy value and fat intake. We observed statistically significant differences between total vitamin E intake (dietary supplements and diet; p = 0.000) and for vitamin A, both dietary and dietary with supplements (p = 0.010 and p = 0.009, respectively). These differences are associated with the higher use of dietary supplements in the third month of lactation compared with the sixth month (90% vs. 81%; p < 0.001). The highest consumption was recorded for lycopene, followed by β-carotene and lutein + zeaxanthin in the lowest amount. We observed no significant differences in carotenoid intake between the months of lactation.
Table 3.
Energy value and selected nutrients and carotenoid intake by participants at the third and sixth month of lactation.
| Nutrient (Unit) |
Dietary Intake Mean Value ± SD 1 or (95% CI 2) Min–Max |
p-Value 3 | |
|---|---|---|---|
| Third Month (n = 49) |
Sixth Month (n = 47) |
||
| Energy (kcal/day) |
2193.7 ± 631.17 1186.5–3914.0 |
2046.2 ± 502.9 1051.4–3317.3 |
0.083 |
| Total fat (g/day) |
84.3 ± 28.5 37.2–185.6 |
76.4 ± 26.1 18.9–135.7 |
0.085 |
| Vitamin E, dietary (mg α-tocopherol Equivalent/day) |
12.6 ± 5.9 4.3–32.7 |
11.4 ± 5.1 4.7–24.5 |
0.258 |
| Vitamin E, dietary & supplements (mg α-tocopherol Equivalent/day) |
21.5 ± 13.9 4.3–57.7 |
13.1 ± 6.7 4.7–27.5 |
0.000 |
| Vitamin A, dietary (µg Retinol Equivalent/day) |
1289.5 ± 591.4 226.4–2447.3 |
1030.1 ± 500.0 213.1–2745.2 |
0.010 |
| Vitamin A, dietary & supplements (µg Retinol Equivalent/day) |
1295.0 ± 588.3 226.4–2447.3 |
1030.1 ± 500.0 213.1–2745.2 |
0.009 |
| β-carotene (µg/day) |
4480.8 (3575.0–5386.7) 319.6–16461.0 |
3441.9 (5037.5–9474.1) 716.2–9552.9 |
0.232 |
| Lycopene (µg/day) |
7897.3 (5465.2–10329.5) 477.2–30472.7 |
7255.8 (5037.5–9474.1) 339.2 – 38852.9 |
0.422 |
| Lutein + zeaxanthin (µg/day) |
2945.2 (1910.8–3979.6) 263.7–16678.9 |
3739.3 (2834.9–4643.7) 128.9–12207.3 |
0.054 |
1 SD—standard deviation; 2 CI—coefficient interval; 3 Wilcoxon matched pairs test.
3.3. Associations between Carotenoid Concentrations, Dietary Intake and Maternal Characteristics
The associations between carotenoid concentrations, maternal factors and dietary intake are shown in Table 4. The intake of all of the analysed carotenoids positively correlated with its carotenoid breastmilk concentrations at the third and sixth months. For lutein, we observed a strong correlation (third month: r = 0.711, sixth month: r = 0.726; p ≤ 0.001), for β-carotene—a weak and moderate correlation (third month: r = 0.442, sixth month: r = 0.532; p ≤ 0.001), whereas for the lycopene, only a weak correlation (third month: r = 0.374; p ≤ 0.01, sixth month: r = 0.338; p ≤ 0.05). We found statistically significant adverse correlations between maternal BMI (kg/m2) and all three carotenoid concentrations in breastmilk at the third month of lactation (β-carotene r = −0.337; lycopene r = −0.286; lutein + zeaxanthin r = −0.355; p ≤ 0.05) and also β-carotene at the sixth month (r = −0.337; p ≤ 0.05). However, the analysis based on BMI categories found significant inverse associations only for β-carotene at the sixth month (r = −0.453; p ≤ 0.05).
Table 4.
Correlations between breastmilk carotenoids and maternal characteristics and dietary intake.
| Variables | Breastmilk β-carotene r Coefficient (p-Value) |
Breastmilk Lycopene r Coefficient (p-Value) |
Breastmilk Lutein + Zeaxanthin r Coefficient (p-Value) |
|||
|---|---|---|---|---|---|---|
| Third Month (n = 49) |
Sixth Month (n = 47) |
Third Month (n = 49) |
Sixth Month (n = 47) |
Third Month (n = 49) |
Sixth Month (n = 47) |
|
| Maternal characteristic | ||||||
| Maternal age (years) 1 | 0.015 | −0.197 | −0.136 | 0.113 | −0.048 | −0.009 |
| Maternal education 2 | 0.022 | −0.091 | 0.026 | 0.046 | −0.029 | −0.020 |
| Mode of delivery 2 | −0.009 | −0.001 | −0.094 | −0.050 | −0.175 | −0.115 |
| Number of children 2 | 0.079 | 0.061 | −0.003 | 0.105 | 0.057 | 0.037 |
| BMI at third or sixth month (kg/m2) 1 | −0.248 * | −0.337 * | −0.286 * | −0.119 | −0.355* | −0.205 |
| Maternal dietary intake | ||||||
| Carotenoid intake (µg) 1,3 | 0.442 *** | 0.532 *** | 0.374 ** | 0.338 * | 0.711 *** | 0.726 *** |
| Energy intake (kcal/day) 1 | 0.041 | 0.182 | −0.097 | −0.005 | −0.078 | 0.169 |
| Fat intake (g/day) 1 | 0.049 | 0.302 * | −0.197 | −0.155 | −0.105 | 0.167 |
| Vitamin E intake (mg/day) 1 | 0.270 | 0.062 | 0.082 | 0.013 | 0.123 | −0.069 |
| Vitamin A intake (µg/day) 1 | 0.008 | 0.136 | 0.175 | 0.241 | −0.027 | −0.001 |
| Breastmilk composition | ||||||
| Breastmilk fat (g/100 mL) 1 | 0.235 | 0.167 | 0.274 | 0.064 | 0.399 * | 0.237 |
1 Spearman rank correlation coefficient; 2 tau Kendall coefficient; 3 intake of respective carotenoid; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
Table 5 presents the results of a linear regression analysis of carotenoid concentrations in breastmilk and its dietary intake. The univariate analysis reveals significant associations between dietary individual carotenoids intake in all analysed compounds. After the adjustments (models 2–4), we also found significant associations between dietary intake and breastmilk lycopene and β-carotene at the third month of lactation, however the models were not significant and explained only 3–14% of the variance in breastmilk carotenoids. However, even after adjustment for maternal age, BMI, education and mode of delivery, fat and vitamin A and E intake (model 4), dietary intake of lutein + zeaxanthin explained 51–68% of the variation at both times (third month, β = 0.730 (95% CI 0.516–0.943), p = 0.000; sixth month β = 0.644 (95% CI 0.448–0.840)) and β-carotene explained 35% of the variation in breastmilk β-carotene at the sixth month (β = 0.428, 95% CI 0.180–0.676).
Table 5.
Univariate and multivariate linear regression models between breastmilk carotenoids and dietary intake and third and sixth months of lactation.
| Model | Dietary Intake | Breastmilk Carotenoids at Third Month of Lactation | Breastmilk Carotenoids at Sixth Month of Lactation | ||||
|---|---|---|---|---|---|---|---|
| β-carotene (n = 49) |
Lycopene (n = 49) |
Lutein + Zeaxanthin (n = 49) |
β-carotene (n = 47) |
Lycopene (n = 47) |
Lutein + Zeaxanthin (n = 47) |
||
| 1 | β (95% CI) p-value |
0.342 (0.066–0.618) 0.016 |
0.364 (0.087–0.640) 0.011 |
0.711 (0.504–0.917) 0.000 |
0.397 (0.121–0.672) 0.016 |
0.364 (0.084–0.643) 0.012 |
0.779 (0.591–0.967) 0.000 |
| Model parameters | R2 = 0.10 | R2 = 0.11 | R2 = 0.49 | R2 = 0.14 | R2 = 0.11 | R2 = 0.60 | |
| 2 | β (95% CI) p-value |
0.325 (0.054–0.596) 0.020 |
0.369 (0.088–0.650) 0.011 |
0.680 (0.468–0.891) 0.000 |
0.391 (0.116–0.665) 0.006 |
0.379 (0.092–0.665) 0.011 |
0.785 (0.593–0.977) 0.000 |
| Model parameters |
R2 = 0.14 0.013 |
R2 = 0.10 0.039 |
R2 = 0.50 0.000 |
R2 = 0.07 0.037 |
R2 = 0.10 0.037 |
R2 = 0.60 0.000 |
|
| 3 | β (95% CI) p-value |
0.325 (0.054–0.596) 0.020 |
0.369 (0.088–0.650) 0.011 |
0.680 (0.468–0.891) 0.000 |
0.391 (0.116–0.665) 0.006 |
0.379 (0.092–0.665) 0.011 |
0.785 (0.593–0.977) 0.000 |
| Model parameters |
R2 = 0.06 0.238 |
R2 = 0.11 0.126 |
R2 = 0.62 0.000 |
R2 = 0.29 0.005 |
R2 = 0.07 0.191 |
R2 = 0.62 0.000 |
|
| 4 | β (95% CI) p-value |
0.407 (0.094–0.721) 0.012 |
0.415 (0.104–0.726) 0.010 |
0.730 (0.516–0.943) 0.000 |
0.428 (0.180–0.676) 0.001 |
0.401 (0.089–0.713) 0.013 |
0.644 (0.448–0.840) 0.000 |
| Model parameters |
R2 = 0.04 0.337 |
R2 = 0.06 0.262 |
R2 = 0.51 0.000 |
R2 = 0.35 0.003 |
R2 = 0.03 0.351 |
R2 = 0.68 0.000 |
|
Model 1: univariate analysis; Model 2: multivariate analysis adjusted for season; Model 3: multivariate analysis adjusted for maternal age, BMI, education and mode of delivery; Model 4: multivariate analysis adjusted for maternal age, BMI, education and mode of delivery, fat and vitamin A and E intake.
4. Discussion
In this study, we found that the breastmilk carotenoid concentrations were unchanged through the first six months of lactation. The carotenoid with the highest concentration in breastmilk was lycopene, whereas the β-carotene and lutein + zeaxanthin contents were similar. In addition, lycopene was the main dietary carotenoid. We observed a strong positive relationship between lutein + zeaxanthin dietary intake and its concentration in breastmilk, even after adjustment for confounders. In addition, we noted adverse associations between the breastmilk carotenoids in the third month of lactation and maternal BMI.
Previous studies investigated the breastmilk carotenoid changes during lactation and found that their concentrations decreased with the duration of lactation [19,31,41,46]. Colostrum contains significantly higher concentrations of carotenoids compared to mature milk, regardless of dietary intake or plasma carotenoid concentration [19,30,31,41,46,47]. However, in our study, we did not find any differences in carotenoid concentration between the first and sixth month of lactation which may be explained by the fact that we analysed only mature milk. According to other studies, the decrease occurs only between colostrum and mature milk and the mature milk then has a stable level of carotenoids [30,31,47]. This difference in carotenoid concentration between colostrum and mature milk suggests the occurrence of a special mechanism of transporting carotenoids into the mammary gland during the first day postpartum [30,41,46,48]. This may be associated with the importance of carotenoids, especially lutein, to the health and development of newborns from the first days of life, including decreasing oxidative stress, as well as protection of the retina and participation in its proper development [6,24,26]. Other studies reported not only longitudinal changes in carotenoid concentration, however also diurnal changes, and even within a feeding session, there were changes as a result of changes in breastmilk fat concentrations [39,40,49].
Previous research has demonstrated that the primary carotenoids in breastmilk are β-carotene, lutein and zeaxanthin, lycopene, α-carotene and β-cryptoxanthin, although differences in their proportions were observed [19,28,29,30,31,39,41,46,50]. In the current study, lycopene was the main breastmilk carotenoid with a concentration of 112.2 at the first month and 110.1 nmol/L at the sixth month. However, studies from other populations reported much lower concentrations of lycopene in mature milk, from 14.0 to 59.8 nmol/L (German [31,41], Brazil [50], China [19,28,30] Australia [28], Canada [28], Chile [28], Japan [28], Mexico [28,30], Philippines [28], UK [28], USA [28,30]). In addition, some studies found much higher concentrations of lutein compared to our results (33.0 and 37.1 nmol/L in the first and sixth month of lactation, respectively), from 44.0–114.4 nmol/L (studies conducted in Chile [28], China [28,30,33], Japan [28], Mexico [28,30]), and some reported lower 6.0–29.0 nmol/L (studies conducted in Germany [41], Brazil [50,51], Australia [28], Canada [28], USA [28], UK [28], China [19]). Similarly, other authors observed higher concentrations of β-carotene 36.2–78.2 nmol/L (Germany [31,41], Australia [28], Canada [28], Chile [28], China [28,30], Japan [28], Mexico [28], UK [28], USA [28,30]), although some studies showed a lower concentration of 16.0–22.0 nmol/L (Philippines [28], Brazil [50,51], China [19]). These differences can be explained by variations in dietary habits between populations, including the availability of fruit and vegetables, as well as preferred dish preparation methods. It is also important to note that other studies use different methods of breastmilk sample collection (for example, total volume of one breast or just 5–12 millilitres of foremilk which had 25% lower concentrations of carotenoids compared to the hindmilk [40]). Breastmilk carotenoids are the only source of carotenoids for newborns and infants because infant formula are not fortified with them [6]. β-carotene has pro-vitamin A properties, so it can be converted, if necessary, to contribute to meeting the nutritional requirement for vitamin A [52]. A growing body of studies highlight the importance of lutein during the neonatal and infancy period due to its role during neuro and visual development, as well as reducing oxidative stress and the risk of disorders associated with prematurity [6,7,24,26]. However, lycopene may also decrease the risk of adverse neonatal outcomes (birth parameters, Respiratory Distress Syndrome, Newborn Intensive Care Unit admission), as shown in a recently published study which assessed the maternal serum lycopene during pregnancy in 180 maternal-infant pairs from the USA [53].
As carotenoids cannot be synthesized by mammals, all breastmilk carotenoids are diet-derived. The source of carotenoids are vegetables and fruits, especially tomatoes and its products for lycopene, green, yellow and orange for lutein and yellow and orange for β-carotene [24]. In our study, lycopene was not only the main carotenoid in breastmilk, however it was also the carotenoid with the highest intake in our study group (7897.3, 7255.8 µg/d, third and sixth month of lactation, respectively), followed by β-carotene (4480.0, 3441.9 µg/day) and lutein + zeaxanthin (2945.2, 3739.3 µg/day). These amounts were similar to the carotenoid intake during the first and second trimester that was reported in a study that was conducted in the USA [54]. β-carotene intake was also similar to the results of a study that was conducted among pregnant Polish women, however the authors reported a lower intake of lycopene and lutein + zeaxanthin [55], although other studies of pregnant women reported a much lower intake of these carotenoids [56,57,58,59]. The NHANES study also showed a much lower lutein intake by women who were at reproductive age [60], although a recent study that was conducted in Canada found a similar intake of lycopene and lutein + zeaxanthin and higher intake of β-carotene [61]. Detailed data on the dietary intake of carotenoids during lactation are limited. Cena et al. [27] calculated lutein consumption in 15 Italian women at the third and 30th day postpartum, which was 1209 ± 157 and 1258 ± 102 µg/day, which is also lower compared to our study. However, this data was obtained almost 10 years ago and dietary habits may have changed since then due to the increasing availability of fruits and vegetables. A study that was conducted among Chinese populations found an intake of lutein + zeaxanthin at the level 3.3 ± 0.41 mg/day [33], which was similar to our study.
Several studies have found that a dietary intake of carotenoids determines the serum carotenoids [24,27,62] and, hence, it determines the breastmilk concentrations [27,30,31,32,33,50,51]. Other studies analysed serum carotenoids and found strong correlations between maternal plasma and breastmilk, where breastmilk concentrations were 10–120 times lower compared to the serum concentrations [30,51]. Cena et al. [27] described the strong associations between the dietary intake of lutein and its concentration in serum (r = 0.94) and breastmilk (r = 0.84), however a more recent study by Xu et al. [33] did not find any associations between maternal dietary lutein + zeaxanthin and serum or breastmilk concentrations. Furthermore, a Brazilian study found no association between pro-vitamin A carotenoids and breastmilk β-carotene [51]. Despite the inconsistency of these results, several interventional studies clearly indicated that the concentration of breastmilk carotenoids increases after the consumption of high-carotenoid food products (e.g., carrot or tomato paste, Chlorella) or dietary supplements [62,63,64,65]. A multinational study by Canfield et al. [28] also found that the major milk carotenoids are consistent with major dietary carotenoids. The current study confirmed the associations between dietary carotenoid intake and the breastmilk concentrations that were observed by other authors. Nonetheless, the strength of the association noted in the current study differed depending on the carotenoid—it was weaker for lycopene (r = 0.374, r = 0.338, third and sixth month, respectively) and β-carotene at the third month of lactation (r = 0.442), moderate at the sixth month (r = 0.532) and was the strongest for lutein (r = 0.711, r = 0.726). After adjustment for potential confounders, we confirmed this observation only for lutein and β-carotene. We hypothesized that stronger associations of lutein compared to β-carotene or lycopene were the result of better nutritional economy of lutein and more efficient uptake to the mammary gland and breastmilk due to its important role during early postnatal development [6,26]. Lutein and zeaxanthin, as xanthophylls, are also more polar compared to carotenes and in vitro studies suggest that xanthophylls may have higher bioavailability [30,48]. Due to their end hydroxyl groups, they have a higher polarity and are easily transferred into lipoproteins, which are responsible for carotenoid transport, and further into milk fat globules [30,48]. Milk fat globules are covered by a membrane trilayer that is derived from the endoplasmic reticulum and apical plasma membrane of mammary epithelial cells [30,48,66]. Lutein and zeaxanthin have a higher membrane solubility and a perpendicular orientation within the membrane bilayer which allows them to be more stably bound within the membrane layer compared to carotenes [67]. A recent study comparing dietary and serum carotenoids in men and non-lactating women showed that women have significantly higher concentrations of serum carotenoids than men despite a lower dietary intake of carotenoids, which indicates a sex-difference in the nutritional economy of carotenoids [61].
Maternal dietary intake is not the only factor influencing the breastmilk carotenoid status. In the current study, we found adverse associations between all breastmilk carotenoids at the third month of lactation and β-carotene in the sixth month and maternal BMI. A recent study by Xue et al. [19] found that overweight women had lower concentrations of β-carotene compared to women who were of normal weight, however they did not find any associations for lutein, similar to Meneses and Trugo [51] who also did not observe any associations between breastmilk carotenoids and maternal BMI. However, studies analysing the impact of body mass on serum carotenoids found adverse associations between them [61,68]. The explanation of this association could be twofold. Firstly, both being overweight and obese are associated with greater body fat storage and the carotenoids may be uptaken and stored in adipose tissue [48]. A previous study by Johnson et al. [69] showed that women have a higher lutein concentration in adipose tissue compared to men despite a lower dietary intake and similar serum concentration, which also indicates more effective carotenoid accumulation in adipose tissue in women. Previous studies found that circulating concentrations of other micronutrients, e.g., folate and vitamin B12, may also be altered in women who are overweight and obese, independent of other maternal factors [70,71]. This observation indicated the possibility of a modification in micronutrient metabolism, including carotenoids, which resulted in reduced plasma levels and increased uptake in other tissues, especially adipose tissue [70,71]. Secondly, being overweight and obese coincide with elevated oxidative stress levels which reduces the carotenoid level, one of the antioxidant compounds [72]. On the other hand, we observed adverse associations only between the BMI category and β-carotene at the sixth month of lactation. However, more research is needed to explain this association during the lactation period as well as the impact of pre-pregnancy and pregnancy diet on the breastmilk carotenoids due to physiological weight loss during the lactation period.
Strengths and Limitations
The strength of this study is that we used twice repeated three-day records to collect the data on maternal nutrition which helped to decrease the risk of underestimating or overestimating long-term dietary habits. Second, we collected breastmilk samples from a 24-hour period, as well as both foremilk and hindmilk which minimized possible errors due to changes in breastmilk composition. Third, we had a low drop-out rate (11%), despite a relatively long follow-up period.
Finally, a number of potential limitations need to be considered. First, we had a sample with a very good socio-economic status and education level that were higher than the national average. Our group also had a moderate number of participants which was generally larger than some studies, mainly prospective (n = 21 [27,31], n = 23 [40], n = 46 [51]) and lower compared to other mainly cross-sectional studies (n = 56 [33]; n = 140 [46]; n = 365 [30]; n = 456 [28]; n = 509 [19]). Second, we used convenience sampling. Due to this factor, precautions must be taken when extrapolating the results. Third, we used three-day records only at the second and third visit, so we cannot analyse the associations between dietary intake and breastmilk carotenoids at the first month of lactation. Fourth, we did not collect maternal serum, so we cannot analyse the relationship between the maternal dietary intake of carotenoids and plasma correlations.
5. Conclusions
The concentrations of β-carotene, lycopene and lutein + zeaxanthin in breastmilk samples, as well as its dietary intake, were studied in the first six months of lactation in healthy women from an urban area of Poland. Overall, our findings reveal that maternal dietary intake of carotenoids positively correlates with breastmilk concentrations, especially for lutein. Furthermore, breastmilk carotenoids in the studied population were moderate (β-carotene and lutein + zeaxanthin) or relatively high (lycopene) compared to other populations and remained stable over time. It was also found that an increase in maternal BMI depletes breastmilk carotenoid concentrations. Our results indicate that the maternal dietary intake of carotenoids is an important factor that influences breastmilk carotenoid concentrations and it is easily modifiable through nutritional intervention. Research should continue to explore the biological impact of such results and improve the knowledge of the unique composition of human milk. These findings may help to determine the nutritional recommendations for the dietary intake of carotenoids for breastfeeding mothers and infants and may also form the basis for the development of nutritional programs or dietary supplements. Moreover, an analysis of breastmilk carotenoids may be useful as a non-invasive method to monitor maternal carotenoid status, as well as its intake by infants.
Acknowledgments
We thank all of the mothers and infants for participating in the study. Thanks are also extended to Holy Family Hospital in Warsaw, especially to Elżbieta Łodykowska for her help in participant recruitment.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/11/1/193/s1, Table S1: Characteristics of the study group, Figure S1: Standard curves for all carotenoids that were used in the presented manuscript.
Author Contributions
M.A.Z., J.H. and A.W. were responsible for the conception and design of this study. J.H. and M.A.Z. were involved in the funding acquisition in respect to the project and managing of the project. M.A.Z. and A.W. were responsible for the data collection of this study. M.A.Z. was responsible for the data cleaning and statistical analysis for this particular paper. M.A.Z. was responsible for writing the manuscript. J.H. and A.W. were responsible for revising the manuscript critically for important intellectual content. The manuscript has been revised by all co-authors.
Funding
This research was partially funded by the Polish Ministry of Science and Higher Education. This research was partially funded by the Faculty of Human Nutrition and Consumer Sciences, Warsaw University of Life Sciences, grant number 505-10-100200-N00322-99.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization/United Nations Children’s Fund . Global Strategy for Infant and Young Child Feeding. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 2.Eidelman A.I., Schanler R.J., Johnston M., Landers S., Noble L., Szucs K., Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 3.Fewtrell M., Bronsky J., Campoy C., Domellöf M., Embleton N., Fidler Mis N., Hojsak I., Hulst J.M., Indrio F., Lapillonne A., et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017;64:119–132. doi: 10.1097/MPG.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 4.Butte N.F., Lopez-Alarcon M.G., Garza C. Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant During the First 6 Months of Life. World Health Organization; Geneva, Switzerland: 2001. [(accessed on 20 November 2018)]. Available online: http://www.who.int/nutrition/publications/infantfeeding/nut_adequacy_of_exc_bfeeding_eng.pdf/ [Google Scholar]
- 5.Ballard O., Morrow A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielińska M.A., Wesołowska A., Pawlus B., Hamułka J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients. 2017;9:838. doi: 10.3390/nu9080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsopmo A. Phytochemicals in human milk and their potential antioxidative protection. Antioxidants. 2018;7:32. doi: 10.3390/antiox7020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victora C.G., Bahl R., Barros A.J., França G.V., Horton S., Krasevec J., Murch S., Sankar M.J., Walker N., Rollins N.C., et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 9.Mitoulas L.R., Kent J.C., Cox D.B., Owens R., Sherriff J.L., Hartmann P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002;88:29–37. doi: 10.1079/BJN2002579. [DOI] [PubMed] [Google Scholar]
- 10.Kent J.C., Mitoulas L.R., Cregan M.D., Ramsay D.T., Doherty D.A. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117:e387. doi: 10.1542/peds.2005-1417. [DOI] [PubMed] [Google Scholar]
- 11.Gidrewicz D.A., Fenton T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. doi: 10.1186/1471-2431-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napierala M., Mazela J., Merritt T.A., Florek E. Tobacco smoking and breastfeeding: Effect on the lactation process, breast milk composition and infant development. A critical review. Environ. Res. 2016;151:321–338. doi: 10.1016/j.envres.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Grote V., Verduci E., Scaglioni S., Vecchi F., Contarini G., Giovannini M., Koletzko B., Agostoni C., European Childhood Obesity Project Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016;70:250–256. doi: 10.1038/ejcn.2015.162. [DOI] [PubMed] [Google Scholar]
- 14.Bzikowska-Jura A., Czerwonogrodzka-Senczyna A., Olędzka G., Szostak-Węgierek D., Weker H., Wesołowska A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients. 2018;10:1379. doi: 10.3390/nu10101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrera C., Valenzuela R., Chamorro R., Bascuñán K., Sandoval J., Sabag N., Valenzuela F., Valencia M.P., Puigrredon C., Valenzuela A. The Impact of Maternal Diet during Pregnancy and Lactation on the Fatty Acid Composition of Erythrocytes and Breast Milk of Chilean Women. Nutrients. 2018;10:839. doi: 10.3390/nu10070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henjum S., Lilleengen A.M., Aakre I., Dudareva A., Gjengedal E.L.F., Meltzer H.M., Brantsæter A.L. Suboptimal Iodine Concentration in Breastmilk and Inadequate Iodine Intake among Lactating Women in Norway. Nutrients. 2017;9:643. doi: 10.3390/nu9070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen L.H. B vitamins in breast milk: Relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 2012;3:362–369. doi: 10.3945/an.111.001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aumeistere L., Ciproviča I., Zavadska D., Bavrins K., Borisova A. Zinc Content in Breast Milk and Its Association with Maternal Diet. Nutrients. 2018;10:1438. doi: 10.3390/nu10101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue Y., Campos-Giménez E., Redeuil K.M., Lévèques A., Actis-Goretta L., Vinyes-Pares G., Zhang Y., Wang P., Thakkar S.K. Concentrations of Carotenoids and Tocopherols in Breast Milk from Urban Chinese Mothers and Their Associations with Maternal Characteristics: A Cross-Sectional Study. Nutrients. 2017;9:1229. doi: 10.3390/nu9111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambati R.R., Phang S.M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini R.K., Nile S.H., Park S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015;76:735–750. doi: 10.1016/j.foodres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory USDA National Nutrient Database for Standard Reference, Legacy. [(accessed on 20 September 2018)]; Available online: https://ndb.nal.usda.gov/ndb/
- 23.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014;72:605–612. doi: 10.1111/nure.12133. [DOI] [PubMed] [Google Scholar]
- 25.Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Giordano E., Quadro L. Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Arch. Biochem. Biophys. 2018;647:33–40. doi: 10.1016/j.abb.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cena H., Castellazzi A.M., Pietri A., Roggi C., Turconi G. Lutein concentration in human milk during early lactation and its relationship with dietary lutein intake. Public Health Nutr. 2009;12:1878–1884. doi: 10.1017/S1368980009004807. [DOI] [PubMed] [Google Scholar]
- 28.Canfield L.M., Clandinin M.T., Davies D.P., Fernandez M.C., Jackson J., Hawkes J., Goldman W.J., Pramuk K., Reyes H., Sablan B., et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur. J. Nutr. 2003;42:133–141. doi: 10.1007/s00394-003-0403-9. [DOI] [PubMed] [Google Scholar]
- 29.Jackson J.G., Zimmer J.P. Lutein and zeaxanthin in human milk independently and significantly differ among women from Japan, Mexico, and the United Kingdom. Nutr. Res. 2007;27:449–453. doi: 10.1016/j.nutres.2007.04.020. [DOI] [Google Scholar]
- 30.Lipkie T.E., Morrow A.L., Jouni Z.E., McMahon R.J., Ferruzzi M.G. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS ONE. 2015;10:e0127729. doi: 10.1371/journal.pone.0127729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweigert F.J., Bathe K., Chen F., Büscher U., Dudenhausen J.W. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur. J. Nutr. 2004;43:39–44. doi: 10.1007/s00394-004-0439-5. [DOI] [PubMed] [Google Scholar]
- 32.Henriksen B.S., Chan G., Hoffman R.O., Sharifzadeh M., Ermakov I.V., Gellermann W., Bernstein P.S. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Investig. Ophthalmol. Vis. Sci. 2013;54:5568–5578. doi: 10.1167/iovs.13-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X., Zhao X., Berde Y., Low Y., Kuchan M. Milk and Plasma Lutein and Zeaxanthin Concentrations in Chinese Breast-Feeding Mother-Infant Dyads with Healthy Maternal Fruit and Vegetable Intake. J. Am. Coll. Nutr. 2018:1–6. doi: 10.1080/07315724.2018.1490934. [DOI] [PubMed] [Google Scholar]
- 34.Wądołowska L., Niedźwiedzka E. Food Frequency Questionnaire FFQ-6. [(accessed on 4 November 2018)]; Available online: www.uwm.edu.pl/edu/lidiawadolowska. (In Polish)
- 35.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 37.Juczyński Z., Ogińska-Bulik N. PSS-10–Perceived Stress Scale. Psychological Test Laboratory of the Polish Psychological Association; Warsaw, Poland: 2009. (In Polish) [Google Scholar]
- 38.Matczak A., Jaworowska A., Ciechanowicz A., Fecenec D., Stańczak J., Zalewska E. DSR—Children Development Scale DSR. Psychological Test Laboratory of the Polish Psychological Association; Warsaw, Poland: 2007. (In Polish) [Google Scholar]
- 39.Giuliano A.R., Neilson E.M., Yap H.H., Baier M., Canfield L.M. Methods of nutritional biochemistry quantitation of and inter/intra-individual variability in major carotenoids of mature human milk. J. Nutr. Biochem. 1994;5:551–556. doi: 10.1016/0955-2863(94)90054-X. [DOI] [Google Scholar]
- 40.Jackson J.G., Lien E.L., White S.J., Bruns N.J., Kuhlman C.F. Major carotenoids in mature human milk: Longitudinal and diurnal patterns. J. Nutr. Biochem. 1998;9:2–7. doi: 10.1016/S0955-2863(97)00132-0. [DOI] [Google Scholar]
- 41.Macias C., Schweigert F.J. Changes in the concentration of carotenoids, vitamin A, alpha-tocopherol and total lipids in human milk throughout early lactation. Ann. Nutr. Metab. 2001;45:82–85. doi: 10.1159/000046711. [DOI] [PubMed] [Google Scholar]
- 42.Szponar L., Wolnicka K., Rychlik E. Album of Photographs of Food Products and Dishes. National Food and Nutrition Institute; Warsaw, Poland: 2000. (In Polish) [Google Scholar]
- 43.Kunachowicz H., Nadolna I., Przygoda B., Iwanow K., editors. Food Composition Tables. PZWL; Warsaw, Poland: 2017. (In Polish) [Google Scholar]
- 44.ISAK . International Standards for Anthropometric Assessment. International Society for the Advancement of Kinanthropometry; Potchefstroom, South Africa: 2001. [(accessed on 18 November 2018)]. Available online: http://www.ceap.br/material/MAT17032011184632.pdf. [Google Scholar]
- 45.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. World Health Organization; Geneva, Switzerland: 2000. (Report of WHO Consultation, WHO Technical Report Series 894). [PubMed] [Google Scholar]
- 46.Xavier A.A.O., Díaz-Salido E., Arenilla-Vélez I., Aguayo-Maldonado J., Garrido-Fernández J., Fontecha J., Sánchez-García A., Pérez-Gálvez A. Carotenoid Content in Human Colostrum is Associated to Preterm/Full-Term Birth Condition. Nutrients. 2018;10:1654. doi: 10.3390/nu10111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song B.J., Jouni Z.E., Ferruzzi M.G. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition. 2013;29:195–202. doi: 10.1016/j.nut.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Bohn T., Desmarchelier C., Dragsted L.O., Nielsen C.S., Stahl W., Rühl R., Keijer J., Borel P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017;61:1600685. doi: 10.1002/mnfr.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S., Prime D.K., Hepworth A.R., Lai C.T., Trengove N.J., Hartmann P.E. Investigation of short-term variations in term breast milk composition during repeated breast expression sessions. J. Hum. Lact. 2013;29:196–204. doi: 10.1177/0890334412470213. [DOI] [PubMed] [Google Scholar]
- 50.De Azeredo V.B., Trugo N.M. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition. 2008;24:133–139. doi: 10.1016/j.nut.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Meneses F., Trugo N.M.F. Retinol, β-carotene, and lutein + zeaxanthin in the milk of Brazilian nursing women: Associations with plasma concentrations and influences of maternal characteristics. Nutr. Res. 2005;25:443–451. doi: 10.1016/j.nutres.2005.03.003. [DOI] [Google Scholar]
- 52.Strobel M., Tinz J., Biesalski H.K. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur. J. Nutr. 2007;46:1–20. doi: 10.1007/s00394-007-1001-z. [DOI] [PubMed] [Google Scholar]
- 53.Hanson C., Lyden E., Furtado J., Van Ormer M., White K., Overby N., Anderson-Berry A. Serum Lycopene Concentrations and Associations with Clinical Outcomes in a Cohort of Maternal-Infant Dyads. Nutrients. 2018;10:204. doi: 10.3390/nu10020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Litonjua A.A., Rifas-Shiman S.L., Ly N.P., Tantisira K.G., Rich-Edwards J.W., Camargo C.A., Jr., Weiss S.T., Gillman M.W., Gold D.R. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am. J. Clin. Nutr. 2006;84:903–911. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamułka J., Sulich A., Zielińska M., Wawrzyniak A. Assessment of carotenoid intake in a selected group of pregnant women. Probl. Hig. Epidemiol. 2015;96:763–768. [Google Scholar]
- 56.Mathews F., Yudkin P., Smith R.F., Neil A. Nutrient intakes during pregnancy: The influence of smoking status and age. J. Epidemiol. Community Health. 2000;54:17–23. doi: 10.1136/jech.54.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scaife A.R., McNeill G., Campbell D.M., Martindale S., Devereux G., Seaton A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br. J. Nutr. 2006;95:771–778. doi: 10.1079/BJN20051718. [DOI] [PubMed] [Google Scholar]
- 58.Watson P.E., McDonald B.W. Seasonal variation of nutrient intake in pregnancy: Effects on infant measures and possible influence on diseases related to season of birth. Eur. J. Clin. Nutr. 2007;61:1271–1280. doi: 10.1038/sj.ejcn.1602644. [DOI] [PubMed] [Google Scholar]
- 59.Miyake Y., Sasaki S., Tanaka K., Hirota Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758–765. doi: 10.1111/j.1398-9995.2009.02267.x. [DOI] [PubMed] [Google Scholar]
- 60.Johnson E.J., Maras J.E., Rasmussen H.M., Tucker K.L. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J. Am. Diet. Assoc. 2010;110:1357–1362. doi: 10.1016/j.jada.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Allore T., Lemieux S., Vohl M.C., Couture P., Lamarche B., Couillard C. Correlates of the difference in plasma carotenoid concentrations between men and women. Br. J. Nutr. 2018:1–10. doi: 10.1017/S0007114518003045. [DOI] [PubMed] [Google Scholar]
- 62.Sherry C.L., Oliver J.S., Renzi L.M., Marriage B.J. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J. Nutr. 2014;144:1256–1263. doi: 10.3945/jn.114.192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lietz G., Mulokozi G., Henry J.C., Tomkins A.M. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J. Nutr. 2006;136:1821–1827. doi: 10.1093/jn/136.7.1821. [DOI] [PubMed] [Google Scholar]
- 64.Nagayama J., Noda K., Uchikawa T., Maruyama I., Shimomura H., Miyahara M. Effect of maternal Chlorella supplementation on carotenoid concentration in breast milk at early lactation. Int. J. Food Sci. Nutr. 2014;65:573–576. doi: 10.3109/09637486.2014.898257. [DOI] [PubMed] [Google Scholar]
- 65.Haftel L., Berkovich Z., Reifen R. Elevated milk β-carotene and lycopene after carrot and tomato paste supplementation. Nutrition. 2015;31:443–445. doi: 10.1016/j.nut.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Koletzko B. Human Milk Lipids. Ann. Nutr. Metab. 2016;69:28–40. doi: 10.1159/000452819. [DOI] [PubMed] [Google Scholar]
- 67.Widomska J., Subczynski W.K. Why has nature chosen lutein and zeaxanthin to protect the retina? J. Clin. Exp. Ophthalmol. 2014;5:326. doi: 10.4172/2155-9570.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L., Gaziano J.M., Norkus E.P., Buring J.E., Sesso H.D. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am. J. Clin. Nutr. 2008;88:747–754. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson E.J., Hammond B.R., Yeum K.J., Qin J., Wang X.D., Castaneda C., Snodderly D.M., Russell R.M. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am. J. Clin. Nutr. 2000;71:1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 70.Knight B.A., Shields B.M., Brook A., Hill A., Bhat D.S., Hattersley A.T., Yajnik C.S. Lower Circulating B12 Is Associated with Higher Obesity and Insulin Resistance during Pregnancy in a Non-Diabetic White British Population. PLoS ONE. 2015;10:e0135268. doi: 10.1371/journal.pone.0135268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maffoni S., De Giuseppe R., Stanford F.C., Cena H. Folate status in women of childbearing age with obesity: A review. Nutr. Res. Rev. 2017;30:265–271. doi: 10.1017/S0954422417000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keaney J.F., Jr., Larson M.G., Vasan R.S., Wilson P.W., Lipinska I., Corey D., Massaro J.M., Sutherland P., Vita J.A., Benjamin E.J., et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.