Abstract
Background: Alternatives in treatment-strategies exist for resectable gastric cancer. Our aims were: (1) to assess the benefit of perioperative, neoadjuvant and adjuvant treatment-strategies and (2) to determine the optimal adjuvant regimen for gastric cancer treated with curative intent. Methods: PubMed, EMBASE, CENTRAL, and ASCO/ESMO conferences were searched up to August 2017 for randomized-controlled-trials on the curative treatment of resectable gastric cancer. We performed two network-meta-analyses (NMA). NMA-1 compared perioperative, neoadjuvant and adjuvant strategies only if there was a direct comparison. NMA-2 compared different adjuvant chemo(radio)therapy regimens, after curative resection. Overall-survival (OS) and disease-free-survival (DFS) were analyzed using random-effects NMA on the hazard ratio (HR)-scale and calculated as combined HRs and 95% credible intervals (95% CrIs). Results: NMA-1 consisted of 9 direct comparisons between strategies for OS (14 studies, n = 4187 patients). NMA-2 consisted of 16 direct comparisons between adjuvant chemotherapy/chemoradiotherapy regimens for OS (37 studies, n = 10,761) and 14 for DFS (30 studies, n = 9714 patients). Compared to taxane-based-perioperative-chemotherapy, surgery-alone (HR = 0.58, 95% CrI = 0.38–0.91) and perioperative-chemotherapy regimens without a taxane (HR = 0.79, 95% CrI = 0.58–1.15) were inferior in OS. After curative-resection, the doublet oxaliplatin-fluoropyrimidine (for one-year) was the most efficacious adjuvant regimen in OS (HR = 0.47, 95% CrI = 0.28–0.80). Conclusions: For resectable gastric cancer, (1) taxane-based perioperative-chemotherapy was the most promising treatment strategy; and (2) adjuvant oxaliplatin-fluoropyrimidine was the most promising regimen after curative resection. More research is warranted to confirm or reproach these findings.
Keywords: stomach neoplasms, chemotherapy, chemoradiotherapy, perioperative
1. Introduction
Gastric adenocarcinoma is one of the leading causes of cancer related mortality on a global scale [1]. Even after a curative resection, relapse-related death remains a major problem. There is no global consensus on the optimum treatment strategy (perioperative, neoadjuvant or adjuvant systemic therapy and/or radiotherapy) to be administered in addition to surgery for resectable gastric cancer. Perioperative chemotherapy is the preferred treatment strategy in many countries in Europe, as there is evidence this will reduce the number of relapses [2]. For a decade, the perioperative anthracycline-based MAGIC regimen, consisting of epirubicin, cisplatin and 5-FU was the preferred option [3]. Recently, the FLOT-4 trial established the superiority of a perioperative taxane-based regimen with docetaxel, oxaliplatin and 5-FU with leucovorin (FLOT) over perioperative epirubicin, cisplatin and a fluoropyrimidine; 5-FU or capecitabine (ECF/ECX) [4]. The FLOT regimen significantly improved survival (median 50 months with FLOT and 35 months with ECF/ECX) and led to a higher number of R0 resections (84% with FLOT and 77% with MAGIC) [4]. In Asian countries, after a curative resection, adjuvant chemotherapy, usually without any neoadjuvant therapy, is the standard of care [5]. For example, adjuvant oxaliplatin combined with capecitabine or S-1 as monotherapy after curative resection are two established treatment regimens [5]. Finally, in the United States adjuvant chemotherapy with radiotherapy after curative resection is a frequently applied treatment strategy, based on the intergroup 0116 trial [6]. However, the American NCCN guideline also acknowledges the benefit of the other treatment strategies, including perioperative and adjuvant chemotherapy [7].
After the landmark MAGIC trial, neoadjuvant and perioperative strategies were more frequently applied to improve overall survival [3]. In perioperative trials, only half of all patients start with adjuvant therapy after a surgical resection [3,4]. The question rises whether administration of neoadjuvant, or adjuvant therapy only would lead to the same survival benefit as a perioperative regimen. Moreover, the optimal adjuvant regimen after a curative resection has not yet been established. Network meta-analysis (NMA) allows for the comparison of more than two treatments at once by introducing a common comparator (e.g., surgery only) and combining direct with indirect estimates into a combined effect size [8,9]. Therefore, NMA can aid clinical decision making by comparing different regimens with one or multiple comparators, even if studies comparing regimens head-to-head are not available. NMA can also aid in finding the optimum treatment backbone for future randomized trials.
We conducted a systematic review, using NMA, with two primary aims regarding efficacy: (1) compare the clinical benefit of perioperative, neoadjuvant and adjuvant treatment strategies; and (2) to establish the optimal adjuvant regimen after a curative resection for gastric cancer. Our secondary aim was to investigate the safety of different chemo(radio)therapy regimens.
2. Results
2.1. Description of the Included Studies
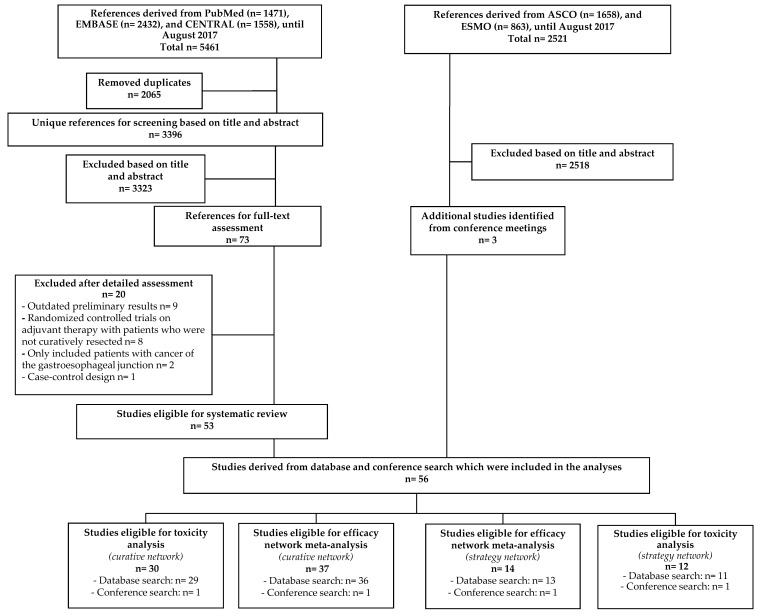
From a total of 5461 unique references, identified by searching PubMed, Embase and Central, 73 references remained after title and abstract screening. 20 references were excluded after full text assessment including the SAMIT trial for the primary analysis as it included R1 resected patients [10]. The results of the SAMIT trial were only used for a sensitivity analysis. By searching the conference meetings of the ASCO and ESMO meetings three additional studies were identified. In total, 56 studies (n = 15,795 patients) could be included in any of the analyses (Figure 1). Two separate networks were created, one comparing different treatment strategies; perioperative, neoadjuvant and adjuvant therapy and one in which different adjuvant regimens were compared after a curative resection. Before merging different treatment strategies or drug classes, a preliminary NMA was conducted for both networks. When taxane-based neoadjuvant and taxane-based adjuvant chemotherapy were separated from non-taxane containing neoadjuvant/adjuvant chemotherapy the network lost the ability to detect any significant difference between comparisons (Figures S1 and S2). Therefore, due to the low amount of studies and patients for each comparison neoadjuvant regimens were pooled together as well as different adjuvant regimens. Based on the FLOT-4 trial, taxane-based perioperative chemotherapy was kept as a separate clinical entity compared to non-taxane containing perioperative chemotherapy [4]. A description of the baseline characteristics (Table 1 and Table 2), pairwise meta-analyses (Figures S3–S5) and risk of bias of both NMAs can be found in the Supplementary Results (Figures S6 and S7).
Figure 1.
Flowchart of references derived from database search (left) and from conference search (right). Due to the absence of enough data to calculate a hazard ratio for survival, three studies on different treatment strategies and two studies on adjuvant therapy after curative resection were only eligible for the toxicity analyses. N = number of studies.
Table 1.
Baseline characteristics of studies included in the treatment-strategy network meta-analysis (NMA-1).
| Studies | No. | Regimen | Node | Stage 1 | D2 or > LND No. (%) | Descent | Age, Median, (Range), y | Men No. (%) |
|---|---|---|---|---|---|---|---|---|
| Perioperative Chemotherapy vs. Surgery | ||||||||
| Ychou 2011 [11] | 113 | Peri + Cis + 5-FU | PC | I–IV | D2 | W | 63 (36-75) | 96 (85) |
| 111 | Surg | S | I–IV | W | 63 (38–75) | 91 (82) | ||
| Cunningham 2006 [3] | 250 | Peri + Epi + Cis + 5-FU | PC | II–III | 93 (37) | W | 62 (29–85) | 205 (82) |
| 253 | Surg | S | II–III | 96 (38) | W | 62 (23–81) | 191 (76) | |
| Perioperative Chemotherapy vs. Perioperative Chemotherapy + Bevacizumab | ||||||||
| Cunningham 2017 [12] | 533 | Peri + Epi + Cis + Cap | PC | II–III | D1+D2 | W | 63 (31–79) | 434 (82) |
| 530 | Peri + Epi + Cis + Cap + BEV | PCB | II–III | W | 64 (28–82) | 425 (80) | ||
| Perioperative Chemotherapy vs. Perioperative Chemotherapy + Radiotherapy | ||||||||
| Verheij 2016 [13] | 393 | Peri + Epi + Cis/Ox + Cap | PC | I–III | 40 (6) | W | 62 | 264 (67) |
| 395 | Peri + Epi + Cis/Ox + Cap + RT | PCR | I–III | W | 265 (67) | |||
| Perioperative Chemotherapy vs. Adjuvant Chemotherapy | ||||||||
| Nio 2004 [14] | 102 | Peri + UFT | PC | I–IV | 58 (57) | A | 64 (±12) | 70 (69) |
| 193 | UFT | AC | I–IV | 95 (49) | A | 65 (±12) | 141 (73) | |
| Perioperative Chemotherapy Taxane Based vs. Perioperative Chemotherapy | ||||||||
| Al-Batran 2017 [4] | 356 | Peri + Dtx + Ox + 5-FU/Lv | PCT | II–III | D2 | W | 62 | 530 (74) |
| 360 | Peri + Epi + Cis + 5-FU/Cap | PC | II–III | W | ||||
| Perioperative Chemotherapy Taxane Based vs. Perioperative Chemotherapy + Bevacuzimab | ||||||||
| Ma 2015 [15] | 40 | Peri + Dtx + Ox + 5-FU/Lv | PCT | II–III | 21 (53) | A | 53 * | 22 (55) |
| 40 | Peri + Dtx + Ox + 5-FU/Lv + BEV | PCB | II–III | 31 (78) | A | 55 * | 24 (60) | |
| Perioperative Chemotherapy Taxane Based vs. Adjuvant Chemotherapy | ||||||||
| Cui 2014 [16] | 48 | Peri + Ptx + Cis + Tgf | PCT | II–III | NR | A | 55 (41–69) * | 19 (40) |
| 48 | Ptx + Cis + Tgf | AC | II–III | NR | A | 56 (39–72) * | 21 (44) | |
| Qu 2010 [17] | 39 | Peri + Ptx + Ox + 5-FU/Lv | PCT | II–III | NA | A | NA | NA |
| 39 | Ptx + Ox + 5-FU/Lv | AC | II–III | NA | A | NA | NA | |
| Neoadjuvant Chemotherapy vs. Surgery | ||||||||
| Imano 2010 [18] | 16 | Neo + Cis + 5-FU | NC | II–III | 16 (100) | A | 58 (±12) | 13 (81) |
| 16 | Surg | S | II–III | 16 (100) | A | 60 (±8) | 9 (56) | |
| Schuhmacher 2010 [19] | 72 | Neo + Cis + 5-FU/Lv | NC | III–IV | 67 (96) | W | 56 (38–70) | 50 (69) |
| 72 | Surg | S | III–IV | 63 (93) | W | 58 (26–69) | 50 (69) | |
| Zhao 2006 [20] | 20 | Neo + 5-FU/Lv | NC | I–IV | NR | A | 58 (32–70) * | NR |
| 20 | Surg | S | I–IV | NR | A | NR | ||
| Hartgrink 2004 [21] | 29 | Neo + Doxo + 5-FU/Lv + MTX | NC | I–IV | D1 | W | 60 (34–75) * | 32 (54) |
| 30 | Surg | S | I–IV | W | ||||
| Neoadjuvant Chemotherapy vs. Adjuvant Chemotherapy | ||||||||
| Fazio 2016 [22] | 34 | Neo + Dtx + Cis + 5-FU | NC | I–IV | 62 (90) | W | 57 (25–75) | 23 (68) |
| 35 | Dtx + Cis + 5-FU | AC | I–IV | W | 59 (39–76) | 24 (69) | ||
1 Staging was done according to the 7th edition of the AJCC and according to the pathological TNM stage [23]. Nio 2004 administered epirubicin, cisplatin and 5-FU to stage IV patients. Qu 2010 and Cui 2014 administered epirubicin, cisplatin and 5-FU after progression. Ma 2015 administered irinotecan, 5-FU and leucovorin when there was no response on initial therapy. * Mean age was given instead of median age. Abbreviations: 5-FU = 5-fluorouracil; A = Asian; AC = adjuvant chemotherapy; BEV = bevacizumab; Cap = capecitabine; Cis = cisplatin; Doxo = doxorubicin; Dtx = docetaxel; Epi = epirubicin; LND = lymph node dissection; Lv = leucovorin; MTX = methotrexate; NA = not available; NC = neoadjuvant chemotherapy; Neo = neoadjuvant; No. = number; NR = not reported; Ox = oxaliplatin; PC = perioperative chemotherapy; PCB = perioperative chemotherapy with bevacizumab; PCT = perioperative taxane-based chemotherapy; PCR = perioperative chemotherapy with adjuvant radiotherapy; Peri = perioperative; Ptx = paclitaxel; S = surgery alone; RT = radiotherapy; Surg = surgery; Tgf = tegafur; UFT = tegafur/uracil; W = western; y = years.
Table 2.
Baseline characteristics of studies included in the adjuvant therapy for curatively resected gastric cancer network meta-analysis (NMA-2).
| Studies | No. | Regimen | Node | Stage 1 | D2 or > LND No. (%) | Descent | Age, Median, (Range), y | Men No. (%) |
|---|---|---|---|---|---|---|---|---|
| Anthracycline + Fluoropyrimidine vs. Observation | ||||||||
| Neri 2001 [24] | 69 | Epi + 5-FU/Lv | AF | II–III | 9 (13) | W | 62 (37–73) | 50 (72.5) |
| 68 | Observation | Obs | II–III | 10 (15) | W | 64 (35–74) | 48 (70.6) | |
| Krook 1991 [25] | 61 | Doxo + 5-FU | AF | I–III | NR | W | 63 (33–77) | 47 (77) |
| 64 | Observation | Obs | I–III | NR | W | 62 (38–78) | 51 (80) | |
| Anthracycline + Doublet vs. Observation | ||||||||
| Kulig 2010 [26] | 141 | Doxo + Eto + Cis | ATr | I–III | 112 (79) | W | 61 (58–67) | 100 (71) |
| 154 | Observation | Obs | I–III | 123 (80) | W | 64 (61–66) | 111 (72) | |
| Di Costanzo 2008 [27] | 130 | Epi + Cis + 5-FU/Lv | ATr | I–III | 71 (55) | W | 59 | 79 (61) |
| 128 | Observation | Obs | I–III | 72 (56) | W | 59 | 78 (61) | |
| De Vita 2007 [28] | 112 | Epi + Eto + 5-FU/Lv | ATr | I–III | 0 | W | 63 (39–70) | 66 (59) |
| 113 | Observation | Obs | I–III | 0 | W | 62 (41–70) | 65 (58) | |
| Tentes 2006 [29] | 20 | Doxo + MMC + 5-FU | ATr | II–III | 20 (100) | W | 65 (±10) * | 14 (70) |
| 20 | Observation | Obs | II–III | 20 (100) | W | 65 (±11) * | 11 (55) | |
| Tsavaris 1996 [30] | 42 | Epi + MMC + 5-FU | ATr | III | NR | W | 53 (41–65) * | 32 (76) |
| 42 | Observation | Obs | III | NR | W | 57 (35–66) * | 25 (60) | |
| Lise 1995 [31] | 155 | Doxo + MMC + 5-FU | ATr | II–III | 84 (27) | W | <71 years | 94 (61) |
| 159 | Observation | Obs | II–III | W | <71 years | 108 (68) | ||
| Coombes 1990 [32] | 133 | Doxo + MMC + 5-FU | ATr | II–III | NR | W | 57 * | 93 (70) |
| 148 | Observation | Obs | II–III | NR | W | 57 * | 98 (68) | |
| Anthracycline + Etoposide + Cisplatin + Fluoropyrimidine vs. Observation | ||||||||
| Bajetta 2002 [33] | 135 | Doxo + Eto + Cis + 5-FU/Lv | AECF | II–III | Maj. | W | 57 (23–70) | 81 (59) |
| 136 | Observation | Obs | II–III | Maj. | W | 57 (31–70) | 93 (68) | |
| Anthracycline + Doublet vs. Fluoropyrimidine | ||||||||
| Cascinu 2007 [34] | 201 | Epi + Cis + 5-FU/Lv | ATr | II–III | 312 (79) | W | 58 | 135 (67) |
| 196 | 5FU/Lv | F | II–III | W | 59 | 120 (61) | ||
| Lee 2004 [35] | 32 | Epi + Cis + 5-FU/Lv | ATr | III | 32 (100) | A | 53 (31–61) | 13 (41) |
| 29 | 5-FU | F | III | 29 (100) | A | 52 (26–66) | 13 (45) | |
| Anthracycline + Fluoropyrimidine vs. Mitomycin C + Fluoropyrimidine vs. Fluoropyrimidine | ||||||||
| Tsujinaka 2000 [36] | 61 | Epi + 5-FU | AF | I–II | 60 (98) | A | ≤75 years | 38 (62) |
| 62 | MMC + 5-FU | MF | I–II | 61 (98) | A | ≤75 years | 44 (71) | |
| 62 | 5-FU | F | I–II | 61 (98) | A | ≤75 years | 44 (71) | |
| Anthracycline + Doublet vs. Mitomycin C + Fluoropyrimidine vs. Fluoropyrimidine | ||||||||
| Chang 2002 [37] | 131 | Doxo + MMC + 5-FU | ATr | I–III | 131 (100) | A | 51 (26–70) | 100 (76) |
| 131 | MMC + 5-FU | MF | I–III | 131 (100) | A | 54 (23–74) | 96 (73) | |
| 133 | 5-FU | F | I–III | 133 (100) | A | 53 (21–75) | 99 (74) | |
| Cisplatin + Fluoropyrimidine vs. Observation | ||||||||
| Bouche 2005 [38] | 127 | Cis + 5-FU | CF | II–III | 70 (27) | W | 60 (32–82) | 93 (73) |
| 133 | Observation | Obs | II–III | W | 62 (31–83) | 93 (70) | ||
| Chipponi 2004 [39] | 93 | Cis + 5-FU/Lv | CF | II–III | D1+D2 | W | 59 * | 58 (62) |
| 103 | Observation | Obs | II–III | W | 63 * | 71 (69) | ||
| Fluoropyrimidine vs. Observation | ||||||||
| Sasako 2011 [40] | 529 | S-1 | F | II–III | 529 (100) | A | 63 (27–80) | 367 (69) |
| 530 | Observation | Obs | II–III | 530 (100) | A | 63 (33–80) | 369 (70) | |
| Nakajima 2007 [41] | 93 | UFT | F | II–III | 93 (100) | A | 63 | 75 (70) |
| 95 | Observation | Obs | II–III | 95 (100) | A | 64 | 77 (73) | |
| Mitomycin C vs. Observation | ||||||||
| Grau 1993 [42] | 68 | MMC | M | I–III | NR | W | 56 * | 44 (65) |
| 66 | Observation | Obs | I–III | NR | W | 57 * | 44 (67) | |
| Mitomycin C + Fluoropyrimidine vs. Observation | ||||||||
| Cirera 1999 [43] | 76 | MMC + Tgf | MF | I–III | 76 (100) | W | 61 * | 52 (68) |
| 72 | Observation | Obs | I–III | 72 (100) | W | 61 * | 42 (58) | |
| Kim 1992 [44] | 77 | MMC + 5-FU | MF | III | 77 (100) | A | (30–70) | NR |
| 94 | Observation | Obs | III | 94 (100) | A | (30–70) | NR | |
| Mitomycin C + Fluoropyrimidine vs. Mitomycin C | ||||||||
| Grau 1998 [45] | 40 | MMC + Tgf | MF | I–III | D1+D2 | W | 62 (36–75) | 27 (68) |
| 45 | MMC | M | I–III | W | 63 (22–75) | 27 (60) | ||
| Mitomycin C + Cisplatin + Fluoropyrimidine vs. Mitomycin C + Fluoropyrimidine | ||||||||
| Kang 2013 [46] | 431 | MMC + Cis + 5DFUR | MCF | II–III | 431 (100) | A | 55 (20–70) | 294 (68) |
| 424 | MMC + 5DFUR | MF | II–III | 424 (100) | A | 56 (29–70) | 294 (69) | |
| Mitomycin C + Cisplatin + Fluoropyrimidine vs. Cisplatin + Fluoropyrimidine | ||||||||
| Shimoyama 1999 [47] | 12 | MMC + Cis + UFT (600 mg) | MCF | I–III | D1+D2 | A | 65 (±8) | 13 (77) |
| 17 | Cis + UFT | CF | I–III | A | 64 (±8) | 8 (67) | ||
| Oxaliplatin + Fluoropyrimidine vs. Observation | ||||||||
| Noh 2014 [48] | 520 | Ox + Cap | OxF | II–III | 520 (100) | A | 56 (±11) * | 373 (72) |
| 515 | Observation | Obs | II–III | 515 (100) | A | 56 (±11) * | 358 (70) | |
| Oxaliplatin + Fluoropyrimidine vs. Fluoropyrimidine | ||||||||
| Zhang 2011 [49] | 42 | Ox + 5-FU/Lv | OxF | II–III | 42 (100) | A | 48 | 25 (60) |
| 38 | 5-FU/Lv | F | II–III | 38 (100) | A | 54 | 24 (63) | |
| Oxaliplatin + Fluoropyrimidine Prolonged vs. Oxaliplatin + Fluoropyrimidine | ||||||||
| Feng 2015 [50] | 152 | Ox + Cap (Prolonged) | OxFPr | II–III | 152 (100) | A | 61 (±11) | 104 (67) |
| 155 | Ox + Cap | OxF | II–III | 155 (100) | A | 60 (±10) | 99 (65) | |
| Radiotherapy + Chemotherapy vs. Observation | ||||||||
| Smalley 2012 [6] | 281 | RT + 5-FU/Lv | RCh | I–III | 54 (10) | W | 60 (25–87) | 202 (72) |
| 275 | Observation | Obs | I–III | W | 59 (23–80) | 195 (71) | ||
| Radiotherapy + Chemotherapy vs. Fluoropyrimidine | ||||||||
| Kim 2012 [51] | 46 | RT + 5-FU/Lv | RCh | III | 46 (100) | A | 9> 60 | 34 (74) |
| 44 | 5-FU/Lv | F | III | 44 (100) | A | 14>60 | 25 (57) | |
| Yu 2012 [52] | 34 | RT + 5-FU/Lv | RCh | II–III | D1+D2 | A | NR | NR |
| 34 | 5-FU/Lv | F | II–III | A | NR | NR | ||
| Zhu 2012 [53] | 186 | RT + 5-FU/Lv | RCh | I–III | 205 (100) | A | 56 (38–73) | 135 (73) |
| 165 | 5-FU/Lv | F | I–III | 175 (100) | A | 59 (42–75) | 126 (76) | |
| Radiotherapy + Chemotherapy vs. Cisplatin + Fluoropyrimidine | ||||||||
| Park 2015 [54] | 230 | RT + Cis + Cap | RCh | I–III | 230 (100) | A | 56 (28–76) | 143 (62) |
| 228 | Cis + Cap | CF | I–III | 228 (100) | A | 56 (22–77) | 153 (67) | |
| Kwon 2010 [55] | 31 | RT + Cis + Cap + 5-FU | RCh | III | 31 (100) | A | 8 ≥ 60 | 21 (68) |
| 30 | Cis + 5-FU | CF | III | 30 (100) | A | 14 ≥ 60 | 23 (77) | |
| Radiotherapy + Chemotherapy vs. Taxane + Cisplatin | ||||||||
| Bamias 2010 [56] | 72 | RT + Dtx + Cis/Car | RCh | II–III | D0+D1+D2 | W | 63 (32–75) | 48 (67) |
| 71 | Dtx + Cis/Car | TC | II–III | W | 62 (41–79) | 52 (73) | ||
| Taxane + Fluoropyrimidine vs. Cisplatin + Fluoropyrimidine | ||||||||
| Lee 2016 [57] | 75 | Dtx + S-1 | TF | III | 75 (100) | A | NR | NR |
| 78 | Cis + S-1 | CF | III | 78 (100) | A | NR | NR | |
| Taxane + Irinotecan + Cisplatin + Fluoropyrimidine vs. Fluoropyrimidine or Mitomycin C | ||||||||
| Bajetta 2014 [58] | 562 | Dtx + IRI + Cis + 5-FU/Lv | TICF | II–III | 796 (72) | W | ≤75 years | NR |
| 538 | 5-FU/Lv | F | II–III | W | ≤75 years | NR | ||
| Di Bartolomeo 2006 [59] | 85 | Dtx + IRI + Cis + 5-FU/Lv | TICF | II–III | 66 (77) | W | 10 ≥ 70 | 60 (71) |
| 81 | MMC | M | II–III | 62 (76) | W | 8 ≥ 70 | 55 (68) | |
1 Staging was done according to the 7th edition of the AJCC and according to the pathological TNM stage [23]. * Mean age was given instead of median age. Abbreviations: 5-DFUR = doxifluridine; 5-FU = 5-fluorouracil; A = anthracycline; A descent = Asian; ATr = anthracycline-based triplet; Cap = capecitabine; Car = carboplatin; C = cisplatin; Cis = cisplatin; Doxo = doxorubicin; Dtx = docetaxel; E = etoposide; Epi = epirubicin; Eto = etoposide; F = fluoropyrimidine; I = irinotecan; IRI = irinotecan; LND = lymph node dissection; Lv = leucovorin; M = mitomycin C; MMC = mitomycin C; No. = number; NR = not reported; Obs = observation; Ox = oxaliplatin; OxFpr = doublet oxaliplatin with an one year treatment with a fluoropyrimidine; RT = radiotherapy; RCh = chemoradiotherapy; T = taxane; Tgf = tegafur; UFT = uracil/tegafur; W = western; y = years.
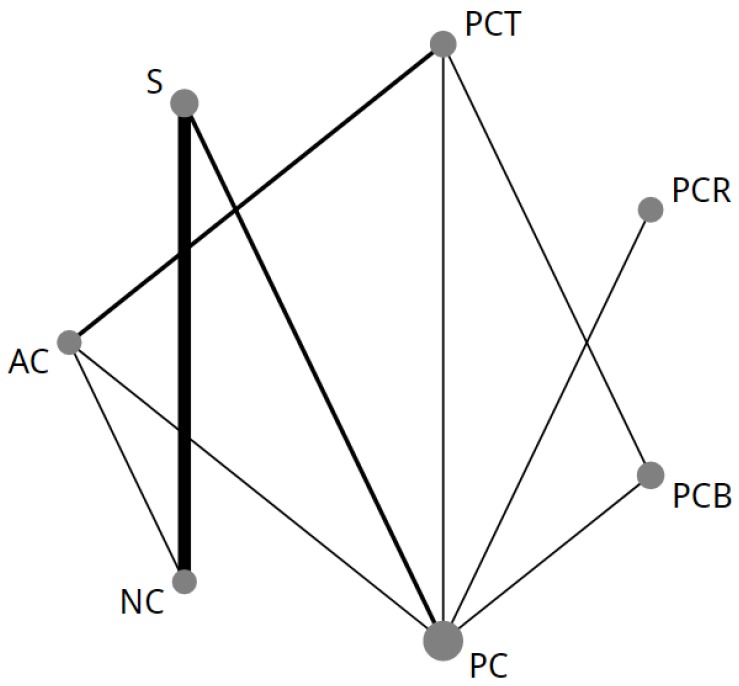
The NMA comparing treatment strategies (NMA-1) consisted of 14 individual studies [3,4,11,12,13,14,15,16,17,18,19,20,21,22] and seven different treatment strategies (Figure 2). For OS there were nine direct comparisons (n = 4187 patients). For one study, the HR for OS was extracted from a previously conducted meta-analysis [17,60]. There was insufficient data available to conduct a NMA for progression free survival or disease free survival.
Figure 2.
First network of all treatments in the strategy network meta-analysis (NMA-1). The size of each node corresponds to the number of patients who were randomly assigned to receive the given regimen. The lines connect the regimens that were directly compared in head-to-head randomized controlled trials (RCTs). The thickness of the lines corresponds to the number of RCTs. AC = adjuvant chemotherapy; NC = neoadjuvant chemotherapy; PC = perioperative chemotherapy without a taxane; PCB = perioperative chemotherapy combined with bevacizumab; PCR = perioperative chemotherapy combined with adjuvant chemoradiotherapy; PCT = taxane-based perioperative chemotherapy; S = surgery only.
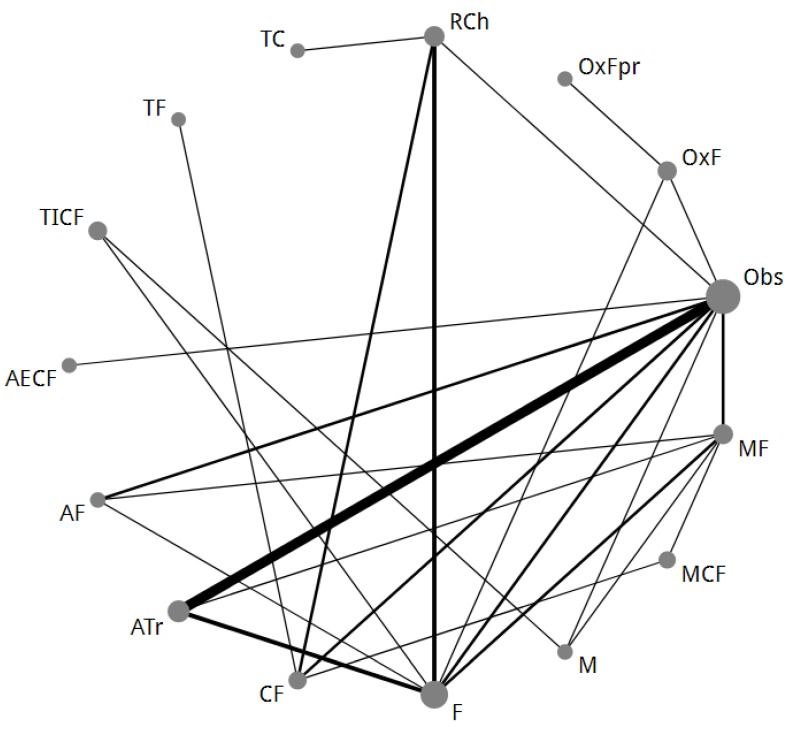
After merging, a total of 37 studies [6,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] were included in the NMA comparing adjuvant therapy after curative resection (NMA-2), with 14 different radio/chemotherapy regimens. For OS, there were 16 direct comparisons between different regimens with in total n = 10,761 patients (Figure 3). For DFS, there were 14 direct comparisons with in total n = 9714 patients. There was not enough data available to calculate the HR for OS and DFS in the published reports of seven RCTs [25,30,31,32,42,51,55] and therefore, HRs were extracted from a previously conducted individual patient data meta-analysis or a meta-analysis [61,62].
Figure 3.
Second network of all different treatment regimens in the adjuvant therapy for curatively resected gastric cancer network meta-analysis (NMA-2). The size of each node corresponds to the number of patients who were randomly assigned to receive the given regimen. The lines connect the regimens that were directly compared in head-to-head randomized controlled trials (RCTs). The thickness of the lines corresponds to the number of RCTs. A = anthracycline; ATr = anthracycline-based triplet; C = cisplatin; E = etoposide; F = fluoropyrimidine; I = irinotecan; M = mitomycin C; Obs = observation; Ox = oxaliplatin; OxFpr = eight cycles of oxaliplatin-fluoropyrimidine thereafter eight cycles of fluoropyrimidine monotherapy; RCh = chemoradiotherapy; T = taxane.
2.2. NMA-1 Comparing Different Treatment Strategies
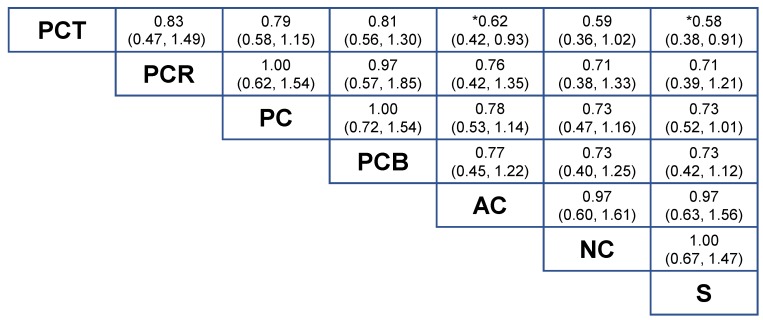
OS could be compared in the strategy-based NMA-1 (Figure 4). Taxane-based perioperative chemotherapy (PCT) was the most effective treatment strategy compared to surgery alone (S), HR = 0.58 (95% CrI = 0.38 to 0.91). Taxane-based perioperative chemotherapy was superior compared to adjuvant chemotherapy (AC), HR = 0.62 (95% CrI = 0.42 to 0.93) and was non-significant compared to neoadjuvant chemotherapy (NC), HR = 0.59 (95% CrI = 0.36 to 1.02) although a clinically-relevant HR was found (HR < 0.80). Compared to perioperative chemotherapy without a taxane (PC), the addition of adjuvant chemoradiotherapy (PCR), HR = 1.00 (95% CrI = 0.62 to 1.54) or bevacizumab to perioperative chemotherapy (PCB), HR = 1.00 (95% CrI = 0.72 to 1.54) did not result in a survival benefit. Compared to surgery-alone, no survival benefit was found for neoadjuvant chemotherapy, HR = 1.00 (95% CrI = 0.67 to 1.47) nor for adjuvant chemotherapy, HR = 0.97 (95% CrI = 0.63 to 1.56).
Figure 4.
Results of the treatment-strategy random effects network meta-analysis (NMA-1) for seven different strategies in terms of overall survival. Relative effects in combined hazard ratios and 95% credible intervals are shown for the combination chemotherapy regimens. The hazard ratio for a given comparison could be read in the intersection of two treatments. The strategies are grouped according to their baseline efficacy compared with surgery-alone. All z-tests to compare two treatments were performed two-sided. * p < 0.05. Abbreviations: AC = adjuvant chemotherapy; NC = neoadjuvant chemotherapy; PC = perioperative chemotherapy regimens without a taxane; PCB = perioperative chemotherapy combined with bevacizumab; PCR = perioperative chemotherapy combined with adjuvant chemoradiotherapy; PCT = taxane-based perioperative chemotherapy; S = surgery only.
2.3. NMA-2 Comparing Adjuvant Regimens after Curative Resection
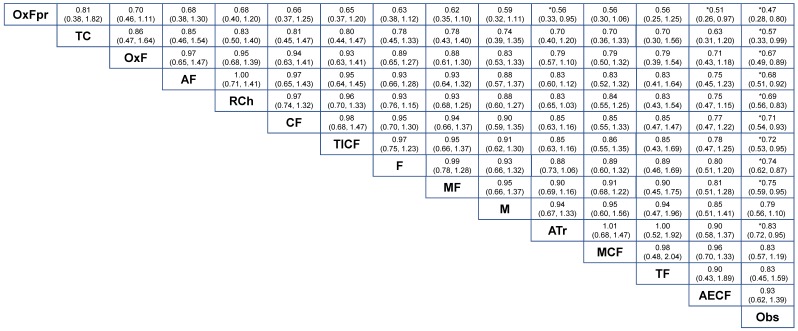
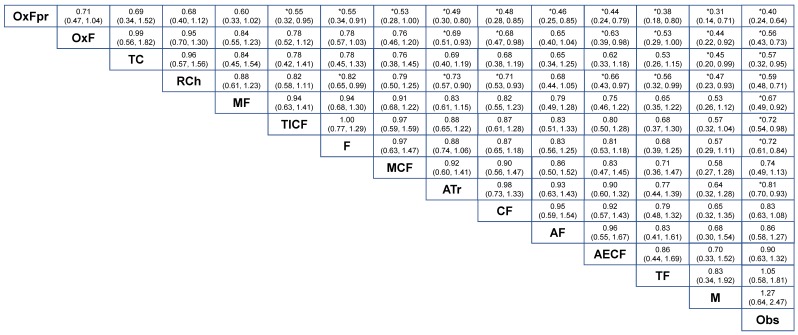
The results for OS and DFS for NMA-2 are summarized in Figure 5 and Figure 6. Compared with observation-alone (Obs), the largest survival benefit was found for oxaliplatin with a prolonged 1-year course of a fluoropyrimidine (OxF-prolonged) which reached a HR = 0.47 (95% CrI = 0.28 to 0.80) for OS and HR = 0.40 (95% CrI = 0.24 to 0.64) for DFS. OxF-prolonged showed a non-significant but clinically-relevant HR to fluoropyrimidine-monotherapy (F), HR = 0.63 (95% CrI = 0.38 to 1.12) in OS. In addition, OxF-prolonged was more effective in terms of DFS compared to fluoropyrimidine monotherapy, HR = 0.55 (95% CrI = 0.34 to 0.91). OxF showed superior efficacy compared to a cisplatin-fluoropyrimidine doublet (CF) in DFS, HR = 0.68 (95% CrI = 0.47 to 0.98) but not in OS (Figure 5). Increased efficacy was found for OxF-prolonged compared to an anthracycline-based triplet (ATr) in terms of both OS, HR = 0.56 (95% CrI = 0.33 to 0.95) and DFS, HR = 0.49 (95% CrI = 0.30 to 0.80). Radiotherapy combined with chemotherapy (RCh) showed no benefit compared to OxF-prolonged, OxF or a taxane-cisplatin doublet (TC) in the OS and DFS analysis (Figure 5 and Figure 6).
Figure 5.
Results of the adjuvant therapy for curatively resected gastric cancer random effects network meta-analysis (NMA-2) for 14 different treatment modalities in terms of overall survival. Relative effects in combined hazard ratios and 95% credible intervals are shown for the combination chemotherapy regimens. The hazard ratio for a given comparison could be read in the intersection of two treatments. The strategies are grouped according to their baseline efficacy compared with observation-alone. All z-tests to compare two treatments were performed two-sided. * p < 0.05. Abbreviations: A = anthracycline; ATr = anthracycline-based triplet; C = cisplatin; E = etoposide; F = fluoropyrimidine; I = irinotecan; M = mitomycin C; Obs = observation; Ox = oxaliplatin; OxFpr = eight cycles of oxaliplatin-fluoropyrimidine thereafter eight cycles of fluoropyrimidine monotherapy; RCh = chemoradiotherapy; T = taxane.
Figure 6.
Results of the adjuvant therapy for curatively resected gastric cancer random effects network meta-analysis (NMA-2) for 14 different treatment modalities in terms of disease free survival. Relative effects in combined hazard ratios and 95% credible intervals are shown for the combination chemotherapy regimens. The hazard ratio for a given comparison could be read in the intersection of two treatments. The strategies are grouped according to their baseline efficacy compared with observation-alone. All z-tests to compare two treatments were performed two-sided. * p < 0.05. Abbreviations: A = anthracycline; ATr = anthracycline-based triplet; C = cisplatin; E = etoposide; F = fluoropyrimidine; I = irinotecan; M = mitomycin C; Obs = observation; Ox = oxaliplatin; OxFpr = eight cycles of oxaliplatin-fluoropyrimidine thereafter eight cycles of fluoropyrimidine monotherapy; RCh = chemoradiotherapy; T = Taxane.
2.4. Network Consistency and Sensitivity Analyses
An extended description of the assessment of network inconsistency and the comparison between direct and combined HRs can be found in the Supplementary Results (Figures S8–S10). Node-split models were non-significant for both NMAs. For NMA-1, perioperative trials were mainly studied in a Western population. Sensitivity analysis for descent, stage and type of lymph node dissection showed the same overall trend indicating perioperative chemotherapy with a taxane is the most promising treatment strategy. However, it must be taken into account the sensitivity analyses for NMA-1 were relatively underpowered due to the low amount of studies per sensitivity analysis. For NMA-2 oxaliplatin containing regimens were only studied in Asian D2 dissected patients. For the other regimens, sensitivity analyses for descent, stage and type of lymph node dissection did not have a major impact on the direction of the HR. For NMA-2, when the results of the comparison between fluoropyrimidine monotherapy and sequential therapy with a fluoropyrimidine and a taxane (TF) were added from the SAMIT trial, which included 7% R1 resected patients, TF reached a significant HR = 0.71 (95% CrI = 0.54 to 0.93) for OS compared to observation.
2.5. Toxicity and Surgical Complications
In total, 12 studies for the treatment strategy NMA-1 contributed to the grade 3–4 toxicity and surgical related adverse events (AEs) pair-wise meta-analyses. For the NMA-2 comparing adjuvant therapy after curative resection 30 studies were included in the grade 3–4 toxicity AEs pair-wise meta-analyses. For NMA-2 only regimens which were significant (p < 0.05) compared to observation-alone were included in the grade 3–4 AE analyses. Preoperative TOxF showed an increased rate of neutropenia compared to preoperative ACF (52.3% and 40.0%, respectively, relative risk [RR] = 1.38, 95% CI = 1.05 to 1.81). However, preoperative ACF was associated with an increased rate of nausea and vomiting (26.3% and 12.5%, respectively, RR = 2.10, 95% CI = 1.23 to 3.60). Patients receiving bevacizumab in combination with perioperative ACF had an increased amount of anastomotic leakages compared to perioperative ACF (15.8% and 6.6%, respectively, RR = 2.40, 95% CI = 1.60 to 3.61). No significant increase in 30-day mortality or surgery related morbidity was found in patients which had received chemotherapy before the operation compared to patients which had received no treatment before surgery.
The pair-wise meta-analyses for adjuvant therapy after curative resection included six comparisons between chemotherapy/chemoradiotherapy and observation alone. Therefore, no RR could be calculated for these comparisons. The doublet oxaliplatin-fluoropyrimidine showed a more tolerable toxicity profile (OxF: neutropenia 22%; thrombocytopenia 8%; nausea/vomiting 15%; stomatitis 1%) than a cisplatin-fluoropyrimidine doublet (CF: neutropenia 26%; thrombocytopenia 14%; nausea/vomiting 30%; stomatitis 17%). S-1 monotherapy for one year had the lowest amount of grade 3–4 AEs (S-1: leukopenia 1%; anemia 1%; diarrhea 3%; stomatitis 0%). The addition of radiotherapy to a chemotherapeutic regimen did not significantly increase the amount of grade 3–4 AEs compared to the same chemotherapeutic backbone without radiotherapy. A full overview of grade 3–4 adverse events and surgical related outcomes can be found in the Supplementary Results (Tables S1–S3).
3. Discussion
Based on the results of our two NMAs for the comparison of treatment strategies and the comparison of adjuvant therapy after curative resection for resectable gastric cancer, three major conclusions can be drawn which may help guide clinical practice and future research. The results are mainly hypothesis-generating and should be interpreted accordingly as there are limitations associated with the performed analyses.
First, taxane-containing perioperative chemotherapy (PCT) was the most effective treatment strategy compared to surgery alone. Therefore, PCT is the preferred treatment strategy when patients have not yet received surgery and are sufficiently fit to start with chemotherapy. A meta-analysis, based in part on individual patient data, of 14 RCTs investigating the benefit of pre/perioperative chemo(radio)therapy for patients with gastroesophageal adenocarcinoma performed by the Cochrane group found a HR = 0.81 (95%CI 0.73–0.89, p < 0.0001) in favor of pre/perioperative therapy compared to surgery alone [63]. The different RCTs used relatively similar regimens based on platinum agents with or without anthracyclines. The Cochrane meta-analysis calculated a combined effect size for perioperative and neoadjuvant trials [63]. In NMA-1 we could separate perioperative from neoadjuvant trials and compared PCT with neoadjuvant therapy. The HR was in favor of PCT but did not reach statistical significance, HR = 0.59 (95% CrI 0.36–1.02). By using the NMA technique we could also compare PCT with adjuvant chemotherapy and found PCT to reach a statically significant survival benefit compared to adjuvant chemotherapy, HR = 0.62 (95% CrI 0.42–0.93). Perioperative chemotherapy without a taxane (PC) did not reach statistical significance in NMA-1 in the random effects model compared to surgery alone, HR = 0.73 (95% CrI 0.52–1.01). Although, it did reach statistical significance in the pairwise comparison between PC and surgery alone, HR = 0.73 (95% CrI 0.61–0.88), Figure S3. Our results do confirm the findings of the Cochrane review in favor of perioperative chemotherapy and we further identified the relative benefit of PCT compared to neoadjuvant or adjuvant chemotherapy. Moreover, perioperative chemotherapy is also an established treatment strategy in the ESMO and NCCN guidelines when patients have not yet received surgery [2,7]. Of note, approximately only 50% of the patients, in perioperative trials will start with adjuvant therapy [3,4]. Potentially, the administration of neoadjuvant chemotherapy could be as effective as perioperative chemotherapy. However, the findings of our NMA-1 suggest survival benefit may not solely be based on the administration of neoadjuvant therapy alone, as neoadjuvant chemotherapy failed to improve survival compared to surgery alone, HR = 1.00 (95% CrI = 0.67 to 1.47). Hypothetically, survival benefit can also be obtained by administering adjuvant chemotherapy after neoadjuvant therapy. Thus, rather than the timing of the chemotherapy, the amount of chemotherapy may be most relevant. Unfortunately, currently available data are insufficient to test this hypothesis and the results of our NMA should be interpreted with caution. The neoadjuvant and adjuvant arms in our strategy NMA-1 were relatively small and might thus be underpowered to detect a survival benefit for these strategies. For now, taxane-containing perioperative chemotherapy is preferable compared to neoadjuvant chemotherapy and adjuvant chemotherapy. A well powered randomized controlled trial should investigate if taxane-containing perioperative chemotherapy is superior compared to taxane-containing neoadjuvant chemotherapy.
Second, after a curative resection, the doublet oxaliplatin-fluoropyrimidine showed the largest survival benefit compared to observation-alone. The ESMO, NCCN and Japanese gastric cancer guidelines highlight the efficacy of a doublet containing oxaliplatin and fluoropyrimidine, based on the CLASSIC trial [2,5,64]. In Japan the use of S-1 as adjuvant therapy is considered to be a viable alternative, based on the ACTS-GS trial [65]. Results from NMA-2 indicated that oxaliplatin was preferable compared to cisplatin. Findings in advanced esophagogastric cancer support the use of oxaliplatin over cisplatin [66,67,68,69,70,71,72,73,74]. Also, the addition of oxaliplatin to a fluoropyrimidine in a prolonged-adjuvant treatment course conveyed survival benefit compared to fluoropyrimidine-monotherapy although OS results were non-significant due to a lack of power. Based on our NMA-2, the doublet oxaliplatin-fluoropyrmidine is preferred for patients in good condition and fluoro-pyrimidine-monotherapy should be reserved for patients with co-morbidity limiting intensive treatment.
Based on NMA-2, the use of anthracycline based chemotherapy is inferior to an oxaliplatin based doublet. This reflects results in advanced esophagogastric cancer where fluoropyrimidine doublets are preferred over cisplatin doublets and anthracycline-based triplets, as first-line treatment option [66,67]. Moreover, also for patients with esophageal cancer whom received neoadjuvant chemotherapy, anthracyclines in a triplet combination with cisplatin-fluoropyrimidine did not improve survival compared to the doublet cisplatin-fluoropyrimidine [75]. In sum, the addition of an anthracycline to a doublet regimen based on a platinum-fluoropyrimidine compound does not lead to additional survival benefit in esophagogastric cancer [76].
Third, currently there is no definitive advantage of incorporating adjuvant chemoradiotherapy in the curative treatment of gastric cancer. In NMA-1 adjuvant chemoradiotherapy combined with perioperative chemotherapy showed similar or even inferior efficacy compared to perioperative-taxane containing chemotherapy. Moreover, after a curative resection chemotherapy combined with radiotherapy did not improve survival compared to oxaliplatin-fluoropyrimidine or a taxane-cisplatin based doublet. The CRITICS study compared perioperative chemotherapy to perioperative chemotherapy combined with post-operative chemoradiotherapy and showed no benefit of post-operative chemoradiotherapy in any of the analyzed subgroups [13,77]. According to NMA-2, after a curative resection, the addition of radiotherapy to a chemotherapeutic regimen does not increase efficacy compared to chemotherapy-alone. However, chemoradiotherapy may be beneficial for patients with an R1 resection, according to data from the National Cancer Database [78]. Also, radiotherapy could be beneficial if an inadequate lymph node dissection (D0 or D1) was performed. In the intergroup 0116 trial, in which only 10% of the patients received a D2-dissection, benefit was observed from chemoradiotherapy compared to observation [6]. After a curative resection with an adequate D2 lymph node dissection, the ARTIST trial did not observe benefit from chemoradiotherapy compared to chemotherapy alone [54]. However, in a sub-analysis in node positive patients DFS was significantly better with chemoradiotherapy [54]. The ARTIST II trial (Clinical-Trials.gov identifier: NCT01761461) with node positive stage II and III gastric cancer patients (with an R0 and D2 lymph node dissection) is a three arm study comparing S-1 vs. Oxaliplatin + S-1 vs. Oxaliplatin+S-1 with radiotherapy and will confirm or reproach the results of our NMA.
Importantly, the CROSS study showed that neoadjuvant chemoradiotherapy in esophageal and GEJ cancer resulted in a significant survival benefit compared to surgery-alone [79]. To date, it is unknown if neoadjuvant chemoradiotherapy could improve outcomes in gastric cancer and results from ongoing randomized trials are eagerly awaited. The TOPGEAR (Clinical-Trials.gov identifier: NCT01924819), ESOPEC (Clinical-Trials.gov identifier: NCT02509286), Neo-AGIS (Clinical-Trials.gov identifier: NCT01726452) and the CRITICS II study (Clinical-Trials.gov identifier: NCT02931890) will all shed more light on the role of neoadjuvant chemoradiotherapy.
Our approach has some limitations. First, oxaliplatin-containing regimens were primarily studied in Asian, D2 lymph node dissected patients. Therefore, the results may only be extrapolated to the Western setting with caution. On the other hand, in Western countries oxaliplatin with a fluoropyrimidine is an established regimen for advanced esophagogastric cancer and curatively resected colon cancer [71,80].
Second, predictive factors could have influenced our results. In the perioperative chemotherapy trials gastric cancer, GEJ and esophageal adenocarcinoma patients were included which could have obscured the degree to which the results could be extrapolated to gastric cancer. Although, in the perioperative trials there was no significant heterogeneity in treatment effect according to tumor location [3,11]. Sensitivity analyses were also conducted to account for three potentially, predictive factors (stage; lymph node dissection; origin) which showed consistent results. Therefore, it seems unlikely our results can solely be related to differences in surgery between Asian and Western countries or the type of lymph node dissection.
Third, for several nodes in both NMAs there were few RCTs available. Therefore, statistical power was lacking for specific comparisons. This might also explain the absent survival benefit of adjuvant chemotherapy in NMA-1 compared to surgery alone contrary to NMA-2 were we found significant benefit for several adjuvant chemo(radio)therapy regimens. Although, the discrepancy could also be related to the amount of R1/R2 resected patients in the adjuvant trials of NMA-1 compared to NMA-2 were all RCTs included R0 resected patients. Another example, is the node taxane-cisplatin in NMA-2 which consisted of 70 patients from one RCT. Moreover, no comparison between the best treatments of both NMAs—such as between taxane-based perioperative chemotherapy and the adjuvant doublet oxaliplatin-fluoropyrimidine—could be made. However, the comparison between taxane-based perioperative chemotherapy and adjuvant chemotherapy in NMA-1 was statistically more robust, due to the fact three out of four adjuvant studies were taxane-based triplet regimens.
Fourth, most RCTs investigating adjuvant therapy compared to observation after curative resection in NMA-2 were conducted between 1990 and 2010. Results must be extrapolated with care to current clinical practice.
4. Methods
4.1. Protocol
The protocol was registered in PROSPERO, the international prospective register of systematic reviews (CRD42017074888).
4.2. Literature Search
Our systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [81]. PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for eligible randomized controlled trials up to August 2017. The search strategy consisted of medical subject headings (MeSH) and text words for gastric cancer and esophageal cancer. The search included the term ‘esophageal cancer’ to not miss studies which included both esophageal and gastric cancer patients. Moreover, the meeting abstracts from the American Society of Clinical Oncology (ASCO) (http://ascopubs.org/search/advanced) and European Society for Medical Oncology (ESMO) (https://academic.oup.com/annonc/advanced-search) were searched up to August 2017. The literature search strategy was established and performed by MM and EtV. Three authors (TvdE, RM, FaN) screened the titles, abstracts and full articles independently. All parts of the search were screened by at least two authors in mutual consultation, and disagreements were discussed with a third arbiter (EtV or HvL) until consensus was reached.
4.3. Study Selection
Eligibility criteria consisted of the following:
-
(1)
Prospective phase II or III randomized controlled trials.
-
(2)
Patients with pathologically proven gastric adenocarcinoma stage I, II and III (T1–4, N1–3, M0).
-
(3)
The treatment of patients with gastric cancer was with curative intent.
-
(4)
Patients were treated with one or more of the following intravenous or oral cytotoxic agents; fluoropyrimidine (F; either 5-fluorouracil [5-FU], capecitabine [Cap], S-1, tegafur/uracil [UFT], tegafur, or doxifluridine). Platinum-based compounds (cisplatin [C] and oxaliplatin [Ox]). Taxanes (T; either paclitaxel, or docetaxel) or anthracyclines (A; either epirubicin, or doxorubicin). Irinotecan based regimens (I), etoposide (E) and mitomycin C (M) or methotrexate (MTX).
-
(5)
Patients treated with radiotherapy combined with one or more cytotoxic agents (RCh).
-
(6)
Patients treated with targeted agents.
Comparator arm in a randomized controlled trial could consist of chemotherapy (with or without radiotherapy), surgery-alone, irrespective of pathological outcome, (S) or observation after a curative resection (Obs: negative microscopic and macroscopic resection margins; R0). All Studies were included with a D0 or > lymph node dissection. Perioperative and neoadjuvant studies were eligible if they included patients which were deemed resectable with curative intent at inclusion. Trials that solely focused on patients with malignancy of the gastroesophageal junction (GEJ) were excluded, as GEJs are considered esophageal cancer according to the 7th and 8th edition of the American Joint Committee on Cancer (AJCC) [23,82].
4.4. Data Extraction and Quality Assessment
Primary outcome was overall survival (OS). Secondary outcomes were disease free survival (DFS), grade 3 to 4 adverse events (AEs) and complications after surgery (30-day mortality, total morbidity, anastomotic leakage, abdominal abscess, sepsis). Quality of the studies was assessed using the Cochrane Risk of Bias tool (version 5.1.0, The Nordic Cochrane Centre, Denmark). Items were scored as low, high or unknown risk of bias.
4.5. Statistical Analysis
Hazard ratios (HR) with 95% confidence intervals (95% CIs) were extracted for OS and DFS to calculate the logHR and standard error based on intention-to-treat study populations. A missing HR was either calculated by the methods described in the paper from Tierney et al. or by digitizing the Kaplan-Meier curves using Plot Digitizer (http://plotdigitizer.sourceforge.net), thereafter the HRs were calculated using the method of Parmar et al. [83,84]. In case neither the HRs nor the Kaplan-Meier curves were provided in the study reports, HRs were extracted from a previously conducted meta-analysis or individual patient data meta-analysis [60,61,62].
For all analyses consisting of at least 10 individual studies, a random effects network meta-analysis (NMA) in the Bayesian framework was conducted, using the GeMTC-standalone version (https://gemtc.drugis.org/signin.html), based on the GeMTC R-package [85]. The model accounted for relative treatment effects in multi-arm trials by manually providing the standard error of the absolute effect in the baseline arm (https://gemtc.drugis.org/manual.html). The number of burn-in iterations was set at 5.000 and the inference iterations at 20,000. To assess a correct posterior distribution, the potential scale reduction factor would be kept below 1.05 and the density plots would provide a smooth regular shape. Run lengths were extended if this was not the case and the Markov chains had not converged [86]. Direct and indirect treatment effects were combined into a single effect size, and the relative effects between all treatments were calculated as combined hazard ratios and 95% credible intervals (95% CrIs). Outcomes were deemed significant at an α-level of 0.05. A point estimate of HRs of 0.80 or less was regarded as clinically relevant [87].
The included randomized controlled trials consisted of different designs. In trials investigating perioperative and neoadjuvant therapy, patients were randomized before undergoing surgery. Most trials investigating adjuvant therapy randomized patients after a curative resection. Thus the patient population of perioperative and neoadjuvant studies is different from adjuvant studies. Therefore, the majority of the trials investigating perioperative and neoadjuvant therapy could not be compared within a single NMA model to adjuvant studies. To be comparable within one NMA, the transitivity assumption has to be fulfilled, which implies that in principle every patient in a study could have been randomized to every treatment in the network [88]. Therefore, we decided to create two networks:
-
(1)
A network comparing different treatment strategies (NMA-1).
-
(2)
A network comparing different adjuvant treatment modalities after curative resection (NMA-2).
Moreover, grade 3–4 toxicity and surgical complications were assessed by pairwise meta-analysis.
4.6. Merging of Treatment Groups
The first network (NMA-1) was created to compare treatment strategies as a whole, i.e., perioperative strategies, neoadjuvant strategies and adjuvant strategies. More specifically, in these type of trials in the model, patients were randomized to either perioperative or neoadjuvant therapy before surgery. In addition, studies investigating adjuvant therapy could be included if patients were randomized to this study arm before undergoing surgery and a head-to-head comparison in a randomized controlled trial was available with either perioperative or neoadjuvant treatment.
The following groups were compared: (1) perioperative chemotherapy with non-taxane-containing cytotoxic regimens; (2) perioperative chemotherapy with taxane-containing cytotoxic regimens; (3) perioperative chemotherapy with adjuvant chemoradiotherapy; (4) perioperative chemotherapy with bevacizumab; (5) neoadjuvant chemotherapy and; (6) adjuvant chemotherapy. The merging of different regimens in the strategy network was performed according to the following insights:
-
(1)
In a preliminary network meta-analysis (NMA) without merging of different neoadjuvant, perioperative or adjuvant regimens, there was no significant difference between the separate original treatment regimens.
-
(2)
Taxane-based perioperative chemotherapy was kept separate from standard anthracycline-based perioperative chemotherapy due to statistically significant direct evidence for superiority of taxane-based chemotherapy provided by the recently presented results of the FLOT-4 study [4].
-
(3)
Bevacizumab combined with perioperative chemotherapy (PCB) was kept as a separate node in the network. The ST03 trial did not show any survival benefit in favor of PCB compared to perioperative anthracycline-based chemotherapy [12]. To establish if this is also the case compared to taxane-based perioperative chemotherapy, PCB was analyzed separately from perioperative chemotherapy.
The second model (NMA-2) included RCTs investigating adjuvant therapy for curatively resected gastric cancer. More specifically, studies were included if patients were randomized to adjuvant therapy arms if a curative resection (R0) was achieved.
Cytotoxic agents from the same drug class were taken together based on previous evidence in metastatic esophagogastric cancer [66]. The following drug classes were identified: (1) fluoropyrimidines (F): 5-FU, S-1, capecitabine, UFT, tegafur and doxifluridine (with or without the co-administration of leucovorin [Lv]); (2) anthracyclines (A): epirubicin and doxorubicin; (3) taxanes (T): docetaxel and paclitaxel.
Radiotherapy in combination with one or more cytotoxic agents was grouped together as chemoradiotherapy (RCh). An anthracycline combined with two other cytotoxic agents (two of the following: a fluoropyrimidine, mitomycin C, etoposide or cisplatin) was grouped together as anthracycline-containing triplet (ATr). Oxaliplatin with capecitabine for eight cycles and thereafter eight extra cycles of capecitabine monotherapy was acknowledged as a separate treatment regimen (OxF-prolonged) [50]. Several subanalyses were performed to examine if the merging of drug classes was justified and showed consistency in treatment efficacy, see Supplementary Methods (Section 1.6).
4.7. Sensitivity Analysis and Assessment of Inconsistency
Sensitivity analyses were performed for stage: in NMA-1, studies that evaluated patients with stage IV-disease [M1] (discovered during treatment or surgery) were omitted and for NMA-2, studies which only included stage III patients were omitted. To account for potential confounding due to differences in surgical techniques between Asia and Western patients, we conducted two sensitivity analyses for the extend of lymph node dissection (D0/D1 versus D2 or higher) and Asian vs. Western patients. Node-split models were created to assess network consistency (between direct and indirect evidence). In case of inconsistency, baseline characteristics were explored for the corresponding studies. Sensitivity analyses were performed, omitting the studies responsible for network inconsistency one by one.
5. Conclusions
Based on currently available data, taxane-containing perioperative chemotherapy is the most promising treatment strategy for resectable gastric adenocarcinoma. If no neoadjuvant treatment has been given, an oxaliplatin-fluoropyrimidine doublet is the most promising adjuvant regimen after a curative resection for resectable gastric adenocarcinoma. The use of adjuvant oxaliplatin has to be further verified in Western gastric cancer patients. Further research is warranted to confirm or reproach our findings.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6694/11/1/80/s1, Supplementary Methods and Results. Figure S1: Results for NMA-1 when neoadjuvant taxane and adjuvant taxane containing chemotherapy were separated from non-taxane containing neoadjuvant or adjuvant chemotherapy compared to surgery alone, Figure S2: Results for NMA-1 when neoadjuvant taxane and adjuvant taxane containing chemotherapy were separated from non-taxane containing neoadjuvant or adjuvant chemotherapy, Figure S3: Overall survival pair wise meta-analysis based on treatment strategy, Figure S4: Overall survival pair wise meta-analysis of adjuvant therapy for curatively resected gastric cancer, Figure S5: Disease free survival pair wise meta-analysis of adjuvant therapy for curatively resected gastric cancer, Figure S6: Overall survival risk of bias assessment for the treatment strategy NMA-1, Figure S7: Overall survival and disease free survival risk of bias assessment for the adjuvant therapy after curative resection NMA-2, Figure S8: Direct and combined hazard ratios for overall survival in the treatment strategy NMA-1, Figure S9: Direct and combined hazard ratios for overall survival in the adjuvant therapy after curative resection NMA-2, Figure S10: Direct and combined hazard ratios for disease free survival in the adjuvant therapy after curative resection NMA-2, Table S1: Grade 3-4 toxicity for the treatment strategy NMA-1, Table S2: Surgical mortality and morbidity for the treatment strategy NMA-1, Table S3: Grade 3-4 toxicity for the adjuvant therapy after curative resection NMA-2.
Author Contributions
T.v.d.E. was involved in the screening of articles, data extraction/analyses and writing the article. E.t.V. provided intellectual guidance and corrected the first draft. M.M. created the search. R.M.A.M. and F.A.A.N. were involved in the screening of articles. L.d.W. and M.L. performed data extraction. S.S.G. and M.C.C.M.H. provided intellectual input and corrected the final draft. M.G.H.v.O. and H.W.M.v.L. provided intellectual guidance and corrected several draft versions.
Funding
This research received no external funding.
Conflicts of Interest
S.S.G. has served as a consultant for Medtronic and has received an unrestricted research grant from Olympus. M.G.H.v.O. has received unrestricted research grants from Bayer, Lilly, Merck Serono, and Roche. H.W.M.v.L. has served as a consultant for Philips, Celgene, Lilly, and Nordic, and has received unrestricted research funding from Philips, Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, and Roche. The other authors have nothing to declare.
References
- 1.Karimi P., Islami F., Anandasabapathy S., Freedman N.D., Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth E.C., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D., Committee E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J., Nicolson M., Scarffe J.H., Lofts F.J., Falk S.J., Iveson T.J., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Al-Batran S.-E., Homann N., Schmalenberg H., Kopp H.-G., Haag G.M., Luley K.B., Schmiegel W.H., Folprecht G., Probst S., Prasnikar N., et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J. Clin. Oncol. 2017;35:4004. doi: 10.1200/JCO.2017.35.15_suppl.4004. [DOI] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smalley S.R., Benedetti J.K., Haller D.G., Hundahl S.A., Estes N.C., Ajani J.A., Gunderson L.L., Goldman B., Martenson J.A., Jessup J.M., et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Gastric Cancer Clinical Practice Guideline Version 1.2018. [(accessed on 4 December 2018)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
- 8.Cipriani A., Barbui C., Rizzo C., Salanti G. What is a multiple treatments meta-analysis? Epidemiol Psychiatr. Sci. 2012;21:151–153. doi: 10.1017/S2045796011000837. [DOI] [PubMed] [Google Scholar]
- 9.Lu G., Ades A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 10.Tsuburaya A., Yoshida K., Kobayashi M., Yoshino S., Takahashi M., Takiguchi N., Tanabe K., Takahashi N., Imamura H., Tatsumoto N., et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15:886–893. doi: 10.1016/S1470-2045(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 11.Ychou M., Boige V., Pignon J.P., Conroy T., Bouche O., Lebreton G., Ducourtieux M., Bedenne L., Fabre J.M., Saint-Aubert B., et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D., Stenning S.P., Smyth E.C., Okines A.F., Allum W.H., Rowley S., Stevenson L., Grabsch H.I., Alderson D., Crosby T., et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): Primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357–370. doi: 10.1016/S1470-2045(17)30043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verheij M., Cats A., Jansen Edwin P.M., Van Grieken Nicole C.T., Aaronson Neil K., Boot H., Lind Pehr A., Meershoek-Klein Kranenbarg E., Nordsmark M., Putter H., et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. Ann. Oncol. 2016;27:ii140. doi: 10.1093/annonc/mdw237.02. [DOI] [Google Scholar]
- 14.Nio Y., Koike M., Omori H., Hashimoto K., Itakura M., Yano S., Higami T., Maruyama R. A randomized consent design trial of neoadjuvant chemotherapy with tegafur plus uracil (UFT) for gastric cancer—A single institute study. Anticancer Res. 2004;24:1879–1887. [PubMed] [Google Scholar]
- 15.Ma J., Yao S., Li X.S., Kang H.R., Yao F.F., Du N. Neoadjuvant Therapy of DOF Regimen Plus Bevacizumab Can Increase Surgical Resection Ratein Locally Advanced Gastric Cancer: A Randomized, Controlled Study. Medicine. 2015;94:e1489. doi: 10.1097/MD.0000000000001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui H.B., Ge H.E., Bai X.Y., Zhang W., Zhang Y.Y., Wang J., Li X., Xing L.P., Guo S.H., Wang Z.Y. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp. Ther. 2014;7:1083–1088. doi: 10.3892/etm.2014.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu J.J., Shi Y.R., Liu F.R., Ma S.Q., Ma F.Y. A clinical study of paclitaxel combined with FOLFOX4 regimen as neoadjuvant chemotherapy for advanced gastric cancer. Zhonghua Wei Chang. Wai Ke Za Zhi. 2010;13:664–667. [PubMed] [Google Scholar]
- 18.Imano M., Itoh T., Satou T., Sogo Y., Hirai H., Kato H., Yasuda A., Peng Y.F., Shinkai M., Yasuda T., et al. Prospective randomized trial of short-term neoadjuvant chemotherapy for advanced gastric cancer. Eur. J. Surg. Oncol. 2010;36:963–968. doi: 10.1016/j.ejso.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Schuhmacher C., Gretschel S., Lordick F., Reichardt P., Hohenberger W., Eisenberger C.F., Haag C., Mauer M.E., Hasan B., Welch J., et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao W.H., Wang S.F., Ding W., Sheng J.M., Ma Z.M., Teng L.S., Wang M., Wu F.S., Luo B. Apoptosis induced by preoperative oral 5′-DFUR administration in gastric adenocarcinoma and its mechanism of action. World J. Gastroenterol. 2006;12:1356–1361. doi: 10.3748/wjg.v12.i9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartgrink H.H., van de Velde C.J., Putter H., Songun I., Tesselaar M.E., Kranenbarg E.K., de Vries J.E., Wils J.A., van der Bijl J., van Krieken J.H., et al. Neo-adjuvant chemotherapy for operable gastric cancer: Long term results of the Dutch randomised FAMTX trial. Eur. J. Surg. Oncol. 2004;30:643–649. doi: 10.1016/j.ejso.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Fazio N., Biffi R., Maibach R., Hayoz S., Thierstein S., Brauchli P., Bernhard J., Stupp R., Andreoni B., Renne G., et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann. Oncol. 2016;27:668–673. doi: 10.1093/annonc/mdv620. [DOI] [PubMed] [Google Scholar]
- 23.Rice T.W., Blackstone E.H., Rusch V.W. 7th edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann. Surg. Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 24.Neri B., Cini G., Andreoli F., Boffi B., Francesconi D., Mazzanti R., Medi F., Mercatelli A., Romano S., Siliani L., et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br. J. Cancer. 2001;84:878–880. doi: 10.1054/bjoc.2000.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krook J.E., O’Connell M.J., Wieand H.S., Beart R.W., Jr., Leigh J.E., Kugler J.W., Foley J.F., Pfeifle D.M., Twito D.I. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer. 1991;67:2454–2458. doi: 10.1002/1097-0142(19910515)67:10<2454::AID-CNCR2820671010>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Kulig J., Kolodziejczyk P., Sierzega M., Bobrzynski L., Jedrys J., Popiela T., Dadan J., Drews M., Jeziorski A., Krawczyk M., et al. Adjuvant chemotherapy with etoposide, adriamycin and cisplatin compared with surgery alone in the treatment of gastric cancer: A phase III randomized, multicenter, clinical trial. Oncology. 2010;78:54–61. doi: 10.1159/000292360. [DOI] [PubMed] [Google Scholar]
- 27.Di Costanzo F., Gasperoni S., Manzione L., Bisagni G., Labianca R., Bravi S., Cortesi E., Carlini P., Bracci R., Tomao S., et al. Adjuvant chemotherapy in completely resected gastric cancer: A randomized phase III trial conducted by GOIRC. J. Natl. Cancer Inst. 2008;100:388–398. doi: 10.1093/jnci/djn054. [DOI] [PubMed] [Google Scholar]
- 28.De Vita F., Giuliani F., Orditura M., Maiello E., Galizia G., Di Martino N., Montemurro F., Carteni G., Manzione L., Romito S., et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: A randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study) Ann. Oncol. 2007;18:1354–1358. doi: 10.1093/annonc/mdm128. [DOI] [PubMed] [Google Scholar]
- 29.Tentes A.A., Markakidis S.K., Karanikiotis C., Fiska A., Tentes I.K., Manolopoulos V.G., Dimitriou T. Intraarterial chemotherapy as an adjuvant treatment in locally advanced gastric cancer. Langenbecks Arch. Surg. 2006;391:124–129. doi: 10.1007/s00423-006-0022-z. [DOI] [PubMed] [Google Scholar]
- 30.Tsavaris N., Tentas K., Kosmidis P., Mylonakis N., Sakelaropoulos N., Kosmas C., Lisaios B., Soumilas A., Mandrekas D., Tsetis A., et al. A randomized trial comparing adjuvant fluorouracil, epirubicin, and mitomycin with no treatment in operable gastric cancer. Chemotherapy. 1996;42:220–226. doi: 10.1159/000239446. [DOI] [PubMed] [Google Scholar]
- 31.Lise M., Nitti D., Marchet A., Sahmoud T., Buyse M., Duez N., Fiorentino M., Dos Santos J.G., Labianca R., Rougier P., et al. Final results of a phase III clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J. Clin. Oncol. 1995;13:2757–2763. doi: 10.1200/JCO.1995.13.11.2757. [DOI] [PubMed] [Google Scholar]
- 32.Coombes R.C., Schein P.S., Chilvers C.E., Wils J., Beretta G., Bliss J.M., Rutten A., Amadori D., Cortes-Funes H., Villar-Grimalt A. A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J. Clin. Oncol. 1990;8:1362–1369. doi: 10.1200/JCO.1990.8.8.1362. [DOI] [PubMed] [Google Scholar]
- 33.Bajetta E., Buzzoni R., Mariani L., Beretta E., Bozzetti F., Bordogna G., Aitini E., Fava S., Schieppati G., Pinotti G., et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann. Oncol. 2002;13:299–307. doi: 10.1093/annonc/mdf040. [DOI] [PubMed] [Google Scholar]
- 34.Cascinu S., Labianca R., Barone C., Santoro A., Carnaghi C., Cassano A., Beretta G.D., Catalano V., Bertetto O., Barni S., et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J. Natl. Cancer Inst. 2007;99:601–607. doi: 10.1093/jnci/djk131. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.J., Kim S.Y., Shin I., Cho K.S., Joo H.Z., Yoon C. Randomized Phase III Trial of Cisplatin, Epirubicin, Leucovorin, 5-Fluorouracil (PELF) Combination versus 5-fluorouracil Alone as Adjuvant Chemotherapy in Curative Resected Stage III Gastric Cancer. Cancer Res. Treat. 2004;36:140–145. doi: 10.4143/crt.2004.36.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujinaka T., Shiozaki H., Inoue M., Furukawa H., Hiratsuka M., Kikkawa N., Takami M., Suzuki T., Monden M. Evaluation of effectiveness of chemotherapy in patients with gastric cancer after curative resection. Int. J. Clin. Oncol. 2000;5:372–379. doi: 10.1007/PL00012066. [DOI] [Google Scholar]
- 37.Chang H.M., Jung K.H., Kim T.Y., Kim W.S., Yang H.K., Lee K.U., Choe K.J., Heo D.S., Bang Y.J., Kim N.K. A phase III randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil and mitomycin C versus 5-fluorouracil alone in curatively resected gastric cancer. Ann. Oncol. 2002;13:1779–1785. doi: 10.1093/annonc/mdf302. [DOI] [PubMed] [Google Scholar]
- 38.Bouche O., Ychou M., Burtin P., Bedenne L., Ducreux M., Lebreton G., Baulieux J., Nordlinger B., Martin C., Seitz J.F., et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801) Ann. Oncol. 2005;16:1488–1497. doi: 10.1093/annonc/mdi270. [DOI] [PubMed] [Google Scholar]
- 39.Chipponi J., Huguier M., Pezet D., Basso N., Hay J.M., Quandalle P., Jaeck D., Fagniez P.L., Gainant A. Randomized trial of adjuvant chemotherapy after curative resection for gastric cancer. Am. J. Surg. 2004;187:440–445. doi: 10.1016/j.amjsurg.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Sasako M., Sakuramoto S., Katai H., Kinoshita T., Furukawa H., Yamaguchi T., Nashimoto A., Fujii M., Nakajima T., Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima T., Kinoshita T., Nashimoto A., Sairenji M., Yamaguchi T., Sakamoto J., Fujiya T., Inada T., Sasako M., Ohashi Y., et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br. J. Surg. 2007;94:1468–1476. doi: 10.1002/bjs.5996. [DOI] [PubMed] [Google Scholar]
- 42.Grau J.J., Estape J., Alcobendas F., Pera C., Daniels M., Teres J. Positive results of adjuvant mitomycin-C in resected gastric cancer: A randomised trial on 134 patients. Eur. J. Cancer. 1993;29A:340–342. doi: 10.1016/0959-8049(93)90381-O. [DOI] [PubMed] [Google Scholar]
- 43.Cirera L., Balil A., Batiste-Alentorn E., Tusquets I., Cardona T., Arcusa A., Jolis L., Saigi E., Guasch I., Badia A., et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer. J. Clin. Oncol. 1999;17:3810–3815. doi: 10.1200/JCO.1999.17.12.3810. [DOI] [PubMed] [Google Scholar]
- 44.Kim J.P., Kwon O.J., Oh S.T., Yang H.K. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann. Surg. 1992;216:269–278; discussion 278–269. doi: 10.1097/00000658-199209000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grau J.J., Estapé J., Fuster J., Filella X., Visa J., Terés J., Soler G., Albiol S., García-Valdecasas J.C., Grande L., et al. Randomized trial of adjuvant chemotherapy with mitomycin plus ftorafur versus mitomycin alone in resected locally advanced gastric cancer. J. Clin. Oncol. 1998;16:1036–1039. doi: 10.1200/JCO.1998.16.3.1036. [DOI] [PubMed] [Google Scholar]
- 46.Kang Y.K., Chang H.M., Yook J.H., Ryu M.H., Park I., Min Y.J., Zang D.Y., Kim G.Y., Yang D.H., Jang S.J., et al. Adjuvant chemotherapy for gastric cancer: A randomised phase 3 trial of mitomycin-C plus either short-term doxifluridine or long-term doxifluridine plus cisplatin after curative D2 gastrectomy (AMC0201) Br. J. Cancer. 2013;108:1245–1251. doi: 10.1038/bjc.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimoyama S., Shimizu N., Kaminishi M. Type-oriented intraoperative and adjuvant chemotherapy and survival after curative resection of advanced gastric cancer. World J. Surg. 1999;23:284–291; discussion 291–282. doi: 10.1007/PL00013186. [DOI] [PubMed] [Google Scholar]
- 48.Noh S.H., Park S.R., Yang H.K., Chung H.C., Chung I.J., Kim S.W., Kim H.H., Choi J.H., Kim H.K., Yu W., et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X.L., Shi H.J., Cui S.Z., Tang Y.Q., Ba M.C. Prospective, randomized trial comparing 5-FU/LV with or without oxaliplatin as adjuvant treatment following curative resection of gastric adenocarcinoma. Eur. J. Surg. Oncol. 2011;37:466–472. doi: 10.1016/j.ejso.2011.01.027. Erratum in 2013, 39, 525. [DOI] [PubMed] [Google Scholar]
- 50.Feng W.M., Tang C.W., Guo H.H., Bao Y., Fei M.Y. Prolonged adjuvant capecitabine chemotherapy improved survival of stage IIIA gastric cancer after D2 gastrectomy. Biomed. Pharmacother. 2015;72:140–143. doi: 10.1016/j.biopha.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Kim T.H., Park S.R., Ryu K.W., Kim Y.W., Bae J.M., Lee J.H., Choi I.J., Kim Y.J., Kim D.Y. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:e585–e592. doi: 10.1016/j.ijrobp.2012.07.2378. [DOI] [PubMed] [Google Scholar]
- 52.Yu C., Yu R., Zhu W., Song Y., Li T. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J. Cancer Res. Clin. Oncol. 2012;138:255–259. doi: 10.1007/s00432-011-1085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu W.G., Xua D.F., Pu J., Zong C.D., Li T., Tao G.Z., Ji F.Z., Zhou X.L., Han J.H., Wang C.S., et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother. Oncol. 2012;104:361–366. doi: 10.1016/j.radonc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 54.Park S.H., Sohn T.S., Lee J., Lim D.H., Hong M.E., Kim K.M., Sohn I., Jung S.H., Choi M.G., Lee J.H., et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J. Clin. Oncol. 2015;33:3130–3136. doi: 10.1200/JCO.2014.58.3930. [DOI] [PubMed] [Google Scholar]
- 55.Kwon H.C., Kim M.C., Kim K.H., Jang J.S., Oh S.Y., Kim S.H., Kwon K.A., Lee S., Lee H.S., Kim H.J. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac. J. Clin. Oncol. 2010;6:278–285. doi: 10.1111/j.1743-7563.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 56.Bamias A., Karina M., Papakostas P., Kostopoulos I., Bobos M., Vourli G., Samantas E., Christodoulou C., Pentheroudakis G., Pectasides D., et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother. Pharmacol. 2010;65:1009–1021. doi: 10.1007/s00280-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 57.Lee C., Jung M., Kim H.S., Jung I., Shin D.B., Kang S.Y., Zang D.Y., Kim K.H., Lee M.H., Kim B.S., et al. An update on the randomized phase III POST trial: S-1 based doublet as an adjuvant chemotherapy for curatively resected stage III gastric cancer. J. Clin. Oncol. 2016;34:4042. doi: 10.1200/JCO.2016.34.15_suppl.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajetta E., Floriani I., Di Bartolomeo M., Labianca R., Falcone A., Di Costanzo F., Comella G., Amadori D., Pinto C., Carlomagno C., et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann. Oncol. 2014;25:1373–1378. doi: 10.1093/annonc/mdu146. [DOI] [PubMed] [Google Scholar]
- 59.Di Bartolomeo M., Buzzoni R., Mariani L., Ferrario E., Katia D., Gevorgyan A., Zilembo N., Bordonaro R., Bochicchio A.M., Massidda B., et al. Feasibility of sequential therapy with FOLFIRI followed by docetaxel/cisplatin in patients with radically resected gastric adenocarcinoma. A randomized phase III trial. Oncology. 2006;71:341–346. doi: 10.1159/000108575. Erratum in 2007, 73, 406. [DOI] [PubMed] [Google Scholar]
- 60.Zhao J.H., Gao P., Song Y.X., Sun J.X., Chen X.W., Ma B., Yang Y.C., Wang Z.N. Which is better for gastric cancer patients, perioperative or adjuvant chemotherapy: A meta-analysis. BMC Cancer. 2016;16:631. doi: 10.1186/s12885-016-2667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou M.L., Kang M., Li G.C., Guo X.M., Zhang Z. Postoperative chemoradiotherapy versus chemotherapy for R0 resected gastric cancer with D2 lymph node dissection: An up-to-date meta-analysis. World J. Surg. Oncol. 2016;14:209. doi: 10.1186/s12957-016-0957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paoletti X., Oba K., Burzykowski T., Michiels S. Benefit of Adjuvant Chemotherapy for Resectable Gastric Cancer A Meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 63.Ronellenfitsch U., Schwarzbach M., Hofheinz R., Kienle P., Kieser M., Slanger T.E., Jensen K., GE Adenocarcinoma Meta-analysis Group Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst. Rev. 2013;31:CD008107. doi: 10.1002/14651858.CD008107.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bang Y.J., Kim Y.W., Yang H.K., Chung H.C., Park Y.K., Lee K.H., Lee K.W., Kim Y.H., Noh S.I., Cho J.Y., et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 65.Sakuramoto S., Sasako M., Yamaguchi T., Kinoshita T., Fujii M., Nashimoto A., Furukawa H., Nakajima T., Ohashi Y., Imamura H., et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. Erratum in 2008, 358, 1977. [DOI] [PubMed] [Google Scholar]
- 66.Ter Veer E., Haj Mohammad N., van Valkenhoef G., Ngai L.L., Mali R.M.A., Anderegg M.C., van Oijen M.G.H., van Laarhoven H.W.M. The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djw166. [DOI] [PubMed] [Google Scholar]
- 67.Wagner A.D., Syn N.L., Moehler M., Grothe W., Yong W.P., Tai B.C., Ho J., Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W.W., Wang F., Xu R.H. Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: A meta-analysis. PLoS ONE. 2013;8:e68974. doi: 10.1371/journal.pone.0068974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrelli F., Zaniboni A., Coinu A., Cabiddu M., Ghilardi M., Sgroi G., Barni S. Cisplatin or not in advanced gastric cancer: A systematic review and meta-analysis. PLoS ONE. 2013;8:e83022. doi: 10.1371/journal.pone.0083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Batran S.E., Hartmann J.T., Probst S., Schmalenberg H., Hollerbach S., Hofheinz R., Rethwisch V., Seipelt G., Homann N., Wilhelm G., et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 71.Cunningham D., Starling N., Rao S., Iveson T., Nicolson M., Coxon F., Middleton G., Daniel F., Oates J., Norman A.R., et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 72.Huang J., Zhao Y., Xu Y., Zhu Y., Huang J., Liu Y., Zhao L., Li Z., Liu H., Wang Q.L., et al. Comparative effectiveness and safety between oxaliplatin-based and cisplatin-based therapy in advanced gastric cancer: A meta-analysis of randomized controlled trials. Oncotarget. 2016;7:34824–34831. doi: 10.18632/oncotarget.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ter Veer E., Creemers A., de Waal L., van Oijen M.G.H., van Laarhoven H.W.M. Comparison of cytotoxic backbones for first line trastuzumab-containing regimens in HER2-positive advanced esophagogastric cancer: A meta-analysis. Ann. Oncol. 2017;28(Suppl. 5):V209–V268. doi: 10.1093/annonc/mdx369.057. [DOI] [PubMed] [Google Scholar]
- 74.Montagnani F., Turrisi G., Marinozzi C., Aliberti C., Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]
- 75.Alderson D., Cunningham D., Nankivell M., Blazeby J.M., Griffin S.M., Crellin A., Grabsch H.I., Langer R., Pritchard S., Okines A., et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): An open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1249–1260. doi: 10.1016/S1470-2045(17)30447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashraf N., Kim R. Treatment of Gastric and Gastroesophageal Cancers-Do We Really Need Anthracyclines? JAMA Oncol. 2017;3:1172–1173. doi: 10.1001/jamaoncol.2016.3064. [DOI] [PubMed] [Google Scholar]
- 77.Cats A., Jansen E.P.M., van Grieken N.C.T., Sikorska K., Lind P., Nordsmark M., Meershoek-Klein Kranenbarg E., Boot H., Trip A.K., Swellengrebel H.A.M., et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:616–628. doi: 10.1016/S1470-2045(18)30132-3. [DOI] [PubMed] [Google Scholar]
- 78.Rhome R.M., Moshier E., Sarpel U., Ohri N., Mazumdar M., Buckstein M.H. Predictors of Positive Margins After Definitive Resection for Gastric Adenocarcinoma and Impact of Adjuvant Therapies. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:1106–1115. doi: 10.1016/j.ijrobp.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 79.Hagen P., Hulshof M.C., Lanschot J.J., Steyerberg E.W., Berge Henegouwen M.I., Wijnhoven B.P., Richel D.J., Nieuwenhuijzen G.A., Hospers G.A., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 80.Andre T., Boni C., Mounedji-Boudiaf L., Navarro M., Tabernero J., Hickish T., Topham C., Zaninelli M., Clingan P., Bridgewater J., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 81.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 82.Rice T.W., Patil D.T., Blackstone E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017;6:119–130. doi: 10.21037/acs.2017.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 84.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Valkenhoef G., Lu G., de Brock B., Hillege H., Ades A.E., Welton N.J. Automating network meta-analysis. Res. Synth. Methods. 2012;3:285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 86.Gelman A., Rubin D.B. Markov chain Monte Carlo methods in biostatistics. Stat. Methods Med. Res. 1996;5:339–355. doi: 10.1177/096228029600500402. [DOI] [PubMed] [Google Scholar]
- 87.Ellis L.M., Bernstein D.S., Voest E.E., Berlin J.D., Sargent D., Cortazar P., Garrett-Mayer E., Herbst R.S., Lilenbaum R.C., Sima C., et al. American Society of Clinical Oncology perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. J. Clin. Oncol. 2014;32:1277–1280. doi: 10.1200/JCO.2013.53.8009. [DOI] [PubMed] [Google Scholar]
- 88.Donegan S., Williamson P., D’Alessandro U., Tudur Smith C. Assessing key assumptions of network meta-analysis: A review of methods. Res. Synth. Methods. 2013;4:291–323. doi: 10.1002/jrsm.1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.