Abstract
Anaplastic lymphoma kinase (ALK) sequencing can identify resistance mechanisms and guide next-line therapy in ALK+ non-small-cell lung cancer (NSCLC), but the clinical significance of other rebiopsy findings remains unclear. We analysed all stage-IV ALK+ NSCLC patients with longitudinally assessable TP53 status treated in our institutions (n = 62). Patients with TP53 mutations at baseline (TP53mutbas, n = 23) had worse overall survival (OS) than patients with initially wild-type tumours (TP53wtbas, n = 39, 44 vs. 62 months in median, p = 0.018). Within the generally favourable TP53wtbas group, detection of TP53 mutations at progression defined a “converted” subgroup (TP53mutconv, n = 9) with inferior OS, similar to that of TP53mutbas and shorter than that of patients remaining TP53 wild-type (TP53wtprogr, 45 vs. 94 months, p = 0.043). Progression-free survival (PFS) under treatment with tyrosine kinase inhibitors (TKI) for TP53mutconv was comparable to that of TP53mutbas and also shorter than that of TP53wtprogr cases (5 and 8 vs. 13 months, p = 0.0039). Fewer TP53wtprogr than TP53mutbas or TP53mutconv cases presented with metastatic disease at diagnosis (67% vs. 91% or 100%, p < 0.05). Thus, acquisition of TP53 mutations at progression is associated with more aggressive disease, shorter TKI responses and inferior OS in ALK+ NSCLC, comparable to primary TP53 mutated cases.
Keywords: anaplastic lymphoma kinase positive (ALK+) non-small cell lung cancer (NSCLC), tumor protein p53 gene (TP53) mutation, tyrosine kinase inhibitor, progression-free survival, overall survival
1. Introduction
Anaplastic lymphoma kinase (ALK) gene fusions are driver genetic alterations in approximately 5% of non-small-cell lung cancers (NSCLC) [1]. Α breakthrough in their treatment was the development of several ALK tyrosine kinase inhibitors (TKI), which in sequential administration have pushed median patient survival to over five years [2]. Analysis of TKI failure has therefore become a main focus of research efforts, because its prediction, mechanistic dissection and individualized treatment is of key importance for further therapeutic advances.
Recent studies combining state-of-the-art molecular profiling with detailed clinical annotation have identified two molecular risk factors associated with TKI failure in ALK-driven NSCLC: echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion variant V3 [3,4,5] and the presence of tumor protein p53 gene (TP53) mutations at initial diagnosis [6,7,8]. They occur independent from each other in about 30–40% and 20% of patients, respectively, have synergistic effects and are both associated with shorter progression-free survival (PFS) after treatment with first- and second-generation ALK TKI and with worse overall survival (OS) [8].
Furthermore, molecular workup of a follow-up tissue or liquid biopsy at the time of TKI failure is gaining importance for the management of ALK+ NSCLC, since it can reveal patient-specific resistance mechanisms and guide subsequent therapeutic decisions [9,10,11]. In particular, detection of ALK resistance mutations can be useful for the selection of suitable next-line TKI based on in vitro sensitivity data [12]. However, the clinical importance of other molecular findings in tumor rebiopsies remains unclear.
Here, we examine the significance of TP53 mutations detected at the time of disease progression in ALK+ NSCLC patients with TP53 wild-type status at initial diagnosis.
2. Results
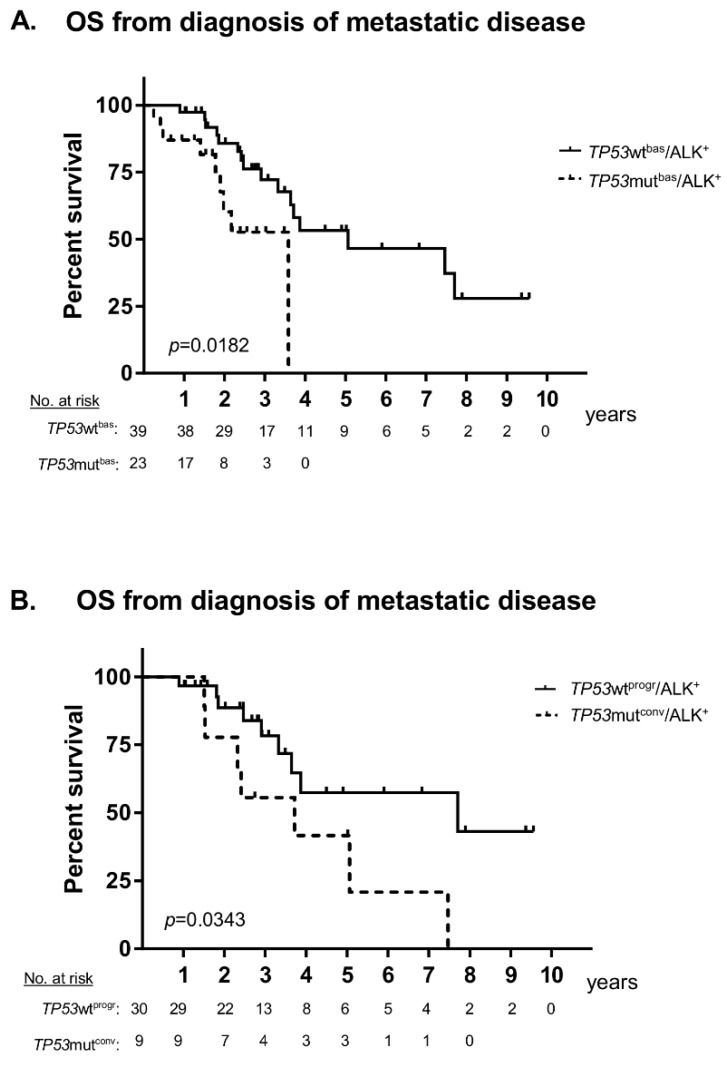
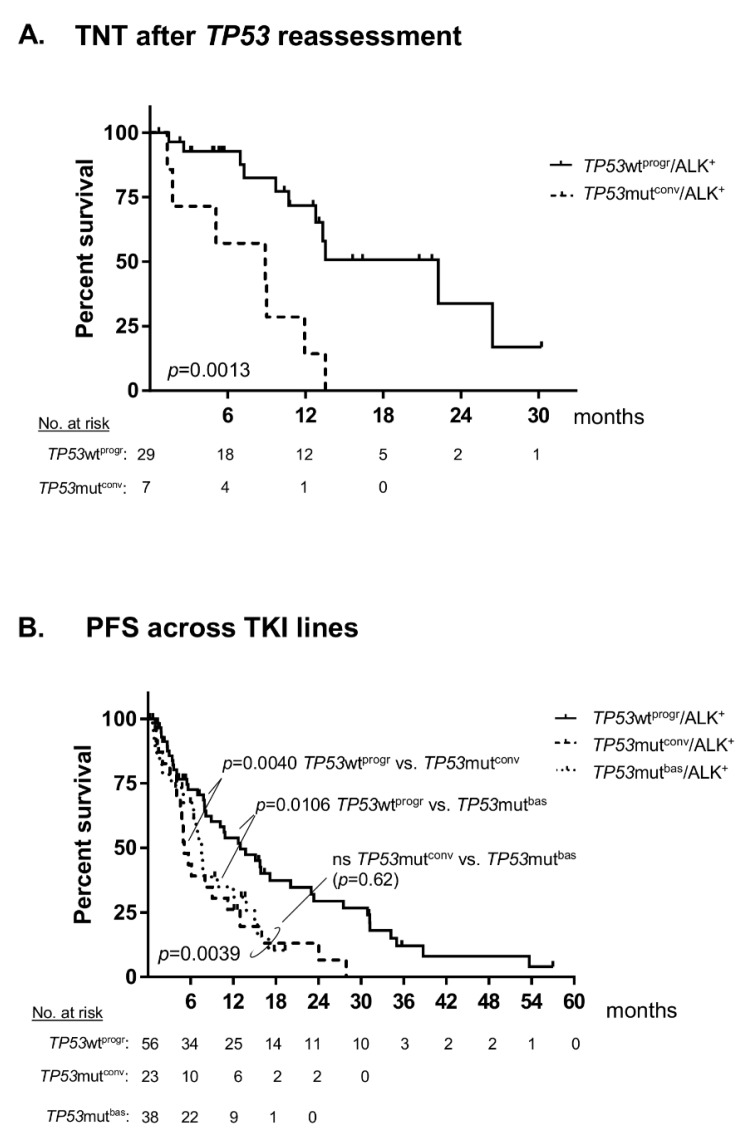
ALK+ NSCLC patients with tumour TP53 mutations at baseline (TP53mutbas) had a worse overall survival (OS) from the diagnosis of metastatic disease than patients with initially wild-type TP53 tumours (TP53wtbas, 44 vs. 62 months in median, p = 0.0182, Figure 1A). Within the generally favourable TP53wtbas patient group, detection of TP53 mutations in a subsequent tissue or liquid biopsy performed at disease progression identified an unfavourable, TP53 “converted” patient subgroup (TP53mutconv, Table 1) with OS comparable to that of TP53mutbas patients and shorter than that of patients retaining TP53 wild-type status after progression (TP53wtprogr, 45 vs. 94 months in median, p = 0.0343, Figure 1B). These comprised 23% (9/39) of initially TP53 wild-type (TP53wtbas) cases in our cohort. The newly acquired TP53 mutations resided in exons 5–10 of TP53 (Supplementary Table S1), i.e., in genetic regions that had already been tested as wild-type at initial diagnosis, because they were included in the NGS panel of both methods used in this study. All of them were pathogenic and resulted in loss-of-function (Supplementary Table S1). The time-to-next-treatment (TNT) for patients treated with TKI after the reassessment of TP53 status, was significantly shorter for cases with a positive (TP53mutconv) than for cases with a negative (TP53wtprogr) result (9 vs. 23 months in median, p = 0.0013, Figure 2A). In addition, PFS under treatment with ALK TKI across treatment lines for patients with secondary detection of TP53 mutations at progression (TP53mutconv) was comparable to that of patients with TP53 mutations at baseline (TP53mutbas), and also shorter than that of patients retaining TP53 wild-type status (TP53wtprogr, 5 and 8 vs. 13 months in median, p = 0.0039, Figure 2B). In contrast, there was no significant difference in the PFS under chemotherapy across treatment lines between patients of the three groups according to TP53 status (7 and 5 vs. 8 months in median, respectively, p = 0.60, Supplementary Figure S1).
Figure 1.
Overall survival of patients with metastatic anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC) according to TP53 status at baseline and progression. (A) The median overall survival (OS) was 44 months for patients with TP53 mutations at baseline (TP53mutbas) vs. 62 months for patients without TP53 mutations at baseline (TP53wtbas logrank p = 0.0182). (B) The median OS was 45 months for patients with initially wild-type status and detection of TP53 mutations in a subsequent biopsy (TP53mutconv) vs. 94 months for patients without subsequent detection of TP53 mutations (TP53wtprogr, logrank p = 0.0343). Treatment details are given in Table 1.
Table 1.
Patient characteristics and details of treatment.
| All Study Patients (N = 62) | TP53wtbas (n = 39) | TP53mutbas (n = 23) | ||
|---|---|---|---|---|
| TP53wtprogr (n = 30) | TP53mutconv (n = 9) | |||
| Age (median; IQR) | 51; 17 | 63; 20 | 65; 19 | |
| Sex (male/female) | 15/16 | 5/4 | 12/11 | |
| ECOG PS at diagnosis of stage IV (median; IQR) | 0; 0 | 0; 0 | 1; 0 | |
| Histology | adenocarcinoma 1 | 29/30 | 9/9 | 23/23 |
| ALK status | positive | all cases by inclusion criteria | ||
| EML4-ALK V3 2 | 8/24 | 5/9 | 8/20 | |
| Stage IV NSCLC | at initial diagnosis | 20/30 * | 9/9 | 21/23 |
| M1a | 7/20 | 1/9 | 5/21 | |
| by relapse of M0 NSCLC | 10/30 | 0/9 | 2/23 | |
| TP53 assessment at baseline + at progression 3 | ||||
| method | FFPE at BL +FFPE at PD 4 | 8/30 (neg + neg) | 2/9 (neg + pos) | See Table S1 |
| FFPE at BL +ctDNA at PD | 11/30 (neg + neg) | 7/9 (neg + pos) | ||
| FFPE at PD 4 | 6/30 (neg) | |||
| only ctDNA at PD | 5/30 (neg) | |||
| TKI line (start) at 2nd assessment (median; IQR) | 2; 1 | 2; 1 | ||
| treatment line at 2nd assessment (median; IQR) | 2; 3 | 4; 1 | ||
| - days after diagnosis of stage IV (median; IQR) | 702; 1056 | 752; 600 | ||
| ALK TKI treatment, next-line | ||||
| crizotinib | 14 | 2 | ||
| ceritinib | 7 | 4 | ||
| alectinib | 6 | 1 | ||
| brigatinib | 2 | - | ||
| - no. of patients 5 | 29/30 (97%) | 7/9 (78%) | ||
| - no. of patients with CBDP | 15/30 | 4/7 | ||
| ALK TKI treatment, all lines (1–8) | ||||
| crizotinib | 23 | 9 | 19 | |
| ceritinib | 12 | 9 | 5 | |
| alectinib | 14 | 4 | 10 | |
| brigatinib | 4 | 0 | 3 | |
| lorlatinib | 3 | 1 | 1 | |
| - no. of patients 5,6 | 29/30 (97%) | 9/9 (100%) | 22/23 (96%) | |
| Chemotherapy, all lines (1–8) | ||||
| platin-doublets | 15 | 8 | 7 | |
| monotherapy | 6 | 4 | 6 | |
| - no. of patients | 14/30 (47%) | 8/9 (89%) | 8/23 (35%) | |
| Summary of the complete treatment | ||||
| no. of treatment lines (mean; SD) | 3.0; 1.5 | 4.0; 1.7 | 2.4; 1.6 | |
| no. of TKI treatment lines (mean; SD) | 1.9; 1.2 | 2.6; 1.0 | 1.7; 1.1 | |
| patients with additional radiotherapy | 18/30 (60%) | 6/9 (67%) | 12/21 (57%) | |
| patients with additional surgical treatment 7 | 5/30 (17%) | 1/9 (11%) | 5/21 (24%) | |
| Follow-up in months (median (25th–75th percentile)) | 36 (28–94) | |||
TP53wtbas: TP53 wild-type at baseline; TP53wtprogr: TP53 wild-type at baseline and after disease progression; TP53mutconv: TP53 wild-type at baseline with detection of TP53 mutations at progression; TP53mutbas: TP53 mutated at baseline; IQR: interquartile range; neg: negative; SD: standard deviation; PS: performance status; BL: baseline; PD: disease progression; no.: number; CBDP: continuation of treatment beyond disease progression due to ongoing clinical benefit; * p < 0.05 compared to TP53mutconv and p < 0.05 compared to TP53mutbas. 1 1/30 TP53wtprogr patients had an EML4-ALK V2 (E20;A20)+ large-cell neuroendocrine lung carcinoma. 2 The ALK fusion could be typed in 53/62 cases. 3 For 3/30 TP53wtprogr cases, TP53 wild-type status at progression was evaluated by analysis of ctDNA samples obtained 24, 29 and 37 months later. 4 For 7/8 TP53wtprogr cases, also ctDNA at PD (neg); for 5/6 TP53wtprogr cases, also ctDNA at PD (neg). 5 One TP53wtprogr patient received definitive local treatment for oligometastatic disease and is still in remission without exposure to TKI; 2/9 TP53mutconv patients did not receive next-line treatment after reassessment of TP53 status due to rapid clinical deterioration (they had received TKI in previous lines). 6 One TP53mutbas patient has ongoing stable disease 18 months after first-line chemotherapy without initiation of next-line treatment. 7 Excluding video-assisted thoracoscopy and pleurodesis for pleural effusion.
Figure 2.
Progression-free survival (PFS) of patients with metastatic anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC) under treatment with tyrosine kinase inhibitors (TKI) according to TP53 status at baseline and progression. (A) The median time-to-next-treatment (TNT) under TKI for patients with initially wild-type TP53 tumours after reassessment of TP53 status was 9 months for cases with a positive result (TP53mutconv) vs. 23 months for cases with negative result (TP53wtprogr, logrank p = 0.0013). Treatment details including continuation of treatment beyond disease progression are given in Table 1. (B) The median PFS under TKI treatment across all treatment lines was 8 months for patients with TP53 mutations at baseline (TP53mutbas) vs. 5 months for patients with initially wild-type status and detection of TP53 mutations in a subsequent biopsy (TP53mutconv) vs. 13 months for patients without subsequent detection of TP53 mutations (TP53wtprogr, logrank p = 0.0039); ns: not significant.
Analysis of initial clinical presentation revealed that patients retaining TP53 wild-type status after disease progression (TP53wtprogr) had featured a lower rate of metastatic disease at initial diagnosis than patients with TP53 mutations either at baseline (TP53mutbas, 67% vs. 91%, p = 0.034, Table 1) or at disease progression (TP53mutconv, 67% vs. 100%, p = 0.045, Table 1). The OS from initial diagnosis was also similar between patients with TP53 mutations detected either at diagnosis (TP53mutbas) or at disease progression (TP53mutconv) and worse than that of patients retaining TP53 wild-type status (TP53wtprogr, 44 and 45 months in median vs. not reached, p = 0.0012, Supplementary Figure S2).
3. Discussion
The results presented here extend the findings of recent studies that demonstrated the major clinical significance of baseline TP53 mutational status in ALK+ NSCLC [6,7,8] As in a previous report [8], presence of TP53 mutations in our patients at initial diagnosis was associated with shorter PFS under TKI (Figure 2B) and worse OS (Figure 1A), but did not apparently affect benefit from chemotherapy (Supplementary Figure S1).
The main novel finding of this study is that secondary detection of TP53 mutations at disease progression in patients with wild-type TP53 at baseline has a similar negative impact. Both PFS under TKI treatment and OS were shorter for initially wild-type patients with TP53 mutations detected later in the course of the disease (TP53mutconv), when compared to patients retaining the TP53 wild-type status (TP53wtther, Figure 2B and Figure 1B, respectively). Thus, among the generally favourable group of initially TP53 wild-type ALK+ tumours, acquisition of TP53 mutations identifies an unfavourable subgroup with a clinical course similar to that of primarily TP53 mutated cases. Emergence of TP53 mutations at the time of disease progression was observed in 23% (9/39) of initially TP53 wild-type patients in our cohort (Table 1). Of note, a similar percentage (20–24%) of metastatic ALK+ NSCLC has been reported to harbor TP53 mutations at baseline in two recent series [7,8], which adds up to TP53 mutations being detectable in approximately 40–50% of TKI-refractory ALK+ NSCLC patients, as had already been noted in an earlier study [12]. It should also be mentioned here, that TP53 mutations are overrepresented among the baseline samples of the current study, because all cases with detectable TP53 mutations at diagnosis were included, but several initially TP53 wild-type tumours had to be excluded, because no TP53 reassessment was available. Acquisition of TP53 alterations with disease progression has also been noted in various hematologic malignancies, like chronic lymphocytic leukemia [13] and multiple myeloma [14], in which it is also associated with worse outcome.
The comparable prognostic and predictive role of TP53 mutations in TP53mutbas and TP53mutconv ALK+ NSCLC suggest a similar adverse biology in these tumours, regardless of the time-point and context of TP53 mutation detection. One possibility is that other, more basic and still unidentified biologic alterations in TP53 mutated tumours might exert an even more important influence on clinical course, and that these could be active already before TP53 mutations become detectable, which may have implications for the ongoing efforts to target mutant TP53 with novel drugs [15]. At the same time, it cannot be excluded that due to intratumour heterogeneity, a tumour might initially be tested as TP53 wild-type on a TP53 wild-type region of the neoplasm, despite having a similar overall TP53 mutation load as tumours with readily detectable TP53 mutations. Indeed, analysis of surgical specimens has shown that TP53 sequencing results can be variable between different regions of the same tumour [16,17].
A noninvasive strategy to overcome the impact of intratumour heterogeneity could be to perform liquid biopsies (ctDNA assays) in addition to tissue biopsies in cases when a more accurate determination of TP53 status is needed, e.g., for purposes of prospective molecular risk stratification. The similarly adverse role of initially and subsequently detected TP53 mutations in our patients (Figure 1 and Figure 2), in combination with the predominance of ctDNA over tissue (i.e., FFPE DNA) assays among TP53 assessments under therapy in our study (Table 1, including footnote 4, and Supplementary Table S1) support the feasibility of this approach. Even though sensitivity of liquid biopsies for the detection of mutations is lower than 100%, for example it was determined as 60–70% regarding EGFR T790M in a recent study [18], we detected a TP53 mutation in the baseline ctDNA sample of a patient with wild-type TP53 in the respective biopsy (Supplementary Table S1), but cannot estimate the frequency of this constellation, because we lack baseline ctDNA samples for the majority of our patients.
In summary, the results of this study extend the picture of adverse clinical outcome associated with TP53 mutations in ALK+ NSCLC, and demonstrate the great potential of ctDNA assays for molecular profiling and longitudinal monitoring in ALK+ NSCLC beyond detection of ALK resistance mutations.
4. Materials and Methods
This study included all patients treated at our institution for histologically confirmed, ALK-driven NSCLC with TP53 status assessment at baseline and/or after disease progression after informed consent and approval by the Heidelberg University ethics committee (S-296/2016). Characteristics of study patients are summarized in Table 1. Biosamples were provided by BioMaterial Bank Heidelberg (BMBH) in accordance with its regulations and after approval by the Heidelberg University ethics committee. Clinical data were collected through a review of patient records and radiological images with chest CT and brain MRI-based restaging every 6–12 weeks. PFS was evaluated according to RECIST v1.1 [19]. The presence of an ALK translocation was ascertained by positive results in at least two of the following assays: ALK immunohistochemistry (D5F3 clone, Roche, Mannheim, Germany), ALK fluorescent in situ hybridisation (ZytoLight SPEC ALK probe, ZytoVision, Bremerhaven, Germany) and RNA-based next-generation sequencing (NGS, ThermoFisher Lung Cancer Fusion Panel, Waltham, MA, USA), as published previously (details are given in the Supplements) [5,20,21]. TP53 status was determined either on formalin-fixed paraffin-embedded (FFPE) tissue samples by DNA-based NGS using a proprietary Lung Cancer Panel that covers the entire TP53 exons 4, 5, 6, 7, 8, 9, 10, as published previously [8,21,22], and/or by plasma DNA genotyping using the AVENIO ctDNA Targeted kit that covers the entire TP53 exons 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, according to the manufacturer’s instructions (Roche, Mannheim, Germany; details are given in the Supplementary Files under supplementary Methods S1–S4). Baseline TP53 status was either directly determined by analysis of tumour samples obtained before treatment start in 51/62 cases or inferred as wild-type based on a negative TP53 result in an assessment performed at the time of disease progression in the remaining cases (Table 1 and Supplementary Table S1). TP53 status under therapy was determined by analysing tissue and/or blood (ctDNA) samples obtained after disease progression (Table 1 and Supplementary Table S1). Patients with detection of TP53 mutations at disease progression, but unknown baseline status, as well as patients with wild-type baseline TP53 status without reassessment after progression, were excluded from this analysis. In contrast, all patients with TP53 mutations at baseline were included. Survival data were analysed according to Kaplan–Meier and compared between patient subgroups with the logrank test. Median follow-up time was calculated by the reverse Kaplan–Meier method. Categorical data were compared with the chi-square test. Statistical calculations were performed with SPSS version 24 (IBM, Armonk, NY, USA) and plots generated with GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA).
5. Conclusions
This study shows that detection of TP53 mutations in tissue of liquid rebiopsies at the time of disease progression in previously negative patients is associated with more aggressive clinical course, shorter TKI responses and inferior OS in ALK+ NSCLC, comparable to primary TP53 mutated cases. These results extend the picture of adverse clinical outcome associated with TP53 mutations in ALK+ NSCLC, and demonstrate the great potential of ctDNA assays for molecular profiling and longitudinal monitoring in ALK+ NSCLC beyond detection of ALK resistance mutations.
Acknowledgments
The authors thank Ingrid Heinzmann-Groth and Saskia Östringer of the Translational Research Unit of the Thoraxklinik Heidelberg for assistance with the collection of patient samples, and they thank all technical assistants in the Genomics Core Facility of the German Cancer Research Center (DKFZ) as well as in the Center for Molecular Pathology (CMP) Heidelberg for expert handling of samples and for technical support. They also thank Gregor Obernosterer (Roche Diagnostics) for logistic support. They also acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Abbreviations
| ALK | anaplastic lymphoma kinase |
| NSCLC | non-small cell lung cancer |
| TKI | tyrosine kinase inhibitor |
| TNT TP53 |
time-to-next-treatment tumor protein p53 |
| PFS | progression-free survival |
| OS | overall survival |
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6694/11/1/124/s1, Figure S1: Progression-free survival of patients with metastatic ALK+ NSCLC under treatment with chemotherapy according to TP53 status at baseline and under therapy; Figure S2: Overall survival of study patients from initial diagnosis; Table S1: TP53 mutations of the study patients; Supplementary Method S1: RNA- and DNA-next-generation sequencing (NGS); Method S2: RT-PCR; Method S3: fluorescence in situ hybridisation (FISH); Method S4: ctDNA analysis.
Author Contributions
P.C., S.D., and M.K. performed the analysis of clinical in conjunction with genetic data, wrote the first draft of the manuscript and finalized it after input from the other coauthors. S.D., A.-L.V., V.E., S.O., T.Z., H.S. and A.S. performed next-generation sequencing analysis of ctDNA samples. M.K., V.E., O.N., J.B., M.A., J.L., P.S. and A.S. performed next-generation sequencing, reverse-transcription polymerase chain reaction, fluorescence in situ hybridisation and immunohistochemistry analysis of formalin-fixed paraffin-embedded tumour samples. C.-P.H., F.J.H., M.E., M.M., H.B. and M.T. analyzed radiological images and/or provided clinical data and patient samples. All authors contributed to writing and review of the manuscript.
Funding
This work was supported by the German Center for Lung Research (DZL), by the German Cancer Consortium (DKTK), and by the Heidelberg Center for Personalized Oncology at the German Cancer Research Center (DKFZ-HIPO).
Conflicts of Interest
P.C. reports speaker’s honoraria from Novartis and Roche. S.D. reports speaker’s honoraria from Roche; V.E. reports advisory board and lecture fees from AstraZeneca and ThermoFisher; C.P.H. reports consultation, lecture and other fees from Novartis, Basilea, Bayer, Grifols, Boehringer, Pierre Fabre, Covidien, Siemens, Chiesi, Intermune, MEDA Pharma, Bracco, Pfizer, MSD, Roche, Lilly, AstraZeneca, Schering-Plough, Essex, Gilead, MeVis, Fresenius, Astellas as well as ownership of GSK stocks; F.J.H. reports advisory board fees and honoraria from Lilly, Roche, AstraZeneca, Novartis, Boehringer, Chiesi, Teva, Pulmonx BTG and Olympus as well as research funding from Lilly, Roche, AstraZeneca, Novartis, Boehringer, Chiesi and Teva; J.L. reports advisory board fees from AstraZeneca. P.S. reports advisory board honoraria from Pfizer, Roche, Novartis, AstraZeneca as well as speaker’s honoraria and research funding from Roche, AstraZeneca and Novartis; M.T. reports advisory board honoraria from Novartis, Lilly, BMS, MSD, Roche, Celgene, Takeda, AbbVie, Boehringer, speaker’s honoraria from Lilly, MSD, Takeda, research funding from AstraZeneca, BMS, Celgene, Novartis, Roche and travel grants from BMS, MSD, Novartis, Boehringer; HS reports advisory board and speaker’s honoraria from Roche; AS reports advisory board honoraria from BMS, AstraZeneca, ThermoFisher, Novartis, speaker’s honoraria from BMS, Illumina, AstraZeneca, Novartis, ThermoFisher, MSD, Roche, as well as research funding from Chugai and BMS.
References
- 1.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S., Watanabe H., Kurashina K., Hatanaka H., et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Duruisseaux M., Besse B., Cadranel J., Perol M., Mennecier B., Bigay-Game L., Descourt R., Dansin E., Audigier-Valette C., Moreau L., et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): A french nationwide cohort retrospective study. Oncotarget. 2017;8:21903–21917. doi: 10.18632/oncotarget.15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo C.G., Seo S., Kim S.W., Jang S.J., Park K.S., Song J.Y., Lee B., Richards M.W., Bayliss R., Lee D.H., et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various alk inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann. Oncol. 2017;28:791–797. doi: 10.1093/annonc/mdw693. [DOI] [PubMed] [Google Scholar]
- 4.Lin J.J., Zhu V.W., Yoda S., Yeap B.Y., Schrock A.B., Dagogo-Jack I., Jessop N.A., Jiang G.Y., Le L.P., Gowen K., et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J. Clin. Oncol. 2018 doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christopoulos P., Endris V., Bozorgmehr F., Elsayed M., Kirchner M., Ristau J., Buchhalter I., Penzel R., Herth F.J., Heussel C.P., et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ NSCLC. Int. J. Cancer. 2018;142:2589–2598. doi: 10.1002/ijc.31275. [DOI] [PubMed] [Google Scholar]
- 6.Aisner D.L., Sholl L.M., Berry L.D., Rossi M.R., Chen H., Fujimoto J., Moreira A.L., Ramalingam S.S., Villaruz L.C., Otterson G.A., et al. The impact of smoking and TP53 mutations in lung adenocarcinoma patients with targetable mutations-the lung cancer mutation consortium (LCMC2) Clin. Cancer Res. 2018;24:1038–1047. doi: 10.1158/1078-0432.CCR-17-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kron A., Alidousty C., Scheffler M., Merkelbach-Bruse S., Seidel D., Riedel R., Ihle M., Michels S., Nogova L., Fassunke J., et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann. Oncol. 2018 doi: 10.1093/annonc/mdy333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christopoulos P., Kirchner M., Bozorgmehr F., Endris V., Elsayed M., Budczies J., Ristau J., Penzel R., Herth F.J., Heussel C.P., et al. Identification of a highly lethal V3+ TP53+ subset in ALK+ lung adenocarcinoma. Int. J. Cancer. 2018 doi: 10.1002/ijc.31893. [DOI] [PubMed] [Google Scholar]
- 9.Lin J.J., Riely G.J., Shaw A.T. Targeting alk: Precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCoach C.E., Blakely C.M., Banks K.C., Levy B., Chue B.M., Raymond V.M., Le A.T., Lee C.E., Diaz J., Waqar S.N., et al. Clinical utility of cell-free DNA for the detection of alk fusions and genomic mechanisms of alk inhibitor resistance in non-small cell lung cancer. Clin. Cancer Res. 2018;24:2758–2770. doi: 10.1158/1078-0432.CCR-17-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amirouchene-Angelozzi N., Swanton C., Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-17-0343. [DOI] [PubMed] [Google Scholar]
- 12.Gainor J.F., Dardaei L., Yoda S., Friboulet L., Leshchiner I., Katayama R., Dagogo-Jack I., Gadgeel S., Schultz K., Singh M., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malcikova J., Stano-Kozubik K., Tichy B., Kantorova B., Pavlova S., Tom N., Radova L., Smardova J., Pardy F., Doubek M., et al. Detailed analysis of therapy-driven clonal evolution of TP53 mutations in chronic lymphocytic leukemia. Leukemia. 2015;29:877–885. doi: 10.1038/leu.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin M., Sive J.I., Allen C., Roddie C., Chavda S.J., Smith D., Blombery P., Jones K., Ryland G.L., Popat R., et al. Prevalence and timing of TP53 mutations in del(17p) myeloma and effect on survival. Blood Cancer J. 2017;7:e610. doi: 10.1038/bcj.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bykov V.J.N., Eriksson S.E., Bianchi J., Wiman K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L.L., Kan M., Zhang M.M., Yu S.S., Xie H.J., Gu Z.H., Wang H.N., Zhao S.X., Zhou G.B., Song H.D., et al. Multiregion sequencing reveals the intratumor heterogeneity of driver mutations in TP53-driven non-small cell lung cancer. Int. J. Cancer. 2017;140:103–108. doi: 10.1002/ijc.30437. [DOI] [PubMed] [Google Scholar]
- 17.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B.K., Veeriah S., Shafi S., Johnson D.H., Mitter R., Rosenthal R., et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 18.Passiglia F., Rizzo S., Di Maio M., Galvano A., Badalamenti G., Listi A., Gulotta L., Castiglia M., Fulfaro F., Bazan V., et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in nsclc: A systematic review and meta-analysis. Sci. Rep. 2018;8:13379. doi: 10.1038/s41598-018-30780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New Response Evaluation Criteria In Solid Tumours: Revised recist guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Pfarr N., Stenzinger A., Penzel R., Warth A., Dienemann H., Schirmacher P., Weichert W., Endris V. High-throughput diagnostic profiling of clinically actionable gene fusions in lung cancer. Genes Chromosomes Cancer. 2016;55:30–44. doi: 10.1002/gcc.22297. [DOI] [PubMed] [Google Scholar]
- 21.Volckmar A.-L., Leichsenring J., Kirchner M., Christopoulos P., Neumann O., Budczies J., de Oliveira C.M.M., Rempel E., Buchhalter I., Brandt R., et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: Analysis of the first 3,000 Heidelberg cases. Int. J. Cancer. 2019 doi: 10.1002/ijc.32133. [DOI] [PubMed] [Google Scholar]
- 22.Endris V., Penzel R., Warth A., Muckenhuber A., Schirmacher P., Stenzinger A., Weichert W. Molecular diagnostic profiling of lung cancer specimens with a semiconductor-based massive parallel sequencing approach: Feasibility, costs, and performance compared with conventional sequencing. J. Mol. Diagn. 2013;15:765–775. doi: 10.1016/j.jmoldx.2013.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.