Abstract
N-acylhomoserine lactones (AHLs), bacterial signaling compounds involved in quorum-sensing, are a structurally diverse group of compounds. We describe here the identification, synthesis, occurrence and biological activity of a new AHL, N-((2E,5Z)-2,5-dodecadienoyl)homoserine lactone (11) and its isomer N-((3E,5Z)-3,5-dodecadienoyl)homoserine lactone (13), occurring in several Roseobacter group bacteria (Rhodobacteraceae). The analysis of 26 strains revealed the presence of 11 and 13 in six of them originating from the surface of the macroalgae Fucus spiralis or sediments from the North Sea. In addition, 18 other AHLs were detected in 12 strains. Compound identification was performed by GC/MS. Mass spectral analysis revealed a diunsaturated C12 homoserine lactone as structural element of the new AHL. Synthesis of three likely candidate compounds, 11, 13 and N-((2E,4E)-2,4-dodecadienoyl)homoserine lactone (5), revealed the former to be the natural AHLs. Bioactivity test with quorum-sensing reporter strains showed high activity of all three compounds. Therefore, the configuration and stereochemistry of the double bonds in the acyl chain seemed to be unimportant for the activity, although the chains have largely different shapes, solely the chain length determining activity. In combination with previous results with other Roseobacter group bacteria, we could show that there is wide variance between AHL composition within the strains. Furthermore, no association of certain AHLs with different habitats like macroalgal surfaces or sediment could be detected.
Keywords: quorum-sensing, structure elucidation, Rhodobacteraceae, autoinducers, bacterial signaling, Heck coupling, mass spectrometry, gas chromatography, sediment, AHL
1. Introduction
N-acylhomoserine lactones (AHLs) are well known signalling compounds used by Gram-negative bacteria for quorum-sensing (QS)-driven cell-to-cell communication. QS is a cell density-dependant mechanism to regulate physiological traits like antibiotic production, cell differentiation or biofilm formation [1,2,3,4,5,6,7]. AHLs constitute a γ-lactone ring and an acyl side chain that is usually even-numbered and unbranched, ranging in chain length from C4–C18 [3]. The stereochemistry of the lactone ring is S. Functional groups like hydroxy- or carbonyl-group can be present at C-3 of the acyl chain. The double bond of unsaturated AHLs is Z-configured and in the position ω-7, with few exceptions [3]. An additional double bond can occur at C-2 in E-configuration, a feature especially occurring in AHLs produced by marine Roseobacter group bacteria (Rhodobacteraceae). Roseobacters are abundant in the ocean, occurring in diverse habitats [8], e. g. in open waters, shore environments, sediments, attached to biotic and abiotic surfaces as well as in symbiosis with higher organisms like algae [9,10,11]. AHLs of this bacterial group have saturated, unsaturated and sometimes oxygenated acyl chains, ranging in length between C8 and C18 [12] with the exception of aromatic p-coumaroylhomoserine lactone produced by Ruegeria pomeroyi DSS-3 [13]. They are involved in various biological traits [14], e.g., in the production of the antibiotic tropodithietic acid in Phaeobacter inhibens [5] or cell differentiation in Dinoroseobacter shibae [4].
In a broader program, we currently look into the inventory of AHLs occurring in Roseobacter group bacteria. A non-targeted analytical approach was developed using extraction of AHLs from bacterial cultures by XAD-16 adsorption, solvent extraction, and direct analysis by GC/MS. This approach combines high-sensitivity with unbiased analysis and allows structural proposals to be made basing on the information rich EI-mass spectra obtained. We could successfully use this approach to identify several previously unknown AHLs [12,15,16] as well as related N-acylalanine methyl esters (NAMEs) [17,18]. We have previously analyzed AHLs from roseobacters isolated from macroalgae surfaces and found a high proportion of strains producing AHLs in various mixtures [12]. A specific AHL signature of macroalgae associated strains was not observed. In an extension of this study we investigated 16 strains obtained from one location of samples of the algae Fucus spiralis, collected from a single location (Neuharlingersiel, German Wadden Sea), nine strains from Norwegian trench sediments [19] and one sea water strain from the German/Danish coast to test for the occurrence of specific AHL signatures. During this investigation we detected two previously unreported AHLs, their identification, synthesis, and biological activities being reported.
2. Results
2.1. Occurrence of N-acylhomoserine Lactones in Roseobacter Group Bacteria of Fucus Spiralis and the Eastern North Sea
Sixteen roseobacters originating from Fucus spiralis from the German Wadden Sea and 10 strains from the eastern North Sea were cultivated in marine broth and analyzed for the presence of AHLs by GC/MS as described previously [12,20]. AHLs were detected in eight of the isolates from F. spiralis (50%) and three of the sediment strains (33%) as well as in the open water strain (Table 1). The highest numbers of individual compounds were detected on the extracts obtained from Octadecabacter sp. (Lw-22) and Loktanella sp. (D15 (40)), 12 and 13 AHLs being detected, exhibiting a distinct qualitative profile. The sediment strain Phaeobacter sp. (SK040) contained nine different AHLs, while the other sediment strains SK013 and SK032 had only one or two AHLs, comparable to the water column strain. The AHL composition of other strains varied between single compounds and mixtures (Table 1).
Table 1.
AHL production of Roseobacter group isolates from Fucus spiralis (German Wadden Sea) and sediments as well as open waters (Eastern North Sea) a.
| Strain | Genus Affiliation | C12:0 | C12:1 | C12:2 (11) | C12:2 (13) | 3-OH-C10 | 3-OH-C14 | C14:0 | C14:1 | C14:2 | C15:0 | C15:1 | C16:0 | C16:1 | C16:2 | C17:1 | C18:0 | C18:1 | C18:2 | C18:2 | C18:2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fucus Spiralis | |||||||||||||||||||||
| D12-1.68 | Roseovarius sp. | 94.4 | 1.3 | 4.3 | |||||||||||||||||
| D3 | Loktanella sp. | 15.7 | 2.4 | 21.2 | 60.7 | ||||||||||||||||
| Lw-22 | Octadecabacter sp. | 6.6 | 3.7 | 0.7 | 4.6 | 72.7 | 4.3 | 1.2 | 1.9 | 0.9 | 0.6 | 1.1 | 1.8 | ||||||||
| D4 (50) | Octadecabacter sp. | 8.3 | 25.8 | 4.4 | 1.1 | 0.7 | 59.8 | ||||||||||||||
| Lw-26b | Loktanella sp. | 100 | |||||||||||||||||||
| D15 (40) | Loktanella sp. | 3.9 | 5.9 | 1.2 | 0.9 | 18.4 | 2.3 | 58.6 | 0.5 | 0.8 | 0.6 | 6.1 | 0.5 | 0.3 | |||||||
| Lw-55a | Loktanella sp. | 100 | |||||||||||||||||||
| MDLw-58 | Dinoroseobacter sp. | 5.4 | 3.9 | 2.5 | 88.2 | ||||||||||||||||
| Sediment | |||||||||||||||||||||
| SK013 | Shimia sp. | 100 | |||||||||||||||||||
| SK032 | Huaishuia sp. | 81.7 | 18.3 | ||||||||||||||||||
| SK040 | Phaeobacter sp. | 5.8 | 3.7 | 1.4 | 7.3 | 4.4 | 4.8 | 64.9 | 3.5 | 4.1 | |||||||||||
| Water Column | |||||||||||||||||||||
| SK038 | Sulfitobacter sp. | 100 | |||||||||||||||||||
a relative amounts of AHLs for each strain in %. No AHLs detected in Puniceibacterium sp. Lw-III1a, Sulfitobacter sp. B15 G2, Pseudooceanicola sp. Lw-13e, Roseobacter sp. B14, Loktanella sp. SK033, Phaeobacter sp. N05I, Sulfitobacter sp. B14 27, A12, D4 55, SK012, Citreicella sp. Lw-41a, Roseovarius pelophilus G5II, Tateyamaria pelophila SAM4, Pseudoruegeria sp. SK021.
Octadecenoylhomoserine lactone (C18:1-HSL) and C16:1-HSL were the most common compounds, produced by six of the 12 bacteria. Roseovarius sp. D12-1.68 displayed a unique profile due to the presence of C12:0 as major AHL with a relatively short chain length compared to most other major AHLs of roseobacters [12,15,21]. Dinoroseobacter shibae MDLw-58 produced three different isomers of the C18:2-HSL, consistent with previous observations of closely related strains [20]. While the major component was 2E,11Z-C18:2-HSL, the location and configuration of the double bonds in the other two isomers remains unknown. Other diunsaturated AHLs with shorter chain lengths, rarely observed in strains taxonomically distant from roseobacters, occurred as well. These include C16:2-HSL in Huaishuia sp. SK032, C14:2-HSL in Octadecabacter sp. Lw-22 and several strains containing C12:2-HSL. Because this AHL was not previously reported and due to its abundant occurrence in the investigated strains, we determined its structure of this new AHL, as reported in Section 2.2. Less abundant were the oxygenated AHLs, 3-OH-C10- and 3-OH-C14-HSL. The only odd numbered AHLs were C15:0-HSL, C15:1-HSL and C17:1-HSL which occurred as minor components in several strains.
2.2. Identification and Synthesis of New Diunsaturated N-acylhomoserine Lactones from the Roseobacter Group
During these analyses two compounds, A as the major component and B in lower concentration, were detected in five of the strains, whose mass spectra showed similarity to those of other AHLs. The spectra of A (Figure 1b) and B (Supplementary Figure S1) were very similar, although the quality of the spectra was often low due to overlapping peaks from other compounds. To elucidate their structure, analysis of mass spectral data and total synthesis were performed.
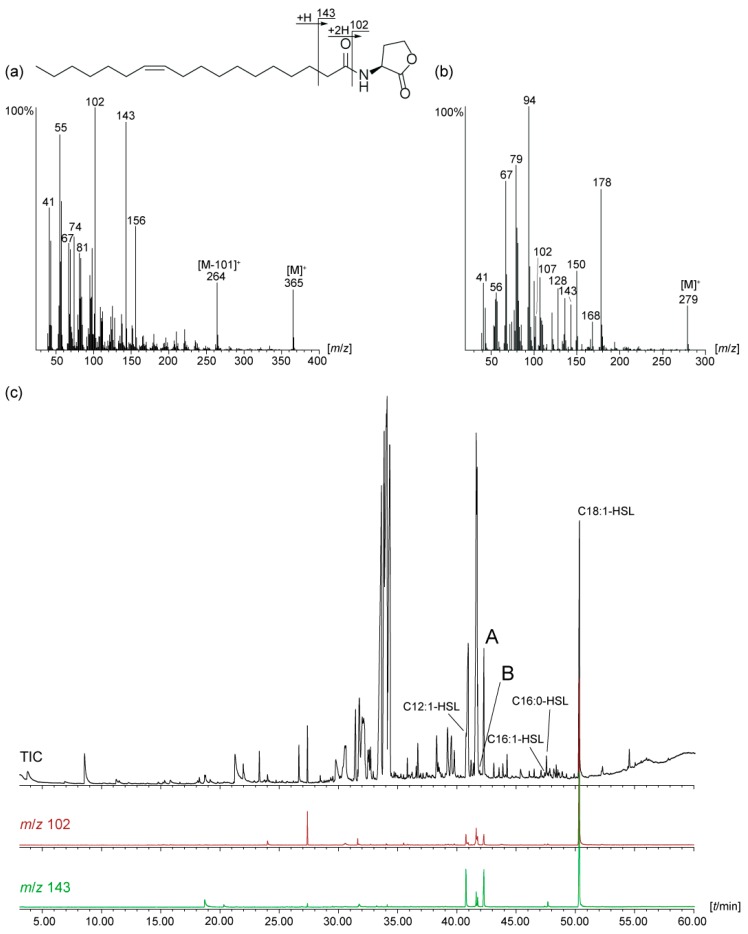
Figure 1.
(a) Mass spectrum and fragmentation pattern of N-((Z)-11-octadecenoyl)homoserine lactone (Z11-C18:1-HSL); (b) mass spectrum of the unknown AHL A; (c) total ion chromatogram of the natural extract of Octadecabacter D4 (50) and characteristic ion traces m/z 102 and 143, indicating potential presence of AHLs.
Electron impact mass spectra of AHLs have a typical fragmentation pattern shown in Figure 1a. AHLs are characterized by the fragment ions m/z 102, 143 and a small [M]+ [15,22]. The ion m/z 102 is formed by α-cleavage of the homoserine lactone unit and transfer of two hydrogens, while McLafferty-rearrangement forms the ion m/z 143. The intensity of m/z 102 is higher in monounsaturated compared to diunsaturated AHLs and the intensities of m/z 102 and 143 decrease with the acyl chain length [15]. Cleavage of the homoserine moiety explains the ion [M--101]+, m/z 264, in the spectrum of (Z)-11-octadecenoylhomoserine lactone (Z11-C18:1-HSL, Figure 1a).
Compound A and B showed both ions m/z 102 and 143 and a putative [M]+ at m/z 279, indicating to be C12:2-homoserine lactones (C12:2-HSL). Additional ions at m/z 94 and 107, untypical for AHLs, were present in high intensity. The gas chromatographic retention index of A was 2422 and that of B 2388. High resolution ESI-MS of A delivered an ion at m/z 280.19071 [M + H]+, consistent with the formula C16H26NO3 (calc. 280.19072) required for an diunsaturated AHL. The homoserine lactone unit is indicated by the ion m/z 102.05496 (C4H8NO2) and the acyl chain by the ion m/z 179.14314 (C12H19O) in the ESI spectrum [18]. The location of the double bonds could not be determined with dimethyl disulfide derivatization [12,20] because of the low concentration of the compound. Nevertheless, the usual ω-7 position of double bonds in AHLs and the previous detection of Z5-C12:1-HSL in roseobacters [12] suggested A to be 2E,5Z-C12:2-HSL, because the second double-bond in all known natural AHLs is located at C-2 with E-configuration. The close proximity of the double bonds might also favor a double bond shift into conjugation during biosynthesis with concomitant double bond isomerization, leading to 2E,4E-C12:2-HSL. Furthermore, deconjugation is a known process known to occur during formation of α,β-unsaturated amides under basic conditions, leading potentially to 3E,5Z-C12:2-HSL [23,24]. Therefore, we decided to synthesize all three compounds to reveal insight into the MS and GC behavior of the isomers and also allowing to perform bioassays to investigate whether slight changes in double bond location and geometry have an influence on the activity in AHL reporter assays.
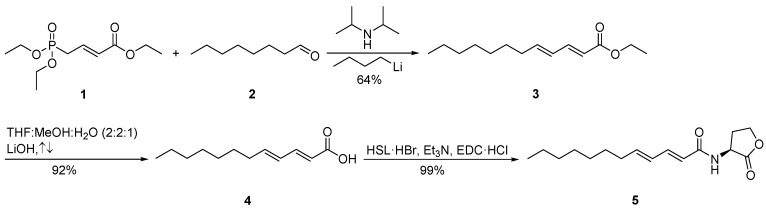
The synthesis of 2E,4E-C12:2-HSL (5) started with a homologous Horner-Wadsworth-Emmons reaction of triethyl (E)-4-phosphonocrotonate (1) with octanal (2) to furnish ethyl (2E,4E)-2,4-dodecadienoate (3) (Scheme 1). After saponification with lithium hydroxide, 4 was coupled with l-homoserine lactone hydrobromide (HSL·HBr) in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) and triethylamine, yielding the desired AHL N-((2E,4E)-2,4-dodecadienoyl)homoserine lactone (5).
Scheme 1.
Synthesis of N-((2E,4E)-2,4-dodecadienoyl)homoserine lactone (2E,4E-C12:2-HSL, 5). l-homoserine lactone hydrobromide (HSL·HBr), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC·HCl).
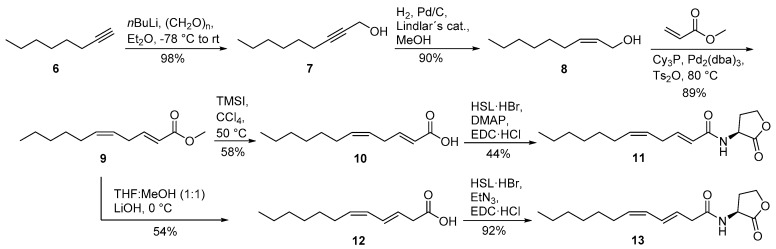
The isomers 2E,5Z-C12:2-HSL (11) and 3E,5Z-C12:2-HSL (13) were synthesized from octyne (6) (Scheme 2). The addition of paraformaldehyde furnished non-2-yn-1-ol (7). The allylic alcohol (Z)-non-2-en-1-ol (8) was obtained after hydrogenation with Lindlar′s catalysts. A Heck reaction according to Tsukuda et al. [25] using methyl acrylate instead of butyl acrylate yielded in excellent yield the desired diastereomerically pure methyl (2E,5Z)-2,5-dodecadienoate (9) that was saponified with lithium hydroxide. The basic conditions lead to a rearrangement of the double bond from C-2 to C-3, forming acid 12. This acid was coupled with HSL·HBr in the presence of EDC·HCl and triethylamine to form N-((3E,5Z)-3,5-dodecadienoyl)homoserine lactone (13). The saponification of ester 9 can also be performed under milder conditions, thus preventing the rearrangement. First, ester 9 was transesterified to the trimethylsilyl ester with trimethylsilyl iodide (TMSI) in CCl4. Aqueous hydrolysis delivers acid 10 without rearrangement [26]. Finally, acid 10 was coupled with HSL·HBr in the presence of EDC·HCl and p-dimethylaminopyridine (DMAP) to furnish N-((2E,5Z)-2,5-dodecadienoyl)homoserine lactone (11). The isomerization of the double bond, leading again to 13, can also be induced by the use of triethylamine in this step instead of the weaker base DMAP [24]. AHL 11 is not stable for prolonged storage under room temperature, leading to the isomerized compound 13 and other stereoisomers.
Scheme 2.
Synthesis of N-((2E,5Z)-2,5-dodecadienoyl)homoserine lactone (2E,5Z-C12:2-HSL, 11) and N-((3E,5Z)-3,5-dodecadienoyl)homoserine lactone (3E,5Z-C12:2-HSL, 13). Tricyclohexylphosphine (Cy3P), tris(dibenzylideneacetone)dipalladium (Pd2(dba)3), l-homoserine lactone hydrobromide (HSL·HBr), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC·HCl), p-dimethylaminopyridine (DMAP).
Comparison of both mass spectra (Figure 2) and gas chromatographic retention indices of the synthesized AHLs with those of the natural compounds indicated the unknown major AHL A to be 2E,5Z-C12:2-HSL (11), while the minor component B is its rearrangement product, 3E,5Z-C12:2-HSL (13). The mass spectrum of 5 differs from those of 11 and 13, showing an ion at m/z 180 instead of m/z 178. The ions m/z 94, 102, 107 and 143 are present, but the intensity of these fragment ions is different to those of the natural compounds, as is the retention index of 2510. HR-MS data showed that the ions m/z 107 and 94, not observed in high intensity in other diunsaturated AHLs with longer acyl chains, are composed of C8H11 and C6H6O, respectively. They seem to be formed by acyl cleavage followed by allylic chain cleavage with or without previous elimination of water.
Figure 2.
Mass spectra of synthetic AHLs: (a) 2E,5Z-C12:2-HSL (11); (b) 2E,4E-C12:2-HSL (5); (c) 3E,5Z-C12:2-HSL (13).
2.3. Activity of N-acylhomoserine Lactones in AHL Reporter Assays
The three C12:2-HSLs 5, 11, and 13 as well as 2E,11Z-C18:2-HSL, a major AHL of the Roseobacter group model strain Dinoroseobacter shibae DFL12 [4,20] were tested for quorum sensing activity with two sensor strains (Table 2). Escherichia coli MT102 (pJBA132) [27] responds primarily to short chain AHLs while Pseudomonas putida F117 (pRK-C12) [28] prefers long chain AHLs. Both strains do not produce AHLs but are able to respond to them through the expression of a LuxR-controlled promoter fused to a gene coding for an easily detectable output signal, fluorescence [15]. In addition, C6:0-, C8:0- and 3-oxo-C8:0-HSL for E.coli MT102 and Z9-C16:1-, 3-oxo-C8:0- and 3-oxo-C12:0-HSL for P. putida F117 were tested as references. The maximum fold induction with sensor strain E.coli MT102 was low for all target AHLs, 5, 11, 13 as well as 2E,11Z-C18:2-HSL, but C6 and C8-HSLs showed activity as expected.
Table 2.
Activity of synthetic AHLs in quorum sensing experiments with the sensor strains E.coli MT102 (pJBA132) and P. putida F117 (pRK-C12). Maximum fold induction.
| AHLa | MT102 | F117 |
|---|---|---|
| 2E,4E-C12:2 (5) | 1.00 ± 0.01 | 81.22 ± 1.26 |
| 2E,5Z-C12:2 (11) | 1.01 ± 0.01 | 78.03 ± 2.05 |
| 3E,5Z-C12:2 (13) | 1.03 ± 0 | 73.64 ± 2.34 |
| 2E,11Z-C18:2 | 1.02 ± 0.01 | 1.32 ± 0.04 |
| 3-oxo-C8:0 | 13.41± 0.49 | 30.46 ± 2.79 |
| C6:0 | 17.09 ± 1.04 | |
| C8:0 | 10.24 ± 0.55 | |
| 3-oxo-C12:0 | 77.75 ± 2.51 | |
| Z9-C16:1 | 72.37 ± 2.47 |
a The final concentration of each tested compound was 10 µM.
In contrast, sensor P. putida F117, most sensitive to C12:0-HSL [16], showed high induction upon exposure to AHLs 5, 11, and 13, as well as 3-oxo-C12:0-HSL and Z9-C16:1-HSL, a lower activity for 3-oxo-C8:0-HSL being noted. The almost identical values exhibited by the four C12 compounds evidence a certain degree of selectivity dependent on chain length, but the reporter strain is insensitive to position and configuration of the double bonds, as well as presence or absence of a 3-oxo functional group.
3. Discussion
The unknown AHL A was identified to be 2E,5Z-C12:2-HSL (11). This new AHL is the smallest AHL with two double-bonds identified so far and occurs in several roseobacters. The minor 3E,5Z-C12:2-HSL (13) seems to be a rearrangement product of 11. Although we cannot exclude that the process took place during work-up, the observed chemical instability of 11 may point to the formation of 13 as a bacterial metabolic product. Different roseobacters are able to produce a bishomologous series of diunsaturated AHLs with double-bonds at C-2 and the ω-7 position [12,15,16,20]. AHL 11 extends this series to a shorter C12 chain length. It is also within the preferred chain-length of major AHLs of roseobacters that ranges from C12 to C18, with the exception of 3-OH-C10-AHL. Although we analyzed many roseobacters for AHL presence using GC/MS as well as HPLC/MS methods, we never found evidence for the presence of p-coumaroyl-HSL reported from Ruegeria pomeroyi DSS-3 [13] in any of these strains.
Although the AHLs 5, 11 and 13 have the same chain length, the shape of the side chain differs. The two (E)-configured double bonds of 2E,4E-C12:2-HSL lead to a straight aliphatic chain, while the (Z)-configured double-bonds in 2E,5Z-C12:2-HSL and 3E,5Z-C12:2-HSL induce a bend in the chain. 3-oxo-C12:0-HSL even has a bend chain due to H-bonding towards the amide carbonyl group [29]. Obviously, there is no influence of the chain configuration on the activity of the reporter strain. Therefore, the configuration of the side chain does not seem to be recognized by the receptor, although the chain-length obviously is. Whether this is also true for the cognate receptors in the roseobacters is unknown. Nevertheless, the (2E)-double bond is not common in fatty acids of roseobacters [30] and thus the prominent occurrence of this structural motif and of diunsaturated AHLs in general indicate the importance of the double bonds for their function as signaling compounds of these bacteria.
No general association of specific AHLs or their mixtures with certain habitats was observed. This includes strains originating from the same host organism as F. spiralis reported here, but also strains from the sediment or the water phase [12,15]. In general, surface-associated strains seem to produce AHLs more often under laboratory conditions compared to those obtained from sediments or the water column.
4. Materials and Methods
4.1. General Experimental Procedures
General conditions: Chemicals were obtained from Sigma-Aldrich (Taufkirchen, Germany), Carl Roth (Karlsruhe, Germany) or from Acros Organics (Schwerte, Germany) and were used without further purification. Solvents were purified by distillation and dried according to standard procedures. Moisture- and/or oxygen-sensitive reactions were carried out under a nitrogen atmosphere in vacuum-heated flasks with dried solvents. Thin-layer chromatography (SiO2, TLC) was performed on 0.20 mm Macherey-Nagel silica gel plates (Polygram SIL G/UV254), and column chromatography was performed with Merck silica gel 60 (0.040–0.063 mm) by using standard flash chromatographic methods. NMR spectra were recorded on DRX-400 (400 MHz), AV III-400 (400 MHz) or AV II-600 (600 MHz) spectrometers (Bruker: Bremen, Germany), and were referenced against TMS (δ = 0.00 ppm), CDCl3 (δ = 7.26 ppm) for 1H-NMR and CDCl3 (δ = 77.01 ppm) for 13C-NMR experiments. GC/MS analyses of extracts were carried out on an GC 7890A gas chromatograph connected to a 5975C mass-selective detector (Agilent; Waldbronn, Germany). Synthetic samples were analyzed on an HP GC 6890 system connected to an HP 5973 mass selective detector fitted with a HP-5 MS fused-silica capillary column (30 m × 0.25 mm i.d., 0.22 µm film; (Agilent; Waldbronn, Germany). Conditions were as follows: carrier gas (He): 1.2 mL min−1; injection volume: 1 mL; injector: 250 °C; transfer line: 300 °C, EI 70 eV. The gas chromatograph was programmed as follows: 50 °C (5 min isothermal), increasing with 5 °C min−1 to 320 °C, and operated in splitless mode for XAD extracts and 50 °C (5 min isothermal), increasing with 10 °C min−1 to 320 °C in split mode (20:1) for synthetic compounds. Gas chromatographic retention indices, I, were determined from a homologous series of n-alkanes. Acids were transformed into volatile trimethylsilyl esters with N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) for analysis by GC/MS [31]. HRMS analyses were carried out on a Thermo Fisher linear iontrap (Thermo Fisher: Bremen, Germany) coupled with an LTQ-Orbitrap mass spectrometer (Thermo Fisaher: Bremen, Germany) using ESI positive mode. ESI measurements were performed by direct infusion mode using a custom-made micro-spray device mounted on a Proxeon nano-spray ion source. All solvents used were of LC/MS grade.
4.2. Strains and Culture Conditions
The bacterial strains were collected at various occasions in the North Sea [19]. Information on bacterial strains investigated is shown in Table 3.
Table 3.
Bacterial isolates with GenBank accession number, genus affiliation and origin.
| Strain | GenBank Acc No (16S) | Genus Affiliation | Strain Origin/Host | Location |
|---|---|---|---|---|
| Lw-III1a | KM268064 | Puniceibacterium sp. | Fucus spiralis | Neuharlingersiel |
| B15 G2 | KM268068 | Sulfitobacter sp. | Fucus spiralis | Neuharlingersiel |
| Lw-13e | KM268063 | Pseudooceanicola sp. | Fucus spiralis | Neuharlingersiel |
| D12-1.68 | KM268065 | Roseovarius sp. | Fucus spiralis | Neuharlingersiel |
| B14 | KM268066 | Roseobacter sp. | Fucus spiralis | Neuharlingersiel |
| B14 27 | KM268072 | Sulfitobacter sp. | Fucus spiralis | Neuharlingersiel |
| A12 | KM268070 | Sulfitobacter sp. | Fucus spiralis | Neuharlingersiel |
| D3 | KC731427 | Loktanella sp. | Fucus spiralis | Neuharlingersiel |
| D4 55 | KM268071 | Sulfitobacter sp. | Fucus spiralis | Neuharlingersiel |
| Lw-22 | KM268073 | Octadecabacter sp. | Fucus spiralis | Neuharlingersiel |
| D4 50 | KM268074 | Octadecabacter sp. | Fucus spiralis | Neuharlingersiel |
| Lw-26b | KM268054 | Loktanella sp. | Fucus spiralis | Neuharlingersiel |
| D15 40 | KM268056 | Loktanella sp. | Fucus spiralis | Neuharlingersiel |
| Lw-55a | KM268057 | Loktanella sp. | Fucus spiralis | Neuharlingersiel |
| MDLw-58 | KM268059 | Dinoroseobacter sp. | Fucus spiralis | Neuharlingersiel |
| Lw-41a | KM268061 | Citreicella sp. | Fucus spiralis | Neuharlingersiel |
| N05I | AJ968647 | Phaeobacter sp. | German North Sea Coast | Tidal-flat sediment |
| G5II | AJ968650 | Roseovarius pelophilus | German North Sea Coast | Tidal-flat sediment |
| SAM4 | AJ968651 | Tateyamaria pelophila a | German North Sea Coast | Tidal-flat sediment |
| SK012 | HG423260 | Sulfitobacter sp. | Danish North Sea | sediment |
| SK013 | LAJH00000000 | Shimia sp. b | Norwegian North Sea | sediment |
| SK021 | HG423263 | Pseudoruegeria sp. b | Norwegian North Sea | sediment |
| SK032 | HG423269 | Huaishuia sp. | German North Sea | sediment |
| SK033 | HG423270 | Loktanella sp. | Norwegian North Sea | sediment |
| SK040 | HG423272 | Phaeobacter sp. | Danish North Sea | sediment |
| SK038 | HG423271 | Sulfitobacter sp. | German North Sea | seawater |
4.3. Bacteria Cultivation and XAD Extraction
Bacterial strains were inoculated from marine broth (MB) medium agar plates into a preculture (50 mL, MB medium) and were cultivated for one to three days at 20/28 °C and 160 rpm. Bacterial cultures (100 mL) were grown from precultures for three to five days in MB medium at 20/28 °C and 160 rpm containing Amberlite XAD-16 (2 g). The XAD-16 resin was cleaned using Soxhlet extraction with acetonitrile, methanol and finally diethyl ether. The adsorbent was separated from the culture by filtration and extracted three times with CH2Cl2/H2O (10:1). The combined organic phases were dried with MgSO4, and the solvent was removed under reduced pressure. The extract was dissolved in CH2Cl2 (50 µL) and analyzed by GC/MS [20].
4.4. AHL Reporter Assays
The sensor strain Pseudomonas putida pRK-C12 [27] was inoculated from plates into a preculture which was grown on LB medium (20 mL with 20 mg/mL gentamycin) at 30 °C with shaking (160 rpm) overnight. The next day fresh medium was added, and the culture was grown on a shaking platform for 1–2 h until an OD620 value of 1.0 was reached. For the test, LB medium (99 µL) and 1 µL of the test compound (final concentration 10 µM, stock solution 1 mg/mL in dichlormethane) were pipetted into 96-well microtiter plates, and the sensor strain (100 µL) was added. Microtiter plates were incubated at 30 °C and shaken. Fluorescence was determined in a Victor 1420 Multilabel Counter (Perkin Elmer; Rodgau, Germany) at an excitation wavelength of 485 nm and a detection wavelength of 535 nm every 60 min for the first 6 h, and finally after 24 h of incubation. The OD620 value was also measured. Dichlormethane was used as negative control, and synthetic 3-oxo-C12:0-HSL was used as positive control. Fold induction of fluorescence was calculated by dividing the specific fluorescence (gfp535/OD620) of the test sample by the specific fluorescence of the negative control. Mean and standard deviation of three biological replicas after 6 h were determined, because fluorescence decreased slightly in the 24 h time point. The sensor strain Escherichia coli MT102 [29] was used as described [15] and the highest values obtained after 24 h in this case are reported in Table 2.
4.5. Synthetic Procedures
4.5.1. l-Homoserine Lactone Hydrobromide
A solution of bromoacetic acid (5.12 g, 36.9 mmol) and l-methionine (5.00 g, 33.5 mmol) in H2O/2-propanol/AcOH (5:5:2, 48.3 mL) was heated to reflux for 8 h. The solvent of the cooled mixture was evaporated. The orange solid was dissolved in dioxane/HCl (2:1, 20.1 mL), heated 10 min at 50 °C and stirred for 5 h at room temperature. The reaction mixture was placed in a fridge over night to evoke precipitation. l-Homoserine lactone hydrobromide was obtained by filtration and washed with cold isopropanol (3.19 g, 17.5 mmol, 52%) [35].
1H-NMR (400 MHz, DMSO-d6): δ = 4.44 (t, J = 8.8 Hz, 1H), 4.36–4.25 (m, 2H), 2.60–2.51 (m, 1H), 2.42–2.31 (m, 1H); 13C-NMR (100 MHz, DMSO-d6): δ = 173.4, 66.3, 47.8, 27.0; MS (70 eV, EI): m/z (%): 43 (100), 57 (90.5), 56 (62.4), 42 (40.1), 44 (16.0), 41 (8.3), 101 (5.0) [M+], 54 (4.8), 39 (4.3), 73 (4.0).
4.5.2. Ethyl (2E,4E)-2,4-Dodecadienoate (3)
N,N-Diisopropylamine (1.64 mL, 11.7 mmol) was dissolved in THF (25 mL) and cooled to −78 °C. n-Butyl lithium in hexane (1.6 M, 4.88 mL, 7.8 mmol) was added slowly to the mixture. The solution was stirred for 10 min at −78 °C, followed by 15 min at 0 °C. (E)-Triethyl-4-phosphonocrotonate (1, 1.30 mL, 5.8 mmol) was added slowly at −78 °C under stirring and after 15 min octanal (2, 0.61 mL, 3.9 mmol) was added at the same temperature, again stirring continued for 15 min. The reaction mixture was allowed to warm up to room temperature and stirred for 2.5 h. Sat. NH4Cl solution was added and the mixture was extracted three times with ethyl acetate. The combined organic phases were dried with MgSO4, filtered and the solvent was evaporated in vacuo. The crude product was purified by flash chromatography on silica [pentane/EtOAc (30:1)] to receive the desired product as a clear oil (280 mg, 1.25 mmol, 64%) [36].
Rf = 0.4 (pentane/EtOAc 30:1); 1H-NMR (400 MHz, CDCl3): δ = 7.29–7.22 (m, 1H), 6.20–6.08 (m, 2H), 5.78 (d, J = 15.4 Hz, 1H), 4.19 (q, J = 7.2 Hz, 2H), 2.16 (q, J = 7.0 Hz, 2H), 1.43 (quin, J = 7.3 Hz, 2H), 1.32–1.24 (m, 11H), 0.88 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 167.3, 145.1, 144.8, 128.3, 119.1, 60.1, 33.0, 31.8, 29.1, 29.1, 28.7, 22.6, 14.3, 14.1; MS (70 eV, EI): m/z (%): 125 (100), 97 (58.4), 81 (51.7), 67 (39.6), 179 (34.9), 98 (30.6), 79 (30.5), 95 (25.3), 127 (22.8), 99 (22.4), 224 (21.0) [M+].
4.5.3. (2E,4E)-2,4-Dodecadienoic Acid (4)
Lithium hydroxide (106.8 mg, 4.46 mmol) was added to a stirred solution of ester 3 (50 mg, 0.22 mmol) in THF/MeOH/H2O (2:2:1) and stirred for 12 h under reflux. The mixture was acidified with 2 m sulfuric acid and extracted three times with dichloromethane. The combined organic phases were dried with Na2SO4, filtered and concentrated in vacuo to get the desired product after purification by flash chromatography on silica [pentane/EtOAc (2:1)] as a white solid (39.9 mg, 0.20 mmol, 92%).
Rf = 0.3 (pentane/EtOAc 2:1); 1H-NMR (400 MHz, CDCl3): δ = 7.35 (dd, J = 15.3, 10.0 Hz, 1H), 6.24–6.16 (m, 2H), 5.78 (d, J = 15.3 Hz, 1H), 2.18 (q, J = 7.3 Hz, 2H), 1.43 (quin, J = 7.3 Hz, 2H), 1.33–1.20 (m, 8H), 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 172.8, 147.5, 146.3, 128.2, 118.2, 33.1, 31.8, 29.1, 29.1, 28.6, 22.6, 14.1; MS (70 eV, EI, trimethylsilyl ester): m/z (%): 169 (100), 253 (64.0), 73 (31.9), 155 (30.1), 170 (20.5), 75 (17.5), 254 (15.7), 268 (13.8) [M+], 81 (13.4), 171 (9.2).
4.5.4. N-((2E,4E)-2,4-Dodecadienoyl)homoserine Lactone (5)
l-Homoserine lactone hydrobromide (53 mg, 0.29 mmol) was dissolved in dry dichloromethane. Triethylamine (0.04 mL, 0.29 mmol) was added to the solution, followed by the addition of acid 4 (57 mg, 2.29 mmol) and EDC·HCl (56 mg, 0.29 mmol). The reaction mixture was stirred for 12 h at room temperature and washed with H2O, sat. NaHCO3 solution, and brine. The organic layers were extracted three times with dichloromethane, dried with Na2SO4, filtered and concentrated. The crude product was purified by flash chromatography on silica [pentane/EtOAc (1:1)] to obtain pure AHL 5 (80.6 mg, 0.28 mmol, 99%).
Rf = 0.3 (pentane/EtOAc 1:1); 1H-NMR (600 MHz, CDCl3): δ = 7.23 (dd, J = 15.0, 9.7 Hz, 1H), 6.25 (br s, 1H), 6.17–6.08 (m, 2H), 5.81 (d, J = 15.1 Hz, 1H), 4.66 (ddd, J = 11.6, 8.6, 6.0 Hz, 1H), 4.48 (td, J = 9.0, 1.1 Hz, 1H), 4.31 (ddd, J = 11.3, 9.3, 5.9 Hz, 1H), 2.87 (dddd, J = 12.5, 8.5, 5.9, 1.2 Hz, 1H), 2.22–2.18 (m, 1H), 2.15 (q, J = 7.0 Hz, 2H), 1.41 (quin, J = 7.0 Hz, 2H), 1.33–1.26 (m, 8H), 0.88 (t, J = 7.2 Hz, 3H); 13C-NMR (150 MHz, CDCl3): δ = 175.5, 166.6, 144.4, 142.7, 127.8, 119.9, 66.0, 49.2, 32.8, 31.5, 30.4, 28.9, 28.9, 28.5, 22.4, 14.0 (Supplementary Figure S4); HRMS (ESI+) m/z: 302.1728 [M + Na]+, calculated for C16H25NO3Na 302.1727 [M + Na]+.
4.5.5. Non-2-yn-1-ol (7)
n-Butyl lithium in hexane (1.6 m, 31.25 mL, 50 mmol) was added at −78 °C to a stirred solution of 1-octyne (6, 5 g, 45 mmol) in dry diethyl ether and stirred for 30 min. Paraformaldehyde (2.77 g, 90 mmol) was added and the mixture was allowed to warm up to room temperature. Sat. NH4Cl solution was added, the phases were separated and extracted three times with diethyl ether. The combined organic phases were washed with brine, dried with Na2SO4, filtered and the solvent was evaporated. The crude product was purified by flash chromatography on silica [pentane/EtOAc (7:1)] to furnish the desired product as a clear oil (6.2 g, 40 mmol, 98%) [37].
Rf = 0.4 (pentane/EtOAc 7:1); 1H-NMR (400 MHz, CDCl3): δ = 4.25 (dt, J = 5.8, 2.1 Hz, 2H), 2.21 (tt, J = 7.1, 2.2 Hz, 2H), 1.51 (quin, J = 7.3 Hz, 2H), 1.41–1.24 (m, 6H), 0.89 (t, J = 6.8 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 86.7, 78.3, 51.4, 31.3, 28.6, 28.5, 22.5, 18.7, 14.0; MS (70 eV, EI): m/z (%): 67 (100), 55 (97.2), 41 (95.0), 93 (73.4), 70 (72.5), 39 (68.8), 43 (64.0), 69 (58.0), 79 (57.4), 83 (56.5), 122 (10.0) [M+-H2O].
4.5.6. Non-2-en-1-ol (8)
Lindlar’s catalyst (50 mg) was added to a solution of ynol 7 (500 mg, 3.57 mmol) and methanol (10 mL). The mixture was stirred for 20 min at room temperature under a H2 atmosphere. The catalyst was filtered through a short pad of silica and the solvent was evaporated. The product was received after purification by flash chromatography on silica [pentane/EtOAc (10:1)] as a colorless oil (454 mg, 3.19 mmol, 90%).
Rf = 0.3 (pentane/EtOAc 10:1); 1H-NMR (400 MHz, CDCl3): δ = 5.63–5.51 (m, 2H), 4.2 (d, J = 6.0 Hz, 2H), 2.07 (q, J = 6.7 Hz, 2H), 1.40–1.23 (m, 8H), 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 133.3, 128.3, 58.6, 31.7, 29.6, 28.9, 27.4, 22.6, 14.1; MS (70 eV, EI): m/z (%): 57 (100), 41 (69.6), 55 (50.7), 43 (49.0), 54 (41.0), 67 (39.4), 82 (38.7), 81 (36.0), 68 (34.6), 95 (33.7), 124 (22.7) [M+-H2O], 142 (0.3) [M+].
4.5.7. Methyl (2E,5Z)-2,5-Dodecadienoate (9)
The allylic alcohol 8 (454 mg, 3.19 mmol) was added to a mixture of tris(dibenzylideneacetone)dipalladium (83 mg, 0.08 mmol), tricyclohexylphosphine (45 mg, 0.16 mmol) and p-toluenesulfonic anhydride (1.25 g, 3.8 mmol) in methyl acrylate (16 mL) and stirred for 12 h at 80 °C in a special high-pressure vial under argon. The catalyst was filtered through a short silica column and washed with diethyl ether. The solvent was evaporated in vacuo and the crude product was purified by flash chromatography on silica [pentane/EtOAc (30:1)] to the desired product (599.7 mg, 2.85 mmol, 89%) [25].
Rf = 0.4 (pentane/EtOAc 30:1); 1H-NMR (400 MHz, CDCl3): δ = 6.97 (dt, J = 15.7, 6.4 Hz, 1H), 5.82 (dt, J = 15.7, 1.8 Hz, 1H), 5.56-5.34 (m, 2H), 3.73 (s, 3H), 2.88 (dd, J = 6.9, 6.3 Hz, 2H), 2.01 (q, J = 6.6 Hz, 2H), 1.38–1.26 (m, 8H), 0.88 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 167.1, 148.1, 133.8, 124.9, 121.1, 51.4, 35.1, 32.6, 31.7, 29.3, 28.8, 22.6, 14.1; MS (70 eV, EI): m/z (%): 79 (100), 111 (90.3), 81 (70.2), 67 (63.6), 41 (60.0), 210 (52.8) [M+], 55 (50.0), 95 (47.4), 80 (47.3), 100 (45.9).
4.5.8. (2E,5Z)-2,5-Dodecadienoic Acid (10)
Trimethylsilyl iodide (0.038 mL, 0.268 mmol) was added to a stirred solution of ester 9 (30 mg, 0.134 mmol) in 0.5 mL CCl4 and warmed to 50 °C for one hour. The mixture was washed with sat. NH4Cl solution followed by sat. sodium thiosulfate solution and extracted three times with dichloromethane. The combined organic phases were dried with MgSO4, filtered and concentrated in vacuo to get the desired product after purification by flash chromatography on silica [pentane/Et2O (5:1)] as a white solid (15 mg, 0.077 mmol, 58%).
Rf = 0.2 (pentane/EtOAc 5:1); 1H-NMR (400 MHz, CDCl3): δ = 7.08–7.01(m, 1H), 5.81 (d, J = 15.6 Hz, 1H), 5.42–5.26 (m, 2H), 2.94 (dd, J = 6.9, 6.3 Hz, 2H), 2.01 (q, J = 6.9 Hz, 2H), 1.35–1.24 (m, 8H), 0.81 (t, J = 7.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 172.8, 148.8, 133.4, 124.4, 121.5, 33.0, 28.9, 28.8, 28.7, 27.8, 22.6, 14.0; MS (70 eV, EI, trimethylsilyl ester): m/z (%): 169 (100), 253 (42.4), 73 (40.2), 155 (21.9), 75 (19.9), 170 (15.1), 81 (13.5), 254 (8.8), 43 (8.7), 268 (8.0) [M+].
4.5.9. N-((2E,5Z)-2,5-Dodecadienoyl)homoserine Lactone (11)
l-Homoserine lactone hydrobromide (22 mg, 0.1224 mmol) was dissolved in dry dichloromethane, followed by the addition of p-dimethylaminopyridine (DMAP) (15 mg, 0.1224 mmol) and acid 10 (24 mg, 0.1224 mmol). EDC·HCl (23.5 mg, 0.1224 mmol) was added at 0 °C, the solution was stirred for 5 min at 0 °C and for 12 h at room temperature. The reaction mixture was washed one time with H2O, sat. NaHCO3 solution and brine. The organic layers were extracted three times with dichloromethane, dried with Na2SO4, filtered and the solvent was evaporated. The crude product was purified by flash chromatography on silica [pentane/EtOAc (1:1)] to the desired AHL (15 mg, 0.054 mmol, 44%).
Rf = 0.2 (pentane/EtOAc 1:1); 1H-NMR (600 MHz, CDCl3): δ = 6.91 (dt, J = 15.4, 6.4 Hz, 1H), 6.06 (br s, 1H), 5.83 (dt, J = 15.3, 1.7 Hz, 1H), 5.53–5.36 (m, 2H), 4.62 (ddd, J = 11.6, 8.6, 5.6 Hz, 1H), 4.50–4.47 (m, 1H), 4.33–4.28 (m, 1H), 2.92–2.87 (m, 3H), 2.20–2.12 (m, 1H), 2.01 (q, J = 7.0 Hz, 2H), 1.39–1.25 (m, 8H), 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (150 MHz, CDCl3): δ = 175.5, 166.3, 145.1, 133.8, 125.0, 122.5, 66.2, 49.4, 34.9, 32.6, 31.7, 30.7, 29.3, 28.9, 22.6, 14.1 (Supplementary Figure S2); HRMS (ESI+) m/z: 280.1909 [M + H]+, calculated for C16H26NO3 280.1907 [M + H]+; 302.1729 [M + Na]+, calculated for C16H25NO3Na 302.1727 [M + Na]+.
4.5.10. (3E,5Z)-3,5-Dodecadienoic Acid (12)
Lithium hydroxide (0.238 mL, 1.5 M) was added to a stirred solution of ester 9 (50 mg, 0.238 mmol) in THF/MeOH (1:1) and stirred for 3 h at 0 °C. The mixture was acidified with 1 m HCl and extracted three times with dichloromethane. The combined organic phases were dried with Na2SO4, filtered and concentrated in vacuo to get the desired product after purification by flash chromatography on silica [pentane/EtOAc (5:1)] as a white solid (25 mg, 0.128 mmol, 54%).
Rf = 0.2 (pentane/EtOAc 5:1); 1H-NMR (400 MHz, CDCl3): δ = 6.19–5.98 (m, 2H), 5.72 (dt, J = 15.1, 7.1 Hz, 1H), 5.39 (dt, J = 10.8, 7.6 Hz, 1H), 3.10 (d, J = 7.2 Hz, 2H), 2.09 (q, J = 7.6, 7.2 Hz, 2H), 1.43–1.26 (m, 8H), 0.86 (t, J = 6.9 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 178.2, 135.4, 134.7, 129.3, 121.3, 37.7, 31.7, 29.2, 28.9, 27.8, 22.6, 14.1; MS (70 eV, EI, trimethylsilyl ester): m/z (%): 73 (100), 75 (23.5), 253 (15.2), 74 (14.4), 268 (12.2) [M+], 79 (10.9), 150 (10.9), 41 (6.4), 67 (6.4), 224 (6.4).
4.5.11. N-((3E,5Z)-3,5-Dodecadienoyl)homoserine Lactone (13)
l-Homoserine lactone hydrobromide (21.8 mg, 0.12 mmol) was dissolved in dry dichloromethane. Triethylamine (0.02 mL, 0.12 mmol) was added to the solution, followed by the addition of acid 12 (24.4 mg, 0.12 mmol) and EDC·HCl (23 mg, 0.12 mmol). The reaction mixture was stirred for 12 h at room temperature and washed successively with H2O, sat. NaHCO3 solution and brine. The organic layers were extracted three times with dichloromethane, dried with Na2SO4, filtered and the solvent was evaporated. The crude product was purified by flash chromatography on silica [pentane/EtOAc (1:1)] to obtain the desired AHL 13 (32 mg, 0.115 mmol, 92%).
Rf = 0.2 (pentane/EtOAc 1:1); 1H-NMR (400 MHz, CDCl3): δ = 6.34 (br s, 1H), 6.12–5.93 (m, 2H), 5.66–5.51 (m, 2H), 4.51 (ddd, J = 11.5, 8.5, 6.5 Hz, 1H), 4.41–4.36 (m, 1H), 4.2 (ddd, J = 11.0, 9.3, 6.0 Hz, 1H), 3.00 (d, J = 7.3 Hz, 2H), 2.74–2.68 (m, 1H), 2.15–2.04 (m, 1H), 2.00 (q, J = 7.2 Hz, 2H), 1.32–1.20 (m, 8H), 0.81 (t, J = 7.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ = 175.4, 171.6, 135.8, 135.7, 129.2, 122.0, 66.0, 49.2, 39.9, 32.6, 31.7, 30.1, 29.1, 28.8, 22.5, 14.0 (Supplementary Figure S3); HRMS (ESIs1/z: 280.1909 [M + H]+, calculated for C16H26NO3 280.1907 [M + H]+; 302.1729 [M + Na]+, calculated for C16H25NO3Na 302.1727 [M + Na]+.
Acknowledgments
We thank the DFG for funding in the framework of TRR/SFB 51 “Roseobacter”.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/17/1/20/s1, Figure S1: Mass spectrum of natural compound B. Figure S2: 1H NMR and 13C NMR spectrum of 2E,5Z-C12:2-HSL (11). Figure S3: 1H NMR and 13C NMR spectrum of 3E,5Z-C12:2-HSL (13). Figure S4: 1H NMR and 13C NMR spectrum of 2E,4E-C12:2-HSL (5).
Author Contributions
Conceptualization, L.Z. and S.S.; Data curation, L.Z.; Methodology, L.Z.; Validation, S.S. and I.W.-D.; Investigation, L.Z. and H.W.; Resources, S.S., L.W., M.P., T.B., B.E. and I.W.-D.; Writing-Original Draft Preparation, L.Z.; Writing-Review & Editing, S.S.; Visualization, S.S.; Supervision, S.S.; Project Administration, S.S.; Funding Acquisition, S.S.
Funding
This research was funded by The Deutsche Forschungsgemeinschaft (DFG) in the framework of TRR/SFB 51 “Roseobacter”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Zan J., Liu Y., Fuqua C., Hill R. Acyl-homoserine Lactone Quorum Sensing in the Roseobacter Clade. Int. J. Mol. Sci. 2014;15:654–669. doi: 10.3390/ijms15010654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchan A., Mitchell A., Nathan Cude N.W., Campagna S. Acyl-homoserine Lactone-Based Quorum Sensing in Members of the Marine Bacterial Roseobacter Clade: Complex Cell-to-Cell Communication Controls Multiple Physiologies. In: de Bruijn F.J., editor. Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria. Wiley Blackwell; Hoboken, NJ, USA: pp. 225–233. [Google Scholar]
- 3.Schulz S., Hötling S. The use of the lactone motif in chemical communication. Nat. Prod. Rep. 2015;32:1042–1066. doi: 10.1039/C5NP00006H. [DOI] [PubMed] [Google Scholar]
- 4.Patzelt D., Wang H., Buchholz I., Rohde M., Gröbe L., Pradella S., Neumann A., Schulz S., Heyber S., Münch K., et al. You are what you talk: Quorum sensing induces individual morphologies and cell division modes in Dinoroseobacter shibae. ISME J. 2013;7:2274–2286. doi: 10.1038/ismej.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger M., Neumann A., Schulz S., Simon M., Brinkhoff T. Tropodithietic acid production in Phaeobacter gallaeciensis is regulated by N-acyl homoserine lactone-mediated quorum sensing. J. Bacteriol. 2011;193:6576–6585. doi: 10.1128/JB.05818-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H., Ziesche L., Frank O., Michael V., Martin M., Petersen J., Schulz S., Wagner-Döbler I., Tomasch J. The CtrA phosphorelay integrates differentiation and communication in the marine alphaproteobacterium Dinoroseobacter shibae. BMC Genomics. 2014;15:130. doi: 10.1186/1471-2164-15-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickschat J.S. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010;27:343–369. doi: 10.1039/b804469b. [DOI] [PubMed] [Google Scholar]
- 8.Giebel H.-A., Kalhoefer D., Lemke A., Thole S., Gahl-Janssen R., Simon M., Brinkhoff T. Distribution of Roseobacter RCA and SAR11 lineages in the North Sea and characteristics of an abundant RCA isolate. ISME J. 2011;5:8–19. doi: 10.1038/ismej.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freese H.M., Methner A., Overmann J. Adaptation of Surface-Associated Bacteria to the Open Ocean. Front. Microbiol. 2017;8:1659. doi: 10.3389/fmicb.2017.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchan A., Gonzalez J.M., Moran M.A. Overview of the Marine Roseobacter Lineage. Appl. Environ. Microbiol. 2005;71:5665–5677. doi: 10.1128/AEM.71.10.5665-5677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkhoff T., Giebel H.-A., Simon M. Diversity, ecology, and genomics of the Roseobacter clade: A short overview. Arch. Microbiol. 2008;189:531–539. doi: 10.1007/s00203-008-0353-y. [DOI] [PubMed] [Google Scholar]
- 12.Ziesche L., Bruns H., Dogs M., Wolter L., Mann F., Wagner-Döbler I., Brinkhoff T., Schulz S. Homoserine Lactones, Methyl Oligohydroxybutyrates, and Other Extracellular Metabolites of Macroalgae-Associated Bacteria of the Roseobacter Clade: Identification and Functions. ChemBioChem. 2015;16:2094–2107. doi: 10.1002/cbic.201500189. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer A.L., Greenberg E.P., Oliver C.M., Oda Y., Huang J.J., Bittan-Banin G., Peres C.M., Schmidt S., Juhaszova K., Sufrin J.R., et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–599. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 14.Cude W.N., Buchan A. Acyl-homoserine lactone-based quorum sensing in the Roseobacter clade: Complex cell-to-cell communication controls multiple physiologies. Front. Microbiol. 2013;4:336. doi: 10.3389/fmicb.2013.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner-Döbler I., Thiel V., Eberl L., Allgaier M., Bodor A., Meyer S., Ebner S., Hennig A., Pukall R., Schulz S. Discovery of complex mixtures of novel long-chain quorum sensing signals in free-living and host-associated marine Alphaproteobacteria. ChemBioChem. 2005;6:2195–2206. doi: 10.1002/cbic.200500189. [DOI] [PubMed] [Google Scholar]
- 16.Thiel V., Kunze B., Verma P., Wagner-Döbler I., Schulz S. New Structural Variants of Homoserine Lactones in Bacteria. ChemBioChem. 2009;10:1861–1868. doi: 10.1002/cbic.200900126. [DOI] [PubMed] [Google Scholar]
- 17.Bruns H., Thiel V., Voget S., Patzelt D., Daniel R., Wagner-Döbler I., Schulz S. N-acylated alanine methyl esters (NAMEs) from Roseovarius tolerans, structural analogs of quorum-sensing autoinducers, N-acylhomoserine lactones. Chem. Biodivers. 2013;10:1559–1573. doi: 10.1002/cbdv.201300210. [DOI] [PubMed] [Google Scholar]
- 18.Bruns H., Herrmann J., Müller R., Wang H., Wagner Döbler I., Schulz S. Oxygenated N-Acyl Alanine Methyl Esters (NAMEs) from the Marine Bacterium Roseovarius tolerans EL-164. J. Nat. Prod. 2018;81:131–139. doi: 10.1021/acs.jnatprod.7b00757. [DOI] [PubMed] [Google Scholar]
- 19.Kanukollu S., Wemheuer B., Herber J., Billerbeck S., Lucas J., Daniel R., Simon M., Cypionka H., Engelen B. Distinct compositions of free-living, particle-associated and benthic communities of the Roseobacter group in the North Sea. FEMS Microbiol. Ecol. 2016;92:fiv145. doi: 10.1093/femsec/fiv145. [DOI] [PubMed] [Google Scholar]
- 20.Neumann A., Patzelt D., Wagner-Döbler I., Schulz S. Identification of new N-acylhomoserine lactone signalling compounds of Dinoroseobacter shibae DFL-12T by overexpression of luxI genes. ChemBioChem. 2013;14:2355–2361. doi: 10.1002/cbic.201300424. [DOI] [PubMed] [Google Scholar]
- 21.Doberva M., Stien D., Sorres J., Hue N., Sanchez-Ferandin S., Eparvier V., Ferandin Y., Lebaron P., Lami R. Large Diversity and Original Structures of Acyl-homoserine Lactones in Strain MOLA 401, a Marine Rhodobacteraceae Bacterium. Front. Microbiol. 2017;8:1152. doi: 10.3389/fmicb.2017.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camara M., Daykin M., Chhabra S.R. Detection, purification, and synthesis of N-acylhomoserine lactone quorum sensing signal molecules. Meth. Microbiol. 1998;27:319–330. [Google Scholar]
- 23.Majewski M., Green J.R., Snieckus V. Stereoselective deprotonation of α,β-unsaturated amides. Tetrahedron Lett. 1986;27:531–534. doi: 10.1016/S0040-4039(00)84032-0. [DOI] [Google Scholar]
- 24.Theodorou V., Gogou M., Philippidou M., Ragoussis V., Paraskevopoulos G., Skobridis K. Synthesis of β,γ-unsaturated primary amides from α,β-unsaturated acids and investigation of the mechanism. Tetrahedron. 2011;67:5630–5634. doi: 10.1016/j.tet.2011.05.096. [DOI] [Google Scholar]
- 25.Tsukada N., Sato T., Inoue Y. Palladium-catalyzed allylic alkenylation of allylic alcohols with n-butyl acrylate. Chem. Commun. 2003;19:2404–2405. doi: 10.1039/b307705e. [DOI] [PubMed] [Google Scholar]
- 26.Jung M.F., Lyster M.A. Quantitative dealkylation of alkyl esters via treatment with trimethylsilyl iodide. A new method for ester hydrolysis. J. Am. Chem. Soc. 1977;99:968–969. doi: 10.1021/ja00445a062. [DOI] [Google Scholar]
- 27.Riedel K., Hentzer M., Geisenberger O., Huber B., Steidle A., Wu H., Hoiby N., Givskov M., Molin S., Eberl L. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001;147:3249–3262. doi: 10.1099/00221287-147-12-3249. [DOI] [PubMed] [Google Scholar]
- 28.Steidle A., Allesen-Holm M., Riedel K., Berg G., Givskov M., Molin S., Eberl L. Identification and characterization of an N-acylhomoserine lactone-dependent quorum-sensing system in Pseudomonas putida strain IsoF. Appl. Environ. Microbiol. 2002;68:6371–6382. doi: 10.1128/AEM.68.12.6371-6382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowe D., Nicholson A., Fleming A., Carey E., Sánchez-Sanz G., Kelleher F. Conformational studies of Gram-negative bacterial quorum sensing 3-oxo N-acyl homoserine lactone molecules. Bioorg. Med. Chem. 2017;25:4285–4296. doi: 10.1016/j.bmc.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Ziesche L., Rinkel J., Dickschat J.S., Schulz S. Acyl-group specificity of AHL synthases involved in quorum-sensing in Roseobacter group bacteria. Beilstein J. Org. Chem. 2018;14:1309–1316. doi: 10.3762/bjoc.14.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz S. Composition of the silk lipids of the spider Nephila clavipes. Lipids. 2001;36:637–647. doi: 10.1007/s11745-001-0768-7. [DOI] [PubMed] [Google Scholar]
- 32.Sass H., Köpke B., Rütters H., Feuerlein T., Dröge S., Cypionka H., Engelen B. Tateyamaria pelophila sp. nov., a facultatively anaerobic alphaproteobacterium isolated from tidal-flat sediment, and emended descriptions of the genus Tateyamaria and of Tateyamaria omphalii. Int. J. Syst. Evol. Microbiol. 2010;60:1770–1777. doi: 10.1099/ijs.0.013524-0. [DOI] [PubMed] [Google Scholar]
- 33.Kanukollu S., Voget S., Pohlner M., Vandieken V., Petersen J., Kyrpides N.C., Woyke T., Shapiro N., Göker M., Klenk H.-P., et al. Genome sequence of Shimia str. SK013, a representative of the Roseobacter group isolated from marine sediment. Stand. Genom. Sci. 2016;11:25. doi: 10.1186/s40793-016-0143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohlner M., Degenhardt J., von Hoyningen-Huene Avril J.E., Wemheuer B., Erlmann N., Schnetger B., Badewien T.H., Engelen B. The Biogeographical Distribution of Benthic Roseobacter Group Members along a Pacific Transect Is Structured by Nutrient Availability within the Sediments and Primary Production in Different Oceanic Provinces. Front. Microbiol. 2017;8:2550. doi: 10.3389/fmicb.2017.02550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angle S.R., Henry R.M. Studies toward the Synthesis of (+)-Palustrine. J. Org. Chem. 1998;63:7490–7497. doi: 10.1021/jo980749g. [DOI] [PubMed] [Google Scholar]
- 36.Clerc J., Li N., Krahn D., Groll M., Bachmann A.S., Florea B.I., Overkleeft H.S., Kaiser M. The natural product hybrid of Syringolin A and Glidobactin A synergizes proteasome inhibition potency with subsite selectivity. Chem. Commun. 2011;47:385–387. doi: 10.1039/C0CC02238A. [DOI] [PubMed] [Google Scholar]
- 37.Kleinbeck F., Toste F.D. Gold (I)-catalyzed enantioselective ring expansion of allenylcyclopropanols. J. Am. Chem. Soc. 2009;131:9178–9179. doi: 10.1021/ja904055z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.