Abstract
Bothrops snakebites usually present systemic bleeding, and the clinical–epidemiological and laboratorial factors associated with the development of this manifestation are not well established. In this study, we assessed the prevalence of Bothrops snakebites with systemic bleeding reported at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado, in Manaus, Amazonas State, Brazil, and the clinical–epidemiological and laboratorial factors associated with systemic bleeding. This is an observational, cross-sectional study carried out between August, 2013 and July, 2016. Patients who developed systemic bleeding on admission or during hospitalization were considered cases, and those with non-systemic bleeding were included in the control group. Systemic bleeding was observed in 63 (15.3%) of the 442 Bothrops snakebites evaluated. Bothrops snakebites mostly occurred in males (78.2%), in rural areas (89.0%) and in the age group of 11 to 30 years old (40.4%). It took most of the patients (59.8%) less than 3 h to receive medical assistance. Unclottable blood (AOR = 3.11 (95% CI = 1.53 to 6.31; p = 0.002)) and thrombocytopenia (AOR = 4.52 (95% CI = 2.03 to 10.09; p < 0.001)) on admission were independently associated with systemic bleeding during hospitalization. These hemostatic disorders on admission increase the chances of systemic bleeding during hospitalization. Prospective studies are needed to clarify the pathophysiology of systemic bleeding in Bothrops snakebites in the Amazon region.
Keywords: hemostatic disorders, unclottable blood, thrombocytopenia, Bothrops atrox
1. Introduction
Snakebite envenomation is a potentially life-threatening injury and a major public health problem in rural areas of tropical and sub-tropical countries of Africa, the Middle-East, Asia, Oceania, and Latin America. Global estimates suggest that at least 400,000 snakebites occur annually, which result in 20,000 deaths [1]. In Brazil, approximately 26,000 snakebites of medical importance are reported each year, with an average lethality rate of 0.4% [2]. The Amazon region reports the highest incidence of snakebite envenomation in Brazil, with 52.6 cases/100,000 inhabitants [3]. In the Amazonas State, an incidence rate of 52.8 cases/100,000 inhabitants and a 0.6% lethality rate can be observed [4]. Injuries occurring at a distance >300 Km from Manaus, the Amazonas State capital, the age of the victim ≥61 years, and indigenous status were factors that were independently associated with case fatality from snakebites [5]. The Bothrops genus is responsible for the highest frequency of snakebites in the Brazilian Amazon, causing 80% to 90% of the cases in the region [6]. Bothrops atrox is the main species of snake involved in these snakebites [6,7]. Bothrops snakebite victims usually present local symptoms (i.e. pain, swelling, ecchymosis, and blistering) and systemic manifestations such as bleeding and coagulation disorders [7,8,9]. Complications such as necrosis, secondary bacterial infection, compartment syndrome, and acute renal failure may also occur [8]. Although cases of death are rare, they are frequently associated with renal and respiratory failure, shock, sepsis, and hemorrhage in the central nervous system [9]. In fatal cases of Bothrops snakebites in the Amazonas State, systemic bleeding, circulatory shock, sepsis, and acute respiratory failure were usually observed [5]. One case of fatal hemorrhagic stroke after a Bothrops snakebite in the Amazonas State, in which the presence of venom in the patient’s brain tissue was identified after death, has been reported. The victim received Bothrops antivenom two and a half hours after the bite [10].
Clinical manifestations observed in Bothrops snakebites appear as result of the action of the snake venom [11]. The components that are most abundant in B. atrox venom are snake venom metalloproteinases (SVMPs), snake venom serine proteinases (SVSPs), and phospholipases A2 (PLA2) [12]. These components of the snake venom can affect hemostasis by activating or inhibiting coagulant factors or platelets, or by disrupting endothelium or by causing thrombosis [13,14]. The association of the coagulant action of venom, with its activity on platelets and vascular endothelium, could lead to a higher incidence of systemic bleeding as a result of the snakebite [15]. Hemodynamic changes, which may result in cardiovascular shock, may occur as a consequence of systemic bleeding [16]. However, the composition and biological activities of snake venoms may vary, not only between different species of snakes (interspecific variation), but also within the same species (intraspecific variation) depending on the geographical origin, ontogenetic stage, habitat, or sex of the snake, thus influencing the clinical manifestations of snakebites [17,18].
It has been observed that between 7% and 53% of Bothrops snakebites develop systemic bleeding [7,15,19,20,21]. It is not clear why some Bothrops snakebite patients develop systemic bleeding and others do not. A better understanding of the development of systemic bleeding in patients who have suffered Bothrops snakebites would lead to improved management and understanding of the pathophysiology of envenomation of snakes that have medical importance in the Brazilian Amazon. Thus, the aim of this study was to assess the prevalence of Bothrops snakebites in which systemic bleeding was reported at a specialist hospital for snakebites in Manaus, Amazonas State, and the clinical–epidemiological and laboratorial factors associated with systemic bleeding in victims of envenomation by Bothrops snakes. The results show that systemic bleeding observed in Bothrops snakebite patients is an important systemic effect of the envenoming. Thrombocytopenia and unclottable blood detected on admission are factors associated with systemic bleeding during hospitalization. Moreover, the effect of other parameters, such as some toxins components of the venom, cannot be ruled out.
2. Results
2.1. Study Population
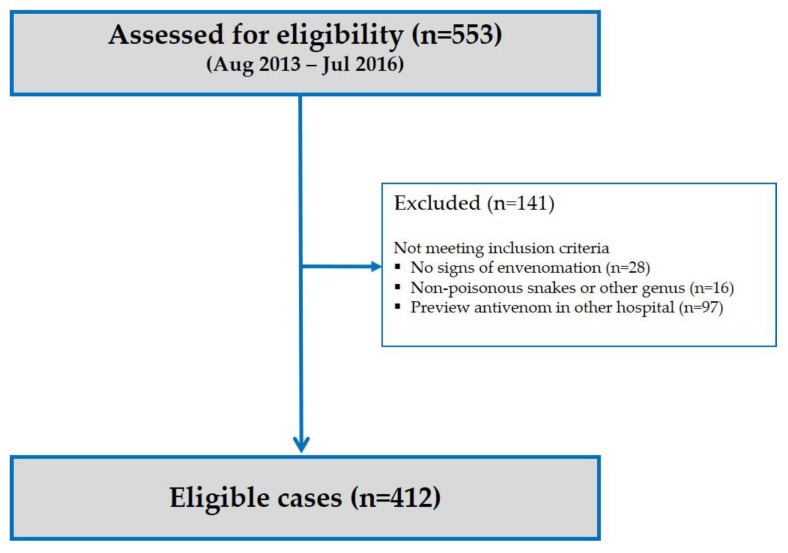
A total of 553 patients with clinical–epidemiological diagnosis of Bothrops envenomation were assessed for eligibility over the three year study period. From this total, 412 were eligible for inclusion in our study (Figure 1). The majority of Bothrops envenomations were in males (78.2%) and in the age group of 11 to 30 years old (40.4%). These snakebites mostly occurred in Manaus (57.4%), Amazonas State capital, in rural areas (89.0%), and only 39.8% were related to work. The majority of patients (59.8%) took less than 3 h to reach medical assistance. The most affected anatomical site was the foot (72.1%). The Bothrops bite was confirmed by snake identification in 32.8% of the included patients’ records. All Bothrops snakes were identified as being Bothrops atrox (Table 1).
Figure 1.
Flow chart for the inclusion of patient with clinical–epidemiological diagnosis of Bothrops envenomation in our study.
Table 1.
Background information of Bothrops snakebite patients obtained on admission.
| Characteristics (n; Completeness) | Number | % |
|---|---|---|
| Gender (n = 412; 100%) | ||
| Male:female | 322:90 | |
| Age group in years (n = 412; 100%) | ||
| 0–10 | 41 | 10.0 |
| 11–20 | 83 | 20.2 |
| 21–30 | 83 | 20.2 |
| 31–40 | 60 | 14.6 |
| 41–50 | 54 | 13.1 |
| 51–60 | 51 | 12.4 |
| >60 | 40 | 9.7 |
| Geographic location of the snakebite incident (n = 411; 99.76%) | ||
| Capital (municipality of Manaus) | 236 | 57.4 |
| Municipalities of the interior of the Amazonas State | 171 | 41.6 |
| Other States | 4 | 1.0 |
| Area of occurrence (n = 410; 99.52%) | ||
| Rural | 365 | 89.0 |
| Urban | 45 | 11.0 |
| Work-related bite (n = 332; 80.58%) | ||
| Yes | 132 | 39.8 |
| Occupation (n = 171; 41.51%) | ||
| Rural worker | 85 | 49.7 |
| Other | 86 | 50.3 |
| Time-taken to reach to medical assistance (h) (n = 410; 99.52%) | ||
| 0–3 | 245 | 59.8 |
| 4–6 | 85 | 20.7 |
| >6 | 80 | 19.5 |
| Anatomical location of bite (n = 412; 100%) | ||
| Foot | 297 | 72.1 |
| Lower Leg | 59 | 14.3 |
| Thigh | 4 | 1.0 |
| Hand | 49 | 11.9 |
| Arm | 3 | 0.7 |
| Pre-hospital treatment | ||
| Tourniquets (n = 314; 76.21%) | 56 | 17.8 |
| Local incisions (n = 313; 75.97%) | 17 | 5.4 |
| Use of topical/oral medicines (n = 314; 76.21%) | 167 | 53.2 |
| Bothrops bite confirmation (n = 412; 100.0%) | ||
| Confirmed by snake identification | 135 | 32.8 |
| Clinical-epidemiological diagnosis | 277 | 67.2 |
Clinical characterization of Bothrops snakebite patients on admission showed that the most frequent local manifestations were pain (93.9%) followed by swelling (93.4%). Among the systemic alterations related to hemostasis, unclottable blood (58.2%), bleeding (12.6%), and thrombocytopenia (8.7%) were observed. Other common systemic manifestations were headache (11.4%), nausea (10.7%), and vomiting (9.7%). Most cases were moderate (54.2%), followed by mild (27.7%) cases. Comorbidities such as arterial hypertension (9.3%) and diabetes (2.6%) were also observed. There were no recorded deaths due to a snakebite in this study period (Table 2).
Table 2.
Clinical characteristics and comorbidities of Bothrops snakebite patients obtained on admission.
| Variable | Number | % |
|---|---|---|
| Local manifestations (n = 412; 100%) | ||
| Pain | 385 | 93.9 |
| Swelling | 382 | 93.4 |
| Bleeding from fang punctures | 120 | 29.3 |
| Redness | 120 | 29.1 |
| Ecchymosis | 36 | 8.7 |
| Paresthesia | 35 | 8.5 |
| Haematoma | 8 | 1.9 |
| Blistering | 7 | 1.7 |
| Regional lymphadenopathy | 4 | 1.0 |
| Systemic alterations related to hemostasis | ||
| Unclottable blood (n = 386; 93.69%) | 224 | 58.2 |
| Bleeding (n = 412; 100%) | 52 | 12.6 |
| Trombocytopenia (n = 379; 91.99%) | 33 | 8.7 |
| Other systemic manifestations (n = 412; 100%) | ||
| Headache | 47 | 11.4 |
| Nausea | 44 | 10.7 |
| Vomiting | 40 | 9.7 |
| Blurred vision | 14 | 3.4 |
| Abdominal pain | 7 | 1.7 |
| Diarrhoea | 5 | 1.2 |
| Clinical severity of envenomation (n = 404; 98.06%) | ||
| Mild | 112 | 27.7 |
| Moderate | 219 | 54.2 |
| Severe | 73 | 18.1 |
| Comorbidities (n = 313; 75.97%) | ||
| Arterial hypertension | 29 | 9.3 |
| Diabetes | 8 | 2.6 |
| Hepatopathy | 4 | 1.3 |
| Cardiopathy | 3 | 1.0 |
| HIV/Aids | 1 | 0.3 |
| Time spent in hospital (days) | ||
| Mean (range) (n = 405, 98.30%) | 7 | (1–41) |
| Outcome (n = 411; 99.76%) | ||
| Discharged | 411 | 100.0 |
2.2. Hemostasis Parameters
Systemic bleeding was observed during hospitalization in 15.3% of the Bothrops snakebite patients. Conjunctival bleeding was the most frequent form of systemic bleeding (6.7%), followed by gingival bleeding (6.3%) (Table 3). The majority of the Bothrops snakebite patients with systemic bleeding during hospitalization showed a platelet count on admission that was higher than 150,000/µL, irrespective of the type of systemic bleeding, except for one patient who presented petechiae (<50,000/µL) (Table 4).
Table 3.
Systemic bleeding of Bothrops snakebite patients during hospitalization.
| Description | Number | % |
|---|---|---|
| Systemic bleeding (n = 412; 100%) | 63 | 15.3 |
| Conjunctival | 27 | 6.7 |
| Gingival | 26 | 6.3 |
| Macrohematuria | 18 | 4.4 |
| Haematemesis | 12 | 2.9 |
| Haemoptysis | 10 | 2.4 |
| Epistaxis | 10 | 2.4 |
| Ecchymosis | 7 | 1.7 |
| Enterorrhage | 5 | 1.2 |
| Metrorrhagia | 3 | 0.7 |
| Petechiae | 1 | 0.2 |
| From recent wound | 1 | 0.2 |
Table 4.
Systemic bleeding of Bothrops snakebite patients during hospitalization versus platelet count on admission.
| Platelet Count | |||
|---|---|---|---|
| <50 × 10³/µL n (%) |
50–150 × 10³/µL n (%) |
>150 × 10³/µL n (%) |
|
| Systemic bleeding (n = 55; 100%) | 5 (9.1) | 8 (14.5) | 42 (76.4) |
| Conjunctival | 0 (0.0) | 2 (8.3) | 22 (91.7) |
| Gingival | 2 (8.3) | 4 (16.7) | 18 (75.0) |
| Macrohematuria | 1 (6.3) | 4 (25.0) | 11 (68.7) |
| Haematemesis | 2 (20.0) | 2 (20.0) | 6 (60.0) |
| Haemoptysis | 1 (11.1) | 3 (33.3) | 5 (55.6) |
| Epistaxis | 0 (0.0) | 1 (11.1) | 8 (88.9) |
| Ecchymosis | 0 (0.0) | 0 (0.0) | 5 (100.0) |
| Enterorrhage | 0 (0.0) | 1 (25.0) | 3 (75.0) |
| Metrorrhagia | 0 (0.0) | 1 (33.3) | 2 (66.7) |
| Petechiae | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| From recent wound | 0 (0.0) | 0 (0.0) | 1 (100.0) |
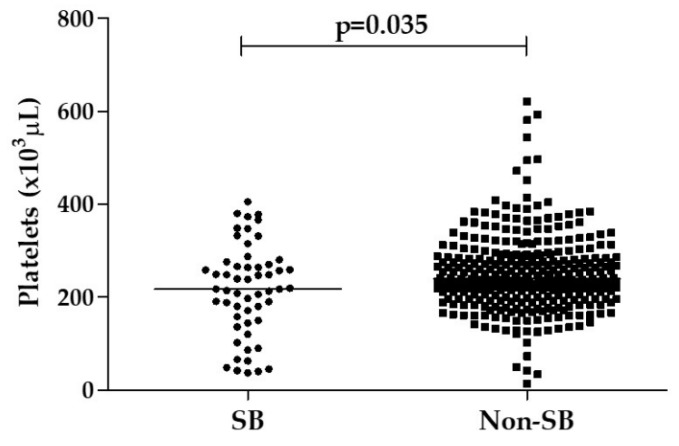
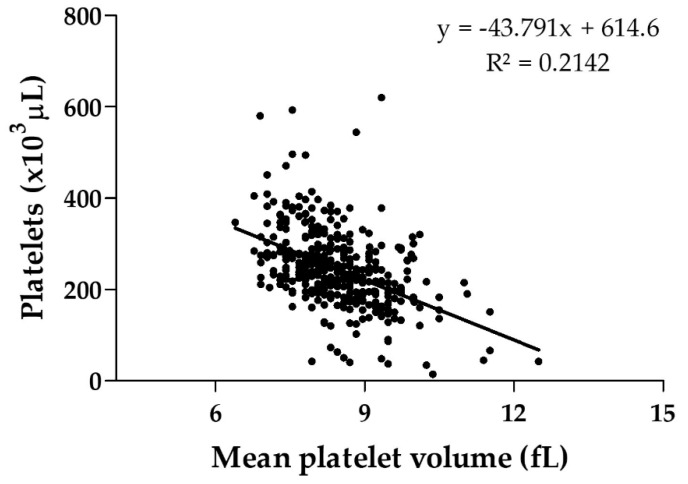
Median platelet count on admission of Bothrops snakebite patients with (217,000/µL) and without (239,500/µL) systemic bleeding during hospitalization showed significant statistical differences (p = 0.035) (Figure 2). Negative (inverse) correlation was found between the variables of number of platelets and mean platelet volume on admission (r = −0.463; p < 0.001) for absolute values during testing (Figure 3).
Figure 2.
Platelet counts obtained on admission for Bothrops snakebite patients that showed systemic bleeding (SB) or not (Non-SB) during hospitalization.
Figure 3.
Platelet counts and mean platelet volume obtained on admission for Bothrops snakebite patients. Pearson correlation coeficiente (r) between the variables was equivalent to −0.463 (p < 0.001).
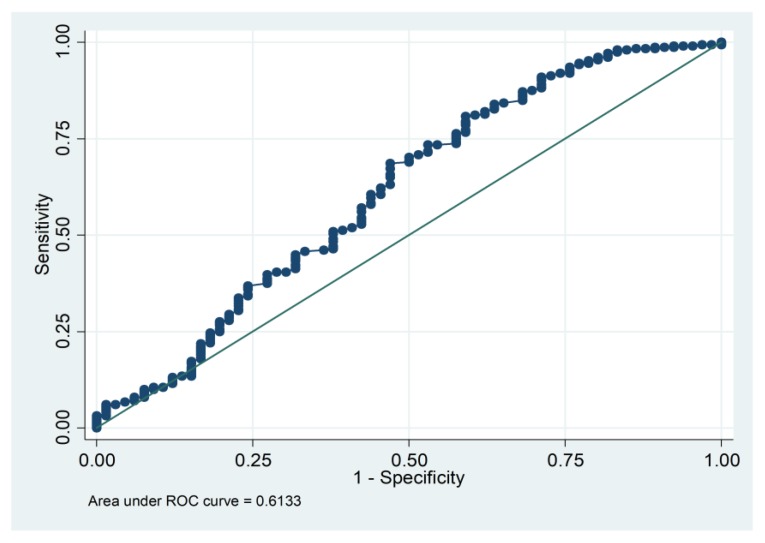
The area under the Receiver operating characteristics (ROC) curve showed that platelet counts obtained on admission presented a poor ability for discriminating Bothrops snakebite patients with systemic bleeding during hospitalization (AUROC = 0.6133; 95% CI = 0.531 to 0.696; p = 0.007) (Figure 4).
Figure 4.
Receiver operating characteristics (ROC) curve depicting the discriminatory performance of the platelet count obtained on admission (AUROC = 0.6133; 95% CI = 0.531 to 0.696; p = 0.007) for systemic bleeding during hospitalization in Bothrops snakebite patients. AUROC, area under ROC curve; continuous line: Reference.
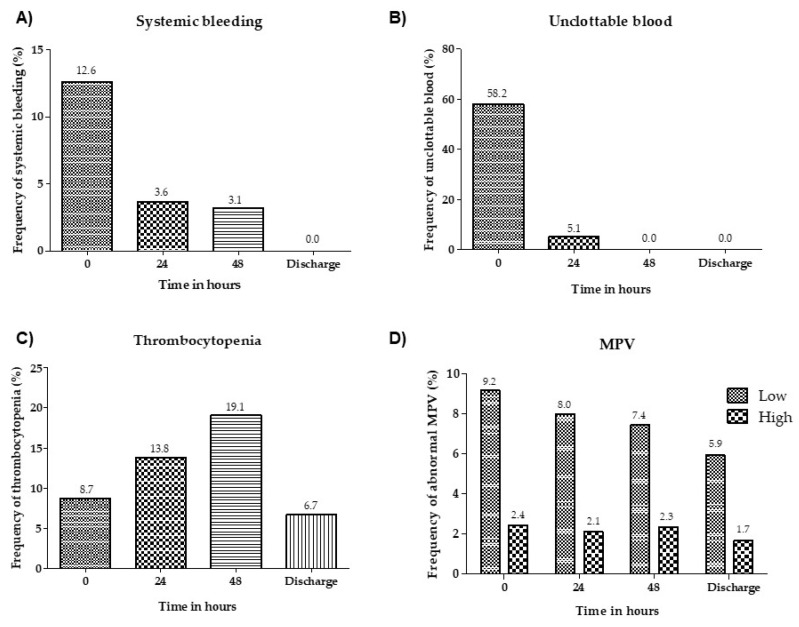
Systemic bleeding and unclottable blood presented an improvement within 24 h in most patients after administration of the specific antivenom, while the frequency of thrombocytopenia increased in the first 48 h after antivenom administration, though it did decrease on discharge, and the frequency of abnormal mean platelet volume showed a decrease until discharge (Figure 5). No bleeding episode was recorded in patients who returned for clinical reassessment after being discharged.
Figure 5.
Frequency of systemic bleeding (A), unclottable blood (B), thrombocytopenia (platelet count <150,000/µL) (C) and abnormal mean platelet volume, low (<7.4 fL) and high (>10.4 fL) (D) on admission, 24 h and 48 h after administration of the specific antivenom and on discharge. MPV: Mean platelet volume.
2.3. Factors Associated with Systemic Bleeding
A total of 63 Bothrops snakebite patients with systemic bleeding during hospitalization were included as cases and 349 Bothrops snakebite patients with non-systemic bleeding were included as controls. Unclottable blood (AOR = 3.11 (95% CI = 1.53 to 6.31; p = 0.002)) and thrombocytopenia (AOR = 4.52 (95% CI = 2.03 to 10.09; p < 0.001)) on admission were independently associated with the risk of developing systemic bleeding after a Bothrops snakebite during hospitalization (Table 5).
Table 5.
Parameters obtained on admission associated with systemic bleeding (SB) of Bothrops snakebite patients during hospitalization.
| Description | SB (n) | % | Non-SB (n) | % | Crude OR (IC 95%) | p | Adjusted OR (IC 95%) | p |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Female | 15 | 16.7 | 75 | 83.3 | 0.88 (0.46–1.65) | 0.682 | ||
| Male | 48 | 14.9 | 274 | 85.1 | ||||
| Age group in years | ||||||||
| 0–10 | 6 | 14.6 | 35 | 85.4 | 1 | |||
| 10–20 | 14 | 16.9 | 69 | 83.1 | 1.18 (0.42–3.35) | 0.751 | ||
| 20–30 | 10 | 12.1 | 73 | 87.9 | 0.79 (0.27–2.37) | 0.687 | ||
| 30–40 | 7 | 11.7 | 53 | 88.3 | 0.77 (0.24–2.48) | 0.662 | ||
| 40–50 | 12 | 22.2 | 42 | 77.8 | 1.66 (0.57–4.90) | 0.353 | ||
| 50–60 | 8 | 15.7 | 43 | 84.3 | 1.08 (0.34–3.42) | 0.889 | ||
| >60 | 6 | 15.0 | 34 | 85.0 | 1.03 (0.30–3.51) | 0.963 | ||
| Area of occurrence | ||||||||
| Urban | 3 | 6.7 | 42 | 93.3 | 2.75 (0.83–9.18) | 0.099 | 2.28 (0.66–7.84) | 0.190 |
| Rural | 60 | 16.4 | 305 | 83.6 | ||||
| Time-taken to reach to medical assistance (h) | ||||||||
| 0–3 | 30 | 12.2 | 215 | 87.8 | 1 | 1 | ||
| 4–6 | 15 | 17.6 | 70 | 82.4 | 1.54 (0.78–3.02) | 0.214 | 0.83 (0.37–1.85) | 0.644 |
| >6 | 18 | 22.5 | 62 | 77.5 | 2.08 (1.08–3.98) | 0.027 | 1.83 (0.88–3.79) | 0.103 |
| Pre-hospital treatment | ||||||||
| Yes | 33 | 14.4 | 197 | 85.6 | 0.92 (0.46–1.84) | 0.802 | ||
| No | 13 | 15.5 | 71 | 84.5 | ||||
| Comorbidities | ||||||||
| Yes | 8 | 17.8 | 37 | 82.2 | 1.24 (0.51–3.01) | 0.637 | ||
| No | 22 | 14.9 | 126 | 85.1 | ||||
| Unclottable blood | ||||||||
| Yes | 48 | 21.4 | 176 | 78.6 | 3.72 (1.86–7.42) | <0.001 | 3.11 (1.53–6.31) | 0.002 * |
| No | 11 | 6.8 | 150 | 93.2 | ||||
| Low hemoglobin | ||||||||
| Yes | 11 | 17.7 | 51 | 82.3 | 1.29 (0.63–2.67) | 0.486 | ||
| No | 44 | 14.3 | 264 | 85.7 | ||||
| Low hematocrit | ||||||||
| Yes | 5 | 11.1 | 40 | 88.9 | 0.68 (0.26–1.81) | 0.444 | ||
| No | 50 | 15.5 | 273 | 84.5 | ||||
| Thrombocytopenia | ||||||||
| Yes | 13 | 39.4 | 20 | 60.6 | 4.71 (2.18–10.15) | <0.001 | 4.52 (2.03–10.09) | <0.001 * |
| No | 42 | 12.1 | 304 | 87.9 | ||||
| Mean platelet volume | ||||||||
| Low | 5 | 14.7 | 29 | 85.3 | 1 | 1 | ||
| Normal | 44 | 13.5 | 282 | 86.5 | 0.91 (0.33–2.46) | 0.845 | 0.70 (0.24–2.00) | 0.503 |
| High | 4 | 44.4 | 5 | 55.6 | 4.64 (0.92–23.48) | 0.064 | 3.46 (0.56–21.55) | 0.182 |
* p < 0.05 was considered significant. Thrombocytopenia: platelet count <150,000/μL; Low hemoglobin: <12.5 g/dL; Low hematocrit: <36%: Low mean platelet volume: <7.4 fL; High mean platelet volume: >10.4 fL.
3. Discussion
Bothrops snake envenomation usually results in systemic effects such as coagulation disorders and systemic bleeding [7,22]. Although systemic bleeding is a common clinical manifestation in Bothrops snakebites, the clinical–epidemiological and laboratorial factors associated with the development of this effect are not well known in relation to the envenomations that occur in the Amazon region. In this study, systemic bleeding was present in 15.3% of the Bothrops snakebite patients during hospitalization, the most common being conjunctival and gingival, in consonance with previous studies from the Brazilian Amazon [7,23,24]. However, this rate was lower than that observed in envenomations by other species of Bothrops in Central and South America [15,21,25], which suggests that other mechanisms may be involved in the development of systemic bleeding.
The majority of Bothrops snakebites occurred in males, between 11 and 30 years old, living in rural areas and were mainly unrelated to work. Most of our patients took less than 3 h to reach medical assistance. These characteristics were also observed in other studies regarding the Brazilian Amazon and other regions of Brazil [4,21,23], as well as in other countries [16,26]. However, there was no significant association between systemic bleeding and epidemiological aspects of Bothrops snakebites in this study, although age and time taken to reach medical assistance were independently associated with severity and mortality in other studies of snakebites conducted in the Brazilian Amazon [4].
In this study, thrombocytopenia was an infrequent event. This observation was similar to that found in another study carried out in Belém, Pará State, Brazil, where B. atrox also causes most of the snakebites [7]. Contrary the this, B. jararaca snakebites showed a higher frequency of thrombocytopenia [15,21,22,27]. Most of the cases of systemic bleeding observed in our study showed a normal platelet count on admission, irrespective of the type of systemic bleeding, except for one case of petechiae. However, although thrombocytopenia is an uncommon finding, in this study thrombocytopenia on admission was independently associated with the development of systemic bleeding during hospitalization.
Indeed, platelets participate in reactions of hemostasis such as in adhesion to the cut end of a blood vessel, spreading of adherent platelets on the exposed subendothelial surface, secretion of stored platelet constituents, and formation of platelet aggregates [28,29]. In addition, platelets interact with proteins of the coagulation through surface receptors and phospholipids [30,31]. Recently, it has been observed that platelets also have roles in immune and inflammatory processes [32,33]. In snakebites, thrombocytopenia probably results from a multifactorial etiology as venom-induced platelet aggregation [34], sequestration to the areas of damage near the site of the bite [35], and venom-induced oxidative stress leading to a drop-in platelet count by apoptosis [36]. Components isolated from the B. atrox venom such as thrombocytin [34], batroxostatin [37], botrocetin [38], and batroxrhagin [39] may be acting on platelets leading to thrombocytopenia or changes in platelet function.
On the other hand, a negative correlation was found between the number of platelets and mean platelet volume on patient admission, which suggests a consequence of peripheral platelet destruction in which the mean platelet volume tends to increase. Studies show large platelets are more reactive than ordinary size platelets, as measured by aggregation and total release of granular content [40]. However, the ROC curve showed that only platelet counts on admission are not a good predictor of systemic bleeding during hospitalization in this specific envenomation, indicating that other parameters are involved in the development of systemic bleeding during hospitalization.
In this study, unclottable blood on admission was observed in more than half of Bothrops snakebite patients. The coagulation disorders observed in B. atrox snakebites patients can be explained by the presence of components of the venom with thrombin-like activity, which directly hydrolyze fibrinogen in fibrin [41,42,43] and pro-coagulants, which activate coagulation factors II and X [44,45], which results in the intravascular thrombin generation. Other coagulation factors activated by components isolated from B. atrox venom are the factors XIII [34] and V [46]. In addition, B. atrox venom components that present fibrin(geno)lytic activity can also contribute to coagulopathy [47]. Unclottable blood on admission was independently associated with the development of systemic bleeding during hospitalization in this study.
We observed a significative association between thrombocytopenia and unclottable blood on admission in Bothrops snakebites patients who developed systemic bleeding during hospitalization. Nonetheless, the poor results found in the discriminative analysis (ROC curve) prompts us to believe that these parameters alone are not the only ones involved in the systemic bleeding phenomena shown here. This corroborates with findings regarding B. jararaca snakebites patients, which suggest that systemic bleeding may occur in patients with coagulable blood and thrombocytopenia and that coagulation disorders are not therefore the primary cause of systemic bleeding [27]. Similar reports have been described with B. atrox snakebites in Colombia [20].
P-III SVMP is the markedly predominant toxin of the B. atrox venom, which comes from the snake responsible for the majority of snakebites in the Brazilian Amazon [12]. Interestingly, a PIII-SVMP called Batroxrhagin, the major component of B. atrox venom, is highly similar to Jarharagin, and is able to inhibit collagen-induced platelet-aggregation, hydrolyze fibrin, and is highly hemorrhagic [39]. P-III hemorrhagic SVMPs induce hemorrhage by their accumulation at the basement membrane by binding to collagens [48,49]. P-I SVMPs, such as batroxase [47] and atroxlysin-I [50], that induce bleeding through hydrolysis of extracellular matrix components have also been isolated from B. atrox venom. In addition, it has been observed in Bothrops snakebites that haemorrhagins present in snake venom cause local haemorrhage and systemic bleeding by the direct action on components of the basement membrane of capillaries [15,51]. Serum haemorraghin levels were significantly higher in patients with clinical signs of systemic bleeding than those without in envenomation by B. jararaca snakes. Haemorraghin levels were also correlated inversely with platelet count in these patients [15]. In our study, high serum haemorraghin levels probably also contributed to the development of systemic bleeding in Bothrops snakebites in the Brazilian Amazon. However, these levels were not determined. Likewise, changes in platelet function were not evaluated.
Another aspect to be considered in this study was antivenom therapy of these Bothrops snakebites. Bothrops antigen used in Brazil to immunize horses in order to produce the antivenom consists of a mixture of venoms of B. jararaca (50%), B. alternatus (12.5%), B. moojeni (12.5%), B. neuwiedi (12.5%), and B. jararacussu (12.5%) (manufacturer’s data). B. atrox venom is not included in the immunization pool used to produce Bothrops antivenom. Nevertheless, the results suggest that venom-induced systemic bleeding and unclottable blood were able to be ceased in most patients after 24 h of antivenom administration. These results were also found in other studies on Bothrops snakebites in the Brazilian Amazon [7,19] and other regions of Brazil [22]. On the other hand, it has been noted that Bothrops antivenom is less effective for neutralizing factor X activation activity of B atrox venom when compared to prothrombin activation activity [52]. In contrast, the frequency of thrombocytopenia increased in the first 48 h after antivenom administration, though decreased on discharge, while the frequency of abnormal mean platelet volume showed a decrease on discharge. Studies propose that venom-induced oxidative stress could lead to thrombocytopenia by apoptosis [36]. In Bothrops jararaca and B. jararacussu snakebites, it was observed that Bothrops envenomation promotes persistent and pronounced oxidative stress in the blood of the victims up to 1 month after hospitalization [53], which could explain the thrombocytopenia observed in our study up to 48 h after antivenom administration.
In conclusion, systemic bleeding observed in Bothrops snakebite patients in this part of the Amazon was an important systemic effect of the envenomation. The prevalence of systemic bleeding in this study was similar to that of others from the same region, but lower than that of other Bothrops spp envenomations. Thrombocytopenia and unclottable blood detected on admission were independently associated with the risk of developing systemic bleeding during hospitalization, which suggests that these hemostatic disorders increase the chances of systemic bleeding. Moreover, the effect of other parameters such as some toxins components of the venom can be involved in the development of systemic bleeding. Prospective studies are needed to elucidate the pathophysiology of systemic bleeding in Bothrops snakebites in the Brazilian Amazon and identify the different factors involved in its development as well as on the therapeutic response of hemostatic disorders.
4. Materials and Methods
4.1. Study Design and Data Source
This was an observational, cross-sectional study designed to assess the prevalence of and factors associated with systemic bleeding in Bothrops snakebite patients. Clinical–epidemiological and laboratorial data were obtained from patients’ records with clinical–epidemiological diagnosis of Bothrops envenomation who were attended to at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD), a specialist hospital for snakebites in Manaus, capital of the Amazonas State, Brazil, between August, 2013 and July, 2016. Eligible cases were those with clinical signs of Bothrops envenomation. Patients that underwent previous antivenom therapy in other health service centers were not included in this study. According to the Brazilian Ministry of Health Guidelines for Snakebite Diagnosis and Treatment [54], envenomation by Bothrops species is classified as follows: (a) Mild, characterized by pain and mild or absent local edema, mild or absent systemic bleeding, with or without change in coagulation time; (b) moderate: Characterized by pain and evident edema involving three or more segments of the affected limb, accompanied or not by systemic bleeding and coagulopathy; (c) severe: Characterized by edema involving the entire affected limb, usually accompanied by severe pain. Systemic manifestations such as hypotension, shock, oliguria/anuria, or severe bleeding are defined as a severe case, regardless of the local effect. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the FMT-HVD, Manaus, Brazil (approval number: 1.433.431, approval date: 2 March 2016). Term of commitment of use of data was signed by the researchers.
4.2. Clinical–Epidemiological and Laboratorial Parameters
The clinical–epidemiological variables analyzed were gender, age (in years), geographical location of the occurrence of the snakebite, type of area where the snakebite occurred (rural or urban), work-related bite, occupation, time taken to reach medical assistance (in hours), anatomical region of the bite, pre-hospital treatment (use of topical or oral medicines, use of tourniquet and other procedures), Bothrops bite confirmation by snake, local and systemic manifestations, clinical severity of envenomation (mild, moderate, or severe according to the Brazilian Health Ministry guidelines), presence of comorbidities, time spent in hospital (in days), and outcome (discharge or death). Analyzed laboratory variables were clotting time (in minutes), hemoglobin (mg/dL), hematocrit (%), platelet count (number/μL), and mean platelet volume (fL). The snakes were identified by a trained biologist at the FMT-HVD. All variables were checked by two independent researchers before analysis and further investigated for possible association with systemic bleeding as a dependent variable.
Snakebite-induced coagulopathy was evaluated by a modification of the Lee–White clotting time (LWCT) [55] which is recommended by the Brazilian Ministry of Health. With the use a plastic unlubricated syringe, 1 mL of venous blood was collected and placed into a new glass tube (13 × 75 mm) without any anticoagulants at 37 °C. Using a stopwatch, the timing started as soon as the blood was drawn into the tube. The glass tube was left undisturbed for 5 min and then checked for clots every following minute by gently tilting the tube. Unclottable blood was defined when the blood was not clotted until 30 min. The sensitivity of the LWCT performed in routine clinical settings for the diagnosis of hypofibrinogenemia is 78% [56]. Thrombocytopenia was defined by a platelet count below 150,000/μL. Low hemoglobin was defined when values were lower than 12.5 g/dL. Low hematocrit was defined when values were below 36%. Low and high mean platelet volume was defined when values were below 7.4 fL and above 10.4 fL, respectively. In order to identify clinical–epidemiological and laboratorial variables collected on admission associated with systemic bleeding, patients evolving to systemic bleeding on admission or during hospitalization were classified as a case, and one with non-systemic bleeding was included as a control. All patients were asked to return to the hospital for clinical reassessment in case of complications at any time, or on day 7 after discharge.
4.3. Statistical Analysis
A database and descriptive analyses were produced using Microsoft Excel Office 365 software which was fed information by two independent individuals. Statistical analyses were made using the software STATA statistical package version 13 (Stata Corp, College Station, TX, USA) and Graphpad Prism version 5 (Graphpad Software, Inc., San Diego, CA, USA). The Mann–Whitney test was used for comparison of medians. Differences in results were considered statistically significant when p < 0.05. The Pearson correlation coefficient was calculated to measure the degree of association between the platelet count and mean platelet volume on admission. The performance of platelet counts on admission to predict systemic bleeding during hospitalization in Bothrops snakebite patients was assessed using ROC curve.
Proportions of systemic bleeding during hospitalization were compared by Chi-square test (corrected by Fisher’ test if necessary); differences were considered statistically significant for p < 0.05. The crude Odds Ratio (OR) with its respective 95% Confidence Interval (95% CI) was determined considering systemic bleeding during hospitalization as the dependent variable. Logistic regression was used for the multivariable analyses and the adjusted Odds Ratios with 95% CI were also calculated. All variables associated with the outcome at a significance level of p < 0.20 in the univariate analysis were included in the multivariable analysis. Statistical significance was considered if p < 0.05 in the Hosmer–Lemeshow goodness-of-fit test.
Acknowledgments
We thank Marcos Sabóia for his technical support with the database.
Author Contributions
Conceptualization, S.S.O., I.S.S.-M. and W.M.M. methodology, S.S.O., I.S.S.-M. and W.M.M.; software, S.S.O.; validation, S.S.O., E.C.A., A.S.S., J.P.T.P., L.K.S.S. and E.F.N.; formal analysis, S.S.O., J.D.d.-B.-S., V.S.S.; investigation, S.S.O., E.C.A., A.S.S., J.P.T.P., L.K.S.S. and E.F.N.; resources, J.A.G.S. and W.M.M.; data curation, S.S.O., E.C.A., A.S.S., J.P.T.P., L.K.S.S. and E.F.N.; writing—original draft preparation, S.S.O.; writing—review and editing, J.A.G.S., M.V.G.L., I.S.S.-M. and W.M.M.; visualization, S.S.O., I.S.S.-M. and W.M.M.; supervision, I.S.S.-M. and W.M.M.; project administration, S.S.O.; funding acquisition, W.M.M.
Funding
This research was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES/PROCAD). M.V.G.L. and W.M.M. are CNPq fellows.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Key Contribution
Systemic bleeding observed after Bothrops snakebites in the Brazilian Amazon is an important systemic effect of the envenomation. Thrombocytopenia and unclottable blood on admission are factors associated with systemic bleeding during hospitalization.
References
- 1.Kasturiratne A., Wickremasinghe A.R., De Silva N., Gunawardena N.K. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan H.W., Monteiro W.M. History and perspectives on how ensure antivenom accessibility in the most remote areas in Brazil. Toxicon. 2018;151:15–23. doi: 10.1016/j.toxicon.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 3.Brazilian Ministry of Health . Guia de Vigilância Epidemiológica. 7th ed. Ministério da Saúde; Brasília, Brazil: 2009. [Google Scholar]
- 4.Feitosa E.L., Sampaio V.S., Salinas J.L., Queiroz A.M., da Silva I.M., Gomes A.A., Sachett J., Siqueira A.M., Ferreira L.C.L., dos Santos M.C., et al. Older Age and Time to Medical Assistance Are Associated with Severity and Mortality of Snakebites in the Brazilian Amazon: A Case-Control Study. PLoS ONE. 2015;10:e0132237. doi: 10.1371/journal.pone.0132237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza A.S., Sachett J.A.G., Alcântara J.A., Freire M., Alecrim M.G.C., Lacerda M., Ferreira L.C.L., Fan H.W., Sampaio V.S., Monteiro W.M. Snakebites as cause of deaths in the Western Brazilian Amazon: Why and who dies? Deaths from snakebites in the Amazon. Toxicon. 2018;145:15–24. doi: 10.1016/j.toxicon.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Wen F.H., Monteiro W.M., Moura da Silva A.M., Tambourgi D.V., Mendonça da Silva I., Sampaio V.S., dos Santos M.C., Sachett J., Ferreira L.C.L., Kalil J., et al. Snakebites and Scorpion Stings in the Brazilian Amazon: Identifying Research Priorities for a Largely Neglected Problem. PLoS Negl. Trop. Dis. 2015;9:e0003701. doi: 10.1371/journal.pntd.0003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardal P.P.O., Souza S.M., Monteiro M.R.C.C., França F.O.S., Tomy S.C., Sano-Martins I.S., Sousa-e-Silva M.C.C., Colombini M., Kodera N.F., Moura-da-Silva A.M., et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004;98:28–42. doi: 10.1016/S0035-9203(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro L.A., Jorge M.T. Epidemiologia e quadro clínico dos acidentes por serpentes Bothrops jararaca adultas e filhotes. Rev. Inst. Med. Trop. São Paulo. 1990;32:436–442. doi: 10.1590/S0036-46651990000600008. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro L.A., Albuquerque M.J., Pires de Campos V.A.F., Katz G., Takacka N.Y., Lebrão M.L., Jorge M.T. Óbitos por serpentes peçonhentas no Estado de São Paulo: Avaliação de 43 casos, 1988/93. Rev. Assoc. Med. Bras. 1998;44:312–318. doi: 10.1590/S0104-42301998000400010. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira S.S., Freitas-de-Sousa L.A., Alves E.C., Ferreira L.C.L., Mendonça-da-Silva I., Lacerda M.V.G., Fan H., Moura-da-Silva A.M., Monteiro W.M. Fatal stroke after Bothrops snakebite in the Amazonas state, Brazil: A case report. Toxicon. 2017;138:102–106. doi: 10.1016/j.toxicon.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld G. Symptomatology, pathology and treatment of snakes bites in South América. In: Bucgerl W., Buckley E.E., editors. Venomous Animals and Their Venoms. Academic Press; New York, NY, USA: 1971. pp. 347–382. [Google Scholar]
- 12.Sousa L.F., Nicolau C., Peixoto P.S., Bernardoni J.L., Oliveira S.S., Portes-Junior J.A., Mourão R.H.V., Lima-dos-Santos I., Sano-Martins I.S., Chalkidis H.M., et al. Comparison of Phylogeny, Venom Composition and Neutralization by Antivenom in Diverse Species of Bothrops Complex. PLoS Negl. Trop. Dis. 2013;7:e2442. doi: 10.1371/journal.pntd.0002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Q., Clemetson J.M., Clemetson K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005;3:1791–1799. doi: 10.1111/j.1538-7836.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 14.Sajevic T., Leonardi A., Križaj I. Haemostatically active proteins in snake venoms. Toxicon. 2011;57:627–645. doi: 10.1016/j.toxicon.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Kamiguti A.S., Rugman F.P., Theakston R.D.G., França F.O.S., Ishii H., Hay C. BIASG The role of venom haemorrhagin in spontaneous bleeding in Bothrops jararaca envenoming. Thromb. Haemost. 1992;67:484–488. [PubMed] [Google Scholar]
- 16.Málaque C.M.S., Gutiérrez J.M. Snakebite envenomation in Central and South America. In: Brent J., editor. Critical Care Toxicology. Springer; Cham, Switzerland: 2015. pp. 1–22. [Google Scholar]
- 17.Chippaux J.P., Whiliams V., White J. Snake venom variability: Methods of study, results and interpretation. Toxicon. 1991;29:1279–1303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- 18.Sousa L.F., Portes-Junior J.A., Nicolau C.A., Bernardoni J.L., Nishiyama-Jr M.Y., Amazonas D.R., Freitas-de-Sousa L.A., Mourão R.H., Chalkidis H.M., Valente R.H., et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017;159:32–46. doi: 10.1016/j.jprot.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Mendonça-da-Silva I., Magela Tavares A., Sachett J., Sardinha J.F., Zaparolli L., Gomes Santos M.F., Lacerda M., Monteiro W.M. Safety and efficacy of a freeze-dried trivalent antivenom for snakebites in the Brazilian Amazon: An open randomized controlled phase IIb clinical trial. PLoS Negl. Trop. Dis. 2017;11:1–21. doi: 10.1371/journal.pntd.0006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otero R., Gutiérrez J.M., Núñez V., Robles A., Estrada R., Segura E., Toro M.F., García M.E., Díaz A., Ramírez E.C., et al. A randomized double-blind clinical trial of two antivenoms in patients bitten by Bothrops atrox in Colombia. Trans. R. Soc. Trop. Med. Hyg. 1996;90:696–700. doi: 10.1016/S0035-9203(96)90442-3. [DOI] [PubMed] [Google Scholar]
- 21.Santoro M.L., Sano-Martins I.S., Fan H.W., Cardoso J.L., Theakston R.D.G., Warrell D.A. Haematological evaluation of patients bitten by the jararaca, Bothrops jararaca, in Brazil. Toxicon. 2008;51:1440–1448. doi: 10.1016/j.toxicon.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso J.L.C., Fan H.W., França F.O.S., Jorge M.T., Leite R.P., Nishioka S.A., Avila A., Sano-Martins I.S., Tomy S.C., Santoro M.L., et al. Randomized comparative trial of three antivenomsin the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q. J. Med. 1993;86:315–325. [PubMed] [Google Scholar]
- 23.Sachett J.A.G., da Silva I.M., Alves E.C., Oliveira S.S., Sampaio V.S., do Vale F.F., Romero G.A.S., dos Santos M.C., Marques H.O., Colombini M., et al. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: A randomized controlled clinical trial. PLoS Negl. Trop. Dis. 2017;11:1–21. doi: 10.1371/journal.pntd.0005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves E.C., Gonc J.D.A., Sousa D.D.B., De Oliveira S., Nascimento F., Santos S., Moura M., Wen F.H., Monteiro W.M., Carlos L., et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE. 2018 doi: 10.1371/journal.pone.0202361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otero-Patiño R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54:998–1011. doi: 10.1016/j.toxicon.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Chaves L.F., Chuang T., Sasa M., Gutiérrez J.M. Snakebites are associated with poverty, weather fluctuations, and El Niño. Sci. Adv. 2015;1:7. doi: 10.1126/sciadv.1500249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiguti A.S., Cardoso J.L.C., Theakston R.D.G., Sano-Martins I.S., Hutton R.A., Rugman F.P., Warrell D.A., Hay C.R.M. Coagulopathy and Haemorrhage in Human Victims. Toxicon. 1991;29:961–972. doi: 10.1016/0041-0101(91)90079-7. [DOI] [PubMed] [Google Scholar]
- 28.Colman R.W., Clowes A.W., Golhaber S.Z., Marder V.J., George J.N. Hemostasis an Thrombosis: Basic Principles and Clinical Practice. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2006. Overview of hemostasis; pp. 1–51. [Google Scholar]
- 29.De Queiroz M.R., de Sousa B.B., da Cunha Pereira D.F., Mamede C.C.N., Matias M.S., de Morais N.C.G., de Oliveira Costa J., de Oliveira F. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon. 2017;133:33–47. doi: 10.1016/j.toxicon.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Roberts H.R., Lozier J.N. New perspective on the coagulation cascade. Hosp. Prat. 1992;27:97–112. doi: 10.1080/21548331.1992.11705345. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman M. Remodeling the blood coagulation cascade. J. Thromb. Thrombolysis. 2003;16:17–20. doi: 10.1023/B:THRO.0000014588.95061.28. [DOI] [PubMed] [Google Scholar]
- 32.Katoh N. Platelets as versatile regulators of cutaneous inflammation. J. Dermatol. Sci. 2009;53:89–95. doi: 10.1016/j.jdermsci.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Von Hundelshausen P., Weber C. Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 34.Niewiarowski S., Kirby E.P., Brudzynski T.M., Stocker K. Thrombocytin, a serine protease from Bothrops atrox venom. 2. Interaction with platelets and plasma-clotting factors. Biochemistry. 1979;18:3570–3577. doi: 10.1021/bi00583a021. [DOI] [PubMed] [Google Scholar]
- 35.Simon T.L., Brace T.G. Envenomation coagulopathy in wounds from pit vipers. N. Engl. J. Med. 1981;305:443–447. doi: 10.1056/NEJM198108203050808. [DOI] [PubMed] [Google Scholar]
- 36.Sunitha K., Hemshekhar M., Thushara R.M., Santhosh M.S., Sundaram M.S., Kemparaju K., Girish K.S. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon. 2015;98:89–97. doi: 10.1016/j.toxicon.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Rucinski B., Niewiarowski S., Holt J.C., Soszka T., Knudsen K.A. Batroxostatin, an Arg-Gly-Asp-containing peptide from Bothrops atrox, is a potent inhibitor of platelet aggregation and cell interaction with fibronectin. Biochim. Biophys. Acta. 1990;1054:257–262. doi: 10.1016/0167-4889(90)90096-V. [DOI] [PubMed] [Google Scholar]
- 38.Read M.S., Shermer R.W., Brinkhous K.M. Venom coagglutinin: An activator of platelet aggregation dependent on von Willebrand factor. Proc. Natl. Acad. Sci. USA. 1978;75:4514–4518. doi: 10.1073/pnas.75.9.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freitas-De-Sousa L.A., Amazonas D.R., Sousa L.F., Sant’Anna S.S., Nishiyama M.Y., Serrano S.M.T., Junqueira-De-Azevedo I.L.M., Chalkidis H.M., Moura-Da-Silva A.M., Mourão R.H.V. Comparison of venoms from wild and long-term captive Bothrops atrox snakes and characterization of Batroxrhagin, the predominant class PIII metalloproteinase from the venom of this species. Biochimie. 2015;118:60–70. doi: 10.1016/j.biochi.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Elsayed A.M., Mohamed G.A. Mean platelet volume and mean platelet volume/platelet count ratio as a risk stratification tool in the assessment of severity of acute ischemic stroke. Alexandria J. Med. 2017;53:67–70. doi: 10.1016/j.ajme.2016.03.003. [DOI] [Google Scholar]
- 41.Blomback B. Studies on the action of thrombic enzymes on bovine fibrinogen as measured by N-terminal analysis. Ark. Kemi. 1958;12:321. [Google Scholar]
- 42.Stocker K., Barlow G.H. The coagulant enzyme from Bothrops atrox venom (Batroxobin) Methods Enzymol. 1976;45:214–223. doi: 10.1016/s0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- 43.Petretski J.H., Kanashiro M., Silva C.P., Alves E.W., Kipnis T.L. Two related thrombin-like enzymes present in Bothrops atrox venom. Braz. J. Med. Biol. Res. 2000;33:1293–1300. doi: 10.1590/S0100-879X2000001100005. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann H., Bon C. Blood Coagulation Induced by the Venom of Bothrops atrox. 1. Identification, Purification, and Properties of a Prothrombin Activator. Biochemistry. 1987;26:772–780. doi: 10.1021/bi00377a018. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann H., Bon C. Blood Coagulation Induced by the Venom of Bothrops atrox. 2. Identification, Purification, and Properties of Two Factor X Activators. Biochemistry. 1987;26:780–787. doi: 10.1021/bi00377a019. [DOI] [PubMed] [Google Scholar]
- 46.Rosing J., Govers-Riemslag J.W., Yukelson L., Tans G. Factor V activation and inactivation by venom proteases. Haemostasis. 2002;31:241–246. doi: 10.1159/000048069. [DOI] [PubMed] [Google Scholar]
- 47.Cintra A.C.O., De Toni L.G.B., Sartim M.A., Franco J.J., Caetano R.C., Murakami M.T., Sampaio S.V. Batroxase, a new metalloproteinase from B. atrox snake venom with strong fibrinolytic activity. Toxicon. 2012;60:70–82. doi: 10.1016/j.toxicon.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Herrera C., Escalante T., Voisin M.B., Rucavado A., Morazán D., Macêdo J.K.A., Calvete J.J., Sanz L., Nourshargh S., Gutiérrez J.M., et al. Tissue Localization and Extracellular Matrix Degradation by PI, PII and PIII Snake Venom Metalloproteinases: Clues on the Mechanisms of Venom-Induced Hemorrhage. PLoS Negl. Trop. Dis. 2015;9:e0003731. doi: 10.1371/journal.pntd.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldo C., Jamora C., Yamanouye N., Zorn T.N., Moura-da-Silva A.M. Mechanisms of vascular danage by hemorrhagic snake venom metalloproteinases: Tissue distribution and in situ hydrolysis. PLoS Negl. Trop. Dis. 2010;4:e727. doi: 10.1371/journal.pntd.0000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez E.F., Schneider F.S., Yarleque A., Borges M.H., Richardson M., Figueiredo S.G., Evangelista K.S., Eble J.A. The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergón) snake venom acts both on blood vessel ECM and platelets. Arch. Biochem. Biophys. 2010;496:9–20. doi: 10.1016/j.abb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Gutiérrez J.M., Rucavado A., Escalante T., Díaz C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005;45:997–1011. doi: 10.1016/j.toxicon.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 52.Sousa L.F., Zdenek C.N., Dobson J.S., Op den Brouw B., Coimbra F., Gillett A., Del-Rei T.H.M., Chalkidis H.D.M., Sant’Anna S., Teixeira-da-Rocha M.M., et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins. 2018;10:411. doi: 10.3390/toxins10100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strapazzon J.D.O., Parisotto E.B., Moratelli A.M., Garlet T.R., Bastos J., Zimermann I.R., Zanin M., Fagundez R., Lino M.R.d.O., Fröde T.S., et al. Systemic oxidative stress in victims of Bothrops snakebites. J. Appl. Biomed. 2015;13:161–167. doi: 10.1016/j.jab.2014.11.002. [DOI] [Google Scholar]
- 54.Brasil Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos. 2nd ed. Fundação Nacional de Saúde; Brasília, Brazil: 2001. pp. 21–25. [Google Scholar]
- 55.Lee R., White P. A clinical study of the coagulation time of blood. Am. J. Med. Sci. 1913;145:495. doi: 10.1097/00000441-191304000-00004. [DOI] [Google Scholar]
- 56.De Brito Sousa J.D., Sachett J.A.G., de Oliveira S.S., Mendonça-da-Silva I., Marques H.O., de Lacerda M.V.G., Fan H.W., Monteiro W.M. Accuracy of the lee—White clotting time performed in the hospital routine to detect coagulopathy in Bothrops atrox envenomation. Am. J. Trop. Med. Hyg. 2018;98:1547–1551. doi: 10.4269/ajtmh.17-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]