Abstract
A detailed chemical investigation of two South China Sea nudibranchs Phyllidiella pustulosa and Phyllidia coelestis, as well as their possible sponge-prey Acanthella cavernosa, led to the isolation of one new nitrogenous cadinane-type sesquiterpenoid xidaoisocyanate A (1), one new naturally occurring nitrogen-containing kalihinane-type diterpenoid bisformamidokalihinol A (16), along with 17 known nitrogenous terpenoids (2–15, 17–19). The structures of all the isolates were elucidated by detailed spectroscopic analysis and by the comparison of their spectroscopic data with those reported in the literature. In addition, the absolute stereochemistry of the previously reported axiriabiline A (5) was determined by X-ray diffraction (XRD) analysis. In a bioassay, the bisabolane-type sesquiterpenoids 8, 10, and 11 exhibited cytotoxicity against several human cancer cell lines.
Keywords: nitrogenous terpenoids, South China Sea, sponge, nudibranch, cytotoxicity
1. Introduction
Sea slugs of the genus Phyllidiella and Phyllidia are prolific in the South China Sea. They are well known for their ability to ingest toxic nitrogenous sesquiterpenoids from their diets, and use either these metabolites themselves or their biosynthetically transformed derivatives as a weapon for chemical defense [1,2,3,4,5,6,7]. An intriguing ecological study showed that when sea slugs are under attack, they release a lot of mucus containing these nitrogenous metabolites to poison their enemies [8]. The dietary origin of nitrogenous sesquiterpenoids has been supported by chemical investigations involving the isolation of such metabolites from both nudibranchs and their sponge-preys [9,10,11,12,13].
Marine sponges of the genus Acanthella are well known as a rich source of diverse diterpenoids and sesquiterpenoids containing nitrogenous functional groups, including cyano, isocyano, isothiocyano, and formamido functionalities [14,15,16,17,18]. Many of these secondary metabolites merit further investigation due to their various biological activities ranging from cytotoxic [15], antimalarial [19,20], and antimicrobial [21,22] to antifouling properties [14,23,24,25,26,27]. Some of them, with novel structures and promising biological activities, have attracted much attention from chemists seeking to perform their total synthesis in parallel with intensive biological studies towards new drug leads [28,29,30,31].
In our previous chemical investigation on South China Sea (Hainan) nudibranchs and sponges, nitrogenous terpenoids were isolated and structurally characterized [1,17,18,32,33,34]. In the course of our continuing project on searching for chemically fascinating and biologically active secondary metabolites from Hainan marine molluscs, as well as the chemical ecology study between nudibranchs and their sponge-preys, we made different collections of two nudibranchs, Phyllidiella pustulosa and Phyllidia coelestis, as well as their sponge-prey Acanthella cavernosa, from the same location (Xidao Island, Hainan Province, China), with the aim of accumulating their nitrogenous metabolites for further study of their bioactivities, as well as studying the dietary relationship between P. pustulos, P. coelestis, and their sponge-prey A. cavernosa.
2. Results
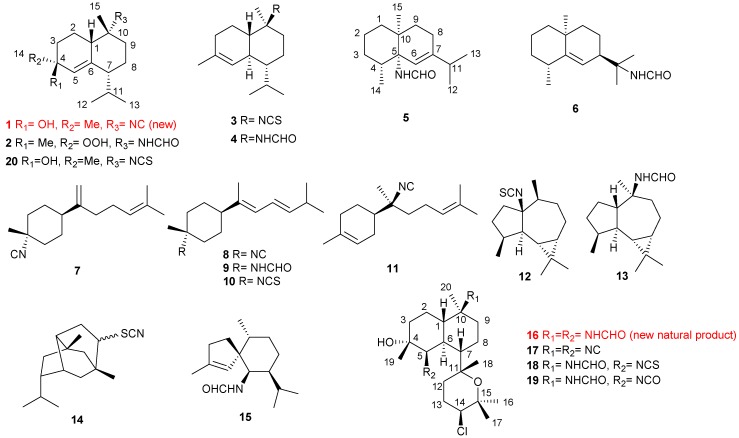
Chemical investigation of the collected two nudibranchs, P. pustulosa and P. coelestis, as well as one sponge, A. cavernosa, led to the isolation of one new cadinane-type sesquiterpenoid (1), one new naturally occurring kalihinane-type diterpenoid (16), along with 14 known sesquiterpenoids (2–15) and three known diterpenoids (17–19) (Figure 1). All the compounds contain nitrogen atoms in different functional groups, such as isocyanate, isothiocyanate, and formamide. Herein, we describe the isolation, structure elucidation, and cytotoxic activity of these compounds, as well as their possible biosynthetic origin influenced by the prey-predator relationship.
Figure 1.
Structures of compounds 1–20.
2.1. Phyllidiella pustulosa
The Et2O soluble portion of the acetone extract of the mollusc P. pustulosa was subjected to silica gel chromatography (petroleum ether/ether gradient). Guided by NMR analysis, the selected terpene-containing fractions were subsequently purified on repeated column chromatography (silica gel, Sephadex LH-20, reversed phase-C18 and RP-HPLC) to afford one new cadinane-type sesquiterpenoid (1), along with nine known metabolites (2, 3, 6–8, 12–14, 17) (Figure 1). The known compounds were identified as two cadinane-type sesquiterpenoids: halichon G (2) [35] and 10-isothiocyanato-4-cadinene (3) [13,28,36,37,38], one eudesmane-type sesquiterpenoid: 11-formamido-7βH-eudesm-5-ene (6) [39,40], two bisabolane-type sesquiterpenoids: Δ7,14-3-isocyanotheonellin (7) [1,41] and 3-isocyanotheonellin (8) [1], two aromadendrane-type sesquiterpenoids: 1-isothiocyanatoaromadendrane (12) [42] and axamide-2 (13) [43,44], one mixture of pupukeanane-type sesquiterpenoids: 9-thiocyanatopupukeanane isomers (14) [6], and one kalihinane-type diterpenoid: kalihinol A (17) [45].
Compound 1, namely xidaoisocyanate A, was obtained as a colorless oil, −3.6 (c 0.1, MeOH). Its molecular formula, C16H25NO, was established by HREIMS (m/z 247.1927, [M]+, calcd. 247.1936), indicating five degrees of unsaturation (Figures S1 and S2). The diagnostic 1H and 13C NMR resonances, as well as coupling constants of the connected protons (Table 1, Figures S3 and S4), indicated the presence of one trisubstituted double bond (δH 5.58, s, δC 130.4, CH; δC 136.5, qC) and four methyl groups (δH 0.97 (3H, d, Me-12); 0.90 (3H, d, Me-13); 1.40 (3H, s, Me-14); 1.42 (3H, t, Me-15)). The typical 13C NMR signal of sp3 quaternary carbon (δC 63.3, qC), bearing in mind the odd molecular weight of 1, suggested the presence of an isocyano group (−NC group). The above functionalities account for three out of the five degrees of unsaturation, suggesting a bicyclic ring system in 1. The above structural features were reminiscent of the co-occurring molecule 2, as well as a previously reported axinisothiocyanate J (20) [46] which was isolated from the sponge Axinyssa sp.
Table 1.
1H and 13C NMR data of 1 and 16, and their model compounds 20 and 17, respectively, recorded in CDCl3 a.
| No. | 1 | 20 | No. | 16 | 17 | ||
|---|---|---|---|---|---|---|---|
| δH Mult (J in Hz) | δ C | δ C | δH Mult (J in Hz) | δ C | δ C | ||
| 1 | 1.96 m | 45.9 CH | 46.9 CH | 1 | 1.37 m | 42.6 CH | 42.3 CH |
| 2a | 1.69 m | 21.6 CH2 | 21.6 CH2 | 2a | 1.47 m | 21.7 CH2 | 21.6 CH2 |
| 2b | 1.94 m | 2b | 1.61 m | ||||
| 3a | 1.53 m | 36.3 CH2 | 36.2 CH2 | 3 | 1.51 m, 2H | 33.7 CH2 | 32.6 CH2 |
| 3b | 1.96 m | ||||||
| 4 | - | 69.5 qC | 69.3 qC | 4 | - | 71.6 qC | 70.5 qC |
| 5 | 5.58 s | 130.4 CH | 130.2 CH | 5 | 4.18 (d, 10.4) | 59.8 CH | 63.7 CH |
| 6 | - | 136.5 qC | 137.2 qC | 6 | 2.35 m | 36.6 CH | 36.0 CH |
| 7 | 1.64 m | 47.3 CH | 47.4 CH | 7 | 1.57 m | 45.8 CH | 48.4 CH |
| 8a | 1.49 m | 21.8 CH2 | 22.5 CH2 | 8a | 1.62 m | 23.1 CH2 | 21.9 CH2 |
| 8b | 1.69 m | 8b | 1.02 m | ||||
| 9a | 1.51 m | 39.4 CH2 | 40.4 CH2 | 9a | 1.72 m | 40.7 CH2 | 39.7 CH2 |
| 9b | 2.01 (d, 10.0) | 9b | 1.55 m | ||||
| 10 | - | 63.3 qC | 66.0 qC | 10 | - | 55.0 qC | 59.0 qC |
| 11 | 2.14 m | 26.8 CH | 26.8 CH | 11 | - | 79.0 qC | 76.8 qC |
| 12 | 0.97 (d, 6.8) | 22.1 CH3 | 22.1 CH | 12a | 1.48 m | 38.1 CH2 | 38.0 CH2 |
| 12b | 1.57 m | ||||||
| 13 | 0.90 (d, 6.8) | 17.5 CH3 | 17.5 CH | 13a | 1.99 m | 27.7 CH2 | 27.4 CH2 |
| 13b | 2.06 m | ||||||
| 14 | 1.40 s | 27.1 CH3 | 26.7 CH3 | 14 | 3.68 (dd, 12.4, 4.4) | 64.4 CH | 64.1 CH |
| 15 | 1.42 (t, 1.8) | 28.9 CH3 | 28.2 CH3 | 15 | - | 76.7 qC | 76.0 qC |
| NC (1) and NCS (20) | - | n.d. b | n.d. b | 16 | 1.37 s | 23.5 CH3 | 22.8 CH3 |
| 17 | 1.31 s | 31.4 CH3 | 30.5 CH3 | ||||
| 18 | 1.27 s | 19.7 CH3 | 19.2 CH3 | ||||
| 19 | 1.19 s | 18.8 CH3 | 29.0 CH3 | ||||
| 20 | 1.18 s | 29.0 CH3 | 20.7 CH3 | ||||
| CHO-1 or NC | 8.25 (d, 12.0) | 163.7 CH | 157.0 qC | ||||
| CHO-2 or NC | 8.10 (d, 11.4) | 167.6 CH | 153.0 qC | ||||
a Assignments were deduced by the analysis of 1D and 2D NMR spectra. b n.d. means not detected.
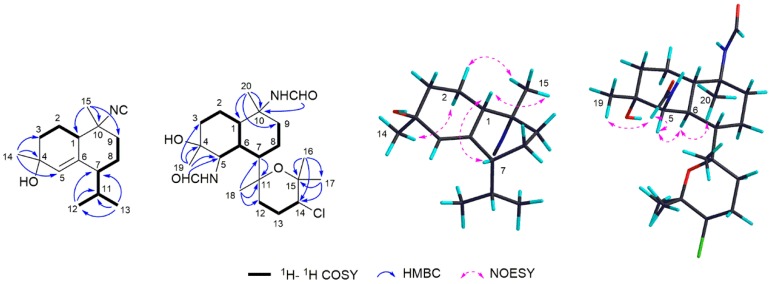
Detailed comparison of the NMR data revealed that 1 should possess the same cadinane ring system as 20. The only significant difference of these two compounds was the presence of an isocyano group at C-10 in 1 instead of the isothiocyano group (−NCS group) in 20. According to this, the 13C NMR data of C-1, C-9, and C-10 in 1 were upfield shifted (δC 45.9, CH, Δδ = −1.0 ppm; δC 39.4, CH2, Δδ = −1.0 ppm; δC 63.3, qC, Δδ = −2.7 ppm), respectively, compared with those in 20. Further 2D NMR spectra, including COSY, HSQC, and HMBC (Figures S5–S7), allowed the unambiguous determination of the planar structure of compound 1 (Figure 2).
Figure 2.
1H-1H COSY, key HMBC and NOESY correlations of compounds 1 and 16.
The relative configuration of 1 was deduced by NOESY spectra (Figure 2 and Figure S8). The NOE correlation between H-5 (δH 5.58, s) and H-11 (δH 2.14, m) indicated the Z-geometry of Δ5,6. The correlations of H-1 (δH 1.96, m) with Me-15 (δH 1.42, t) and H-7 (δH 1.64, m) indicated that these protons were on the same side of the molecule and were tentatively assigned to be α-oriented. Furthermore, the obvious NOE correlation between Me-15 and H-2b (1.94, m), and between Me-14 (1.40, s) and H-2a (1.69, m) suggested the α-orientation of Me-14. Therefore, the structure of compound 1 was determined as shown in Figure 1, which was further confirmed by its similar NMR data to those of axinisothiocyanate J (20) based on a biogenetic consideration [46]. In fact, compound 1 was identified as a C-10 epimer of a known isocyanosesquiterpene alcohol, which was first isolated from the nudibranch Phyllidia pustulosa [12].
2.2. Phyllidia coelestis
The abovementioned usual workup of the Et2O-soluble portion of the acetone extract of the animals of P. coelestis yielded six pure compounds: 6, 8–11, and 14 (Figure 1). The known compounds were identified as one eudesmane-type sesquiterpenoid: 6 [39,40], four bisabolane-type sesquiterpenoids: 8 [1], theonellin formamide (9) [33], theonellin isothiocyanate (10) [33], and 7-isocyano-7,8-dihydro-α-bisabolene (11) [42], and one mixture of pupukeanane-type sesquiterpenoids: 14 [6] by direct comparison of its NMR data and specific rotation with those reported in the literature.
2.3. Acanthella cavernosa
The frozen A. cavernosa animals were cut into pieces and exhaustively extracted by acetone. The Et2O-soluble portion of the acetone extract was repeatedly chromatographed to yield pure compounds 4, 5, 15, 16, 18, and 19 (Figure 1). The known compounds were readily identified as one cadinane-type sesquiterpenoid: 10-formamido-4-cadinene (4) [24], one eudesmane-type sesquiterpenoid: axiriabiline A (5) [32], one spiroaxane-type sesquiterpenoid: axamide-3 (15) [27], along with two kalihinane-type diterpenoids: 10β-formamido-5β-isothiocyanatokalihinol-A (18) [14] and 10β-formamido-5-isocyanatokalihinol-A (19) [14] by comparing their NMR spectroscopic data and specific optical rotation with those reported in the literature.
Compound 16 was isolated as an optically active colorless oil, +19 (c 0.1, CHCl3). Its molecular formula was determined as C22H37N2O4Cl by HRESIMS (m/z 429.2522 [M+H]+, calcd. 429.2515), indicating five degrees of unsaturation (Figure S9). The IR spectrum (Figure S10) of 16 showed absorptions at νmax 1665 cm−1 and 3440 cm−1, indicating the presence of the amide carbonyl and hydroxy groups, respectively. The 13C NMR and DEPT spectra of 16 displayed 22 carbon signals, including five sp3 methyls, six sp3 methylenes, five sp3 methines, four sp3 quaternary carbons, and two sp2 methines. The spectroscopic data (Table 1, Figures S11 and S12) showed highly similarity to those of co-occurring related known compounds 18 and 19, indicating that 16 is also a kalihinane-type diterpenoid. In fact, they differed from each other only by the substitution at C-5 position of the kalihinane ring. Bearing in mind the two additional protons present in its molecular formula in comparison to 19, a −NHCHO group (δH 8.10 s, δC 167.6, CH) should be attached to the C-5 of compound 16. Intriguingly, resonances for both formamides were observed as a plethora of signals between δH 8.0 and 8.3. These included eight signals arising from the four isomeric arrangements possible for the two formamides at C-5 and C-10 [47]. Detailed analysis of the 1D and 2D NMR spectra, including 1H-1H COSY, HSQC, and HMBC (Figures S13–S15), allowed the establishment of the planar structure of 16 (Figure 2), the same as a known compound named bisformamidokalihinol A, which was obtained from the hydrolysis of kalihinol A with acetic acid [48].
The relative configuration of 16 was also determined to be the same as co-occurring compounds 17–19 by careful interpretation of its NOESY spectrum with the clear NOE correlations of H-1/H-7, H-5/H-6/H3-20, and H3-19/NHCHO at C-5 (Figure 2 and Figure S16). Since the absolute configuration of 17 has been previously determined by total synthesis [29], from a biogenetic point of view, the absolute configuration of compound 16 was tentatively assigned as 1S,4R,5R,6S,7S,10S,11R,14S.
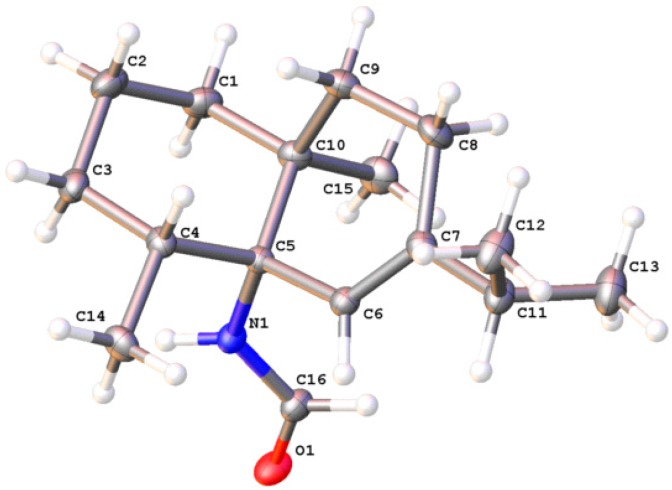
It is worth noting that compound 5 was previously isolated from the Hainan sponge Axinyssa variabilis, and its absolute configuration was determined by a combination of ROESY experiment and time dependent density functional theory-electronic circular dichroism (TDDFT-ECD) calculation [32]. In this work, we obtained a single crystal of 5, and X-ray diffraction (XRD) analysis on a suitable crystal of 5 by employing Ga Kα radiation (λ = 1.34139 Å) with small Flack parameter 0.02 (16) allowed not only the unambiguous definition of the planar structure as illustrated in Figure 3, but also the revision of its absolute configuration from 4S,5R,10S to 4R,5S,10S.
Figure 3.
Perspective Oak Ridge Thermal Ellipsoid Plot (ORTEP) drawing of the X-ray structure of 5.
Aware of the potent cytotoxicity exhibited by marine nitrogenous terpenoids, we performed in vitro biological evaluation of all the isolated metabolites on several tumor cell lines. The results (Table 2) showed that compounds 8, 10, and 11 exhibited strong cytotoxicity against human cancer cell line SNU-398 with IC50 values of 0.50, 2.15, and 0.50 μM, respectively. In addition, compound 8 also displayed broad cytotoxicity against the other three cancer cell lines, including A549, HT-29, and Capan-1, with IC50 values of 8.60, 3.35, and 1.98 μM, respectively. It is interesting to note that, although only three compounds showed cytotoxicity, they are all of the same bisabolane type. Therefore, a preliminary structure-activity relationship could be addressed, that is, the bisbolane skeleton might be good for activity, while regarding the inactive compounds 7 and 9, the terminal olefin or the formamide group might be harmful for activity. More diverse bisabolanes should be discovered and tested for cytotoxicity to support our proposal.
Table 2.
Cytotoxicity of compounds 1–19 against four human cancer cell lines.
| Compounds a | A549 | HT-29 | SNU-398 | Capan-1 |
|---|---|---|---|---|
| IC50 (μM) | ||||
| 8 | 8.60 ± 6.36 | 3.35 ± 3.12 | 0.50 ± 0.46 | 1.98 ± 1.76 |
| 10 | >50 | >50 | 2.15 ± 0.93 | >50 |
| 11 | >50 | >50 | 0.50 ± 0.35 | >50 |
| VCR | 10.13 nM | 0.23 nM | 0.04 nM | 0.30 nM |
a Compounds 1–7, 9, 12–19 were considered to be inactive with IC50 values of more than 50 μM; VCR: vincristine.
3. Discussion
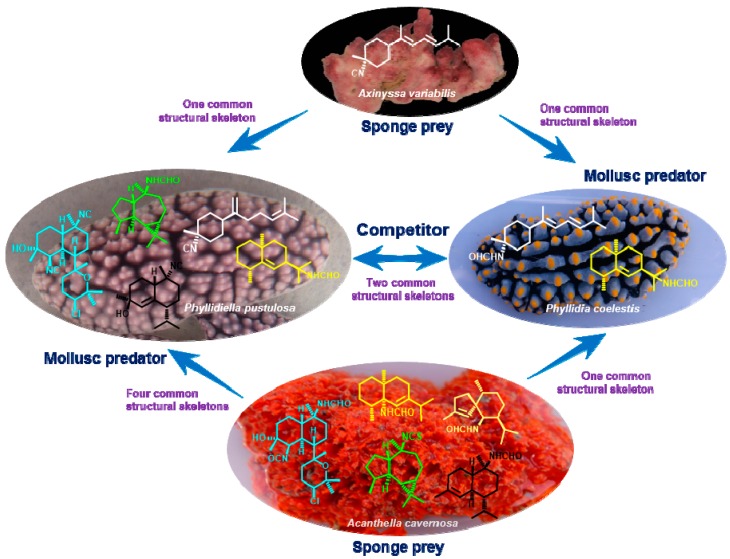
In recent years, several marine molluscs were found by our group to contain the same or similar secondary metabolites as those in marine corals or sponges, which was further proved to be due to the predator–prey relationship between these animals. For example, isoquinolinequinones were discovered from both the nudibranch Jorunna funebris and its sponge-prey Xestospongia sp. [49,50], while cladiellane-type diterpenoids were isolated from both the nudibranch Tritoniopsis elegans and its soft coral prey Cladiella krempfi [51]. In this study, similar results were observed by the chemical investigation of the three title animals. As shown in Figure 4, by comparison of the typical nitrogenous terpenoids in the two nudibranchs P. pustulosa and P. coelestis with those in the sponge A. cavernosa, four common structural skeletons were observed in both P. pustulosa and A. cavernosa, including cadinane, eudesmane, aromadendrane, and kalihinane, whereas one common eudesmane skeleton was found in all three animals. In addition, our previous chemical investigation of the marine sponge A. variabilis from the same water area in the South China Sea revealed the main secondary metabolites as bisabolene sesquiterpenoids [52], which was the common skeleton found in both P. pustulosa and P. coelestis (Figure 4). Therefore, on the basis of these research observations, we hold the belief that the two nudibranchs P. pustulosa and P. coelestis feed on the sponges A. cavernosa and A. variabilis and accumulate the useful dietary metabolites from the sponges, especially those toxic isocyanide derivatives, to be employed as their own chemical defensive agents for surviving in the harsh marine living environment. More intriguingly, it is obvious that one nudibranch can feed on various sponges to obtain diverse isocyanide metabolites, so as to use them as specially appointed chemical weapons on particular occasions.
Figure 4.
The common structural skeletons of the nudibranchs and their sponge-preys.
In summary, the chemical investigation of the two nudibranchs P. pustulosa and P. coelestis, as well as the sponge A. cavernosa, led to the isolation and determination of 19 nitrogenous terpenoids with high chemical diversity. In fact, a total of seven different chemical skeletons were observed: four cadinane-type sesquiterpenoids (1–4), two eudesmane-type sesquiterpenoids (5–6), five bisabolene-type sesquiterpenoids (7–11), two aromadendrane-type sesquiterpenoids (12 and 13), one pupukeanane-type sesquiterpenoid (14), one spiroaxane-type sesquiterpenoid (15), and four kalihinane-type diterpenoids (16–19). Their structures including relative stereochemistry were elucidated by comprehensive NMR analyses. The absolute configuration of two new metabolites (1 and 16) were tentatively assigned based on the biogenetic consideration, whereas that of the known compound 5 was revised by the XRD analysis. In bioassay, the bisabolane-type sesquiterpenoids 8, 10, and 11 displayed considerable cytotoxicity against several cancer cell lines, which is worth further pharmacological study. Further chemical ecological research on the basis of the predator–prey relationship to prove our hypothesis would be interesting to be conducted in the future.
4. Materials and Methods
4.1. General Experimental Procedures
Optical rotations were measured in CHCl3 on a Perkin-Elmer 241MC polarimeter (PerkinElmer Inc., Waltham, MA, USA). IR spectra were recorded on a Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA) with KBr pellets; peaks are reported in cm−1. 1D and 2D NMR spectra were measured on a Bruker DRX-400 or Bruker DRX-500 spectrometer (Bruker Biospin AG, Fällanden, Germany), using the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H NMR and CDCl3 (δC 77.00 ppm) for 13C NMR. Chemical shifts are expressed in δ (ppm) and coupling constants (J) in Hz. 1H and 13C NMR assignments were supported by 1H–1H COSY, HSQC, HMBC, and NOESY experiments. EIMS and HREIMS spectra were recorded on a Finnigan-MAT-95 mass spectrometer (FinniganMAT, San Jose, CA, USA). HRESIMS spectra were recorded on an Agilent G6250 Q-TOF (Agilent, Santa Clara, CA, USA). Reversed-phase (RP) HPLC purification was carried out on an Agilent 1260 series liquid chromatography equipped with a DAD G1315D detector at 210 and 254 nm and with a semi-preparative ODS-HG-5 column (5 μm, 250 × 9.4 mm). Commercial silica gel (Qingdao Haiyang Chemical Group Co., Ltd., Qingdao, China, 200–300 and 300–400 mesh) was used for column chromatography, and precoated silica gel plates (Yan Tai Zi Fu Chemical Group Co., Yantai, China, G60 F-254) were used for analytical Thin-layer chromatography (TLC). Spots were detected on TLC under UV light or by heating after spraying with anisaldehyde H2SO4 reagent. All the chemicals were obtained from commercial sources. All solvents used for column chromatography (CC) were of analytical grade, and solvents used for HPLC were of HPLC grade.
4.2. Biological Material, Extraction, and Isolation
4.2.1. Biological Material
The molluscs and sponges were collected using scuba at Xidao Island, Hainan Province, China, in March 2014, at a depth of −15 to −20 m, and identified by Professor Xiu-Bao Li from Hainan University. The voucher sample is deposited at the Shanghai Institute of Materia Medica, CAS.
4.2.2. Extraction and Isolation of 1–19
The lyophilized bodies of P. pustulosa (24 specimens, 11.1 g, dry weight) were carefully dissected into internal organs and mantle that were separately extracted by acetone using ultrasound. Filtration of the two homogenates gave an aqueous-Me2CO filtrate that was concentrated in vacuo to give a gummy residue. The residue was suspended in H2O and extracted sequentially with diethyl ether and n-BuOH. The mantle ether extract (431.3 mg) was subjected to a silica gel column eluting with light petroleum ether/diethyl ether gradient to yield 11 fractions (A-K), including pure compounds 3 (5.3 mg), 12 (2.6 mg), and 13 (1.0 mg). A less polar fraction E was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2:1:1), followed by silica gel CC (PE/Et2O, 100:1 to 50:1) to afford 7 (2.0 mg), 8 (2.2 mg), and 14 (2.6 mg). A middle polar fraction I was separated by a column of Sephadex LH-20 eluting with CHCl3/MeOH (1:1), followed by ODS CC (MeOH/H2O, 60:40) to afford 1 (1.5 mg) and 2 (1.0 mg). Fraction J was chromatographed over Sephadex LH-20 eluting with CHCl3/MeOH (1:1), followed by silica gel CC (PE/Et2O, 6:4), and was further purified by ODS CC (MeOH/H2O, 50:50) to yield 5 (2.0 mg) and 17 (3.1 mg). The digestive gland ether extract (60.0 mg) was purified by a silica gel column eluting with light petroleum ether/diethyl ether gradient, followed by a similar procedure as above, to give compounds 3 (1.3 mg), 5 (0.5 mg), 7 (1.3 mg), 8 (1.8 mg), 14 (0.9 mg), and 17 (1.9 mg).
The lyophilized bodies of P. coelestis (seven specimens, 25.5 g, dry weight) were extracted by acetone using ultrasound. The extracts of both internal organs and mantle were combined due to the similar TLC results, to give 700 mg extract. An approach similar to the abovementioned fractional method was applied to give a total of seven fractions (A–G). Compounds 8 (5.2 mg) and 9 (3.4 mg) were obtained directly from fractions B and G after purification by HPLC, respectively. Fraction B was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2:1:1), followed by HPLC purification to give compounds 10 (1.5 mg) and 11 (1.2 mg). Fraction F was treated by the same procedure as above to give compound 6 (1.7 mg).
The frozen A. cavernosa sponges (55 g, dry weight) were cut into pieces and extracted exhaustively with acetone at room temperature (6 × 2.0 L). The organic extract was evaporated to give a brown residue, which was then partitioned between H2O and Et2O. The upper layer was concentrated under reduced pressure to give a red residue (1.0 g). The resultant residue was separated into six fractions (A–F) by gradient silica gel column chromatography. The resulting fractions were then fractionated into sub-fractions by Sephadex LH-20. The sub-fraction F6 was purified by semi-preparative HPLC (70% MeOH to 100% MeOH in 20 min), yielding compounds 16 (4.0 mg), 18 (2.0 mg), and 19 (1.9 mg). The sub-fraction E4 of fraction E gave compounds 4 (3.1 mg), 6 (4.1 mg), and 15 (2.7 mg).
Xidaoisocyanate A (1), colorless oil, −3.6 (c 0.1, MeOH); for 1H and 13C NMR spectroscopic data, see Table 1; HREIMS: m/z calcd for C16H25NO [M]+: 247.1936; found: 247.1927.
Bisformamidokalihinol A (16), colorless oil, +19 (c 0.1, CHCl3); for 1H and 13C NMR spectroscopic data, see Table 1; HRESIMS: m/z calcd for C22H38N2O4Cl [M+H]+: 429.2515; found: 429.2522.
Axiriabiline A (5), colorless crystal, m.p. 105−107 °C, −123 (c 0.1, CHCl3); X-ray crystal data for compound 5: C16H27NO M = 249.38, orthorhombic, a = 11.5594(2) Å, b = 12.0694(2) Å, c = 21.2049(4) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 2958.40(9) Å3, T = 170.01 K, space group P2(1)2(1)2(1), Z = 8, 28095 reflections measured, 5616 independent reflections (Rint = 0.0569). The final R1 values were 0.0416 (I > 2σ(I)). The final wR(F2) values were 0.1051 (I > 2σ(I)). The final R1 values were 0.0446 (all data). The final wR(F2) values were 0.1081 (all data). The structure was solved by direct methods (SHELXS97) and refined using full-matrix least-squares difference Fourier techniques. All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were placed in idealized positions and refined as riding atoms with their related isotropic parameters. Crystallographic data (excluding structure factors) for the structure in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication no. CCDC 1880256. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
4.3. Bioassay Procedures
Cytotoxic Activity
Compounds 1–19 were evaluated for their cytotoxic activity against four human cancer cell lines (A549, HT-29, SNU-398, and Capan-1) using the sulforhodamine B (SRB, Sigma, St. Louis, MO, USA) method. Four cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cytotoxic activity in vitro was indicated in terms of IC50 (μM), that is, the concentration of a compound that inhibited the proliferation rate of tumor cells by 50% as compared to the untreated control cells. Vincristine was used as a reference drug.
Acknowledgments
This research work was financially supported by the National Key Research and Development Program of China (No. 2018YFC0310903), the National Natural Science Foundation of China (NSFC) (Nos. 81520108028 and 41676073), the NSFC/CNRS joint project (No. 81811530284), and the SKLDR/ SIMM Project (No. SIMM1705ZZ-01). X.-W. Li is thankful for the financial support of the “Youth Innovation Promotion Association” (No. 2016258) from the Chinese Academy of Sciences and the SA-SIBS Scholarship Program. M. Gavagnin thanks MIUR-ITALY PRIN2015 (Project No. 2015MSCKCE_004) for partial funding. We thank X.-B. Li from Hainan University for the taxonomic identification of the title animal material.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/17/1/56/s1, Figure S1. LREIMS spectrum of compound 1. Figure S2. HREIMS spectrum of compound 1. Figure S3. 1H NMR spectrum of compound 1 in CDCl3. Figure S4. 13C NMR spectrum of compound 1 in CDCl3. Figure S5. HSQC spectrum of compound 1 in CDCl3. Figure S6. 1H-1H COSY spectrum of compound 1 in CDCl3. Figure S7. HMBC spectrum of compound 1 in CDCl3. Figure S8. NOESY spectrum of compound 1 in CDCl3. Figure S9. HRESIMS spectrum of compound 16. Figure S10. IR spectrum of compound 16. Figure S11. 1H NMR spectrum of compound 16 in CDCl3. Figure S12. 13C NMR spectrum of compound 16 in CDCl3. Figure S13. HSQC spectrum of compound 16 in CDCl3. Figure S14. 1H-1H COSY spectrum of compound 16 in CDCl3. Figure S15. HMBC spectrum of compound 16 in CDCl3. Figure S16. NOESY spectrum of compound 16 in CDCl3.
Author Contributions
Y.-W.G. and X.-W.L. conceived and designed the experiments; Q.W., W.-T.C., S.-W.L., J.-Y.Y., and X.-J.H. performed the experiments; Q.W., W.-T.C., and Z.-H.M. analyzed the data; L.-G.Y. contributed materials; Y.-W.G., X.-W.L., Q.W., and W.-T.C. wrote the paper. M.G. and H.W. analyzed the chemical ecology relationship.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Manzo E., Ciavatta M.L., Gavagnin M., Mollo E., Guo Y.-W., Cimino G. Isocyanide terpene metabolites of Phyllidiella pustulosa, a nudibranch from the South China Sea. J. Nat. Prod. 2004;67:1701–1704. doi: 10.1021/np0400961. [DOI] [PubMed] [Google Scholar]
- 2.Jomoria T., Shibutani T., Ahmadi P., Suzuka T., Tanaka J. A New Isocyanosesquiterpene from the nudibranch Phyllidiella pustulosa. Nat. Prod. Commun. 2015;10:1913–1914. [PubMed] [Google Scholar]
- 3.Jaisamut S., Prabpai S., Tancharoen C., Yuenyongsawad S., Hannongbua S., Kongsaeree P., Plubrukarn A. Bridged tricyclic sesquiterpenes from the tubercle nudibranch Phyllidia coelestis Bergh. J. Nat. Prod. 2013;76:2158–2161. doi: 10.1021/np4007074. [DOI] [PubMed] [Google Scholar]
- 4.White A.M., Pierens G.K., Skinner-Adams T., Andrews K.T., Bernhardt P.V., Krenske E.H., Mollo E., Garson M.J. Antimalarial isocyano and isothiocyanato sesquiterpenes with tri- and bicyclic skeletons from the nudibranch Phyllidia ocellata. J. Nat. Prod. 2015;78:1422–1427. doi: 10.1021/acs.jnatprod.5b00354. [DOI] [PubMed] [Google Scholar]
- 5.Sim D.C., Wayan M.I., White A.M., Martiningsih N.W., Loh J.J.M., Cheney K.L., Garson M.J. New sesquiterpenoid isonitriles from three species of Phyllidid nudibranchs. Fitoterapia. 2017;126:69–73. doi: 10.1016/j.fitote.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Yasman Y., Edrada R.A., Wray V., Proksch P. New 9-thiocyanatopupukeanane sesquiterpenes from the nudibranch Phyllidia varicosa and its sponge-prey Axinyssa aculeata. J. Nat. Prod. 2003;66:1512–1514. doi: 10.1021/np030237j. [DOI] [PubMed] [Google Scholar]
- 7.Dumdei E.J., Flowers A.E., Garson M.J., Moore C.J. The biosynthesis of sesquiterpene isocyanides and isothiocyanates in the marine sponge Acanthella cavernosa (Dendy); Evidence for dietary transfer to the dorid nudibranch Phyllidiella pustulosa. Comp. Biochem. Phys. 1997;118:1385–1392. doi: 10.1016/S0300-9629(97)00051-0. [DOI] [Google Scholar]
- 8.Thompson J.E., Walker R.P., Wratten S.J., Faulkner D.J. A chemical defense mechanism for the nudibranch Cadlina luteomarginata. Tetrahedron. 1982;38:1865–1873. doi: 10.1016/0040-4020(82)80035-5. [DOI] [Google Scholar]
- 9.Gulavita N.K., de Silva E.D., Hagadone M.R., Karuso P., Scheuer P.J. Nitrogenous bisabolene sesquiterpenes from marine invertebrates. J. Org. Chem. 1986;51:5136–5139. doi: 10.1021/jo00376a015. [DOI] [Google Scholar]
- 10.Fusetani N., Wolstenholme H.J., Matsunaga S. Two new sesquiterpene isonitriles from the nudibranch, Phyllida pustulosa. Tetrahedron Lett. 1991;32:7291–7294. doi: 10.1016/0040-4039(91)80501-V. [DOI] [Google Scholar]
- 11.Kassuhlke K.E., Potts B.C.M., Faulkner D.J. New nitrogenous sesquiterpenes from two Philippine nudibranchs, Phyllidia pustulosa and P. varicosa, and from a Palauan sponge, Halichondria cf. lendenfeldi. J. Org. Chem. 1991;56:3747–3750. doi: 10.1021/jo00011a065. [DOI] [Google Scholar]
- 12.Hirota H., Okino T., Yoshimura E., Fusetani N. Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron. 1998;54:13971–13980. doi: 10.1016/S0040-4020(98)00867-9. [DOI] [Google Scholar]
- 13.Wright A.D. GC-MS and NMR analysis of Phyllidiella pustulosa and one of its dietary sources, the sponge Phakellia carduus. Comp. Biochem. Physiol. 2003;134:307–313. doi: 10.1016/S1095-6433(02)00265-9. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., Li N., Jiao W.-H., Wang R.-P., Peng Y., Qi S.-H., Song S.-J., Chen W.-S., Lin H.-W. Antifouling and cytotoxic constituents from the South China Sea sponge Acanthella cavernosa. Tetrahedron. 2012;68:2876–2883. doi: 10.1016/j.tet.2012.01.084. [DOI] [Google Scholar]
- 15.Xu Y., Lang J.-H., Jiao W.-H., Wang R.-P., Peng Y., Song S.-J., Zhang B.-H., Lin H.-W. Formamido-diterpenes from the South China Sea sponge Acanthella cavernosa. Mar. Drugs. 2012;10:1445–1458. doi: 10.3390/md10071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright A.D., McCluskey A., Robertson M.J., MacGregor K.A., Gordon C.P., Guenther J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org. Biomol. Chem. 2011;9:400–407. doi: 10.1039/C0OB00326C. [DOI] [PubMed] [Google Scholar]
- 17.Sun J.-Z., Chen K.-S., Yao L.-G., Liu H.-L., Guo Y.-W. A new kalihinol diterpene from the Hainan sponge Acanthella sp. Arch. Pharm. Res. 2009;32:1581–1584. doi: 10.1007/s12272-009-2110-4. [DOI] [PubMed] [Google Scholar]
- 18.Yan X.-H., Zhu X.-Z., Yu J.-L., Jin D.-Z., Guo Y.-W., Mollo E., Cimino G. 3-Oxo-axisonitrile-3, a new sesquiterpene isocyanide from the Chinese marine sponge Acanthella sp. J. Asian Nat. Prod. Res. 2006;8:579–584. doi: 10.1080/10286020410001721096. [DOI] [PubMed] [Google Scholar]
- 19.Miyaoka H., Shimomura M., Kimura H., Yamada Y. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge Acanthella sp. Tetrahedron. 1998;54:13467–13474. doi: 10.1016/S0040-4020(98)00818-7. [DOI] [Google Scholar]
- 20.Angerhofer C.K., Pezzuto J.M., König G.M., Wright A.D., Sticher O. Antimalarial activity of sesquiterpenes from the marine sponge Acanthella klethra. J. Nat. Prod. 1992;55:1787–1789. doi: 10.1021/np50090a014. [DOI] [PubMed] [Google Scholar]
- 21.Trimurtulu G., Faulkner D.J. Six new diterpene isonitriles from the sponge Acanthella cavernosa. J. Nat. Prod. 1994;57:501–506. doi: 10.1021/np50106a009. [DOI] [PubMed] [Google Scholar]
- 22.Capon R.J., MacLeod J.K. New isothiocyanate sesquiterpenes from the Australian marine sponge Acanthella pulcherrima. Aust. J. Chem. 1988;41:979–983. doi: 10.1071/CH9880979. [DOI] [Google Scholar]
- 23.Yang L.-H., Lee O.O., Jin T., Li X.-C., Qian P.-Y. Antifouling properties of 10beta-formamidokalihinol-A and kalihinol A isolated from the marine sponge Acanthella cavernosa. Biofouling. 2006;22:23–32. doi: 10.1080/08927010500498623. [DOI] [PubMed] [Google Scholar]
- 24.Nogata Y., Yoshimura E., Shinshima K., Kitano Y., Sakaguchi I. Antifouling substances against larvae of the barnacle Balanus amphitrite from the marine sponge, Acanthella cavernosa. Biofouling. 2003;19:193–196. doi: 10.1080/0892701031000065963. [DOI] [PubMed] [Google Scholar]
- 25.Okino T., Yoshimura E., Hirota H., Fusetani N. New antifouling kalihipyrans from the marine sponge Acanthella cavernosa. J. Nat. Prod. 1996;59:1081–1083. doi: 10.1021/np960496r. [DOI] [Google Scholar]
- 26.Fusetani N., Hiroto H., Okino T., Tomono Y., Yoshimura E. Antifouling activity of isocyanoterpenoids and related compounds isolated from a marine sponge and nudibranchs. J. Nat. Toxins. 1996;5:249–259. [Google Scholar]
- 27.Hirota H., Tomono Y., Fusetani N. Terpenoids with antifouling activity against barnacle larvae from the marine sponge Acanthella cavernosa. Tetrahedron. 1996;52:2359–2368. doi: 10.1016/0040-4020(95)01079-3. [DOI] [Google Scholar]
- 28.Nishikawa K., Umezawa T., Garson M.J., Matsuda F. Confirmation of the configuration of 10-isothiocyanato-4-cadinene diastereomers through synthesis. J. Nat. Prod. 2012;75:2232–2235. doi: 10.1021/np300439e. [DOI] [PubMed] [Google Scholar]
- 29.Miyaoka H., Abe Y., Sekiya N., Mitome H., Kawashima E. Total synthesis of antimalarial diterpenoid (+)-kalihinol A. Chem. Commun. 2012;48:901–903. doi: 10.1039/C1CC16468F. [DOI] [PubMed] [Google Scholar]
- 30.White R.D., Keaney G.F., Slown C.D., Wood J.L. Total synthesis of (+/-)-kalihinol C. Org. Lett. 2004;6:1123–1126. doi: 10.1021/ol049918b. [DOI] [PubMed] [Google Scholar]
- 31.White R.D., Wood J.L. Progress toward the total synthesis of kalihinane diterpenoids. Org. Lett. 2001;3:1825–1827. doi: 10.1021/ol015828k. [DOI] [PubMed] [Google Scholar]
- 32.Li X.-W., Chen S.-H., Ye F., Mollo E., Zhu W.-L., Liu H.-L., Guo Y.-W. Axiriabilines A–D, uncommon nitrogenous eudesmane-type sesquiterpenes from the Hainan sponge Axinyssa variabilis. Tetrahedron. 2017;73:5239–5243. doi: 10.1016/j.tet.2017.07.027. [DOI] [Google Scholar]
- 33.Liu H.-L., Xue D.-Q., Chen S.-H., Li X.-W., Guo Y.-W. New highly oxidized formamidobisabolene-derived sesquiterpenes from a Hainan sponge Axinyssa variabilis. Helv. Chim. Acta. 2016;99:650–653. doi: 10.1002/hlca.201600077. [DOI] [Google Scholar]
- 34.Wu Q., Nay B., Yang M., Ni Y., Wang H., Yao L., Li X. Marine sponges of the genus Stelletta as promising drug sources: Chemical and biological aspects. Acta Pharm. Sin. B. 2018 doi: 10.1016/j.apsb.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prawat H., Mahidol C., Kaweetripob W., Prachyawarakorn V., Tuntiwachwuttikul P., Ruchirawat S. Sesquiterpene isocyanides, isothiocyanates, thiocyanates, and formamides from the Thai sponge Halichondria sp. Tetrahedron. 2016;72:4222–4229. doi: 10.1016/j.tet.2016.05.060. [DOI] [Google Scholar]
- 36.Clark R.J., Stapleton B.L., Garson M.J. New isocyano and isothiocyanato terpene metabolites from the tropical marine sponge Acanthella cavernosa. Tetrahedron. 2000;56:3071–3076. doi: 10.1016/S0040-4020(00)00226-X. [DOI] [Google Scholar]
- 37.Nishikawa K., Nakahara H., Shirokura Y., Nogata Y., Yoshimura E., Umezawa T., Okino T., Matsuda F. Total synthesis of 10-isocyano-4-cadinene and determination of its absolute configuration. Org. Lett. 2010;12:904–907. doi: 10.1021/ol9027336. [DOI] [PubMed] [Google Scholar]
- 38.Nishikawa K., Nakahara H., Shirokura Y., Nogata Y., Yoshimura E., Umezawa T., Okino T., Matsuda F. Total synthesis of 10-isocyano-4-cadinene and its stereoisomers and evaluations of antifouling activities. J. Org. Chem. 2011;76:6558–6573. doi: 10.1021/jo2008109. [DOI] [PubMed] [Google Scholar]
- 39.Petrichtcheva N.V., Duque C., Dueñas A., Zea S., Hara N., Fujimoto Y. New nitrogenous eudesmane-type compounds isolated from the Caribbean sponge Axinyssa ambrosia. J. Nat. Prod. 2002;65:851–855. doi: 10.1021/np0104471. [DOI] [PubMed] [Google Scholar]
- 40.Ciminiello P., Fattorusso E., Magno S., Mayol L. Nitrogenous sesquiterpenes based on allo-aromadendrane and epi-eudesmane skeletons from the marine sponge Axinella cannabina. Can. J. Chem. 1987;65:518–522. doi: 10.1139/v87-090. [DOI] [Google Scholar]
- 41.Zubía E., Ortega M.J., Carballo J.L. Sesquiterpenes from the sponge Axinyssa isabela. J. Nat. Prod. 2008;71:2004–2010. doi: 10.1021/np800465n. [DOI] [PubMed] [Google Scholar]
- 42.Jumaryatno P., Stapleton B.L., Hooper J.N.A., Brecknell D.J., Blanchfield J.T., Garson M.J. A comparison of sesquiterpene scaffolds across different populations of the tropical marine sponge Acanthella cavernosa. J. Nat. Prod. 2007;70:1725–1730. doi: 10.1021/np070156d. [DOI] [PubMed] [Google Scholar]
- 43.Prawat H., Mahidol C., Wittayalai S., Intachote P., Kanchanapoom T., Ruchirawat S. Nitrogenous sesquiterpenes from the Thai marine sponge Halichondria sp. Tetrahedron. 2011;67:5651–5655. doi: 10.1016/j.tet.2011.05.094. [DOI] [Google Scholar]
- 44.Zhang W., Gavagnin M., Guo Y.-W., Mollo E., Ghiselinc M.T., Cimino G. Terpenoid metabolites of the nudibranch Hexabranchus sanguineus from the South China Sea. Tetrahedron. 2007;63:4725–4729. doi: 10.1016/j.tet.2007.03.082. [DOI] [Google Scholar]
- 45.Okino T., Yoshimura E., Hirota H., Fusetani N. Antifouling kalihinenes from the marine sponge Acanthella cavernosa. Tetrahedron Lett. 1995;36:8637–8640. doi: 10.1016/0040-4039(95)01861-B. [DOI] [Google Scholar]
- 46.Zubía E., Ortega M.J., Hernández-Guerrero C.J., Carballo J.L. Isothiocyanate sesquiterpenes from a sponge of the genus Axinyssa. J. Nat. Prod. 2008;71:608–614. doi: 10.1021/np070593s. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez J., Nieto R.M., Hunter L.M., Diaz M.C., Crews P., Lobkovsky E., Clardy J. Variation among known kalihinol and new kalihinene diterpenes from the sponge Acanthella cavernosa. Tetrahedron. 1994;50:11079–11090. doi: 10.1016/S0040-4020(01)89411-4. [DOI] [Google Scholar]
- 48.Shimomura M., Miyaoka H., Yamada Y. Absolute configuration of marine diterpenoid kalihinol A. Tetrahedron Lett. 1999;40:8015–8017. doi: 10.1016/S0040-4039(99)01664-0. [DOI] [Google Scholar]
- 49.He W.-F., Li Y., Feng M.-T., Gavagnin M., Mollo E., Mao S.C., Guo Y.-W. New isoquinolinequinone alkaloids from the South China Sea nudibranch Jorunna funebris and its possible sponge-prey Xestospongia sp. Fitoterapia. 2014;96:109–114. doi: 10.1016/j.fitote.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Huang R.-Y., Chen W.-T., Kurtán T., Mándi A., Ding J., Li J., Li X.-W., Guo Y.-W. Bioactive isoquinolinequinone alkaloids from the South China Sea nudibranch Jorunna funebris and its sponge-prey Xestospongia sp. Future Med. Chem. 2016;8:17–27. doi: 10.4155/fmc.15.169. [DOI] [PubMed] [Google Scholar]
- 51.Ciavatta M.L., Manzo E., Mollo E., Mattia C.A., Tedesco C., Irace C., Guo Y.-W., Li X.-B., Cimino G., Gavagnin M. Tritoniopsins A–D, cladiellane-based diterpenes from the South China Sea nudibranch Tritoniopsis elegans and its prey Cladiella krempfi. J. Nat. Prod. 2011;74:1902–1907. doi: 10.1021/np200342k. [DOI] [PubMed] [Google Scholar]
- 52.Mao S.-C., Guo Y.-W., van Soest R., Cimino G. New nitrogenous bisabolene-type sesquiterpenes from a Hainan sponge Axinyss aff. Variabilis. Helv. Chim. Acta. 2007;90:588–593. doi: 10.1002/hlca.200790059. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.