Abstract
Germline mutations in succinate dehydrogenase subunit B and D (SDHB and SDHD) are predisposed to hereditary paraganglioma (PGL) and pheochromocytoma (PHEO). The phenotype of pathogenic variants varies according to the causative gene. In this retrospective study, we estimate the mortality of a nationwide cohort of SDHB variant carriers and that of a large cohort of SDHD variant carriers and compare it to the mortality of a matched cohort of the general Dutch population. A total of 192 SDHB variant carriers and 232 SDHD variant carriers were included in this study. The Standard Mortality Ratio (SMR) for SDHB mutation carriers was 1.89, increasing to 2.88 in carriers affected by PGL. For SDHD variant carriers the SMR was 0.93 and 1.06 in affected carriers. Compared to the general population, mortality seems to be increased in SDHB variant carriers, especially in those affected by PGL. In SDHD variant carriers, the mortality is comparable to that of the general Dutch population, even if they are affected by PGL. This insight emphasizes the significance of DNA-testing in all PGL and PHEO patients, since different clinical risks may warrant gene-specific management strategies.
Keywords: SDHB, SDHD, mortality, paraganglioma, pheochromocytoma
1. Introduction
Paragangliomas (PGL) are rare tumors that originate from cells of neural crest origin in the paraganglia associated with the autonomic nervous system. PGL can be subdivided into head and neck paragangliomas (HNPGL), pheochromocytomas (PHEO), and thoracic and abdominal extra-adrenal PGL (sympathetic PGL; sPGL). An increasing number of genes are associated with hereditary PGL/PHEO. Most frequently, hereditary PGL syndrome is caused by genes encoding subunits or cofactors of succinate dehydrogenase (SDH), such as SDHA/B/C/D/AF2. Other associated genes are RET, NF1, VHL, HIF2A, FH, TMEM127, and MAX [1,2]. In the Netherlands, pathogenic variants in SDHD are the most prevalent cause of PGL syndrome, followed by variants in SDHB and SDHA [3,4]. Although all SDHx genes encode subunits of the same SDH complex and pathogenic variants all disrupt its enzymatic function, different genes are associated with different phenotypes. The reported lifelong penetrance of pathogenic SDHB variants (22–42%) [5,6] is considerably lower than the penetrance of paternally inherited SDHD mutations (88–100%) [7,8,9,10].
When pathogenic SDHB variants cause disease, the clinical outcome is reported to be less favorable than that in SDHD-linked disease. SDHB mutation carriers are reported to develop metastatic PGL more frequently and patients with metastatic disease associated with SDHB variants are reported to have a poor 5-year survival rate compared to patients with metastatic disease associated with other causative genes [11]. The mortality of SDHB variant carriers is currently unknown [12]. In this study we estimate the mortality for a nationwide cohort of SDHB variant carriers and compare this risk with the mortality of SDHD variant carriers and that of the general Dutch population.
2. Subjects and Methods
2.1. Eligibility Criteria
The cohort of pathogenic germline variant carriers (hereafter variants) in SDHB included in this study has been described in detail previously [6,13]. The mortality of this nationwide SDHB-linked cohort was compared with the mortality of the general Dutch population and with the mortality of an updated cohort of SDHD variant carriers, which has been described previously [12]. Only SDHD variant carriers with paternal inheritance were included. Carriers of SDHD variants were identified using the database of the Laboratory for Diagnostic Genome Analysis (LDGA) at the Leiden University Medical Center (LUMC), a tertiary referral center for patients with PGL. Screening for SDH variants was performed in all persons diagnosed with PGL who agreed to genetic testing.
Screening for SDHB and SDHD variants was performed by direct sequencing of peripheral blood leucocytes using the Sanger method on an ABI 377 Genetic Analyzer (Applied Biosystems, Carlsbad, California) and by multiplex ligation-dependent probe amplification (MLPA) using the P226 MLPA kit (MRC Holland, Amsterdam, the Netherlands). Family members of index patients were tested for the family-specific variant. All variants described in this study were submitted to the Leiden Open (source) Variation Database LOVD database (http://chromium.liacs.nl/lovd_sdh). SDHB and SDHD germline variants were classified according to the international guidelines put forth by Plon et al. [14]. SDHD variants were described using the reference sequence NG_012340.1 covering SDHB transcript NM_003000.2, and NG_012337.1 covering SDHD transcript NM_003002.2, available from the TCA Cycle Gene Variant Database LOVD database. In this manuscript we report pathogenic or likely pathogenic variants, including missense mutations in highly conserved regions that are determined to be likely pathogenic as germline mutations based partly on mutation prediction analyses. Information on amino acid conservation can be found in the LOVD database (http://chromium.liacs.nl/lovd_sdh). Further information including mutation prediction analyses can be obtained on request.
The study was approved by the Medical Ethics Committee of the Leiden University Medical Center; participating centers complied with their local Medical Ethics Committee requirements. Written informed consent was obtained from the parents/guardians of individuals under 18 years of age.
2.2. Clinical Characteristics
Clinical data were retrieved from medical records. Pathogenic variant carriers were investigated for occurrences of PGL and/or PHEO according to the structured protocols used for standard care in the Netherlands for PGL or PHEO patients [15,16]. Patients were offered clinical surveillance for PGL/PHEO at the departments of otorhinolaryngology and endocrinology. For asymptomatic SDHB and SDHD variant carriers older than 18 years of age, surveillance consisted of magnetic resonance imaging (MRI) of the head and neck region once every 2–3 years, and MRI or computed tomography (CT) scans of the thorax, abdomen, and pelvis once every 1–2 years in SDHB variant carriers. Biochemical screening was performed annually on SDHB variant carriers, and every 1–2 years on SDHD variant carriers. This screening measured levels of (nor)epinephrine, vanillylmandelic acid, dopamine, (nor)metanephrine, and/or 3-methoxytyramine in two 24-hour urinary samples (depending on the Academic Center in which urinary measurement(s) were performed), and/or plasma free (nor)metanephrine and 3-methoxytyramine. In cases of excessive catecholamine secretion (i.e., any value above the upper reference limit), radiological assessment by MRI or CT scans of the thorax, abdomen, and pelvis, and/or 123I metaiodobenzylguanidine (MIBG) scans, positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG PET) scans, 18F-L-dihydroxyphenylalanine (18F-DOPA) PET-scans, or positron emission tomography with 1,4,7,10-tetraazacyclododecane-NI, NII, NIII, NIIII-tetraacetic acid (D)-Phe1-thy3-octreotide (68Ga-DOTATOC PET) scans were performed to identify potential sources of excessive catecholamine production. In cases without available tumor histology, tumors were classified as paraganglionic based on their specific characteristics in CT and/or MRI scans. When in doubt, additional nuclear medicine imaging studies were performed in order to confirm the diagnosis. At the time of this study, there were no national, structured protocols for surveillance in SDHB mutation carriers younger than 18 years of age. Therefore, the method and interval of surveillance in this age category varied between centers.
In case of a diagnosis of HNPGL, PHEO or sPGL, intensified surveillance or treatment was offered. Surgical resection was generally the preferred treatment option for PHEO or sPGL. In cases of HNPGL, the management strategy was guided by clinical symptoms, tumor characteristics such as localization, size, and growth rate, and patient characteristics such as age, comorbidity, and patient preferences. A wait and scan policy, radiotherapy, or surgical resection were possible treatment options.
2.3. Mortality and Survival
For this study, follow-up data from SDHB and SDHD variant carriers were included from the date of the DNA test. In cases where clinical follow-up was available for the period before the DNA test, this period was not considered in the mortality analysis because it would have introduced immortal time bias [17]. Follow-up was defined as the time between the DNA test and the last clinical follow-up date before the end of the study period. Patients who were alive at the last clinical follow-up were classified as alive. Follow-up ended at the end of the study period, at the date of death or, in case of emigration, at the date of emigration [13]. To compare mortality between SDHB and SDHD variant carriers and the general population, the standardized mortality ratio (SMR) was estimated. Mortality rates for the Dutch population were obtained from Statistics Netherlands (CBS, The Netherlands) [18], using rates stratified by sex, age (per 1 year) and date (1-year periods). The SMR was calculated by dividing the observed number of deaths in the SDHB and SDHD cohorts. The expected number of deaths was calculated as the sum of the stratified number of expected deaths (stratum-specific mortality rates from the general population times follow-up time at risk).
Survival was graphically displayed for SDHB and SDHD variant carriers by plotting survival in the carriers against the expected survival based on matched data from the general population. STATA 14.0 (Stata Corp, Texas, USA) was used for statistical analysis.
3. Results
In total, 192 SDHB variant carriers and 232 SDHD variant carriers were included in this study. The clinical characteristics are depicted in Table 1. The mean age at identification of the pathogenic gene variant was 46 years (range 9–77) in SDHB variant carriers and 44 years (range 16–73) in SDHD variant carriers. In total, 53 SDHB variant carriers (27.6%) and 198 SDHD variant carriers (85.3%) were diagnosed with HNPGL, either at time of presentation or during follow-up. Four SDHB patients (2.1%) and 16 SDHD patients (6.9%) developed PHEO and 26 SDHB patients (13.5%) and 18 (7.8%) SDHD patients developed sPGL. Malignant PGL, defined as metastatic PGL in non-paraganglionic tissue, were diagnosed in 14 SDHB (7.3%) and four SDHD patients (1.7%). Most SDHB variant carriers (110/193; 57.3%) were not affected at the time of DNA testing or during follow-up. In contrast, the majority of SDHD variant carriers was diagnosed with SDHD-associated disease (203/232; 87.5%). Details of the specific SDHB and SDHD variants are included in Appendix A.
Table 1.
Clinical characteristics of carriers of pathogenic variants in succinate dehydrogenase subunits B and D (SDHB and SDHD).
| Clinical Characteristics |
SDHB n = 192 |
SDHD n = 232 |
|---|---|---|
| Male (%)/female (%) | 81 (42.2)/111 (57.8) | 123 (53.0)/109 (47.0) |
| Mean age at genetic testing | 46 years (range 9–77) | 44 years (range 16–73) |
| HNPGL (%) | 53 (27.6) | 198 (85.3) |
| sPGL (%) | 26 (13.5) | 18 (7.8) |
| Pheochromocytoma (%) | 4 (2.1) | 16 (6.9) |
| Malignant PGL (%) | 14 (7.3) | 4 (1.7) |
| Unaffected (%) | 110 (57.3) | 30 (12.9) |
HNPGL = head and neck paraganglioma, sPGL = sympathetic paraganglioma, PGL = paraganglioma.
Mortality and SMR
Mortality data were available for all SDHB and SDHD variant carriers. The mean follow-up period was 3.0 (range 0–14.5) and 5.1 (range 0–12.5) years, respectively, for SDHB and SDHD variant carriers. In total, 6/192 (3.1%) SDHB variant carriers died at age 32, 37, 49, 52, 62, and 63. In three patients the cause of death was directly related to progressive PGL disease. In contrast, 5/232 (2.2%) SDHD variant carriers died at age 41, 43, 71, 71, and 74. In two cases the cause of death was most likely associated with PGL disease. Clinical characteristics of the variant carriers who died during the study period are listed in Table 2.
Table 2.
Details of six SDHB and five SDHD variant carriers who died during follow-up.
| Sex | Mutation | Predicted Protein Change | Location of PGL | Age at PGL Diagnosis (years) | Age at Diagnosis of Malignant Disease (years) | Age at Death (years) | Location of Metastases | Cause of Death |
|---|---|---|---|---|---|---|---|---|
| M |
SDHB exon 3 deletion |
p.? | Presacral | 28 | 28 | 32 | Bone | Progressive malignant PGL |
| F |
SDHB c.654G > A |
p.(Trp218*) | Bladder | 19 | 58 | 62 | Lymph nodes, bone | Progressive malignant PGL |
| F |
SDHB exon 3 deletion |
p.? | Para-vertebral abdominal | 33 | 33 | 37 | Lymph nodes, bone | Progressive malignant PGL |
| F |
SDHB c.727T > A |
p.(Cys243Ser) | Retroperitoneal (para-aortic) | 52 | 55 | 63 | Bone | Myocardial infarction, heart failure and acute respiratory distress syndrome |
| F |
SDHB c.423 + 1G > A |
p.? | n.a. | 49 | n.a. | 52 | n.a. | Respiratory insufficiency due to lung bleeding after chemoradiotherapy for lung cancer |
| F |
SDHB c.423 + 1G > A |
p.? | n.a. | 42 | n.a. | 49 | n.a. | Metastatic breast cancer |
| F |
SDHD c.274G > T |
p.(Asp92Tyr) | Bladder | 42 | 42 | 43 | Lymph nodes, bone marrow | Progressive malignant PGL |
| F |
SDHD c.274G > T |
p.(Asp92Tyr) | Mediastinal | 67 | 67 | 74 | Lymph nodes, bone | Unknown, however the patient was known to have progressive malignant PGL |
| F |
SDHD c.274G > T |
p.(Asp92Tyr) | Bilateral CBT, VBT | 55 | n.a. | 71 | n.a. | Cardiac arrest |
| F |
SDHD c.242C > T |
p.(Pro81Leu) | CBT | 38 | n.a. | 41 | n.a. | Breast cancer |
| M |
SDHD c.274G > T |
p.(Asp92Tyr) | CBT, jugular PGL, retroperitoneal | 52 | n.a. | 71 | n.a. | Prostate cancer |
PGL = paraganglioma, CBT = carotid body tumor, VBT = vagal body tumor, n.a. = not applicable.
A direct comparison between SDHB and SDHD variant carriers is hampered by the limited number of carriers and the heterogeneity between both groups. We performed an adjusted Poisson regression, adjusting for age, sex, and calendar time. The rate ratio comparing SDHB to SDHD variant carriers was 0.48 (95% confidence interval (CI) 0.15–1.62). However, the power for this analysis is low. As both groups have few events, we cannot draw conclusions from the non-significant p-value.
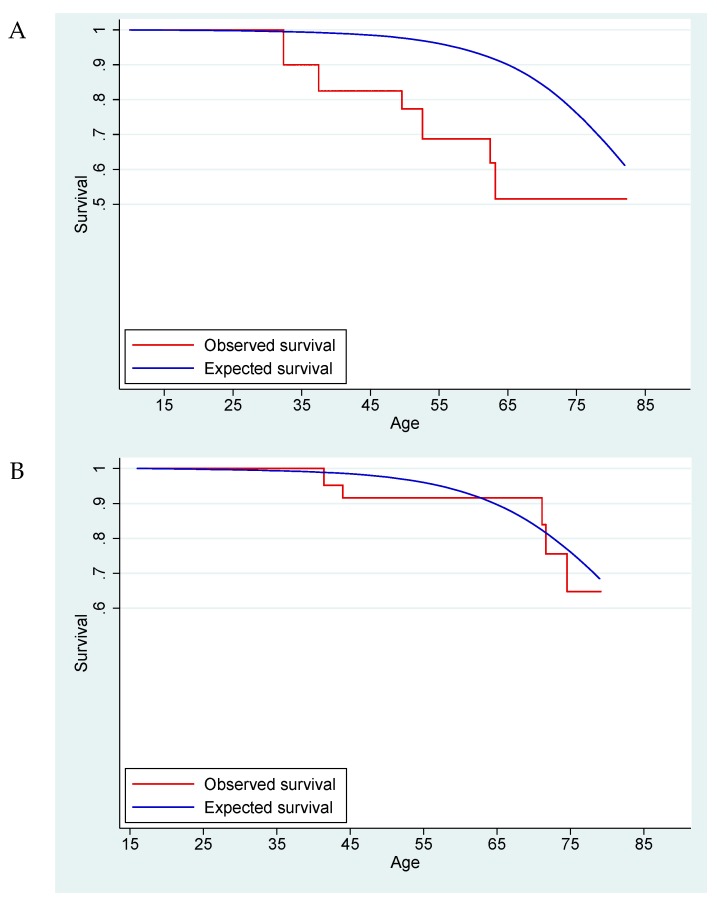
For the comparison of both the SDHB- and SDHD-linked cohorts with normative data of the Dutch population, a total of 1781 person-years were available (SDHB 590 and SDHD 1191 years, respectively). The SMR for SDHB mutation carriers was 1.89 (95% confidence interval (CI) 0.85–4.21) (Figure 1). A separate analysis including only symptomatic SDHB variant carriers—i.e., those with manifest disease—showed a higher SMR at 2.88 (95% CI 1.08–7.68). These results suggest an increased mortality risk for SDHB variant carriers compared to the general Dutch population, especially for carriers affected by SDHB-associated disease. For SDHD variant carriers, the SMR was 0.93 (95% CI 0.39–2.23), increasing only slightly to 1.06 (95% CI 0.44–2.54) in affected carriers, suggesting that mortality is not increased in SDHD variant carriers.
Figure 1.
The Kaplan–Meier survival curve for SDHB variant carriers (A) and SDHD variant carriers (B) compared with the expected survival based on the general Dutch population.
4. Discussion
In this study we estimated the mortality for SDHB and SDHD pathogenic variant carriers. Whereas the mortality for SDHD variant carriers is comparable with a matched cohort of the general Dutch population (SMR = 0.93), SDHB variant carriers show a higher mortality (SMR = 1.89, meaning a 1.89 times higher risk of death than the matched cohort of the general Dutch population).
These mortality ratios should be interpreted with some caution. First, not all deaths in our cohort are directly attributable to PGL-linked disease. However, a comparison is made with the mortality of the general Dutch population. Therefore, eliminating other causes of death would be inappropriate.
Second, even though the SDHB variant carriers represent a nationwide cohort, PGL is a rare disease and patient numbers are inevitably limited. As a result, the study estimates have broad confidence intervals. In addition, the follow-up of the start of this study is defined as the time of DNA testing and not PGL/PHEO diagnosis. As the genetic causes of hereditary PGL syndromes have been determined only recently, follow-up is relatively limited. However, the differences between SDHB and SDHD variant carriers are remarkable, all the more so when considering that SDHD variants are characterized by a high penetrance of PGL (88–100%), and SDHB variants by a much lower lifelong PGL risk (22–42%) [5,6,7,8,9,10]. In SDHD variant carriers, the occurrence of often multiple associated (HN)PGL seems to have no clear impact on survival [12]. In contrast, SDHB variant carriers seem to face increased mortality even though they are under more intensive surveillance and, in our study, have a shorter follow-up. This decreased survival of SDHB variant carriers is attributable to the higher mortality of affected SDHB patients (SMR = 2.88). Moreover, the majority of deceased SDHB-linked patients suffered from progressive malignant PGL (Table 2). Unaffected SDHB variant carriers have a mortality ratio that is more in line with the general Dutch population (SMR = 1.12).
It is intriguing that the causative gene seems to determine variation in the prognoses for PGL/PHEO patients, even though pathogenic variants in SDHB and SDHD cause PGL/PHEO syndrome through defects in the same protein complex (succinate dehydrogenase, SDH). We speculate that this could be the result of intrinsic properties of the SDHB-associated PGL/PHEO syndrome, a deleterious effect of SDHB variants on other factors that influence survival, or differences between SDHB and SDHD variants in the potential to induce other types of malignancy. Interestingly, other types of malignancies (i.e. prostate cancer, lung cancer, breast cancer) are listed as causes of death both in the SDHB- and SDHD-linked cohorts (see Table 2). Although the SDHx-associated tumor spectrum is expanding, none of these malignancies have been directly linked to SDHB or SDHD variants. Even so, SDHD and/or SDHB variants could alter the susceptibility to certain types of malignancy other than PGL/PHEO. Indeed, 0.25% and 0.05% of breast cancer exomes carry somatic SDHB and SDHD variants, respectively [19,20].
The finding that all deceased SDHB-related PGL patients had metastatic PGL suggests that the occurrence of metastatic disease in SDHB-linked PGL syndrome particularly impacts survival, and that metastases may be either more prevalent in SDHB-linked cases, as suggested before [7,10,21,22,23,24], or more aggressive than metastatic diseases associated with other SDHx genes, a finding that is in line with the very poor 5-year survival rate of SDHB-linked metastatic disease reported by Amar et al. [11]. Another explanation might be that metastases from sPGL behave more aggressively than those of parasympathetic HNPGL, and that these sPGL are more prevalent in SDHB-linked disease [13,25]. Indeed, the PGL patients that died of progressive PGL disease both in the SDHB- and SDHD-linked cohorts all suffered from primary sPGL tumors.
The difference in the mortality between SDHB and SDHD variant carriers is another clear indication that causative genetic alteration is of critical importance to the outcome and risks of an individual PGL patient. This is important in counseling PGL/PHEO patients, but may also warrant gene-specific management strategies for PGL patients. In the present study, however, we did not evaluate the effect of PGL follow-up protocols or treatment on survival. From the patients that died of SDHB-related disease (n = 3), two already had proven metastatic disease at the time of diagnosis. Surgical resection with tumor-free margins seems to be a logical treatment strategy when trying to avoid progression of the disease, but there may be undetected metastases already present at the time of surgery [26,27]. The observation that the higher mortality associated with SDHB variant carriers seems to be attributable to patients that are affected by metastatic sPGL may warrant a more aggressive surgical strategy towards sPGL tumors in SDHB-linked patients. The risk of the malignant transformation of an sPGL tumor left untreated is, however, unknown. This unknown risk of disease progression must be weighed against the risk of surgical morbidity [28].
5. Conclusion
In conclusion, compared to a matched cohort of the general population, mortality is increased in SDHB variant carriers but not in SDHD variant carriers. This insight emphasizes the significance of DNA-testing; gene-specific clinical risks may warrant tailored management strategies. Further research is necessary to demonstrate the effect of (early) intervention of PGL/PHEO on mortality rates, especially in SDHB variant carriers.
Appendix A. SDHB Variants and SDHD Variants
| DNA Mutation | Predicted Protein Change | Number of Subjects (%) |
| Exon 3 deletion | p.? | 59 (30.7) |
| c.423 + 1G > A | p.? | 45 (23.4) |
| c.654G > A | p.(Trp218*) | 19 (9.9) |
| c.653G > C | p.(Trp218Ser) | 11 (5.7) |
| c.574T > C | p.(Cys192Arg) | 8 (4.2) |
| c.200 + 1G > A | p.? | 6 (3.1) |
| c.137G > A | p.(Arg46Gln) | 4 (2.1) |
| c.328A > C | p.(Thr110Pro) | 4 (2.1) |
| c.418G > T | p.(Val140Phe) | 4 (2.1) |
| c.725G > A | p.(Arg242His) | 3 (1.6) |
| c.649C > T | p.(Arg217Cys) | 3 (1.6) |
| c.590C > G | p.(Pro197Arg) | 3 (1.6) |
| c.686_725del | p.(Glu229fs) | 3 (1.6) |
| c.343C > T | p.(Arg115*) | 3 (1.6) |
| c.292T > C | p.(Cys98Arg) | 2 (1.0) |
| Deletion promoter and exon 1 | p.? | 1 (0.5) |
| Deletion promoter till exon 8 | p.0 | 2 (1.0) |
| Exon 2 deletion | p.? | 2 (1.0) |
| Exon 1 deletion | p.? | 2 (1.0) |
| c.713delT | p.(Phe238fs) | 1 (0.5) |
| c.727T > A | p.(Cys243Ser) | 1 (0.5) |
| c.761C > T | p.(Pro254Leu) | 1 (0.5) |
| c.626C > T | p.(Pro209Leu) | 1 (0.5) |
| c.380T > C | p.(Ile127Thr) | 1 (0.5) |
| c.325A > C | p.(Asn109His) | 1 (0.5) |
| c.1A > G | p.? | 1 (0.5) |
| c.119A > C | p.(Lys40Thr) | 1 (0.5) |
| c.274G > T | p.(Asp92Tyr) | 175 (74.7) |
| c.416T > C | p.(Leu139Pro) | 34 (14.6) |
| c.284T > C | p.(Leu95Pro) | 6 (2.6) |
| Deletion promoter, exon 1 and 2 | p.? | 4 (1.7) |
| c.242C > T | p.(Pro81Leu) | 3 (1.3) |
| c.337_340delGACT | p.(Asp113fs) | 2 (0.9) |
| c.122dupC | p.(Glu42fs) | 2 (0.9) |
| Exon 1. c.3G > C | p.(Met1Ile) | 1 (0.4) |
| Exon 2: c.169_169 + 9del10, splice donor mutation | p.? | 1 (0.4) |
| Intron 2 c.169_169 + 9del | p.? | 1 (0.4) |
| Specific SDHD variant unknown (tested elsewhere) | unknown | 3 (1.3) |
Author Contributions
Conceptualization, J.A.R., L.T.v.H., O.M.D., F.J.H., J.C.J., E.P.M.C., and E.F.H.; Data curation, J.A.R., L.T.v.H., N.D.N., C.R.L., K.E., A.N.A.v.d.H.-S., M.N.K., A.v.B., H.J.L.M.T., H.P.M.K., P.H.L.T.B, K.M.A.D, M.F.D., F.J.H., J.C.J., E.P.M.C., and E.F.H.; Formal analysis, J.A.R., L.T.v.H., O.M.D., N.D.N., F.J.H., J.C.J., E.P.M.C., and E.F.H.; Investigation, K.M.A.D.; Methodology, J.A.R., L.T.v.H., O.M.D., A.v.B., F.J.H., E.P.M.C., and E.F.H.; Supervision, J.A.R., E.P.M.C., and E.F.H.; Visualization, J.C.J. and E.F.H.; Writing—original draft, J.A.R., L.T.v.H., O.M.D., N.D.N., C.R.L., K.E., A.N.A.v.d. H.-S., M.N.K., A.v.B., H.J.L.M.T., H.P.M.K., P.H.L.T.B., K.M.A.D., M.F.D., F.J.H., J.C.J., E.P.M.C., and E.F.H.; Writing—review and editing, J.A.R., L.T.v.H., E.P.M.C., and E.F.H.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cascon A., Comino-Mendez I., Curras-Freixes M., de Cubas A.A., Contreras L., Richter S., Peitzsch M., Mancikova V., Inglada-Perez L., Perez-Barrios A., et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J. Natl. Cancer Inst. 2015;107:djv053. doi: 10.1093/jnci/djv053. [DOI] [PubMed] [Google Scholar]
- 2.Dahia P.L. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer. 2014;14:108–119. doi: 10.1038/nrc3648. [DOI] [PubMed] [Google Scholar]
- 3.Hensen E.F., van Duinen N., Jansen J.C., Corssmit E.P., Tops C.M., Romijn J.A., Vriends A.H., van der Mey A.G., Cornelisse C.J., Devilee P., et al. High prevalence of founder mutations of the succinate dehydrogenase genes in the Netherlands. Clin. Genet. 2012;81:284–288. doi: 10.1111/j.1399-0004.2011.01653.x. [DOI] [PubMed] [Google Scholar]
- 4.Van der Tuin K., Mensenkamp A.R., Tops C.M.J., Corssmit E.P.M., Dinjens W.N., van de Horst-Schrivers A.N., Jansen J.C., de Jong M.M., Kunst H.P.M., Kusters B., et al. Clinical Aspects of SDHA-Related Pheochromocytoma and Paraganglioma: A Nationwide Study. J. Clin. Endocrinol. Metab. 2018;103:438–445. doi: 10.1210/jc.2017-01762. [DOI] [PubMed] [Google Scholar]
- 5.Andrews K.A., Ascher D.B., Pires D.E.V., Barnes D.R., Vialard L., Casey R.T., Bradshaw N., Adlard J., Aylwin S., Brennan P., et al. Tumour risks and genotype–phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J. Med. Genet. 2018;55:384–394. doi: 10.1136/jmedgenet-2017-105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rijken J.A., Niemeijer N.D., Jonker M.A., Eijkelenkamp K., Jansen J.C., van Berkel A., Timmers H.J.L.M., Kunst H.P.M., Bisschop P.H.L.T., Kerstens M.N., et al. The penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin. Genet. 2018;93:60–66. doi: 10.1111/cge.13055. [DOI] [PubMed] [Google Scholar]
- 7.Neumann H.P., Pawlu C., Peczkowska M., Bausch B., McWhinney S.R., Muresan M., Buchta M., Franke G., Klisch J., Bley T.A., et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 8.Kunst H.P., Rutten M.H., de Mönnink J.P., Hoefsloot L.H., Timmers H.J.L.M., Marres H.A.M., Jansen J.C., Kremer H., Bayley J.-P., Cremers C.W.R.J. SDHAF2 (PGL2-SDH5) and hereditary head and neck paraganglioma. Clin. Cancer Res. 2011;17:247–254. doi: 10.1158/1078-0432.CCR-10-0420. [DOI] [PubMed] [Google Scholar]
- 9.Hensen E.F., Jansen J.C., Siemers M.D., Oosterwijk J.C., Vriends A.H., Corssmit E.P., Bayley J.-P., van der Mey A.G., Cornelisse C.J., Devilee P. The Dutch founder mutation SDHD.D92Y shows a reduced penetrance for the development of paragangliomas in a large multigenerational family. Eur. J. Hum. Genet. 2010;18:62–66. doi: 10.1038/ejhg.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benn D.E., Gimenez-Roqueplo A.P., Reilly J.R., Bertherat J., Burgess J., Byth K., Croxson M., Dahia P.L., Elston M., Gimm O., et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J. Clin. Endocrinol. Metab. 2006;91:827–836. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- 11.Amar L., Baudin E., Burnichon N., Peyrard S., Silvera S., Bertherat J., Bertagna X., Schlumberger M., Jeunemaitre X., Gimenez-Roqueplo A., et al. Succinate Dehydrogenase B Gene Mutations Predict Survival in Patients with Malignant Pheochromocytomas or Paragangliomas. J. Clin. Endocrinol. Metab. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 12.Van Hulsteijn L.T., Heesterman B., Jansen J.C., Bayley J.P., Hes F.J., Corssmit EPMDekkers O.M. No evidence for increased mortality in SDHD variant carriers compared with the general population. Eur. J. Hum. Genet. 2015;23:1713–1716. doi: 10.1038/ejhg.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemeijer N.D., Rijken J.A., Eijkelenkamp K., van der Horst-Schrivers A.N.A., Kerstens M.N., Tops C.M.J., van Berkel A., Timmers H.J.L.M., Kunst H.P.M., Leemans C.R., et al. The phenotype of SDHB germline mutation carriers; a nationwide study. Eur. J. Endocrinol. 2017;177:115–125. doi: 10.1530/EJE-17-0074. [DOI] [PubMed] [Google Scholar]
- 14.Plon S.E., Eccles D.M., Easton D., Foulkes W.D., Genuardi M., Greenblatt M.S., Hogervorst F.B.L., Hoogerbrugge N., Spurdle A.B., Tavtigian S.V. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutch Guideline for Detecting Hereditary Tumors. [(accessed on 17 March 2017)];2010 Available online: https://www.stoet.nl.
- 16.Dutch Guidelines for Oncology Care. [(accessed on 17 March 2017)];2016 Available online: http://www.oncoline.nl/familiair-paraganglioom.
- 17.Suissa S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 18.Statistics Netherlands. [(accessed on 17 March 2017)]; Available online: https://www.cbs.nl/
- 19.Tate J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., Boutselakis H., Cole C.G., Creatore C., Dawson E., et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oudijk L., Gaal J., de Krijger R.R. The Role of Immunohistochemistry and Molecular Analysis of Succinate Dehydrogenase in the Diagnosis of Endocrine and Non-Endocrine Tumors and Related Syndromes. Endocr. Pathol. 2018 doi: 10.1007/s12022-018-9555-2. [DOI] [PubMed] [Google Scholar]
- 21.Timmers H.J., Kozupa A., Eisenhofer G., Raygada M., Adams K.T., Solis D., Lenders J.W., Pacak K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J. Clin. Endocrinol. Metab. 2007;92:779–786. doi: 10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- 22.Srirangalingam U., Walker L., Khoo B., MacDonald F., Gardner D., Wilkin T.J., Skelly R.H., George E., Spooner D., Monson J.P., et al. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin. Endocrinol. 2008;69:587–596. doi: 10.1111/j.1365-2265.2008.03274.x. [DOI] [PubMed] [Google Scholar]
- 23.Amar L., Bertherat J., Baudin E., Ajzenberg C., Bressac-de Paillerets B., Chabre O., Chamontin B., Delemer B., Giraud S., Murat A., et al. Genetic testing in pheochromocytoma or functional paraganglioma. J. Clin. Oncol. 2005;23:8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 24.Van Hulsteijn L.T., Dekkers O.M., Hes F.J., Smit J.W., Corssmit E.P. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: A systematic review and meta-analysis. J. Med. Genet. 2012;49:768–776. doi: 10.1136/jmedgenet-2012-101192. [DOI] [PubMed] [Google Scholar]
- 25.Hulsteijn L.T., den Dulk A.C., Hes F.J., Bayley J.P., Jansen J.C., Corssmit E.P.M. No difference in phenotype of the main Dutch SDHD founder mutations. Clin. Endocrinol. 2013;79:824–831. doi: 10.1111/cen.12223. [DOI] [PubMed] [Google Scholar]
- 26.Kapetanakis S., Chourmouzi D., Gkasdaris G., Katsaridis V., Eleftheriadis E., Givissis P. Functional extra-adrenal paraganglioma of the retroperitoneum giving thoracolumbar spine metastases after a five-year disease-free follow-up: A rare malignant condition with challenging management. Pan Afr. Med. J. 2017;28:94. doi: 10.11604/pamj.2017.28.94.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valadea S., Chazeraina P., Khaninea V., Lazardb T., Baudinc E., Zizaa J.M. Late bone metastases of a pheochromocytoma. Rev. Med. Interne. 2010;31:772–775. doi: 10.1016/j.revmed.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Papaspyrou K., Mann W.J., Amedee R.G. Management of head and neck paragangliomas: Review of 120 patients. Head Neck. 2009;31:381–387. doi: 10.1002/hed.20967. [DOI] [PubMed] [Google Scholar]