Abstract
Atherosclerotic cardiovascular diseases are preferential targets of healthy diet and preventive medicine partially through strategies to improve lipid profile and counteract oxidative metabolites. Ninety individuals with cardiovascular disease (CVD) risk factors were randomized (1:1:1 ratio) to three arms, according to a four-week run-in, eight-week intervention, and four-week follow up study, testing the effects of a lactofermented Annurca apple puree (lfAAP), compared to unfermented apple puree (AAP) or probiotic alone (LAB) on plasma lipid profile and trimethylamine-N-oxide (TMAO) levels. By comparing the treatments, data indicated for the subjects tested with lfAAP a higher variation of the following serum parameters, in respect to the other treatment groups: high density lipoprotein cholesterol (HDL-C), +61.8% (p = 0.0095); and TMAO levels, −63.1% (p = 0.0042). The present study would suggest lfAAP as an effective functional food for beneficial control of plasma HDL-C and TMAO levels.
Keywords: functional food, Annurca apple puree, Lactobacillus, fermentation, plasma lipids, trimethylamine-N-oxide
1. Introduction
Polyphenols are a major group of bioactive substances found in fruits and vegetables with potential health benefits. As polyphenols characteristically have radical-scavenging activities, they exhibit antioxidant, as well as anti-inflammatory and anticarcinogenic behaviours [1,2]. Epidemiological studies show that dietary consumption of foods containing these compounds can result in a decreased risk of the development of certain lifestyle-related chronic diseases such as cardiovascular disease and colorectal cancer [2,3].
In order for polyphenols in fruits and vegetables to be available for absorption (i.e., become bioavailable) within the human gastrointestinal tract, these compounds must be released from the plant cell (i.e., be bioaccessible). When cells break during processing or oral mastication of fruits and vegetables, lipid membranes and the cellulose-pectin based plant cell wall (PCW) are fractured, allowing the contents within the cell to be released [4,5]. During exit from the cell, polyphenols come into contact with PCW for the first time, with the potential for binding interactions to take place, before they become available for uptake. Any binding interactions would be expected to be relevant for processed forms of fruits and vegetables, such as purees and sauces, where cell walls are deliberately ruptured during processing, thereby allowing polyphenol–PCW interactions to occur over a longer time [4,5]. Previous authors have demonstrated that specific apple polyphenols, the oligomeric procyanidins, are capable of selective interactions with individual cell wall constituents, with the neutral procyanidins interacting more extensively with pectin than cellulose [6,7]. Consequently, interactions between different polyphenols and the PCW, are likely to affect intestinal bioaccessibility of fruit and vegetable polyphenols. At first sight, non-bioaccessible polyphenols (e.g., by being bound to PCW material) would appear desirable, since they would survive to the large intestine, thus, being important for maintaining good gut health. In fact, epidemiological studies show that diets high in fruits and vegetables are associated with lower rates of colorectal cancer [8]. However, bioaccessible polyphenols represent the only form of antioxidant compounds, which being absorbed by the small intestine, can reach the systemic circulation and exert protective effects at the cardiovascular level [2].
Over the last decades, fermented foods are the object of a renewed interest in Western countries [9]. Mainly of vegetable origin, these products are strongly appreciated for several reasons, primarily nutrition–health approaches, followed by food safety, desirable organoleptic modification, shelf life extension, simplicity of preparation, valorisation of unused raw vegetal material, and sustainable development [10,11,12]. Among the wide range of microbial strains responsible for the fermentation of vegetable and fruits, in many cases lactic acid bacteria (LAB) are involved. Lactobacillus species are among the most frequent species of LAB involved in fruits or vegetables fermentation, specifically, L. plantarum, L. brevis, L. rhamnosus, and L. acidophilus [13]. Lactic fermentation modifies the composition of the fermented materials and can improve the beneficial health benefits of food by several mechanisms. Of major interest, it is reported that lactic fermentation can enhance the intestinal bioaccessibility of polyphenols through hydrolysis of esters with PCW polymeric constituents. Specifically, some authors have demonstrated that the decrease in soluble and insoluble fibre during fermentation processes in vegetables could be attributed to the hydrolysis of pectic compounds and the use of cellulose and hemicellulose as substrates by the microorganisms responsible for the fermentation [14,15,16].
Our research group has previously evaluated the polyphenolic composition of Annurca apples, the only apple cultivar native to the Campania region (Southern Italy), listed as a Protected Geographical Indication (PGI) product (Commission Regulation (EC) No. 417/2006)) [17]. By comparing experimental data with those from more common commercial apple cultivars (i.e., Red Delicious, Pink Lady, Fuji, Golden Delicious), Annurca apples exhibited the highest polyphenolic concentration, especially as regards oligomeric procyanidins. Moreover, our previous clinical study has demonstrated that a daily consumption of Annurca apples beneficially affects the serum cholesterol levels in healthy subjects, providing the most appreciable results among different apple cultivars tested [18]. In the light of what is stated above, it can be hypothesised that lactic acid fermentation may play a major role in enhancing the potential functional properties of this food product. Thus, the aims of the present work were to: i) evaluate the effects of lactic acid fermentation on the levels of free polyphenolic compounds in Annurca apple puree; and ii) compare the effects of lactofermented Annurca apple puree, unfermented Annurca apple puree, and probiotic alone, on human plasma lipid profile and oxidative amines.
2. Materials and Methods
2.1. Reagents and Standards
All chemicals and reagents used were either analytical-reagent or HPLC grade. The water was treated in a Milli-Q water purification system (Millipore, Bedford, MA, USA) before use. The standards used for the identification and quantification of phenolic acids and flavonoids were: chlorogenic acid, (+)-catechin, (−)-epicatechin, isorhamnetin, myricetin, phloretin, phloridzin (phloretin-2-O-glucoside), procyanidin B2, quercetin, quercitrin (quercetin-3-O-rhamnoside), rutin (quercetin-3-O-rutinoside), isoquercitrin (quercetin-3-O-glucoside), hyperin (quercetin-3-O-galactoside), and cyanidin-3-O-galactoside chloride (Sigma Chemical Co., St. Louis, MO, USA). Acetonitrile and methyl alcohol were of HPLC grade (Carlo Erba, Milano, Italy). Sodium taurocholate, phosphatidylcholine, cholesterol, NaCl, and sodium phosphate were purchased from Sigma Chemical Co.
2.2. Fruit Collection and Annurca Apple Puree (AAP) Preparation
Annurca (M. pumila Miller cv Annurca) apple fruits were collected in Valle di Maddaloni (Caserta, Italy) in October 2016 when fruits had just been harvested (green peel). Fruits were reddened, following the typical treatment for about 30 days, and then analysed [19]. Large-scale production of AAP was accomplished by Masseria Giòsole (Caserta, Italy). Apples were milled with hot (90 °C) water to a fine puree which was hermetically closed into sterile glass jars and submitted to sterilisation (120 °C for 15 min).
2.3. Lactofermented AAP (lfAAP) Preparation
Inoculation of AAP with the probiotic strains Lactobacillus rhamnosus LRH11 and Lactobacillus plantarum SGL07 (Farmalabor, Canosa, Italy) was performed as previously reported [20,21] with slight modifications. Probiotic strains were reactivated by culturing twice in MRS broth (meat peptone, 10.0 g/L; dextrose, 20.0 g/L; yeast extract, 5.0 g/L; beef extract 10.0 g/L; disodium phosphate 2.0 g/L; sodium acetate, 5.0 g/L; ammonium citrate, 2.0 g/L; magnesium sulphate 0.1 g/L; manganese sulphate 0.05 g/L; Tween 80 1.0 g/L) (Thermo scientific, Whalthan, MA, USA) at 37 °C for 18 h. Pellets were washed in sterile physiological solution (NaCl 8.5 g/L) and suspended in 5 mL of the same solution. The sterilised jars containing AAP were opened in sterile conditions and inoculated with one or the other probiotic strain to obtain a bacterial load of about 2.5 × 106 CFU/g. Both types of samples were incubated at room temperature for 48 h and stored at 4 °C for 4 weeks. At each of the three steps of fermentation (0–24–48 h), aliquots of the lactofermented AAP (lfAAP) were collected for microbiological, chemical-physical, and chemical analyses.
2.4. Enumeration of Probiotic Microorganisms in lfAAP
Viability of probiotic cultures in lfAAP was determined and expressed as colony forming units (CFU)/mL on MRS agar (Oxoid, Milan, Italy). Serial dilutions were prepared in sterile physiological solution before plating onto MRS agar. Plates were incubated at 37 °C for 48 h in an Oxoid anaerobic system.
2.5. Preparation of Polyphenolic Extracts from AAP and lfAAP Samples
Lyophilized samples (10 g) were treated with 100 mL of 80% methanol (0.5% formic acid) for 24 h at 4 °C to extract phenolic compounds. After centrifugation, the supernatant was slowly filtered through an Amberlite XAD-2 column packed as follows: resin (10 g; pore size 9 nm; particle size 0.3–1.2 mm; Supelco, Bellefonte, PA, USA) was soaked in methanol, stirred for 10 min and then packed into a glass column (10 × 2 cm). The column was washed with 100 mL of acidified water (pH 2) and 50 mL of deionised water for sugar, and other polar compound removal. The adsorbed phenolic compounds were extracted from the resin by elution with 100 mL of methanol, which was evaporated by flushing with nitrogen. Samples were stored at −20 °C until HPLC analysis.
2.6. HPLC-DAD/ESI-MS Analysis of Polyphenolic Extracts
Polyphenolic extracts were solubilized with 1% formic acid. Analyses were run on a Jasco Extrema LC-4000 system (Jasco Inc., Easton, MD, USA) provided with photodiode array detector (DAD). The column selected was a Kinetex® C18 column (250 mm × 4.6 mm, 5 μm; Phenomenex, Torrance, CA, USA). The analyses were performed at a flow rate of 1 mL/min, with solvent A (2% acetic acid) and solvent B (0.5% acetic acid in acetonitrile and water 50:50, v/v). After a 5 min hold at 10% solvent B, elution was performed according to the following conditions: from 10% (B) to 55% (B) in 50 min and to 95% (B) in 10 min, followed by 5 min of maintenance. Flavonols, procyanidins, dihydrochalcones, flavanols, and hydroxycinnamic acids were monitored at 280 nm and anthocyanins at 520 nm. For quantitative analysis, standard curves for each polyphenol standard were prepared over a concentration range of 0.1–1.0 μg/μL with six different concentration levels and duplicate injections at each level. The identity of polyphenols was confirmed by LC-ESI/MS experiments and data were compared with those of commercial standards. The same chromatographic apparatus and conditions (HPLC system, gradient elution, column, temperature) were coupled to an Advion Expression mass spectrometer (Advion Inc., Ithaca, NY, USA) equipped with an electrospray (ESI) source. Mass spectra were recorded from m/z = 50 to 1200, both in negative and in positive ionization mode. The capillary voltage was set at −28 V, the spray voltage was at 3 kV, and the tube lens offset was at −10 V in negative ion mode, while the capillary voltage was set at 34 V, the spray voltage was at 3.5 kV, and the tube lens offset was at 55 V in positive ion mode. The capillary temperature was 275 °C. Data were acquired in full scan and SIM modes.
2.7. Study Population and Protocol
Study participants were recruited by the Samnium Medical Cooperative (Benevento, Italy). Subjects were enrolled in January 2017. Subjects aged 18–70 years were eligible for enrolment if they had the following values of serum parameters at baseline: total cholesterol (TC), 200–260 mg/dL; HDL-C, 30–45 mg/dL; LDL-C, 150–182 mg/dL; glucose, 90–125 mg/dL; TG, 170–280 mg/dL.
Exclusion criteria were: smoking, obesity (BMI > 30 kg/m2), diabetes, serious hepatic disease (cirrhosis, hepatitis), serious renal disorders (serum creatinine > 2.8 mg/dL), heart disease, family history of chronic diseases, drug therapy or supplement intake for hypercholesterolemia, drug therapy or supplement intake containing apple polyphenols, heavy physical exercise (>10 h/week), pregnant women, women suspected of being pregnant, women who hoped to become pregnant, breastfeeding, birch pollen allergy, use of vitamin/mineral supplements 2 weeks prior to entry into the study, and donation of blood less than 3 months before the study.
The subjects received oral and written information concerning the study before they gave their written consent. Protocol, letter of intent of volunteers, and synoptic document about the study were submitted to the Scientific Ethics Committee of AO Rummo Hospital (Benevento, Italy). The study was approved by the committee (protocol 10/2016/PR of 22/10/2016) and carried out in accordance with the Helsinki declaration of 1964 (as revised in 2000). The subjects were asked to make records in an intake-checking table for the intervention study and side effects in daily reports. The study was a randomised, single centre trial conducted at the Samnium Medical Cooperative (Benevento, Italy).
The study duration was 16 weeks. The subjects were randomly divided into three groups (AAP, lfAAP, and LAB). Each group underwent 4 weeks of run-in period (no treatment), followed by 8 weeks of intervention, and 4 weeks of follow-up. All of the subjects were provided all 8-weeks of the treatment doses at randomization. During the intervention periods, subjects were administered according to the belonging group, as follows: AAP group, Annurca apple puree (125 g/day); lfAAP group, lactofermented Annurca apple puree (125 g/day); LAB group, 1 capsule/day, containing the exact amount of Lactobacillus strain occurring in 125 g of lfAAP (about, 3.0 × 108 CFU). Subjects were instructed to take AAP, lfAAP, or LAB, at one of the meals. Subjects were asked to keep their dietary habits unchanged throughout the entire study. To this regard, they were provided with a food diary on which annotate their daily dietary habits and were instructed to maintain their habitual patterns of physical activity throughout the entire study period.
Both the examinations and the study treatment were performed in an outpatient setting. Clinic visits, and blood and faecal swab sampling were performed after 12 h of fasting at weeks 0, 4, 8, 12, and 16. Subjects were informed not to drink alcohol or perform hard physical activity 48 h prior to blood sampling. All blood samples were taken in the morning and immediately after measurement of heart rate and blood pressure. Blood samples were collected in 10-mL EDTA-coated tubes (Becton–Dickinson, Plymouth, UK) and plasma was isolated by centrifugation (20 min, 2.200 g, 4 °C). All samples were stored at −80 °C until analysis.
Plasma TC, HDL-C, LDL-C, glucose, and TG levels were determined using commercially available kits from Diacron International (Grosseto, Italy). Analyses were performed on a Diacron International Free Carpe Diem. The assay sensitivity for the individual analytical determination was determined as follows: TC, 1 mg/dL; HDL-C, 1 mg/dL; LDL-C, 5 mg/dL; glucose, 4 mg/dL; TG, 3 mg/dL. Intra- and inter-day variations were 1.4% and 1.6% for TC, 1.6% and 2.2% for LDL-C, 2.0% and 2.3% for HDL-C, 1.1% and 1.7% for glucose, and 1.3% and 1.8% for TG, respectively.
Trimethylamine-N-oxide (TMAO) was quantified in blood samples as previously reported [22], with slight modifications. An aliquot of 80 µL of plasma was added to 160 µL of methanol. The mixture was vortexed for 2 min, and then centrifuged at 16.128 rcf. The supernatant was collected and analysed by HPLC/ESI-MS technique. The HPLC system Jasco Extrema LC-4000 system (Jasco Inc., Easton, MD, USA) was coupled to an Advion Expression mass spectrometer (Advion Inc., Ithaca, NY, USA). An electrospray ionization (ESI) source was used in positive ion mode. Source and capillary temperatures were 150 and 400 °C, respectively. Capillary voltage was +0.60 kV, and desolvation and cone gas (both N2) flow rates were 800 and 20 L/h, respectively. For the separation of the analytes, a Luna Hilic (5µ particle size, 150 × 3 mm) and security guard colon both supplied by Phenomenex (Torrance, CA, USA,) were used. The column temperature was maintained at 60 °C during analysis. Mobile phase composition comprised (A) 0.15% formic acid in water containing a final concentration of 10 mM ammonium acetate, and (B) 100% methanol (LC/MS grade) in the ratio 80:20 (A:B), run isocratically at a flow rate of 0.35 mL/min for 6 min, with a 5 µl injection volume. After use, the column was stored in 100% acetonitrile and was routinely cleaned according to the manufacturer’s instructions. Trimethylamine-N-oxide was identified and quantified by analytical standard (Sigma–Aldrich St. Louis, MO, USA) and calibration curve.
Faecal swabs were analysed for their microbial composition. Specifically, the following BD (Becton Dickinson, Heidelberg, Germany) ready-for-use culture plates were purchased and employed according to instructions: Bifidobacterium agar, modified; Bacteroides bile esculin agar with amikacin; Lactobacillus (LBS) selection agar; Enterococcus agar.
In addition to the five clinic visits, six standardised telephone interviews were performed every 14 days starting from the first clinic visit, to verify compliance and increase protocol adherence. In particular, these interviews reminded subjects to complete their intake-checking table for the intervention study and to record any treatment discontinuation, or adverse events they might have experienced in the meantime (which were also documented regularly on the case report forms during each telephone and clinic visit).
All subjects underwent a standardised physical examination, assessment of medical history (up to five years before enrolment), laboratory examination, measurement of blood pressure and heart rate, and evaluation of Body mass index (BMI). Body mass index was calculated from body height and body weight. Body fat percentage was measured using a body composition analyser (TBF-310, Tanita Corp., Tokyo, Japan) and systolic blood pressure, diastolic blood pressure, and heart rate were measured using an HBP-9020 (OMRON COLIN Corp., Tokyo, Japan). At each clinic visit, subjects had to complete three self-administered questionnaires on quality of life aspects, and their diaries were checked for data completeness and quality of documentation to ensure subject comprehension of the diary items.
2.8. Randomisation, Concealment, and Blinding
A total of 90 eligible subjects (51 men and 39 women, 18–70 years of age) were randomly assigned to three sub-groups (each one of 30 subjects). If a subject dropped out before the intervention period, he or she was replaced by the next eligible subject enrolled at the same centre. The concealed allocation was performed by an internet-based randomisation schedule, stratified by study site. The random number list was generated by an investigator with no clinical involvement in the trial. Subjects, clinicians, core laboratories, and trial staff (data analysts, statisticians) were blind to treatment allocation.
2.9. Study Outcomes and Data Collection
2.9.1. Primary and Secondary Efficacy Outcomes
Primary endpoints measured were plasma level variations of TC, HDL-C, LDL-C, glucose, TG, and TMAO, while key secondary outcomes were microbial composition of faecal swabs and parameters collected during clinic visits, such as blood pressure, heart rate, and evaluation of BMI. All raw subject ratings were evaluated in a blinded manner at the site of the principal investigator.
2.9.2. Safety
We assessed safety from reports of adverse events as well as laboratory parameters concerning the hepatic and renal function, vital signs (i.e., blood pressure, pulse, height, weight, and body mass index), and physical or neurological examinations. Safety was assessed over the entire treatment period at weeks 0, 4, 8, 12, and 16, including adverse events occurring in the first three weeks after cessation of treatments.
2.10. Statistics
2.10.1. Methodology
During the trial, it became apparent that dropouts and incomplete diary documentation created missing data that could not be adequately handled by the intended robust comparison. To deal with the missing data structure, we used a negative binomial, generalised linear mixed effects model (NB GLMM) that not only yields unbiased parameter estimates when missing observations are missing at random (MAR) [23], but also provides reasonably stable results even when the assumption of MAR is violated [24,25]. Subjects who did not provide any diary data (leading to zero evaluable days) were excluded from the MAR-based primary efficacy analysis, according to an “all observed data approach” as proposed by previous authors [26]. This approach is statistically efficient without using multiple imputation techniques. Data retrieved after withdrawal of randomised study treatment were also included in the analysis.
Unless otherwise stated, all of the experimental results were expressed as mean ± SD of at least five replications. Statistical analysis of data was performed by the Student’s t-test or two-way ANOVA followed by the Tukey–Kramer multiple comparison test to evaluate significant differences between a pair of means. The statistic heterogeneity was assessed by using Cochran’s test (p < 0.1). The I2 statistic was also calculated, and I2 > 50% was considered as significant heterogeneity across studies. A random-effects model was used if significant heterogeneity was shown among the trials. Otherwise, results were obtained from a fixed-effects model. Percent change in mean and SD values were excluded when extracting SD values for an outcome. Standard deviation values were calculated from standard errors, 95% CIs, p-values, or t if they were not available directly. Previously defined subgroup analyses were performed to examine the possible sources of heterogeneity within these studies and included health status, study design, type of intervention, duration, total polyphenols dose, and Jadad score. Treatment effects were analysed using PROC MIXED with treatment and period as fixed factors, subject as random factor, and baseline measurements as covariates, and defined as weighted mean difference and 95% CIs calculated for net changes in faecal and serum parameters, and blood pressure values. Data that could not meet the criteria of variance homogeneity (Levenes test) and normal distribution (determined by residual plot examination and Shapiro–Wilks test) even after log transformation were analysed by a nonparametric test (Friedman). The level of significance (α-value) was 95% in all cases (p < 0.05).
2.10.2. Analysis Sets
The full analysis set population included all randomised subjects, and subjects who did not fail to satisfy a major entry criterion. We excluded subjects who provided neither primary nor secondary efficacy data from efficacy analyses. The per protocol set consisted of all subjects who did not substantially deviate from the protocol; they had two characteristics. Firstly, this group included subjects for whom no major protocol violations were detected (for example, poor compliance, errors in treatment assignment). Secondly, they had to have been on treatment for at least 50 days counting from day of first intake (completion of a certain pre-specified minimal exposure to the treatment regimen). Hence, subjects who prematurely discontinued the study or treatment were excluded from the per protocol sample.
2.10.3. Determination of Sample Size
In order to determine whether this study provided sufficient power to detect statistically significant differences using this design, a post-hoc power analysis was performed on the primary endpoints measured for all treatments (one-way repeated measures ANOVA, observed F = 0.6596, α = 0.05, 1 group, n = 20, 5 measurements, observed correlation among measurements = 0.7977, Geisser–Greenhouse sphericity ε = 0.4991). Based on these values, the statistical power was 100%, indicating that the sample size was sufficient to detect statistically significant differences if they were indeed present. This resulted in n = 20 subjects, which was increased to n = 30.
2.11. Subject Involvement
No subjects were involved in setting the research question or the outcome measures, nor were they involved in developing plans for participant recruitment, or the design and implementation of the study. There are no plans to explicitly involve subjects in dissemination. Final results will be sent to all participating sites.
3. Results
3.1. Polyphenolic Composition of AAP and lfAAP
Quantitative results from LC-MS analyses of polyphenols in AAP and lfAAP samples are reported in Table 1. Data show that fermentation of AAP for 24 h by using L. rhamnosus increased the amount of individual free polyphenols from a minimum of 20% up to 45% (total polyphenols: +32.7%), while at 48 h results were significantly lower (from a minimum of 10% up to 20%; total polyphenols: +14.9%), than non-fermented AAP, respectively. Conversely, fermentation of AAP using L. plantarum provided lower, but still significant results both at 24 and 48 h of incubation, than what was observed for AAP fermented using L. rhamnosus, respectively. As control, AAP samples were handled in the same incubation conditions (times and temperature) of lfAAP, but without the inoculation of any Lactobacillus strain: no significant variation of AAP polyphenolic compositions were registered. In the light of these results, lfAAP obtained by 24 h incubation with L. rhamnosus was selected as the best candidate sample to be administered to subjects.
Table 1.
Polyphenolic composition of Annurca apple puree (AAP) and its lactofermented versions (lfAAP) obtained by incubation (at room temperature) with different Lactobacillus strains at different times.
| AAP | lfAAP (by L. rhamnosus at 24 h) | lfAAP (by L. rhamnosus at 48 h) | lfAAP (by L. plantarum at 24 h) | lfAAP (by L. plantarum at 48 h) | |
|---|---|---|---|---|---|
| Chlorogenic acid | 8.98 ± 0.02 a | 10.78 ± 0.03 b | 9.88 ± 0.02 c | 9.16 ± 0.04 a | 9.07 ± 0.05 a |
| [+]-Catechin | 1.20 ± 0.04 a | 1.52 ± 0.03 b | 1.34 ± 0.05 c | 1.25 ± 0.04 a | 1.22 ± 0.06 a |
| [−]-Epicatechin | 2.80 ± 0.06 a | 3.48 ± 0.05 b | 3.14 ± 0.04 c | 2.91 ± 0.03 a | 2.86 ± 0.07 a |
| Procyanidin B1 | 0.70 ± 0.02 a | 1.01 ± 0.03 b | 0.84 ± 0.05 c | 0.78 ± 0.03 a | 0.75 ± 0.02 a |
| Procianidin B2 | 1.78 ± 0.02 a | 2.58 ± 0.04 b | 2.14 ± 0.02 c | 1.99 ± 0.03 d | 1.88 ± 0.05 a,d |
| Procyanidin trimer | 1.28 ± 0.02 a | 1.87 ± 0.04 b | 1.54 ± 0.02 c | 1.43 ± 0.03 d | 1.36 ± 0.01 e |
| Cyanidin-3-O-galactoside | 0.04 ± 0.02 a | 0.05 ± 0.02 a | 0.05 ± 0.02 a | 0.04 ± 0.03 a | 0.04 ± 0.02 a |
| Rutin (Quercetin-3-O-rutinoside) | 0.80 ± 0.02 a | 1.08 ± 0.03 b | 0.93 ± 0.04 c | 0.86 ± 0.02 d | 0.83 ± 0.05 a |
| Hyperin (Quercetin-3-O-galactoside) | 8.90 ± 0.04 a | 12.1 ± 0.06 b | 10.3 ± 0.04 c | 9.61 ± 0.05 d | 9.26 ± 0.03 e |
| Isoquercitrin (Quercetin-3-O-glucoside) | 3.52 ± 0.02 a | 4.75 ± 0.03 b | 4.08 ± 0.06 c | 3.80 ± 0.05 d | 3.66 ± 0.03 e |
| Reynoutrin (Quercetin-3-O-xyloside) | 2.04 ± 0.08 a | 2.75 ± 0.05 b | 2.37 ± 0.07 c | 2.20 ± 0.06 d | 2.12 ± 0.08 a |
| Guajaverin (Quercetin 3-O-arabinopyranoside) | 1.74 ± 0.02 a | 2.35 ± 0.04 b | 2.02 ± 0.03 c | 1.88 ± 0.06 d | 1.81 ± 0.05 a |
| Avicularin (Quercetin 3-O-arabinofuranoside) | 3.96 ± 0.02 a | 5.35 ± 0.07 b | 4.59 ± 0.02 c | 4.28 ± 0.09 d | 4.12 ± 0.08 e |
| Quercetin-O-pentoside | 1.22 ± 0.02 a | 1.65 ± 0.04 b | 1.39 ± 0.03 c | 1.32 ± 0.02 d | 1.27 ± 0.05 a |
| Quercitrin (Quercetin-3-O-rhamnoside) | 2.34 ± 0.02 a | 3.16 ± 0.02 b | 2.71 ± 0.04 c | 2.53 ± 0.06 d | 2.43 ± 0.07 a |
| Phloretin-2-O-xyloglucoside | 2.70 ± 0.02 a | 3.78 ± 0.05 b | 3.19 ± 0.08 c | 2.97 ± 0.04 d | 2.83 ± 0.02 e |
| Phloridzin (phloretin-2-O-glucoside) | 3.02 ± 0.02 a | 4.23 ± 0.07 b | 3.56 ± 0.06 c | 3.32 ± 0.04 d | 3.17 ± 0.02 e |
| Total polyphenols | 47.02 ± 0.09 a | 62.40 ± 0.12 b | 54.07 ± 0.14 c | 50.33 ± 0.11 d | 46.25 ± 0.10 a |
Values are expressed as mg/125 g puree and are the means ± SD (n = 5; p < 0.01). abcde Mean values in rows with different superscript letters are significantly different by the Tukey–Kramer multiple comparison test. AAP = Annurca apple puree; lfAAP = lactofermented Annurca apple puree.
3.2. Enrolment and Subject Attrition
Subjects were enrolled in January 2017. A total of 122 subjects were screened for eligibility; 32 subjects (26.2%) did not pass the screening stage; 90 subjects were randomised. The most common reason was that subjects did not meet the inclusion criteria regarding values of serum parameters at baseline (n = 14), followed by general refusal to participate for no specific reasons (n = 4), and concerns about the protocol, especially fear of placebo (n = 2). Some fulfilled exclusion criteria (n = 12).
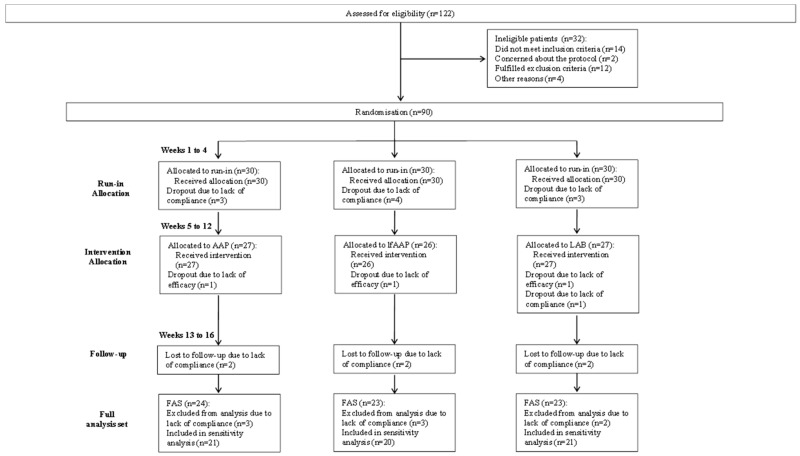
Overall, 90 subjects were assigned to the study. Subjects underwent a run-in period during the first four weeks before the treatment period of eight weeks. The follow-up period lasted another four weeks. Figure 1 shows the flow of participants through the trials together with the completeness of diary information over the entire treatment period.
Figure 1.
Study flowchart according to the consolidated standards of reporting trials (CONSORT). The diagram shows enrolment and primary efficacy endpoints based on subject diaries, from pre-screening to data collection; and the extent of exclusions, loss to follow-up, and completeness of diary documentation available across the entire trial period. AAP = subgroup administered with Annurca apple puree (125 g/day); lfAAP = subgroup administered with lactofermented Annurca apple puree (125 g/day); LAB = subgroup administered with 1 capsule/day, containing Lactobacillus rhamnosus (3.0 × 108 CFU); FAS = full analysis set.
No subject prematurely terminated study participation before allocation to treatment. Figure 1 follows the CONSORT PRO reporting guideline [27] and reveals that within the assessment period, the following percentage of subjects provided data for the primary endpoint: subgroup AAP, 77.8% (21 of 27 subjects); subgroup lfAAP, 76.9% (20 of 26 subjects); subgroup LAB, 77.8% (21 of 27 subjects). In each group, a few subjects did not submit any diaries, giving no specific reason for this. Completeness of the subject diaries did not differ between the treatment groups.
3.3. Participants’ Baseline Characteristics
Table 2 shows the demographic and clinical characteristics assessed at the baseline visits of all 90 subjects randomised. Overall, about half of the randomised subjects were female; the total age range was 18–70 years. The groups were well balanced for demographics and clinical factors.
Table 2.
Baseline characteristics of intention to treat sample according to study treatment.
| Run-in | |||
|---|---|---|---|
| Characteristics | AAP (n = 30) | lfAAP (n = 30) | LAB (n = 30) |
| Demographics | |||
| Age (years) | 46.9 ± 10.6 | 45.8 ± 11.1 | 47.6 ± 10.4 |
| Male sex (No (%)) | 17 (56.7%) | 18 (60.0%) | 16 (53.3%) |
| White ethnicity (No (%)) | 30 (100%) | 30 (100%) | 30 (100%) |
| Clinical parameters | |||
| TC (mg/dL) | 234.1 ± 13.2 | 238.5 ± 12.1 | 236.2 ± 11.8 |
| LDL-C (mg/dL) | 154.0 ± 11.1 | 155.7 ± 12.4 | 166.9 ± 12.0 |
| HDL-C (mg/dL) | 37.8 ± 6.3 | 38.3 ± 7.4 | 40.5 ± 6.6 |
| Glucose (mg/dL) | 100.5 ± 8.2 | 102.2 ± 9.3 | 110.2 ± 9.1 |
| Triglycerides (mg/dL) | 178.1 ± 9.6 | 188.1 ± 11.7 | 197.6 ± 11.6 |
| TMAO (μM) | 2.01 ± 0.05 | 2.68 ± 0.06 | 1.72 ± 0.06 |
| Treatment | |||
| Characteristics | AAP (n = 27) | lfAAP (n = 26) | LAB (n = 27) |
| Demographics | |||
| Age (years) | 45.1 ± 10.3 | 46.2 ± 10.7 | 48.2 ± 10.2 |
| Male sex (No (%)) | 15 (55.5%) | 16 (61.5%) | 15 (55.5%) |
| White ethnicity (No (%)) | 27 (100%) | 26 (100%) | 27 (100%) |
| Clinical parameters | |||
| TC (mg/dL) | 235.5 ± 13.3 | 237.6 ± 14.3 | 239.1 ± 11.9 |
| LDL-C (mg/dL) | 152.1 ± 11.1 | 156.4 ± 11.6 | 165.8 ± 11.7 |
| HDL-C (mg/dL) | 36.7 ± 7.5 | 37.4 ± 6.7 | 38.5 ± 7.2 |
| Glucose (mg/dL) | 99.1 ± 2.7 | 100.0 ± 8.9 | 107.2 ± 8.5 |
| Triglycerides (mg/dL) | 180.4 ± 16.8 | 185.2 ± 18.3 | 200.1 ± 19.0 |
| TMAO (μM) | 2.37 ± 0.04 | 3.01 ± 0.05 | 2.02 ± 0.06 |
Values are means ± SD (n = 5). Results were significantly different at a level of p = 0.001. AAP = subgroup to be administered with Annurca apple puree; lfAAP = subgroup to be administered with lactofermented Annurca apple puree; LAB = subgroup to be administered with Lactobacillus rhamnosus. TC: total cholesterol.
3.4. Primary Efficacy Outcome Measures
No significant variations of plasma TC, LDL-C, HDL-C, glucose, TG, and TMAO levels, with respect to the baseline values, were registered in subjects of all subgroups, at the end of the run-in period (Table 2). Analysing results regarding the variation of plasma lipid values at the end of the intervention period (Table 3), we can assert that the most significant achievements were registered as regards HDL-C and TMAO levels in all subgroups. Specifically, the lfAAP sample exerted the highest influence on both parameters, increasing HDL-C levels by 61.8% (p = 0.0095) and lowering TMAO levels by 63.1% (p = 0.0042), at the end of the intervention period, followed by AAP sample (HDL-C: +48.4%, p = 0.0042; TMAO: −42.3%, p = 0.0032) and LAB sample (HDL-C: +17.7%, p = 0.0036; TMAO: −25.8%, p = 0.0029). It is noteworthy that all of the experimental results were achieved already after one month of intervention study and were confirmed at the end of the second month. Comparing the LC-MS results, regarding the effects of lactofermentation on the bioaccessibility of polyphenols in AAP samples (Table 1), with the clinical values, concerning the influence of lfAAP sample on plasma HDL-C and TMAO levels (Table 3), a significant correlation was found. Specifically, for the lfAAP sample exhibiting the highest bioaccessibility of polyphenols (Table 1), it was the most effective in increasing HDL-C levels and lowering TMAO levels in human plasma (Table 3). As regards the results at the end of the follow-up period, HDL-C and TMAO levels registered slightly significant variations in respect to the end of the treatment period (Table 3). As a general trend, a loss of efficacy was observed, as regards both parameters, at the end of the follow-up period, in respect to the end of the treatment period (t 60). The LAB treatment demonstrated the highest stability of efficacy (HDL-C, −6.6% (p = 0.0076); TMAO, −8% (p = 0.0058)), followed by the other two treatments ((lfAAP: HDL-C, −13.3% (p = 0.0061); TMAO, −12.9% (p = 0.0042); AAP: HDL-C, −10.4% (p = 0.0049); TMAO, −7.2% (p = 0.0037)).
Table 3.
Effects of Annurca apple puree (AAP) and its lactofermented version (lfAAP) on plasma cholesterol, glucose, triglyceride, and TMAO levels.
| AAP | Δ (%) | lfAAP | Δ (%) | LAB | Δ (%) | ||
|---|---|---|---|---|---|---|---|
| TC (mg/dL) | t 0 | 235.5 ± 13.3 | 237.6 ± 14.3 | 239.1 ± 11.9 | |||
| t 30 | 246.1 ± 14.2 | +4.5 | 257.1 ± 12.8 | +8.2 | 242.2 ± 13.5 | +1.3 | |
| t 60 | 246.8 ± 13.6 | +4.8 | 259.0 ± 12.1 | +9.0 | 243.4 ± 13.8 | +1.8 | |
| t 90 | 248.2 ± 14.0 | +5.4 | 259.5 ± 16.7 | +9.2 | 253.1 ± 14.2 | +5.8 | |
| LDL-C (mg/dL) | t 0 | 152.1 ± 11.1 | 156.4 ± 11.6 | 165.8 ± 11.7 | |||
| t 30 | 157.3 ± 11.3 | +2.7 | 163.5 ± 10.9 | +3.6 | 168.7 ± 10.8 | +1.5 | |
| t 60 | 158.6 ± 13.6 | +3.4 | 164.8 ± 11.2 | +4.3 | 169.7 ± 11.2 | +2.0 | |
| t 90 | 160.0 ± 12.5 | +5.2 | 166.2 ± 11.1 | +6.3 | 172.6 ± 10.7 | +4.1 | |
| HDL-C (mg/dL) | t 0 | 36.7 ± 7.5 | 35.4 ± 6.7 | 38.5 ± 7.2 | |||
| t 30 | 53.6 ± 7.5 * | +46.1 # | 56.2 ± 7.9 * | +58.9 # | 44.3 ± 8.0 * | +15.2 # | |
| t 60 | 54.5 ± 7.0 * | +48.4 # | 57.3 ± 8.1 * | +61.8 # | 45.3 ± 8.3 * | +17.7 # | |
| t 90 | 49.6 ± 6.8 * | +35.1 # | 53.6 ± 7.5 * | +51.4 # | 42.8 ± 7.6 * | +11.1 # | |
| Glucose (mg/dL) | t 0 | 99.1 ± 8.7 | 100.0 ± 8.9 | 107.2 ± 8.5 | |||
| t 30 | 102.0 ± 10.8 | +2.9 | 103.8 ± 12.0 | +3.8 | 109.4 ± 12.4 | +2.1 | |
| t 60 | 102.5 ± 11.6 | +3.4 | 104.5 ± 13.1 | +4.5 | 109.9 ± 10.3 | +2.5 | |
| t 90 | 103.0 ± 12.1 | +3.9 | 105.1 ± 12.4 | +5.1 | 110.2 ± 11.9 | +2.8 | |
| Triglycerides (mg/dL) | t 0 | 180.4 ± 16.8 | 185.2 ± 18.3 | 200.1 ± 19.0 | |||
| t 30 | 185.1 ± 19.3 | +2.6 | 192.2 ± 16.4 | +3.8 | 203.3 ± 21.1 | +1.6 | |
| t 60 | 186.2 ± 14.7 | +3.2 | 193.5 ± 17.3 | +4.5 | 204.5 ± 15.2 | +2.2 | |
| t 90 | 189.0 ± 16.2 | +4.8 | 194.6 ± 17.0 | +5.1 | 206.9 ± 18.7 | +3.4 | |
| TMAO (μM) | t 0 | 2.37 ± 0.04 | 3.01 ± 0.05 | 2.02 ± 0.06 | |||
| t 30 | 1.45 ± 0.02 * | −38.9 # | 1.24 ± 0.06 * | −58.7 # | 1.56 ± 0.07 * | −22.9 # | |
| t 60 | 1.36 ± 0.05 * | −42.3 # | 1.11 ± 0.04 * | −63.1 # | 1.49 ± 0.04 * | −25.8 # | |
| t 90 | 1.54 ± 0.04 * | −35.1 # | 1.50 ± 0.06 * | −50.2 # | 1.66 ± 0.04 * | −17.8 # |
AAP = subgroup administered with Annurca apple puree (125 g/day); lfAAP = subgroup administered with lactofermented Annurca apple puree (125 g/day); LAB = subgroup administered with 1 capsule/day, containing Lactobacillus rhamnosus (3.0 × 108 CFU). *: Significantly different from baseline at p < 0.01 (PROC MIXED). #: Significantly different from the other treatments at p < 0.05 (PROC MIXED).
3.5. Secondary Efficacy Outcome Measures
Results of the faecal swab microbial composition are reported in Table 4. On average, all of the samples (AAP, lfAAP, and LAB) determined a strong increase in Bifidobacterium and Lactobacillus population, and a slightly significant reduction of Bacteroides and Enterococcus genera, at the intestinal level. Very interestingly, AAP revealed the leading effects of increasing Bifidobacterium and Lactobacillus by about 74 and 300 times, respectively, followed by LAB (26 and 40 times, respectively), while lfAAP exerted a minor influence (3.5 and 2 times, respectively). Concerning all of these results, slightly significant variations, registered as a general decrease of efficacy, were revealed at the end of the follow-up period, respect to the end of the treatment period (Table 4). The lfAAP treatment demonstrated the highest stability of efficacy ((Bifidobacterium, −61.0% (p = 0.0055); Lactobacillus, −21.5% (p = 0.0079); Bacteroides, −9.0% (p = 0.0085); Enterococcus, −4.1% (p = 0.0034)), followed by the other two treatments (LAB: Bifidobacterium, −800% (p = 0.0028); Lactobacillus, −1050% (p = 0.0066); Bacteroides, −15.8% (p = 0.0043); Enterococcus, −14.8% (p = 0.0042); AAP: Bifidobacterium, −1460% (p = 0.0038); Lactobacillus, −12110% (p = 0.0053); Bacteroides, −15.0% (p = 0.0077); Enterococcus, −2.4% (p = 0.09)).
Table 4.
Effects of Annurca apple puree (AAP) and its lactofermented version (lfAAP) on intestinal microbial composition.
| AAP | Δ (%) | lfAAP | Δ (%) | LAB | Δ (%) | ||
|---|---|---|---|---|---|---|---|
| Bifidobacterium (CFU/mL) | t 0 | 10,499 ± 2458 | 20,341 ± 4316 | 15,239 ± 3104 | |||
| t 30 | 637,289 ± 82,567 * | +6970 # | 62,040 ± 5018 * | +205 # | 335,258 ± 45,128 * | +2100 # | |
| t 60 | 776,961 ± 74,329 * | +7300 # | 70,379 ± 6894 * | +246 # | 396,214 ± 45,219 * | +2500 # | |
| t 90 | 623,640 ± 77,241 * | +5840 # | 57,971 ± 5746 * | +185 # | 274,302 ± 39,489 * | +1700 # | |
| Lactobacillus (CFU/mL) | t 0 | 3584 ± 532 | 2989 ± 387 | 3467 ± 478 | |||
| t 30 | 748,339 ± 64,651 * | +20,780 # | 6127 ± 597 * | +105 # | 124,812 ± 49,731 * | +3500 # | |
| t 60 | 1,078,784 ± 109,657 * | +30,000 # | 6486 ± 529 * | +117 # | 140,413 ± 14,521 * | +3950 # | |
| t 90 | 644,761 ± 58,452 * | +17,890 # | 5843 ± 509 * | +95.5 # | 104,010 ± 23,464 * | +2900 # | |
| Bacteroides (CFU/mL) | t 0 | 7843 ± 755 | 5671 ± 487 | 6891 ± 337 | |||
| t 30 | 3529 ± 550 * | −55.1 # | 4139 ± 364 * | −27.5 # | 5168 ± 499 * | −25.8 # | |
| t 60 | 2901 ± 351 * | −63.2 # | 3856 ± 405 * | −32.1 # | 4410 ± 461 * | −36.0 # | |
| t 90 | 4062 ± 259 * | −48.2 # | 4360 ± 422 * | −23.1 # | 5499 ± 415 * | −20.2 # | |
| Enterococcus (CFU/mL) | t 0 | 6914 ± 419 | 8317 ± 575 | 7819 ± 561 | |||
| t 30 | 6706 ± 731 | −3.7 # | 7651 ± 645 * | −8.4 # | 5082 ± 484 * | −35.5 # | |
| t 60 | 6568 ± 457 | −5.3 # | 7402 ± 534 * | −11.6 # | 4456 ± 379 * | −43.0 # | |
| t 90 | 6713 ± 610 | −2.9 # | 7693 ± 625 * | −7.5 # | 5614 ± 404 * | −28.2 # |
Data are expressed as CFU/mL of faecal swab sample. Values are means ± SD (n = 5). * Significantly different from baseline at p < 0.01 (PROC MIXED). # Significantly different from the other treatments at p < 0.05 (PROC MIXED). t 0: 1st day of treatment; t 30: 30th day of treatment; t 60: 60th day of treatment; t 90: 30th day of follow-up. AAP = subgroup administered with Annurca apple puree (125 g/day); lfAAP = subgroup administered with lactofermented Annurca apple puree (125 g/day); LAB = subgroup administered with 1 capsule/day, containing Lactobacillus rhamnosus (3.0 × 108 CFU).
3.6. Study Strength and Limitations
The major strengths of the clinical trial herein presented reside in the originality of the study and in the evaluation of the treatment effects in real-world settings. The positive results, herein reported, can inform physicians about a novel treatment/intervention, which can represent a valuable alternative in the clinical practice. Conversely, the main limitations of our study include the short-term assessment for the treatment of a chronic condition, the choice of exclusively white race, and the wide age range due to the availability of such individuals at the stage of the recruitment. Moreover, the lack of a placebo group, and the intention to make a treatment comparison, are clear indicators of the explorative nature of the present work, confirmed by the absence of a power analysis, which intends to define the bases of a more structured and statistically robust clinical study.
4. Discussion
Our experimental results regarding the lactic acid fermentation of Annurca apple puree indicated a general increase of the polyphenolic compound levels in the final product, although this effect may strongly vary according to the type of Lactobacillus strain used and the incubation conditions (time and temperature) (Table 1). Polyphenols would be released from soluble and insoluble fibre during the fermentation process thanks to the action of bacterial hydrolases, thus, potentially enhancing their intestinal bioaccessibility [14,15,16]. Previous authors have demonstrated that ingestion of dietary polyphenols is followed by their absorption into the circulatory system through the small intestine [2]. However, it is well known that the bioavailability of polyphenols in the small intestine is quite low, mainly due to their general high polarity and molecular weight [2]. Nevertheless, a wide range of biochemical reactions occur along the entire intestinal tract which can significantly increase the possibility of polyphenols being absorbed: i) hydrolysis of glycosylated polyphenols by lactase and cytosolic β-glucosidases, releasing more lipophilic aglycones which may then enter the epithelial cells by passive diffusion [28]; ii) metabolism (sulfation, glucuronidation and/or methylation) of the same aglycones prior to passage into the blood stream [29]; and iii) further fermentation by colonic microflora, which can cleave conjugating moieties and subject the resultant aglycones to ring fission, leading to the production of phenolic acids and hydroxycinnamates, which can be absorbed in the large intestine [29]. All of these metabolites can be absorbed and ultimately excreted in urine in substantial quantities that, in most instances, are well in excess of the flavonoid metabolites that entered the circulatory system via the small intestine [2]. Therefore, it is evident that the increase in intestinal bioaccessibility of food polyphenols by lactic acid fermentation could lead to the formation of bioavailable antioxidant compounds with potential beneficial effects at systemic level.
Fermentation of AAP for 24 h using L. rhamnosus represented the best incubation conditions in terms of increase in polyphenol bioaccessibility (Table 1). Thus, this product (lfAAP) was chosen to be administered to subjects in order to evaluate its effects on plasma primary outcomes. Specifically, lfAAP exerted a significant influence on HDL-C levels, allowing a higher increase of its values than what was assessed in subjects assuming AAP and LAB (Table 3). Our research group previously evaluated the influence of daily consumption of Annurca apples on the cholesterol levels of mildly hypercholesterolemic healthy subjects [18].
Interestingly, a significant effect was also obtained by lfAAP on plasma TMAO values, decreasing its levels by 63.1% (p = 0.0042) in respect to what was revealed in subjects belonging to AAP (−42.3%; p = 0.0032) and LAB groups (−25.8%; p = 0.0029) (Table 3). Trimethylamine-N-oxide is currently recognized as a prognostic marker of oxidative stress for incident cardiovascular events beyond traditional risk factors [30,31]. Normal TMAO blood levels range from 0.5 to 5 µM [32,33], and increased TMAO serum levels are associated with a greater CVD risk [31]. Primarily, TMAO derives from the metabolism of choline and L-carnitine by the gut microbiota (mainly Clostridia, Shigella, Proteus, and Aerobacter), producing trimethylamine (TMA) which is oxidised by flavin-containing monooxygenase-3 (FMO3) in the liver [30,33,34,35,36]. In this context, the microbiota remodelling has been recognised as a novel and useful approach for the management of high TMAO levels-related diseases [37]. Particularly, evidence has shown that polyphenols are able to act as bactericide and bacteriostatic agents against some specific bacterial strains, mainly Clostridia and Bacteroides [38,39], and to favour the growth of non-TMA producing species, including Prevotella and Firmicutes [40], suggesting that a diet rich in polyphenols, or food supplements, may represent a strategy for microbiota remodelling. Actually, a further interpretation regarding plasma TMAO-reducing effect observed in this study may be formulated. Polyphenols are well-known as powerful antioxidant molecules, and it has been established that TMAO can act as an electron acceptor metabolite [41]. At the serum level, thus, TMAO and polyphenols may be involved in redox reactions, resulting in the generation of TMA. Very few recent studies have evaluated the effects of specific supplementations on plasma TMAO levels in humans. In the trial of Angiletta et al. [42], obese subjects at risk for insulin resistance, having elevated TMAO levels (≥5 μM) compared to levels previously measured in healthy subjects (∼1 μM), consumed tea or cocoa flavanols in a randomized crossover design while consuming a controlled diet [43]. Another study assessed the influence of inulin supplementation on plasma TMAO concentrations in individuals at risk for type 2 diabetes (TMAO levels ∼3 μM) [42]. Both studies concluded that the respective interventions did not significantly affect plasma TMAO levels in the enrolled subjects. Thus, our study is the first evidence of an effective dietary intervention on plasma TMAO levels in healthy subjects.
According to our secondary efficacy outcome measures, AAP was supposed to be the most effective in positively remodelling intestinal microbiota (Table 4). Although faecal swabs should be regarded as mere indicators of intestinal microbial population, and the four microbial genera (Bifidobacterium, Lactobacillus, Bacteroides, and Enterococcus), objects of our analyses are not fully representative of human gut microbiota, subjects assuming AAP revealed, in particular, a higher increase of fermentative bacteria than those administered with lfAAP. It could be hypothesised that AAP, richer in intact pectic compounds than lfAAP, may have provided subjects with substances of prebiotic interest which are well-known to strongly address the gut microflora balance in favour of the fermentative bacteria [44,45]. Furthermore, faecal swabs from subjects assuming AAP revealed a higher increase of fermentative bacteria even than those from LAB subgroup. Such results are of major interest since it would corroborate that prebiotics may play a major role in manipulating gut microbial composition with an efficacy even higher than what is possible through an attempt of direct gut colonisation with probiotics [46]. Undoubtedly, our results need to be deepened and supported by further experimental intervention.
5. Conclusions
The present study indicates lfAAP as an effective functional food for beneficial control of plasma HDL-C and TMAO levels, with clinical relevance in primary cardiovascular disease prevention. Obviously, further in vitro, in vivo, and human trials are needed to corroborate our results. Particularly, specific investigations will be necessary to identify the main constituents responsible for the clinical results observed in this study, and to evaluate their contribution to the described effects.
Acknowledgments
The authors wish to thank Medical Doctors: Antonio Albanese, Maria Capozzi, Giovanni Liviero, Gabriele Monteforte, Umberto Iuliano, Bernardo Salerno, Rosa Servodio, Nicola Zeoli, Alessandra De Vita, Elisa Panello, Pompea Pascucci (UCCP—Unità Complessa Cure Primarie, Via Manzoni, San Giorgio del Sannio, Benevento, Italy); and Laboratory Analysts: A. Capozzi (UNILAB SANNIO S.C.A.R.L. Via Dei Sanniti, San Giorgio del Sannio, Benevento, Italy). Their contribution is gratefully appreciated.
Author Contributions
G.C.T., D.C., G.B., M.D., B.B., and E.N. were responsible for study concept and design. G.C.T., D.C., G.B., M.D., R.C., M.M., C.S., B.B., and E.N. acquired the clinical data. G.C.T., R.C., C.S., and M.M. acquired the in vitro data. All authors analysed and interpreted the data and drafted the manuscript. All authors critically revised the manuscript for important intellectual content. E.N. obtained funding. E.N. provided administrative, technical, or material support. All authors supervised the study. All authors, external and internal, had full access to all of the data. E.N. is the guarantor.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the ethics committee at the Hospital AO Rummo of Benevento, Italy.
Transparency statement
The lead author (the manuscript’s guarantor) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Cheynier V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005;81:223s–229s. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 2.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 3.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005;81:215s–217s. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 4.Padayachee A., Netzel G., Netzel M., Day L., Zabaras D., Mikkelsen D., Gidley M. Binding of polyphenols to plant cell wall analogues-Part 1: Anthocyanins. Food Chem. 2012;134:155–161. doi: 10.1016/j.foodchem.2012.02.082. [DOI] [PubMed] [Google Scholar]
- 5.Padayachee A., Netzel G., Netzel M., Day L., Zabaras D., Mikkelsen D., Gidley M.J. Binding of polyphenols to plant cell wall analogues-Part 2: Phenolic acids. Food Chem. 2012;135:2287–2292. doi: 10.1016/j.foodchem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Le Bourvellec C., Bouchet B., Renard C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Bba-Gen Subj. 2005;1725:10–18. doi: 10.1016/j.bbagen.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Renard C.M.G.C., Baron A., Guyot S., Drilleau J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001;29:115–125. doi: 10.1016/S0141-8130(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 8.Beliveau R. Role of nutrition in preventing cancer. Can. Fam. Physician. 2007;53:1905–1911. [PMC free article] [PubMed] [Google Scholar]
- 9.Ebner S., Smug L.N., Kneifel W., Salminen S.J., Sanders M.E. Probiotics in dietary guidelines and clinical recommendations outside the European Union. World J. Gastroenterol. 2014;20:16095–16100. doi: 10.3748/wjg.v20.i43.16095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligne B., Ganzle M., Kort R., Pasin G., Pihlanto A., et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotech. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Ray R.C., Ward O.P. Microbial Biotechnology in Horticulture. Volume 1 CRC Press; Plymouth, UK: 2006. [Google Scholar]
- 12.Motarjemi Y. Impact of small scale fermentation technology on food safety in developing countries. Int. J. Food Microbiol. 2002;75:213–229. doi: 10.1016/S0168-1605(01)00709-7. [DOI] [PubMed] [Google Scholar]
- 13.Septembre-Malaterre A., Remize F., Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018;104:86–99. doi: 10.1016/j.foodres.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Granito M., Frias J., Doblado R., Guerra M., Champ M., Vidal-Valverde C. Nutritional improvement of beans (Phaseolus vulgaris) by natural fermentation. Eur. Food Res. Technol. 2002;214:226–231. doi: 10.1007/s00217-001-0450-5. [DOI] [Google Scholar]
- 15.Martin-Cabrejas M.A., Sanfiz B., Vidal A., Molla E., Esteban R., Lopez-Andreu F.J. Effect of fermentation and autoclaving on dietary fiber fractions and anti nutritional factors of beans (Phaseolus vulgaris L.) J. Agric. Food Chem. 2004;52:261–266. doi: 10.1021/jf034980t. [DOI] [PubMed] [Google Scholar]
- 16.Siebenhandl S., Lestario L.N., Trimmel D., Berghofer E. Studies on tape ketan-an Indonesian fermented rice food. Int. J. Food Sci. Nutr. 2001;52:347–357. doi: 10.1080/09637480120057585. [DOI] [PubMed] [Google Scholar]
- 17.Tenore G.C., Campiglia P., Stiuso P., Ritieni A., Novellino E. Nutraceutical potential of polyphenolic fractions from Annurca apple (M. pumila Miller cv Annurca) Food Chem. 2013;140:614–622. doi: 10.1016/j.foodchem.2012.10.112. [DOI] [PubMed] [Google Scholar]
- 18.Tenore G.C., Caruso D., Buonomo G., D’Urso E., D’Avino M., Campiglia P., Marinelli L., Novellino E. Annurca (Malus pumila Miller cv. Annurca) apple as a functional food for the contribution to a healthy balance of plasma cholesterol levels: Results of a randomized clinical trial. J. Sci. Food Agric. 2017;97:2107–2115. doi: 10.1002/jsfa.8016. [DOI] [PubMed] [Google Scholar]
- 19.Lo Scalzo R., Testoni A., Genna A. ‘Annurca’ apple fruit, a southern Italy apple cultivar: Textural properties and aroma composition. Food Chem. 2001;73:333–343. doi: 10.1016/S0308-8146(00)00306-X. [DOI] [Google Scholar]
- 20.Nazzaro F., Fratianni F., Sada A., Orlando P. Synbiotic potential of carrot juice supplemented with Lactobacillus spp. and inulin or fructooligosaccharides. J. Sci. Food Agric. 2008;88:2271–2276. doi: 10.1002/jsfa.3343. [DOI] [Google Scholar]
- 21.Garbetta A., D’Antuono I., Sisto A., Minervini F., Cardinali A., Lavermicocca P. Effect of artichoke fermentation by probiotic strain Lactobacillus paracasei LMG P-22043 and of digestion process on polyphenols and antioxidant activity. J. Funct. Foods. 2018;45:523–529. doi: 10.1016/j.jff.2018.02.020. [DOI] [Google Scholar]
- 22.Beale R., Airs R. Quantification of glycine betaine, choline and trimethylamine N-oxide in seawater particulates: Minimisation of seawater associated ion suppression. Anal. Chim. Acta. 2016;938:114–122. doi: 10.1016/j.aca.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Little R.J.A., Rubin D.B. Statistical Analysis with Missing Data. John Wiley & Sons, Inc.; New York, NY, USA: 2002. [Google Scholar]
- 24.Molenberghs G., Thijs H., Jansen I., Beunckens C., Kenward M.G., Mallinckrodt C., Carroll R.J. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5:445–464. doi: 10.1093/biostatistics/kxh001. [DOI] [PubMed] [Google Scholar]
- 25.O’Kelly M., Ratitch B. Clinical Trials with Missing Data: A Guide for Practitioners. John Wiley & Sons, Inc.; New York, NY, USA: 2014. [Google Scholar]
- 26.White I.R., Carpenter J., Horton N.J. Including all individuals is not enough: Lessons for intention-to-treat analysis. Clin. Trials. 2012;9:396–407. doi: 10.1177/1740774512450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvert M., Blazeby J., Altman D.G., Revicki D.A., Moher D., Brundage M.D., Grp C.P. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. J. Am. Med. Assoc. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 28.Day A.J., Canada F.J., Diaz J.C., Kroon P.A., Mclauchlan R., Faulds C.B., Plumb G.W., Morgan M.R.A., Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/S0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 29.Crozier A. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Blackwell Publishing Ltd.; Hoboken, NJ, USA: 2007. [Google Scholar]
- 30.Li X.S.M., Obeid S., Klingenberg R., Gencer B., Mach F., Raber L., Windecker S., Rodondi N., Nanchen D., Muller O., et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramaniam S., Fletcher C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018;175:1344–1353. doi: 10.1111/bph.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ufnal M., Zadlo A., Ostaszewski R. TMAO: A small molecule of great expectations. Nutrition. 2015;31:1317–1323. doi: 10.1016/j.nut.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z.N., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X.M., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisel S.H., Wishnok J.S., Blusztajn J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 1983;225:320–324. [PubMed] [Google Scholar]
- 35.Bennett B.J., Vallim T.Q.D., Wang Z.N., Shih D.M., Meng Y.H., Gregory J., Allayee H., Lee R., Graham M., Crooke R., et al. Trimethylamine-N-Oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koeth R.A., Levison B.S., Culley M.K., Buffa J.A., Wang Z.N., Gregory J.C., Org E., Wu Y.P., Li L., Smith J.D., et al. gamma-Butyrobetaine Is a proatherogenic intermediate in gut microbial metabolism of L-Carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyu M., Wang Y.F., Fan G.W., Wang X.Y., Xu S.Y., Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selma M.V., Espin J.C., Tomas-Barberan F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 39.Lee H.C., Jenner A.M., Low C.S., Lee Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 40.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 41.Barrett E.L., Kwan H.S. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 1985;39:131–149. doi: 10.1146/annurev.mi.39.100185.001023. [DOI] [PubMed] [Google Scholar]
- 42.Baugh M.E., Steele C.N., Angiletta C.J., Mitchell C.M., Neilson A.P., Davy B.M., Hulver M.W., Davy K.P. Inulin supplementation does not reduce plasma trimethylamine n-oxide concentrations in individuals at risk for type 2 diabetes. Nutrients. 2018;10 doi: 10.3390/nu10060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angiletta C.J., Griffin L.E., Steele C.N., Baer D.J., Novotny J.A., Davy K.P., Neilson A.P. Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Func. 2018;9:5350–5361. doi: 10.1039/C8FO00962G. [DOI] [PubMed] [Google Scholar]
- 44.Islamova Z.I., Ogai D.K., Abramenko O.I., Lim A.L., Abduazimov B.B., Malikova M.K., Rakhmanberdyeva R.K., Khushbaktova Z.A., Syrov V.N. Comparative assessment of the prebiotic activity of some pectin polysaccharides. Pharm. Chem. J. 2017;51:288–291. doi: 10.1007/s11094-017-1600-9. [DOI] [Google Scholar]
- 45.Chung W.S.F., Meijerink M., Zeuner B., Hoick J., Louis P., Meyer A.S., Wells J.M., Flint H.J., Duncan S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017;93 doi: 10.1093/femsec/fix127. [DOI] [PubMed] [Google Scholar]
- 46.Vieira A.T., Teixeira M.M., Martins F.S. The role of probiotics and prebiotics in inducing gut immunity. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]