Abstract
About 20% of patients with high grade serous epithelial ovarian carcinoma (HGSOC) are intrinsically resistant to standard first-line platinum-based combination therapy. There is no marker yet available to identify these patients. In that context, all patients with HGSOC initially receive the same standard first-line platinum-based therapy, and those with intrinsically resistant diseases can only be identified retrospectively after they experienced early relapse to therapy. The aim of this study was to evaluate serum or ascites CA125 and ascites leptin in patients with intrinsic resistance and to compare them with those of sensitive patients. To this end, we enrolled 80 women with HGSOC who underwent cytoreductive surgery. Thirty seven were considered to have baseline clinical resistance to first-line therapy with progression-free survival < 6 months despite treatment. Serum were collected preoperatively and ascites samples were collected at the time of the surgery. The levels of CA125 and leptin were measured by ELISA. Patients with baseline clinical resistance to first-line therapy had a significantly poorer outcome compared to patients with sensitive HGSOC with an OS of 21 months versus 43 months. Median levels of serum CA125, ascites CA125 and ascites leptin were not significantly different between patients with sensitive and resistant HGSOC. Serum CA125/ascites leptin ratio was found to be significantly elevated in resistant patients compared to patients with drug-sensitive diseases. In ROC analysis, the AUC for serum CA125/ascites leptin ratio was higher than CA125 or leptin alone to differentiate patients with resistance from those with sensitive HGSOC. Elevated serum CA125/ascites leptin ratio was a predictor of poor OS in HGSOC patients. Thus, serum CA125/ascites leptin is a potential novel biomarker to predict baseline clinical resistance to first-line treatment and poor outcome in patients with HGSOC.
Keywords: Ascites, ovarian cancer, biomarkers, leptin, CA125, drug resistance
Introduction
Despite extensive efforts to improve treatments, ovarian cancer (OC) remains a deadly disease with a median progression-free survival (PFS) of only 16-22 months and a 5-year survival rate of < 30% [1]. This high mortality is related to the often asymptomatic OC progression (most women present with an advanced metastatic disease) and the frequent occurrence of drug resistance [2,3]. Management of these patients include a complete cytoreductive surgery followed by chemotherapy. Platinum-based combination chemotherapy is the standard first-line treatment for advanced stage OC. Although overall initial response rates to first-line platinum based chemotherapy are good (80%), 20% of patients will not respond to the initial chemotherapy and will be considered to have baseline clinical resistance [4,5]. Baseline clinical resistance (the so-called refractory patients) is defined by the absence of response to platinum-based therapy or progression during the course of therapy, or if the clinical progression-free survival (PFS) is less than 6 months [6]. Furthermore, the majority of patients who respond to first-line therapy will ultimately develop recurrent disease. High-grade serous OC (HGSOC) is the most common histotype, which is associated with spread into the peritoneal cavity and late onset of symptoms [7].
Predictive biomarkers are urgently needed to improve diagnosis, guide new molecular targeted therapy and monitor activity and therapeutic response across a wide spectrum of cancers. In particular, the identification of novel surrogate biomarkers to identify HGSOC patients with baseline clinical drug resistance would significantly decrease the mortality and the morbidity associated with unnecessary chemotherapy toxicity. Currently, patients with baseline clinical resistant diseases can only be identified retrospectively after they experienced a lack of response to first-line therapy.
Increased adiposity is associated with increased cancer incidence, morbidity and mortality [8]. Furthermore, epidemiological studies have shown that obesity is associated with an increased risk of OC as a high body mass index (BMI) strongly correlates with the occurrence of OC [9,10]. Cancer-associated adipocytes support the growth and metastasis of cancer cells, contributing to an invasive and aggressive phenotype [8]. Leptin is a pleiotropic adipokine that is produced primarily in adipose tissue. Leptin is expressed at high levels in ascites of patients with OC [11] and is known to stimulate tumor cell proliferation, migration and invasion [12]. Recent studies have suggested that high circulating levels of leptin correlate with a worse outcome in OC [13,14]. Tumor expression of leptin is associated with cisplatin resistance [15]. The tumor antigen CA125, encoded by MUC16 mucin gene, is currently used in clinic for disease monitoring and assessing response and relapse to treatment [16-21]. The N-terminal extracellular region of MUC16 is cleaved and released into the serum of patients with HGSOC [16,17]. Recent studies suggest that a Risk of Ovarian Malignancy Algorithm (ROMA) incorporating CA125 and HE4 levels in serum shows a high potential for discriminating ovarian cancer from benign gynecological diseases [22,23]. Several studies have demonstrated that MUC16 C-terminal domain enhanced proliferation, migration, invasion and tumorigenicity of ovarian, breast and pancreatic cancer cell lines [25,26]. Ectopic expression of MUC16 C-terminal domain has also been shown to confer enhanced resistance to cisplatin and taxol [27]. A recent study suggested that the combination of CA125 and leptin could be a potential predictor of baseline clinical resistance in OC [11]. These data suggest that quantification of CA125-leptin ratio might be useful as a predictive and prognostic biomarker for OC.
Ascites is an attractive biofluid for biomarker discovery as it is easy and minimally invasive to obtain. Proximal fluids such as ascites-as opposed to serum-might reflect events in ovarian tumorigenesis earlier than in peripheral blood circulation [28]. Furthermore, the concentration of soluble factors is usually much higher in ascites compared to serum [29]. Thus, the accessibility of ascites-a simple non-invasive puncture-provides an excellent source of potential diagnostic and prognostic biomarkers.
Accordingly, in the present study, we aimed to evaluate the clinical significance of CA125 levels in both serum and ascites and leptin levels in ascites and whether their expression levels in HGSOC patients could serve as a biomarker for predicting baseline clinical resistance and outcome.
Material and methods
Patients
The study population consisted of 80 patients with newly diagnosed HGSOC admitted at the Centre Hospitalier Universitaire de Sherbrooke (CHUS). All patients received three to six courses of adjuvant chemotherapy consisting of carboplatin and taxol. This study was approved by the Institutional Review Board of the Centre de Recherche of CHUS. Informed consent was obtained from women that underwent surgery by the gynecologic oncology service between 2000 and 2018. All samples were reviewed by an experienced pathologist. Baseline characteristics and serum CA125 levels were collected for all patients. All patients had a follow up ≥ 12 months. Disease progression was defined by either serum CA125 ≥ 2 × nadir value on two occasions, documentation of lesion progression or appearance of new lesions on CT-scan or death [30]. Patient’s conditions were staged according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO). PFS was defined by the time from the initial surgery to evidence of disease progression. Baseline drug resistance was defined as those with PFS < 6 months or lack of response to initial platinum-based chemotherapy or progression during initial treatment based on serum CA125 values, or lack of response, progression or appearance of new lesions on CT-scan or ultrasound, and death within the first 6 months [6].
Peritoneal fluid specimens
Ascites is routinely obtained at the time of the initial cytoreductive surgery for ovarian cancer patients treated at the Centre Hospitalier Universitaire de Sherbrooke. Ascites were centrifuged at 1000 rpm for 15 min and cell-free supernatants were stored at -80°C until assayed. All acellular fluids were supplied by the Banque de tissus et de données of the Réseau de Recherche en Cancer of the Fonds de la Recherche du Québec en Santé affiliated to the Canadian Tumor Repository Network (CTRNet).
ELISA measurements
Leptin levels in ascites samples were determined by ELISA using the commercially available human Quantikine kits from R&D Systems (Minneapolis, MN). The assays were performed in duplicate according to the manufacturer’s protocols. The detection threshold for leptin is 7.8 pg/ml and the intra-assay variability is 3-3.2%. The inter-assay variability is 3.5%. The median values were used for statistical analysis.
CA125 measurements
CA125 was determined at Centre Hospitalier Universitaire de Sherbrooke laboratory in serum samples by EIA using the Elecsys 2010 analyzer and CA125 II reagents (Roche Diagnostics, Québec, Canada). The reference range was 0-35 kUI/L.
Statistical analysis
Comparison between unpaired groups was made using the Mann-Whitney test or the Kruskal-Wallis test. Statistical differences in PFS were determined by the log-rank test, and Kaplan-Meier survival curves were made. PFS was defined as the interval between the date of the initial debulking surgery and the time of disease progression or the last date of follow up. Receiver-operator characteristic (ROC) analysis was performed to determine the discriminating value of CA125 and leptin in distinguishing patients with baseline clinical resistance to first-line treatment from the sensitive patients. Sensitivity against 1-specificity was plotted at each cutoff threshold, and the area under the curve (AUC) values were determined. The optimal cutoff threshold for discriminating patients with sensitive and resistant diseases was obtained by the Youden index [31], which is the point on the ROC curve at which the Youden index was maximal. Progression-free survival (PFS) and overall survival (OS) curves were analyzed using the Kaplan-Meier method, and differences were examined using the log-rank tests. The threshold for statistical significance is P < 0.05.
Results
Comparison of CA125 and leptin levels between drug resistant and drug sensitive patients
Between 1998 and 2017, 422 newly diagnosed patients with various sub-types of OC were enrolled in our biobank, among whom 164 were high grade (2/3 or 3/3) serous type (8% stage I/II and 92% stage III/IV). Out of the 164 patients, 37 (22.5%) were resistant to first-line chemotherapy. All resistant patients were included in this study. The patient baseline characteristics are shown in Table 1. There was no significant differences between patients with sensitive OC and those with baseline clinical resistance with regards to age, optimal cytoreductive surgery and body mass index (BMI). Patients with baseline clinical resistance to first-line therapy had a median progression-free survival (PFS) of 4 months and an overall survival (OS) of 21 months compared to patients with drug-sensitive OC who had a PFS of 13 months (P < 0.0001) and an OS of 43 months respectively (P = 0.0003) (Figure 1).
Table 1.
Baseline characteristics of study patients
| Variables | Sensitive (N = 43)No (%) | Resistant (N = 37)No (%) |
|---|---|---|
| Age (years) | ||
| Median | 63 | 62 |
| Range | 36-79 | 27-85 |
| BMI* | ||
| Median | 24.8 | 24.3 |
| Range | 19.4-40.9 | 14.9-37.4 |
| Stage (FIGO) | ||
| I/II | 1 (2.3) | 0 (0) |
| III/IV | 42 (97.3) | 37 (100) |
| Grade | ||
| 1 | 0 (0) | 0 (0) |
| 2 | 8 (18.6) | 9 (24.3) |
| 3 | 35 (81.4) | 28 (75.7) |
| Histologic subtype | ||
| Serous | 43 (100) | 37 (100) |
| Mucinous | 0 (0) | 0 (0) |
| Endometriod | 0 (0) | 0 (0) |
| Mixed cells | 0 (0) | 0 (0) |
| Debulking status | ||
| Optimal < 2 cm | 34 (79.1) | 28 (75.7) |
| Suboptimal > 2 cm | 9 (20.9) | 9 (24.3) |
| Prior chemotherapy | ||
| Yes | 30 (69.8) | 28 (76.7) |
| No | 13 (30.2) | 9 (24.3) |
| Median serum CA125 at surgery (KUI/L) | 43.6 | 144.8 |
| Range | 9.6-4382 | 9-14180 |
BMI = body mass index.
FIGO = International Federation of Gynecology and Obstetrics.
Figure 1.
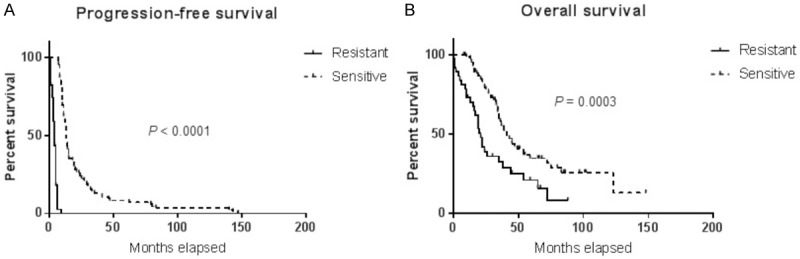
Outcome of patients with baseline clinical resistance versus those with drug resistant diseases. Kaplan-Meier curves for (A) PFS and (B) OS according to the patient resistance status.
The median CA125 levels in serum of patients with resistant diseases was 144.8 KIU/L compared to 43.6 KIU/L for patients with sensitive OC, a difference that was not significantly different (P = 0.3205) (Figure 2A). Furthermore, median ascites CA125 levels were 295 KIU/L and 168 KIU/L for patients with resistant and sensitive diseases respectively with P = 0.6907 (Figure 2B). Leptin ascites levels were also not statistically different (P = 0.0545) with a median of 672 pg/ml for resistant patients and 724 pg/ml for sensitive patients (Figure 2C). When combining two biomarkers by calculating serum CA125/ascites leptin ratio, it was found to be significantly higher in resistant patients compared to patients with drug-sensitive diseases (P = 0.0230) (Figure 2D). In contrast, ascites CA125/ascites leptin ratio was not significantly different between the two groups (data not shown).
Figure 2.
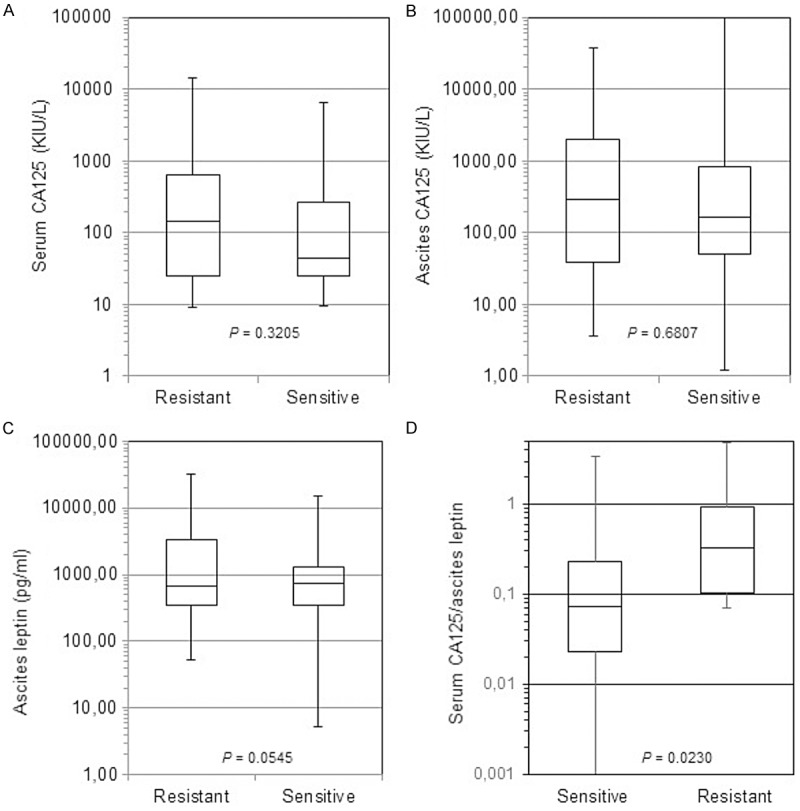
Serum and ascites levels of CA125 and ascites levels of leptin in HGSOC patients with baseline clinically resistance to first-line therapy compared to those with sensitive HGSOC. Box plots representing serum levels of CA125 (A), ascites CA125 (B), leptin (C), serum CA125/leptin ratio (D). The P value is indicated for each factor.
AUC values for CA125 and leptin
We next performed ROC analyses to determine the potential usefulness of CA125 and leptin as biomarkers for baseline clinical resistance in OC. Our ROC curves revealed that taken individually, serum CA125, ascites CA125 and leptin did not discriminate patients with baseline clinical resistance from those with sensitive diseases with AUC values ranging from 0.5089 to 0.5534 (Figure 3A-C). Ascites CA125/ascites leptin was also unable to discriminate between the two groups with AUC values of 0.5555 (Figure 3D). In contrast, serum CA125/ascites leptin was more robust in discriminating patients with baseline clinical resistance to first-line therapy from patients with sensitive diseases, with AUC values of 0.846 (CI: 0.759-0.934; P < 0.0001) (Figure 3E). At a cutoff value of 0.3, the sensitivity and specificity of serum CA125/ascites leptin ratio to identify an OC patient with baseline clinical resistance were 61.8% and 88.4%, respectively.
Figure 3.
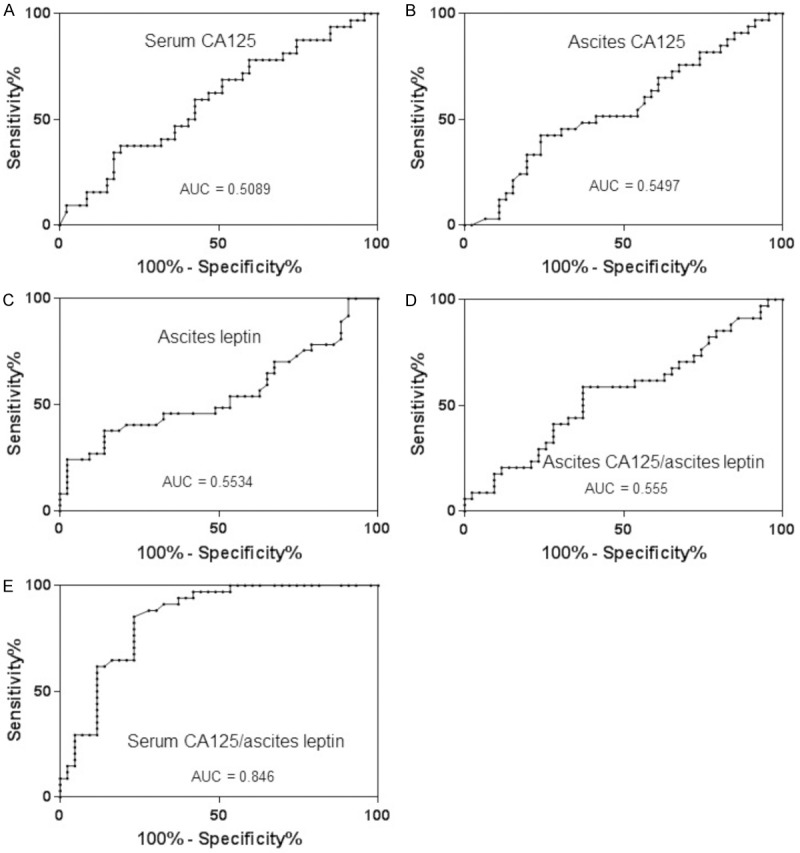
ROC curves analysis and AUC values for distinguishing patients with baseline resistance to first-line therapy versus those with initially sensitive HGSOC. Serum CA125 (A), ascites CA125 (B), ascites leptin (C), ascites CA125/ascites leptin ratio (D), and serum CA125/ascites leptin ratio (E).
Prognostic significance of CA125 and leptin
We next examined whether serum or ascites CA125 and ascites leptin in patients with HGSOC could serve as predictor of outcome. Towards this end, we performed Kaplan-Meier survival analysis. At the end of the follow up, 64 patients in our cohort had died (80%). The median value was used as a cutoff to separate patients into low and high producers. As shown in Figure 4, CA125 levels, either in the serum or ascites, were not predictive of PFS or OS in our cohort of patients. Although not significant (P = 0.6049), there was a trend towards a shorter PFS in patients with high leptin levels in ascites. Furthermore, higher ascites leptin levels were predictive of significantly poor OS, with a median OS of 28 months for high leptin levels versus 49 months for patients with low ascites leptin levels (P = 0.0195). Importantly, high serum CA125/ascites leptin ratio was a significant predictor of poor PFS and OS with 10 months versus 6 months (P = 0.0369) and 37 months versus 21 months (P = 0.0374), respectively (Figure 5).
Figure 4.
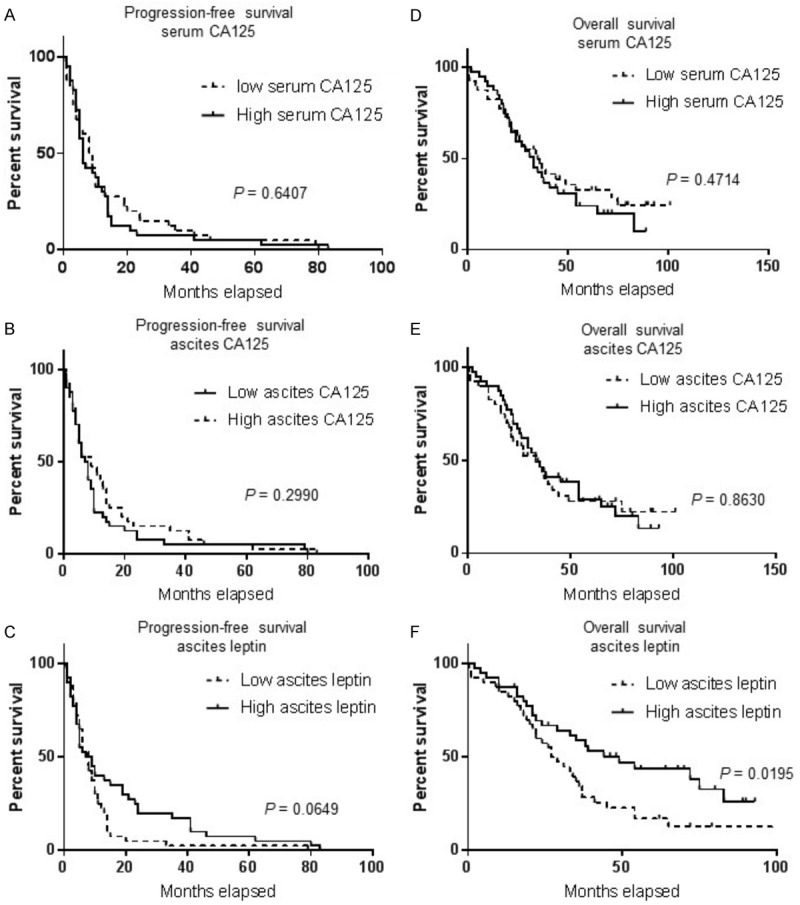
Overall survival (OS) and progression-free survival (PFS) curves of the 80 patients with HGSOC plotted against CA125 and leptin. A. PFS curve for serum CA125. B. PFS curve for ascites CA125. C. PFS curve for ascites leptin. D. OS curve for serum CA125. E. OS curve for ascites CA125. F. OS curve for ascites leptin. The median levels of each factor were taken as cutoff points. The P value is indicated for each factor.
Figure 5.
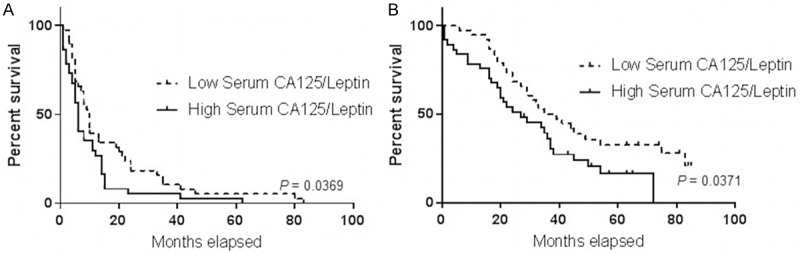
Overall survival (OS) and progression-free survival (PFS) curves of the 80 patients with HGSOC plotted against serum CA125/ascites leptin ratio. PFS (A) and OS (B) curves.
Discussion
To the best of our knowledge, this is the first study in which serum and ascites levels of CA125 along with ascites levels of leptin were measured in patients with HGSOC and linked to a poor outcome and to baseline clinical resistance to first-line platinum-based treatment. To ensure a homogenous group of patients for this study, and as it is the most common histotype, we selected patients with HGSOC, which is often diagnosed at an advanced stage with spread into the peritoneal cavity. In this context, the conclusions of this study may not apply to other ovarian cancer sub-types, which have distinct genetic backgrounds. In addition, we used a strict definition of resistance and only HGSOC patients that were refractory to first-line platinum-based treatment were considered as having baseline clinical resistance. With no surprise, we found that patients presenting with baseline clinical resistance to first-line treatment had a shorter OS compared to patients that were initially sensitive to first-line treatment (21 months versus 43 months; P = 0.0003).
Predictive tools are urgently needed to improve diagnosis, guide new molecular targeted therapy and monitor activity and therapeutic response across a wide spectrum of cancers. In particular, the identification of novel surrogate biomarkers to identify patients with baseline drug resistant OC would significantly decrease the mortality and the morbidity associated with as 22% of OC patients in this study were intrinsically resistant to first-line standard chemotherapy. Currently, all patients with HGSOC initially receive the same standard first-line platinum-based therapy, and those with baseline clinical resistance can only be identified retrospectively after they experienced early relapse to therapy. Thus, biomarkers that could identify those with resistant diseases before they have to go through inefficient and toxic first-line chemotherapy are highly needed. The identification of biomarkers that can predict clinical responses to the initial platinum-based therapy would provide a practical approach to identify patients who could benefit from alternative or novel experimental therapies. In that context, we demonstrated for the first time the potential role of the ratio of serum levels of CA125 and ascites leptin for the identification of patients with baseline clinical resistance to standard first-line therapy. This is supported by the high AUC value derived from comparisons between patients that were refractory to first-line therapy and those that responded to this initial treatment (AUC = 0.846). These findings are in line with those of a smaller pilot study which suggested the discriminating potential of CA125 and leptin for patients with drug resistant OC [11]. In addition, we observed that high serum CA125/ascites leptin ratios in HGSOC patients were associated a shorter PFS and a poorer OS.
Ectopic expression of MUC16 c-terminal domain has been associated with resistance to cisplatin and death receptor ligand in ovarian and breast cancer cell lines by altering the expression of pro- and anti-apoptotic proteins [26,27,32]. MUC16 overexpression in lung cancer cells induced resistance to cisplatin and gemcitabine by downregulation of p53 [33]. In gastro-oesophageal carcinomas, tumor leptin expression was associated with chemoresistance [34]. Leptin contributes to the protection of human leukemic cells from cisplatin toxicity [35]. These reports are consistent with our data, suggesting a previously unrecognized role and clinical significance for serum CA125/ascites leptin ratio in imparting aggressiveness in HGSOC.
In this study, higher ascites leptin levels were associated with a poor OS in patients with HGSOC. In women with OC, obesity worsens the prognosis which is in line with our findings [8]. However, the role of leptin as a prognostic marker for cancer remains controversial. For example, in patients with gastric carcinoma, serum leptin alone had no apparent prognostic role in clinical outcome or responsiveness to chemotherapy [36]. In another study, serum leptin levels were not associated with renal cell carcinoma pathology or outcome [37]. A meta-analysis examining the role of serum leptin in lung cancer found no relationship between levels of serum leptin and lung cancer [38]. In contrast, leptin and leptin/adiponectin ratios have been associated with more aggressive prostate cancer histology [39]. Elevated leptin levels have been found to correlate with poor outcome in breast and colon cancer [40,41]. Therefore, although we found that higher ascites levels were associated with a worse prognosis, its role as a prognostic biomarker for cancer remains to be confirmed.
Advanced stage, high grade and sub-optimal surgery are known to be poor prognostic factors in OC patients [36]. All these prognostic factors were well balance between patients with baseline clinical resistance and those with sensitive diseases. In fact, nearly all patients in this study were advanced stage, with high grade tumor and underwent optimal cytoreductive surgery. Furthermore, the median BMI between the two groups in our cohort was similar (24). The limitations of our study include the retrospective nature of the analysis and our relatively small sample size. It is also unclear how our results would differ in a more obese patient population.
In conclusion, this is the first study to demonstrate the clinical significance of serum CA125/ascites leptin ratio for distinguishing HGSOC patients that have baseline clinical resistance to first-line therapy. This ratio may be a predictive biomarker for OC and its potential should further be evaluated in an independent retrospective cohort as well as in a prospective manner to confirm its clinical usefulness.
Acknowledgements
This work was supported by a grant from the Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke (Axe: Cancer, prognostic et diagnostic). We wish to thank the Banque de tissus et de données du Réseau de Recherche en Cancer du Fond de Recherche du Québec en Santé (FRQS), affiliated to the Canadian Tumor Repository Network (CTRNet) for providing the ascites samples.
Disclosure of conflict of interest
None.
References
- 1.Partridge EE, Barnes MN. Epithelial ovarian cancer: prevention, diagnosis, and treatment. CA Cancer J Clin. 1999;49:297–320. doi: 10.3322/canjclin.49.5.297. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, Xu X, Hamilton TC. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 5.Cannistra SA. Cancer of the ovary. N Eng J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 6.Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011;12:1169–1174. doi: 10.1016/S1470-2045(11)70123-1. [DOI] [PubMed] [Google Scholar]
- 7.Le Page C, Rahimi K, Köbel M, Tonin PN, Meunier L, Portelance L, Bernard M, Nelson BH, Bernardini MQ, Barlett JMS, Bachvarov D, Gotlieb W, Gilks B, McAlpine JN, Nachtigal MW, Piché A, Watson PH, Vanderhyden B, Huntsman DG, Provencher DM, Mes-Masson AM. Characteristics and outcome of the COEUR Canadian validation cohort for ovarian cancer biomarkers. BMC Cancer. 2018;18:347. doi: 10.1186/s12885-018-4242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parks J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer mechanisms underlying tumor progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, Schatzkin A, Lacey JV Jr. Body mass index and risk of ovarian cancer. Cancer. 2009;115:812–822. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schouten LJ, Rivera C, Hunter DJ, Spiegelman D, Adami HO, Arslan A, Beeson WL, van den Brandt PA, Buring JE, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Hankison SE, Lacey JV Jr, Leitzmann M, Lukanova A, Marshall JR, Miller AB, Patel AV, Rodriguez C, Rohan TE, Ross JA, Wolk A, Zhang SM, Smith-Warner SA. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2008;17:902–912. doi: 10.1158/1055-9965.EPI-07-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane D, Matte I, Garde-Granger P, Laplante C, Carignan A, Rancourt C, Piché A. Inflammation-regulating factors in ascites as predictive biomarkers of drug resistance and progression-free survival in serous epithelial ovarian cancers. BMC Cancer. 2015;15:492. doi: 10.1186/s12885-015-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- 13.Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, Al-Kyraya KS. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer. 2009;8:74. doi: 10.1186/1476-4598-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129:353–357. doi: 10.1016/j.ygyno.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Bain GH, E Collie-Duguid E, Murray GI, Gilbert FJ, Denison A, Mckiddie F, Ahearn T, Fleming I, Leeds J, Phull P, Park K, Nanthakumaran S, Matula KM, Grabsch HI, Tan P, Welch A, Schweiger L, Dahle-Smith A, Urquhart G, Finegan M, Petty RD. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer. 2014;110:1525–1534. doi: 10.1038/bjc.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 17.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–740. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 19.Canney PA, Moore M, Wilkinson PM, James RD. Ovarian cancer antigen CA125: a prospective clinical assessment of its role as a tumour marker. Br J Cancer. 1984;50:765–769. doi: 10.1038/bjc.1984.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergote IB, Bormer OP, Abeler VM. Evaluation of serum CA 125 levels in the monitoring of ovarian cancer. Am J Obstet Gynecol. 1987;157:88–92. doi: 10.1016/s0002-9378(87)80352-6. [DOI] [PubMed] [Google Scholar]
- 21.Bast RC Jr, Klug TL, St-John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR Jr, Knapp RC. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Eng J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 22.Moore RG, Miller MC, DiSilvestro P, Landrum LM, Gajewski W, Ball JJ, Skates SJ. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011;118:280–288. doi: 10.1097/AOG.0b013e318224fce2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC Jr, Skates SJ. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with pelvic mass. Gynecol Oncol. 2009;112:40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina R, Escudero JM, Augé JM, Filella X, Foj L, Torné A, Lejarcegui J, Pahisa J. HE4 a novel tumour marker for ovarian cancer: comparison with CA125 and ROMA algorithm in patients with gynaecological diseases. Tumor Biol. 2011;32:1087–1095. doi: 10.1007/s13277-011-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thériault C, Pinard M, Comamala M, Migneault M, Beaudin J, Matte I, Boivin M, Piché A, Rancourt C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol Oncol. 2011;121:434–443. doi: 10.1016/j.ygyno.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Lakshmanan I, Ponnusamy MP, Das S, Chakraborty S, Haridas D, Mukhopadhyay P, Lele SM, Batra SK. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene. 2012;31:805–817. doi: 10.1038/onc.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boivin M, Lane D, Piché A, Rancourt C. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol. 2009;115:407–413. doi: 10.1016/j.ygyno.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 29.Giuntoli RL, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29:2875–2884. [PubMed] [Google Scholar]
- 30.Rustin GJ, Timmers P, Nelstrop A, Shreeves G, Bentzen SM, Baron B, Piccart MJ, Bertelsen K, Stuart G, Cassidy J, Eisenhauer E. Comparison of CA-125 and standard definitions of progression of ovarian cancer in the intergroup trial of cisplatin and paclitaxel versus cisplatin and cyclophosphamide. J. Clin. Oncol. 2006;24:45–51. doi: 10.1200/JCO.2005.01.2757. [DOI] [PubMed] [Google Scholar]
- 31.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matte I, Lane D, Boivin M, Rancourt C, Piché A. MUC16 mucin (CA125) attenuates TRAIL-induced apoptosis by decreasing TRAIL receptor R2 expression and increasing c-FLIP expression. BMC Cancer. 2014;14:234. doi: 10.1186/1471-2407-14-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakshmanan I, Salfity S, Seshacharyulu P, Rachagani S, Thomas A, Das S, Majhi PD, Nimmakayala RK, Vengoji R, Lele SM, Ponnusamy MP, Batra SK, Ganti AK. MUC16 regulates TSPYL5 for lung cancer cell growth and chemoresistance by suppressing p53. Clin Cancer Res. 2017;23:3906–3917. doi: 10.1158/1078-0432.CCR-16-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bain GH, Collie-Duguid E, Murray GI, Gilbert FJ, Denison A, McKiddie F, Ahearn T, Fleming I, Leeds J, Phull P, Park K, Nanthakumaran S, Matula KM, Grabsch HI, Tan P, Welch A, Schweiger L, Dahle-Smith A, Urquhart G, Finegan M, Petty RD. Tumor expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer. 2014;110:1525–1534. doi: 10.1038/bjc.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efferth T, Fabry U, Osieka R. Leptin contributes to the protection of human leukemic cells from cisplatinum cytotoxicity. Anticancer Res. 2000;20:2541–2546. [PubMed] [Google Scholar]
- 36.Tas F, Karabulut S, Erturk K, Duranyildiz D. Clinical significance of serum leptin level in patients with gastric cancer. Eur Cytokine Netw. 2018;29:52–58. doi: 10.1684/ecn.2018.0408. [DOI] [PubMed] [Google Scholar]
- 37.de Martino M, Leitner CV, Hofbauer SL, Lucca I, Haitel A, Shariat SF, Klatte T. Serum adiponectin predicts cancer-specific survival of patients with renal cell carcinoma. Eur Urol Focus. 2016;2:197–203. doi: 10.1016/j.euf.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Tong X, Ma Y, Zhou Q, He J, Peng B, Liu S, Yan Z, Yang X, Fan H. Serum and tissue leptin in lung cancer: a meta-analysis. Oncotarget. 2017;8:19699–19711. doi: 10.18632/oncotarget.14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Sebastiano KM, Pinthus JH, Duivenvoorden WC, Patterson L, Dubin JA, Mourtzakis M. Elevated C-peptides, abdominal obesity, and abnormal adipokine profile are associated with higher gleason scores in prostate cancer. Prostate. 2017;77:211–221. doi: 10.1002/pros.23262. [DOI] [PubMed] [Google Scholar]
- 40.Guadagni F, Roselli M, Martini F, Spila A, Riondino S, D’Alessandro R, Del Monte G, Formica V, Laudisi A, Protarena I, Palmirotta R, Ferroni P. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009;29:3321–3327. [PubMed] [Google Scholar]
- 41.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou MF, Yuan SS. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]


