Abstract
Integrin-linked kinase (ILK), which is an ankyrin repeat-containing serine/threonine protein kinase, interacts with integrin β1 and the β3 cytoplasmic domain and phosphorylates integrin β1. ILK has multiple functions in cells, such as cell-extracellular matrix interactions, cell cycle, apoptosis, cell proliferation and cell motility, which are associated with the interacting partners of ILK and downstream signaling pathways. Upregulation of ILK is frequently observed in cancer tissues compared to corresponding normal tissues. Emerging evidence has demonstrated that ILK plays an important role in biological processes associated with tumorigenesis, including cancer cell proliferation, angiogenesis, metastasis, and drug resistance. Furthermore, inhibition of ILK expression and activity using siRNA or chemical inhibitors has shown a significant suppressive effect on cancer development and progression, implicating the potential of ILK as a target for cancer treatment. In this review, we summarized the functional role of ILK in tumorigenesis, with the expectation that targeting ILK could provide more evidence for cancer therapy.
Keywords: ILK, tumorigenesis, diagnostic and prognostic biomarker, therapeutic target
Introduction
Since its discovery in 1996 as an interaction partner of the β1 integrin cytoplasmic domain, ILK has been reported as a serine/threonine protein kinase, which plays a central role in fundamental processes, including the regulation of cell shape, motility, growth, survival, differentiation and gene expression [1]. Under normal conditions, ILK overexpression overrides the adhesion-dependent regulation of cell cycle progression and regulates cell growth and survival [2]. ILK regulates cell-cell adhesion and cell-matrix interactions through the loss of E-cadherin expression and increase in fibronectin matrix assembly [3]. Moreover, ILK modulates actin rearrangement, regulates chondrocyte shape and proliferation, and regulates the processes of myogenic differentiation [4-7]. In addition, ILK also inhibits anoikis and apoptosis through activation of phosphatidylinositide 3-kinase/protein kinase B (PI3K/AKT) signaling and stimulation of downstream anti-apoptotic pathways [8]. Since the discovery that overexpression of ILK induces the transformation of epithelial cells in vitro and in vivo, the important role of ILK in cancer proliferation, invasion, metastasis, angiogenesis, and chemoresistance has been extensively studied [9-14]. It is becoming clear that ILK exerts its biological functions through various signaling pathways, including PI3K/AKT, glycogen synthase kinase 3-beta (GSK3β), nuclear factor-kappa B (NF-κB), cell division control protein 42 homolog (Rac/Cdc42), mammalian target of rapamycin (mTOR), vascular endothelial growth factor (VEGF), and Snail1/E-cadherin [15-20]. More importantly, upregulation of ILK is frequently observed in human malignancies, and high ILK overexpression is associated with poor prognosis of cancer patients, suggesting its implication in cancer diagnosis and prognosis.
In this review, we summarized the physiological and pathological role of ILK in human cancer, including its biological functions, interaction proteins, downstream signaling, and upstream regulation. Current progress on the development of specific small molecule inhibitors of ILK is described, and the potential of pharmacological inhibition of ILK for cancer treatment is discussed.
Structure and interaction proteins of ILK
ILK, which is localized on chromosome 11p15.5-p15.49 in humans [21], encodes a 59k serine/threonine protein kinase [1]. ILK consists of a COOH-terminal catalytic domain, a central pleckstrin homology (PH)-like domain, and an N-terminal domain consisting of four ankyrin-like repeats [22] (Figure 1). The main function of the ankyrin repeats is the regulation of protein-protein interactions [23]. Particularly interesting new cysteine-histidine-rich protein (PINCH), a widely expressed and evolutionarily conserved protein comprising five LIM domains, is a binding protein of ILK [24]. ILK-associated protein (ILKAP), a protein phosphatase 2C (PP2C) family protein phosphatase, binds to ILK to negatively regulate its downstream signaling [22]. Furthermore, engulfment and cell motility 2 (ELMO2) also interacts with ILK via the N-terminal domain to modulate cell polarity [25]. A PH-like domain in the central region mediates the interaction between ILK and 3’-phosphorylated inositol lipids, which is required for the PI3K-dependent activation of ILK [22]. The C-terminal domain, which is the catalytic domain of ILK, interacts with integrins [23], paxillin, a focal adhesion adapter protein [26], calponin homology domain-containing integrin-linked kinase binding protein CH-ILKBP [26], Affixin [27], Rictor [28], Mig-2 [29], and Kindlin [30,31].
Figure 1.
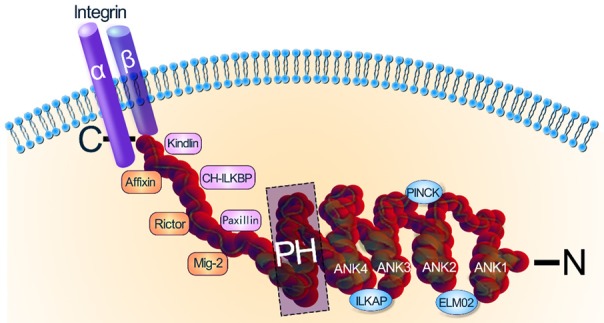
Structure and interacting proteins of ILK. ILK consists of three components: a COOH-terminal catalytic domain, a central pleckstrin homology (PH)-like domain, and an N-terminal domain. PINCH, ILKAP and ELMO2 interact with ILK via the N-terminal domain to modulate cell function. The C-terminal domain of ILK, which is the catalytic domain, directly interacts with Kindlin, Affixin, CH-ILKBP, Rictor, Paxillin and Mig-2. PH, pleckstrin homology; PINCH, particularly interesting new cysteine-histidine-rich protein; ILKAP, ILK-associated protein; ELMO2, engulfment and cell motility 2; CH-ILKBP, calponin homology domain-containing integrin-linked kinase (ILK)-binding protein.
Role of ILK in tumorigenesis
Since the discovery in 1998 that ILK overexpression induces tumorigenic transformation of epithelial cells in vitro and in vivo, which is accompanied by upregulation of fibronectin matrix assembly and downregulation of E-cadherin expression [12], accumulating evidence has demonstrated the role of ILK in the characteristics of cancer, including cell proliferation, cell survival, angiogenesis, metastasis and drug resistance (Figure 2), which is summarized according to different cancer types in Table 1. In addition to the findings that ILK is widely overexpressed in different cancers, ILK upregulation is more importantly associated with tumor grade and survival (Table 2). These results clearly indicate that ILK plays an important role in cancer development and progression.
Figure 2.

Role of ILK in regulating signaling pathways and cancer phenotypes. ILK is a central regulator of signaling cascades that control a series of biological processes that are crucial to cancer progression. ILK is activated by integrins and regulates downstream molecules, such as NF-κB, Hippo, Wnt1 and GSK3β. GSK3β, glycogen synthase kinase 3-beta; NF-κB, nuclear factor-kappa B; mTOR, mammalian target of rapamycin; STAT3, signal transducer and activator of transcription 3; FOXO1, forkhead box O1; JNK, c-Jun N-terminal kinase; PKB/AKT, phosphatidylinositide 3-kinase/protein kinase B; Wnt1, wingless-type MMTV integration site family, member 1; ERK, extracellular regulated MAP kinase; EMT, epithelial-to-mesenchymal transition; IL6, interleukin 6; PDGF, platelet-derived growth factor; HIF-1, hypoxia inducible factor 1 subunit alpha; MRP1, multidrug resistance-associated protein 1.
Table 1.
Function of ILK in different cancers
| Cancer Type | Observed Functions of ILK | Involved Pathway | References |
|---|---|---|---|
| Prostate cancer | Survival, Growth, Motility, Apoptosis | AKT | [81,92] |
| Multiple myeloma | Angiogenesis, Survival | HIF-2α-ILK | [46,93,94] |
| Colorectal cancer | EMT, Proliferation, Invasion, Chemoresistance, Migration | NF-κB/p65 | [16,17,55] |
| Pancreatic cancer | EMT, Proliferation, Migration, Invasion | MUC1-C | [11,95] |
| Glioma cells | Proliferation, Migration, Invasion, Temozolomide resistance | Caspase3, E-cadherin, NF-κB, Cyclin D1 | [14,15,96] |
| Bladder cancer | EMT, Proliferation, Morphology | ILK/PI3K/AKT | [97,98] |
| Lung cancer | EMT, Migration, Invasion, Drug resistance | MRP1, NF-κB, MMP-9 | [18,52] |
| Gastric cancer | Growth, Migration, Survival, Multidrug resistance, Cell cycle | NF-κB, ERK1/2, E-cadherin, AP-1, MMP-2/9, Cystatin B, p-AKT | [54,99,100] |
| Tongue cancer | EMT, Proliferation, Migration, Invasion | AKT, GSK3β, MMP2, MMP9 | [58] |
| Ovarian cancer | Migration, Invasion | Rac1, AKT | [101] |
| Breast tumors | Proliferation | PI3K/AKT | [102] |
| CLL | Proliferation | NF-κB | [103] |
| Thyroid cancer | EMT, Migration | AKT | [104] |
| OSCC | EMT, Growth, Metastasis, Adhesion | AKT, GSk3β | [105] |
| RCC | EMT, Migration, Invasion | Snail, Zeb-1 | [59] |
| Retinoblastoma cells | Proliferation, Cytokinesis, Mitosis, Cytoskeleton dynamics | Unknown | [43] |
| Phyllodes breast tumors | EMT, Metastasis | E-cadherin, ZEB1, β-catenin, Twist, N-cadherin, Snail, Vimentin | [106] |
CLL: Chronic Lymphocytic Leukemia; OSCC: Oral squamous cell carcinoma; RCC: Renal Cell Carcinoma.
Table 2.
ILK expression in different cancers
| Cancer Type | Comments | Methods | References |
|---|---|---|---|
| Prostate cancer | Overexpression of ILK in 57.1% of prostate cancer samples and 18.2% of benign prostatic hyperplasia (BPH) samples | IHC | [81] |
| Breast tumors | Greater ILK expression with increasing tumor grade | IHC | [106] |
| Colorectal cancer | Upregulated ILK mRNA and protein expression in primary CRC cells; high expression of ILK in 42.2% of primary CRC samples | IHC, WB, RT-PCR | [55] |
| ISCC | Increased expression level of ILK is associated with lymph node metastases and patient survival rate | IHC | [57] |
| CLL | ILK overexpression in patient samples, particularly in tumor cells harboring prognostic high-risk markers | IHC | [103] |
| NSCLC | Increased ILK overexpression in 46.4% of NSCLC tumors | IHC | [107] |
| Gastric cancer | ILK overexpression in 47.4% of gastric cancer tumor tissues | IHC | [108] |
| Breast cancer | Upregulated ILK1 mRNA expression in primary breast cancer tissues; 54.6% of patients are classified with ILK1 overexpression | IHC | [109] |
| Osteosarcoma | ILK overexpression is correlated with distant metastasis and it is an independent prognostic factor for poor overall survival | IHC | [47] |
| BTCC | Overexpression of ILK protein in BTCC tissue (53.6%) | IHC, RT-PCR | [110] |
| Pancreatic cancer | Increased ILK expression level in pancreatic cancer | IHC | [11] |
| CCRC | Upregulated ILK expression in high-grade CCRCs compared to low-grade CCRCs | IHC | [111] |
| RCC | ILK underexpression in normal cells and low-stage RCC cells and ILK overexpression in advanced and metastatic cells | IHC, WB | [59] |
IHC, Immunocytochemistry; WB, Western blot; RT-PCR, Real-time PCR; ISCC, laryngeal squamous cell carcinoma; CLL, Chronic Lymphocytic Leukemia; NSCLC, Non-small cell lung cancer; BTCC, Bladder transitional cell carcinoma; CCRC, Clear cell renal carcinoma; RCC, Renal Cell Carcinoma.
ILK promotes cancer cell proliferation
Analysis of the expression pattern and regulation of ILK in mouse skin provided the first in vivo evidence of the role of ILK in the regulation of cell proliferation [32], and the effect of ILK on the proliferation of cancer cells has since become a popular topic. It has been reported that inhibition of catalytic activity of ILK suppresses tumor growth by inhibiting the PI3K/mTOR, signal transducer and activator of transcription 3 (STAT3) and forkhead box O1 (FKHR) pathways [33] or decreasing the phosphorylation of protein kinase B/AKT and GSK3β [34-36]. Similarly, knockdown of ILK with siRNA in colorectal cancer cells has been shown to decrease the expression levels of cyclin D1, Snail, matrix metallopeptidase 9 (MMP9) and fibronectin, and ILK intestinal knockout has been shown to result in a smaller tumor volume when mice are treated with azoxymethane and dextran sodium sulfate [37]. In addition, overexpression of ILK could stabilize β-catenin and increase β-catenin/Tcf transcriptional activity [38]. ILK cooperates with Wnt1 to stimulate the expression of β-catenin and cyclin D1 and accelerates breast tumor development [39]. In neuroblastoma cells, inhibition of ILK expression with an antisense oligonucleotide interferes with the regulation of ILK on PTEN (phosphatase and tensin homolog)-AKT signaling and tumor growth [40]. Additionally, ILK represses apoptosis and induces cell proliferation through regulation of the c-Jun N-terminal kinase (JNK) signaling pathway [41]. In human breast, prostate and colon cancer cells, ILK inhibits the Hippo pathway through inactivation of Merlin by direct phosphorylation of protein phosphatase 1 regulatory subunit 12A (MYPT1) [42]. Interestingly, Sikkema et al. found that ILK not only promotes cell proliferation but also regulates mitotic cytoskeleton dynamics and cytokinesis [43]. Therefore, ILK interacts with multiple pathways to affect cancer cell proliferation.
ILK increases tumor angiogenesis
Oxygen and nutrients are required for the growth and survival of mammalian cells. Angiogenesis occurs during development, wound healing, pregnancy and other physiological processes. However, angiogenesis is also an essential step in the conversion of a tumor from a benign to a malignant and metastatic phenotype. Tumor growth is a complex and multistep process involving recruitment of neighboring blood vessels or endothelial cells to deliver oxygen and nutrients into the tumor microenvironment to construct a favorable environment for tumor growth [44]. Tumors cannot grow beyond a critical size or metastasize to another organ without a sufficient nutrient supply [44]. VEGF, a secreted protein, is one of the most important proangiogenic factors involved in tumor angiogenesis [45]. The regulation of VEGF occurs at the gene transcription, translation, and posttranslation levels. Tan et al. found that ILK regulates VEGF expression by inducing hypoxia inducible factor 1 subunit alpha (HIF-1α) protein expression in a PKB/AKT- and mTOR-dependent manner and increases VEGF-stimulated blood vessel formation [19]. Additionally, overexpression of ILK in melanoma cells stimulates VEGF via NF-κB-mediated upregulation of interleukin 6 (IL-6), which is a pleiotropic cytokine closely associated with cancer development [20]. Genetic or pharmacological inhibition of ILK using siRNA or small molecule inhibitors has been shown to successfully decrease the expression and secretion of VEGF and suppress cancer progression [46-48]. ILK has been reported to be induced by hypoxia-inducible factor-2 alpha (HIF-2α) and VEGF and mediate their effect on angiogenesis in a positive feedback manner [46,49].
ILK enhances the resistance of cancer cells to chemotherapeutic drugs
Overexpression of ILK could suppress stress-induced cell apoptosis [8] and regulate resistance to hyperthermia by inhibiting the activity of stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) and p38 mitogen-activated protein kinase (p38 MAPK kinase) [50]. Moreover, knockdown of ILK makes cells more sensitive to EGFR inhibitors [51]. ILK is speculated to be associated with resistance to chemotherapeutic drugs in multiple cancer types. In pancreatic adenocarcinoma cells, overexpression of ILK increases gemcitabine chemoresistance by increasing phosphorylation of AKT and GSK3β, whereas inhibition of ILK induces apoptosis caused by gemcitabine [13]. Significantly greater expression levels of ILK have been observed in a gemcitabine-resistant subline derived from the lung cancer cell line A549 compared with the parental cells, and downregulation of ILK was shown to sensitize the cells to treatment by repressing epithelial-to-mesenchymal transition (EMT) and cellular drug efflux [52]. Similarly, ILK silencing has been reported to make A549 cells more sensitive to cisplatin, which is one of the most commonly used chemotherapeutic drugs for cancer treatment [53]. In gastric carcinoma SGC7901/DDP cells, not only AKT and extracellular signal-regulated kinase-1/2 (ERK) but also activator protein 1 (AP-1) and NF-κB pathways have been shown to be involved in the multidrug resistance caused by the upregulation of ILK [54]. Overexpression of ILK in glioma cells decreases the sensitivity to temozolomide, which is accompanied by the upregulation of the anti-apoptotic protein Bcl-2 and downregulation of the proapoptotic protein Bax [14]. Moreover, the expression correlation between ILK and markers of cancer stem cells, as well as the functional role of ILK in the sensitivity of cells to 5-FU and oxaliplatin, have been reported in colorectal cancer cells [55]. These findings support the important role of ILK in cancer chemoresistance.
ILK promotes cancer metastasis
Tumor metastasis is defined as the spread of cancer from one organ/site to distant sites. Metastatic cancers are largely incurable, and greater than 90% of mortality from cancer is attributed to metastasis [56,57]. Identification of the key proteins and signaling pathways that promote cancer invasion and metastasis could facilitate the development of new treatment strategies. Emerging evidence has suggested that the expression level of ILK is closely correlated with cancer metastasis and poor prognosis (Table 1). For example, downregulation of ILK decreases N-cadherin, Vimentin, Snail, Slug and Twist expression levels, as well as cell migration and invasion in human tongue cancer cells [58]. A similar phenomenon has also been observed in adenoid cystic carcinoma of salivary glands and renal cell carcinoma [59,60]. Recent studies have confirmed that NF-κB is crucial for the activation of metastasis mediated by ILK. In lung cancer, ILK stimulates matrix metalloproteinase-9 to promote cell migration and invasion through NF-κB [18]. Overexpression of ILK promotes cell migration and invasion of glioma cells, which is related to downregulation of E-cadherin via the NF-κB pathway [15]. Some studies have also reported that ILK could induce migration and invasion of colorectal cancer cells by promoting NF-κB-mediated EMT [16,17].
Upstream regulation of ILK
ILK is regulated at the transcriptional level
The ILK protein is encoded by 15 exons, with the major transcriptional start site located 138 bp upstream of exon 1 and the translation initiation codon (ATG) located within the second exon. The ILK gene has the features of housekeeping genes, especially a TATA-less and GC-rich promoter region [61], and there are multiple transcription factor binding sites within the promoter, such as AP-2, Sp1 and NF-κB [62]. In NSCLC cells, PGE2 stimulates cell growth through upregulation of ILK promoter activity, which is dependent on the binding of Sp1 to the ILK gene promoter [62]. Researchers have shown that rosiglitazone and metformin repress nasopharyngeal carcinoma (NPC) cell growth by reducing AP-2α-dependent ILK transcription [63]. Integrin αVβ3 has been reported to increase ILK promoter activity in human ovarian cancer, and the binding of Ets-1 to the second Ets DNA motif is critical for this process [64,65]. A recent study demonstrated that KRAS regulates ILK expression through E2F1-mediated transcriptional activation, which induces KRAS expression as a regulatory loop to promote aggressive phenotypes in pancreatic cancer [66]. Moreover, it has been shown in the kidney that integrin α3 increases ILK expression via the Src/p-β-catenin/p-Smad2 regulatory axis [67]. ILK has also been shown to be activated by Twist, which is the conserved basic helix-loop-helix transcription factor, and mediates the effect of Twist in EMT and tumor metastasis [68]. In addition, hypoxia has been reported to stimulate ILK transcriptional expression in an HIF-1α-dependent manner, and ILK, in turn, induces HIF-1α expression in prostate and breast cancer cells [69]. However, it remains unclear which transcription factors are responsible for ILK transcription.
ILK is regulated at the posttranscriptional level
Recent studies have demonstrated that miRNAs can function as tumor suppressors or oncogenes in various cancers [70]. Researchers have shown that miR-542-3p inhibits ILK gene expression by binding to its 3’-UTR in oral squamous cell carcinoma cells [71], which has been confirmed in gastric adenocarcinoma cells [72]. It has been reported that miR-625 represses ILK expression by directly targeting its 3’-UTR to suppress lymphatic metastasis of human gastric cancer cells in vitro and in vivo [73]. Moreover, ILK has been shown to be targeted by miR-145, which functions synergistically with miR-143 to inhibit the growth of bladder cancer cells [74].
Protein modification of ILK
Although direct phosphorylation of ILK has not been reported, the kinase activity of ILK is not only stimulated by integrin and growth factors but also activated by PI3K via a PH-domain-mediated interaction with phosphatidylinositol 3,4,5-trisphosphate (PIP3) and regulated by the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10), which acts as an antagonist of PI3K signaling to dephosphorylate PIP3 to PIP2 [75,76]. Moreover, ILKAP, a serine/threonine phosphatase of the PP2C (protein phosphatase 2C) family, has been shown to negatively regulate ILK activity and signaling [77].
Potential of ILK-targeting agents for cancer therapy
Chemotherapy is one of the standard treatment options for cancer patients. However, limited treatment efficiency, side effects and development of resistance to the current chemotherapeutic drugs remain serious challenges in the management of human cancer. For example, epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) drugs have been shown to significantly prolong the lifetime of patients with non-small cell lung cancer [78,79]. However, chemoresistance is a substantial obstacle in the use of the first-generation EGFR inhibitor drugs, and there are 3 generations of EGFR-TKI drugs [80]. Therefore, the development of novel strategies that target different oncoproteins or kinases to improve treatment outcomes is urgently needed. As summarized above, ILK is frequently overexpressed in cancer cells compared with the surrounding normal cells and plays a crucial role in regulating various cellular processes, including proliferation, survival, invasion, angiogenesis and metastasis, suggesting that ILK may be a promising target for cancer therapy.
The expression of ILK in cancer cells can be inhibited by antisense oligonucleotides and siRNA. Knockdown of ILK using siRNA or shRNA has been shown to markedly inactivate the PI3K/AKT pathway and repress EMT, tumor growth and metastasis of tongue and prostate cancer cells in vitro and in vivo [58,81]. The antisense oligonucleotide targeting ILK has been reported to delay tumor formation of human ovarian carcinoma cells in nude mice [82] and exhibit a synergistic effect with either the Raf-1 inhibitor or the MEK inhibitor to kill glioblastoma cells [83].
By screening a compound library, Lee et al. identified N-methyl-3-(1-(4-(piperazin-1-yl) phenyl)-5-(40-(trifluoromethyl)-[1,10-biphenyl]-4-yl)-1H-pyrazol-3-yl)propanamide (compound 22) as an ILK inhibitor. This compound was shown to exert strong inhibitory effects on the proliferation of a panel of prostate and breast cancer cells through inactivation of the AKT pathway and inhibition of the transcription factor Y-box binding protein-1 (YB-1). More importantly, the in vivo treatment efficacy of compound 12 as a single agent used to suppress the growth of prostate tumor xenografts suggested its potential use as a lead compound to develop more I LK inhibitors [84]. Moreover, researchers have reported that treatment with QLT0267, a new ILK inhibitor, not only inhibits the kinase activity of ILK and PI3K/AKT signaling and leads to cell growth arrest in vitro but also suppresses tumor angiogenesis and reduces tumor volume of thyroid cancer and glioblastoma xenografts in vivo [85,86]. In addition, the combination of QLT0267 with docetaxel has been shown to produce a synergistic effect in inhibiting the PI3K/AKT pathway and VEGF secretion, as well as in enhancing the treatment outcome in an orthotopic breast cancer model [87]. A recent study indicated that inhibition of ILK with QLT0267 could reduce acquired resistance to 5-FU and decrease the expression levels of EMT and cancer stem cell (CSC) markers in human colon cancer cells [55]. These findings suggest that ILK represents a valid therapeutic target for cancer treatment, and more ILK-targeting agents with improved efficacy and minimal toxicity need be developed to provide more therapeutic strategies for cancer treatment.
Conclusion and future perspectives
Since the discovery of ILK in 1996 as a new protein defined as a receptor-proximal protein kinase, its biological functions and mechanisms of activity have become popular topics in the fields of biochemistry and cancer biology. Accumulating studies have indicated that ILK plays an important role in various characteristics of cancer, including cell proliferation, migration, invasion, angiogenesis, chemoresistance and metastasis. ILK functions through multiple signaling pathways, such as PI3K/AKT, Hippo, NF-κB, ERK and Bcl-2. The expression of ILK is regulated at the transcriptional, posttranscriptional, and posttranslational levels. However, the role of ILK in some cancer types remains unclear, and more importantly, whether there are some crucial upstream regulators of ILK warrants in-depth investigation.
Cancer is a substantial threat to human life, and increasing numbers of patients have been reported to have drug resistance when treated with chemotherapeutic drugs for long periods of time [88]. Tumor metastasis remains largely incurable, and up to 90% of cancer-related deaths are caused by metastatic disease rather than primary tumors [89-91]. All of these factors cause more difficulties for the treatment of cancer and result in poor clinical outcomes. Therefore, the investigation of new targets for cancer therapy is urgently needed. ILK is overexpressed in tumors compared to adjacent normal tissues, and genetic and pharmacological inhibition of ILK has been reported to inhibit a series of oncogenic signaling pathways and suppress tumor development and progression in several cancer types. These findings support the potential of ILK as an ideal target for cancer therapy. Future studies should be performed to combine the in silico design of ILK-targeting lead compounds and function-oriented high-throughput screening, as well as drug repositioning, for the development of novel therapeutic strategies with improved efficacy and decreased toxicity to improve the treatment outcomes of this lethal disease.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Project 81773085, 81672953), the National Key Research and Development Program of China (2017YFA0505100), Guangzhou Science and Technology Project (201707010260), Guangdong Natural Science Research Grant (2016A030313838), and the Fundamental Research Funds for the Central Universities (21617434).
Disclosure of conflict of interest
None.
References
- 1.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 2.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 3.Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci U S A. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grashoff C, Aszodi A, Sakai T, Hunziker EB, Fassler R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 2003;4:432–438. doi: 10.1038/sj.embor.embor801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Li J, Zhang Y, Wu C. The roles of integrin-linked kinase in the regulation of myogenic differentiation. J Cell Biol. 2000;150:861–872. doi: 10.1083/jcb.150.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpstra L, Prud’homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- 9.Mi Z, Guo H, Wai PY, Gao C, Kuo PC. Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis. 2006;27:1134–1145. doi: 10.1093/carcin/bgi352. [DOI] [PubMed] [Google Scholar]
- 10.Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XY, Liu N, Liu W, Song SW, Guo KJ. Silencing of the integrin-linked kinase gene suppresses the proliferation, migration and invasion of pancreatic cancer cells (Panc-1) Genet Mol Biol. 2012;35:538–544. doi: 10.1590/S1415-47572012005000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 13.Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. RNA interference demonstrates a novel role for integrin-linked kinase as a determinant of pancreatic adenocarcinoma cell gemcitabine chemoresistance. Clin Cancer Res. 2005;11:3433–3438. doi: 10.1158/1078-0432.CCR-04-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang F, Wang B, Bao L, Zhao YS, Zhang SM, Zhang SQ. Overexpression of ILK promotes temozolomide resistance in glioma cells. Mol Med Rep. 2017;15:1297–1304. doi: 10.3892/mmr.2017.6157. [DOI] [PubMed] [Google Scholar]
- 15.Liang F, Zhang S, Wang B, Qiu J, Wang Y. Overexpression of integrin-linked kinase (ILK) promotes glioma cell invasion and migration and down-regulates E-cadherin via the NF-kappaB pathway. J Mol Histol. 2014;45:141–151. doi: 10.1007/s10735-013-9540-5. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Ma JL, Zhang Y, Deng GL, Qu YL, Wu XL, He JX, Zhang S, Zeng S. Integrin-linked kinase overexpression promotes epithelial-mesenchymal transition via nuclear factor-kappaB signaling in colorectal cancer cells. World J Gastroenterol. 2016;22:3969–3977. doi: 10.3748/wjg.v22.i15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Z, Yin H, Wang R, Wu D, Sun W, Liu B, Su Q. Overexpression of integrin-linked kinase (ILK) promotes migration and invasion of colorectal cancer cells by inducing epithelial-mesenchymal transition via NF-kappaB signaling. Acta Histochem. 2014;116:527–533. doi: 10.1016/j.acthis.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Gao Y, Wang L, Liu S, Han B, Ma L, Ling Y, Mao S, Wang X. Overexpression of integrin-linked kinase promotes lung cancer cell migration and invasion via NF-kappaB-mediated upregulation of matrix metalloproteinase-9. Int J Med Sci. 2013;10:995–1002. doi: 10.7150/ijms.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 20.Wani AA, Jafarnejad SM, Zhou J, Li G. Integrin-linked kinase regulates melanoma angiogenesis by activating NF-kappaB/interleukin-6 signaling pathway. Oncogene. 2011;30:2778–2788. doi: 10.1038/onc.2010.644. [DOI] [PubMed] [Google Scholar]
- 21.Hannigan GE, Bayani J, Weksberg R, Beatty B, Pandita A, Dedhar S, Squire J. Mapping of the gene encoding the integrin-linked kinase, ILK, to human chromosome 11p15.5-p15.4. Genomics. 1997;42:177–179. doi: 10.1006/geno.1997.4719. [DOI] [PubMed] [Google Scholar]
- 22.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Wu C. Integrin-linked kinase and associated proteins (review) Int J Mol Med. 1999;3:563–572. doi: 10.3892/ijmm.3.6.563. [DOI] [PubMed] [Google Scholar]
- 24.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho E, Irvine T, Vilk GJ, Lajoie G, Ravichandran KS, D’Souza SJ, Dagnino L. Integrin-linked kinase interactions with ELMO2 modulate cell polarity. Mol Biol Cell. 2009;20:3033–3043. doi: 10.1091/mbc.E09-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolopoulos SN, Turner CE. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J Biol Chem. 2001;276:23499–23505. doi: 10.1074/jbc.M102163200. [DOI] [PubMed] [Google Scholar]
- 27.Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, Mohri H, Ohno S, Ishigatsubo Y. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol. 2001;153:1251–1264. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–1624. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 29.Papachristou DJ, Gkretsi V, Rao UN, Papachristou GI, Papaefthymiou OA, Basdra EK, Wu C, Papavassiliou AG. Expression of integrin-linked kinase and its binding partners in chondrosarcoma: association with prognostic significance. Eur J Cancer. 2008;44:2518–2525. doi: 10.1016/j.ejca.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qadota H, Luo Y, Matsunaga Y, Park AS, Gernert KM, Benian GM. Suppressor mutations suggest a surface on PAT-4 (Integrin-linked Kinase) that interacts with UNC-112 (Kindlin) J Biol Chem. 2014;289:14252–14262. doi: 10.1074/jbc.M114.556308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadry YA, Huet-Calderwood C, Simon B, Calderwood DA. Kindlin-2 interacts with a highly conserved surface of ILK to regulate focal adhesion localization and cell spreading. J Cell Sci. 2018:131. doi: 10.1242/jcs.221184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie W, Li F, Kudlow JE, Wu C. Expression of the integrin-linked kinase (ILK) in mouse skin: loss of expression in suprabasal layers of the epidermis and up-regulation by erbB-2. Am J Pathol. 1998;153:367–372. doi: 10.1016/S0002-9440(10)65580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yau CY, Wheeler JJ, Sutton KL, Hedley DW. Inhibition of integrin-linked kinase by a selective small molecule inhibitor, QLT0254, inhibits the PI3K/PKB/mTOR, Stat3, and FKHR pathways and tumor growth, and enhances gemcitabine-induced apoptosis in human orthotopic primary pancreatic cancer xenografts. Cancer Res. 2005;65:1497–1504. doi: 10.1158/0008-5472.CAN-04-2940. [DOI] [PubMed] [Google Scholar]
- 34.Chan J, Ko FC, Yeung YS, Ng IO, Yam JW. Integrin-linked kinase overexpression and its oncogenic role in promoting tumorigenicity of hepatocellular carcinoma. PLoS One. 2011;6:e16984. doi: 10.1371/journal.pone.0016984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Zhu J, Li HY, Pan XY, Jiang R, Chen JX. Small interfering RNA targeting integrin-linked kinase inhibited the growth and induced apoptosis in human bladder cancer cells. Int J Biochem Cell Biol. 2011;43:1294–1304. doi: 10.1016/j.biocel.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Zhang S, Hu L, Liu L, Guo W, Zhang J. HMGA1 participates in MHCC97H cell proliferation and invasion through the ILK/Akt/GSK3beta signaling pathway. Mol Med Rep. 2017;16:9287–9294. doi: 10.3892/mmr.2017.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assi K, Mills J, Owen D, Ong C, St Arnaud R, Dedhar S, Salh B. Integrin-linked kinase regulates cell proliferation and tumour growth in murine colitis-associated carcinogenesis. Gut. 2008;57:931–940. doi: 10.1136/gut.2007.142778. [DOI] [PubMed] [Google Scholar]
- 38.Oloumi A, McPhee T, Dedhar S. Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta. 2004;1691:1–15. doi: 10.1016/j.bbamcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Oloumi A, Maidan M, Lock FE, Tearle H, McKinney S, Muller WJ, Aparicio SA, Dedhar S. Cooperative signaling between Wnt1 and integrin-linked kinase induces accelerated breast tumor development. Breast Cancer Res. 2010;12:R38. doi: 10.1186/bcr2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor CJ, Qiao J, Colon NC, Schlegel C, Josifi E, Chung DH. Integrin-linked kinase regulates phosphatase and tensin homologue activity to promote tumorigenesis in neuroblastoma cells. Surgery. 2011;150:162–168. doi: 10.1016/j.surg.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Yang A, Xu C, Xing Y, Gong W, Li J. c-Jun N-terminal kinase is involved in the regulation of proliferation and apoptosis by integrin-linked kinase in human retinoblastoma cells. Graefes Arch Clin Exp Ophthalmol. 2011;249:1399–1407. doi: 10.1007/s00417-010-1607-3. [DOI] [PubMed] [Google Scholar]
- 42.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sikkema WK, Strikwerda A, Sharma M, Assi K, Salh B, Cox ME, Mills J. Regulation of mitotic cytoskeleton dynamics and cytokinesis by integrin-linked kinase in retinoblastoma cells. PLoS One. 2014;9:e98838. doi: 10.1371/journal.pone.0098838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 45.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Xu Y, Liu H, Zhao P, Chen Y, Yue Z, Zhang Z, Wang X. HIF-2alpha-ILK is involved in mesenchymal stromal cell angiogenesis in multiple myeloma under hypoxic conditions. Technol Cancer Res Treat. 2018;17:1533033818764473. doi: 10.1177/1533033818764473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee SH, Han I, Lee MR, Cho HS, Oh JH, Kim HS. Role of integrin-linked kinase in osteosarcoma progression. J Orthop Res. 2013;31:1668–1675. doi: 10.1002/jor.22409. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Zhang Z, Yao C. Targeting integrin-linked kinase increases apoptosis and decreases invasion of myeloma cell lines and inhibits IL-6 and VEGF secretion from BMSCs. Med Oncol. 2011;28:1596–1600. doi: 10.1007/s12032-010-9616-y. [DOI] [PubMed] [Google Scholar]
- 49.Kaneko Y, Kitazato K, Basaki Y. Integrin-linked kinase regulates vascular morphogenesis induced by vascular endothelial growth factor. J Cell Sci. 2004;117:407–415. doi: 10.1242/jcs.00871. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Li Y, Huang Q, Wang H, Yan B, Dewhirst MW, Li CY. Increased resistance of tumor cells to hyperthermia mediated by integrin-linked kinase. Clin Cancer Res. 2003;9:1155–1160. [PubMed] [Google Scholar]
- 51.Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, Tanabe KK. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 52.Jia Z. Role of integrin-linked kinase in drug resistance of lung cancer. Onco Targets Ther. 2015;8:1561–1565. doi: 10.2147/OTT.S81447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X, Xu Z, Wang Z, Wu Z, Gong Y, Zhou L, Xiang Y. RNA silencing of integrin-linked kinase increases the sensitivity of the A549 lung cancer cell line to cisplatin and promotes its apoptosis. Mol Med Rep. 2015;12:960–966. doi: 10.3892/mmr.2015.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song W, Jiang R, Zhao CM. Role of integrin-linked kinase in multi-drug resistance of human gastric carcinoma SGC7901/DDP cells. Asian Pac J Cancer Prev. 2012;13:5619–5625. doi: 10.7314/apjcp.2012.13.11.5619. [DOI] [PubMed] [Google Scholar]
- 55.Tsoumas D, Nikou S, Giannopoulou E, Champeris Tsaniras S, Sirinian C, Maroulis I, Taraviras S, Zolota V, Kalofonos HP, Bravou V. ILK expression in colorectal cancer is associated with emt, cancer stem cell markers and chemoresistance. Cancer Genomics Proteomics. 2018;15:127–141. doi: 10.21873/cgp.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 57.Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, Cao X, Rabban E, Kumar-Sinha C, Raymond V, Schuetze S, Alva A, Siddiqui J, Chugh R, Worden F, Zalupski MM, Innis J, Mody RJ, Tomlins SA, Lucas D, Baker LH, Ramnath N, Schott AF, Hayes DF, Vijai J, Offit K, Stoffel EM, Roberts JS, Smith DC, Kunju LP, Talpaz M, Cieslik M, Chinnaiyan AM. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. doi: 10.1038/nature23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing Y, Qi J, Deng S, Wang C, Zhang L, Chen J. Small interfering RNA targeting ILK inhibits metastasis in human tongue cancer cells through repression of epithelial-to-mesenchymal transition. Exp Cell Res. 2013;319:2058–2072. doi: 10.1016/j.yexcr.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Han KS, Li N, Raven PA, Fazli L, Ettinger S, Hong SJ, Gleave ME, So AI. Targeting integrin-linked kinase suppresses invasion and metastasis through downregulation of epithelial-to-mesenchymal transition in renal cell carcinoma. Mol Cancer Ther. 2015;14:1024–1034. doi: 10.1158/1535-7163.MCT-14-0771. [DOI] [PubMed] [Google Scholar]
- 60.Zhao D, Yang K, Tang XF, Lin NN, Liu JY. Expression of integrin-linked kinase in adenoid cystic carcinoma of salivary glands correlates with epithelial-mesenchymal transition markers and tumor progression. Med Oncol. 2013;30:619. doi: 10.1007/s12032-013-0619-3. [DOI] [PubMed] [Google Scholar]
- 61.Melchior C, Kreis S, Janji B, Kieffer N. Promoter characterization and genomic organization of the gene encoding integrin-linked kinase 1. Biochim Biophys Acta. 2002;1575:117–122. doi: 10.1016/s0167-4781(02)00247-6. [DOI] [PubMed] [Google Scholar]
- 62.Zheng Y, Ritzenthaler JD, Sun X, Roman J, Han S. Prostaglandin E2 stimulates human lung carcinoma cell growth through induction of integrin-linked kinase: the involvement of EP4 and Sp1. Cancer Res. 2009;69:896–904. doi: 10.1158/0008-5472.CAN-08-2677. [DOI] [PubMed] [Google Scholar]
- 63.Hahn SS, Tang Q, Zheng F, Zhao S, Wu J, Chen J. Repression of integrin-linked kinase by antidiabetes drugs through cross-talk of PPARgamma- and AMPKalpha-dependent signaling: role of AP-2alpha and Sp1. Cell Signal. 2014;26:639–647. doi: 10.1016/j.cellsig.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Hapke S, Kessler H, Luber B, Benge A, Hutzler P, Hofler H, Schmitt M, Reuning U. Ovarian cancer cell proliferation and motility is induced by engagement of integrin alpha(v)beta3/Vitronectin interaction. Biol Chem. 2003;384:1073–1083. doi: 10.1515/BC.2003.120. [DOI] [PubMed] [Google Scholar]
- 65.Lossner D, Abou-Ajram C, Benge A, Aumercier M, Schmitt M, Reuning U. Integrin alphavbeta3 upregulates integrin-linked kinase expression in human ovarian cancer cells via enhancement of ILK gene transcription. J Cell Physiol. 2009;220:367–375. doi: 10.1002/jcp.21774. [DOI] [PubMed] [Google Scholar]
- 66.Chu PC, Yang MC, Kulp SK, Salunke SB, Himmel LE, Fang CS, Jadhav AM, Shan YS, Lee CT, Lai MD, Shirley LA, Bekaii-Saab T, Chen CS. Regulation of oncogenic KRAS signaling via a novel KRAS-integrin-linked kinase-hnRNPA1 regulatory loop in human pancreatic cancer cells. Oncogene. 2016;35:3897–3908. doi: 10.1038/onc.2015.458. [DOI] [PubMed] [Google Scholar]
- 67.Zheng G, Zhang J, Zhao H, Wang H, Pang M, Qiao X, Lee SR, Hsu TT, Tan TK, Lyons JG, Zhao Y, Tian X, Loebel DAF, Rubera I, Tauc M, Wang Y, Wang Y, Wang YM, Cao Q, Wang C, Lee VWS, Alexander SI, Tam PPL, Harris DCH. alpha3 integrin of cell-cell contact mediates kidney fibrosis by integrin-linked kinase in proximal tubular E-cadherin deficient mice. Am J Pathol. 2016;186:1847–1860. doi: 10.1016/j.ajpath.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu L, Tang X, Du YE, Hu P, Liu M. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int J Biochem Cell Biol. 2016;71:62–71. doi: 10.1016/j.biocel.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Chou CC, Chuang HC, Salunke SB, Kulp SK, Chen CS. A novel HIF-1alpha-integrin-linked kinase regulatory loop that facilitates hypoxia-induced HIF-1alpha expression and epithelial-mesenchymal transition in cancer cells. Oncotarget. 2015;6:8271–8285. doi: 10.18632/oncotarget.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-beta1/smads and mir-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiao B, Cai JH, King-Yin Lam A, He BX. MicroRNA-542-3p inhibits oral squamous cell carcinoma progression by inhibiting ILK/TGF-beta1/Smad2/3 signaling. Oncotarget. 2017;8:70761–70776. doi: 10.18632/oncotarget.19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oneyama C, Morii E, Okuzaki D, Takahashi Y, Ikeda J, Wakabayashi N, Akamatsu H, Tsujimoto M, Nishida T, Aozasa K, Okada M. MicroRNA-mediated upregulation of integrin-linked kinase promotes Src-induced tumor progression. Oncogene. 2012;31:1623–1635. doi: 10.1038/onc.2011.367. [DOI] [PubMed] [Google Scholar]
- 73.Wang M, Li C, Nie H, Lv X, Qu Y, Yu B, Su L, Li J, Chen X, Ju J, Yu Y, Yan M, Gu Q, Zhu Z, Liu B. Down-regulated miR-625 suppresses invasion and metastasis of gastric cancer by targeting ILK. FEBS Lett. 2012;586:2382–2388. doi: 10.1016/j.febslet.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 74.Noguchi S, Yasui Y, Iwasaki J, Kumazaki M, Yamada N, Naito S, Akao Y. Replacement treatment with microRNA-143 and -145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett. 2013;328:353–361. doi: 10.1016/j.canlet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–6. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leung-Hagesteijn C, Mahendra A, Naruszewicz I, Hannigan GE. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 2001;20:2160–2170. doi: 10.1093/emboj/20.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 79.Otsuka K, Hata A, Takeshita J, Okuda C, Kaji R, Masago K, Fujita S, Katakami N. EGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76:835–41. doi: 10.1007/s00280-015-2867-8. [DOI] [PubMed] [Google Scholar]
- 80.Sun W, Yuan X, Tian Y, Wu H, Xu H, Hu G, Wu K. Non-invasive approaches to monitor EGFR-TKI treatment in non-small-cell lung cancer. J Hematol Oncol. 2015;8:95. doi: 10.1186/s13045-015-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan Y, Xiao Y, Li Q, Liu Z, Zhang X, Qin C, Xie J, Wang X, Xu T. In vitro and in vivo effects of short hairpin RNA targeting integrin-linked kinase in prostate cancer cells. Mol Med Rep. 2013;8:419–424. doi: 10.3892/mmr.2013.1532. [DOI] [PubMed] [Google Scholar]
- 82.Li Q, Li C, Zhang YY, Chen W, Lv JL, Sun J, You QS. Silencing of integrin-linked kinase suppresses in vivo tumorigenesis of human ovarian carcinoma cells. Mol Med Rep. 2013;7:1050–1054. doi: 10.3892/mmr.2013.1285. [DOI] [PubMed] [Google Scholar]
- 83.Edwards LA, Verreault M, Thiessen B, Dragowska WH, Hu Y, Yeung JH, Dedhar S, Bally MB. Combined inhibition of the phosphatidylinositol 3-kinase/Akt and Ras/mitogen-activated protein kinase pathways results in synergistic effects in glioblastoma cells. Mol Cancer Ther. 2006;5:645–654. doi: 10.1158/1535-7163.MCT-05-0099. [DOI] [PubMed] [Google Scholar]
- 84.Lee SL, Hsu EC, Chou CC, Chuang HC, Bai LY, Kulp SK, Chen CS. Identification and characterization of a novel integrin-linked kinase inhibitor. J Med Chem. 2011;54:6364–6374. doi: 10.1021/jm2007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Younes MN, Kim S, Yigitbasi OG, Mandal M, Jasser SA, Dakak Yazici Y, Schiff BA, El-Naggar A, Bekele BN, Mills GB, Myers JN. Integrin-linked kinase is a potential therapeutic target for anaplastic thyroid cancer. Mol Cancer Ther. 2005;4:1146–1156. doi: 10.1158/1535-7163.MCT-05-0078. [DOI] [PubMed] [Google Scholar]
- 86.Edwards LA, Woo J, Huxham LA, Verreault M, Dragowska WH, Chiu G, Rajput A, Kyle AH, Kalra J, Yapp D, Yan H, Minchinton AI, Huntsman D, Daynard T, Waterhouse DN, Thiessen B, Dedhar S, Bally MB. Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK) Mol Cancer Ther. 2008;7:59–70. doi: 10.1158/1535-7163.MCT-07-0329. [DOI] [PubMed] [Google Scholar]
- 87.Kalra J, Warburton C, Fang K, Edwards L, Daynard T, Waterhouse D, Dragowska W, Sutherland BW, Dedhar S, Gelmon K, Bally M. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res. 2009;11:R25. doi: 10.1186/bcr2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 92.Hu MB, Hu JM, Jiang LR, Yang T, Zhu WH, Hu Y, Wu XB, Jiang HW, Ding Q. Differential expressions of integrin-linked kinase, beta-parvin and cofilin 1 in high-fat diet induced prostate cancer progression in a transgenic mouse model. Oncol Lett. 2018;16:4945–4952. doi: 10.3892/ol.2018.9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao W, Zhang X, Zang L, Zhao P, Chen Y, Wang X. ILK promotes angiogenic activity of mesenchymal stem cells in multiple myeloma. Oncol Lett. 2018;16:1101–1106. doi: 10.3892/ol.2018.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steinbrunn T, Siegmund D, Andrulis M, Grella E, Kortum M, Einsele H, Wajant H, Bargou RC, Stuhmer T. Integrin-linked kinase is dispensable for multiple myeloma cell survival. Leuk Res. 2012;36:1165–1171. doi: 10.1016/j.leukres.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Huang HL, Wu HY, Chu PC, Lai IL, Huang PH, Kulp SK, Pan SL, Teng CM, Chen CS. Role of integrin-linked kinase in regulating the protein stability of the MUC1-C oncoprotein in pancreatic cancer cells. Oncogenesis. 2017;6:e359. doi: 10.1038/oncsis.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng K, Wang G, Li C, Shan X, Liu H. Knockdown of ILK inhibits glioma development via upregulation of E-cadherin and downregulation of cyclin D1. Oncol Rep. 2015;34:272–278. doi: 10.3892/or.2015.3983. [DOI] [PubMed] [Google Scholar]
- 97.Gil D, Ciolczyk-Wierzbicka D, Dulinska-Litewka J, Laidler P. Integrin-linked kinase regulates cadherin switch in bladder cancer. Tumour Biol. 2016;37:15185–15191. doi: 10.1007/s13277-016-5354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhuang X, Lv M, Zhong Z, Zhang L, Jiang R, Chen J. Interplay between intergrin-linked kinase and ribonuclease inhibitor affects growth and metastasis of bladder cancer through signaling ILK pathways. J Exp Clin Cancer Res. 2016;35:130. doi: 10.1186/s13046-016-0408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng PC, Chen CL, Shan YS, Chang WT, Liu HS, Hong TM, Hsieh CY, Lin SH, Lin CF. An increase in integrin-linked kinase non-canonically confers NF-kappaB-mediated growth advantages to gastric cancer cells by activating ERK1/2. Cell Commun Signal. 2014;12:69. doi: 10.1186/s12964-014-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song W, Zhao C, Jiang R. Integrin-linked kinase silencing induces a S/G2/M phases cell cycle slowing and modulates metastasis-related genes in SGC7901 human gastric carcinoma cells. Tumori. 2013;99:249–256. doi: 10.1177/030089161309900221. [DOI] [PubMed] [Google Scholar]
- 101.Choi YP, Kim BG, Gao MQ, Kang S, Cho NH. Targeting ILK and beta4 integrin abrogates the invasive potential of ovarian cancer. Biochem Biophys Res Commun. 2012;427:642–648. doi: 10.1016/j.bbrc.2012.09.114. [DOI] [PubMed] [Google Scholar]
- 102.Qu Y, Hao C, Xu J, Cheng Z, Wang W, Liu H. ILK promotes cell proliferation in breast cancer cells by activating the PI3K/Akt pathway. Mol Med Rep. 2017;16:5036–5042. doi: 10.3892/mmr.2017.7180. [DOI] [PubMed] [Google Scholar]
- 103.Krenn PW, Hofbauer SW, Pucher S, Hutterer E, Hinterseer E, Denk U, Asslaber D, Ganghammer S, Sternberg C, Neureiter D, Aberger F, Wickstrom SA, Egle A, Greil R, Hartmann TN. ILK induction in lymphoid organs by a TNFalpha-NF-kappaB-regulated pathway promotes the development of chronic lymphocytic leukemia. Cancer Res. 2016;76:2186–2196. doi: 10.1158/0008-5472.CAN-15-3379. [DOI] [PubMed] [Google Scholar]
- 104.Shirley LA, McCarty S, Yang MC, Saji M, Zhang X, Phay J, Ringel MD, Chen CS. Integrin-linked kinase affects signaling pathways and migration in thyroid cancer cells and is a potential therapeutic target. Surgery. 2016;159:163–170. doi: 10.1016/j.surg.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Que L, Zhao D, Tang XF, Liu JY, Zhang XY, Zhan YH, Zhang L. Effects of lentivirus-mediated shRNA targeting integrin-linked kinase on oral squamous cell carcinoma in vitro and in vivo. Oncol Rep. 2016;35:89–98. doi: 10.3892/or.2015.4374. [DOI] [PubMed] [Google Scholar]
- 106.Akrida I, Nikou S, Gyftopoulos K, Argentou M, Kounelis S, Zolota V, Bravou V, Papadaki H. Expression of EMT inducers integrin-linked kinase (ILK) and ZEB1 in phyllodes breast tumors is associated with aggressive phenotype. Histol Histopathol. 2018;33:937–949. doi: 10.14670/HH-11-987. [DOI] [PubMed] [Google Scholar]
- 107.Abd El-Rehim DM, Abd-Elghany MI, Nazmy MH. Integrin-linked kinase, snail and multidrug resistance protein 1: three concordant players in the progression of non-small cell lung cancer. J Egypt Natl Canc Inst. 2015;27:129–137. doi: 10.1016/j.jnci.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Gu X, Xing X, Yang W, Hu J, Dai D. High expression of integrin-linked kinase predicts aggressiveness and poor prognosis in patients with gastric cancer. Acta Histochem. 2014;116:758–762. doi: 10.1016/j.acthis.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Yang HJ, Zheng YB, Ji T, Ding XF, Zhu C, Yu XF, Ling ZQ. Overexpression of ILK1 in breast cancer associates with poor prognosis. Tumour Biol. 2013;34:3933–3938. doi: 10.1007/s13277-013-0981-y. [DOI] [PubMed] [Google Scholar]
- 110.Wang DL, Lan JH, Chen L, Huang B, Li Z, Zhao XM, Ma Q, Sheng X, Li WB, Tang WX. Integrin-linked kinase functions as a tumor promoter in bladder transitional cell carcinoma. Asian Pac J Cancer Prev. 2012;13:2799–2806. doi: 10.7314/apjcp.2012.13.6.2799. [DOI] [PubMed] [Google Scholar]
- 111.Engelman Mde F, Grande RM, Naves MA, de Franco MF, de Paulo Castro Teixeira V. Integrin-linked kinase (ILK) expression correlates with tumor severity in clear cell renal carcinoma. Pathol Oncol Res. 2013;19:27–33. doi: 10.1007/s12253-012-9554-4. [DOI] [PubMed] [Google Scholar]


