Abstract
Intrahepatic and extrahepatic metastases are responsible for the majority of hepatocellular carcinoma (HCC)-related mortalities. Long noncoding RNAs (lncRNAs) exert important functions in modulating various tumor behaviors. However, the functions and mechanisms of lncRNAs in HCC metastasis remain largely unknown. In this study, downregulation of lncRNA growth arrest-specific 5 (GAS5) was observed in HCC tissues and cells, and predicted poor prognosis of patients with HCC. Through performing gain- and loss-of-function experiments, we found that GAS5 could obviously inhibit migration and invasion of HCC cells in vitro, and suppress tumor metastasis in vivo. Mechanistically, GAS5 functioned as a tumor suppressor in HCC metastasis through directly interacting with miR-182 and abrogating its oncogenic function in this setting. Moreover, GAS5 acted as a competing endogenous RNA (ceRNA) for miR-182 to upregulate the expression of anti-metastasis protein ANGPTL1. Finally, we demonstrated that using ultrasound targeted microbubble destruction (UTMD)-mediated GAS5 transfection could significantly decrease migratory and invasive abilities of HCC cells. Collectively, our study first reveals the mechanism of GAS5/miR-182/ANGPTL1 axis in suppressing HCC metastasis, which provides promising new avenues for therapeutic intervention against HCC progression.
Keywords: lncRNA, ceRNA, ANGPTL1, metastasis, HCC
Introduction
Human hepatocellular carcinoma (HCC) is the sixth most common cancer and one of the main causes for cancer-associated deaths worldwide [1]. Even though great improvements of comprehensive therapy for HCC have been made, the 5-year survival rate remains low [2]. Distant metastasis and high rate of tumor recurrence is the main cause of the poor prognosis of HCC patients [3]. However, the molecular mechanisms underlying HCC metastasis are not well revealed.
The long noncoding RNAs (lncRNAs) are a class of noncoding RNA larger than 200 nucleotides without protein-coding ability, which can regulate gene expression at transcriptional and posttranscriptional levels and modulate the modification of proteins [4]. Increasing evidence has shown that dysregulation of lncRNAs may influence cell proliferation, migration, invasion, angiogenesis and drug resistance. For example, loss of lncRNA MIR22HG in HCC promotes proliferation, invasion, and metastasis in vitro and in vivo and predicts poor prognosis outcome [5]. ZFAS1 functions as an oncogene in HCC progression by sponging miR-182 and abrogating its tumor-suppressive function [6]. lnc-DILC can suppress liver cancer stem cell expansion by inhibiting IL-6 transcription and STAT3 activation [7]. lncRNA growth arrest-specific 5 (GAS5), locates at 1q25.1, is typically reduced in various cancers and associated with clinicopathological characteristics, such as tumor size, invasion and lymph node metastasis [8]. In glioma, GAS5 sponges miR-18a to inhibit cell proliferation, migration and invasion [9]. GAS5 also interacts with proteins to exert suppressive effect on tumor prgression. For instance, GAS5 interacts with E2F4 and recruits it to EZH2 promoter, which induces EZH2 silence and cell apoptosis in bladder cancer cells [10]. Moreover, GAS5 promotes cell cycle arrest through interacting with YBX1 and regulating p21 expression in gastric cancer [11]. However, the functional significance and underlying mechanism of GAS5 as a potential tumor suppressor in HCC metastasis remains to be elucidated.
miRNAs are highly conserved small single-stranded non-coding RNAs, which could regulate gene expression through translational inhibition or inducing the degradation of mRNA. Recent studies have demonstrated an oncogenic role of miR-182 in various cancers, such as lung cancer, ovarian cancer and HCC, while miR-182 acts as a tumor suppressor in renal cancer [12-15]. In HCC, miR-182 facilitates HCC metastasis by suppressing metastasis suppressor 1 (MTSS1) and FOXO3a, and enhances drug resistance by targeting tumor protein 53-induced nuclear protein 1 (TP53INP1) [12,16,17]. Moreover, increased miR-182 expression can be used as a diagnostic and prognostic predictor in HCC patients [18]. The oncogenic influence of miR-182 in HCC metastasis deserves further investigation.
Downregulation of tumor suppressor genes plays an important role in the pathogenesis of human cancers. The downregulated transcription level of Angiopoietin-like protein 1 (ANGPTL1) has been observed in various cancers. ANGPTL1 functions as a tumor suppressor by inhibiting migration, invasion, metastasis, sorafenib resistance and cancer stemness [19-21]. Although GAS5, miR-182 and ANGPTL1 have been proved to play crucial roles in cancer progression, their regulatory mechanism and interaction still need to be further studied.
In this study, we demonstrated a downregulation of GAS5 expression and its function in HCC metastasis. Also, we showed the regulatory relationship among GAS5, miR-182, and ANGPTL1. This study developed a novel pathway of GAS5 and displayed a new therapeutic target for HCC treatment.
Materials and methods
Patients and tissue samples
Sixty-four cases of HCC tissue and paired nontumor tissue specimens were collected from HCC patients admitted to The First Affiliated Hospital of Jinzhou Medical University from January 2012 and December 2017. All tissues were directly preserved in liquid nitrogen and stored at -80°C. Written informed consents for the use of these clinical materials in research were signed by all patients. All experiments were approved by the Ethic Committee of The First Affiliated Hospital of Jinzhou Medical University.
Cell culture
Six HCC cell lines (SMMC-7721, Hep3B, MHCC-97h, PLC/PRF/5) and two normal hepatic cell lines (LO2, QSG-7701) were obtained from the cell bank of the typical culture deposit committee of Chinese academy of sciences (Shanghai, China). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco), 100 IU/mL penicillin (Gibco), and 100 mg/mL streptomycin (Gibco) in a humidified atmosphere containing 5% CO2 at 37°C.
Lentivirus production and infection
To construct GAS5 overexpressing cells, the lentiviral vector pLV expressing nothing or full-length GAS5 and pLV/helper packaging plasmids was transfected into 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). To construct GAS5 knockdown cells, the lentiviral vector pLKO.1 expressing scramble (shNC) or GAS5 shRNAs (shGAS5) and pLV/helper packaging plasmids was transfected into 293T cells. Supernatants were collected at 48 h post transfection, and filtered through 0.45 μm filters to remove cells and debris. HCC cells were infected with above lentiviral particles. Stable cell clones were selected by using 2 μg/ml puromycin for one week. The overexpression and knockdown efficiency was determined by qRT-PCR assay. The target sequences of GAS5 and ANGPTL1 shRNAs were provided as follow: shGAS5-1: GTCCTAAAGAGCAAGCCTA, shGAS5-2: GGATGACTTGCTTGGGTAA, shANGPTL1: TGCAGAGGTGGACAATTCA.
RNA immunoprecipitation (RIP)
RIP assay was performed according to previous study [6]. Hep3B and SMMC-7721 cells were transfected with pcDNA-GAS5-MS2 or pcDNA-GAS5-mut-MS2 or pcDNA-MS2 and pMS2-GFP (Addgene). After 48 hours, cells were used to perform RIP using an anti-GFP antibody (Abcam) and the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) according to the manufacturer’s instructions. Then the miR-182 level was analyzed by qRT-PCR.
For anti-AGO2 RIP, Hep3B and SMMC-7721 cells were transfected with miR-NC or miR-182 mimics. After 48 hours, cells were used to perform RIP using an anti-AGO2 antibody (Millipore) as described above, and then the GAS5 associated with AGO2 was detected by qRT-PCR.
RNA pull-down assay
GAS5 or GAS5-mut was in vitro transcribed respectively from vector pSPT19-GAS5 or pSPT19-GAS5-mut, and biotin-labeled with the Biotin RNA Labeling Mix (Roche) and T7 RNA polymerase (Roche), treated with RNase-free DNase I (Roche). The bound RNAs were purified using TRIzol. One milligram of whole-cell lysates from SMMC-7721 and Hep3B cells were incubated with 3ug of purified biotinylated transcripts for 1 hr at 25°C; complexes were isolated with streptavidin agarose beads (Invitrogen). The GAS5 level in the pull-down material was analyzed by qRT-PCR.
Luciferase reporter assay
ANGPTL1 3’-untranslational region (3’-UTR) and mutant ANGPTL1 3’-UTR (ANGPTL1-mut) was cloned into pmirGLO luciferase reporter vector (Promega). SMMC-7721 and Hep3B stable clones were cotransfected with miR-182 or miR-NC and recombinant plasmids by using Lipofectamine 2000 (Invitrogene). After 48 hrs, the luciferase activity was detected by using Dual-Luciferase Reporter Assay System (Promega). The relative luciferase activity was normalized to Renilla luciferase activity.
RNA isolation and quantitative real-time (qRT-PCR)
Total RNAs were extracted using TRIzol reagent (Invitrogen). cDNA was synthesized using a PrimeScript RT reagent Kit with gDNA Eraser kit (Takara, Dalian, China) according to the manufacturer’s instruction. PCR reactions were followed using SYBR Premix Ex Taq II (Takara) with the StepOne Plus Real Time PCR System (Life Technologies). The GAPDH were used as internal controls. The sequences of the primers were as follows: GAS5-forward: TATGGAGAGTCGGCTTGACTA, GAS5-reverse: TAACAGGTCTGCCTGCATTT; ANGPTL1-forward: CACCACACTGGACAGAGATAAA, ANGPTL1-reverse: CTTGGTGCTTGCTTCTGTAATG.
Western blot
Cell protein was extracted using RIPA Protein Extraction Reagent (Beyotime, Beijing, China) supplemented with 1% protease inhibitor cocktails (Roche). The protein samples were separated by SDS-PAGE, transferred to a PVDF membrane (Millipore) and then incubated with primary antibodies overnight at 4°C. After wash, the membrane was incubated with secondary antibodies. Protein bands were visualized by ECL chemiluminescence kit (Millipore). The primary antibodies used were rabbit anti-ANGPTL1 polyclonal antibody (1:1000, Abcam, USA) and mouse anti-GAPDH monoclonal antibody (1:1000, Proteintech, Wuhan, China).
Isolation of cytoplasmic and nuclear RNA
Cytoplasmic and nuclear RNA were isolated and purified using the Cytoplasmic & Nuclear RNA Purification Kit (Norgen, Belmont, CA) according to the manufacturer’s instructions.
Transient transfection
Transfections were performed using the Lipofectamine 2000 kit (Invitrogen) according to the manufacturer’s instructions. The double-stranded microRNA mimics and their respective negative control RNAs (GenePharma) or their inhibitors were introduced into cells at a final concentration of 50 nM. The cells were harvested or used for further experiments at 48 hour after transfection. The sequences of microRNA inhibitors was shown as follow: inh: CCUAAGGUUAAGUCGCCCUCGCUC, inh-182: AGUGUGAGUU CUACCAUUGCCAAA.
Migration and invasion assay
Migration and invasion assays were performed using the Transwell system (24-wells, 8-μm pore size, Corning Costar, USA). 1 × 105 cells in 200 μL serum-free DMEM were seeded in the upper chamber coated (for invasion assays) or uncoated (for migration assays) with Matrigel. The medium supplemented with 10% serum was used as a chemoattractant in the bottom chamber. The cells were incubated at 37°C for 24 h. The cells in the top chambers were removed with cotton swabs, whereas the migrated and invaded cells on the lower membrane surface were fixed in paraformaldehyde for 10 min, and then stained with 0.2% crystal violet and counted in at least 5 random microscope fields.
Ultrasound-targeted microbubble destruction (UTMD) exposure
A therapeutic ultrasound machine (PHYSIOSON Basic) was used to make ultrasound at the frequency of 1 MHz. The ultrasound transducer was placed at the bottom of dishes with coupling medium on the surface of the transducer. Microbubbles (SonoVue) were lipid-shelled ultrasound contrast agents containing sulfur hexafluoride gas (diameter 3-6.0 μm) and used at a concentration of 1 × 108 bubbles/ml. UTMD parameters were as follow: ultrasound intensity, 0.8 W/cm2; exposure time, 70 s; pulse frequency, 100 Hz; duty cycle, 20%; volumetric ratio of microbubbles: medium, 1:6.
In vivo animal studies
To detect the effect of GAS5 on metastasis in vivo, 1 × 106 stable HCC cells with GAS5 knockdown or overexpression were injected into nude mice (seven mice per group). Intrasplenic injection model was used for detecting the liver colonization of HCC cells. A tail vein injection model was used for detecting the pulmonary metastasis of HCC cells. After 6 weeks, all the mice were sacrificed. The liver and lung tissues were collected and fixed in 10% formalin and embedded in paraffin. H&E staining was performed on sections. The number of hepatic or pulmonary metastasis nodes was counted using an Olympus light microscope. One metastasis nodes should contain more than 50 cells. All animal studies were approved by the animal institute of The First Affiliated Hospital of Jinzhou Medical University.
Statistical analyses
Data were expressed as mean ± standard deviation (SD). Paired Student’s t test, Chi-square test, or One-Way ANOVA test was performed to compare the differences among different groups. The Kaplan-Meier method and log-rank test was used to estimate overall survival. Differences with P<0.05 were considered statistically significant.
Results
GAS5 expression is decreased in HCC and correlated with poor outcomes
To detect the differential expression of GAS5, the expression levels of GAS5 in 60 paired HCC and adjacent non-tumor tissues were measured by qRT-PCR. As shown in Figure 1A, GAS5 expression in HCC tissues was dramatically decreased compared with adjacent non-tumor tissues. It was also observed that GAS5 expression was significantly downregulated in aggressive HCC tissues compared with non-aggressive HCC tissues (Figure 1B). Moreover, the Kaplan-Meier analysis and the log-rank test revealed that the overall survival in HCC patients with a high-level GAS5 was notably longer than that in those with low-level GAS5 (Figure 1C). Additionally, we examined the GAS5 expression in different HCC cells. Downregulation of GAS5 expression could be observed in all the six HCC cell lines, compared to two normal hepatic cell lines, LO2 and QSG-7701 cells (Figure 1D). SMMC-7721 cells expressed the highest level and Hep3B cells showed the lowest expression of GAS5, thus these two cell lines were applied for further loss- and gain-of-function assays, respectively. In conclusion, these data suggested that GAS5 may play a suppressive role in HCC progression.
Figure 1.
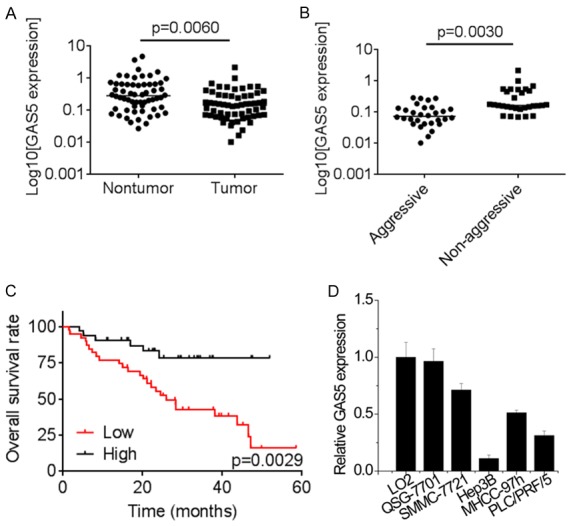
GAS5 expression is decreased in HCC and correlated with poor outcomes. A. The expression of GAS5 in 60 paired HCC tissues and adjacent non-tumor tissues was measured by qRT-PCR. B. The comparison of GAS5 levels in 30 aggressive and 30 non-aggressive HCC tissue samples was determined by qRT-PCR. C. The Kaplan-Meier analysis and the log-rank test was used to evaluate the relationship between GAS5 expression of HCC tissues and overall survival rate of HCC patients. The median expression level of GAS5 in HCC tissues was used as the cutoff. D. The relative expression of four HCC cell lines and two normal liver cell lines was detected by qRT-PCR.
GAS5 suppresses cell migration, invasion and tumor metastasis in vitro and in vivo
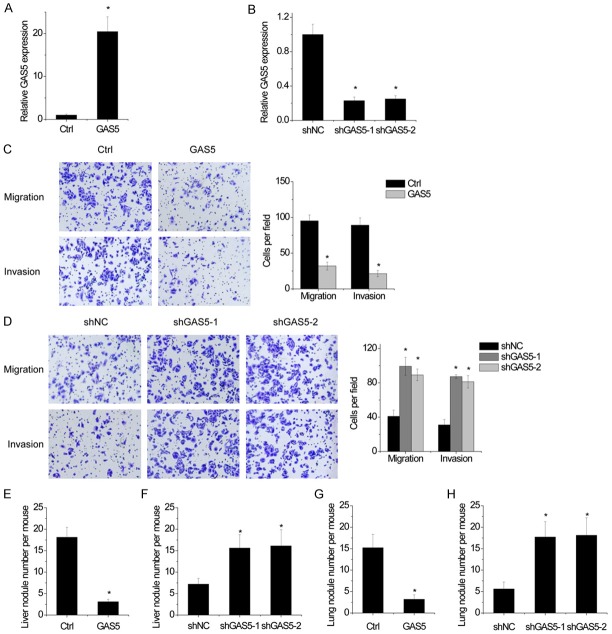
Next, the functional roles of GAS5 in HCC cells were investigated. Hep3B cells were infected with lentiviral particles expressing GAS5 (Figure 2A), while SMMC-7721 cells were infected with lentiviral particles expressing GAS5 shRNAs (Figure 2B). Migration and invasion is critical for tumor metastasis. Transwell assays showed that overexpression of GAS5 significantly attenuated the migration and invasion abilities of Hep3B cells (Figure 2C). Conversely, the cell migration and invasion was enhanced by GAS5 silencing in SMMC-7721 cells (Figure 2D).
Figure 2.
GAS5 suppresses cell migration, invasion and tumor metastasis in vitro and in vivo. A. The GAS5 was overexpressed in Hep3B cells by being infected lentiviral particles expressing GAS5, then the GAS5 expression was detected by qRT-PCR. B. The GAS5 expression was silenced in SMMC-7721 cells by being infected lentiviral particles expressing GAS5 shRNAs, then the GAS5 expression was detected by qRT-PCR. C. A transwell assay was conducted to examine the migratory and invasive and capabilities of control and GAS5 overexpressing Hep3B cells. Statistical results of the transwell assay (three independent experiments) was shown. D. A transwell assay was conducted to examine the migratory and invasive and capabilities of control and GAS5 knockdown SMMC-7721 cells. Statistical results of the transwell assay (three independent experiments) was shown. E. Number of liver metastases in each group 6 weeks after intrasplenic injection with control and GAS5 overexpressing Hep3B cells. F. Number of liver metastases in each group 6 weeks after intrasplenic injection with control and GAS5 knockdown SMMC-7721 cells. G. Number of lung metastases in each group 6 weeks after tail vein injection with control and GAS5 overexpressing Hep3B cells. H. Number of lung metastases in each group 6 weeks after tail vein injection with control and GAS5 knockdown SMMC-7721 cells. Bars represent mean ± SD. *P<0.05.
We further validate the effects of GAS5 on HCC metastasis in vivo. To explore the influence of GAS5 on liver colonization, different stable cells with GAS5 alteration were intrasplenically injected into nude mice. Overexpression of GAS5 resulted in less liver metastases burden formed by Hep3B cells (Figure 2E). In contrast, the depletion of GAS5 significantly increased the liver metastases burden of SMMC-7721 cells (Figure 2F). The role of GAS5 in lung colonization was further explored by injecting stable cells into the tail veins of nude mice. The results revealed that the number of lung metastasis modules in the GAS5 overexpressing Hep3B group is significantly decreased compared with the control group (Figure 2G). Reciprocally, GAS5-knockdown SMMC-7721 cells exhibited more pulmonary metastasis compared to control group (Figure 2H). Taken together, these results indicated that GAS5 inhibits cell migration, invasion and tumor metastasis both in vitro and in vivo.
GAS5 is associated with miR-182
Using cytoplasmic and nuclear RNA fractions from SMMC-7721 cells, we observed that GAS5 is expressed in relative abundance in the cytoplasm (Figure 3A), indicating that GAS5 may interact with microRNAs. To reveal the potential mechanisms of GAS5 functions in HCC cells, bioinformatics tools (TargetScan and miRBase) were used to predict the potential target microRNAs of GAS5. Bioinformatics tools analysis revealed the potential binding sites for miR-182 on GAS5 (Figure 3B). SMMC-7721 and Hep3B cells were transfected miR-182 mimics and inhibitors, respectively. The results of luciferase reporter assays showed that miR-182 mimics significantly decreased the luciferase activity of the wild-type GAS5 but not empty vector or mutant GAS5 (GAS5-mut) in SMMC-7721 cells (Figure 3C). Conversely, miR-182 inhibitor increased the luciferase activity of the GAS5 in Hep3B cells (Figure 3D). Furthermore, an RIP with MS2-binding protein (MS2bp) which specifically binds RNA containing MS2-binding sequences (MS2bs) was carried out to confirm the direct interaction between GAS5 and miR-182. The results demonstrated that the GAS5 RIP in both SMMC-7721 and Hep3B cells was significantly enriched for miR-182 compared to the empty vector (MS2), IgG, GAS5 with mutations in miR-182 binding sites (GAS5-mut) (Figure 3E). The RNA pull-down assay also confirmed the association of GAS5 and miR-182 in both SMMC-7721 and Hep3B cells (Figure 3F). The microRNAs inhibit their targets in an AGO2-dependent manner. To validate whether GAS5 was regulated by miR-182 in such a manner, anti-AGO2 RIP was carried out in Hep3B and SMMC-7721 cells transiently transfected with miR-182 mimics. Endogenous GAS5 was specifically enriched in miR-182-transfected cells (Figure 3G), indicating that GAS5 is recruited to AGO2-related RNA-induced silencing complexes and functionally interacts with miR-182. Moreover, overexpression GAS5, but not mutant GAS5 (Figure 3H), reduced the miR-182 expression in Hep3B cells, while GAS5 silencing increased miR-182 level in SMMC-7721 cells (Figure 3I). However, overexpression or depletion of miR-182 did not change GAS5 expression (Figure 3J and 3K). Collectively, these results indicated that GAS5 physically interacts with miR-182.
Figure 3.
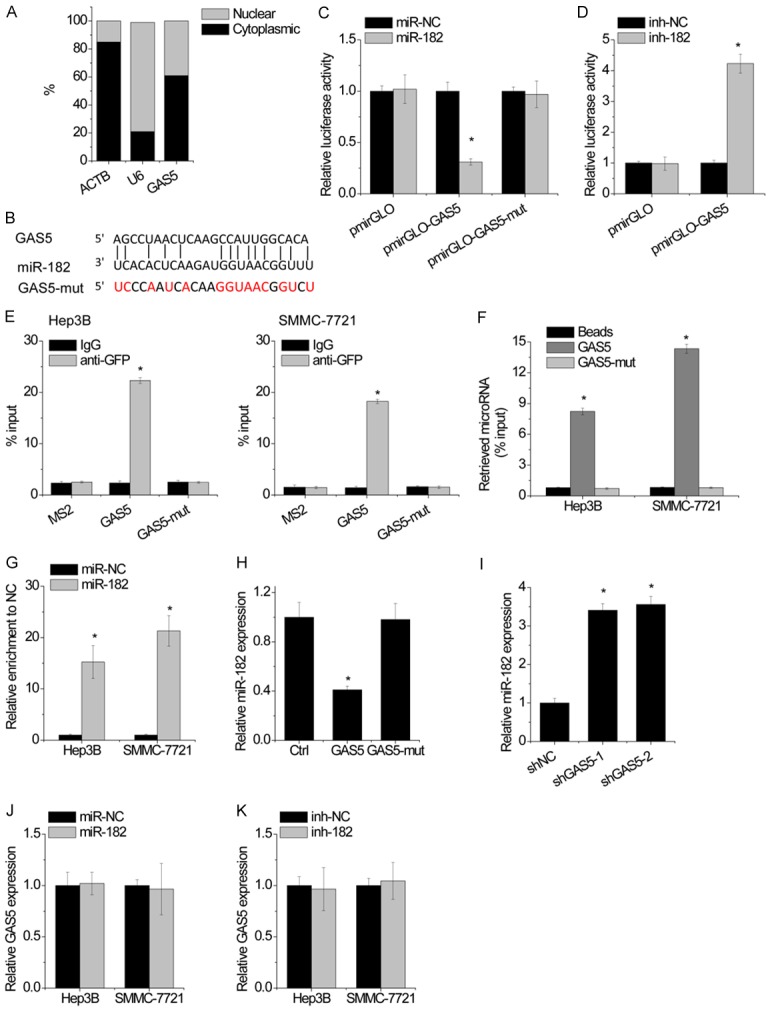
GAS5 is associated with miR-182. A. GAS5 mainly located in cytoplasm of SMMC-7721 cells shown by qRT-PCR. B. Regions of putative interaction between GAS5 and miR-182. C. Luciferase activity in SMMC-7721 cells cotransfected with miR-NC or miR-182 and luciferase reporters containing nothing, GAS5 or mutant GAS5. Data are presented as the relative ratio of firefly luciferase activity to renilla luciferase activity. D. Luciferase activity in Hep3B cells cotransfected with miR-NC (inh-NC) or miR-182 inhibitor (inh-182) and luciferase reporters containing nothing or GAS5. Data are presented as the relative ratio of firefly luciferase activity to renilla luciferase activity. E. MS2-RIP was performed to detect miR-182 endogenously interacted with GAS5 in SMMC-7721 and Hep3B cells. F. SMMC-7721 and Hep3B cell lysates were incubated with biotin-labeled GAS5; after pull-down, miR-182 was assessed by qRT-PCR. G. Anti-AGO2 RIP was performed in SMMC-7721 and Hep3B cells transiently overexpressing miR-NC or miR-182, followed by qRT-PCR to detect GAS5 associated with AGO2. H. The expression of miR-182 was detected by qRT-PCR in SMMC-7721 cells with overexpression of wild-type or mutant GAS5. I. The expression of miR-182 was detected by qRT-PCR in Hep3B cells with GAS5 knockdown. J. The expression of GAS5 was detected by qRT-PCR in SMMC-7721 and Hep3B cells transfected with miR-NC or miR-182. K. The expression of GAS5 was detected by qRT-PCR in SMMC-7721 and Hep3B cells transfected with miR-NC or miR-182 inhibitor. Bars represent mean ± SD. *p<0.05.
miR-182 directly targets ANGPTL1
The biologic consequences of miR-182 in regulating HCC cell migration and invasion were then detected using Transwell assay. Transfection of miR-182 mimic increased miR-182 expression, while miR-182 level is reduced in Hep3B cells after treatment with miR-182 inhibitor (Figure 4A and 4B). Functionally, miR-182 overexpression markedly promoted cell migration and invasion, whereas miR-182 knockdown exerted opposite effect (Figure 4C and 4D).
Figure 4.
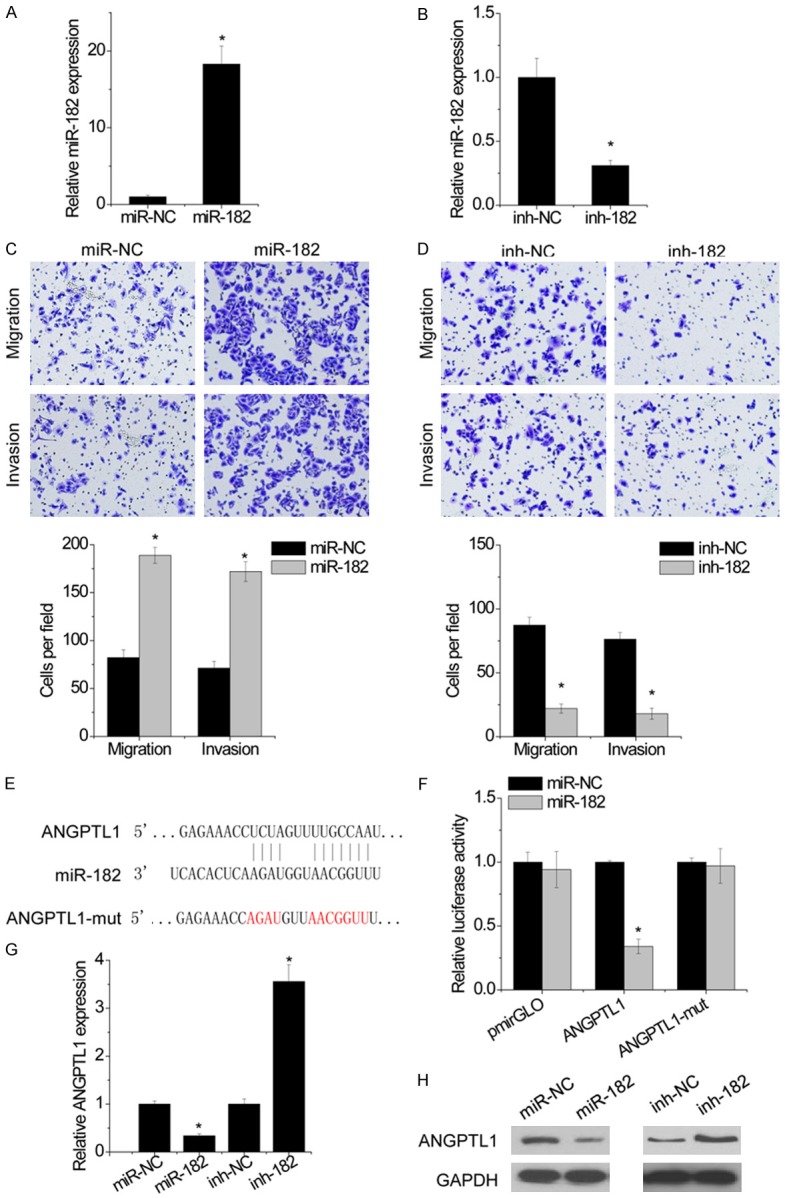
miR-182 directly targets ANGPTL1. A. Hep3B cells were transfected with miR-182. The miR-182 level was detected by qRT-PCR. B. Hep3B cells were transfected with miR-182 inhibitor. The miR-182 level was detected by qRT-PCR. C. The effect of miR-182 overexpression on migration and invasion in Hep3B cells was detected by transwell assay. Statistical results of the transwell assay (three independent experiments) was shown. D. The effect of miR-182 knockdown on migration and invasion in Hep3B cells was detected by transwell assay. Statistical results of the transwell assay (three independent experiments) was shown. E. Regions of putative interaction between ANGPTL1 3’UTR and miR-182. F. Luciferase activity in Hep3B cells cotransfected with miR-NC or miR-182 and luciferase reporters containing nothing, ANGPTL1 3’UTR or its mutant. G. ANGPTL1 mRNA levels in indicated Hep3B cells was detected by qRT-PCR. H. ANGPTL1 protein levels in indicated Hep3B cells was detected by western blot. Bars represent mean ± SD. *P<0.05.
To elucidate the underlying molecular mechanism about how miR-182 exerted its effects on HCC cells, we searched for potential targets of miR-182. Interestingly, through TargetScan and miRcode, we predicted ANGPTL1 as a target of miR-182 (Figure 4E). We therefore constructed luciferase reporter vectors containing 3’-UTR of ANGPTL1. The luciferase reporter assay demonstrated that miR-182 significantly decreased the luciferase activity of ANGPTL1 3’UTR, whereas mutation of miR-182-targeting sites (ANGPTL1-mut) results in complete abrogation of the suppressive effect (Figure 4F). The results of both qRT-PCR and western blot showed that miR-182 negatively modulate ANGPTL1 expression (Figure 4G and 4H). Together, these results demonstrated that miR-182 targets the tumor suppressor ANGPTL1.
GAS5 upregulates ANGPTL1 expression to suppress aggressiveness
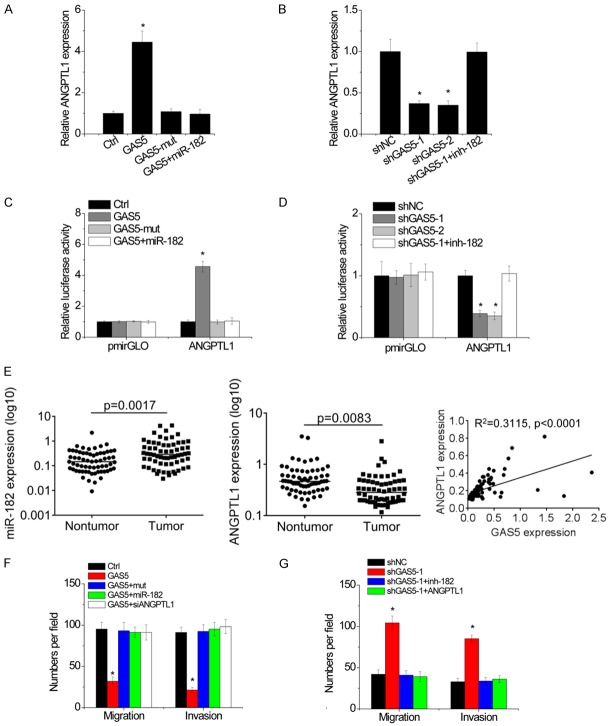
GAS5 directly binds miR-182 and inhibits its activity, and miR-182 targets ANGPTL1. Hence, we speculated GAS5 may regulate ANGPTL1 expression through suppression of miR-182. Overexpression of GAS5, but not GAS5-mut, led to the increase of ANGPTL1 level in Hep3B cells. miR-182 overexpression abrogated this increase (Figure 5A). Conversely, knockdown of GAS5 reduced ANGPTL1 expression level in SMMC-7721 cells, while inhibition of miR-182 overcame the downregulation of ANGPTL1 (Figure 5B).
Figure 5.
GAS5 upregulates ANGPTL1 expression to suppress aggressiveness. A. miR-182 overexpression abolished the GAS5-mediated upregulation of ANGPTL1 mRNA level. B. miR-182 knockdown rescued GAS5 shRNA-mediated downregulation of ANGPTL1 mRNA level. C. Luciferase activity in stable Hep3B cell clones transfected with luciferase reporters containing ANGPTL1 3’UTR. D. Luciferase activity in stable SMMC-7721 cell clones transfected with luciferase reporters containing ANGPTL1 3’UTR. E. The expression of miR-182 and ANGPTL1 in 60 paired HCC tissues and adjacent non-tumor tissues was measured by qRT-PCR. The correlation between ANGPTL1 and GAS5 RNA levels in HCC tissues was then analyzed. F. The stable Hep3B cell clones were transfected with miR-182 or ANGPTL1 siRNA. After 48 hours, the migration and invasion was detected by transwell assay. G. The stable SMMC-7721 cell clones were transfected with inh-182 or ANGPTL1. After 48 hours, the migration and invasion was detected by transwell assay. Bars represent mean ± SD. *P<0.05.
To further verify whether this observed effect depends on the role miR-182 in regulating of the ANGPTL1 3’UTR, luciferase reporter assay was carried out. As shown in Figure 5C, overexpression of GAS5, but not its mutant, increased the luciferase activity of ANGPTL1 3’UTR, whereas overexpression of miR-182 abolished this increase in Hep3B cells. Inversely, GAS5 knockdown repressed the luciferase activity of ANGPTL1 3’UTR, which was rescued by inhibition of miR-182 in SMMC-7721 cells (Figure 5D). We then determined the pathological correlation between GAS5 and ANGPTL1 in HCC tissues. A significant increase of miR-182 expression and decrease of ANGPTL1 expression was found in HCC tissues compared to that in adjacent non-tumor tissues. Moreover, it was observed that GAS5 transcript level was positively correlated with ANGPTL1 mRNA expression (R2=0.3115, P<0.0001; Figure 5E). These results suggest that GAS5 positively regulates ANGPTL1 expression by competitively binding miR-182.
We next tried to investigate whether GAS5 exert its tumor suppressive effects on migration and invasion of HCC cells through GAS5/miR-182/ANGPTL1 axis. The transwell assays demonstrated that overexpression of GAS5, but not the mutant GAS5, facilitated migration and invasion of Hep3B cells, whereas overexpression of miR-182 or depletion of ANGPTL1 abolished these increases (Figure 5F). Conversely, downregulation of GAS5 enhanced the migratory and invasive abilities of SMMC-7721 cells, whereas inhibition of miR-182 or ectopic expression of ANGPTL1 attenuated these effects (Figure 5G). Collectively, our data demonstrated that GAS5/miR-182/ANGPTL1 axis play a suppressive effect on HCC metastasis.
Evaluation of anti-metastatic effect of GAS5 delivered using ultrasound targeted microbubble destruction
Ultrasound targeted microbubble destruction (UTMD) technology has been recognized as a promising technology for gene delivery [22]. We then examined the antimetastatic effect of GAS5 delivered using UTMD. UTMD-mediated GAS5 overexpression was tested by qRT-PCR in Hep3B and SMMC-7721 cells. As shown in Figure 6A, the expression of GAS5 was increased by transfection of GAS5, and GAS5+UTMD group showed more effective upregulation than GAS5 group. Transwell assays showed that GAS5 overexpression inhibited migration and invasion in both SMMC-7721 and Hep3B cells, while UTMD-mediated delivery of GAS5 augmented this effect (Figure 6B). Taken together, these results indicated that UTMD-mediated delivery of GAS5 significantly reduced the migratory and invasive capacities of HCC cells and showed better suppressive effect than lipidosome-mediated transfection.
Figure 6.
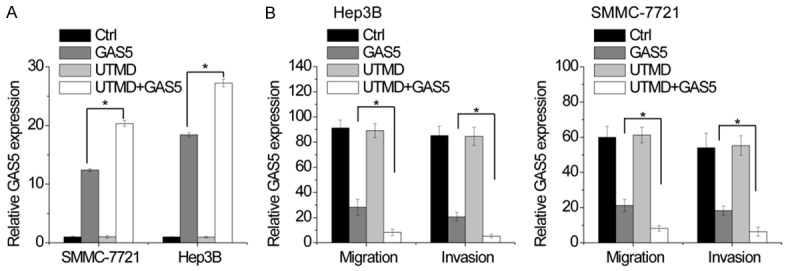
Evaluation of anti-metastatic effect of GAS5 delivered using ultrasound targeted microbubble destruction. A. The vector expressing GAS5 was transfected into Hep3B and SMMC-7721 cells by using lipidosome or UTMD. The GAS5 expression in indicated group was detected by qRT-PCR. B. The migration and invasion of SMMC-7721 and Hep3B cells with different treatment was detected by transwell assay. Statistical results of the transwell assay (three independent experiments) was shown. Bars represent mean ± SD. *P<0.05.
Discussion
LncRNAs have been reported to be connected with the progression of HCC [23,24]. For instance, lncRNA-ATB promotes HCC metastasis via acting as miRNA-200s sponge [25]. Accordingly, GAS5 has been identified as a bona fide tumor suppressor in several human cancers [26]. In the present study, we demonstrated the tumor-suppressive role of GAS5 in both clinical HCC tissues and HCC cells. First, we identified a remarkable reduced level of GAS5 expression in HCC tissue samples compared with corresponding adjacent normal tissues. Clinical observation further showed that downregulation of GAS5 significantly associated with tumor aggression and shorter overall survival time in HCC patients. Functional assays demonstrated that GAS5 suppressed HCC cell migration and invasion in vitro, and inhibited metastasis in vivo. Further investigation showed that GAS5 regulated the HCC cells via suppressing miR-182/ANGPTL1 axis, as GAS5 could regulate miR-182 expression and further modulate its target, ANGPTL1. Thus, based on the above results, we concluded that lncRNA GAS5 functions as a tumor suppressor in HCC metastasis.
Many lncRNAs function as competing endogenous RNAs (ceRNA) to modulate HCC development by competitively binding common microRNAs, such as lncRNA-HCAL, ZFAS1 and CASC2 [6,27,28]. In this study, bioinformatics analysis, luciferase reporter assay, MS2 RIP and RNA pull-down assays confirmed that miR-182 was a target of GAS5 in HCC cells. It was reported that miR-182 enhanced migratory and invasive abilities of HCC cells through targeting FOXO3a [12]. Consistently, knockdown and overexpression approaches here demonstrated that miR-182 facilitated HCC migration and invasion. Hence, we speculated that GAS5 exerted suppressive effect in HCC metastasis through association with miR-182. ANGPTL1 has been confirmed to exert anti-metastatic effect in HCC [29]. ANGPTL1 suppressed tumor metastasis by targeting JAK2/STAT3 signaling, upregulation of miR-138 and suppression of Slug [20,21,29]. However, whether ANGPTL1 is regulated by lncRNAs or microRNAs remain unknown. Interestingly, bioinformatics prediction tools indicated that ANGPTL1 might be a downstream target of miR-182. Subsequently, we revealed that GAS5 could upregulate ANGPTL1 expression via targeting miR-182 in HCC cells. Moreover, miR-182/ANGPTL1 mediated the anti-metastatic effects of GAS5 in HCC cells. In accordance, overexpression of miR-182 or restoration of ANGPTL1 abolished the suppressive effects of GAS5 on HCC cell migration and invasion. To sum up, these data demonstrated that GAS5/miR-182/ANGPTL1 axis negatively regulates HCC progression.
UTMD has recently been used for gene delivery [30-32]. Using UTMD can stimulate sonoporation and change the permeability of cell membrane, which makes the delivery of RNA or DNA into cells more efficient [33]. Our previous study demonstrated that UTMD-mediated siRNA transfection against lncRNA-ATB significantly inhibited lncRNA-ATB expression and ZEB1 and ZEB2 expression and suppressed cell migration and invasion [34]. Here, UTMD-mediated GAS5 overexpression induced significant inhibition of cell migration and invasion. Nevertheless, whether UTMD-mediated GAS5 overexpression works in vivo system needs further investigation. It is possible that UTMD combined with other suppressive lncRNA may be developed as a potential therapeutic approach for HCC treatment.
In conclusion, we demonstrated that GAS5 was downregulated in HCC tissues and cells, and could inhibit migration and invasion of HCC cells via GAS5/miR-182/ANGPTL1 axis. Thus, these findings will improve understanding of mechanism involved in HCC metastasis and provide novel targets for the molecular treatment of HCC.
Acknowledgements
This study was supported by the Natural Science Foundation of Liao Ning Province (No. 2014022013). This study was supported by the Natural Science Foundation of Liao Ning Province (No. 2014022013).
Disclosure of conflict of interest
None.
References
- 1.Donadon M, Solbiati L, Dawson L, Barry A, Sapisochin G, Greig PD, Shiina S, Fontana A, Torzilli G. Hepatocellular carcinoma: the role of interventional oncology. Liver Cancer. 2016;6:34–43. doi: 10.1159/000449346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercado MA, Medina H, Rossano A, Acosta E, Rodriguez M, Chan C, Orozco H. Metastatic disease of the liver: surgical perspective. Rev Gastroenterol Mex. 1997;62:235–238. [PubMed] [Google Scholar]
- 3.Panera N, Crudele A, Romito I, Gnani D, Alisi A. Focal adhesion kinase: insight into molecular roles and functions in hepatocellular carcinoma. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aird J, Baird AM, Lim MCJ, McDermott R, Finn SP, Gray SG. Carcinogenesis in prostate cancer: the role of long non-coding RNAs. Noncoding RNA Res. 2018;3:29–38. doi: 10.1016/j.ncrna.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang DY, Zou XJ, Cao CH, Zhang T, Lei L, Qi XL, Liu L, Wu DH. Identification and functional characterization of long non-coding RNA MIR22HG as a tumor suppressor for hepatocellular carcinoma. Theranostics. 2018;8:3751–3765. doi: 10.7150/thno.22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z, Deng X, Chen H, Shen B, Peng C, Li H, Zhan Q, Zhu Z. Amplification of long noncoding RNA ZFAS1 promotes metastasis in hepatocellular carcinoma. Cancer Res. 2015;75:3181–3191. doi: 10.1158/0008-5472.CAN-14-3721. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Sun W, Shen W, Xia M, Chen C, Xiang D, Ning B, Cui X, Li H, Li X, Ding J, Wang H. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol. 2016;64:1283–1294. doi: 10.1016/j.jhep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Pickard MR, Williams GT. Molecular and cellular mechanisms of action of tumour suppressor GAS5 LncRNA. Genes (Basel) 2015;6:484–499. doi: 10.3390/genes6030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Yu W, Zhu S, Cheng K, Xu H, Lv Y, Long X, Ma L, Huang J, Sun S, Wang K. Long noncoding RNA GAS5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating miR-18a-5p. J Cell Physiol. 2018;234:757–768. doi: 10.1002/jcp.26889. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Guo C, Wang L, Luo G, Huang C, Li Y, Liu D, Zeng F, Jiang G, Xiao X. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis. 2018;9:238. doi: 10.1038/s41419-018-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zhao J, Zhang W, Gan J, Hu C, Huang G, Zhang Y. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci Rep. 2015;5:10159. doi: 10.1038/srep10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao MQ, You AB, Zhu XD, Zhang W, Zhang YY, Zhang SZ, Zhang KW, Cai H, Shi WK, Li XL, Li KS, Gao DM, Ma DN, Ye BG, Wang CH, Qin CD, Sun HC, Zhang T, Tang ZY. miR-182-5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J Hematol Oncol. 2018;11:12. doi: 10.1186/s13045-018-0555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Zhang H, Gong H, Yuan Y, Li Y, Wang C, Li W, Zhang Z, Liu M, Liu H, Chen J. miR-182 suppresses invadopodia formation and metastasis in non-small cell lung cancer by targeting cortactin gene. J Exp Clin Cancer Res. 2018;37:141. doi: 10.1186/s13046-018-0824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu JH, Yang S, Nan CJ, Zhou CC, Lu DQ, Li S, Mu HQ. MiR-182 affects renal cancer cell proliferation, apoptosis, and invasion by regulating PI3K/AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:351–357. doi: 10.26355/eurrev_201801_14179. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Ayub B, Liu Z, Serna VA, Qiang W, Liu Y, Hernando E, Zabludoff S, Kurita T, Kong B, Wei JJ. Anti-miR182 reduces ovarian cancer burden, invasion, and metastasis: an in vivo study in orthotopic xenografts of nude mice. Mol Cancer Ther. 2014;13:1729–1739. doi: 10.1158/1535-7163.MCT-13-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Li J, Shen J, Wang C, Yang L, Zhang X. MicroRNA-182 downregulates metastasis suppressor 1 and contributes to metastasis of hepatocellular carcinoma. BMC Cancer. 2012;12:227. doi: 10.1186/1471-2407-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin J, Luo M, Qian H, Chen W. Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene. 2014;538:342–347. doi: 10.1016/j.gene.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Chu F, Cao Y, Shao J, Wang F. Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol. 2015;36:7439–7447. doi: 10.1007/s13277-015-3430-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST, Yen CJ, Yang CY, Sung SY, Su JL. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology. 2016;64:1637–1651. doi: 10.1002/hep.28773. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Xiao Q, Hu Y, Chen L, Jiang K, Tang Y, Tan Y, Hu W, Wang Z, He J, Liu Y, Cai Y, Yang Q, Ding K. ANGPTL1 attenuates colorectal cancer metastasis by up-regulating microRNA-138. J Exp Clin Cancer Res. 2017;36:78. doi: 10.1186/s13046-017-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo TC, Tan CT, Chang YW, Hong CC, Lee WJ, Chen MW, Jeng YM, Chiou J, Yu P, Chen PS, Wang MY, Hsiao M, Su JL, Kuo ML. Angiopoietin-like protein 1 suppresses SLUG to inhibit cancer cell motility. J Clin Invest. 2013;123:1082–1095. doi: 10.1172/JCI64044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZY, Lin Y, Yang F, Jiang L, Ge S. Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles. Cardiovasc Ultrasound. 2013;11:11. doi: 10.1186/1476-7120-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzafame M, Bianco G, Terracciano LM, Ng CKY, Piscuoglio S. The role of long non-coding RNAs in hepatocarcinogenesis. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Khodiry A, Afify M, El Tayebi HM. Behind the curtain of non-coding RNAs; long non-coding RNAs regulating hepatocarcinogenesis. World J Gastroenterol. 2018;24:549–572. doi: 10.3748/wjg.v24.i5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh PC, Oesterling JE, Epstein JI, Bruzek DJ, Rock RC, Chan DW. The value of prostate-specific antigen in the management of localized prostatic cancer. Prog Clin Biol Res. 1989;303:27–33. [PubMed] [Google Scholar]
- 26.Ma C, Shi X, Zhu Q, Li Q, Liu Y, Yao Y, Song Y. The growth arrest-specific transcript 5 (GAS5): a pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol. 2016;37:1437–1444. doi: 10.1007/s13277-015-4521-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang C, Dou C, Xu M, Liu Q, Tu K. Long non-coding RNA CASC2 suppresses epithelial-mesenchymal transition of hepatocellular carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer. 2017;16:123. doi: 10.1186/s12943-017-0702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie CR, Wang F, Zhang S, Wang FQ, Zheng S, Li Z, Lv J, Qi HQ, Fang QL, Wang XM, Yin ZY. Long noncoding RNA HCAL facilitates the growth and metastasis of hepatocellular carcinoma by acting as a ceRNA of LAPTM4B. Mol Ther Nucleic Acids. 2017;9:440–451. doi: 10.1016/j.omtn.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Q, Jiang L, Liu M, Yu D, Zhang Y, Li Y, Fang S, Li Y, Zhu YH, Yuan YF, Guan XY. ANGPTL1 interacts with integrin alpha1beta1 to suppress HCC angiogenesis and metastasis by inhibiting JAK2/STAT3 signaling. Cancer Res. 2017;77:5831–5845. doi: 10.1158/0008-5472.CAN-17-0579. [DOI] [PubMed] [Google Scholar]
- 30.Park DH, Jung BK, Lee YS, Jang JY, Kim MK, Lee JK, Park H, Seo J, Kim CW. Evaluation of in vivo antitumor effects of ANT2 shRNA delivered using PEI and ultrasound with microbubbles. Gene Ther. 2015;22:325–332. doi: 10.1038/gt.2014.120. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Chang S, Sun J, Zhu S, Pu C, Li Y, Zhu Y, Wang Z, Xu RX. Targeted microbubbles for ultrasound mediated short hairpin RNA plasmid transfection to inhibit survivin gene expression and induce apoptosis of ovarian cancer A2780/DDP cells. Mol Pharm. 2015;12:3137–3145. doi: 10.1021/mp500835z. [DOI] [PubMed] [Google Scholar]
- 32.Wang HH, Song YX, Bai M, Jin LF, Gu JY, Su YJ, Liu L, Jia C, Du LF. Ultrasound targeted microbubble destruction for novel dual targeting of HSP72 and HSC70 in prostate cancer. Asian Pac J Cancer Prev. 2014;15:1285–1290. doi: 10.7314/apjcp.2014.15.3.1285. [DOI] [PubMed] [Google Scholar]
- 33.Yin T, Wang P, Li J, Zheng R, Zheng B, Cheng D, Li R, Lai J, Shuai X. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials. 2013;34:4532–4543. doi: 10.1016/j.biomaterials.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 34.Chen F, Li Y, Feng Y, He X, Wang L. Evaluation of antimetastatic effect of lncRNA-ATB siRNA delivered using ultrasound-targeted microbubble destruction. DNA Cell Biol. 2016;35:393–397. doi: 10.1089/dna.2016.3254. [DOI] [PubMed] [Google Scholar]