Abstract
Induction of cancer stem cell (CSC) characters and epithelial mesenchymal transition (EMT) features are crucial in tumor initiation, progression and metastasis. However, underlying mechanisms remain incompletely understood. Here, we showed that ENPP1 plays an important role in inducing and maintaining EMT phenotypes and CSC features in lung cancer. ENPP1 is upregulated in lung cancer cells. ENPP1-knockdown in lung cancer HCC827 cells and A549 cells resulted in suppressed colonogenic formation, anchorage-independent growth in vitro, and tumorigenicity in vivo. ENPP1-knockdown also reduced expression of CSC makers, including ABCG2, SOX2, NANOG, and CD44. Moreover, ENPP1-knockdown reversed TGFβ-induced EMT phenotypes, including cell migration, E-cadherin repression and vimentin induction. Finally, upregulated ENPP1 was identified in majority of human lung tumor tissues compared to adjacent normal lung tissues. Taken together, our study demonstrates that dysregulated ENPP1 contributes to increased malignancy of human lung cancer by inducing CSC-features, and EMT-like phenotypes.
Keywords: ENPP1, lung cancer, EMT, stemness, migration
Introduction
Lung cancer is leading cause of cancer death worldwide [1]. Despite numerous progress in surgery-, radiation- and chemo-therapy, the 5-year survival rate of lung cancer remains low, about 4-17% [2]. The major therapeutic challenges for lung cancer treatment are its heterogeneity and relapse. Thus, identification of key oncogenic drivers for cancer stemness-related and metastatic features would be critical for designing effective therapeutic strategy [3].
EMT, epithelial-mesenchymal transition, is a process of conversing epithelial cells to mesenchymal cells, which is important for embryonic development, but also for wound healing and tumor metastasis. Cells undergoing EMT loss cell-cell adhesion, cell polarity, while acquire migratory and invasive properties [4]. EMT is considered to be a key step during cancer invasion and metastasis. Metastatic tumor cells not only gain migrating and invasive activities but also stemness-related properties, such as increased self-renewal ability and increased capacity to form 3D-spheres [5]. Thus, EMT plays a critical role in the processes of carcinomas acquiring cancer stem cell phenotype [6]. However, the molecular mechanisms that govern the regulation of EMT and cancer stem cell phenotype in lung cancer are incompletely understood.
Enpp1, ectonucleotide pyrophosphatase/phosphodiesterase 1, is a type II transmembrane glycoprotein with pyrophosphatase and phosphodiesterase activity, expressed highly in bone and cartilage. ENPP1 hydrolysis of pyrophosphate bonds (such as in ATP) and phosphodiester bonds (such as in oligonucleotides) to produce nucleoside 5’-monophosphates, as part of the functions mediated by nucleotide pyrophosphatases/phosphodiesterase (NPPs), which is necessary in a wide range of cellular processes including nucleotide recycling, purinergic receptor signaling and ATP-mediated apoptosis [7,8]. Recent study shows that, ENPP1 upregulates ABCG2 transporter, increase tumor seeding ability and resistant to conventional chemotherapy in breast cancer [9]. Knockdown of ENPP1 in glioblastoma stem-like cells downregulates stem cell-associated genes, induces tumor differentiation, cell death, and sensitization to chemotherapeutic treatment [10]. Overall, these studies suggest that ENPP1 plays an important role in acquisition of cancer stem cell properties. However, whether ENPP1 plays a role in regulation of the malignancy and metastatic features in lung cancer is unknown.
In this study, we investigated the roles of ENPP1 in human lung cancer. Our study demonstrates that ENPP1 may play a significant role in increasing the malignancy and metastatic features of lung cancer.
Materials and methods
Cell lines and reagents
Human non-small cell lung cancer cell line HCC827 and A549 were obtained from ATCC, cultured in RPMI 1640 or DMEM (Hyclone) medium been fortified with 10% fetal bovine serum. Human cell lines HBEC, BEAS-2B, H1792, H661, H1975, H460, H1299, A549 were obtained from ATCC cultured in RPMI 1640 medium and DMEM supplemented with 10% fetal bovine serum respectively. Human embryonic kidney cells HEK293T from ATCC were cultured in DMEM medium supplemented with 10% fetal bovine serum. TGF-β (P01137) were purchased from novoprotein, Antibodies against human-ENPP1 purchased from Santa Cruz (Santa Cruz, CA) (sc-393419), and abcam (ab40003), ABCG2 (#42078), Sox2 (#4900), NAOAG (#4893), E-cadherin (#3195), Vimentin (#5741), CD44 (#3570) were purchased from CST; GAPDH (sc-365062) were purchased from Santa Cruz (Santa Cruz, CA). HRP-ACTIN (HRP-60008) was purchased from proteintech. Human lung tumor tissue (NSCLC) were provided by Shanghai Chest Hospital (Shanghai, China) and all informed consents were obtained.
Westernblot analysis
Cells were lysed with RIPA buffer (150 mM NaCl, 50 mM Tris (PH 8.0), 25 mM NaF, 2 mM Na3VO4, 0.5% deoxycholate, 1% NP40, 0.1% SDS) and add protease inhibitor cocktail (biomark.cn). 30 ug protein of cell lysates were mixed 5 × SDS-PAGE reduced loading buffer and boiled at 95°C for 5 min. Cell lysates were separated in SDS-PAGE and transferred onto a nitrocellulose membrane. Then non-specific binding site were blocked with 5% non-fat milk at room temperature for 1 h. Next, the nitrocellulose membrane washed by PBS twice and added primary antibodies incubated at 4°C overnight. In order to reduce background, membranes were washed three times and 5 minutes for per wash in TBST (0.05% Tween-20). Finally, second antibody conjugated with HRP were incubated at room temperature for 1 hour. After above, membranes washed three times in TBST and Chemiluminescence by ECL kit (Millipore).
Colonogenic formation assay
500 cells were cultured on 6-cm culture dish in 3 ml culture medium, cultivated in humidified incubator (37°C, 5% CO2) for 15 days. During this process, the fresh medium changed every other day. Colony of cells were washed by PBS twice time and dyed with 0.1% crystal violet for at least 40 min. And lastly, unwanted dye were washed softly by running water and the picture were captured.
Anchorage-independent growth assay
First, put 500 ml of 2% low-melting temperature agarose in growth medium at 1:3 ratio in each well of 24-well plate and placed in 4°C until solidification. Second, cells were suspended in growth medium and counted, take out 500 cells per 50 ul. Put the cell on top of a previously cast semisolid layer. Third, put 500 ml 2% low-melting temperature agarose in growth medium at 1:6 ratio and added Matrigel (Collaborative Biomedical Products, Becton Dickinson Labware, Bedford, MA) at 1:30 on the top of cells. The picture captured two week later.
Stem cell maker analysis by flow cytometry
Cells were trypsinized and counted, take out 1 × 106 cells washed by PBS for three times. Then suspended with 200 ml PBS and added 5 ml CD44 antibody incubated at 4°C in the dark for 30 min. After these steps, cells analyzed by flow cytometry (Becton-Dickinson).
Immunoflurosence
Cells were seeded onto cover slips in 6-well plate, two days later cells were treated with TGF-β (5 µM/ml) for 4 days. The procedure of immunofluorescence was performed as described previously [12,23].
Wound healing assay
Cells cultured in 6-well plate, when the cells overspread the culture plate scratch the cells with micro pipette tips. Then the picture captured as needed.
Xenograft model
Human NSCLC cells HCC827 stably transfected with non-specific (sh-NS) or ENPP1sh-RNA (sh-2#) were injected subcutaneously 1 × 106 cell in 0.2 ml serum free 1640 medium supplemented with 30% matrigel) in nude mice. After 3 weeks, the tumor volume was measured twice a week. When the HCC827-shNS tumor volume reached 2000 mm3, the nude mice were sacrificed and the tumor volume (mm3) was calculated by the formula: (a × b2)/2, where “a” is the long diameter and “b” is the short diameter (mm).
Immunohistochemistry (IHC)
NSCLC patients tissue microarray supplied by Jiong Deng’s Lab. Tissue samples were stained to identify ENPP1 proteins. The IHC protocol and score method were performed as previously described [24]. All antibodies were diluted for use according to manufacturers’ instructions.
Statistical analyses
The data were analyzed by the software GraphPad Prism Version 5.01. Data are presented as the mean + SD. A two-tailed unpaired t-test was used to compare results. A value of P<0.05 was considered statistically significant.
Results
ENPP1 knockdown inhibits the growth of lung tumors
To investigate if ENPP1 is upregulated in human lung cancer samples, we first analyzed data from public database from Oncomine (Figure 1A-D), and found that ENPP1 is significantly upregulated in lung adenocarcinoma tissues compared to normal lung tissues in four resources (Okayama lung, Selamat lung, Landi lung, and Su lung).
Figure 1.
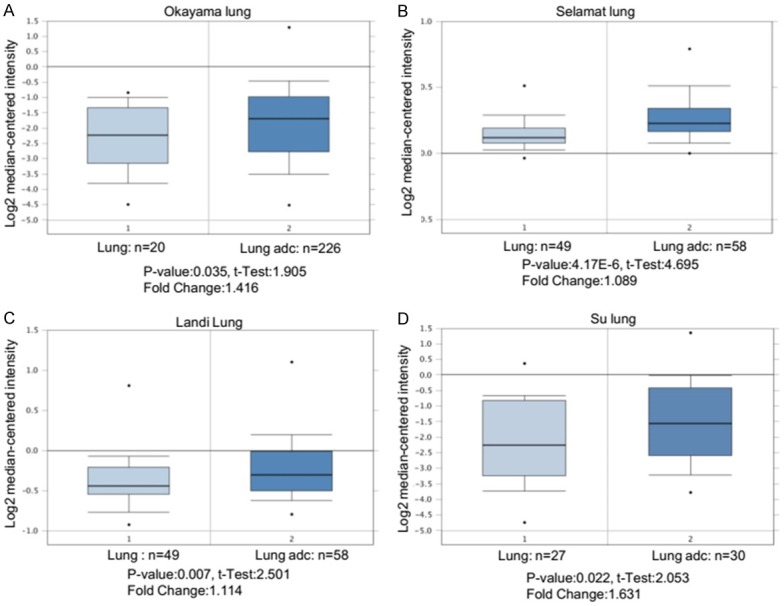
ENPP1 is dysregulated in human lung cancer. A-D. Relative mRNA expression levels of ENPP1 in human lung adenocarcinoma vs normal lung tissues based on data from ONCOMINE.
Next, we analyzed ENPP1 expression levels in a panel of human lung cancer cell lines by immunoblot. The result showed that ENPP1 was high expressed in subset of human lung cancer cell lines, including H1792, A549 and HCC827 (Figure 2A). In comparison, ENPP1 expression is low in HBEC cells, a telomerase transformed normal human bronchi epithelial cell line although it is high in BEAS-2B cells, a virus transformed lung epithelial cell line (whether it is due to viral protein is unclear). Thus, upregulated ENPP1 may contribute to the transformed phenotypes of lung cancer cells. To determine the role of ENPP1 in lung cancer, we selected HCC827 and A549 cell lines for further biochemical and biological characterization. HCC827 and A549 cell line is tumorigenic, which is convenient for characterization of biological activities both in vitro and in vivo. We established four ENPP1-knockdown cell lines (ENPP1-sh2# and ENPP1-sh3#) in HCC827 cells (Figure 2B) and (ENPP1-sh1# and ENPP1-sh2#) in A549 cells (Figure 2C) using shRNA, as confirmed by immunoblot analysis. The sequences of shRNA targeting ENPP1 described as (Table 1). To determine the effects of ENPP1 knockdown, we examined colony formation activities of these cells. The colonogenic assay showed that ENPP1 knockdown, in HCC827-shENPP1 (2#, 3#), resulted in significantly suppressed colony numbers compared to those in control, HCC827-shNS cells (Figure 2D). Consistently, HCC827-shENPP1 cells also exhibited the reduced ability to form colonies in soft agarose assay (Figure 2E). Similarly, the same results were obtained in A549-shENPP1 and A549-shNS (Figure 2F). Taken together, these observations suggest that ENPP1 is crucial for colony forming and anchorage-independent grow activities of lung cancer cells.
Figure 2.
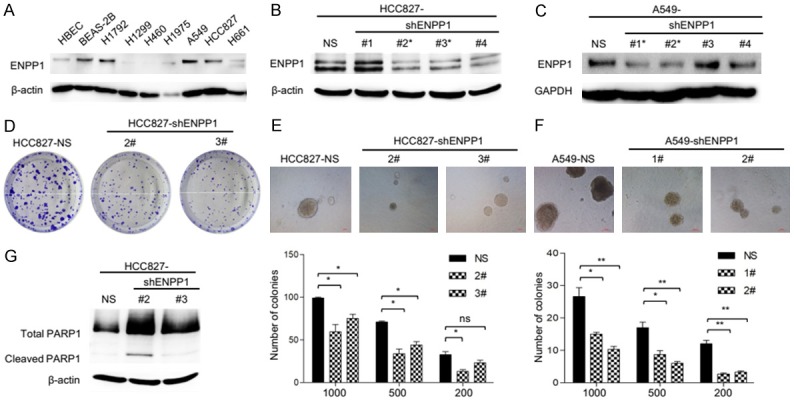
ENPP1 knockdown inhibits the growth of lung tumors. A. Immunoblot analysis of cell lysates from six NSCLC cell lines (H1792, H1299, H460, H1975, A549, HCC827, H661) and two normal human epithelial cell lines (HBEC, BEAS-2B) with indicated antibodies were performed, relative expression levels are expressed as the ratio below the bands that are the intensities of indicated proteins verse β-actin. B, C. HCC827 cells and A549 cells were stably transfected with non-specific sh-NS or ENPP1 shRNA cells, the whole cell lysates were subject to immunoblot analysis as indicated. D. Colony formation analysis of HCC827-shNS and HCC827-shENPP1 cells. E, F. Anchorage independent colony formation analysis of HCC827-shNS, A549-shNS and HCC827-shENPP1, A549-shENPP1 cells. G. Western blot analysis of PARP-1 protein level in HCC827-shNS and HCC827-shENPP1 cell lines.
Table 1.
Sequences of shRNA targeting ENPP1
| Genes | Sequence (5’→3’) |
|---|---|
| ENPP1NC | 5’-CAACAAGATGAAGAGCACCAA-3’ |
| ENPP1sh1 | 5’-GCTGTTTCGAGAGAACATTTG-3’ |
| ENPP1sh2 | 5’-GGATTCAGGGCAGAATATTTA-3’ |
| ENPP1sh3 | 5’-GAACTCATGGAAGTCTTAACC-3’ |
| ENPP1sh4 | 5’-TGAGGGACGATCTTTGAATAT-3’ |
ENPP1 knockdown leads to reduced colony forming activities, suggesting that it might be due to increased susceptibility to apoptosis. Cleavage of PARP that facilitates cellular disassembly serves as a marker of cells undergoing apoptosis [11]. To determine if the susceptibility to apoptosis is involved in, we next examined the cleaved PARP-1 by immunoblot analysis. The result showed that HCC827-shENPP1 cells have increased cleaved PARP compared to HCC827-shNS cells (Figure 2G). Taken together, these results suggest that ENPP1 knockdown increases the apoptotic susceptibility and suppresses the transformed phenotypes in lung cancer cells.
ENPP1 knockdown reduces the stem cell phenotype of lung tumors
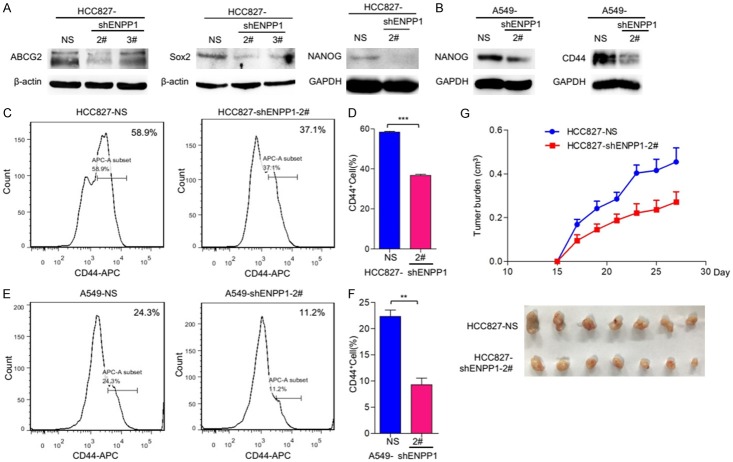
Cancer stem cell (CSC) features are critical for tumor malignancy and relapse. To determine if dysregulated ENPP1 contributes to CSC features in lung cancer cells, we examined the stem cell markers in these lung cancer cells. First, we examined the stemness-related proteins by immunoblot. The results showed that CSC markers, NANOG, ABCG2, and SOX2, were significantly reduced in HCC827-shENPP1 cells, as compared to parental cells, HCC827-shNS (Figure 3A); and similarly, NANOG and CD44 were significantly reduced in A549-shENPP1 cells, as compared to parental cells, A549-shNS (Figure 3B). To extend this characterization further, we also examined stem cell marker CD44 by flow cytometry analysis. The results showed that HCC827-shENPP1 and A549-shENPP1 exhibited significantly reduced level of CD44 compared to parental HCC827-shNS cells and A549-shNS cells (Figure 3C-F). Taken together, these results show that upregulated ENPP1 is essential for maintaining the stem-like phenotype in lung cancer cells.
Figure 3.
ENPP1 knockdown reduce the stem cell phenotype of lung tumors. A, B. Immunoblot analysis of the cancer stem cell makers of ABCG2, SOX2, NANOG in HCC827-shNS, HCC827-shENPP1 and A549-shNS, A549-shENPP1 cell lines. C-F. The expression of CD44 analysis by flow cytometry as the protocol indicated. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001. G. ENPP1 knockdown reduces tumorigenesis in vivo. HCC827-shNS and HCC827shENPP1-2# cells were inoculated subcutaneously on nude mice, after 2 weeks, representative images of tumors and the tumor size was measured at indicated time points.
To determine the role of ENPP1 in tumorigenesis in vivo, we examined tumor growth of HCC827-shNS and HCC827-shENPP1 cells in vivo. Tumor cells were subcutaneously injected at 1 × 106 in nude mice. Tumor size was measured about 3 times a week after tumor appeared. The results showed that the tumor volume of HCC827-shENPP1 was significantly smaller than that of HCC827sh-NS (Figure 3G), suggesting that ENPP1 is required for tumorigenicity of lung cancer HCC827 cells. Thus, upregulated ENPP1 is essential for both increased stemness and tumorigenicity of lung cancer cells.
ENPP1 knockdown reduces the EMT phenotypes induced by TGF-β
It has been reported that over 90% of cancer death was due to local invasion and distant metastasis of tumor cells, whereas EMT play a key role in this process [12]. Moreover, EMT has been shown to promote stemness-related features. To determine whether ENPP1-mediated phenotype changes were due to the effect on EMT, we examined the migratory activity, an EMT-associated biological activity, of HCC827 ENPP1-deficient cells. The results showed that HCC827 cells moved to heal the wound within 48 hours, while HCC827-shENPP1 cells did not (Figure 4A, 4B). This suggests that the migratory activity was significantly suppressed in HCC827-shENPP1 cells as compared to parental cells, HCC827-shNS.
Figure 4.
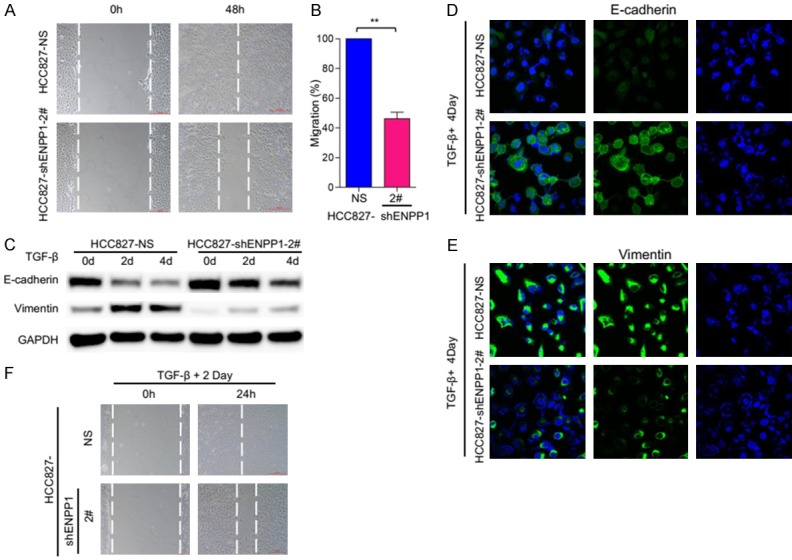
ENPP1 knockdown reduce the EMT induced by TGF-β. A. Wound healing and migratory analysis of HCC827-shNS and HCC827-shENPP1 cell lines. Scale bar = 200 μm. B. Graph demonstrates the migratory ability of HCC827 and HCC827-shENPP1 cell lines (mean ± SD) from 3 separate experiments. C. HCC827-shNS and HCC827-shENPP1 cell lines were treated with TGF-β (5 ng/ml) for the indicated time periods, and expression of E-cadherin and vimentin was analyzed by immunoblot. D, E. HCC827 and HCC827-shENPP1 cell lines were treated with TGF-β (5 ng/ml) for 4 days. Expression of E-cadherin and vimentin, (green) was analyzed by immunofluorescence staining and DAPI (blue) were imaged by confocal microscopy. F. Wound healing and migratory analysis of HCC827-shNS and HCC827-shENPP1 cell lines treated with TGF-β (5 ng/ml) treatment for the indicated time periods. Scale bar = 200 μm.
TGF-β has a dual role in inducing EMT, promoting tumor cell invasion in the late stage of tumor, combination of TGF-β and other oncogenic signaling pathways, such as Ras, can complete EMT [13]. To further explore this character, we examined the migratory activities of HCC827-shNS and HCC827-shENPP1 with or without TGF-β treatment (5 ng/ml) at 2, 4 days respectively. Immunoblot analysis showed that epithelial cell marker, E-cadherin was gradually decreased as TGF-β treatment extended from 0 day, to 2, and 4 day, whereas mesenchymal cell marker vimentin was increased as TGF-β treatment lasted in HCC827-shNS cells. However, the TGF-β induced effects were significantly reduced in HCC827-shENPP1 cells (Figure 4C). This suggests that ENPP1-deficiency reduces the cellular response to TGF-β induced EMT phenotype. Consistently, immunoflourescent (IF) analysis showed that TGF-β repressed E-cadherin in HCC827-shNS cells, while this effect was greatly reduced in HCC827-shENPP1 cells (Figure 3D). Similarly, TGF-β induced vimentin in HCC827-shNS cells, but this effect was significantly reduced in HCC827-shENPP1 (Figure 3E). Moreover, TGF-binduced migration was also significantly reduced in HCC827-shENPP1 cells as compared to parental HCC827-shNS cells (Figure 3F).
To further determine if the ENPP1-deficiency-induced phenotype changes were due to the effect on EMT, we examined the migratory activities of A549-shNS and A549-shENPP1. The results showed that A549-shNS cells moved to heal the wound within 30 hours, while A549-shENPP1 cells did not (Figure 5A, 5B). We also examined the migratory activities of A549-shNS and A549-shENPP1 with or without TGF-β treatment (5 ng/ml) at 2, 4 days respectively. Immunoblot analysis showed that TGF-β treatment increased the expression of mesenchymal cell marker vimentin in A549-shNS cells; whereas it did not in A549-shENPP1 cells (Figure 5C). Consistently, IF analysis showed that TGF-β repressed E-cadherin in A549-shNS cells, whereas this effect was greatly reduced in A549-shENPP1 cells (Figure 5D). Similarly, TGF-β induced vimentin in A549-shNS cells, but this effect was moderately reduced in A549-shENPP1 (Figure 5E). Biologically, TGF-β treatment induced migration in A549-shNS cells, but this effect was significantly reduced in A549-shENPP1 cells (Figure 5F). Taken together, these results demonstrate that ENPP1 is required for TGF-β induced EMT marker changes and migratory activities in lung cancer cells. Thus, dysregulated ENPP1 contributes to the EMT characters of lung cancer cells.
Figure 5.
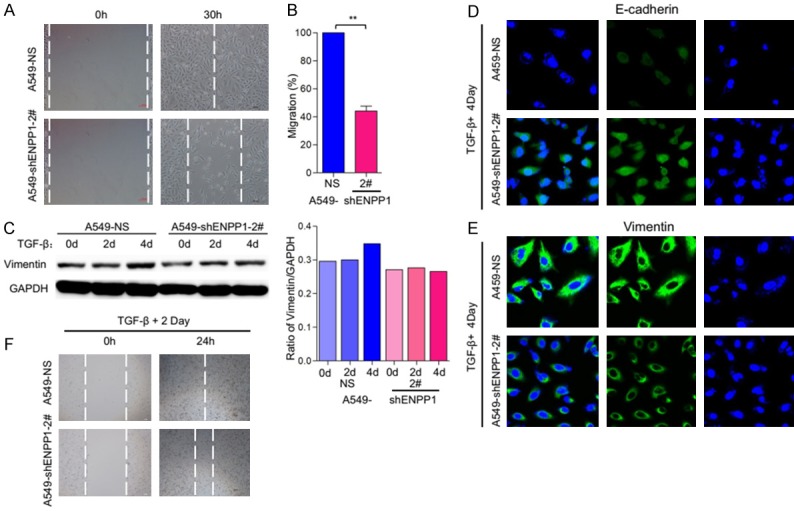
ENPP1 knockdown reduce the EMT induced by TGF-β. A. Wound healing and migratory analysis of A549-shNS and A549-shENPP1 cell lines. Scale bar = 100 μm. B. Graph demonstrates the migratory ability of A549-shNS and A549-shENPP1 cell lines (mean ± SD) from 3 separate experiments. C. A549-shNS and A549-shENPP1 cell lines were treated with TGF-β (5 ng/ml) for the indicated time periods, and expression of vimentin was analyzed by immunoblot. D, E. A549-shNS and A549-shENPP1 cell lines were treated with TGF-β (5 ng/ml) for 4 days. Expression of E-cadherin and vimentin, (green) was analyzed by immunofluorescence staining and DAPI (blue) were imaged by confocal microscopy. F. Wound healing and migratory analysis of HCC827-shNS and HCC827-shENPP1 cell lines treated with TGF-β (5 ng/ml) treatment for the indicated time periods. Scale bar = 100 μm.
ENPP1 is dysregulated in human lung cancer tissues
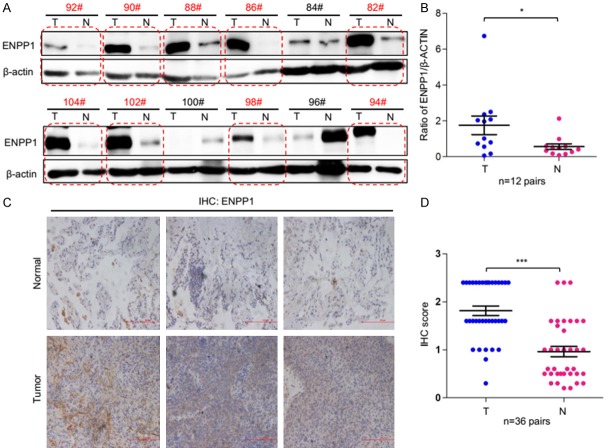
To further investigate the expression profile of ENPP1 in lung cancer, we screened ENPP1 expression in a panel of 12 pairs of lung tumor tissues and adjacent normal lung tissues. The results showed that ENPP1 is significantly upregulated 9 of 12 lung tumor tissues compared to normal lung tissues (as red circular boxes indicated) (Figure 6A, 6B). To extend this analysis further, we examined the protein level of ENPP1 in 36 pairs of lung tissues from normal and tumor tissues by immunohistochemical (IHC) staining assay. The results showed that ENPP1 protein level was significantly upregulated in lung tumor tissues (T) compared to that in adjacent normal lungs (N) (Figure 6C, 6D). These results strongly support that ENPP1 overexpression promotes lung tumor development and progression.
Figure 6.
ENPP1 is dysregulated in human lung cancer. A, B. Representative results of Western blot analysis of ENPP1 in human lung tumor and adjacent normal tissues. C, D. Representative images and IHC score of immunohistochemical staining (IHC) of ENPP1 in the tumor-containing lungs tissues (T) and the normal lung tissues (N) (n = 36 pairs).
Discussion
In this study, we showed that ENPP1 overexpression is important for increased transforming activities, stemness-related features, migration activity and EMT phenotypes. We also found that ENPP1 is frequently upregulated in lung tumor tissues compared to normal lung tissues. These results support that ENPP1 is important for lung tumor development.
Consistent with our observations, previous studies have shown that ENPP1 mRNA expression was high in glioblastoma stem-like cells (GSCs) compared to normal brain, and this is related to increased stem-like phenotype in glioblastoma [10]. In this study, we showed that ENPP1 is important in induction of stemness-like markers and mediation of TGFβ-induced EMT phenotype. This activity has clinic importance since cancer stem cells play a critical role in tumor initiation, growth, metastasis and chemo-resistance [14-16]. In support, previous studies showed that ENPP1 plays a role in maintenance of stem-like phenotype in glioblastoma [10]. However, whether ENPP1-mediated functions in lung cancer and glioblastoma via a similar mechanism remain uncharacterized.
ENPP1 is a membrane protein that hydrolyzes nucleoside tri-, di-, and monophosphates and dinucleoside polyphosphates and produce nucleoside diphosphates, nucleoside monophosphates, nucleosides, phosphate, and inorganic pyrophosphate (PPi) [17]. ENPP1 is expressed in mouse B-lymphocytes [18], and mineralizing cells, such as osteoblasts and chondrocytes, and also on osteoblast- and chondrocyte-derived matrix vesicles [19]. Here, we showed that ENPP1 is highly expressed in lung cancer tissues, implying a biological link. Cancer stem cells play a critical role in tumor relapse, resistance to chemo- and radio-therapy, and metastasis [20], whereas EMT is crucial for induction and maintenance of stem cell-like characteristics [5,21,22]. It remains to determine what molecular mechanism underlying ENPP1-mediated induction of transformation, stemness, and EMT and there is a common mechanism of ENPP1 up regulation between lung cancer and glioblastoma. Taken together, our findings strongly suggest that ENPP1 play an important role in regulation of stem-like phenotype and metastatic in lung cancer.
Acknowledgements
This study was supported by grants from National Nature Science Foundation of China 81620108022 (JD), 91729302 (JD), 81572759 (JD), and 81572693 (FY).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Chellappan S. Lung cancer stem cells: nolecular features and therapeutic targets. Mol Aspects Med. 2014;39:50–60. doi: 10.1016/j.mam.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 8.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–50. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi RU, Miyazaki H, Takeshita F, Yamamoto Y, Minoura K, Ono M, Kodaira M, Tamura K, Mori M, Ochiya T. Loss of microRNA-27b contributes to breast cancer stem cell generation by activating ENPP1. Nat Commun. 2015;6:7318. doi: 10.1038/ncomms8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bageritz J, Puccio L, Piro RM, Hovestadt V, Phillips E, Pankert T, Lohr J, Herold-Mende C, Lichter P, Goidts V. Stem cell characteristics in glioblastoma are maintained by the ecto-nucleotidase E-NPP1. Cell Death Differ. 2014;21:929–40. doi: 10.1038/cdd.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–9. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 12.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 13.Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–50. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Xiao Z, Wong SK, Tin VP, Ho KY, Wang J, Sham MH, Wong MP. Lung cancer tumorigenicity and drug resistance are maintained through ALDH(hi)CD44(hi) tumor initiating cells. Oncotarget. 2013;4:1698–711. doi: 10.18632/oncotarget.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanner RJ, Remke M, Gallo M, Selvadurai HJ, Coutinho F, Lee L, Kushida M, Head R, Morrissy S, Zhu X, Aviv T, Voisin V, Clarke ID, Li Y, Mungall AJ, Moore RA, Ma Y, Jones SJ, Marra MA, Malkin D, Northcott PA, Kool M, Pfister SM, Bader G, Hochedlinger K, Korshunov A, Taylor MD, Dirks PB. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell. 2014;26:33–47. doi: 10.1016/j.ccr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang WC, Chin TM, Yang H, Nga ME, Lunny DP, Lim EK, Sun LL, Pang YH, Leow YN, Malusay SR, Lim PX, Lee JZ, Tan BJ, Shyh-Chang N, Lim EH, Lim WT, Tan DS, Tan EH, Tai BC, Soo RA, Tam WL, Lim B. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun. 2016;7:11702. doi: 10.1038/ncomms11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Old LJ, Boyse EA. Surface alloantigens of plasma cells. J Exp Med. 1970;131:1325–41. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato K, Nishimasu H, Okudaira S, Mihara E, Ishitani R, Takagi J, Aoki J, Nureki O. Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc Natl Acad Sci U S A. 2012;109:16876–81. doi: 10.1073/pnas.1208017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 21.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, Radisky DC, Ferrone S, Knutson KL. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–95. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu MH, Gao X, Luo D, Zhou XD, Xiong W, Liu GX. EMT and acquisition of stem cell-like properties are involved in spontaneous formation of tumorigenic hybrids between lung cancer and bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9:e87893. doi: 10.1371/journal.pone.0087893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun B, Guo W, Hu S, Yao F, Yu K, Xing J, Wang R, Song H, Liao Y, Wang T, Jiang P, Han B, Deng J. Gprc5a-knockout mouse lung epithelial cells predicts ceruloplasmin, lipocalin 2 and periostin as potential biomarkers at early stages of lung tumorigenesis. Oncotarget. 2017;8:13532. doi: 10.18632/oncotarget.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]