Abstract
The tartrate-resistant acid phosphatase (TRAP/ACP5) correlated with tumor progression in many malignancies. However, the role of ACP5 in colorectal cancer (CRC) has not been thoroughly elucidated. In this study, we sought to identify the role for ACP5 in CRC progression. Immunohistochemistry revealed that high ACP5 expression is positively associated with tumor size, tumor classification, lymph node metastasis, distant metastasis and advanced stage cancer in 285 CRC patients. Moreover, high ACP5 expression was significantly associated with poor overall survival and disease-free survival. Then, ectopic expression of ACP5 promoted tumor cell proliferation and invasion, whereas suppression of ACP5 expression resulted in decreased cell proliferation and invasion in colorectal cell lines in vitro. And, inhibition of ACP5 also inhibited growth of engrafted tumors in vivo. Furthermore, we found that ACP5 overexpression positively regulated p-FAK, p-PI3K and p-AKT in CRC cells. ACP5 depletion showed the opposite effects. What’s more, overexpression of FAK in CRC cells could restore the reduced abilities of cell proliferation and invasion caused by siRNAs-ACP5. Finally, we found the inhibition of activity by Akt inhibitors, MK2206, could partially decrease the positive effects of ACP5 on CRC cell proliferation and invasion. In conclusion, our results suggest that overexpressed ACP5 might serve as an indicator for poor prognosis in colorectal cancer patients through regulation of FAK/PI3K/AKT signaling pathway, which might be a potential therapeutic approach for colorectal cancer therapy.
Keywords: Colorectal cancer, ACP5, FAK/PI3K/AKT signaling pathway, prognosis
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed malignancies worldwide and the third leading cause of cancer-related death in the world [1,2]. The development and progression of CRC involves numbers of genetic and epigenetic alterations of tumor suppressor and tumor-related genes, until now we know little about the molecular mechanism [3,4]. Owing to the absence of specific symptoms in the early stages, the patients are usually detected at an advanced stage and the total prognosis of CRC patient is poor [5]. So, it is critically important to illustrate the molecular mechanism of CRC and find specific and sensitive molecular targets for early diagnosis, effective treatment and predictive prognosis.
Tartrate-resistant acid phosphatase (TRAP/ACP5) is a metalloproteinase enzyme that maps to chromosome 19p13.2 and spans approximately 3 kb, belonging to the acid phosphatase family that catalyzes the conversion of orthophosphoric monoester to alcohol and orthophosphate [6,7]. Furthermore, it consists of an open reading frame of 975 bp that encodes an iron-containing glycoprotein of 35-37 kDa [8]. Accumulating studies have demonstrated that ACP5 broadly participates in various malignant processes including uncontrolled proliferation [9], chemotherapy resistance [9], cellular invasion and metastasis [10]. ACP5 expression has been shown to be significantly up-regulated in ovarian cancer, breast cancer and melanoma, and is a useful serum marker for extensive bone metastasis [10-14]. Recent studies have reported that overexpressed ACP5 promotes the distal metastasis of liver and gastric cancer [15,16]. However, the functional role and underlying molecular mechanism of ACP5 in CRC has not been investigated. These prompted us to explore the role of ACP5 in human colorectal cancer.
Materials and methods
Clinical specimen collection
The present research has been approved by the Ethics Committee of Ren Ji Hospital, Shanghai Jiaotong University School of Medicine. The cohort of 317 colorectal cancer patients (32 freshly-frozen and 285 paraffin section pairs of CRC patients) are enrolled in our study. All the tumor tissues and matched adjacent non-tumor tissues contained in this cohort were collected from patients undergoing operation from January 2007 to December 2017. Clinical and pathological data including age, gender, tumor location, tumor size, serum carcinoembryonic antigen (CEA) level and TNM stage were collected. All the patients who had received preoperative treatment, such as radiation or chemotherapy, were excluded. Disease-free survival (DFS) is defined as the time elapsed from surgery to the first occurrence of CRC recurrence and distant metastasis. The follow-up time was calculated from the date of surgery to the date of death, or the last known follow-up. The follow-up data were ceased in April 2018. All patients were well informed, the process was approved by Ethics Committee of Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from each patient.
Cell culture and transfection
The human CRC cell lines (HT-29, HCT116, SW480, SW1116 and SW620) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and kept in our laboratory. All of these cells were cultured in specific medium supplemented with 10% (volume/volume) fetal bovine serum (FBS) and 1% antibiotics (penicillin and streptomycin) at 37°C in a humidified incubator under 5% CO2 condition.
Small interfering RNA (siRNA) targeting ACP5 (siRNAs-ACP5) or FAK (siRNAs-FAK) and negative controls (siRNAs-Control) were specifically synthesized by GenePharma (Shanghai, China). The over-expressing plasmids (pcDNA3.1-ACP5 and pcDNA3.1-FAK) were purchased from Genearray Biotechnology (Shanghai, China). Cells were cultured in 70% confluence and then siRNA was transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The CRC cells were treated with a specific AKT inhibitors, MK2206 (CST Danvers, MA), added at 1 μmol/L for 48 h. Cells were harvested 48 h after transfection for RT-qPCR, western blotting or other following assays.
Total RNA extraction and quantitative real-time PCR
The total RNA was extracted from CRC tissues or cell lines with Trizol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. The cDNA was synthesized using a PrimeScript RT Reagent Kit (Takara, Japan) in accordance with the protocol of manufacturer. StepOne Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA) was applied to detect the expression level of target gene using the SYBR Premix Ex Taq II (Takara, Japan), and GAPDH acted as an internal control. The data were calculated by the 2-ΔΔCt method. The primers for ACP5 were as follows: forward: 5’-GGGAGATCTGTGAGCCAGTG-3’; reverse: 5’-GGGAGCGGTCAGAGAATACG-3’. The primers for GAPDH were as follows: forward: 5’-GCATTGCCCTCAACGACCAC-3’, reverse: 5’-CCACCACCCTGTTGCTGTAG-3’.
Immunohistochemical staining
Immunohistochemistry was performed as previously described [17]. Briefly, after being dewaxed and hydrated, the tissue was antigen-retrieved by microwaving in citrate buffer (10 mM citric acid, pH 6.0), blocked in 5% animal serum, and incubated with anti-ACP5 primary antibody (dilution, 1:250) overnight at 4°C. Next detection was stained for 10 min at 37°C by using secondary biotinylated anti-rabbit antibodies at a 1:100 dilution followed by streptavidin-HRP. Specimens were developed by DAB and the nuclei were counterstained by Hematoxylin. The sections were photographed under a microscope.
Immunohistochemical score
Two researchers who were blinded to patients’ outcome evaluated immunoreactivity independently. Intensity of immunostaining was scored as 0 (no immunostaining), 1 (weak immunostaining), 2 (moderate immunostaining) and 3 (strong immunostaining). The percentage of immunoreactive cells scoring was documented as 0 (none), 1 (< 20%), 2 (20-50%), 3 (51-75%) and 4 (> 75%). And a final score was created to determine the cut-off value for low and high expression group by using grades of the extent × grades of intensity staining. The low expression was defined as a final score < 6 and high expression with a final score ≥ 6.
Western blotting
The tissues and cells were lysed in RIPA buffer with protease inhibitor cocktail. Total proteins, measured by BCA protein assay kit, were separated on 10% SDS-polyacrylamide gels and electrically transferred onto PVDF membrane (Millipore). Membrane was blocked for 1 h at room temperature prior to incubation with monoclonal rabbit anti-ACP5 (Abcam, ab191406), monoclonal rabbit total anti-FAK (Abcam, ab40794), monoclonal rabbit anti-FAK (phospho Y397) (Abcam, ab81298), monoclonal rabbit total anti-PI3K (Abcam, ab151549), monoclonal rabbit anti-PI3K (phospho Y607) (Abcam, ab182651), polyclonal rabbit total anti-AKT (Abcam, ab8805), polyclonal rabbit anti-AKT (phospho S473) (Abcam, ab18206) at 4°C overnight. After washed with TBST, membrane was incubated in horseradish peroxidase-conjugated anti-rabbit antibody for 1 h, and developed by SuperSignal West Dura Extended Duration Substrate (Thermo Scientific).
Cell proliferation assay
Cells were cultured in 96-well plates and treated with Cell Counting Kit-8 (CCK8, Dojindo, Japan) for 2 h at the time points of 24 h, 48 h, 72 h and 96 h after the initial planting. Cell absorbance was detected at 450 nm with a microplate reader.
Transwell chamber assay
After the chamber was coated with fresh Matrigel (diluted in 1:4 with serum-free medium) (BD Bioscience San Jose, CA, USA), 2-3 × 105 cells suspended in serum-free medium were plated on the top of each chamber, while medium containing 20% FBS was put in the lower chamber, used as a chemo-attractant. After 48 h incubation, cells that did not pass through the filter were removed by a cotton swab, whereas cells on the lower surface of the filter were fixed and stained with methanol and crystal violet, respectively. Finally, cells across through the membrane were counted using a microscope.
Establishment of ACP5-knockdown CRC cell lines by lentivirus
HCT116 cells with stable shRNA-ACP5 using the lentiviral expression system. Briefly, lentiviruses containing shRNA-ACP5 or negative control (Scramble-shRNA) were constructed by the GeneChem Company (Shanghai, China). All the lentiviral vectors expressed enhanced green fluorescent protein (GFP) and puromycin resistance gene, which allowed for measuring the infection efficiency and selecting the specific cells of interest. Cells were infected with shRNA-ACP5 or Scr-shRNA vectors, followed by selection for stable lines using 5 μg/ml of puromycin (Clontech, Palo Alto, CA, USA). The resulting stable lines were used for further analysis.
Tumorigenesis in nude mice
Xenograft tumors were generated by subcutaneous injection of 4 × 106 cells (shRNA-ACP5-HCT116 cells or Scr-shRNA-HCT116 cells) in each BALB/C (4-6 weeks, 18-22 g) nude mice, which obtained from the Animal Center of Shanghai Jiao Tong University. All mice were housed and maintained under specific pathogen-free (SPF) conditions and used in accordance with institutional guidelines and approved by the Use Committee for Animal Care.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 and GraphPad Prism 6 software. Briefly, student’s t test or chi-squared test was used to analyze the data. Survival curves were constructed by using the Kaplan-Meier method and analyzed by the log-rank test. Results are given as mean ± SD unless otherwise indicated. P < 0.05 was considered statistically significant.
Results
ACP5 is significantly up-regulated in CRC patients
To evaluate the expression status of ACP5 in CRC tissues, we first analyzed two independent microarray datasets from GEO databases (GSE9689, GSE21510) [18,19]. The results showed that ACP5 expression was significantly increased in tumor tissues compared with normal colorectal tissues (Figure 1A, 1B). Then, the RT-qPCR results showed that ACP5 mRNA was increased in CRC tissues (n=32) compared with matched cancer-adjacent tissues (n=32) (Figure 1C). Furthermore, Western blotting data showed that ACP5 protein was obviously increased in CRC tissues (n=12) compared with matched cancer-adjacent tissues (n=12) (Figure 1D). Finally, we also found that the immunoreactivity of ACP5 was low or high expressed in 113 (39.65%) or 172 (60.35%) of the 285 colorectal cancer samples, respectively (Figure 2A-C). These results indicated that both the mRNA and protein level of ACP5 were significantly up-regulated in CRC tissues.
Figure 1.
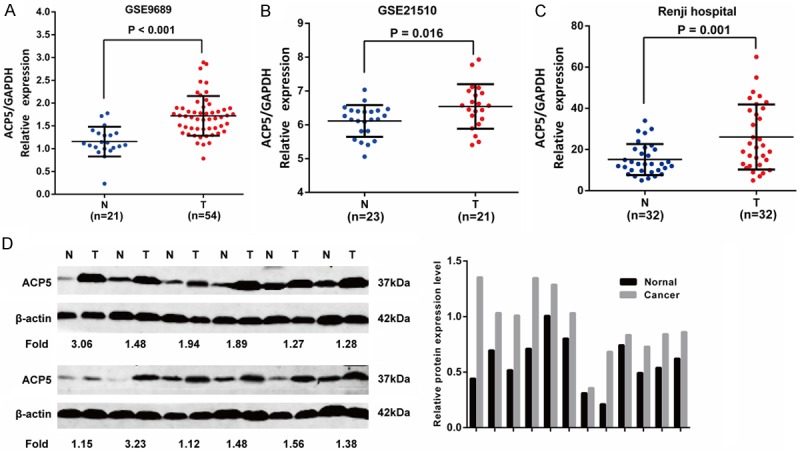
The expression of ACP5 in CRC tissues. A, B. Detection of ACP5 expression in dataset GSE9689, GSE21510. C. ACP5 mRNA expression in 32 pairs of CRC tissues via RT-qPCR. D. ACP5 protein expression in 12 pairs of CRC tissues by western blotting.
Figure 2.

ACP5 expression in CRC tissues and matched adjacent non-tumor tissues. A. ACP5 protein level was measured by immunohistochemical analysis in normal tissues. B. ACP5 protein level was measured by immunohistochemical analysis in CRC tissues. Representative images are shown at 200 × and 400 × magnifications, respectively. C. ACP5 protein level in 285 CRC tissues and matched normal tissues.
ACP5 is correlated with clinicopathological features and prognosis in CRC patients
In order to determine the clinical significance of ACP5, we analyzed the relationship between ACP5 expression and clinicopathological characteristics of CRC patients. As summarized in Table 1, ACP5 expression was prominently correlated with tumor size (P=0.031), tumor classification (P=0.027), lymphatic metastasis (P=0.001), distant metastasis (P=0.003) and TNM stage (P=0.005); whereas no significant relevance was found with age, gender, tumor location and CEA level. ACP5 expression was increased in advanced CRC and was positively associated with TNM stage and metastasis, indicating ACP5 may be implicated in the progression of CRC.
Table 1.
Correlations between ACP5 expression and clinicopathologic features in 285 colorectal cancer patients
| Clinicopathological feature | Total 285 | ACP5 expression | P value (χ2 test) | |
|---|---|---|---|---|
|
| ||||
| Low (n=113, 39.65%) | High (n=172, 60.35%) | |||
| Age (years) | ||||
| < 65 | 122 | 47 (41.59) | 75 (43.60) | 0.737 |
| ≥ 65 | 163 | 66 (58.41) | 97 (56.40) | |
| Gender | ||||
| Male | 170 | 69 (61.06) | 101 (58.72) | 0.694 |
| Female | 115 | 44 (38.94) | 71 (41.28) | |
| Tumor location | ||||
| Rectum | 176 | 73 (64.60) | 103 (59.88) | 0.423 |
| Colon | 109 | 40 (35.40) | 69 (40.12) | |
| Tumor size | ||||
| ≤ 5 cm | 149 | 68 (60.18) | 81 (47.09) | 0.031 |
| > 5 cm | 136 | 45 (39.82) | 91 (52.91) | |
| CEA level | ||||
| ≤ 5 ng/ml | 141 | 59 (52.21) | 82 (47.67) | 0.454 |
| > 5 ng/ml | 144 | 54 (47.79) | 90 (52.33) | |
| Tumor classification | ||||
| T1-2 | 92 | 45 (39.82) | 47 (27.33) | 0.027 |
| T3-4 | 193 | 68 (60.18) | 125 (72.67) | |
| Lymph node metastasis | ||||
| Absent | 144 | 71 (62.83) | 73 (42.44) | 0.001 |
| Present | 141 | 42 (37.17) | 99 (57.56) | |
| Distant metastasis | ||||
| Absent | 233 | 102 (90.27) | 131 (76.16) | 0.003 |
| Present | 52 | 11 (9.73) | 41 (23.84) | |
| TNM stage(AJCC) | ||||
| Stage I | 85 | 44 (38.94) | 41 (23.84) | 0.005 |
| Stage II | 59 | 24 (21.24) | 35 (20.34) | |
| Stage III | 89 | 34 (30.09) | 55 (31.98) | |
| Stage IV | 52 | 11 (9.73) | 41 (23.84) | |
Values in parentheses indicate percentage values. The bold number represents the P-values with significant differences.
To evaluate the prognostic significance of ACP5 in CRC patients, we measured the correlation of ACP5 expression with overall survival (OS) and disease-free survival (DFS) using Kaplan-Meier analysis and log-rank test. As shown in Figure 3A, high ACP5 expression was remarkably associated with decreased OS (P=0.001) and DFS (P=0.001), which indicates that the OS and DFS are better in CRC patients with low ACP5 expression than in those with high ACP5 expression.
Figure 3.
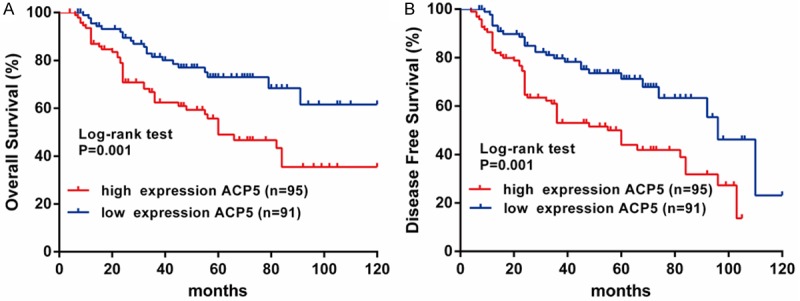
ACP5 expression invested associated the prognosis in CRC patients. A. Kaplan-Meier analysis of overall survival in CRC patients. B. Kaplan-Meier analysis of disease-free survival in CRC patients.
Furthermore, univariate and multivariate analyses were conducted to identify the risk factors correlated with the prognosis of CRC patients. Univariate analysis showed that ACP5 expression, tumor size and TNM stage were significantly associated with OS and DFS (Tables 2, 3). Meanwhile, multivariate analysis using the Cox proportional hazards model revealed that ACP5 expression was a significant independent prognostic factor (Table 2) and recurrent factor (Table 3) for CRC patients. Taken together, these data indicated that high ACP5 expression may be a predictor for poor prognosis and recurrence in CRC patients.
Table 2.
Univariate and multivariate analyses of prognostic parameters for survival in 186 colorectal cancer patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Prognostic parameter | HR | 95% CI | P value | HR | 95% CI | P value |
| ACP5 expression (low vs. high) | 2.258 | 1.353-3.769 | 0.002 | 2.497 | 1.485-4.197 | 0.001 |
| Age (≤ 65 vs. > 65) | 1.243 | 0.761-2.031 | 0.384 | - | - | - |
| Gender (male vs. female) | 0.689 | 0.410-1.159 | 0.160 | - | - | - |
| Tumor Size (≤ 5 cm vs. > 5 cm) | 1.808 | 1.097-2.979 | 0.002 | 1.710 | 1.034-2.829 | 0.037 |
| CEA level (≤ 5 ng/ml vs. > 5 ng/ml) | 1.641 | 1.007-2.674 | 0.047 | 1.177 | 0.715-1.938 | 0.521 |
| TNM stage (I vs. II vs. III vs. IV stage) | 2.150 | 1.530-3.023 | 0.000 | 2.278 | 1.586-3.273 | 0.000 |
HR: Hazard ratio; CI: Confidence interval. The bold number represents the P-values with significant differences.
Table 3.
Univariate and multivariate analyses of recurrent factors for survival in 186 colorectal cancer patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Prognostic parameter | HR | 95% CI | P value | HR | 95% CI | P value |
| ACP5 expression (low vs. high) | 2.228 | 1.392-3.566 | 0.001 | 2.463 | 1.533-3.957 | 0.001 |
| Age (≤ 65 vs. > 65) | 1.283 | 0.817-2.016 | 0.279 | - | - | - |
| Gender (male vs. female) | 0.823 | 0.521-1.300 | 0.404 | - | - | - |
| Tumor Size (≤ 5 cm vs. > 5 cm) | 1.653 | 1.054-2.592 | 0.029 | 1.570 | 0.997-2.471 | 0.052 |
| CEA level (≤ 5 ng/ml vs. > 5 ng/ml) | 1.465 | 0.937-2.291 | 0.093 | - | - | - |
| TNM stage (I vs. II vs. III vs. IV stage) | 2.433 | 1.769-3.345 | 0.000 | 2.596 | 1.865-3.616 | 0.000 |
HR: Hazard ratio; CI: Confidence interval. The bold number represents the P-values with significant differences.
ACP5 promotes cell proliferation and invasion in CRC cells
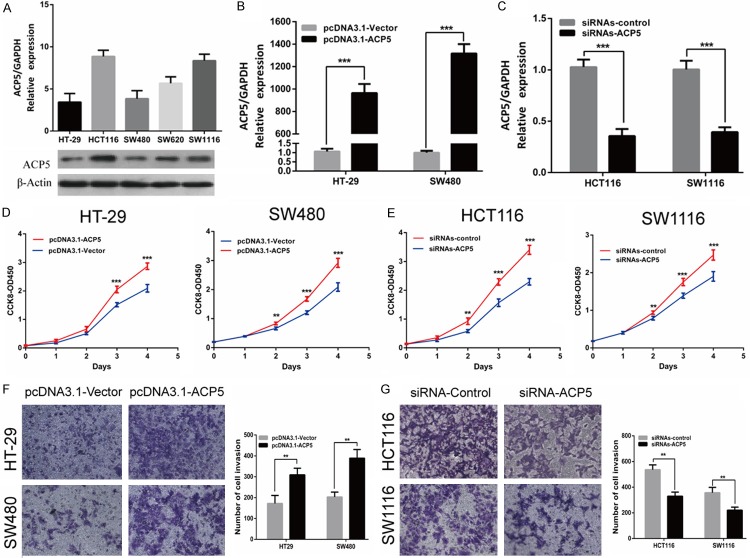
To explore the biological functions of ACP5 in CRC progression and metastasis, we measured the ACP5 mRNA and protein level in five CRC cell lines (Figure 4A). Then, we transfected transiently pcDNA3.1-ACP5 or siRNAs-ACP5 and measured cellular functions into HT-29 and SW480 cells or HCT116 and SW1116 cells. The transfection efficiency was confirmed by RT-qPCR (Figure 4B, 4C) and Western blotting (Figure 5A, 5B). Using CCK-8 assays, we observed that the growth rate of pcDNA3.1-ACP5 treated cells was promoted compared with pcDNA3.1-vector cells, whereas cells with siRNAs-ACP5 had a prominently lower proliferative capacity than negative control in CRC cells (Figure 4D, 4E). To determine whether ACP5 can affect CRC cell invasive capacity, the transwell assay results showed that pcDNA3.1-ACP5 significantly enhanced the invasive potential of HT-29 and SW480 cells (Figure 4F). While the invasion of HCT116 and SW1116 cells with siRNAs-ACP5 were significantly decreased (Figure 4G). These results showed that ACP5 promoted proliferation and invasion in CRC cells.
Figure 4.
ACP5 promoted proliferation and invasion in CRC cells. (A) RT-qPCR and western blotting showed the ACP5 expression in five CRC cell lines. (B, C) ACP5 overexpression (B) or knockdown (C) efficiency was confirmed by RT-qPCR in CRC cells. (D, E) Effects of ACP5 overexpression (D) or knockdown (E) on proliferation was evaluated by CCK-8 assay. (F, G) Effects of ACP5 overexpression (F) or knockdown (G) on invasion was measured by transwell assay. Results shown are the mean ± SD (**P < 0.01, ***P < 0.001) of triplicate determination from three independent experiments.
Figure 5.
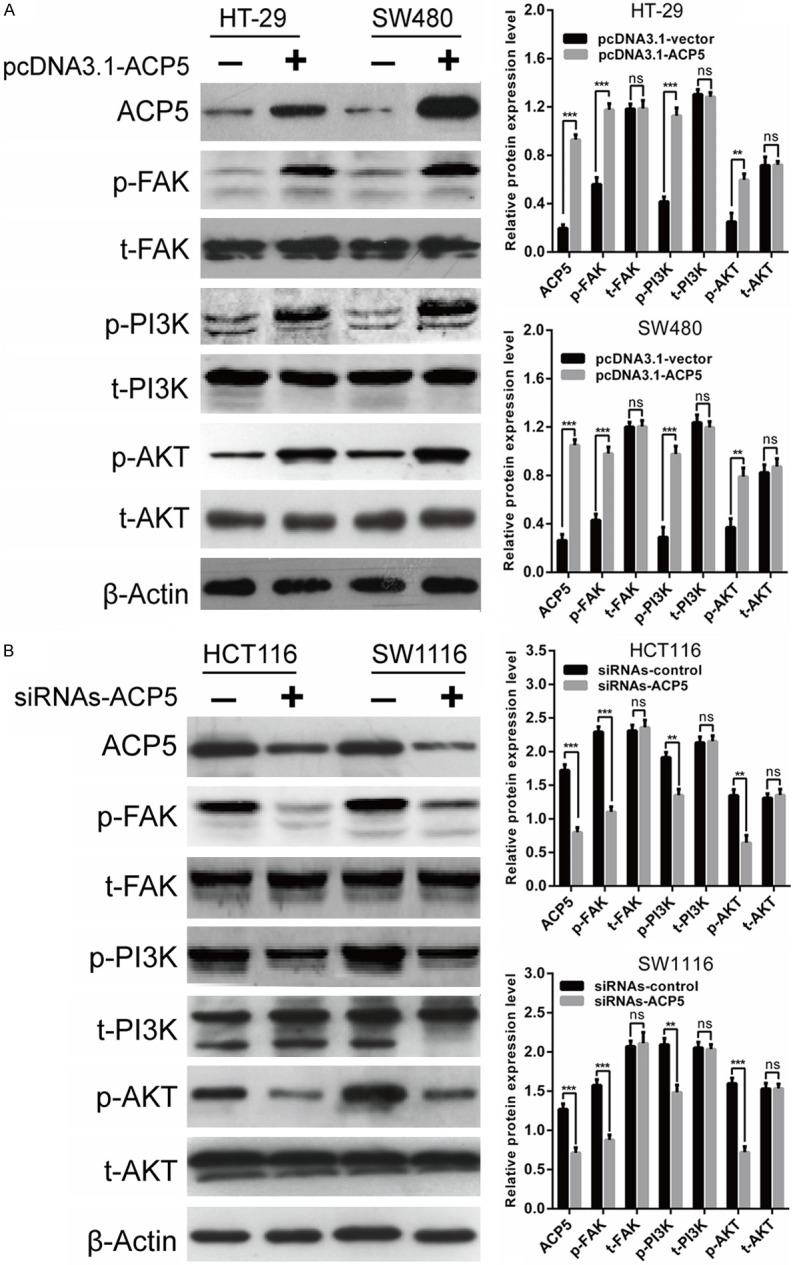
ACP5 regulated FAK/PI3K/AKT signaling pathway in CRC cells. A. Expression of total-FAK, phospho-FAK, total-PI3K, phospho-PI3K, total-AKT, phospho-AKT proteins were determined in HT-29 and SW480 cells transfected with pcDNA3.1-ACP5. B. Expression of total-FAK, phospho-FAK, total-PI3K, phospho-PI3K, total-AKT, phospho-AKT proteins were determined in HCT116 and SW1116 cells transfected with siRNAs-ACP5. Results shown are the mean ± SD (**P < 0.01, ***P < 0.001) of triplicate determination from three independent experiments.
ACP5 up-regulates FAK expression and activates FAK/PI3K/AKT signaling pathway in CRC cells
Since overexpression of ACP5 could activate focal adhesion kinase (FAK) phosphorylation in melanoma cells, which promotes cell proliferation and invasion [10], we attempted to determine the effect of ACP5 on FAK signaling in CRC cells. As a result, we revealed that phospho-FAK, phospho-PI3K and phospho-Akt are marked increased in cells by pcDNA3.1-ACP5, while total-FAK, total-PI3K and total-Akt protein level were unaffected, compared with controls (Figure 5A). Conversely, the phospho-FAK, phospho-PI3K and phospho-Akt protein are marked decreased in cells by siRNA-ACP5 (Figure 5B).
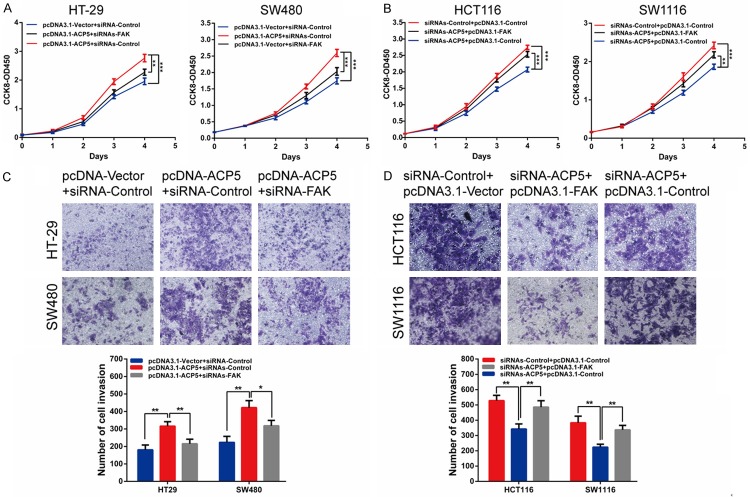
Then we silenced FAK to illustrate the proliferative and invasive potential of CRC cells. Indeed, silencing of FAK suppressed the proliferative and invasive ability of CRC cells and also compromised the role of pcDNA3.1-ACP5 (Figure 6A, 6C). And overexpression of FAK completely recovered the proliferative and invasive potential of siRNAs-ACP5 cells (Figure 6B, 6D). Furthermore, the inhibition of activity by MK2206 (Akt phosphorylation inhibitors) could partially decrease the positive effects of ACP5 on CRC cell proliferation (Figure 7A, 7B) and invasion (Figure 7C). These results suggest that ACP5 promoted CRC progression through the activation of the FAK/PI3K/AKT signaling pathway.
Figure 6.
Knock-down of FAK abolishes the oncogenic role of ACP5 in CRC cells. (A, C) CCK-8 assay (A) or transwell assay (C) showed that ACP5 overexpression induced cell proliferation or invasion, which was largely abolished by siRNAs-FAK. (B, D) CCK-8 assay (B) or transwell assay (D) showed that knock-down of ACP5 could inhibit cell proliferative or invasive ability, which was largely reversed by pcDNA3.1-FAK. Results shown are the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001) of triplicate determination from three independent experiments.
Figure 7.
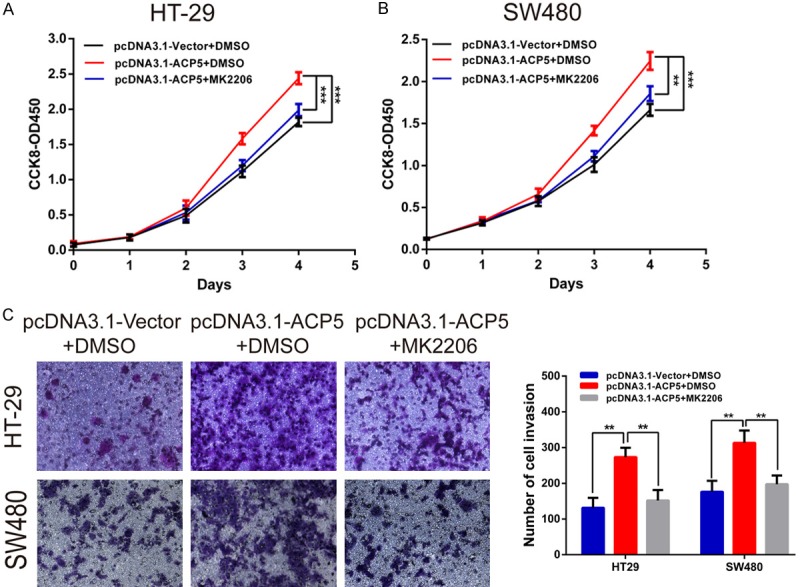
The Akt inhibitors could abolish the oncogenic role of ACP5 in CRC cells. A, B. Proliferation of HT-29 and SW480 cells treated with pcDNA3.1-ACP5 and (or) 1 μmol/L MK2206 by CCK-8 assay. C. Invasion of HT-29 and SW480 cells treated with pcDNA3.1-ACP5 and (or) 1 μmol/L MK2206 by transwell assay. Results shown are the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001) of triplicate determination from three independent experiments.
ShRNA-ACP5 decreases tumor growth in mouse xenograft
To determine whether ACP5 could promote tumor growth in vivo, shRNA-ACP5-HCT116 cells were implanted subcutaneously into the flanks of nude mice. The growth of tumor cells in mice injected with shRNA-ACP5 cells slower than control animals at different time points (Figure 8A, 8B). And the average tumor weight in shRNA-ACP5 group was 0.4-fold smaller than in control animals (Figure 8C). Furthermore, the expression of downstream targets of ACP5, and FAK/PI3K/AKT signaling pathway were markedly decreased in shRNA-ACP5 group compared with that in control mice (Figure 8D, 8E). Together, these results demonstrated that ACP5 may activate FAK/PI3K/AKT signaling pathway to promote tumor progression.
Figure 8.
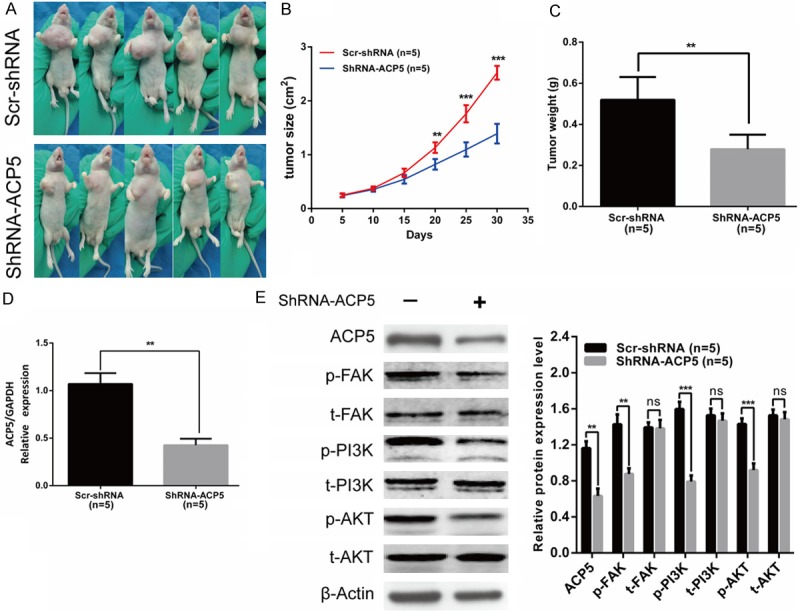
ShRNA-ACP5 inhibits tumor growth by FAK/PI3K/AKT signaling in vivo. (A-C) Tumor xenograft volume (A), tumor size (B) and weight (C) in shRNA-ACP5-treated nude mice were smaller than that in the mock group. (D) ACP5 mRNA expression was decreased in shRNA-ACP5-treated nude mice, compared with control group. (E) Expression of phospho-FAK, phospho-PI3K, phospho-AKT proteins were decreased in shRNA-ACP5-treated nude mice, compared with control group. Results shown are the mean ± SD (**P < 0.01, ***P < 0.001).
Discussion
This report is the first direct investigation of the function and mechanism of ACP5 in colorectal cancer. Firstly, we found that ACP5 was frequently up-regulated in colorectal cancer, and was correlated with worse OS and DFS of the patients. Then, we illustrated ACP5 as an oncogene that promoted cell proliferation and invasion of colorectal cancer, whereas down-regulated ACP5 had the opposite affection. Mechanistically, overexpression ACP5 may activate FAK/PI3K/AKT signaling pathway to promote tumor progression.
Accumulating evidence has reported that ACP5 could play critical roles in tumor progression of many human malignant cancers [20,21]. For example, Kawamura et al indicated that ACP5 expression was up-regulated in gastric cancer. Moreover, patients with positive ACP5 expression had poorer prognosis than those with negative expression after surgery [16]. Xia et al demonstrated that high ACP5 expression predicted poor prognosis of patients with hepatocellular carcinoma, and ACP5 promoted malignant progression of hepatocellular carcinoma through FoxM1/ACP5 signaling pathway [15]. Furthermore, Gao et al proved that ACP5 expression in clinical lung adenocarcinoma was an independent prognostic factor of overall survival for the patients [20]. However, the functional role of ACP5 in colorectal cancer has remained unknown.
In this study, we demonstrated that ACP5 expression was markedly increased in CRC tissues. Moreover, the expression of ACP5 was positively associated with the aggressiveness and recurrence of CRC. Thus, it can be seen that ACP5 acts an oncogene of CRC tumorigenesis, which is consistent with existing literatures [20]. We also illustrated that ACP5 increased CRC cell viability and invasion, indicating the oncogenic role of ACP5 in CRC cell.
Although ACP5 has been proved to promote cell proliferation and invasion, the intrinsic molecular mechanisms are still unclear. It has been proved that the accumulated levels of ACP5 promoted tumorigenesis and metastases by binding to the promoter of FAK in melanoma cells [10]. Interestingly, FAK is a core modulator of PI3K/AKT signaling mainly through forming complex with PI3K [22,23]. It is well known that AKT plays an essential role in downstream of the PI3K pathway, and is involved in multiple cellular processes through dysregulation of signal transduction and subsequently aberrant activation or inactivation of important effectors in colorectal cancer, such as p21, p53, mTOR, GSK-3β [23-25]. Besides, the PI3K/AKT signaling is usually hyperactive in multiple cancers, including colorectal cancer, and promotes tumor growth and metastasis [24,26]. Thereby, we hypothesized and partially validated that abnormal expression of ACP5 promoted cell proliferation and tumor growth possibly through activating the FAK/PI3K/AKT signaling pathway in CRC. Our research revealed that ACP5 overexpression could significantly reduce expression levels of phospho-FAK, phospho-PI3K and phospho-Akt, and knockdown of ACP5 had the opposite affection. We also found overexpression of FAK completely recovered the proliferative and invasive potential of siRNAs-ACP5 cells. And the inhibition of activity by Akt inhibitors could partially decrease the positive effects of ACP5 on CRC cell proliferation and invasion.
In conclusion, our results illustrated that ACP5 functions as an oncogene to facilitate the tumorigenesis and progression of colorectal cancer enhancing the FAK/PI3K/AKT signaling pathway. The present results elucidate a potential mechanism underlying the tumor-oncogenic role of ACP5 in colorectal cancer, and indicate that ACP5 could be a useful marker and potential therapeutic target in colorectal cancer.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81672347, No. 81802308), the Cultivating Project of Ren Ji hospital, China (No. PYMDT-011), and the Municipal Health and Planning Committee of Shanghai, China (No. 201540031).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sunkara V, Hebert JR. The colorectal cancer mortality-to-incidence ratio as an indicator of global cancer screening and care. Cancer. 2015;121:1563–1569. doi: 10.1002/cncr.29228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci. 2015;16:2472–2496. doi: 10.3390/ijms16022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sideris M, Papagrigoriadis S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014;34:2061–2068. [PubMed] [Google Scholar]
- 5.Hamabe A, Yamamoto H, Konno M, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Nishida N, Kawamoto K, Koseki J, Doki Y, Mori M, Ishii H. Combined evaluation of hexokinase 2 and phosphorylated pyruvate dehydrogenase-E1alpha in invasive front lesions of colorectal tumors predicts cancer metabolism and patient prognosis. Cancer Sci. 2014;105:1100–1108. doi: 10.1111/cas.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makrypidi G, Damme M, Muller-Loennies S, Trusch M, Schmidt B, Schluter H, Heeren J, Lubke T, Saftig P, Braulke T. Mannose 6 dephosphorylation of lysosomal proteins mediated by acid phosphatases Acp2 and Acp5. Mol Cell Biol. 2012;32:774–782. doi: 10.1128/MCB.06195-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu R, Sharma SM, Bronisz A, Srinivasan R, Sankar U, Ostrowski MC. Eos, MITF, and PU.1 recruit corepressors to osteoclast-specific genes in committed myeloid progenitors. Mol Cell Biol. 2007;27:4018–4027. doi: 10.1128/MCB.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granholm S, Henning P, Lindholm C, Lerner UH. Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone. 2013;52:83–92. doi: 10.1016/j.bone.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Lausch E, Janecke A, Bros M, Trojandt S, Alanay Y, De Laet C, Hubner CA, Meinecke P, Nishimura G, Matsuo M, Hirano Y, Tenoutasse S, Kiss A, Rosa RF, Unger SL, Renella R, Bonafe L, Spranger J, Unger S, Zabel B, Superti-Furga A. Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet. 2011;43:132–137. doi: 10.1038/ng.749. [DOI] [PubMed] [Google Scholar]
- 10.Scott KL, Nogueira C, Heffernan TP, van Doorn R, Dhakal S, Hanna JA, Min C, Jaskelioff M, Xiao Y, Wu CJ, Cameron LA, Perry SR, Zeid R, Feinberg T, Kim M, Vande Woude G, Granter SR, Bosenberg M, Chu GC, DePinho RA, Rimm DL, Chin L. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell. 2011;20:92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey DW, Dunlap KA, Erikson DW, Patel AK, Bazer FW, Burghardt RC, Johnson GA. Effects of long-term progesterone exposure on porcine uterine gene expression: progesterone alone does not induce secreted phosphoprotein 1 (osteopontin) in glandular epithelium. Reproduction. 2010;140:595–604. doi: 10.1530/REP-10-0169. [DOI] [PubMed] [Google Scholar]
- 12.Capeller B, Caffier H, Sutterlin MW, Dietl J. Evaluation of tartrate-resistant acid phosphatase (TRAP) 5b as serum marker of bone metastases in human breast cancer. Anticancer Res. 2003;23:1011–1015. [PubMed] [Google Scholar]
- 13.Lyubimova NV, Pashkov MV, Tyulyandin SA, Gol’dberg VE, Kushlinskii NE. Tartrate-resistant acid phosphatase as a marker of bone metastases in patients with breast cancer and prostate cancer. Bull Exp Biol Med. 2004;138:77–79. doi: 10.1023/b:bebm.0000046945.95479.d6. [DOI] [PubMed] [Google Scholar]
- 14.Wu YY, Janckila AJ, Ku CH, Yu CP, Yu JC, Lee SH, Liu HY, Yam LT, Chao TY. Serum tartrate-resistant acid phosphatase 5b activity as a prognostic marker of survival in breast cancer with bone metastasis. BMC Cancer. 2010;10:158. doi: 10.1186/1471-2407-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia L, Huang W, Tian D, Chen Z, Zhang L, Li Y, Hu H, Liu J, Chen Z, Tang G, Dou J, Sha S, Xu B, Liu C, Ma J, Zhang S, Li M, Fan D, Nie Y, Wu K. ACP5, a direct transcriptional target of FoxM1, promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Oncogene. 2014;33:1395–1406. doi: 10.1038/onc.2013.90. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura M, Tanaka K, Toiyama Y, Okugawa Y, Okigami M, Yasuda H, Saigusa S, Ohi M, Inoue Y, Uchida K, Mohri Y, Kusunoki M. Clinical significance of tartrate-resistant acid phosphatase type-5 expression in human gastric cancer. Anticancer Res. 2014;34:3425–3429. [PubMed] [Google Scholar]
- 17.Luo Y, Ye GY, Qin SL, Mu YF, Zhang L, Qi Y, Qiu YE, Yu MH, Zhong M. High expression of Rab3D predicts poor prognosis and associates with tumor progression in colorectal cancer. Int J Biochem Cell Biol. 2016;75:53–62. doi: 10.1016/j.biocel.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar C, Cardoso J, Franken P, Molenaar L, Morreau H, Moslein G, Sampson J, Boer JM, de Menezes RX, Fodde R. Cross-species comparison of human and mouse intestinal polyps reveals conserved mechanisms in adenomatous polyposis coli (APC)-driven tumorigenesis. Am J Pathol. 2008;172:1363–1380. doi: 10.2353/ajpath.2008.070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukamoto S, Ishikawa T, Iida S, Ishiguro M, Mogushi K, Mizushima H, Uetake H, Tanaka H, Sugihara K. Clinical significance of osteoprotegerin expression in human colorectal cancer. Clin Cancer Res. 2011;17:2444–2450. doi: 10.1158/1078-0432.CCR-10-2884. [DOI] [PubMed] [Google Scholar]
- 20.Gao YL, Liu MR, Yang SX, Dong YJ, Tan XF. Prognostic significance of ACP5 expression in patients with lung adenocarcinoma. Clin Respir J. 2018;12:1100–1105. doi: 10.1111/crj.12637. [DOI] [PubMed] [Google Scholar]
- 21.Reithmeier A, Panizza E, Krumpel M, Orre LM, Branca RMM, Lehtio J, Ek-Rylander B, Andersson G. Tartrate-resistant acid phosphatase (TRAP/ACP5) promotes metastasis-related properties via TGFβ2/TβR and CD44 in MDA-MB-231 breast cancer cells. BMC Cancer. 2017;17:650. doi: 10.1186/s12885-017-3616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Yu Z, Chen F, Xu H, Shen S, Chen W, Chen L, Su Q, Zhang L, Bi J, Zeng W, Li W, Huang X, Wang Q. miR-300 regulates the epithelial-mesenchymal transition and invasion of hepatocellular carcinoma by targeting the FAK/PI3K/AKT signaling pathway. Biomed Pharmacother. 2018;103:1632–1642. doi: 10.1016/j.biopha.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Yao JF, Deng XF, Zheng XD, Jia M, Wang YQ, Huang Y, Zhu JH. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin alphavbeta3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:23. doi: 10.1186/s13046-018-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moench R, Grimmig T, Kannen V, Tripathi S, Faber M, Moll EM, Chandraker A, Lissner R, Germer CT, Waaga-Gasser AM, Gasser M. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget. 2016;7:68749–68767. doi: 10.18632/oncotarget.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Feng CC, Chu SJ, Zhang R, Lu YM, Zhu JS, Zhang J. Toosendanin inhibits growth and induces apoptosis in colorectal cancer cells through suppression of AKT/GSK-3beta/beta-catenin pathway. Int J Oncol. 2015;47:1767–1774. doi: 10.3892/ijo.2015.3157. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Kuang H, Xue J, Liao L, Yin F, Zhou X. LncRNA AB073614 regulates proliferation and metastasis of colorectal cancer cells via the PI3K/AKT signaling pathway. Biomed Pharmacother. 2017;93:1230–1237. doi: 10.1016/j.biopha.2017.07.024. [DOI] [PubMed] [Google Scholar]