Abstract
To investigate the chemotherapy-induced emotion regulation impairment and its neural correlates in breast cancer (BC) patients by event-related potentials (ERP), seventeen BC patients were investigated on emotion regulation paradigms while undergoing the recording of an event-related potential (ERP) both before and after chemotherapy. The performance of behavioral and ERP was compared for the data collected before and after chemotherapy (BCB and BCA, respectively). The correlation of the difference between the peak and the latency of each component before and after chemotherapy were compared with the difference in behavior (RT and AR). BC patients showed a lower accuracy rate in both explicit and implicit emotion identification in BCA compared to that in BCB. Further, both the N1 and P2 components were significantly delayed. The peak values of the N1 and P2 in BCA were significantly higher than those in BCB, whereas the peak value of the N2 in BCA was significantly lower than that in BCB. There was a positive correlation between the difference in latency at the CZ (r=0.88), F3 (r=0.97) and FZ (r=0.85) points in the N1 component and the RT. The difference in latency at the FCZ point in the N2 (r=0.88) component is positively correlated with the AR. The difference in peak value at the CPZ (r=0.89) point in the N1 component is positively correlated with the RT. Both the implicit and explicit emotional processing was compromised in BC patients following chemotherapy. These emotional processing deficits may be related to the changes of the N1, N2 and P2 of the ERP.
Keywords: Breast cancer, emotion, chemotherapy, event-related potential
Introduction
Breast cancer is the second most common cancer [1]. Most patients diagnosed with breast cancer are treated with chemotherapy. With the development of new chemotherapy regimens and increased odds of survival, the side effects of chemotherapy are reported frequently [2]. In addition to nausea, vomiting, bone marrow suppression and other adverse physical reactions, chemotherapy can also lead to profound psychological side effects. The central nervous system (CNS) toxicity of chemotherapeutic agents affects cognition, emotion and directly impacts the quality of life [3]. Although the blood-brain barrier (BBB) provides some protection for the CNS, studies have accumulated to show that many drugs, such as antimetabolites (e.g., methotrexate or fluorouracil) and platinum-based agents, could cross the BBB and cause damage to the CNS [4]. Chemotherapy-induced cognitive impairment may affect memory, learning, attention, reasoning, and other executive functions and persist even after discontinuation of chemotherapy [5,6].
Emotion is critical to mental health, and emotional distress has been called the “sixth vital sign” in clinical practice [7]. Previous studies have demonstrated cognitive impairment after chemotherapy, particularly in memory and attention, in breast cancer (BC) patients. Cancer patients also experience negative emotions, such as anxiety and depression [8]. Emotional distress may reduce treatment compliance [9,10] or even increase the risk of disease progression [11,12]. Emotion perception and regulation can transpire both explicitly and implicitly. According to a dual-process framework, explicit emotion regulation is defined as a process that requires conscious effort and awareness. Implicit emotion regulation is evoked automatically by the stimulus without one’s awareness [13]. However, it is less clear whether or how chemotherapy may influence implicit and explicit emotional processing in BC patients. Here, we addressed the issue in an electrophysiological study of BC patients.
Event-related potentials (ERP) provide a noninvasive measure of cognitive and emotional functions. Previous studies showed that chemotherapy disrupted attention and information processing along with altered ERP in BC patients [14,15]. Studies also suggested that emotional distress, compared with neural stimuli, elicited greater early posterior negativity (EPN) [16-19], P2 [20,21], late posterior negativity (LPN) [17,19], and/or late positive potential (LPP) [22,23]. Here, we examined explicit and implicit emotional processing in BC patients before and after chemotherapy and the changes in these ERP in association with emotional processing deficits.
Materials and methods
Participants and assessments
Seventeen BC patients were recruited from the Second Affiliated Hospital of Anhui Medical University, Hefei, China, and were hospitalized from January 2017 to March 2018 in the Department of Oncology. All were diagnosed with stage II through IV breast cancer and treated with standard chemotherapy regimens. The study was approved by the Research Ethics Committee of the Affiliated Second Hospital of Anhui Medical University, and all the subjects provided informed consents in accordance with the Helsinki Declaration and the research protocol.
BC patients were recruited according to the following criteria: (1) completion of at least 6 cycles of chemotherapy; (2) ability to complete simple calculations; (3) no use of psychotropic medications; (4) no history of neurologic or psychiatric illnesses; (5) no primary or secondary brain tumor; (6) no abuse of alcohol or drugs; (7) MoCA score ≥26 (see below); (8) HAMD and HAMA scores <7 (see below).
The general cognitive function was evaluated with the Beijing version of the Montreal Cognitive Assessment (MoCA). The MoCA assessed visuospatial/executive, naming, memory, attention, language, abstraction, delayed recall and orientation [24]. Patients were also evaluated with the Hamilton Depression Rating Scale (HAMD) and the Hamilton Anxiety Rating Scale (HAMA), as well as the Emotion Regulation Questionnaire-Chinese Revised Version (ERQ-CRV). The 14-item ERQ-CRV contains two conceptually distinct subscales: the cognitive reappraisal (CR) and the expression inhibition (EI). The item responses were structured by a seven-point Likert scale ranging from 1 to 7 (absolutely never to absolutely always). The results of the clinical assessments are shown in Table 1.
Table 1.
Summary of the clinical assessments across the time points
| HAMA | HAMD | MoCA | ERS* | ||
|---|---|---|---|---|---|
|
| |||||
| CR | EI | ||||
| BCB | 4.3±0.9 | 4.9±1.0 | 27.4±1.0 | 34.7±7.7 | 29.4±8.4 |
| BCA | 4.1±1.2 | 4.5±0.9 | 27.0±1.0 | 38.8±4.4 | 25.4±5.1 |
Note: BCB: breast cancer patients before chemotherapy; BCA: breast cancer patients after chemotherapy; ERS: emotion regulation strategies; CR: cognitive reappraisal; EI: expression inhibition.
Significant strategy main effect and the strategy by time point interaction effect in ANOVA (see text).
Emotion detection paradigm
The emotion detection paradigm was illustrated as follows: the experiment ran in two consecutive blocks of implicit emotion detection, where patients were instructed to judge the correctness of an arithmetic equation as quickly and accurately as possible; it also ran in two blocks of explicit emotion detection, where patients were to determine the valence of a picture (negative or neutral) as quickly and accurately as possible. The order of the arithmetic and valence blocks was counterbalanced across subjects. In both blocks, the stimuli consisted of one simple addition or subtraction equation superimposed on a background picture. Pictures were selected from the International Emotional Picture System (IAPS). The 30 neutral and negative pictures were matched in mean spatial frequency and luminance [25]. Each picture was presented for 3 s and participants were allowed 3 s to respond in each trial, with 30 trials in each block. Participants were allowed a short break in between blocks.
ERP recording
The electroencephalography (EEG) was recorded from 64 Ag-AgCl electrodes placed on the scalp according to the extended International 10/20 system (Waveguard64 cap, Cephalon A/S). The vertical electrooculograms (EOG) were recorded both supraorbitally and infraorbitally from the left eye. The horizontal EOG was recorded as the left versus right orbital rim. The EEG activity was recorded using a left mastoid reference electrode and re-referenced off-line to the mean of the bilateral mastoid electrodes. All electrode impedances were maintained below 5 kV. The EEG and EOG activity was amplified with a DC 0.01-100 Hz band-pass filter and continuously sampled at 512 Hz (64 channel high-speed amplifier, Advanced Neuro Technology, Enschede, Netherlands). The trials with the remaining EOG artifacts (mean EOG voltage exceeding ±100 mV), the amplifier clipping artifacts, or the peak-to-peak deflections exceeding ±100 mV were excluded from the averaging. The ERP waveforms were time-locked to the onset of the face stimuli, and the averaging epoch was 1200 ms, including a 200 ms pre-stimulus baseline.
Measures and statistical analyses
The Statistical Package for Social Sciences (SPSS) 16.0 was used for all statistical analyses.
The differences in questionnaire scores obtained before and after chemotherapy were analyzed using paired-samples t-tests. For reaction time (RT) and accuracy rate (AR) of behavioral data, repeated-measures ANOVAs with-subject effects of time point (BCB, BCA), task (implict ER, explict ER) and valence of emotion (negative, neutral). For the peak amplitude and latency of each components in ERP data, repeated-measures ANOVAs with-subject effects of time point (BCB, BCA), task (implict ER, explict ER) and valence of emotion (negative, neutral). The correlation of the difference between the peak and latency of each component before and after chemotherapy was compared with the difference in behavior (RT and AR) by the Pearson’s correlation analysis.
The ERP data were submitted to an open source toolbox EEGLAB [26], as implemented in MATLAB 7.1 (Math Works) for off-line analysis. The time windows of the N1, N2, P2 and P3 latency and peak value analyses were established based on the grand-averaged potentials of each task condition. The window was 55-155 ms for N1, 200-260 ms for N2, 130-230 ms for P2 and 230-330 ms for P3. The FCZ, FZ, CPZ, CZ, F3, F4 (1 frontal, 3 central and 2 parietal electrodes) were further selected for statistical analysis of the ERP. We focused on the task type (implicit vs. explicit), valence (negative vs. neutral) and time point (BCB vs. BCA) and examined the interaction effects for the ERP amplitude and latency in the repeated-measures ANOVA. The degrees of freedom of the F-test were corrected according to the Greenhouse-Geisser method.
Results
Clinical assessments
The results of the clinical assessments are presented in Table 1. None of the patients had significant anxiety or depression, and there was no statistically significant difference in the HAMA or HAMD scores between BCB and BCA. All patients enrolled in the study had no significant cognitive dysfunction, all had a MoCA score ≥26, and there was no significant difference between BCB and BCA.
In the assessment of emotion regulation strategy, the repeated-measures analysis of variance (ANOVA) of strategy (cognitive reappraisal or CR vs. expression inhibition or EI) x time point (BCB vs. BCA) showed a significant strategy effect (F(1,16)=44.367, P<0.001), but no significant time point effect (F(1,16)=0.100, P=0.756). There was also a significant interaction between the strategy and the time point (F(2,32)=11.271, P<0.05). Thus, the participants were more likely to use CR than EI both before and after chemotherapy, and the participants used more CR than EI after chemotherapy.
Emotion processing: behavioral data
The behavioral data, including the response time (RT) and the accuracy rate (AR), are shown in Table 2, and the results of the repeated-measures analysis of variance are shown in Table 3. The RT of BCA was significantly slower than that of BCB (F(1,16)=4.565, P=0.048), but there were no other main and interaction effects. The AR of BCA was significantly lower than that of BCB (F(1,16)=4.661, P=0.046). For the AR, both task (F(1,16)=5.023, P=0.038) and emotion (F(1,16)=4.521, P=0.049) main effects were significant, with lower AR in implicit vs. explicit tasks and higher AR for negative vs. neutral emotions. There were no significant two-way or three-way interaction effects.
Table 2.
Behavioral data of the emotion detection task
| Outcome | Time point | Implicit task | Explicit task | ||
|---|---|---|---|---|---|
|
| |||||
| Negative | Neutral | Negative | Neutral | ||
| AR (%) | BCB | 79±19 | 73±15 | 85±10 | 86±10 |
| BCA | 73±16 | 67±17 | 75±17 | 73±15 | |
| RT (ms) | BCB | 1373±327 | 1333±299 | 1324±238 | 1262±246 |
| BCA | 1511±191 | 1537±244 | 1323±227 | 1333±204 | |
Note: AR: accuracy rate; RT: reaction time; BCB/BCA: before/after chemotherapy.
Table 3.
Statistics of the behavioral data
| Task | Within-subject effects (DF=1, 16) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Time | Task | Emotion | Time × task | Time × emotion | Task × emotion | Time × Task × emotion | |
| AR | F=4.661 | F=5.029 | F=4.521 | F=3.102 | F=0.079 | F=1.443 | F=0.308 |
| P=0.046* | P=0.039* | P=0.049* | P=0.097 | P=0.783 | P=0.247 | P=0.587 | |
| RT | F=4.565 | F=4.153 | F=0.402 | F=2.593 | F=4.028 | F=0.166 | F=0.006 |
| P=0.048* | P=0.058 | P=0.535 | P=0.127 | P=0.062 | P=0.698 | P=0.941 | |
Note: AR: accuracy rate; RT: response time; BCB/BCA: before/after chemotherapy.
P<0.05.
Emotional processing: ERP
The ERP of the implicit and explicit emotion regulation of BCA and BCB and for the negative and neutral emotion trials are shown in Figure 1.
Figure 1.
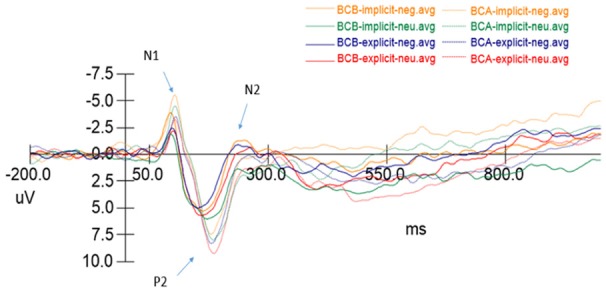
ERP results during implicit and explicit emotion detection.
As shown in Tables 4 and 5, the multivariate repeated-measures ANOVA was computed on the N1, P2, N2 and P3 spatial factor scores using the time points, task and emotion as within-subject factors. The latency of the N1 and P2 components of two time points were significantly different; the N1 and P2 latencies were significantly longer in BCA relative to BCB (F(1,16)=4.568, P<0.05 and F(1,16)=12.528, P<0.01, respectively). In addition, the N1, P2 and N2 components’ emotional main effect was significant (F(1,16)=10.524, P<0.01, F(1,16)=12.952, P<0.01 and F(1,16)=4.425, P<0.05, respectively). The three components showed more prolonged latencies in the neutral emotional pictures than in the negative emotional pictures. None of the above components have other main effects and interaction effects.
Table 4.
Statistics of the Peak Amplitude of the N1, P2, N2, and P3: within-subject effects (df=1, 16)
| ERP | Time point | Task | Emotion | Time point × Task | Time point × Emotion | Task × Emotion | Time point × Task × Emotion |
|---|---|---|---|---|---|---|---|
| N1 | F=6.660 | F=7.138 | F=2.657 | F=0.799 | F=0.526 | F=0.288 | F=0.455 |
| P=0.020* | P=0.017* | P=0.123 | P=0.358 | P=0.479 | P=0.599 | P=0.510 | |
| P2 | F=5.154 | F=0.001 | F=8.878 | F=1.296 | F=0.060 | F=0.314 | F=0.962 |
| P=0.025* | P=0.982 | P=0.009** | P=0.272 | P=0.810 | P=0.583 | P=0.341 | |
| N2 | F=5.614 | F=3.829 | F=25.971 | F=0.022 | F=0.898 | F=1.319 | F=0.742 |
| P=0.031* | P=0.068 | P<0.001*** | P=0.883 | P=0.337 | P=0.268 | P=0.402 | |
| P3 | F=1.701 | F=1.071 | F=1.033 | F=1.981 | F=0.604 | F=0.200 | F=0.028 |
| P=0.201 | P=0.309 | P=0.317 | P=0.169 | P=0.443 | P=0.658 | P=0.868 |
P<0.05;
P<0.01;
P<0.001.
Table 5.
Statistics of the Peak Latency of the N1, P2, N2, and P3: within-subject effects (df=1, 16)
| ERP | Time point | Task | Emotion | Time point × Task | Time point × Emotion | Task × Emotion | Time point × Task × Emotion |
|---|---|---|---|---|---|---|---|
| N1 | F=4.658 | F=0.880 | F=10.524 | F=0.576 | F=0.156 | F=0.062 | F=0.020 |
| P=0.046* | P=0.362 | P=0.005** | P=0.548 | P=0698 | P=0.806 | P=0.890 | |
| P2 | F=12.518 | F=2.037 | F=12.952 | F=003 | F=6.206 | F=0.277 | F=1.643 |
| P=0.003** | P=0.173 | P=0.002** | P=0.958 | P=0.024 | P=0.606 | P=0.434 | |
| N2 | F=3.481 | F=1.326 | F=4.425 | F=0.011 | F=3.326 | F=0.612 | F=1.142 |
| P=0.081 | P=0.267 | P=0.042* | P=0.918 | P=0.097 | P=0.446 | P=0.301 | |
| P3 | F=1.418 | F=0.930 | F=1.808 | F=1.217 | F=0.688 | F=0.231 | F=0.025 |
| P=0.251 | P=0.349 | P=0.197 | P=0.286 | P=0.426 | P=0.637 | P=0.877 |
P<0.05;
P<0.01.
In the peak item, the peak values of the N1 and P2 components in the BCA patients’ time points were significantly higher than those in BCB (F(1,16)=6.660, P<0.05 and F(1,16)=6.154, P<0.05). In the N1 component, the main effect of the task is significant (F(1,16)=7.138, P<0.05), and the implicit ER task induced a higher N1 peak. In the P2 component, the emotional main effect is significant (F(1,16)=8.878, P<0.01), and the neutral emotional pictures induced a greater P2 peak. However, in the N2 component, the peak value of the BCB was significantly higher than that of BCA, and there was a statistically significant difference (F(1,16)=5.614, P<0.05). In the N2 components, the main effect of the emotion was significant (F(1,16)=25.971, P<0.001); it showed a higher peak in the negative emotion than in the neutral emotion. In the P3 component, BCP had no differences between the time points before and after chemotherapy, but the main emotional effect was significant (F(1,16)=6.712, P<0.05), and the neutral emotional picture induced a greater P3 amplitude. None of the above components have other main effects and interaction effects.
Correlation of the difference between the peak and latency of each component and the difference in behavior (RT and AR)
There was a positive correlation between the difference in latency at the CZ (r=0.88), F3 (r=0.97) and FZ (r=0.85) points in the N1 component and the RT, as shown in Figure 2.
Figure 2.
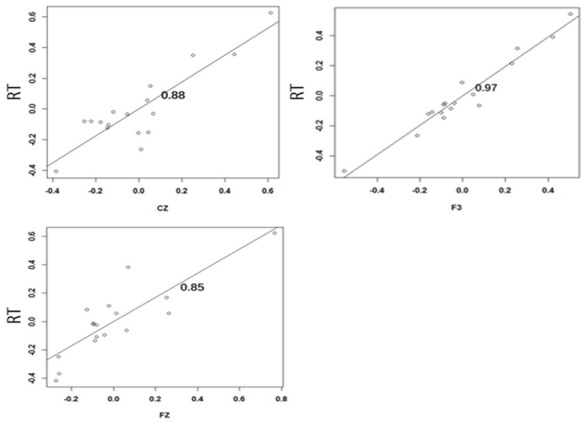
Correlation between the difference in latency at the CZ (r=0.88), F3 (r=0.97) and FZ (r=0.85) points in the N1 component and the RT.
The difference in latency at the FCZ point in the N2 component is positively correlated with the AR (r=0.88), as shown in Figure 3.
Figure 3.
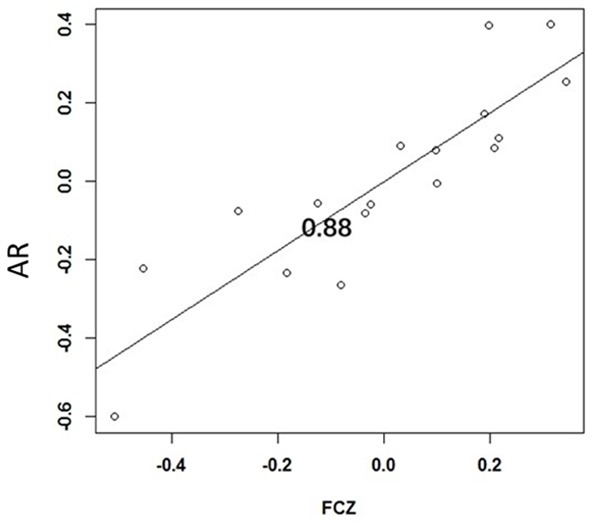
Correlation between the difference of latency of the N2 component before and after chemotherapy and the AR.
The difference in peak value at the CPZ point in the N1 component is positively correlated with the RT (r=0.89), as shown in Figure 4.
Figure 4.
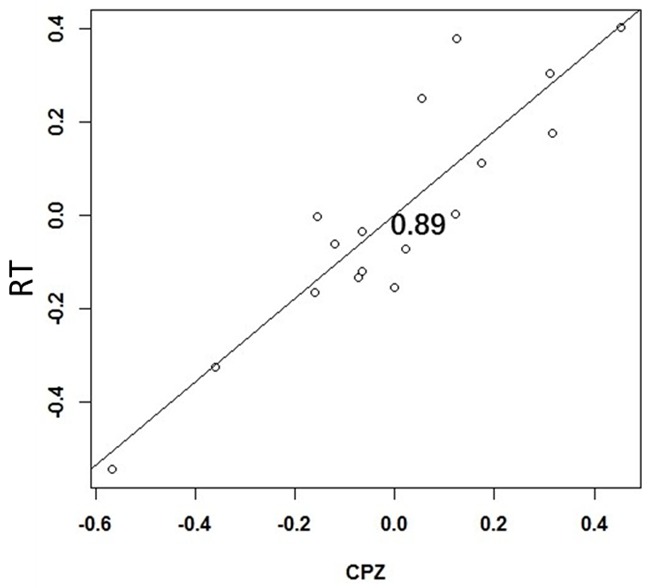
Correlation between the difference of the peak of the N1 component before and after chemotherapy and the RT.
Discussion
The important role of emotion regulation and expression in adaptation to breast cancer is now widely recognized, but it is less clear in chemotherapy-induced emotion impairment in BC patients. The present results show that BC patients showed a lower accuracy rate in both explicit and implicit emotion identification after chemotherapy treatment. The ERP study indicated that the latency of the N1 and P2 components showed a significant delay in BCA, and the peak amplitudes of the N1 and P2 were significantly higher, whereas the peak amplitude of the N2 was lower in BCA. There was a positive correlation between the difference in latency at the CZ (r=0.88), F3 (r=0.97), and FZ (r=0.85) points in the N1 component and the RT. The difference in latency at the FCZ point in the N2 component was positively correlated with the AR (r=0.88). The difference in the peak value at the CPZ point in the N1 component is positively correlated with the RT (r=0.89).
It is important to highlight that there are reliable and valid measures widely used to assess emotion regulation and emotional expression in the larger field of psychology and medicine that have seldom been used in oncology studies. In this study, we use the ERQ-CRV scale to assess the emotion regulation strategy. Reappraisal strategies are antecedent-focused strategies, while suppression strategies are response-focused strategies.
The reappraisal strategy was a more effective emotion adjustment strategy [27]. The results showed that BC patients were more likely to use CR than EI both before and after chemotherapy.
The behavioral results showed that BCA had a poor performance in the response time and the correct rate tasks. Previous studies have shown that BC patients cannot sustain attention, have poor executive functions and have other cognitive disorders [15,28,29] after chemotherapy. In the explicit emotion regulation task, the correct rate of emotion recognition in BCA was significantly lower than that in BCB; this is consistent with previous studies [30]. Moyal et al. found that the effective recognition of emotions is essential for successful reappraisal. Reappraisal is a validated way of emotion regulation [31], and BCA may have poor recognition of mood due to impairment of emotion recognition. BC patients who received chemotherapy respond slowly to event stimuli, and this may affect their performance on emotional regulation.
The EPR results indicated that there was a significant delay in the latency of the N1 and P2 components in BCA. The N1 component is a sign of early visual selective attention [32]. The delay in latency of the N1 component may indicate that BCA had a significant delay in visual attention to emotion. The P2 component is thought to be involved in the processing of stimulus features [33,34]. The significant delay in the P2 component may indicate a delay in the perception of emotions in BCA. Due to the limited resources, the delay in the processing of the task in the N1 and P2 components of BCA may lead to interference with the subsequent task control process [35,36]. In the N1 and P2 components, the emotional main effect is significant. The negative emotion pictures elicit a faster response than that of the neutral emotion pictures on both implicit and explicit emotion regulation. This is in line with the previous findings [37] that people respond faster to negative stimuli in response to environmental threats and adaptation to their existence. It may be because the response time reflects the duration of multiple cognitive processes, where the ERP that are separated from the EEG provide the strength and duration of the individual process [14].
The peaks of the N1 and P2 components in BCA were higher than those in BCB. The BCA will invest more resources into the emotional visual attention and perception, which may improve the implicit and explicit emotional regulation. The main effect of the N1 component task is significant. It is speculated that the implicit ER consumes more cognitive resources to early visual selective attention. This is similar to the analysis of behavioral results, where BC patients need to invest more cognitive resources in implicit ER tasks. Previous studies have found that negative stimulation induces a greater P2 amplitude [20,37], but the peak of the P2 induced by neutral emotion is higher than that induced by negative emotion in the present result. It may be because BC patients need to devote more resources to perceive and identify the neutral stimuli than the negative stimuli. The N2 component in BCB had a greater peak than that in BCA. The N2 amplitude is considered to be a direct indicator of the allocation of attention in conflict monitoring, and the latency represents the speed of the perceived conflict [38,39]. Therefore, the results suggest that BCB patients will invest more resources in the reaction of conflict monitoring to effectively overcome the emotional dimension of the processing bias. The results also show the emotional main effect is significant. The negative stimulation will induce a greater N2 amplitude, and there is a positive correlation between the difference of the ERP component before and after chemotherapy and between the differences in behavior (RT and AR).
The present study has also a limitation which involved a relatively small sample. Thus, further studies should include more patients to confirm the present findings.
In summary, in the present study, we have identified that there is implicit and explicit emotion impairment in BCA patients. They may be related to the changes in the N1, N2 and P2 in the ERP of BC patients who have undergone the chemotherapy.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81872504; 81372487).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kovalchuk A, Ilnytskyy Y, Rodriguez-Juarez R, Shpyleva S, Melnyk S, Pogribny I, Katz A, Sidransky D, Kovalchuk O, Kolb B. Chemo brain or tumor brain - that is the question: the presence of extracranial tumors profoundly affects molecular processes in the prefrontal cortex of TumorGraft mice. Aging (Albany NY) 2017;9:1660–1676. doi: 10.18632/aging.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012;21:1141–8. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simó M, Rifà-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1311–21. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JA, Griffin L, Balcueva EP, Groteluschen DL, Samuel TA, Lesser GJ, Naughton MJ, Case LD, Shaw EG, Rapp SR. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10:176–84. doi: 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JH, Bae SH, Jung YS, Jung YM. Prevalence and characteristics of chemotherapy-related cognitive impairment in patients with breast cancer. J Korean Acad Nurs. 2015;45:118–28. doi: 10.4040/jkan.2015.45.1.118. [DOI] [PubMed] [Google Scholar]
- 7.Bultz BD, Carlson LE. Emotional distress: the sixth vital sign--future directions in cancer care. Psychooncology. 2006;15:93–5. doi: 10.1002/pon.1022. [DOI] [PubMed] [Google Scholar]
- 8.Conley CC, Bishop BT, Andersen BL. Emotions and emotion regulation in breast cancer survivorship. Healthcare (Basel) 2016;4 doi: 10.3390/healthcare4030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 10.Greer JA, Pirl WF, Park ER, Lynch TJ, Temel JS. Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. J Psychosom Res. 2008;65:549–52. doi: 10.1016/j.jpsychores.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 13.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 2011;25:400–12. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, van Dam FS. Electrophysiological correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2005;94:53–61. doi: 10.1007/s10549-005-7093-3. [DOI] [PubMed] [Google Scholar]
- 15.Kam JWY, Brenner CA, Handy TC, Boyd LA, Liu-Ambrose T, Lim HJ, Hayden S, Campbell KL. Sustained attention abnormalities in breast cancer survivors with cognitive deficits post chemotherapy: an electrophysiological study. Clin Neurophysiol. 2016;127:369–378. doi: 10.1016/j.clinph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Kissler J, Herbert C, Peyk P, Junghofer M. Buzzwords: early cortical responses to emotional words during reading. Psychol Sci. 2007;18:475–80. doi: 10.1111/j.1467-9280.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- 17.Schacht A, Sommer W. Emotions in word and face processing: early and late cortical responses. Brain Cogn. 2009;69:538–50. doi: 10.1016/j.bandc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Kissler J, Herbert C, Winkler I, Junghofer M. Emotion and attention in visual word processing: an ERP study. Biol Psychol. 2009;80:75–83. doi: 10.1016/j.biopsycho.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Palazova M, Mantwill K, Sommer W, Schacht A. Are effects of emotion in single words non-lexical? Evidence from event-related brain potentials. Neuropsychologia. 2011;49:2766–75. doi: 10.1016/j.neuropsychologia.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Herbert C, Kissler J, Junghöfer M, Peyk P, Rockstroh B. Processing of emotional adjectives: evidence from startle EMG and ERPs. Psychophysiology. 2006;43:197–206. doi: 10.1111/j.1469-8986.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 21.Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Res. 2007;1148:138–48. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–61. [PubMed] [Google Scholar]
- 23.Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 24.Hobson J. The montreal cognitive assessment (MoCA) Occup Med (Lond) 2015;65:764–5. doi: 10.1093/occmed/kqv078. [DOI] [PubMed] [Google Scholar]
- 25.Delplanque S, N’diaye K, Scherer K, Grandjean D. Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J Neurosci Methods. 2007;165:144–50. doi: 10.1016/j.jneumeth.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Sheppes G, Gross JJ. Emotion regulation effectiveness: what works when. John Wiley & Sons, Inc.; 2012. pp. 330–333. [Google Scholar]
- 28.Ando-Tanabe N, Iwamitsu Y, Kuranami M, Okazaki S, Yasuda H, Nakatani Y, Yamamoto K, Watanabe M, Miyaoka H. Cognitive function in women with breast cancer receiving adjuvant chemotherapy and healthy controls. Breast Cancer. 2014;21:453–62. doi: 10.1007/s12282-012-0405-7. [DOI] [PubMed] [Google Scholar]
- 29.Piccirillo JF, Hardin FM, Nicklaus J, Kallogjeri D, Wilson M, Ma CX, Coalson RS, Shimony J, Schlaggar BL. Cognitive impairment after chemotherapy related to atypical network architecture for executive control. Oncology. 2015;88:360–8. doi: 10.1159/000370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirkner J, Weymar M, Löw A, Hamm C, Struck AM, Kirschbaum C, Hamm AO. Cognitive functioning and emotion processing in breast cancer survivors and controls: an ERP pilot study. Psychophysiology. 2017;54:1209–1222. doi: 10.1111/psyp.12874. [DOI] [PubMed] [Google Scholar]
- 31.Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J, He Y, Lei Y, Yang J, Li H. Event-related potential correlates of the extraverts’ sensitivity to valence changes in positive stimuli. Neuroreport. 2009;20:1071–6. doi: 10.1097/WNR.0b013e32832e7d55. [DOI] [PubMed] [Google Scholar]
- 33.Schulz C, Kaufmann JM, Kurt A, Schweinberger SR. Faces forming traces: neurophysiological correlates of learning naturally distinctive and caricatured faces. Neuroimage. 2012;63:491–500. doi: 10.1016/j.neuroimage.2012.06.080. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann JM, Schulz C, Schweinberger SR. High and low performers differ in the use of shape information for face recognition. Neuropsychologia. 2013;51:1310–9. doi: 10.1016/j.neuropsychologia.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. Int J Psychophysiol. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J, Zhang Q, Chen A, Li H, Wang Q, Zhuang Z, Jia S. Are we sensitive to valence differences in emotionally negative stimuli? Electrophy-siological evidence from an ERP study. Neuropsychologia. 2007;45:2764–71. doi: 10.1016/j.neuropsychologia.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Kong F, Zhang Y, Chen H. ERP differences between processing of physical characteristics and personality attributes. Behav Brain Funct. 2012;8:49. doi: 10.1186/1744-9081-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy E, Potts GF, Loveland KA. Sex-related ERP differences in deviance detection. Int J Psychophysiol. 2003;48:285–92. doi: 10.1016/s0167-8760(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J, He Y, Qinglin Z, Chen A, Li H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. 2008;45:986–93. doi: 10.1111/j.1469-8986.2008.00693.x. [DOI] [PubMed] [Google Scholar]


