Abstract
The purpose of the present study was to investigate the effects of phytic acid (IP6) on morphological and immunohistochemical parameters and oxidative stress response in intestinal explants of pigs exposed to fumonisin B1 (FB1) and/or deoxynivalenol (DON). The jejunal explants were exposed to the following treatments: vehicle, IP6 5 mM, DON 10 µM, FB1 70 µM, DON 10 µM + FB1 70 µM, DON 10 µM + IP6 5 mM, FB1 70 µM + IP6 5 mM, and DON 10 µM + FB1 70 µM + IP6 5 mM. The decrease in villus height and goblet cell density was more evident in DON and DON + FB1 treatments. In addition, a significant increase in cell apoptosis and cell proliferation and a decrease in E-cadherin expression were observed in the same groups. DON and FB1 exposure increased cyclooxygenase-2 expression and decreased the cellular antioxidant capacity. An increase in lipid peroxidation was observed in DON- and FB1-treated groups. IP6 showed beneficial effects, such as a reduction in intestinal morphological changes, cell apoptosis, cell proliferation, and cyclooxygenase-2 expression, and an increase in E-cadherin expression when compared with DON, FB1 alone, or DON and FB1 in association. IP6 inhibited oxidative stress and increased the antioxidant capacity in the explants exposed to mycotoxins.
Keywords: mycotoxins, IP6, reactive oxygen species, morphology, jejunum, pigs
1. Introduction
Fumonisin B1 (FB1) and deoxynivalenol (DON) are the most frequently occurring mycotoxin contaminants in agricultural commodities worldwide, and represent a risk for human and animal health [1]. Exposure to mycotoxins is inevitable, therefore effective strategies to mitigate or even eliminate their harmful impacts are required. In recent years, research involving nutraceutical substances and compounds has increased, due to the excellent preventive and therapeutic action of these compounds on the mycotoxins’ toxic effects [2].
FB1 and DON are mycotoxins, mainly produced by Fusarium spp., that commonly contaminate maize, wheat, barley, and oats [3]. The intracellular action of these mycotoxins has been elucidated, and the induction of oxidative stress and generation of radical oxygen species (ROS) play an important role in their toxic effects, as observed in vivo [4,5] and in vitro [6,7]. Other toxic effects, such as altered intestinal morphology (expression of cell junction proteins, cell proliferation and apoptosis, production of mucin) and inflammatory response (deregulation of anti and pro-inflammatory cytokines and overexpression of cyclooxygenase-2 (cox-2), have also been reported [8,9,10,11,12]. However, the association between intestinal lesions and oxidative stress induced by FB1 and DON has not been elucidated.
Phytic acid (IP6) is a natural antioxidant widely present in cereals and legumes [13]. Several studies have demonstrated the preventive and therapeutic effects of IP6 in diseases associated with mineral and endocrine disturbances, chronic inflammation, and cancer development [14,15,16]. Specifically in models of intestinal inflammation and cancer, IP6 has shown protective effects by decreasing aberrant crypt formation, increasing cell viability, and downregulating pro-inflammatory cytokine, chemokine and cox-2 expression [17,18,19].
Swine are one of the most sensitive species to the toxic effects of FB1 and DON [9]. Furthermore, the similarities with the human immune system and intestinal physiology, as well as with the absorption of IP6 [20], make pigs the ideal experimental model to study the toxic effects induced by mycotoxins, and strategies to mitigate the toxicity using IP6. However, most studies focusing on the effects of FB1 and DON on oxidative stress have been performed in vitro or using laboratory mammals and chickens [21].
In previous in vitro [22] and ex vivo [12] studies, we have shown that IP6 exposure decreases the intestinal lesions induced by FB1 and DON. However, the effect of IP6 on oxidative stress response produced by mycotoxins in the intestine remains to be determined, as well as the effect of IP6 on the combined effects of FB1 and DON. Therefore, the aim of the present study was to investigate the action of IP6 on the toxic effects induced by FB1 and DON alone, or in FB1 and DON in association on the intestine, focusing on the oxidative stress response, morphology, goblet cell density, cell proliferation and apoptosis, and E-cadherin and cox-2 expression in jejunal explants of pigs.
2. Results
2.1. Morphological Assessment
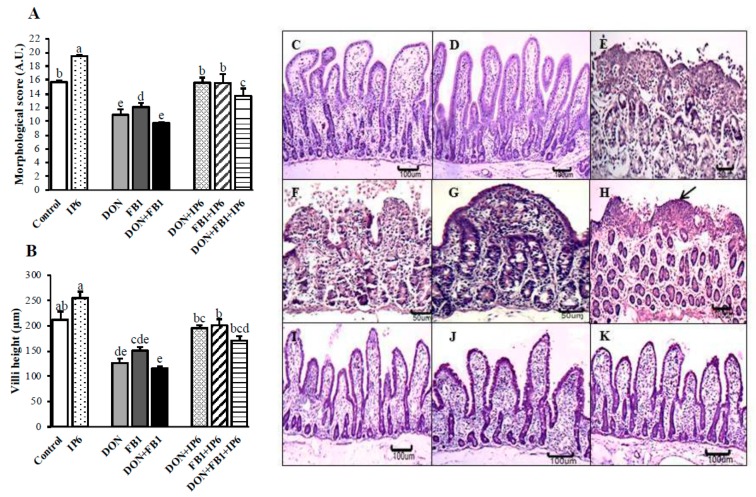
After 3 h of exposure to DON and/or FB1, the explants exhibited moderate to severe jejunal lesions, mainly in the DON + FB1 treatment group (Figure 1A). The main histological changes observed included multifocal to diffuse cytoplasmic vacuolation and flattening of enterocytes, atrophy and villi fusion, lack of apical epithelium, and necrotic debris (Figure 1E–G). Morphological scores decreased significantly in the explants exposed to DON (30.2%), FB1 (23%), and DON + FB1 (38%) compared with the control group. The jejunum exposed to DON presented a significant decrease of 9.4% in the histological score compared to FB1 alone (p ≤ 0.05), and the treatment DON + FB1 showed a significant decrease of 23.3% compared to FB1 (p ≤ 0.05).
Figure 1.
Effects of deoxynivalenol (DON) and fumonisin FB1 (FB1), alone or in association, and phytic acid (IP6) on histological morphology in jejunal explants. (A) Morphological score (AU—Arbitrary Units). (B) Villi height (µm). Explants exposed to control treatment (□); IP6 5 mM ( ); DON (
); DON ( ); FB1 (
); FB1 ( ); DON + FB1 (■); DON + IP6 (
); DON + FB1 (■); DON + IP6 ( ); FB1 + IP6 (
); FB1 + IP6 ( ); DON + FB1 + IP6 (
); DON + FB1 + IP6 ( ). Mean values with different superscript letters were significantly different (p ≤ 0.05). (C) Control treatment. Hematoxylin-eosin (HE), bar 100 µm. (D) IP6 5 mM treatment. HE, bar 100 µm. (E) DON 10 µM alone: severe villi atrophy and fusion, and loss of the apical enterocytes. HE, bar 50 µm. (F) FB1 alone: severe loss of apical enterocytes. HE, bar 50 µm. (G) DON 10 µM + FB1: severe villi fusion. HE, bar 50 µm. (H) DON 10 µM + FB1: flattened enterocytes (arrow), villi atrophy and loss of enterocytes. HE, bar 100 µm. (I) DON 10 µM + IP6 5 mM: histological aspects similar to the control group. HE, bar 100 µm. (J) FB1 + IP6 5 mM: histological aspects similar to the control group. HE, bar 100 µm. (K) DON 10 µM plus FB1 + IP6 5 mM: histological aspects similar to the control group. HE, bar 100 µm.
). Mean values with different superscript letters were significantly different (p ≤ 0.05). (C) Control treatment. Hematoxylin-eosin (HE), bar 100 µm. (D) IP6 5 mM treatment. HE, bar 100 µm. (E) DON 10 µM alone: severe villi atrophy and fusion, and loss of the apical enterocytes. HE, bar 50 µm. (F) FB1 alone: severe loss of apical enterocytes. HE, bar 50 µm. (G) DON 10 µM + FB1: severe villi fusion. HE, bar 50 µm. (H) DON 10 µM + FB1: flattened enterocytes (arrow), villi atrophy and loss of enterocytes. HE, bar 100 µm. (I) DON 10 µM + IP6 5 mM: histological aspects similar to the control group. HE, bar 100 µm. (J) FB1 + IP6 5 mM: histological aspects similar to the control group. HE, bar 100 µm. (K) DON 10 µM plus FB1 + IP6 5 mM: histological aspects similar to the control group. HE, bar 100 µm.
Alternatively, when explants were pre-treated with IP6, the histological scores exhibited a significant increase when compared with explants exposed to DON or FB1 alone, or DON and FB1 in association (Figure 1A). Explants subjected to IP6 alone presented a significant improvement (p ≤ 0.05) of 24.2% in the morphological score when compared to the control (Figure 1A,C,D). In addition, explants exposed to IP6 plus mycotoxins exhibited increases of 42.7% (DON + IP6), 28.6% (FB1 + IP6), and 40.4% (DON + FB1 + IP6) compared to the respective treatments with the mycotoxins alone (p ≤ 0.05). The main histological lesions observed in the explants subjected to IP6 pre-treatment were cytoplasmic vacuolation and interstitial edema. The jejunal explants subjected to DON + IP6 or FB1 + IP6 showed lesional scores similar to the control treatment (p ≤ 0.05) (Figure 1A).
The jejunal explants exposed to mycotoxins exhibited a significant decrease (p ≤ 0.05) in villi height compared with the control (DON—40.4%, FB1—30%, and DON + FB1—45.4%). However, explants exposed to mycotoxins and treated with IP6 exhibited a significant increase (p ≤ 0.05) of 54.6% compared to DON alone, 33% compared to FB1 alone, and 47% compared to DON + FB1 (Figure 1B). The jejunal explants exposed to mycotoxins plus IP6 showed a villi height similar to the control explants (p ≤ 0.05) (Figure 1B,I–K).
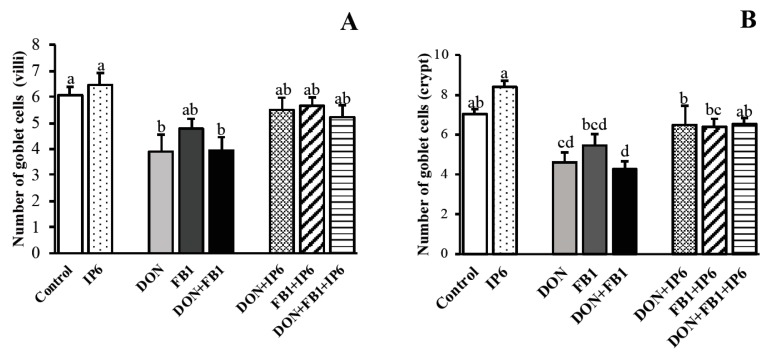
The number of goblet cells in the villi of explants exposed to DON alone and DON + FB1 decreased significantly (p ≤ 0.05) when compared to the control (35.6% and 35%, respectively) (Figure 2). In the crypt region, the number of goblet cells decreased in DON (34.4%) and DON + FB1 (39%) treatments (p ≤ 0.05). Explants exposed to DON + IP6 and DON + FB1 + IP6 showed a significant increase (p ≤ 0.05) in goblet cell density in the crypt region, compared to DON alone (40.7%) and DON + FB1 (53%).
Figure 2.
Mean goblet cell density on jejunal explants. (A) Number of goblet cells on the villi of jejunal explants. (B) Number of goblet cells on the crypts of jejunal explants. Explants exposed to control treatment (□); IP6 5 mM ( ); deoxynivalenol (DON) (
); deoxynivalenol (DON) ( ); fumonisin B1 (FB1) (
); fumonisin B1 (FB1) ( ); DON + FB1 (■); DON + IP6 (
); DON + FB1 (■); DON + IP6 ( ); FB1 + IP6 (
); FB1 + IP6 ( ); DON + FB1 + IP6 (
); DON + FB1 + IP6 ( ). Mean values with different superscript letters were significantly different (p ≤ 0.05).
). Mean values with different superscript letters were significantly different (p ≤ 0.05).
The morphological evaluation showed that the pre-treatment with IP6 in the jejunal explants exposed to mycotoxins resulted in an improvement in the histological score, villi height and goblet cell density. These explants remained histologically similar to the control group (p > 0.05) (Figure 1 and Figure 2).
2.2. Caspase-3, Ki-67, E-cadherin and cox-2 Expression
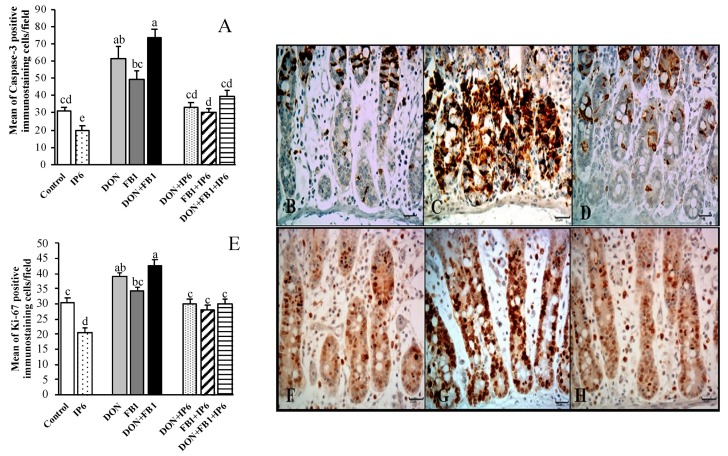
The expression of caspase-3 (Ccasp-3), an indicator of cell apoptosis, decreased 37.3% in the jejunal explants exposed to IP6 compared to the control treatment (p ≤ 0.05). A significant increase in Ccasp-3 expression was observed in explants exposed to DON (95.5%) and DON + FB1 (135.5%) when compared to the control group (p ≤ 0.05). In addition, an increase of 49.6% in cell apoptosis (p ≤ 0.05) was verified in the DON + FB1 group compared to FB1 alone. Nonetheless, a significant decrease in cell apoptosis was observed in DON + IP6 (46.8%), FB1 + IP6 (39%) and DON/FB1 + IP6 (46.8%) groups compared to the respective treatments with the mycotoxins alone (p ≤ 0.05) (Figure 3A–D).
Figure 3.
Effects of DON and FB1, alone or in association, and IP6 on apoptosis (Ccasp-3) and cell proliferation (Ki-67) in jejunal explants. Explants exposed to control treatment (□); IP6 5 mM ( ); deoxynivalenol (DON) (
); deoxynivalenol (DON) ( ); fumonisin B1 (FB1) (
); fumonisin B1 (FB1) ( ); DON + FB1 (■); DON + IP6 (
); DON + FB1 (■); DON + IP6 ( ); FB1 + IP6 (
); FB1 + IP6 ( ); DON + FB1 + IP6 (
); DON + FB1 + IP6 ( ). (A) Mean number of Ccasp-3 immunostained cells per field on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (B) Control treatment: mild Ccasp-3 cytoplasmic immunostaining in crypt cells. (C) DON 10 µM + FB1 70 µM: diffuse Ccasp-3 cytoplasmic immunostaining in crypt cells. (D) DON 10 µM + FB1 70 µM + IP6 5 mM: decrease in Ccasp-3 cytoplasmic immunostaining in crypt cells. (E) Mean number of Ki-67 immunostained cells per field on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (F) Control treatment: mild Ki-67 nuclear immunostaining in crypt cells. (G) DON 10 µM + FB1 70 µM: diffuse Ki-67 nuclear immunostaining in crypt cells. (H) DON 10 µM + FB1 70 µM + IP6 5 mM: decrease in Ki-67 nuclear immunostaining in crypt cells. (B–D; F–H: immunoperoxidase method, bar 25 µm).
). (A) Mean number of Ccasp-3 immunostained cells per field on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (B) Control treatment: mild Ccasp-3 cytoplasmic immunostaining in crypt cells. (C) DON 10 µM + FB1 70 µM: diffuse Ccasp-3 cytoplasmic immunostaining in crypt cells. (D) DON 10 µM + FB1 70 µM + IP6 5 mM: decrease in Ccasp-3 cytoplasmic immunostaining in crypt cells. (E) Mean number of Ki-67 immunostained cells per field on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (F) Control treatment: mild Ki-67 nuclear immunostaining in crypt cells. (G) DON 10 µM + FB1 70 µM: diffuse Ki-67 nuclear immunostaining in crypt cells. (H) DON 10 µM + FB1 70 µM + IP6 5 mM: decrease in Ki-67 nuclear immunostaining in crypt cells. (B–D; F–H: immunoperoxidase method, bar 25 µm).
Explants exposed to IP6 exhibited a decrease of 32.5% (p ≤ 0.05) in Ki-67 expression compared to the control samples. Meanwhile, an increase in cell proliferation (p ≤ 0.05) was observed in explants exposed to DON (28.4%) and DON + FB1 (40%) when compared to the control explants. Jejunal explants subjected to DON + FB1 treatment showed a significant increase (p ≤ 0.05) of 24.7% in cell proliferation compared to FB1 alone. The presence of IP6 induced a significant decrease (p ≤ 0.05) in the cell proliferation of the jejunal explants exposed to DON (30.3%) and DON + FB1 (29.6%), compared to explants exposed only to DON and DON + FB1 (Figure 3E–H).
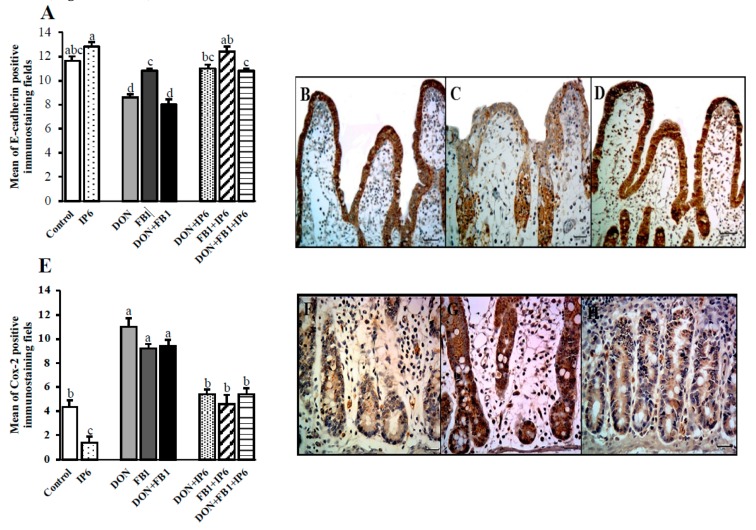
In general, exposure to DON, alone or in association with FB1, induced a significant reduction in E-cadherin expression (25.8% and 31%, respectively compared to the control; 20.3% and 26%, respectively compared to the FB1 treatment). However, when explants were pre-incubated with IP6 and subsequently exposed to the mycotoxins, an increase in E-cadherin immunostaining was observed compared to explants exposed to DON (28%), FB1 (15%), and DON + FB1 (35%) (p ≤ 0.05) alone (Figure 4A–D).
Figure 4.
Effects of DON and FB1, alone or in association, and IP6 on E-cadherin and cox-2 expression in jejunal explants. Explants exposed to control treatment (□); IP6 5 mM ( ); deoxynivalenol (DON) (
); deoxynivalenol (DON) ( ); fumonisin B1 (FB1) (
); fumonisin B1 (FB1) ( ); DON + FB1 (■); DON + IP6 (
); DON + FB1 (■); DON + IP6 ( ); FB1 + IP6 (
); FB1 + IP6 ( ); DON + FB1 + IP6 (
); DON + FB1 + IP6 ( ). (A) Mean of E-cadherin positive immunostaining per fields on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (B) Control treatment: strong and homogeneous E-cadherin immunostaining in epithelial cells. Bar 50 µm. (C) DON 10 µM: mild and non-homogeneous E-cadherin immunostaining in epithelial cells. Bar 25 µm. (D) DON 10 µM + IP6 5 mM: strong and homogeneous E-cadherin immunostaining in epithelial cells similar to control treatment. Bar 50 µm. (B–D: immunoperoxidase method). (E) Mean of cox-2 positive immunostaining per fields on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (F) Control treatment: mild cox-2 cytoplasmic immunostaining in crypt cells. (G) DON 10 µM: diffuse and strong cox-2 cytoplasmic immunostaining in crypt cells. (H) DON 10 µM + IP6 5 mM: decrease in cox-2 cytoplasmic immunostaining in crypt cells similar to control treatment. (F–H: immunoperoxidase method, bar 25 µm).
). (A) Mean of E-cadherin positive immunostaining per fields on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (B) Control treatment: strong and homogeneous E-cadherin immunostaining in epithelial cells. Bar 50 µm. (C) DON 10 µM: mild and non-homogeneous E-cadherin immunostaining in epithelial cells. Bar 25 µm. (D) DON 10 µM + IP6 5 mM: strong and homogeneous E-cadherin immunostaining in epithelial cells similar to control treatment. Bar 50 µm. (B–D: immunoperoxidase method). (E) Mean of cox-2 positive immunostaining per fields on explants exposed to different treatments. Mean values with different superscript letters were significantly different (p ≤ 0.05). (F) Control treatment: mild cox-2 cytoplasmic immunostaining in crypt cells. (G) DON 10 µM: diffuse and strong cox-2 cytoplasmic immunostaining in crypt cells. (H) DON 10 µM + IP6 5 mM: decrease in cox-2 cytoplasmic immunostaining in crypt cells similar to control treatment. (F–H: immunoperoxidase method, bar 25 µm).
The immunoexpression of cox-2 (parameter used to evaluate the inflammatory response) showed a decrease of 68% in the jejunum treated with IP6, compared with the control samples. In spite of this, explants subjected to the mycotoxins showed a significant increase in cox-2 expression (p ≤ 0.05) (150% for DON, 109% for FB1, and 113% for DON + FB1 compared to the control). The presence of IP6 induced a significant decrease (p ≤ 0.05) of 51%, 50%, and 45.2% in explants exposed to DON, FB1, and DON + FB1, respectively (Figure 4E–H).
Overall, explants exposed to mycotoxins and treated with IP6 presented Ccasp-3, Ki-67, E-cadherin, and cox-2 expression similar to the control explants, indicating a beneficial effect of this product in reducing the toxic effects of mycotoxins.
2.3. Oxidative Stress Evaluation
Jejunal exposure to DON and FB1 induced a significant increase in thiobarbituric acid reactive substance (TBARS) levels (62.8% and 54.2%, respectively), while exposure to IP6 resulted in decreased levels when compared to the control explants (40%). When the explants were exposed to IP6 prior to mycotoxins (DON, FB1, and DON+ FB1), the TBARS levels remained similar to those of control explants (Table 1).
Table 1.
Effects of FB1, DON, and IP6 on oxidative stress in jejunal explants of swine.
| Treatment | GSH (nmol/mg of Protein) |
TBARS (ΔOD A535 − A532/mg Protein) |
ABTS (nmol/Trolox Eq/mg of Protein) |
FRAP (nmol Trolox Eq/mg of Protein) |
|---|---|---|---|---|
| Control | 21.79 ± 0.49 a | 0.35 ± 0.04 bc | 60.70 ± 6.65 c | 60.23 ± 1.68 bcd |
| IP6 | 23.24 ± 2.39 a | 0.21 ± 0.02 c | 64.00 ± 8.19 bc | 57.14 ± 2.77 bcd |
| DON | 8.27 ± 2.25 b | 0.58 ± 0.05 a | 40.26 ± 5.93 d | 51.40 ± 2.90 d |
| DON + IP6 | 19.17 ± 2.37 a | 0.19 ± 0.02 c | 67.60 ± 4.19 abc | 66.15 ± 2.31 abc |
| FB1 | 10.50 ± 3.66 b | 0.54 ± 0.03 a | 39.14 ± 8.66 d | 58.99 ± 5.25 bcd |
| FB1 + IP6 | 21.56 ± 1.67 a | 0.22 ± 0.03 c | 81.00 ± 2.64 ab | 71.77 ± 1.91 a |
| DON + FB1 | 11.67 ± 1.64 b | 0.43 ± 0.06 ab | 41.83 ± 1.09 d | 55.77 ± 5.35 cd |
| DON + FB1 + IP6 | 19.26 ± 1.52 a | 0.23 ± 0.02 c | 85.75 ± 5.60 a | 67.71 ± 8.86 ab |
Mean values with their standard errors (n: 5 animals). Duncan’s test. Mean values with different superscript letters (column) were significantly different for each test (p ≤ 0.05). GSH—reduced glutathione; TBARS—thiobarbituric acid reactive substances; ABTS—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; FRAP—ferric-reducing antioxidant power.
The capacity to respond to oxidative stress was evaluated by determining the levels of reduced glutathione (GSH) and ferric-reducing antioxidant power (FRAP) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays. The jejunal explants exposed to mycotoxins showed a significant decrease in endogenous GSH levels compared to the control (DON 62%; FB1 51.8%; DON + FB1 46.4%) (Table 1). The presence of IP6 induced an increase in GSH levels of 131% and 105.5% compared to DON and FB1 alone; in the explants exposed to DON + FB1, IP6 increased the GSH content by 65.1%.
Explants exposed to mycotoxins presented a significant decrease in ABTS scavenging ability (DON 33.6%; FB1 35.5%; DON + FB1 31.1%) compared to the control explants, while pre-exposure to IP6 promoted a significant increase in ABTS scavenging ability in explants exposed to DON (67.9%), FB1 (107%), and DON + FB1 (105.4%) (Table 1). The ferric reducing ability was significantly increased in explants exposed to IP6 + FB1 (20.6%) and IP6 + DON + FB1 (21.4%), compared to explants exposed to these same mycotoxins (Table 1). Collectively, these data indicate that pre-treatment with IP6 inhibits mycotoxin-induced lipid peroxidation and reduction in cellular antioxidant capacity in jejunal explants.
3. Discussion
This study investigated the protective effect of IP6 on jejunal explants of pigs exposed to FB1 and DON. Intestinal morphological changes induced by these mycotoxins alone [12] or in combination [9] have been previously established, and were similar to those observed in the present study. The intestinal epithelial cell (IEC) changes, such as cytoplasmic vacuolation, flattening, necrosis, and loss of microvilli observed following exposure to mycotoxins were probably induced by an increase in cytoplasmic and mitochondrial permeability, which may be associated with ROS generation and resultant lipid peroxidation, as observed in vitro [6,7] and in vivo [4,5].
The reductions in the number of goblet cells and villi height, evidenced in DON and FB1 + DON groups, are probably linked to increased levels of apoptosis. This association is supported by increased caspase-3 immunostaining in these groups. DON induces apoptosis by direct lesion to the mitochondria, or by the extrinsic pathway of apoptosis through increased TNF-α as a consequence of intestinal inflammation [6]. Considering the concentration of DON used and the short period of incubation, the elevated apoptotic index observed may be associated with the activation of the intrinsic apoptosis pathway, i.e., oxidative stress and changes in mitochondrial membrane potential (MMP), deregulation of Bcl-2/Bax expression, release of cytochrome C, and consequent activation of caspase-3. In addition, it has been established that DON can modulate cell proliferation and apoptosis through activation of mitogen-activated protein kinases (MAPKs), mainly ERK1/2, JNK ½, and p38 [23,24,25,26]. This modulation in cell proliferation was observed in the present study. The explants exposed to DON alone and FB1 + DON showed discrete elevation of cell proliferation in the crypt region, which likely occurred as a tissue response to the IEC lesions and high apoptosis level induced by DON, and is evidenced by the increase in Ki-67 expression.
Alterations in cell junction proteins [10], trans-epithelial electrical resistance (TEER), and paracellular permeability [8] were associated with DON intestinal toxicity. In the present study, explants exposed to DON and FB1, alone or in combination, showed decreased E-cadherin expression compared to the control treatment. This reduction is possibly associated with oxidative stress and the morphological changes observed in the IEC, since DON-induced ROS inhibits protein synthesis [27] and MAPKs activation [8].
Besides deregulation of cell proliferation and apoptosis, oxidative stress can induce an overexpression of cox-2 [28]. Cox-2 is an enzyme involved in the metabolism of arachidonic acid, which is strongly induced by p38 MAPK activation and proinflammatory stimuli such as TNF-α, IL-1, IL-6, and IL-8 [28]. In previous studies in pigs, an upregulation in inflammatory cytokines [9,29] and an increase in the expression of cox-2 [12] was associated with DON and FB1 exposure. In the present study, the expression of cox-2 increased significantly in all mycotoxin treatments (DON, FB1, and DON + FB1) demonstrating its role as a biomarker of intestinal oxidative stress and inflammatory response in this model. Cox-2 itself can produce superoxide anions as a by-product of prostanoid synthesis [30]. Analysis for oxidative stress involves free radical generation (resulting in lipid peroxidation) and depletion of antioxidant compounds. In this study, mycotoxins, alone or in combination, affected both mechanisms. An increase in lipid peroxidation was observed mainly in the single agent treatments (~1.5-fold). On the other hand, the GSH, ABTS, and FRAP levels decreased after mycotoxin exposure (~2.2-, 1.5-, and 1.1-fold, respectively).
Reductions in superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and GSH levels have been reported in in vitro and in vivo studies after DON [29,31,32] and FB1 exposure [5,7]. DON toxicity is associated with ROS generation and secondary changes to the lysossomal membrane, and increase in mitochondrial membrane permeability with consequent deregulation of Bcl-2/Bax genes, release of cytochrome C, and activation of caspase-3 [6,33]. Similar to DON, oxidative stress induced by FB1 results in mitochondrial lesion and activation of caspase-3 [6,34,35]. In general, the effects of DON on intestinal epithelial cells were more evident than those of FB1, however, no significant difference was verified in the oxidative stress response. We hypothesize that explants exposed to FB1 present an oxidative stress response similar to DON. However, we also consider that the high cell apoptosis index in explants exposed to DON left less cells to suffer oxidative stress. Therefore, the real potential of oxidative stress induced by DON was masked, since several studies have demonstrated that DON rapidly induces ribotoxic stress and ROS generation, mainly affecting cells with a high cell division index, such as intestinal epithelial cells [36]. The oxidative stress induced by FB1 occurs indirectly via the intracellular accumulation of sphingolipids [29], a toxic mechanism with slower progression compared to DON’s toxic effect at the ribosomal level.
The presence of IP6 induced a protective effect on all histological parameters evaluated. This effect could be observed by the increased histological score, villi height, and goblet cell density compared to the jejunal explants exposed to the mycotoxins alone and associated treatments. The beneficial effects of IP6 are associated with its antioxidant capacity, mainly its ability to inhibit the Fenton reaction and formation of hydroxyl radicals [37]. The evaluation of cell proliferation, apoptosis, and E-cadherin expression suggests that IP6 modulates the toxic effects by decreasing ROS generation and, consequently, cell permeability and MAPKs activation, which results in the maintenance of protein synthesis. The reduction in mycotoxin-induced apoptosis by IP6 demonstrates its potent antioxidant effect, since ROS generation is directly linked to apoptosis activation. Similar modulation of cell viability has been observed in inflammatory bowel studies [19,38,39]. IP6 induced a significant reduction in cox-2 expression compared to the explants exposed to all mycotoxin treatments. The reduction in cox-2 expression observed in the present study can be associated with the ability of IP6 to inhibit ROS production, lipid peroxidation, and inflammatory stimuli [40] induced by DON and FB1. Moreover, studies have demonstrated that IP6 reduces the expression of cox-2 by inhibition of p38 MAPK, as well as the conversion of arachidonic acid into prostaglandins and suppression of β-catenin activity [19,40,41].
By the evaluation of oxidative stress, the presence of IP6 decreased the levels of TBARS in the explants subjected to DON and FB1 treatments by 66.6% and 48.8%, respectively. The presence of IP6 reduced TBARS level by 48.8% compared with the combined mycotoxin treatment. Furthermore, IP6 promoted a significant increase in GSH level and antioxidant capacity in the jejunal explants exposed to mycotoxins. In agreement, the ability of IP6 to protect cells against oxidative stress has been associated with the inhibition of ROS generation, increase of GSH level, CAT, GPx, and SOD content, and decrease of lipid peroxidation (MDA) in hepatocarcinogenesis studies in rats [42,43,44]. Nevertheless, data on the effects of IP6 on intestinal oxidative stress are still scarce. The present results demonstrate that the protective effect of IP6 on the intestinal oxidative stress is associated with its capacity to mitigate lipid peroxidation and increase the antioxidant capacity of the tissue.
The morphological changes and oxidative stress response were more evident in explants exposed to DON and DON + FB1 treatments, and were associated with the rapid and direct induction of oxidative stress by DON- compared to FB1-induced injury. Although FB1 and DON co-contamination of food and feed has been reported worldwide, the effects observed in mycotoxin multi-contaminations depend on the concentration, period of exposition, experimental model, and species susceptibility [45].
4. Conclusions
The mycotoxins DON and FB1, alone or in combination, induced changes in the morphology, cell proliferation, apoptosis, and expression of proteins associated with cell junctions and inflammation of the intestine. These toxic effects are associated with an oxidative stress response, including changes in lipid peroxidation and antioxidant capacity. However, more studies are necessary to elucidate the mechanisms and intracellular signaling pathways that trigger oxidative stress induced by these mycotoxins at the intestinal level. Phytic acid exerts beneficial effects upon the jejunum, modulating the changes induced by the mycotoxins and protecting cells against oxidative stress. In this context, IP6 antioxidant additives may represent an efficient approach in mitigating and preventing the intestinal toxic effects of DON and FB1.
5. Materials and Methods
5.1. Animals and Reagents (FB1, DON, and Phytic Acid)
Five 24-day-old crossbred (Landrace × Large White × Duroc) piglets (7.9 kg ± 0.72) were used in the present study. The purified DON (Molecular weight (MW): 296.32; Sigma–Aldrich, St. Louis, MO, USA) and FB1 (MW: 721.83; Cayman Chemical Company, Ann Arbor, MI, USA) were dissolved in ultrapure water at final concentrations of 10 μM for DON and 70 μM for FB1, and stored at 4 °C. The concentrations of FB1 (70 µM) and DON (10 µM) used are equivalent to 50.5 and 3 mg/kg of feed, respectively.
The phytic acid salt (MW: 819; Sigma–Aldrich, St. Louis, MO, USA) was dissolved in distilled water to the concentration of 15 mM, and the pH adjusted to 7.2. The solution was stored at −20 °C before dilution in the explant culture media. The IP6 (5 mM), DON (10 μM), and FB1 (70 μM) concentrations used were chosen according to previous studies [10,12,23].
5.2. Ex Vivo Experimental Model
The experimental procedures on animals were approved by the ethics commission (CEUA/UEL/Brazil-process n° 4173.2014.05, Date of approval: 09 March 2014). The piglets were euthanized (acepromazine 1%, sodium pentobarbital 40 mg/Kg and KCl 15%) and fragments of the jejunum (5 cm) were sampled, washed with a buffer solution, and opened longitudinally. For each treatment, six explants were sampled using an 8 mm punch, resulting in 48 explants per piglet. The explants were incubated in 6-well plates (three explants/well) at 37 °C in a chamber under CO2-controlled conditions with orbital shaking. The following treatments were applied: culture media [DMEM, (Gibco-BRL Life Technologies, Carlsbad, CA, USA) plus penicillin/streptomycin (1.25 µL/mL, Gibco-BRL Life Technologies, Carlsbad, CA, USA), gentamicin (10 µL/mL, Novafarma, São Paulo, SP, Brazil), fetal bovine serum (100 µL/mL, Invitrogen, São Paulo, SP, Brazil), and l-glutamine (0.4 µL/mL, Sigma Aldrich, St. Louis, MO, USA)] (A, B, C and D treatments), or culture media with IP6 5 mM (E, F, G, and H treatments). The mycotoxins were added to the wells after one hour: DON (10 µM) in the B, D, F, and H treatments, and FB1 (70 µM) in the C, D, G, and H treatments. After a total period of incubation of four hours, the explants (three from each treatment) were fixed in 10% neutral buffered formalin solution, dehydrated in alcohols and embedded in paraffin for histological and immunohistochemical evaluation. Three explants were immediately frozen in liquid nitrogen and posteriorly stored at −80 °C for the quantification of reduced glutathione (GSH), thiobarbituric acid reactive substances (TBARS), and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and ferric-reducing antioxidant power (FRAP) assays.
5.3. Histological and Immunohistochemical Assessment
Sections of 3 µm were stained with hematoxylin and eosin (H&E) for histopathological evaluation and scoring [12]. The microscopic changes and the frequency of the lesions were compared between the treatments as previously described [12]. The following morphological and lesional criteria were included in the score: flattening of enterocytes, villi atrophy and fusion, interstitial edema, lymphatic vessel dilation, loss of apical enterocytes, cell vacuolation, and necrotic debris. The lesional score was calculated by assessing the extent of each lesion (according to intensity or frequency observed, scored from 0 to 2; 0—diffuse, 1—moderate and 2—absent).
The measurement of the villi height was performed randomly in 10 villi per explant (100× magnification), using an image analysis system (MOTIC Image Plus Motic Instruments, Richmond, BC, Canada). Sections of the jejunum samples were subjected to periodic acid-Schiff (PAS) staining to evaluate the goblet cell density. The number of PAS stained goblet cells was counted randomly in 10 villi per explant and their respective bilateral crypts (200× magnification).
Sections from the same explants used for histopathological examination were subjected to immunohistochemical assay. Evaluations of apoptosis, cell proliferation, cell junction expression, and cox-2 expression were performed using antibodies against cleaved caspase-3 (Ccasp3) (anti-Asp 175, 1:200 dilution, Cell Signaling Technology, Beverly, MA, USA), Ki-67 (anti-7B11, 1:50 dilution, Zymed, Waltham, MA, USA), E-cadherin (anti-4A2C7, 1:50, Zymed, Waltham, MA, USA), and cox-2 (anti-CX-294, 1:100 dilution, Dako, Santa Clara, CA, USA), respectively. The protocols and positive and negative controls used were according to the manufacturer’s instructions.
A standard immunohistochemical procedure was performed. Briefly, an EDTA buffer heat-mediated (microwave oven, 750 W) antigen retrieval was used for Ki-67 and Ccasp3, and a citrate buffer for cox-2 and E-cadherin. Incubation of the sections with the primary antibody (overnight at 4 °C) was followed by incubation with the polymer secondary antibody (30 min) (Nichirei Biosciences, Tokyo, Japan), addition of the chromogen (3,3-diaminobenzidine, Invitrogen, São Paulo, SP, Brazil), and counterstaining with hematoxylin.
Immunostaining of the cytoplasm (Ccasp3) and nucleus (Ki-67) was used to assess the index of cell apoptosis (Ccasp3) and proliferation (Ki-67) in five random fields in the crypt region/explant (400× magnification). In addition, E-cadherin expression in villi enterocytes was evaluated in five fields (200× magnification). Only enterocytes showing strong, homogeneous basolateral membrane staining were considered positive. The expression of cox-2 was evaluated in the crypt region in five fields (200× magnification). Positive fields were considered when 50% or more of the cells were immunostained. The total number of fields evaluated in the three explants per treatment/animal was 15.
5.4. GSH Levels Measurement
GSH levels were determined spectrophotometrically by an adapted method described previously [46]. The frozen intestinal samples were homogenized using Tissue Tearor (Bjospec, São Paulo, SP, Brazil) in cold EDTA buffer (0.02 M). The homogenate was treated with 50% trichloroacetic acid (50% w/v) and centrifuged (1500× g for 15 min), and the supernatant was mixed with 0.4 M Tris-HCl solution (pH 8.9) and 10 mM dithiobisnitrobenzoic acid. Posteriorly, the samples were allowed to stand for 5 min before being read at 412 nm (Multiskan GO Microplate Spectrophotometer, ThermoScientific, Vantaa, Finland). A standard curve was prepared using different concentrations of GSH, in addition to the other reagents mentioned before. Results were presented as nmol GSH/mg of protein.
5.5. Lipid Peroxidation Measurement (TBARS)
Lipid peroxidation in the jejunum explants was assessed by determining TBARS levels, using an adapted method described by Guedes et al. [47]. For this assay, trichloroacetic acid (10%) was added to the homogenate to precipitate proteins, followed by centrifugation (1000× g, 3 min, 4 °C). The protein-free supernatant was separated and mixed with thiobarbituric acid (0.67%). The mixture was kept in a water bath (15 min, 100 °C). Malondialdehyde (MDA), an intermediate product of lipid peroxidation, was determined by the difference between absorbance at 535 and 572 nm using a microplate spectrophotometer reader. The results were presented as TBARS (nmol MDA/mg of protein).
5.6. ABTS and FRAP Assays
The ability of intestinal explants in the different treatments to resist oxidative damage was determined by the capacity to reduce 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate radical cation; ABTS+) (ABTS assay) and ferric-reducing antioxidant power (FRAP) assay. The frozen jejunal samples collected were homogenized in ice-cold 1.15% KCl buffer solution. Samples were centrifuged (200× g for 10 min at 4 °C) and the supernatants were used in both assays (ABTS and FRAP), according to Katalinic et al. [48]. The diluted ABTS solution (200 µL) was mixed with 10 µL of sample in each well. After 6 min of incubation at 25 °C, the absorbance was measured at 730 nm (Multiskan GO Microplate Spectrophotometer, ThermoScientific, Vantaa, Finland). For the FRAP assay, the supernatants (10 μL) were mixed with the prepared FRAP reagent (150 µL). The reaction mixture was incubated at 37 °C for 30 min, and the absorbance was measured at 595 nm. The results from ABTS and FRAP assays were equated using a standard Trolox curve (0.02–20 nmol). Considering these are Trolox-equivalent antioxidant capacity (TEAC) assays, results were presented as μmol Trolox-equivalent/mg of protein.
5.7. Statistical Analysis
The data (mean ± standard error) were analyzed using the free software Action 2.3 (Campinas, SP, Brazil). The lesional score, intestinal morphometry, goblet cells density, Ki-67, Ccasp3, E-cadherin, and cox-2 positive cells were compared by one-way analysis of variance (ANOVA), followed by Tukey’s test for multiple comparisons (p values ≤ 0.05). The effect of treatments on the oxidative stress response (GSH, TBARS, ABTS, and FRAP assays) was analyzed by ANOVA, followed by Duncan’s test (p values ≤ 0.05).
Acknowledgments
E.O.d.S. was supported by a fellowship from Brazil’s foundation CAPES. W.A.V.J. and A.P.F.R.L.B received a fellowship from Brazil’s foundation CNPq.
Author Contributions
Conceptualization, E.O.d.S., A.P.F.R.L.B.; Data curation, E.O.d.S., J.R.G., M.S.N.H., A.P.F.R.L.B.; Formal analysis, J.R.G., M.S.N.H., W.A.V.J.; Funding acquisition, A.P.F.R.L.B.; Investigation, E.O.d.S., J.R.G., M.S.N.H., A.P.F.R.L.B.; Methodology, E.O.d.S., J.R.G., M.S.N.H., W.A.V.J., A.P.F.R.L.B. Project administration, E.O.d.S., A.P.F.R.L.B.; Resources, E.O.d.S., J.R.G., M.S.N.H.; Supervision, W.A.V.J., A.P.F.R.L.B.; Validation, E.O.d.S., W.A.V.J., A.P.F.R.L.B.; Visualization, E.O.d.S., A.P.F.R.L.B.; Writing—original draft, E.O.d.S., A.P.F.R.L.B.; Writing—review & editing, E.O.d.S., W.A.V.J., A.P.F.R.L.B.
Funding
This research was supported by grants from Brazil’s foundation CNPq number 474691/2012-8.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
FB1 and DON induce oxidative stress, morphological, enterocyte proliferation, and apoptosis changes, and affect the expression of cell junction and inflammatory-associated proteins in the jejunum of pigs. Phytic acid modulates the morphological and immunohistochemical changes and the oxidative stress induced by FB1 and DON.
References
- 1.Escrivá L., Font G., Manyes L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015;78:185–206. doi: 10.1016/j.fct.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Adhikari M., Negi B., Kaushik N., Adhikari A., Al-Khedhairy A.A., Kaushik N.K., Choi E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget. 2017;8:33933–33952. doi: 10.18632/oncotarget.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alshannaq A., Yu J.-H. Occurrence, toxicity, and analysis of the major mycotoxins in food. Int. J. Environ. Res. Public Health. 2017;14:632. doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osselaere A., Santos R., Hautekiet V., Backer P.D., Chiers K., Ducatelle R., Croubels S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE. 2013;8:e69014. doi: 10.1371/journal.pone.0069014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbes S., Ben Salah-Abbes J., Jebali R., Younes R.B., Queslati R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J. Immunotoxicol. 2016;13:46–54. doi: 10.3109/1547691X.2014.997905. [DOI] [PubMed] [Google Scholar]
- 6.Li D., Ye Y., Lin S., Deng L., Fan X., Zhang Y., Deng X., Li Y., Haikuo M.H.Y. Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: Cellcycle arrest, oxidative stress, and apoptosis. Environ. Toxicol. Pharmacol. 2014;37:141–149. doi: 10.1016/j.etap.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Domijan A.M., Gajski G., Jovanovic I.N., Geric M., Garaj-Vrhovac V. In vitro genotoxicity of mycotoxin ochratoxin A and fumonisin B (1) could be prevented by sodium copper chlorophylin-implication to their genotoxic mechanism. Food Chem. 2015;170:455–462. doi: 10.1016/j.foodchem.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Pinton P., Braicu C., Nougayred J.-P., Laffitte J., Taranu I., Oswald I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen activated protein kinase-dependent mechanism. J. Nutr. 2010;140:1956–1962. doi: 10.3945/jn.110.123919. [DOI] [PubMed] [Google Scholar]
- 9.Bracarense A.P.F.R.L., Lucioli J., Grenier B., Pacheco G.D., Molls W.-D., Schtzmayr G., Oswald I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012;107:1776–1786. doi: 10.1017/S0007114511004946. [DOI] [PubMed] [Google Scholar]
- 10.Basso K., Gomes F., Bracarense A.P. Deoxynivanelol and fumonisin, alone or in combination, induce changes on intestinal junction complexes and in E-cadherin expression. Toxins. 2013;5:2341–2352. doi: 10.3390/toxins5122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan L.Y., Woo C.S., Turner P.C., Wan J.M., El-Nezanmi H. Individual and combined effects of Fusarium toxins on the mRNA expression of pro-inflammatory cytokines in swine jejunal epithelial cells. Toxicol Lett. 2013;220:238–246. doi: 10.1016/j.toxlet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Silva E.O., Gerez J.R., Drape T.C., Bracarense A.F.R.L. Phytic acid decreases deoxynivalenol and fumonisin B1-induced changes on swine jejunal explants. Toxicol. Rep. 2014;1:284–292. doi: 10.1016/j.toxrep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva E.O., Bracarense A.P.F.R.L. Phytic acid: From antinutritional to multiple protection of organic systems. J. Food Sci. 2016;81:R135–R1362. doi: 10.1111/1750-3841.13320. [DOI] [PubMed] [Google Scholar]
- 14.Onomi S., Okazak Y., Katayama T. Effect of dietary level of phytic acid on heptic and serum lipid status in rats fed a high-sucrose diet. Biosci. Biotecnol. Biochem. 2004;68:1379–1381. doi: 10.1271/bbb.68.1379. [DOI] [PubMed] [Google Scholar]
- 15.Kapral M., Wawszyk J., Jurzak M., Hollek A., Weglarz L. The effect of inositol hexaphosphato on the expression of selected metalloproteinase and their tissue inhibitors in Il-1β-stimulated colon. Int. J. Colorectal Dis. 2012;27:1419–1428. doi: 10.1007/s00384-012-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuster J.M.B., Cortes S.P., Bestard J.P., Freixedas F. Plant phosphates, phytate and pathological calcifications in chronic kidney disease. Nefrologia. 2017;37:20–28. doi: 10.1016/j.nefro.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Cholewa K., Parfiniewicz B., Bednarek I., Swiatkowska L., Jezienicka E., Kierot J., Weglarz L. The influence of phytic acid on TNF-α and its receptors genes expression in colon cancer caco-2 cells. Acta Polon. Pharm.-Drug Res. 2008;65:75–79. [PubMed] [Google Scholar]
- 18.Saad N., Esa N.M., Ithnin H. Suppression of β-catenin and cyclooxygenase-2 expression and cell proliferation in azoxymethane-induced colonic cancer in rats by rice bran phytic acid (PA) Asian Pac. J. Cancer Prev. 2013;14:3093–3099. doi: 10.7314/APJCP.2013.14.5.3093. [DOI] [PubMed] [Google Scholar]
- 19.Kapral M., Wawszczyk J., Sośnicki S., Węglarz L. Down-regulation of inducible nitric oxide synthase expression by inositol hexaphosphate in human colon cancer cells. Acta Pol. Pharm. 2015;72:705–711. [PubMed] [Google Scholar]
- 20.Schlemmer U., Jany K.D., Berk A., Schulz E., Rechkemmer G. Degradation of phytate in the gut of pigs-pathway of gastrointestinal inositol phosphate hydrolysis and enzyme involved. Arch. Anim. Nutr. 2001;55:255–280. doi: 10.1080/17450390109386197. [DOI] [PubMed] [Google Scholar]
- 21.Silva E.O., Bracarense A.P.F.R.L., Oswald I.P. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018;11:113–133. doi: 10.3920/WMJ2017.2267. [DOI] [Google Scholar]
- 22.Pacheco G.D., Silva C.A., Pinton P., Oswald I.P., Bracarense A.P. Phytic acid protects porcine intestinal epithelial cells from deoxynivalenol (DON) cytotoxicity. Exp. Toxicol. Pathol. 2012;64:345–347. doi: 10.1016/j.etp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Lucioli J., Pinton P., Patrick C., Laffitte J., Grosjean F., Kolf-Clauw M., Oswald I.P., Bracarense A.P.F.R.L. he food contaminant deoxynivlanol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon. 2013;66:31–36. doi: 10.1016/j.toxicon.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Yang G.-H., Jarvis B.B., Vhung Y.-J., Pestka J.J. Apoptosis induction by the Satratoxins and other trichothecene mycotoxin: Relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000;164:149–160. doi: 10.1006/taap.1999.8888. [DOI] [PubMed] [Google Scholar]
- 25.Pestka J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. Part A. 2008;25:1123–1140. doi: 10.1080/02652030802056626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strasser A., Carra M., Ghareeb K., Awad W., Bohm J. Protective effects of antioxidants on deoxynivalenol-induced damage in murine lymphoma cells. Mycotoxin Res. 2013;29:203–208. doi: 10.1007/s12550-013-0170-2. [DOI] [PubMed] [Google Scholar]
- 27.Reuter S., Gupta S.C., Vhaturvedi M.M., Agarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuenda A., Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Grenier B., Bracarense A.P.F.L., Schwartz H.E., Trumel C., Cossalter A.M., Schatzmayr G., Oswald I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012;83:1465–1473. doi: 10.1016/j.bcp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Beak S.M., Lee Y.S., Kim J.A. NADPH oxidase and cyclooxygenase mediate the ultraviolet B-induced generation of reactiveoxygen species and activation of nuclear factor-kappaB in HaCaT human keratinocytes. Biochimie. 2004;86:425–429. doi: 10.1016/j.biochi.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Hou Y.J., Zhao Y.Y., Xiong B., Cui X.S., Kim N.H., Xu Y.X., Sun S.C. Mycotoxin-containing diet causes oxidative stress in the mouse. PLoS ONE. 2013;8:e60374. doi: 10.1371/journal.pone.0060374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zbynovska K., Petruška P., Capcarova M. Effect of deoxynivalenol on some haematological, biochemical and antioxidant parameters of porcine blood in vitro. J. Microbiol. Biotechnol. Food Sci. 2013;2:1611–1628. [Google Scholar]
- 33.Sun L.H., Lei M., Zhang N.Y., Gao X., Li C., Krumm C.S., Qi D.S. Individual and combined cytotxic effects of aflotoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL3A rat liver cell. Toxicon. 2015;96:6–12. doi: 10.1016/j.toxicon.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Wu Q., Wan D., Liu Q., Chen D., Liu Z., Matinez-Larranaga M.R., Martinez M.A., Anadon A., Yuan Z. Fumonisins: Oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch. Toxicol. 2016;90:81–101. doi: 10.1007/s00204-015-1604-8. [DOI] [PubMed] [Google Scholar]
- 35.Mary V.S., Theumer M.G., Otaiza S.N., Velez P.A., Rubinstein H.R., Theumer M.G. The aflatoxina B1-fumonisin B1 toxicity in BRL-3A Hepatocytes is associated to induction of cytochrome P450 activity and arachidonic acid metabolism. Environ. Toxicol. 2017;32:1711–1724. doi: 10.1002/tox.22395. [DOI] [PubMed] [Google Scholar]
- 36.Pestka J.J., Smolinski A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 37.Graf E., Eaton J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990;8:61–69. doi: 10.1016/0891-5849(90)90146-A. [DOI] [PubMed] [Google Scholar]
- 38.Challa A., Rao D.R., Reddy B. Interactive suppression of aberrant crypt foci induced by azoxymethene in rat coln by phytic acid and green tea. Carcinogenesis. 1997;18:2023–2026. doi: 10.1093/carcin/18.10.2023. [DOI] [PubMed] [Google Scholar]
- 39.Jenab P.M., Thompson L.U. Phytic acid in wheat bran affects colon morphology, cell proliferation and apopstosis. Carcinogenesis. 2000;21:1547–1552. doi: 10.1093/carcin/21.8.1547. [DOI] [PubMed] [Google Scholar]
- 40.Norazalina S., Norhaizan M.E., Hairuszah I., Norashareena M.S. Anticarcinogeninc efficacy of Phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp. Toxicol. Pathol. 2010;62:259–268. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Shafie N.H., Esa N.M., Ithnin H., Akim A.M., Saad N., Pandurangan A.K. Preventive inositol hxaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/β-catenin and cox-2 pathways. Biomed. Res. Int. 2013;2013:681027. doi: 10.1155/2013/681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H.J., Lee S.A., Choi H. Dietary administration of inositol and/or inositol-6-phosphate prevents chemically-induced rat hepatocarcinogenesis. Asian Pac. J. Cancer Prev. 2005;6:41–47. [PubMed] [Google Scholar]
- 43.Abdel-Hamid N.M., Faddah L.M., Al-Rehany M.A., Ali A.H., Bakeet A.A. New role of antinutritional factors, phytic acid and catechin in the treatment of CCl4 intoxication. Ann. Hepatol. 2007;6:262–266. [PubMed] [Google Scholar]
- 44.Foster S.R., Dilworth L.L., Thompson R.K., Alexander-Lindo R.L., Omoruyi F.O. Effects of combined inositol hexakisphosphate and inositol supplement on antioxidant activity and metabolic enzymes in the liver of streptozotocin-induced type 2 diabetic rats. Chem. Biol. Interact. 2017;275:108–115. doi: 10.1016/j.cbi.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Grenier B., Oswald I.P. Mycotoxin co-contamination of foods and feeds: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4:285–313. doi: 10.3920/WMJ2011.1281. [DOI] [Google Scholar]
- 46.Sedlak J., Lindsay R. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagente. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 47.Guedes R.P., Dal Bosco L., Teixeira C.M., Llesuy S., Belló-Klein A., Ribeiro M.F., Partata W.A. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem. Res. 2006;31:603–609. doi: 10.1007/s11064-006-9058-2. [DOI] [PubMed] [Google Scholar]
- 48.Katalinic V., Modun D., Music I., Boban M. Gender differences in antioxidant capacity of rat tissues determined by 2,2’-azinobis (3-ethylbenzothialoine 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015;140:47–52. doi: 10.1016/j.cca.2005.01.005. [DOI] [PubMed] [Google Scholar]