Abstract
New analogs of nortopsentin, a natural 2,4-bis(3′-indolyl)imidazole alkaloid, in which the central imidazole ring of the natural lead was replaced by a 1,2,4-oxadiazole moiety, and in which a 7-azaindole portion substituted the original indole moiety, were efficiently synthesized. Among all derivatives, prescreened against the HCT-116 colon rectal carcinoma cell line, the two most active compounds were selected and further investigated in different human tumor cells showing IC50 values in the micromolar and submicromolar range. Flow cytometric analysis of propidium iodide-stained MCF-7 cells demonstrated that both the active derivatives caused cell cycle arrest in the G0–G1 phase. The cell death mechanism induced by the compounds was considered to be apoptotic by measuring the exposure of phosphatidylserine to the outer membrane and observed morphological evaluation using acridine orange/ethidium bromide double staining. Moreover, further tested on intestinal normal-like differentiated Caco-2 cell line, they exhibited preferential toxicity towards cancer cells.
Keywords: marine alkaloids; nortopsentin analogs; 1,2,4-oxadiazole derivatives; anti-cancer agents; antiproliferative activity
1. Introduction
Natural products (NP) constitute a significant source of bioactive molecules and potential drug leads due to their high chemical diversity, biochemical specificity, binding efficiency with biological targets, and broad panel of bioactivities. About 60% of drugs currently on the market are of natural origin and natural products screening still plays a fundamental role in the drug discovery process [1]. Marine natural products (MNP), in particular marine sponge-derived compounds, have attracted considerable attention due to their unique biodiversity and structural differences as compared to terrestrial natural products. Among MNP or marine-derived molecules, eight compounds are currently on the market with application in different therapeutic areas and several compounds are in different phases of the clinical pipeline, showing promising anticancer activity. Only a few of them are original MNPs, while the majority are derivatives obtained through molecular lead optimization [2].
Considering the successful results obtained using MNP as leads for drug discovery and the growing number of marine-derived molecules entering into clinical trials, researchers are still inspired by their scaffold for the design of new active molecules.
Among MNPs used as lead compounds for the synthesis of new anticancer agents, nortopsentin, an alkaloid isolated from deep-sea sponge Spongsorites ruetzleri having a characteristic 2,4-bis(3′-indolyl)imidazole skeleton, attracted remarkable attention due to its significant antiproliferative activity against the P388 murine leukemia cell line [3]. Many derivatives in which the central imidazole ring was replaced by several five-membered heterocycles were reported, most of them showing antiproliferative activity, often reaching GI50 values in the low micromolar range or even at the sub-micromolar level [3,4,5,6,7,8,9,10,11,12]. The thiazole nortopsentin analogs in particular, in which the structural modification of the lead natural molecule also involved one or both indole portions, led to potent compounds with effect against a wide range of cell lines, including diffuse malignant peritoneal mesothelioma (DMPM), a fatal disease, poorly responsive to conventional therapies, and acted as CDK1 inhibitors [13,14,15,16]. Moreover, the most active thiazole derivatives also bearing a 7-azaindole substitution, in the mouse model, by intraperitoneal administration were effective with a significant reduction of the DMPM and two complete responses at well-tolerated doses [13].
On the other hand, the 1,2,4-oxadiazole ring system is a five-membered heterocycle ring found in many molecules with significant biological activity, especially antitumor [17,18,19], despite its uncommon presence in MNP. To the best of our knowledge, phidianidines, isolated from the marine opisthobranch mollusk Phidiana militaris, are a unique example of MNP possessing a 1,2,4-oxadiazole ring system in their structure, and together with their derivatives they exhibit significant cytotoxic, DAT inhibitory, or neuroprotective activities [20,21,22]. Moreover, the 1,2,4 oxadiazole ring is a bioisoster of amides and esters and for this reason could improve bioavailability and physiochemical properties of compounds bearing it.
Continuing our search for new anticancer compounds [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], herein we report the synthesis of a new 1-methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridine nortopsentin analog 1, designed on the basis of the potent activity shown by thiazole nortopsentin analogs with a 7-azaindole portion and considering the important characteristics of the 1,2,4-oxadiazole ring found in compounds with promising biological activity. The new compounds, in which the central imidazole ring of the natural lead was replaced by a 1,2,4-oxadiazole moiety, and in which a 7-azaindole portion substituted the original indole, were prescreened against the HCT-116 colon rectal carcinoma cell line and the two most active compounds were selected and further investigated in different human tumor cell lines.
2. Results and Discussion
2.1. Chemistry
1-Methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridines 1 were synthesized by reaction between two different key intermediates, N′-hydroxy-1-methyl-1H-indole-3-carboximidamides 2 and methyl-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylates 3 (Scheme 1, Table 1).
Scheme 1.
Synthesis of 1-methyl-3- [3-(1-methyl-1H-indol-3-yl)- 1,2,4-oxadiazol-5-yl]-1H-pyrrolo [2,3-b] pyridines 1a–o. Reagents and conditions: (i) (a) CSI, MeCN, 0 °C, 2 h; (b) DMF, 0 °C, 1 h, 90–98%; (ii) NH2NH2 HCl, DIPEA, EtOH, reflux, 4 h, 65–82%; (iii) trichloroacetyl chloride, AlCl3, DCM, 6 h, 80–99%; (iv) KOH 20%, rt, 90–99%; (v) NaH, EtOH, reflux, 4–6 h, 55–85%.
Table 1.
New 1,2,4-oxadiazole derivatives 1a–o.
| Compound | R1 | R2 | Yield (%) |
|---|---|---|---|
| 1a | H | H | 75 |
| 1b | H | Br | 72 |
| 1c | H | F | 62 |
| 1d | Br | H | 75 |
| 1e | Br | Br | 82 |
| 1f | Br | F | 75 |
| 1g | F | H | 72 |
| 1h | F | Br | 60 |
| 1i | F | F | 55 |
| 1j | Cl | H | 65 |
| 1k | Cl | Br | 75 |
| 1l | Cl | F | 85 |
| 1m | OCH3 | H | 70 |
| 1n | OCH3 | Br | 65 |
| 1o | OCH3 | F | 80 |
N′-hydroxy-1-methyl-1H-indole-3-carboximidamides 2a–e, commercially unavailable, were synthesized from the corresponding 1-methyl-indoles of type 4, prepared as previously reported [16]. The reaction of compounds 4a–e with chlorosulfonyl isocyanate (CSI) in acetonitrile, followed by the addition of N,N-dimethylformamide (DMF), led to the corresponding carbonitriles 5a–e (90–98%), easily converted (65–82%) to the corresponding carboximidamides 2a–e through reaction with hydroxylamine hydrochloride in ethanol (EtOH), in the presence of diisopripylenethylamide (DIPEA).
Methyl-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylates 3a–c were in turn prepared from their 1-methyl-7-azaindoles 6a–c [10,16] converted into the corresponding 2,2,2-trichloro-1-(1-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanones 7a–c (80–99%) by reaction with trichloroacetyl chloride in dichloromethane (DCM) in the presence of aluminum chloride. Once obtained, compounds 7 were subjected to basic hydrolysis with potassium hydroxide solution (KOH 20%) to afford the desired derivatives 3 (90–99%).
Finally, the carboximidamides 2a–e and the carboxylates 3a–c were reacted in the presence of sodium hydride in tetrahydrofuran (THF) to afford the final compounds 1a–o (55–85%).
2.2. Biology
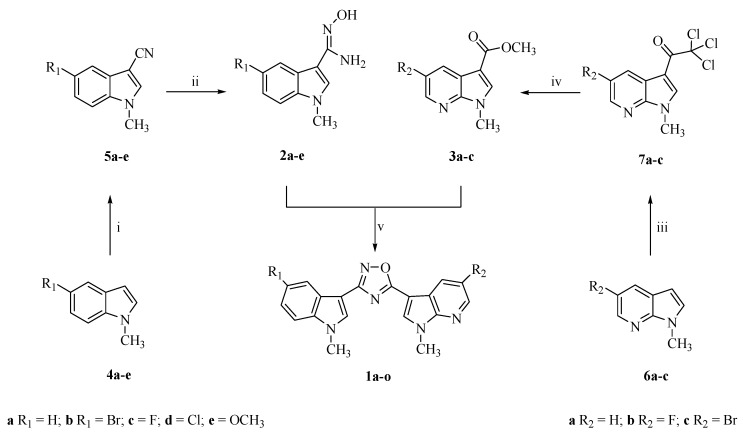
In vitro cytotoxicity of the synthesized compounds 1a–o was prescreened against the HCT-116 colon rectal carcinoma cell line. Monolayer cultures treated for 72 h with 10−8–10−4 µM concentrations of the compounds were examined by MTT assay for the cell viability.
Among the synthesized nortopsentin analogs, the compounds bearing the 5-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridine moiety (see compounds 1k and 1n) showed the highest cytotoxic activity (Figure 1B). Substitution of the bromine atom in this portion with a fluorine (see compounds 1o and 1l) or absence of the halogen atom (see compounds 1j and 1m) resulted in a drop of the antiproliferative effect of the derivatives. The remaining compounds appeared poorly effective.
Figure 1.
Effect of 1-methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b] pyridines 1a–o. on the growth of human tumor cells HCT-116. (A) Compounds bearing the 1-methyl-1H-pyrrolo[2,3-b]pyridine moiety; (B) compounds bearing the 5-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridine moiety; (C) compounds bearing the 5-fluoro-1-methyl-1H-pyrrolo[2,3-b]pyridine moiety. Cells were treated with the compounds and cell viability was measured after 72 h by MTT assay in comparison to cells treated with vehicle alone (control). Values are the mean ± SD of three separate experiments in triplicate.
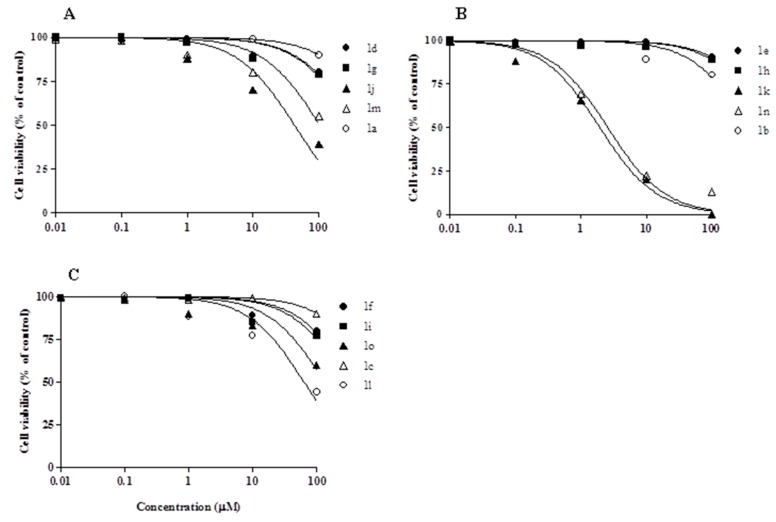
The most active derivatives 1k and 1n were further investigated against MCF-7 (human breast cancer), HeLa (cervix adenocarcinoma), and CaCo2 (colorectal carcinoma) cell lines (Figure 2), and IC50 values in the micromolar and submicromolar range were calculated (Table 2). Compared to 1n, 1k derivative, with the exception of HeLa cell line, appeared 2–4-fold more effective.
Figure 2.
Effect of compounds 1k (left) and 1n (right) on the viability of different human tumor cell lines. Cells were treated with the compounds and cell survival was measured after 72 h by MTT assay in comparison to cells treated with vehicle alone (control), as reported in Section 3.2. Values are the mean ± SD of three separate experiments carried out in triplicate.
Table 2.
Anti-proliferative activity of compounds 1k and 1n against different human cell lines (IC50).
| Compound | IC50 (µM) a | |||
|---|---|---|---|---|
| MCF-7 | HCT-116 | HeLa | CaCo2 | |
| 1k | 0.65 ± 0.05 | 1.93 ± 0.06 | 10.56 ± 0.98 | 1.06 ± 0.09 |
| 1n | 2.41 ± 0.23 | 3.55 ± 0.1 | 13.96 ± 1.41 | 3.33 ± 0.25 |
a IC50 value was calculated by plotting the percentage viability versus concentration on a logarithmic graph. Results are the mean values SD (standard deviation) of three separated experiment carried out in triplicate.
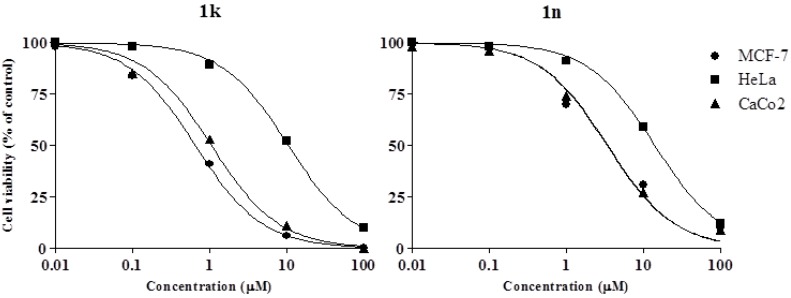
Further studies on the mechanism of the cytotoxic activity of the active compounds were carried out on MCF-7 cells, the tumor cell line more sensitive to both compounds. In order to investigate the effect on the cell cycle, flow cytometric analysis on PI stained MCF-7 cells was carried out after 24 h treatment with compounds 1k and 1n at their relevant IC50 values. As shown in Figure 3A, both the synthesized derivatives caused an accumulation of treated cells in subG0/G1 phase and induced their marked arrest (more than 60%) in G0–G1 phase of the cycle. Although changes in distribution within mitotic phases cannot provide precise information on the mechanism of drug’s activity, these findings suggest that compounds 1k and 1n can affect the cell machinery promoting DNA duplication.
Figure 3.
Effect of compounds 1k and 1n on cell cycle distribution (A) and PS externalization of MCF-7 cells (B). Cell monolayers were incubated for 24 h in the absence (control) or in the presence of individual compounds and submitted to flow cytometric analysis after propidium iodide (A) or AnnexinV/PI double staining (B) as reported in 3.2 Paragraph. (A) Percentage of viable cells in the different phases are given in each picture. Values are the mean ± SD of three separate experiments in triplicate. Representative images of three experiments with comparable results; (B) BU3, viable cells (AnnexinV−/PI−); BU4, cells in early apoptosis (AnnexinV+/PI−); BU2, cells in tardive apoptosis (AnnexinV+/PI+); BU1, necrotic cells (AnnexinV−/PI+). Representative images of three experiments with comparable results.
In order to assess the cell death mechanism, apoptosis induction caused by compounds 1k and 1n in MCF7 cells, after 24 h treatment, was investigated by means of Annexin V/PI dual staining method followed by cytofluorimetric analysis. The biparametric analysis showed that both the compounds induced early apoptosis without causing death by necrosis (Figure 3B).
It is of interest to emphasize that other previously synthesized nortopsentin analogs bearing a 7-azaindole substitution and in which a thiazole ring substituted the central ring of the lead compound, showed cytotoxic activity towards MCF-7 cells associated with mitotic failure and accumulation of cells in phase G2/M [12,16]. The substitution of the central ring with a 1,2,4 oxadiazole one provides molecules of different biological activity that deserves to be investigated.
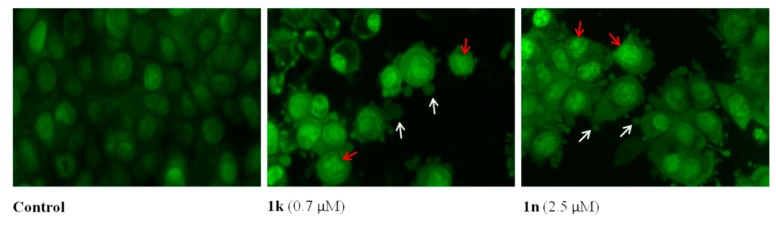
To further verify the pro-apoptotic effects of the synthesized derivatives, AO/EB staining was also used. Morphological evaluation of the cells using AO and EB double staining allows to detect early apoptotic cells stained green with yellow dots, with blebbing cytoplasm, while late apoptotic cells stain orange with fragmented nuclei. Non-apoptotic cells stain green. As shown in Figure 4, MCF-7 cells treated for 24 h with 1k or 1n showed morphological changes typical for early apoptosis with condensation of nuclear material and formation of membrane blebbing.
Figure 4.
Apoptosis by compounds 1k and 1n in MCF-7 cells assessed by acridine orange/ethidium bromide (AO/EB) staining. MCF-7 cells were treated with the compounds for 24 h and compared with the untreated cells. Fluorescence micrographs represent one of three independent experiments. Red arrow: chromatin condensation; white arrow: membrane blebbing.
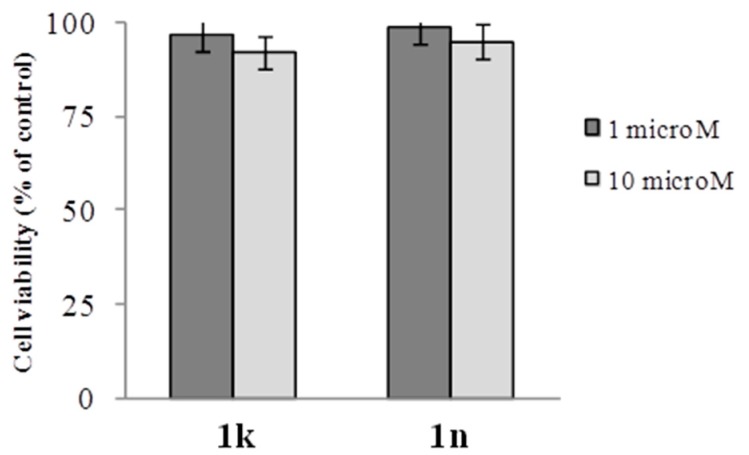
Additional experiments were conducted on intestinal normal-like differentiated Caco-2 cells. As shown in Figure 5, at the concentrations effective to inhibit the growth of tumor cells, the compounds 1k and 1n did not affect to a large extent the viability of the normal-like cells showing selectivity towards cancer cells (Figure 4).
Figure 5.
Effect of compounds 1k and 1n on the viability of intestinal normal-like differentiated Caco-2 cells. Fifteen-days post-confluence monolayers of Caco-2 were incubated for 24 h in the absence (control) or in the presence of the compounds at the indicated concentrations and cell viability was assessed by MTT test as reported in Section 3.2. Values are the mean ± SD of two separate experiments carried out in triplicate.
3. Materials and Methods
3.1. Chemistry
3.1.1. General
All melting points were taken on a Büchi-Tottoly capillary apparatus (Büchi, Cornaredo, Italy) and are uncorrected. IR spectra were determined in bromoform with a Shimadzu FT/IR 8400S spectrophotometer (Shimadzu Corporation, Milan, Italy). 1H and 13C NMR spectra were measured at 200 and 50.0 MHz, respectively, in DMSO-d6 solution, using a Bruker Avance II series 200 MHz spectrometer (Bruker, Milan, Italy). Column chromatography was performed with Merck silica gel 230–400 mesh ASTM or with a Büchi Sepacor chromatography module (prepacked cartridge system). Elemental analyses (C, H, N) were within ± 0.4% of theoretical values and were performed with a VARIO EL III elemental analyzer (Elementar, Langenselbold, Germany). Purity of all the tested compounds was greater than 95%, determined by HPLC (Agilent 1100 Series). Mass spectra of final compounds were performed using a Mariner™ mass spectrometer, Applied Biosystems (Foster City, CA, USA). A Harvard model 11 syringe pump (Holliston, MA, USA) was used to infuse the sample solutions. The ESI source was operated in positive ion mode with an electrospray voltage of 4.5 kV. Compounds 1a–o were characterized only by 1H NMR spectra due to their poor solubility.
General Procedure for the Synthesis of 1-methyl-1H-indole-3-carbonitriles (5a–e)
The appropriate indole 4a–e (1.8 mmol) solubilized in anhydrous acetonitrile (2.0 mL), was reacted with chlorosulfonyl isocyanate (CSI) (0.16 mL, 1.8 mmol, added dropwise at 0 °C. The resulting reaction mixture was stirred at 0 °C for 2 h after which anhydrous dimethylformamide (DMF) (1.0 mL, 106.3 mmol) was added dropwise. The mixture was stirred at 0 °C for 1 h and then it was poured into ice-water. The obtained precipitate was filtered off, dried (Na2SO4), and purified by column chromatography using dichloromethane (DCM) as the eluent.
1-Methyl-1H-indole-3-carbonitrile (5a). White solid; yield 90% m.p. 61–62 °C; spectroscopic data are in accordance to those reported in literature [41].
5-Bromo-1-methyl-1H-indole-3-carbonitrile (5b). White solid; yield 92%; m.p. 103–104 °C; spectroscopic data are in accordance to those reported in literature [41].
5-Fluoro-1-methyl-1H-indole-3-carbonitrile (5c). White solid; yield 90%; m.p. 76.5–78 °C; spectroscopic data are in accordance to those reported in literature [41].
5-Chloro-1-methyl-1H-indolo-3-carbonitrile (5d). White solid; yield: 91%; m.p. 103.8–104.5 °C; spectroscopic data are in accordance to those reported in literature [42].
5-Metoxy-1-metil-1H-indolo-3-carbonitrile (5e). White solid; yield 98%; m.p. 106.5–107.4 °C; spectroscopic data are in accordance to those reported in literature [41].
General Procedure for the Synthesis of N′-hydroxy-1-methyl-1H-indole-3-carboximidamides (2a–e)
To a solution of the appropriate 1-methyl-1H-indolo-3-carbonitrile 5 (0.9 mmol) in ethanol (15 mL) diisopripylenethylamide (DIPEA, 1.32 mL) and hydroxylamine hydrochloride (2.3 mmol, 158.0 mg) were added and the resulting reaction mixture was heated under reflux for 4 h. The solvent was evaporated at reduced pressure and the obtained crude was suspended in water and extracted with ethyl acetate (3 × 30 mL). The organic phases were dried (Na2SO4) and evaporated under reduced pressure. The residue was purified by column chromatography using ethyl acetate as the eluent.
N′-hydroxy-1-methyl-1H-indole-3-carboximidamide (2a). solid; yield 65%; m.p. 110.2–111.6 °C; spectroscopic data are in accordance to those reported in literature [41].
5-Bromo-N′-hydroxy-1-methyl-1H-indole-3-carboximidamide (2b). White solid; yield 75%; m.p. 103.0–104.0 °C IR (cm−1): 3465 (OH), 3360 (NH2), 1669 (C=N); 1H NMR (200 MHz, DMSO-d6) δ: 3.79 (s, 3H, CH3), 5.64 (s, 2H, NH2), 7.30 (d, 1H, J = 8.7 Hz, H-6), 7.44 (d, J = 8.7 Hz, 1H, H-7), 7.79 (s, 1H, H-4), 8.27 (s, 1H, H-2), 9.34 (1H, s, OH); 13C NMR (50 MHz, DMSO-d6) δ: 32.9 (q), 107.8 (s), 111.9 (d), 112.4 (s), 124.0 (d), 124.4 (d), 126.4 (s), 130.0 (d), 135.6 (s), 148.6 (s); Anal. Calculated for C10H10BrN3O (MW: 268.11): C, 44.80; H, 3.76; N, 15.67%. Found: C, 45.11; H, 4.00; N, 15.48%.
5-Fluoro-N′-hydroxy-1-methyl-1H-indole-3-carboximidamide (2c). Gray solid; yield 80%; m.p. 114.0–115.0 °C; IR (cm−1): 3465 (OH), 3370 (NH2), 1640 (C=N); 1H NMR (200 MHz, DMSO-d6) δ: 3.79 (s, 3H, CH3), 5.62 (s, 2H, NH2), 6.98–7.09 (td, J = 9.2, 9.1, 2.5 Hz, 1H, H-6), 7.45 (dd, J = 9.1, 4.4 Hz, 1H, H-7), 7.74–7.80 (m, 2H, H-4 and H-2), 8.27 (s, 1H, OH); 13C NMR (50 MHz, DMSO-d6) δ:33.0 (q), 106.7 (d, JC4-F = 24.8 Hz), 108.5 (s, JC7a-F = 4.6 Hz), 109.7 (d, JC6-F = 26.1 Hz), 111.0 (d, JC7-F = 9.9 Hz), 125.0 (s, JC3a-F = 11.0 Hz), 130.3 (d), 133.6 (s), 148.8 (s), 157.3 (s, JC5-F = 231.9 Hz); Anal. Calculated for C10H10FN3O (MW: 207.20): C, 57.97; H, 4.86; N, 20.28%. Found: C, 57.88; H, 4.62; N, 20.31%.
5-Chloro-N′-hydroxy-1-methyl-1H-indole-3-carboximidamide (2d). Gray solid; yield 82%; m.p. 170.7–172.0 °C; IR (cm−1): 3461 (OH), 3364 (NH2), 1652 (C=N); 1H NMR (200 MHz, DMSO-d6) δ 3.79 (s, 3H, CH3), 5.66 (s, 2H, NH2), 7.19 (dd, J = 8.7, 2.2 Hz, 1H, H-6), 7.47 (d, J = 8.7 Hz, 1H, H-7), 7.81 (s, 1H, H-2), 8.13 (d, J = 2.2 Hz, 1H, H-4), 9.35 (s, 1H, OH). 13C NMR (50 MHz, DMSO-d6) δ 32.89 (q), 107.84 (s), 111.47 (d), 121.31 (d), 121.52 (d) 124.38 (s), 125.75 (s), 130.15 (d), 135.36 (s), 148.63 (s). Anal. Calculated for C10H10ClN3O (MW: 223.66): C, 53.70; H, 4.51; N, 18.79%. Found: C, 53.82; H 4.67; N, 19.00%.
5-Methoxy-N′-hydroxy-1-methyl-1H-indole-3-carboximidamide (2e). White solid; yield 75%; m.p. 165.9–166.5 °C; IR (cm−1): 3447 (OH), 3356 (NH2), 1652 (C=N); 1H NMR (200 MHz, DMSO-d6) δ 3.75 (s, 3H, CH3x2), 5.58 (s, 2H, NH2), 6.82 (dd, J = 8.9, 2.5 Hz, 1H, H-6), 7.33 (d, J = 8.9 Hz, 1H, H-7), 7.59 (d, J = 2.5 Hz, 1H, H-4), 7.69 (s, 1H, H-2), 9.28 (s, 1H, OH).13C NMR (50 MHz, DMSO-d6) δ 32.79 (q), 55.22 (q), 103.67 (d), 107.64 (s), 110.49 (d), 111.77 (d), 125.22 (s), 129.07 (d), 132.09 (s), 149.29 (s), 153.86 (s). Anal. Calculated for C10H10N2O (MW: 219.24): C, 60.26; H, 5.98; N, 19.17%. Found: C, 60.22; H, 5.67; N, 19.25%.
General Procedure for the Synthesis of 2,2,2-trichloro-1-(1-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanones (7a–c)
To a solution of the appropriate 1-methyl-1H-pyrrolo[2,3-b]pyridine 6 (10.15 mmol) in DCM (24 mL), aluminum chloride (35 mmol, 4.7 g) and a solution of trichloroacetyl chloride in DCM (10.15 mmol, 1.1 mL in 5 mL di DCM), were added. The resulting mixture was stirred at room temperature for 6 h, poured into ice-water and extracted with DCM (3 × 20 mL). The organic phases were washed with HCl 10% (2 × 30 mL), dried (Na2SO4), and evaporated under reduced pressure, to afford the desired derivatives as pure compounds.
2,2,2-Trichloro-1-(1-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanone (7a). White solid; yield 80%; m.p. 99–100 °C; IR cm−1: 1690 (CO);1H NMR (200 MHz, DMSO-d6) δ 4.00 (s, 3H, CH3). 7.42 (dd, J = 8.0, 4.8 Hz, 1H, H-5), 8.49 (dt, J = 4.8, 2.0 Hz, 1H, H-6), 8.54 (d, J = 2.0 Hz, 1H, H-4), 8.90 (s, 1H, H-2). 13C NMR (50 MHz, DMSO-d6) δ 31.95 (q), 95.84 (s), 102.37 (s), 119.40 (d), 119.82 (s), 129.97 (d), 140.09 (d), 144.90 (d), 147.64 (s), 176.30 (s). Anal. Calculated for C10H7Cl3N2O (MW: 277.53): C, 43.28; H, 2.54; N, 10.09%. Found: C, 43.32; H 2.67; N, 9.85%.
2,2,2-Trichloro-1-(5-fluoro-1-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanone (7b). Yellow solid; yield 99%; m.p. 110–111 °C; IR cm−1: 1710 (CO); 1H NMR (200 MHz, DMSO-d6) δ 3.98 (s, 3H, CH3). 8.29 (dd, J = 9.0, 2.8 Hz, 1H, H-4), 8.51 (dd, J = 2.8, 1.7 Hz, 1H, H-6), 8.99 (s, 1H, H-2). 13C NMR (50 MHz, DMSO-d6) δ 32.31 (q), 99.49 (s), 120.43 (s), 102.29 (s), 115.74 (d, JC4-F = 29.3 Hz), 133.54 (d, JC6-F = 29.3 Hz), 141.80 (d), 140.22 (s), 144.40 (s), 160.20 (d, JC5-F = 70.4 Hz). Anal. Calculated for C11H6Cl3FN2O (MW: 295.52): C, 40.64; H, 2.05; N, 9.48%. Found: C, 40.55; H 1.98; N, 9.68%.
2,2,2-Trichloro-1-(5-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanone (7c). Orange solid; yield 99%; m.p. 123–124 °C; IR cm−1: 1725 (CO);1H NMR (200 MHz, DMSO-d6) δ 3.97 (s, 3H, CH3), 8.57 (d, J = 2.2 Hz, 1H, H-4), 8.63 (d, J = 2.2 Hz, 1H, H-6), 8.96 (s, 1H, H-2).13C NMR (50 MHz, DMSO-d6) δ 32.21 (s), 101.97 (s), 115.00 (s), 121.41 (s), 131.69 (d), 141.40 (d), 145.23 (d), 146.24 (s), 176.32 (s). Anal. Calculated for C10H6BrCl3N2O (MW: 356.43): C, 33.70; H, 1.70; N, 7.86%. Found: C, 34.01; H 1.55; N, 7.68%.
General Procedure for the Synthesis of methyl-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (3a–c)
To a mixture of the appropriate ethanone 7 (1.1 mmol) in methanol, KOH 20% solution (0.33 mL) was added. The reaction mixture was stirred at room temperature for 3 h. Then HCl 6N (0.2 mL) was added and the mixture was extracted in ethyl acetate (3 × 30 mL).
Methyl-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (3a). White solid; yield 99%; m.p. 135–136 °C; IR cm−1: 1730 (CO); 1H NMR (200 MHz, DMSO-d6) δ 3.83 (s, 3H, CH3), 3.88 (s, 3H, OCH3), 7.30 (dd, J = 7.2, 4.8 Hz, 1H, H-5), 8.35 (m, 3H, H-2, H-4, H-6). 13C NMR (50 MHz, DMSO-d6) δ 31.41 (q), 50.91 (q), 103.75 (s), 117.87 (d), 118.23 (s), 128.95 (d), 136.38 (d), 143.73 (d), 147.56 (s), 163.99 (s). Anal. Calculated for C10H10N2O2 (MW: 190.20): C, 63.15; H, 5.30; N, 14.73%. Found: C, 63.28; H 5.15; N, 14.58%.
Methyl-5-fluoro-1-methyl-1H-pyrrolo[2,3-b]pyridine-3- carboxylate (3b). White solid; yield 90%; m.p. 128–129 °C; IR cm−1: 1738 (CO); 1H NMR (200 MHz, DMSO-d6) δ 3.83 (s, 3H, CH3), 3.88 (s, 3H, OCH3), 8.05 (dd, J = 9.2, 2.8 Hz, 1H, H-4), 8.39 (dd, J = 2.8, 1.8 Hz, 1H, H-6), 8.43 (s, 1H, H-2). 13C NMR (50 MHz, DMSO-d6) δ 31.73 (q), 51.02 (q), 103.76 (d, JC7a-F = 4.1 Hz), 114.48 (d, JC4-F = 21.8 Hz), 118.41 (d, JC3-F = 7.9 Hz), 132.19 (d, JC6-F = 29.4 Hz), 138.27 (d), 144.32 (s), 156.26 (d, JC5a-F = 242.4 Hz), 163.66 (s). Anal. Calculated for C10H9FN2O2 (MW: 208.19): C, 57.69; H, 4.36; N, 13.46%. Found: C, 57.53; H 4.15; N, 13.38%.
Methyl-5-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridine-3- carboxylate (3c). White solid; yield 99%; m.p. 144–145 °C; IR cm−1: 1715 (CO); 1H NMR (200 MHz, DMSO-d6) δ 3.84 (s, 3H, CH3), 3.86 (s, 3H, CH3), 8.39 (d, J = 2.0 Hz, 2H, H-2, H-4), 8.45 (d, J = 2.0 Hz, 1H, H-6). 13C NMR (50 MHz, DMSO-d6) δ 31.64 (q), 51.10 (q), 103.45 (s), 113.36 (s), 119.73 (s), 130.63 (d), 137.85 (d), 143.88 (d), 146.03 (s) 163.57 (s). Anal. Calculated for C10H9BrN2O2 (MW: 269.09): C, 44.63; H, 3.37; N, 10.41%. Found: C, 44.87; H 3.51; N, 10.12%.
General Procedure for the Synthesis of 1-methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridines (1a–o)
To a solution of the proper derivative 2 (2.6 mmol) in tetrahydrofuran (10 mL), sodium hydride (3.0 mmol, 79.3 mg) and molecular sieves were added. The reaction mixture was heated at 60 °C for 30 min. Then, the appropriate derivative 3 (1.3 mmol) was added and the mixture was heated under reflux for 4–6 h. The organic solvent was evaporated at reduced pressure and the resulting crude was purified by column chromatography using dichloromethane/ethyl acetate 1:1 as the eluent.
1-Methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridine (1a). White solid; yield 75%; m.p. 203.2–203.9 °C;1H NMR (200 MHz, DMSO-d6) δ 3.94 (s, 3H, CH3), 3.98 (s, 3H, CH3), 7.24–7.36 (m, 2H, H-6″, H-7″), 7.40–7.46 (m, 1H, H-4″), 7.58–7.62 (m, 1H, H-5″), 8.12–8.16 (m, 1H, H-5), 8.24 (s, 1H, H-2″), 8.48–8.51 (m, 1H, H-6), 8.60 (dd, J = 2.1, 8.0 Hz, 1H, H-4), 8.65 (s, 1H, H-2). Anal. Calculated for C19H15N5O (MW: 329.36): C, 69.29; H, 4.59; N, 21.26%. Found: C, 69.55; H 4.51; N, 21.21%. HRMS: [MH]+, found 330.1351 C19H15N5O requires 330.1349.
5-Bromo-1-methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridine (1b). White solid; yield 72% m.p. 230.7–231.9 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.95 (s, 6H, 2xCH3), 7.31 (s, 2H, H-6″, H-7″), 7.59–7.63 (m, 1H, H-5″), 8.11–8.30 (m, 2H, H-2″, H-4″), 8.58–8.71 (m, 3H, H-2, H-4, H-6). Anal. Calculated for C19H14BrN5O (MW: 408.25): C, 55.90; H, 3.46; N, 17.15%. Found: C, 56.13; H 3.25; N, 16.84%. HRMS: [MH]+, found 408.0449 C19H14BrN5O requires 408.0454.
5-Fluoro-1-methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridine (1c). White solid; yield 62 %; m.p. 227.4–228.4 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.94 (s, 3H, CH3), 3.97 (s, 3H, CH3), 7.24–7.36 (m, 2H, H-6″, H-7″), 7.60 (d, J = 7.4 Hz, 1H, H-4″), 8.12 (d, J = 7.4 Hz, 1H, H-5″), 8.30 (s, 1H, H-2″), 8.38 (m, 1H, H-4) 8.51 (s, 1H, H-6), 8.74 (s, 1H, H-2). Anal. Calculated for C19H14FN5O (MW: 347.35): C, 65.70; H, 4.06; N, 20.16%. Found: C, 65.87; H 4.32; N, 20.44%. HRMS: [MH]+, found 348.1259 C19H14FN5O requires 348.1255.
1-Methyl-3-[3-(5-bromo-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridine (1d). Light brown solid; yield 75%; m.p. 248.2–248.9 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.95 (s, 3H, CH3), 3.99 (s, 3H, CH3), 7.40–7.47 (m, 2H, H-4″, H-6″), 7.61 (d, J = 8.8 Hz, 1H, H-7″) 8.25 (s, 1H, H-4), 8.30 (s, 1H, H-2″), 8.50 (d, J = 6.2 Hz, 1H, H-5), 8.60 (d, J = 6.2 Hz, 1H, H-6), 8.68 (s, 1H, H-2). Anal. Calculated for C19H14BrN5O (MW: 408.25): C, 55.90; H, 3.46; N, 17.15%. Found: C, 56.08; H 3.31; N, 17.25%. HRMS: [MH]+, found 408.0456 C19H14BrN5O requires 408.0454.
5-Bromo-3-[3-(5-bromo-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1e). White solid; yield 82%; m.p. 264.2–265.9 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.94 (s, 3H, CH3), 3.96 (s, 3H, CH3), 7.46 (d, J = 8.1 Hz, 1H, H-6″) 7.61 (d, J = 8.1 Hz, 1H, H-7″), 8.25 (s, 1H, H-4″), 8.36 (s, 1H, H-2″), 8.58 (s, 1H, H-2), 8.72 (m, 2H, H-4, H-6). Anal. Calculated for C19H13Br2N5O (MW: 487.15): C, 46.84; H, 2.69; N, 14.38%. Found: C, 46.97; H 3.00; N, 14.12%. HRMS: [MH]+, found 485.9561 C19H13Br2N5O requires 485.9559.
5-Fluoro-3-[3-(5-bromo-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1f). White solid; yield 75%; m.p. 255.3–256.2 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.94 (s, 3H, CH3), 3.97 (s, 3H, CH3), 7.46 (d, J = 8.7 Hz, 1H, H-6″), 7.61 (d, J = 8.7 Hz, 1H, H-7″), 8.23 (s, 1H, H-2″), 8.35–8.37 (m, 2H, H-4″, H-4), 8.51 (s, 1H, H-6), 8.76 (s, 1H, H-2). Anal. Calculated for C19H13BrFN5O (MW: 426.24): C, 53.54; H, 3.07; N, 16.43%. Found: C, 53.68; H 2.98; N, 16.71%. HRMS: [MH]+, found 426.0358 C19H13BrFN5O requires 426.0360.
3-[3-(5-Fluoro-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1g). Light brown solid; yield 72%; m.p. 236.0–236.7 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.95 (s, 3H, CH3), 3.99 (s, 3H, CH3), 7.19 (t, J = 8.8 Hz, 1H, H-6″), 7.41–7.48 (m, 1H, H-7″), 7.63 (m, 1H, H-4″), 7.80 (d, J = 8.0 Hz, 1H, H-4), 8.33 (s, 1H, H-2″), 8.51 (d, J = 3.4 Hz, 1H, H-6), 8.60 (d, J = 8.0 Hz, 1H, H-5), 8.68 (s, 1H, H-2). Anal. Calculated for C19H14FN5O (MW: 347.35): C, 65.70; H, 4.06; N, 20.16%. Found: C, 65.55; H 4.31; N, 20.25%. HRMS: [MH]+, found 348.1253 C19H14FN5O requires 348.1255
5-Bromo-3-[3-(5-fluoro-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1h). Light brown solid; yield 60%; m.p. 255.2–256.8 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.95 (s, 3H, CH3), 3.96 (s, 3H, CH3), 7.19 (t, J = 8.3 Hz, 1H, H-7″), 7.64 (m, 1H, H-4″), 7.77 (d, J = 8.3, 1.7 Hz, 1H, H-6″), 8.37 (s, 1H, H-2″), 8.58 (s, 1H, H-2), 8.72 (m, 2H, H-4, H-6). Anal. Calculated for C19H13BrFN5O (MW: 426.24): C, 53.54; H, 3.07; N, 16.43%. Found: C, 53.28; H 3.15; N, 16.26%. %. HRMS: [MH]+, found 426.0363 C19H13BrFN5O requires 426.0360
5-Fluoro-3-[3-(5-fluoro-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1i). White solid; yield 55%; m.p. 265.2–266.8 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.95 (s, 3H, CH3), 3.98 (s, 3H, CH3), 7.20 (td, J = 2.6, 9.0 Hz, 1H, H-6″), 7.61–7.68 (m, 1H, H-4″), 7.79 (d, J = 2.6 Hz, 1H, H-6″), 8.35–8.41 (m, 2H, H-2″, H-4), 8.51–8.53 (m, 1H, H-6), 8.76 (s, 1H, H-2). Anal. Calculated for C19H13F2N5O (MW: 365.34): C, 62.46; H, 3.59; N, 19.17%. Found: C, 62.68; H 3.38; N, 19.42%. HRMS: [MH]+, found 366.1157 C19H13F2N5O requires 366.1160
3-[3-(5-Chloro-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1j). White solid; yield 65%; m.p. 242.8–243.7 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.94 (s, 3H, CH3), 3.97 (s, 3H, CH3), 7.51–7.20 (m, 1H, H-6″), 7.64 (d, J = 9.3 Hz, 1H, H-7″),8.65 (s, 1H, H-4″), 8.57 (d, J = 7.1 Hz, 1H, H-5), 8.49 (d, J = 2.5 Hz, 1H, H-4), 8.30 (s, 1H, H-2″), 8.09 (s, 1H, H-2), 7 3.95 (d, J = 7.1 Hz, 1H, H-6). Anal. Calculated for C19H14ClN5O (MW: 363.80): C, 62.73; H, 3.88; N, 19.25%. Found: C, 62.58; H 3.71; N, 19.37%. HRMS: [MH]+, found 364.0962 C19H14ClN5O requires 364.0960.
5-Bromo-3-[3-(5-chloro-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1k). White solid; yield 75%; m.p. 270.2–271.3 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.95 (s, 3H, CH3), 3.97 (s, 3H, CH3), 7.35 (dd, J = 8.7, 2.0 Hz, 1H, H-6″), 7.66 (d, J = 8.7 Hz, 1H, H-7″), 8.09 (d, J = 2.0 Hz, 1H, H-4″), 8.38 (s, 1H, H-2″), 8.58 (d, J = 2.2 Hz, 1H, H-4), 8.70 (d, J = 2.2 Hz, 1H, H-6), 8.74 (s, 1H, H-2). Anal. Calculated for C19H13BrClN5O (MW: 442.70): C, 51.55; H, 2.96; N, 15.82%. Found: C, 51.28; H 3.11; N, 16.02%. HRMS: [MH]+, found 442.0068 C19H13BrClN5O requires 442.0065.
5-Fluoro-3-[3-(5-chloro-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1l). White solid; yield 85%; m.p. 258–259 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.94 (s, 3H, CH3), 3.97 (s, 3H, CH3), 7.34 (d, J = 8.3 Hz, 1H, H-6″), 8.07 (m, 1H, H-7″), 7.64 (m, 1H, H-4″), 8.39 (m, 2H, H-6, H-4’), 8.52 (s, 1H, H2″), 8.77 (s, 1H, H-2). Anal. Calculated for C19H13ClFN5O (MW: 381.79): C, 59.77; H, 3.43; N, 18.34%. Found: C, 59.68; H 3.25; N, 18.56%. HRMS: [MH]+, found 382.0867 C19H13ClFN5O requires 382.0865.
3-[3-(5-Methoxy-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1m). White solid; yield 70%; m.p. 197–198 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.86 (s, 3H, CH3), 3.90 (s, 3H, CH3), 3.98 (s, 3H, OCH3), 6.96 (d, J = 9.0 Hz, 1H, H-6″), 7.61 (s, 1H, H-2″), 7.55–7.32 (m, 2H, H-7″, H-4″), 8.17 (s, 1H, H-2), 8.49 (d, J = 4.0 Hz, 1H, H-6), 8.60 (m, 2H, H-4, H-5). Anal. Calculated for C20H17N5O2 (MW: 359.38): C, 66.84; H, 4.77; N, 19.49%. Found: C, 66.59; H 4.89; N, 19.23%. HRMS: [MH]+, found 360.1456 C20H17N5O2 requires 360.1455.
5-Bromo-3-[3-(5-methoxy-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1n). White solid; yield 65%; m.p. 229.6–230.2 °C; 1H NMR (200 MHz, DMSO-d6) δ 3.88 (s, 3H, CH3). 3.90 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 6.95 (d, J = 8.3 Hz, 1H, H-6″), 7.50 (d, J = 8.3 Hz, 1H, H-7″), 7.62 (d, J = 1.3 Hz, 1H, H-4″), 8.21 (s, 1H, H-2″), 8.58 (s, 1H, H-2), 8.71 (m, 2H, H-4, H-6). Anal. Calculated for C20H16BrN5O2 (MW: 438.28): C, 54.81; H, 3.68; N, 15.98%. Found: C, 54.78; H 3.95; N, 15.78%. HRMS: [MH]+, found 438.0562 C20H16BrN5O2 requires 438.0560.
5-Fluoro-3-[3-(5-methoxy-1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1-methyl-1H-pyrrolo[2,3-b]pyridine (1o). White solid; yield 80%; m.p. 218–219 °C 1H NMR (200 MHz, DMSO-d6) δ 3.78 (s, 3H, CH3), 3.90 (s, 3H, CH3), 3.96 (s, 3H, OCH3), 6.95 (dd, J = 8.9, 2.4 Hz, 1H, H-6″), 7.54 (m, 2H, H-7″, H-4″), 8.22 (m, 1H, H-4), 8.50 (s, 1H, H-2″), 8.37 (dd, J = 8.9, 2.7 Hz, 1H, H-6), 8.72 (s, 1H, H-2). Anal. Calculated for C20H16FN5O2 (MW: 377.37): C, 63.65; H, 4.27; N, 18.56%. Found: C, 63.78; H 4.10; N, 18.63%. HRMS: [MH]+, found 378.1359 C20H16FN5O2 requires 378.1361.
3.2. Biology
Compounds 1a–o, prepared as described above, were dissolved in dimethyl sulfoxide (DMSO) and then diluted in culture medium to have a DMSO concentration not exceeding 0.1%. MCF-7 (human breast cancer), HeLa (cervix adenocarcinoma), HCT-116 (human colorectal carcinoma), and CaCo2 (colorectal carcinoma) cell lines were obtained from American Type Culture Collection, Rockville, MD, USA and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal, 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL), and gentamicin (5 μg/mL). All the cells were maintained in log phase by seeding twice a week at a density of 3 × 108 cells/L in a humidified 5% CO2 atmosphere, at 37 °C. In all experiments, cells were left to incubate overnight to allow adhesion before treatment with the compounds or vehicle alone (control cells). In selected experiments CaCo2 cells were treated 15 days after confluence, at which time the cells are differentiated in normal intestinal-like cells [43].
No differences were found between cells treated with DMSO 0.1% and untreated cells in terms of cell number and viability.
3.2.1. Viability Assay in Vitro
Cytotoxic activity of compounds 1k and 1n against human tumor cell lines (MCF-7, HeLa, Caco-2, and HCT-116) and intestinal-like differentiated cells was determined by the MTT colorimetric assay commonly used to study inhibition of cellular proliferation. Briefly, cells were seeded at 2 × 104 cells/well in 96-well plates containing 200 μL DMEM. When appropriated, cells were treated with vehicle alone (0.1% DMSO, control) or various concentrations (0.01–100 μM) of the drugs in DMEM and let them incubate for 72 h. Then cells were washed, and 50 μL of FBS-free medium containing 5 mg/mL MTT was added. The medium was discarded after 2 h incubation at 37 °C by centrifugation, and formazan blue formed in the cells was dissolved in DMSO. The absorbance, measured at 570 nm on a microplate reader (Bio-RAD, Hercules, CA, USA), of MTT formazan of control cells were taken as 100% of viability.
Cytotoxicity of the compounds was defined as the IC50 value which represents the molar concentration of the compound that inhibits 50% cell viability. IC50 values were calculated by the dose-response inhibition model using GraphPad Prism 5.02 from GraphPad Software (San Diego, CA, USA). Each experiment was repeated at least three times in triplicate to obtain the mean values.
3.2.2. Cell Cycle Analysis
MCF-7 cells (5.0 × 104 cells/cm2) were seeded in triplicate in 24-wells culture plates. After an overnight incubation, the cells were washed with fresh medium and incubated with the compounds or vehicle alone (control cells) in DMEM for 24 h. After trypsinization, aliquots of 1.0 × 106 cells were washed with PBS and incubated in the dark in PBS containing 20 μg/mL propidium iodide (PI) and 200 μg/mL RNase, for 30 min, at room temperature. Then samples of at least 1.0 × 104 cells were subjected to fluorescence-activated cell sorting (FACS) analysis by Epics XL™ flow cytometer using Expo32 software (Beckman Coulter, Fullerton, CA, USA).
3.2.3. Cell Death Detection
Cell death was assessed by staining the cells with AnnexinV-FITC and propidium iodide (PI) (Sigma-Aldrich, Steinheim, Germany) according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA) and subsequent analysis by flow cytometry. MCF-7 cells (5.0 × 104 cells/cm2) were seeded in triplicate in 24-wells culture and after an overnight incubation, washed with fresh medium and incubated for 24 h with the compounds or vehicle alone (control cells) in DMEM. After trypsinization, 1.0 × 106 cells/mL in combining buffer were incubated with Annexin V-FITC and PI solution at room temperature in the dark for 15 min. Then samples of at least 1.0 × 104 cells were subjected to FACS analysis using appropriate 2-bidimensional gating method.
Acridine orange/ethidium bromide (AO/EB) fluorescence staining was also used to identify cell apoptosis. After 24 h treatment, the cells were washed with PBS and stained with AO/EB solution (100 μg/mL, 1:1). After 20 s the AO/EB solution was discarded and cells were immediately visualized by fluorescence microscopy (Leica; Wetzlar, Germany). Multiple photos were taken at randomly-selected areas of the well to obtain representative data.
4. Conclusions
New analogs of nortopsentin, in which the central imidazole ring of the natural lead was replaced by a 1,2,4-oxadiazole moiety and in which a 7-azaindole portion substituted the original indole moiety, were efficiently synthesized. Among the synthesized derivatives, 1k and 1n showed the best cytotoxic activity against several human cancer cell lines. The mechanism of the anti-proliferative effect on MCF-7 was pro-apoptotic, being associated with externalization of plasma membrane phosphatidylserine, chromatin condensation, and membrane blebbing. Finally, the compounds induced an accumulation of cells in G0–G1 phase suggesting that they can affect the cell mechanisms promoting the DNA duplication.
Acknowledgments
The authors acknowledge the 2014–2020 PON Ricerca e Innovazione grant from the Italian Ministry of Education, University and Research, entitled “PROGEMA—Processi Green per l’Estrazione di Principi Attivi e la Depurazione di Matrici di Scarto e Non” (ARS01_00432) to P.C.
Author Contributions
S.C., V.D.S., S.M., and B.P. performed the chemical research and analyzed the data. A.A. and L.T. performed the biological research and analyzed the data. G.C., P.D., L.T., and B.P. participated in the design of the research and the writing of the manuscript. All authors read and approved the final manuscript.
Funding
This project was supported by a 2014–2020 PON Ricerca e Innovazione grant from the Italian Ministry of Education, University and Research, entitled “PROGEMA—Processi Green per l’Estrazione di Principi Attivi e la Depurazione di Matrici di Scarto e Non” (ARS01_00432) to P.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins A., Vieira H., Gaspar H., Santos S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs. 2014;12:1066–1101. doi: 10.3390/md12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakemi S., Sun H.H. Nortopsentins A, B and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991;56:4304–4307. doi: 10.1021/jo00013a044. [DOI] [Google Scholar]
- 4.Diana P., Carbone A., Barraja P., Montalbano A., Martorana A., Dattolo G., Gia O., Dalla Via L., Cirrincione G. Synthesis and antitumor properties of 2,5-bis(3′-indolyl)thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 2007;17:2342–2346. doi: 10.1016/j.bmcl.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 5.Diana P., Carbone A., Barraja P., Martorana A., Gia O., Dalla Via L., Cirrincione G. 3,5-Bis(3′-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorg. Med. Chem. Lett. 2007;17:6134–6137. doi: 10.1016/j.bmcl.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 6.Diana P., Carbone A., Barraja P., Kelter G., Fiebig H.H., Cirrincione G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010;18:4524–4529. doi: 10.1016/j.bmc.2010.04.061. [DOI] [PubMed] [Google Scholar]
- 7.Carbone A., Parrino B., Barraja P., Spanò V., Cirrincione G., Diana P., Maier A., Kelter G., Fiebig H.H. Synthesis and antiproliferative activity of 2,5-bis(3′-indolyl)pyrroles, analogues of the marine alkaloid nortopsentin. Mar. Drugs. 2013;11:643–654. doi: 10.3390/md11030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar D., Kumar N.M., Chang K.H., Gupta R., Shah K. Synthesis and in vitro anticancer activity of 3,5-bis(indolyl)-1,2,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2011;21:5897–5900. doi: 10.1016/j.bmcl.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 9.Carbone A., Parrino B., Cusimano M.G., Spanò V., Montalbano A., Barraja P., Schillaci D., Cirrincione G., Diana P., Cascioferro S. New thiazole nortopsentin analogues inhibit bacterial biofilm formation. Mar. Drugs. 2018;16:274. doi: 10.3390/md16080274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone A., Parrino B., Di Vita G., Attanzio A., Spanò V., Montalbano A., Barraja P., Tesoriere L., Livrea M.A., Diana P., et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo[2,3-b]pyridines and indolyl-thiazolyl-pyrrolo[2,3-c]pyridines, nortopsentin analogues. Mar. Drugs. 2015;13:460–492. doi: 10.3390/md13010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrino B., Carbone A., Di Vita G., Ciancimino C., Attanzio A., Spanò V., Montalbano A., Barraja P., Tesoriere L., Diana P., et al. 3-[4-(1H-Indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b]pyridines, nortopsentin analogues with antiproliferative activity. Mar. Drugs. 2015;13:1901–1924. doi: 10.3390/md13041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spanò V., Attanzio A., Cascioferro S., Carbone A., Montalbano A., Barraja P., Tesoriere L., Cirrincione G., Diana P., Parrino B. Synthesis and antitumor activity of new thiazole nortopsentin analogs. Mar. Drugs. 2016;14:226. doi: 10.3390/md14120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone A., Pennati M., Parrino B., Lopergolo A., Barraja P., Montalbano A., Spanò V., Sbarra S., Doldi V., de Cesare M., et al. Novel 1H-pyrrolo[2,3-b]pyridine derivatives nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013;56:7060–7072. doi: 10.1021/jm400842x. [DOI] [PubMed] [Google Scholar]
- 14.Diana P., Carbone A., Barraja P., Montalbano A., Parrino B., Lopergolo A., Pennati M., Zaffaroni N., Cirrincione G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem. 2011;6:1300–1309. doi: 10.1002/cmdc.201100078. [DOI] [PubMed] [Google Scholar]
- 15.Carbone A., Pennati M., Barraja P., Montalbano A., Parrino B., Spanò V., Lopergolo A., Sbarra S., Doldi V., Zaffaroni N., et al. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]pyridines, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014;21:1654–1666. doi: 10.2174/09298673113206660307. [DOI] [PubMed] [Google Scholar]
- 16.Parrino B., Attanzio A., Spanò V., Cascioferro S., Montalbano A., Barraja P., Tesoriere L., Diana P., Cirrincione G., Carbone A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017;138:371–383. doi: 10.1016/j.ejmech.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D., Patel G., Chavers A.K., Chang K.-H., Shah K. Design and synthesis of 3,5-disubstituted boron-containing 1,2,4-oxadiazoles as potential combretastatin A-4 (CA-4) analogs. Eur. J. Med. Chem. 2011;46:3085–3092. doi: 10.1016/j.ejmech.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H.-Z., Kasibhatla S., Kuemmerle J., Kemnitzer W., Ollis-Mason K., Qiu L., Crogan-Grundy C., Tseng B., Drewe J., Cai S.X. Discovery and structure-activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J. Med. Chem. 2005;48:5215–5223. doi: 10.1021/jm050292k. [DOI] [PubMed] [Google Scholar]
- 19.Krasavin M., Sosnov A.V., Karapetian R., Konstantinov I., Soldatkina O., Godovykh E., Zubkov F., Bai R., Hamel E., Gakh A.A. Antiproliferative 4-(1,2,4-oxadiazol-5-yl)piperidine-1-carboxamides, a new tubulin inhibitor chemotype. Bioorg. Med. Chem. Lett. 2014;24:4477–4481. doi: 10.1016/j.bmcl.2014.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H.Y., Snider B.B. Synthesis of phidianidines A and B. J. Org. Chem. 2012;77:4832–4836. doi: 10.1021/jo300449n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brogan J.T., Stoops S.L., Lindsley C.W.T. Total synthesis and biological evaluation of phidianidines A and B uncovers unique pharmacological profiles at CNS targets. ACS Chem. Neurosci. 2012;3:658–664. doi: 10.1021/cn300064r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C.S., Fu Y., Zhang L., Gong J.X., Wang Z.Z., Xiao W., Zhang H.Y., Guo Y.W. Synthesis and biological evaluation of novel marine-derived indole-based 1,2,4-oxadiazoles derivatives as multifunctional neuroprotective agents. Bioorg. Med. Chem. Lett. 2015;25:216–220. doi: 10.1016/j.bmcl.2014.11.068. [DOI] [PubMed] [Google Scholar]
- 23.Montalbano A., Parrino B., Diana P., Barraja P., Carbone A., Spanò V., Cirrincione G. Synthesis of the new oligopeptide pyrrole derivative isonetropsin and its one pyrrole unit analogue. Tetrahedron. 2013;69:2550–2554. doi: 10.1016/j.tet.2013.01.076. [DOI] [Google Scholar]
- 24.Barraja P., Caracausi L., Diana P., Spanò V., Montalbano A., Carbone A., Parrino B., Cirrincione G. Synthesis and Antiproliferative Activity of the Ring System [1,2]Oxazolo[4,5-g]indole. ChemMedChem. 2012;7:1901–1904. doi: 10.1002/cmdc.201200296. [DOI] [PubMed] [Google Scholar]
- 25.Parrino B., Ullo S., Attanzio A., Spanò V., Cascioferro S., Montalbano A., Barraja P., Tesoriere L., Diana P., Cirrincione G. New tripentone analogs with antiproliferative activity. Molecules. 2017;22:2005. doi: 10.3390/molecules22112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diana P., Stagno A., Barraja P., Montalbano A., Carbone A., Parrino B., Cirrincione G. Synthesis of the new ring system pyrrolizino[2,3-b]indol-4(5H)-one. Tetrahedron. 2011;67:3374–3379. doi: 10.1016/j.tet.2011.03.060. [DOI] [Google Scholar]
- 27.Barraja P., Spanò V., Giallombardo D., Diana P., Montalbano A., Carbone A., Parrino B., Cirrincione G. Synthesis of [1,2]oxazolo[5,4-e]indazoles as antitumour agents. Tetrahedron. 2013;69:6474–6477. doi: 10.1016/j.tet.2013.05.083. [DOI] [Google Scholar]
- 28.Spanò V., Montalbano A., Carbone A., Parrino B., Diana P., Cirrincione G., Castagliuolo I., Brun P., Issinger O.-G., Tisi S., et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur. J. Med. Chem. 2014;74:340–357. doi: 10.1016/j.ejmech.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Parrino B., Carbone A., Muscarella M., Spanò V., Montalbano A., Barraja P., Salvador A., Vedaldi D., Cirrincione G., Diana P. 11H-Pyrido[3′,2′:4,5]pyrrolo[3,2-c]cinnoline and pyrido[3′,2′:4,5]pyrrolo[1,2-c][1,2,3]benzotriazine: Two new ring systems with antitumor activity. J. Med. Chem. 2014;57:9495–9511. doi: 10.1021/jm501244f. [DOI] [PubMed] [Google Scholar]
- 30.Parrino B., Carbone A., Ciancimino C., Spanò V., Montalbano A., Barraja P., Cirrincione G., Diana P., Sissi C., Palumbo M., et al. Water-soluble isoindolo[2,1-a]quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015;94:149–162. doi: 10.1016/j.ejmech.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Parrino B., Carbone A., Spanò V., Montalbano A., Giallombardo D., Barraja P., Attanzio A., Tesoriere L., Palumbo M., Sissi C., et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015;94:367–377. doi: 10.1016/j.ejmech.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Spanò V., Parrino B., Carbone A., Montalbano A., Salvador A., Brun P., Vedaldi D., Diana P., Cirrincione G., Barraja P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015;102:334–351. doi: 10.1016/j.ejmech.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Diana P., Stagno A., Barraja P., Carbone A., Parrino B., Dall’Acqua F., Vedaldi D., Salvador A., Brun P., Castagliuolo I., et al. Synthesis of triazeno-azaindoles a new class of triazenes with antitumor activity. ChemMedChem. 2011;6:1291–1299. doi: 10.1002/cmdc.201100027. [DOI] [PubMed] [Google Scholar]
- 34.Spanò V., Pennati M., Parrino B., Carbone A., Montalbano A., Cilibrasi V., Zuco V., Lopergolo A., Cominetti D., Diana P., et al. Preclinical activity of new [1,2]oxazolo[5,4-e]isoindole derivatives in diffuse malignant peritoneal mesothelioma. J. Med. Chem. 2016;59:7223–7238. doi: 10.1021/acs.jmedchem.6b00777. [DOI] [PubMed] [Google Scholar]
- 35.Barraja P., Diana P., Spanò V., Montalbano A., Carbone A., Parrino B., Cirrincione G. An efficient synthesis of pyrrolo[3′,2′:4,5]thiopyrano[3,2-b]pyridin-2-one: A new ring system of pharmaceutical interest. Tetrahedron. 2012;68:5087–5094. doi: 10.1016/j.tet.2012.04.041. [DOI] [Google Scholar]
- 36.Spanò V., Frasson I., Giallombardo D., Doria F., Parrino B., Carbone A., Montalbano A., Nadai M., Diana P., Cirrincione G., et al. Synthesis and antiproliferative mechanism of action of pyrrole[3′,2′:6,7]cyclohepta[1,2-d]pyrimidin-2-amines as singlet oxygen photosensitizers. Eur. J. Med. Chem. 2016;123:447–461. doi: 10.1016/j.ejmech.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Parrino B., Ciancimino C., Carbone A., Spanò V., Montalbano A., Barraja P., Cirrincione G., Diana P. Synthesis of isoindolo[1,4]benzoxazinone and isoindolo[1,5]benzoxazepine: Two new ring systems of pharmaceutical interest. Tetrahedron. 2015;71:7332–7338. doi: 10.1016/j.tet.2015.04.083. [DOI] [Google Scholar]
- 38.Spanò V., Pennati M., Parrino B., Carbone A., Montalbano A., Lopergolo A., Zuco V., Cominetti D., Diana P., Cirrincione G., et al. [1,2]oxazolo[5,4-e]isoindoles as promising tubulin polymerization inhibitors. Eur. J. Med. Chem. 2016;124:840–851. doi: 10.1016/j.ejmech.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Parrino B., Ullo S., Attanzio A., Cascioferro S., Spanò V., Carbone A., Montalbano A., Barraja P., Cirrincione G., Tesoriere L. Synthesis of 5H-pyrido[3,2-b]pyrrolizin-5-one tripentone analogs with antitumor activity. Eur. J. Med. Chem. 2018;158:236–246. doi: 10.1016/j.ejmech.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Spanò V., Giallombardo D., Cilibrasi V., Parrino B., Carbone A., Montalbano A., Frasson I., Salvador A., Richter S.N., Doria F., et al. Pyrrolo[3′,2′:6,7]cyclohepta[1,2-b]pyridines with potent photo-antiproliferative activity. Eur. J. Med. Chem. 2017;128:300–318. doi: 10.1016/j.ejmech.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Ren X., Chen J., Chen F., Cheng J. The palladium-catalyzed cyanation of indole C-H bonds with the combination of NH4HCO3 and DMSO as a safe cyanide source. Chem. Commun. 2011;47:6725–6727. doi: 10.1039/c1cc11603g. [DOI] [PubMed] [Google Scholar]
- 42.Zhao M., Zhang W., Shen Z. Cu-Catalyzed Cyanation of Indoles with Acetonitrile as a Cyano Source. J. Org. Chem. 2015;80:8868–8873. doi: 10.1021/acs.joc.5b01419. [DOI] [PubMed] [Google Scholar]
- 43.Sun D.X., Lennernas H., Welage L.S., Barnett J.L., Landowski C.P., Foster D., Fleischer D., Lee K.D., Amidon G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequence tags and correlation with permeability of 26 drugs. Pharm. Res. 2002;19:1400–1416. doi: 10.1023/A:1020483911355. [DOI] [PubMed] [Google Scholar]