Abstract
Tetrodotoxin (TTX) is a potent marine neurotoxin with bacterial origin. To date, around 28 analogs of TTX are known, but only 12 were detected in marine organisms, namely TTX, 11-oxoTTX, 11-deoxyTTX, 11-norTTX-6(R)-ol, 11-norTTX-6(S)-ol, 4-epiTTX, 4,9-anhydroTTX, 5,6,11-trideoxyTTX, 4-CysTTX, 5-deoxyTTX, 5,11-dideoxyTTX, and 6,11-dideoxyTTX. TTX and its derivatives are involved in many cases of seafood poisoning in many parts of the world due to their occurrence in different marine species of human consumption such as fish, gastropods, and bivalves. Currently, this neurotoxin group is not monitored in many parts of the world including in the Indian Ocean area, even with reported outbreaks of seafood poisoning involving puffer fish, which is one of the principal TTX vectors know since Egyptian times. Thus, the main objective of this review was to assess the incidence of TTXs in seafood and associated seafood poisonings in the Indian Ocean and the Red Sea. Most reported data in this geographical area are associated with seafood poisoning caused by different species of puffer fish through the recognition of TTX poisoning symptoms and not by TTX detection techniques. This scenario shows the need of data regarding TTX prevalence, geographical distribution, and its vectors in this area to better assess human health risk and build effective monitoring programs to protect the health of consumers in Indian Ocean area.
Keywords: Indian Ocean, Red Sea, tetrodotoxin, pufferfish poisoning
1. Introduction
The tropical and subtropical climates predominant in the Indian Ocean zone, accompanied by industrialization and population increase, are pointed to as the main factors that, together with eutrophication, contribute to the development of toxic phytoplankton blooms—harmful algal blooms (HABs) and bacteria [1]. HABs and some bacteria are marine toxin (MT) producers, turning the Indian Ocean zone vulnerable to this phenomenon [2,3,4,5]. One of the main Indian Ocean MTs is tetrodotoxin (a neurotoxin) and its analogs (TTXs), of which the main producers were reported to belong to different bacteria genera [6,7,8,9,10,11,12,13,14,15]. Cases of human poisoning are recurrent, especially after consumption of TTX-contaminated fish, with the puffer fish as the most common vector reported since Egyptian times [16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Due to the lack of TTX monitoring programs, the episodes of human seafood poisoning are still common in the Indian Ocean area, since seafood is the most common food for many people living along coastal zones [16,17,18,19,20,21,22,24,26,28,29,30,31,32,33,34,35,36,37,38]. Thus, the objective of this paper was to review the incidence of TTX in the Indian Ocean and the Red Sea zones and associated human seafood poisoning incidents. The monitoring of TTXs in this geographic zone is also recommended.
2. Tetrodotoxin
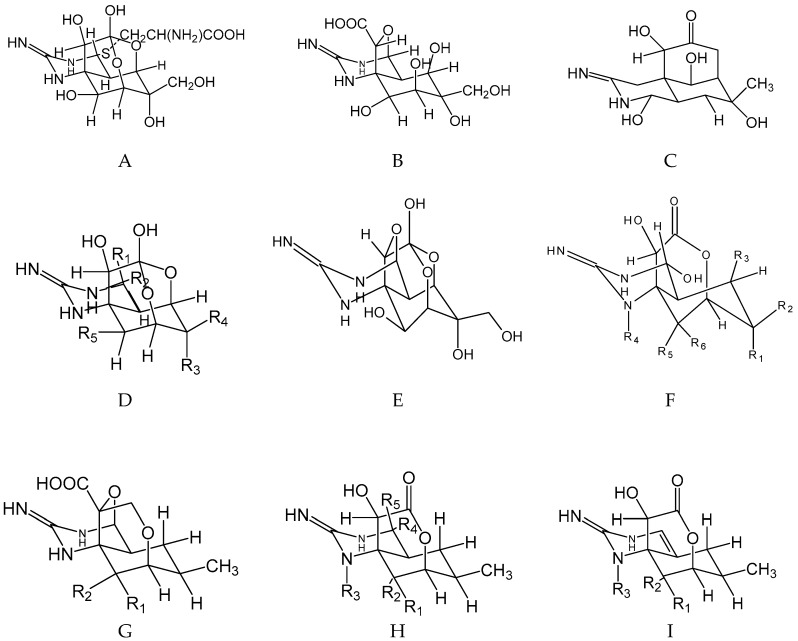
TTX (Figure 1) is a potent neurotoxin group [39] that can provoke severe poisoning after consumption of contaminated seafood. Several species of distinct marine organisms of human consumption were identified as TTX vectors: puffer fish [16,17,18,19,20,21,22,23,24,25,26,27,28,29], gastropods [40], crustaceans [41,42,43,44], and bivalves [45]. Also, the occurrence of TTXs in terrestrial vertebrates such as Polypedates sp., Atelopus sp., Taricha granulosa, [46] and Cynops ensicauda popei [47] was reported [48,49]. TTX is an alkaloid isolated for the first time in 1909 by Tahara and Hirata from the ovaries of globefish [50]. In the marine environment, bacteria are pointed to as the main producers of this group of toxins, namely Serratia marcescens [51], Vibrio alginolyticus, V. parahaemolyticus, Aeromonas sp. [52], Microbacterium arabinogalactanolyticum [13], Pseudomonas sp. [14], Shewanella putrefaciens [6], Alteromonas sp. [8], Pseudoalteromonas sp. [10], and Nocardiopsis dassonvillei [12]. Physicochemically, TTXs are colorless, crystalline weak heterocyclic basic compounds (Figure 1 and Table 1), highly hydro-soluble and also heat-stable [45]; thus, the toxin is not destroyed by cooking procedures.
Figure 1.
Tetrodotoxin (TTX) and analogs modified from European Food Safety Authority (EFSA) 2017 [45] and Yotsu-Yamasshita et al. (2007) [15,53,54]. (*) indicates TTX analogs that occur in marine organisms with known relative toxicity. (A) 4-cysTTX(*), (B) tetrodonic acid, (C) 4,9-anhydroTTX(*), (D) 1-hydroxy-5,11-dideoxyTTX, (E) TTX and 12 analogs, (F) 5-deoxyTTX(*) and three analogs, (G) trideoxyTTX and two analogs, (H) 4-epi-5,6,11-trideoxyTTX and another analog, and (I) 4,4a-anhydro-5,6,11-trideoxyTTX and 1-hydroy-4,4a-anhydro-8-epi-5,5,11-trideooxyTTX (see radicals of the analogs in the Table 1).
Table 1.
Tetrodotoxin (TTX) and analogs shown in Figure 1 and modified from European Food Safety Authority (EFSA) 2017 [45] and Yotsu-Yamasshita et al. (2007) [15,53].
| E | R1 | R2 | R3 | R4 | R5 | |
| TTX (*) | H | OH | OH | CH2OH | OH | |
| 4-epiTTX (*) | OH | H | OH | CH2OH | OH | |
| 6-epiTTX (*) | H | OH | CH2OH | OH | OH | |
| 11-deoxyTTX (*) | H | OH | OH | CH3 | OH | |
| 6,11-dideoxyTTX | H | OH | H | CH3 | OH | |
| 8,11-dideoxyTTX | H | OH | OH | CH3 | H | |
| 11-oxoTTX (*) | H | OH | OH | CH(OH)2 | OH | |
| 11-norTTX-6,6-diol | H | OH | OH | OH | OH | |
| 11-norTTX-6(R)-ol (*) | H | OH | H | OH | OH | |
| 11-norTTX-6(S)-ol (*) | H | OH | OH | H | OH | |
| Chiriquitoxin | H | OH | OH | CH(OH)CH(NH3+)COO− | OH | |
| TTX-8-O-hemisuccinate | H | OH | OH | CH2OH | OOC(CH2)2COO− | |
| TTX-11-carboxylic acid | H | OH | OH | COO− | OH | |
| TTX (*) | H | OH | OH | CH2OH | OH | |
| F | R1 | R2 | R3 | R4 | R5 | R6 |
| 5-deoxyTTX(*) | OH | CH2OH | H | H | OH | H |
| 5,11-dideoxyTTX (*) | OH | CH3 | H | H | OH | H |
| 5,6,11-trideoxyTTX (*) | H | CH3 | H | H | OH | H |
| 8-epi-5,6,11-trideoxyTTX | H | CH3 | H | H | H | OH |
| G | R1 | R2 | ||||
| 4,9-anhydro-5,6,11-trideoxyTTX | H | OH | ||||
| 4.9-anhydro-8-epi-5,6,11-trideoxyTTX | OH | H | ||||
| H | R1 | R2 | R3 | R4 | R5 | |
| 1-hydroxy-8-epi-5,6,11-trideoxyTTX | OH | H | OH | OH | H | |
| 4-epi-5,6,11-trideoxyTTX | H | OH | H | H | OH | |
| I | R1 | R2 | R3 | |||
| 4,4a-anhydro-5,6,11-trideoxyTTX | H | OH | H | |||
| 1-hydroxy-4,4a-anhydro-8-epi-5,5,11-trideooxyTTX | OH | H | OH |
To date, around 28 analogs of TTX were described (Figure 1 and Table 1) and some of them were detected in marine organisms [53], with their relative toxicity well known [45] (chemical structures pointed with asterisks in Figure 1): TTX, 11-oxoTTX, 11-deoxyTTX, 11-norTTX-6(R)-ol, 11-norTTX-6(S)-ol, 4-epiTTX, 4,9-anhydroTTX, 5,6,11-trideoxyTTX [45], 4-CysTTX, 5-deoxyTTX, 5,11-dideoxyTTX, and 6,11-dideoxyTTX [54,55,56,57] (Table 1). Their relative toxicity ranges from 0.01 to 1.0, with 5,6,11-trideoxyTTX and TTX as the least and most toxic, respectively [45], and there are still no available data regarding the toxicity for 4-CysTTX and 5,11-dideoxyTTX. Chemical abstract numbers (CAS) are also listed in Table 2.
Table 2.
| TTX Analogs | TEF | CAS Number |
|---|---|---|
| TTX | 1.0 | 4368-28-9 |
| 11-oxoTTX | 0.75 | 123665-88-3 |
| 11-deoxyTTX | 0.14 | - |
| 11-norTTX-6(R)-ol | 0.17 | - |
| 11-norTTX-6(S)-ol | 0.19 | - |
| 4-epiTTX | 0.16 | 98242-82-1 |
| 4,9-anhydroTTX | 0.02 | 13072-89-4 |
| 6,11-dideoxyTTX | 0.02 | - |
| 5-deoxyTTX | 0.01 | - |
| 5,6,11-trideoxyTTX | 0.01 | - |
| 4-CysTTX | - | - |
| 5,11-dideoxyTTX | - | - |
* TEF—toxic equivalency factor.
The action mechanism of TTXs occurs through the occlusion of the external pore of site 1 of voltage-gated sodium channels on the surface of nerve membranes, blocking cellular communication and causing death by cardio-respiratory paralysis [60]. Paralysis occurs by affecting the respiratory system, the diaphragm, skeletal muscles, and tissues in the digestive tract in humans [39]. TTXs normally accumulate in skin, intestines, liver, muscle, gonads, viscera, and ovaries in different species of puffer fish [16,21,22,29,33,34,35,36,37,61,62,63,64,65]. The symptoms that can be used partially as an indication of TTX human poisoning (wt = 50 kg and TTX amount = 2 mg) were grouped into four levels depending on the amount ingested [66] and are described in Table 3. These symptoms normally appear 40 min after consumption of contaminated food and, in some cases, even six hours after [67].
Table 3.
Characteristic symptoms of TTX human poisoning modified from Noguchi and Ebesu (2001) [66].
| Level | Affected System | Specific Symptoms |
|---|---|---|
| 1 | Neuromuscular | Paresthesia of lips, tongue, and pharynx, taste disturbance, dizziness, headache, diaphoresis, pupillary constriction |
| Gastrointestinal | Salivation, hypersalivation, nausea, vomiting, hyperemesis, hematemesis, hypermotility, diarrhea, abdominal pain | |
| 2 | Neuromuscular | Advanced general paresthesia, paralysis of phalanges and extremities, pupillary dilatation, reflex changes |
| 3 | Neuromuscular | Dysarthria, dysphagia, aphagia, lethargy, incoordination, ataxia, floating sensation, cranial nerve palsies, muscular fasciculation |
| Cardiovascular/pulmonary | Hypotension or hypertension, vasomotor blockade, cardiac arrhythmias, atrioventricular node conduction abnormalities, cyanosis, pallor, dyspnea | |
| Dermatologic | Exfoliative dermatitis, petechiae, and blistering | |
| 4 | Respiratory failure, impaired mental faculties, extreme hypotension, seizures, loss of deep tendon and spinal reflexes | |
Currently, there is no antidote for TTX; however, some studies indicate that the application of activated charcoal could help in reversing the clinical stage of poisoning victims since it reduces the toxin free amount [68]. Also, alkaline gastric lavage with sodium bicarbonate (2%) is indicated as a treatment within the first hour of the incident, due to TTX instability in alkaline media [69]. Another clinical intervention recommendation is the use of cholinesterase inhibitors such as neostigmine [28], and mechanical respiratory help may reduce mortality probability by muscle paralysis [38].
3. TTX Detection Methods
Several methodologies were developed to analyze TTXs and, in recent years, chemical methods became more popular due to their sensitivity with limits of detection (LODs) ranging from 0.9 ng to 0.063 μg. Liquid chromatography with tandem mass spectrometry (LC–MS/MS) techniques, the first choice compared to mouse bioassays (MBAs) and enzymatic methods due to their greater sensitivity and specificity, have the capacity to detect and determine TTXs in complex matrices [70]. Also, due to ethical reasons and lack of specificity, MBA fell into disuse, with the latter reason also attributed to the enzymatic methods. When a poisoning case occurs, it is recommended, when available, to screen the liver, muscle, skin, gonads, and ovaries of the suspected poisoning marine vector samples [28,36,40,41,42,53,54,55,56,62,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. Human urine and plasma should also be analyzed for TTX in these cases [80].
Methods for TTX analysis and their respective limits of quantification (LOQs) and detection (LODs) are described in Table 4 and include the mouse bioassay [12,36,52,89], receptor-based assay [90], immunoassay [31,36,52,73,77,82,89,91,92,93], thin-layer chromatography [13,72], high-performance liquid chromatography [84,94,95], gas chromatography–mass spectrometry [76,84,95], liquid chromatography coupled to mass spectrometry [33,40,96,97,98], surface plasmon resonance [30], and liquid chromatography with fluorescence detection (FLD) [15,32,89].
Table 4.
TTX detection methods, their limits of quantification (LOQs), limits of detection (LODs), and toxicity equivalency factors (TEFs) according to the European Food Safety Authority (EFSA). MBA—mouse bioassay; FLD—fluorescence detection; RB—receptor-based; LC—liquid chromatography; MS—mass spectrometry; HPLC—high-performance liquid chromatography; UVD—ultraviolet detection; SPR—surface plasmon resonance; TLC—thin-layer chromatography; GC—gas chromatography.
| Analysis Method | LOD | LOQ |
|---|---|---|
| MBA [12,36,52,89] | 1.1 μg·g−1 [89] | - |
| Enzymatic assays [31,36,52,73,77,82,89,91,92,93] | 2 ng·mL−1 [92] | - |
| TLC–MS [13,72] | 0.1 μg [72] | - |
| HPLC–FLD [84,94,95] | 1.27 μg·g−1 [94] | |
| GC–MS [76,84,95] | 0.5 μg·g−1 [76] | 1.0 μg·g−1 [76] |
| LC–MS/MS/UPLC–MS/MS [33,40,96,97,98] | 0.09–16 ng·mL−1 [33,40,96,97,98] | 5–63 ng·mL−1 [40] |
| SPR [30] | 0.3–20 ng·mL−1 [30] | - |
| HPLC–FLD [15,32,99] | 40-100 ng·g−1 [15] | - |
4. Geographic Occurrence and Incidence of TTXs in the Indian Ocean and the Red Sea
As described in the introduction section, TTXs were reported in several marine organisms [71], regarding poisoning incidents [71]; the main TTX vectors involved in the Indian Ocean and the Red Sea (Table 4) belong to the Tetraodontidae family: Arothron hispidus in India [65], Takifugu oblongus in Bangladesh [16,33] and India [35,62], Lageocephalus scitalleratus in Singapure [20], Pleuranacanthus sceleratus in Egypt [21,34,37], Reunion Island [29], and Australia [23,24], Chelonodon pataca, Sphaeroides oblongus, Lagocephalus inermis, and Lagocephalus lunaris in India [35,62], Xenopterus naritus in Malaysia [63], Arothron stellatus in India [64], Tetractenos hamiltoni in Australia [80,100], and Tetroadon sp. [17], Tetraodon nigroviridis, and Arothron reticularis in Thailand [99]. The records of TTX occurrence in other marine species such as mollusks are scarce in the Indian Ocean. Gastropods were reported as TTX vectors in other locations: Charonia lampas [85], Gibbula umbilicalis, and Monodonta lineata on the Portuguese coast [40], Nassarius spp. in China [94], Polinices didyma, Natica lineata [84,101], Oliva miniacea, O. mustelina, and O. nirasei [95] in Taiwan, Charonia sauliae [102], Babylonia japonica [86], Niotha spp. [75,81], and Tutufa lissostoma [103] in Japanese crabs, Demania cultripes, Demania toxica, Demania reynaudi, Lophozozymus incises, Lophozozymus pictor, Atergatis floridus [104], and Atergatopsis germaini [83], highlightinh these organisms as potential indicator species [11]. Data on these groups are scarce in the Indian Ocean area, suggesting that further studies and monitoring programs for TTXs are needed. Available data regarding this geographic region are displayed in Table 5.
Table 5.
The incidence of TTXs in the Indian Ocean. NPI—no poisoning incidents, MBA—mouse bioassay; FLD—fluorescence detection; LC—liquid chromatography; MS—mass spectrometry; HPLC—high-performance liquid chromatography; UVD—ultraviolet detection; TLC—thin-layer chromatography; GC—gas chromatography.
| Producing Species | Vector | Sample Tissue | Location | Country | Poisoning Date | TTX | Detection | Maximum Concentration | Poisoning Victims | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Australia | ||||||||||
| Unknown | Puffer fish Lagocephalus scleratus | Close to Fremantle Hospital | Australia | 13 May 1996 | TTX | Symptomatology | - | 3 people | [23] | |
| Unknown | Puffer fish Lagocephalus scleratus | Port Hedland | Australia | 1998 | TTX | Symptomatology | - | 1 person | [24] | |
| Unknown | Toad fish Tetractenos hamiltoni | New South Wales | Australia | 1 January 2001 to 13 April 2002 | TTX | Symptomatology | - | 11 people | [100] | |
| Unknown | Toad fish Tetractenos hamiltoni | Urine | Australia | 2004 | TTX | HPLC–UVD | 5 ng/mL | 7 people | [80] | |
| Serum | 20 ng/mL | |||||||||
| Asian countries | ||||||||||
| Unknown | Puffer fish | Khulna | Bangladesh | April 18 2002 | TTX | Symptomatology | - | 45 people | [27] | |
| Unknown | Puffer fish Takifugu oblongus | Skin | Khulna | Bangladesh | 18 May 2002 | TTX | MBA | 18.9 MU/g | 36 people, 7 deaths | [16] |
| Muscle | 4.4 MU | |||||||||
| Liver | 4.9 MU/g | |||||||||
| Gonads | 132.0 MU/g | |||||||||
| Viscera categories | 37.0 MU/g | |||||||||
| Natore | - | |||||||||
| Dhaka | ||||||||||
| Unknown | Puffer fish | Liver | Khulna | Bangladesh | 24 July 2005 | TTX | Symptomatology | - | 6 people | [22] |
| Unknown | Skin | Khulna | Bangladesh | 25 March 2006 | TTX | LC–MS/MS | 25.35 μg·g−1 | NPI | [33] | |
| Anhydro | 7.71 μg·g−1 | |||||||||
| 11-Deoxy | 1.12 μg·g−1 | |||||||||
| Trideoxy | 15.31 μg·g−1 | |||||||||
| Muscle | TTX | 1.64 μg·g−1 | ||||||||
| Anhydro | - | |||||||||
| 11-Deoxy | - | |||||||||
| Trideoxy | - | |||||||||
| Liver | TTX | 45.71 μg·g−1 | ||||||||
| Anhydro | 29.17 μg·g−1 | |||||||||
| 11-Deoxy | - | |||||||||
| Trideoxy | 9.09 μg·g−1 | |||||||||
| Ovary | TTX | 356.00 μg·g−1 | ||||||||
| Anhydro | 85.87 μg·g−1 | |||||||||
| 11-Deoxy | 26.00 μg·g−1 | |||||||||
| Trideoxy | 2,929.70 μg·g−1 | |||||||||
| Unknown | Puffer fish | Dhaka | Bangladesh | 2008 | TTX | Symptomatology | - | 11 people | [25] | |
| Unknown | Puffer Fish | Narshingdi | Bangladesh | April and June 2008 | TTX | Symptomatology | - | 95 people, 14 deaths | [26] | |
| Natore | ||||||||||
| Dhaka | ||||||||||
| Unknown | Puffer Fish | Dhaka City | Bangladesh | October 2014 | TTX | Symptomatology | - | 11 people, 4 deaths | [18] | |
| Unknown | Puffer fish | - | Khulna | Bangladesh | - | TTX | Symptomatology | - | 37 people, 8 deaths | [28] |
| Unknown | Puffer fish Chelonodon patoca | Liver | Bay of Bengal | India | June 1998 to March 2001 | TTX | MBA | 25.9 MU/g | NPI | [61] |
| Ovary | 183 MU/g | |||||||||
| Sphaeroides oblongus | Liver | 16 MU/g | ||||||||
| Ovary | 7.9 MU/g | |||||||||
| Lagocephalus inermis | Liver | 5.5 MU/g | ||||||||
| Ovary | 28.9 MU/g | |||||||||
| Lagocephalus lunaris | Liver | 5.9 MU/g | ||||||||
| Ovary | 16.6 MU/g | |||||||||
| Unknown | Puffer fish Chelenodon potoca | Liver | Bengal coast | India | June 2000–March 2001 | TTX | MBA | 27.8 MU/g | NPI | [35] |
| Ovary | 156.7 MU/g | |||||||||
| Takifugu oblongus | Liver | 11.75 MU/g | ||||||||
| Ovary | 29.1 MU/g | |||||||||
| Lagocephalus lunaris | Liver | 9 MU/g | ||||||||
| Ovary | 30.1 MU/g | |||||||||
| Lagocephalus inermis | Liver | 5.7 MU/g | ||||||||
| Ovary | 9.64 MU/g | |||||||||
| Kytococcus sedentarius | Puffer fish Arothron hispidus | Skin | Annankil fish landings at Parangipettai | India | 2010 | TTX | MBA | - | NPI | [65] |
| Intestine | - | |||||||||
| Liver | - | |||||||||
| Cellulomonas fimi | Muscle | 4.4 MU | ||||||||
| Liver | 4.9 MU/g | |||||||||
| Gonads | 132.0 MU/g | |||||||||
| Bacillus lentimorbus | Viscera categories | 37.0 MU/g | ||||||||
| Natore | - | |||||||||
| Dhaka | - | |||||||||
| Unknown | Puffer fish Arothron stellatus | Muscles | Parangipettai | India | 2016 | TTX | HPLC–FLD, TLC–UVD | Qualitative | NPI | [64] |
| Gonads | 4-epi | |||||||||
| Liver | anhydro | |||||||||
| Unknown | Puffer fish Takifugu oblongus | Skin | Kasimedu fishing harbor, Chennai, Tamil Nadu | India | 2016 | TTX | MBA | 75.88 MU/g | NPI | [62] |
| GC–MS | 16.5 MU/g | |||||||||
| HPLC | 18 MU/g | |||||||||
| Liver | MBA | 143.33 MU/g | ||||||||
| GC–MS | 32.5 MU/g | |||||||||
| HPLC | 48 MU/g | |||||||||
| Ovary | MBA | 163 MU/g | ||||||||
| GC–MS | 34.5 μg | |||||||||
| HPLC | 51 μg | |||||||||
| Unknown | Puffer fish | - | Johor | Malaysia | May 2008 | TTX | Symptomatology | - | 34 people | [68] |
| Unknown | Carcinoscorpius rotundicauda | Urine | Kota Marudu | Malaysia | June–August 2011 | TTX | GC–MS | 1.3–602 ng/mL | 30 people | [88] |
| Unknown | Puffer fish Xenopterus naritus | Muscle | Manggut | Malaysia | February and July 2013 | TTX | LC–MS/MS | 27.19 μg/g | NPI | [63] |
| Kaong | 16.09 μg/g | |||||||||
| Unknown | Puffer fish Lageocephalus scitalleratus | Alexandra Hospital | Singapore | 2013 | TTX | Symptomatology | 1 person | [20] | ||
| Unknown | Tetraodon nigroviridis | Reproduc tive tissue | Satun | Thailand | April to July 2010 | TTX | LC–MS/MS, MBA | 63.57 MU/g | NPI | [36] |
| Liver | 97.08 MU/g | |||||||||
| Digestive tissue | 43.33 MU/g | |||||||||
| Muscle | 22.12 MU/g | |||||||||
| Arothron reticularis | Reproductive tissue | - | ||||||||
| Liver | 2.08 MU/g | |||||||||
| Digestive tissue | 3.16 MU/g | |||||||||
| Muscle | 4.02 MU/g | |||||||||
| African countries | ||||||||||
| Unknown | Puffer fish Lagocephalus lunaris | Gonads | National Research Center, Dokki, Cairo, | Egypt | September 1990 through May 1991 | TTX | TLC–UVD, MBA | 752 MU/g | NPI | [34] |
| Liver | 246 MU/g | |||||||||
| Muscles | 127 MU/g | |||||||||
| Digestive tract | 221 MU/g | |||||||||
| Skin | 119 MU/g | |||||||||
| Unknown | Puffer fish Lagocephalus sceleratus | Gonads | Attaka fishing harbor | Egypt | October 2002 and June 2003 | TTX | MBA | 3950 MU/g | NPI | [37] |
| Unknown | Puffer fish Lagocephulus scleratus | Muscle | Suez Gulf | Egypt | 23 December 2004 | TTX | 7 people | [21] | ||
| Unknown | Puffer fish | Nosy Be Island | Madagascar | July 1998 | TTX | MBA | 16 UM/g | 3 people, 1 death | [19] | |
| Unknown | Puffer fish Lagocephalus sceleratus | Liver | Reunion Island | Reunion Island | 10 September 2013 | TTX | MBA, LC–MS/MS | 95 MU/g | 10 people | [29] |
| Flesh | 5 MU/g | |||||||||
| Unknown | Puffer fish, Tetraodontidae family | Zanzibar | Tanzania | TTX | Symptomatology | - | 1 death | [17] | ||
5. Final Considerations
TTX data in the Indian Ocean and Red Sea are usually related to fatal outbreaks due to seafood poisoning and not to scientific research, indicating the lack of MT monitoring programs. The symptomatology reports and MBA are used to identify seafood poisoning caused by TTX and analogs, indicating the need for analytical methods such as liquid chromatography to obtain better quantitative data. Both symptomatology and MBA in isolation are not enough to conclude that TTXs are the causative agent of seafood poisoning, since there are other toxins (PSTs) with similar action mechanism that overlap in symptomatology with TTX poisoning. Additionally, MBA cannot discriminate between the different TTX analogs. MBA and symptomatology are used in countries of the Indian Ocean and the Red Sea to identify TTX poisoning due to the lack of availability and accessibility to chemical methods and the absence of TTX monitoring programs.
Thus, the implementation of monitoring programs using chemical analytical methods such as LC–MS/MS instead of MBA in the Indian Ocean and the Red Sea is urgently needed in different species of shellfish and puffer fish, including Arothron hispidus, Takifugu oblongus, Lageocephalus scitalleratus, Pleuranacanthus sceleratus, Chelonodon patoca, Sphaeroides oblongus, Lagocephalus inermis, Lagocephalus lunaris, Xenopterus naritus, Arothron stellatus, Tetractenos hamiltoni, Tetraodon nigroviridis, Arothron reticularisand, Charonia sauliae, Babylonia japonica, Niotha spp., and Tutufa lissostoma, since they are most consumed and are already confirmed to be vectors of TTX in the Indian Ocean and the Red Sea. These species can be used as indicators for monitoring programs using the maximum limit permitted of 2 mg·kg−1 (from Japan).
Acknowledgments
We acknowledge the project H2020 RISE project EMERTOX—Emergent Marine Toxins in the North Atlantic and Mediterranean: New Approaches to Assess their Occurrence and Future Scenarios in the Framework of Global Environmental Changes—Grant Agreement No. 778069.
Author Contributions
V.V. and M.S. conceived the idea. I.J.T. drafted the manuscript. The final version of the manuscript was approved by all authors.
Funding
This research was partially supported by the framework of the Atlantic Interreg project ALERTOXNET—Network for introduction of Innovative Toxicity Alert Systems for safer seafood products—EAPA_317/2016.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hallegraeff G.M. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. doi: 10.2216/i0031-8884-32-2-79.1. [DOI] [Google Scholar]
- 2.Onuma Y., Satake M., Ukena T., Roux J., Chanteau S., Rasolofonirina N., Ratsimaloto M., Naoki H., Yasumoto T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon. 1999;37:55–65. doi: 10.1016/S0041-0101(98)00133-0. [DOI] [PubMed] [Google Scholar]
- 3.Mbaé S.B.A., Mlindassé M., Mihidjaé S., Seyler T. Food-poisoning outbreak and fatality following ingestion of sea turtle meat in the rural community of Ndrondroni, Mohéli Island, Comoros, December 2012. Toxicon. 2016;120:38–41. doi: 10.1016/j.toxicon.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Ranaivoson G., de Ribes Champetier G., Mamy E.R., Jeannerod G., Razafinjato P., Chanteau S. Mass food poisoning after eating sea turtle in the Antalaha district. Arch. Inst. Pasteur Madagascar. 1994;61:84–86. [PubMed] [Google Scholar]
- 5.Boisier P., Ranaivoson G., Rasolofonirina N., Roux J., Chanteau S., Takeshi Y. Fatal mass poisoning in Madagascar following ingestion of a shark (Carcharhinus leucas): Clinical and epidemiological aspects and isolation of toxins. Toxicon. 1995;33:1359–1364. doi: 10.1016/0041-0101(95)00051-M. [DOI] [PubMed] [Google Scholar]
- 6.Auawithoothij W., Noomhorm A. Shewanella putrefaciens, a major microbial species related to tetrodotoxin (TTX)-accumulation of puffer fish Lagocephalus lunaris. J. Appl. Microbiol. 2012;113:459–465. doi: 10.1111/j.1365-2672.2012.05339.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng C.A., Hwang D.F., Tsai Y.H., Chen H.C., Jeng S.S., Noguchi T., Ohwada K., Hasimoto K. Microflora and tetrodotoxin-producing bacteria in a gastropod, Niotha clathrata. Food Chem Toxicol. 1995;33:929–934. doi: 10.1016/0278-6915(95)00061-6. [DOI] [PubMed] [Google Scholar]
- 8.Hwang D.F., Arakawa O., Saito T., Noguchi T., Simidu U., Tsukamoto K., Shida Y., Hashimoto K. Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus. Mar Biol. 1989;100:327–332. doi: 10.1007/BF00391147. [DOI] [Google Scholar]
- 9.Lee M.J., Jeong D.Y., Kim W.S., Kim H.D., Kim C.H., Park W.W., Park Y.H., Kim K.S., Kim H.M., Kim D.S. A tetrodotoxin-producing Vibrio strain, LM-1, from the puffer fish Fugu vermicularis radiatus. Appl. Environ. Microbiol. 2000;66:1698–1701. doi: 10.1128/AEM.66.4.1698-1701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie K.B., Nagelkerken I., James S., Smith G.W. Environmental microbiology: A tetrodotoxin-producing marine pathogen. Nature. 2000;404:354. doi: 10.1038/35006168. [DOI] [PubMed] [Google Scholar]
- 11.Silva M., Pratheepa V.K., Botana L.M., Vasconcelos V. Emergent toxins in North Atlantic temperate waters: A challenge for monitoring programs and legislation. Toxins. 2015;7:859–885. doi: 10.3390/toxins7030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z., Xie L., Xia G., Zhang J., Nie Y., Hu J., Wang S., Zhang R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon. 2005;45:851–859. doi: 10.1016/j.toxicon.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Yu C.-F., Yu P.H.-F., Chan P.-L., Yan Q., Wong P.-K. Two novel species of tetrodotoxin-producing bacteria isolated from toxic marine puffer fishes. Toxicon. 2004;44:641–647. doi: 10.1016/j.toxicon.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Yotsu M., Yamazaki T., Meguro Y., Endo A., Murata M., Naoki H., Yasumoto T. Production of tetrodotoxin and its derivatives by Pseudomonas sp. isolated from the skin of a pufferfish. Toxicon. 1987;25:225–228. doi: 10.1016/0041-0101(87)90245-5. [DOI] [PubMed] [Google Scholar]
- 15.Yotsu-Yamashita M., Mebs D., Kwet A., Schneider M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp.; Urodela, Salamandridae) from southern Germany. Toxicon. 2007;50:306–309. doi: 10.1016/j.toxicon.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed S. Puffer fish tragedy in Bangladesh: An incident of Takifugu oblongus poisoning in Degholia, Khulna. Afr. J. Mar. Sci. 2006;28:457–458. doi: 10.2989/18142320609504197. [DOI] [Google Scholar]
- 17.Chopra S.A. A case of fatal puffer-fish poisoning in a Zanzibari fisherman. East. Afr. Med. J. 1967;44:493–496. [PubMed] [Google Scholar]
- 18.Rafiqui Islam M., Chowdhury F.R., Das S.K., Rahman S., Mahmudur M.D., Amin M.D.R. Outbreak of Puffer Fish Poisoning in Dhaka City. J. Med. 2018;19:30–34. doi: 10.3329/jom.v19i1.34837. [DOI] [Google Scholar]
- 19.Ravaonindrina N., Andriamaso T.H., Rasolofonirina N. Puffer fish poisoning in Madagascar: Four case reports. Arch. Inst. Pasteur Madagascar. 2001;67:61–64. [PubMed] [Google Scholar]
- 20.Yong Y.S., Quek L.S., Lim E.K., Ngo A. A case report of puffer fish poisoning in Singapore. Case Rep. Med. 2013;2013 doi: 10.1155/2013/206971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaki M.A., Mossa A.E.W. Red Sea puffer fish poisoning: Emergency diagnosis and management of human intoxication. Egypt. J. Aquat. Res. 2005;31:370–378. [Google Scholar]
- 22.Chowdhury F.R., Ahasan H.A.M.N., Al Mamun A., Rashid A.K.M.M., Al Mahboob A. Puffer fish (Tetrodotoxin) poisoning: An analysis and outcome of six cases. Trop. Dr. 2007;37:263–264. doi: 10.1258/004947507782333017. [DOI] [PubMed] [Google Scholar]
- 23.Ellis R., Jelinek G.A. Never eat an ugly fish: Three cases of tetrodotoxin poisoning from Western Australia. Emerg. Med. 1997;9:136–142. doi: 10.1111/j.1442-2026.1997.tb00369.x. [DOI] [Google Scholar]
- 24.Field J. Puffer fish poisoning. Emerg. Med. J. 1998;15:334–336. doi: 10.1136/emj.15.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghose A., Ahmed H., Basher A., Amin M.R., Sayeed A.A., Faiz M.A. Tetrodotoxin poisoning in Blangadesh: A case study. J. Med. Toxicol. 2008;4:216. [Google Scholar]
- 26.Homaira N., Rahman M., Luby S.P., Rahman M., Haider M.S., Faruque L.I., Khan D., Parveen S., Gurley E.S. Multiple outbreaks of puffer fish intoxication in Bangladesh, 2008. Am. J. Trop. Med. Hyg. 2010;83:440–444. doi: 10.4269/ajtmh.2010.10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NàzmuíAhêsan H.A.M., AbdutfàhAíMâmun C.H.R. Puffer fish poisoning: A clinical analysis. Pak. J. Med. Sci. 2003;19:29–32. [Google Scholar]
- 28.Nazmul A., Al Mamun A., Rasul C.H., Roy P.K. Puffer fish poisoning (tetrodotoxin) in Bangladesh: Clinical profile and role of anticholinesterase drugs. Trop. Dr. 2005;35:235–236. doi: 10.1258/004947505774938549. [DOI] [PubMed] [Google Scholar]
- 29.Puech B., Batsalle B., Roget P., Turquet J., Quod J.P., Allyn J., Idoumbin J.P., Chane-Ming J., Villefranque J., Mougin-Damour K., et al. Family tetrodotoxin poisoning in Reunion Island (Southwest Indian Ocean) following the consumption of Lagocephalus sceleratus (Pufferfish) Bull. Soc. Pathol. Exot. 2014;107:79–84. doi: 10.1007/s13149-014-0340-2. [DOI] [PubMed] [Google Scholar]
- 30.Taylor A.D., Vaisocherová H., Deeds J., DeGrasse S., Jiang S. Tetrodotoxin detection by a surface plasmon resonance sensor in pufferfish matrices and urine. J. Sens. 2011;2011 doi: 10.1155/2011/601704. [DOI] [Google Scholar]
- 31.Brillantes S., Samosorn W., Faknoi S., Oshima Y. Toxicity of puffers landed and marketed in Thailand. Fish. Sci. 2003;69:1224–1230. doi: 10.1111/j.0919-9268.2003.00749.x. [DOI] [Google Scholar]
- 32.Islam Q.T., Razzak M.A., Islam M.A., Bari M.I., Basher A., Chowdhury F.R., Sayeduzzaman A.B.M., Ahasan H.A.M.N., Faiz M.A., Arakawa O., et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011;105:74–80. doi: 10.1016/j.trstmh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Diener M., Christian B., Ahmed M.S., Luckas B. Determination of tetrodotoxin and its analogs in the puffer fish Takifugu oblongus from Bangladesh by hydrophilic interaction chromatography and mass-spectrometric detection. Anal. Bioanal. Chem. 2007;389:1997. doi: 10.1007/s00216-007-1602-7. [DOI] [PubMed] [Google Scholar]
- 34.El-Sayed M., Yacout G.A., El-Samra M., Ali A., Kotb S.M. Toxicity of the Red Sea pufferfish Pleuranacanthus sceleratus “El-Karad”. Ecotoxicol. Environ. Saf. 2003;56:367–372. doi: 10.1016/S0147-6513(02)00142-2. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh S., Hazra A.K., Banerjee S., Mukherjee B. The Seasonal Toxicological Profile of Four Puffer Fish Species Collected Along Bengal Coast, India. Indian J. Mar. Sci. 2004;33:276–280. [Google Scholar]
- 36.Chulanetra M., Sookrung N., Srimanote P., Indrawattana N., Thanongsaksrikul J., Sakolvaree Y., Chongsa-Nguan M., Kurazono H., Chaicumpa W. Toxic marine puffer fish in Thailand seas and tetrodotoxin they contained. Toxins. 2011;3:1249–1262. doi: 10.3390/toxins3101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabrah M.M., El-Ganainy A.A., Zaky M.A. Biology and toxicity of the pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the Gulf of Suez. Egypt. J. Aquat. Res. 2006;32:283–297. [Google Scholar]
- 38.Haque M.A., Islam Q.T., Ekram A.R.M.S. Puffer fish poisoning. TAJ J. Teach. Assoc. 2008;21:199–202. doi: 10.3329/taj.v21i2.3806. [DOI] [Google Scholar]
- 39.Vasconcelos V., Azevedo J., Silva M., Ramos V. Effects of marine toxins on the reproduction and early stages development of aquatic organisms. Mar. Drugs. 2010;8:59–79. doi: 10.3390/md8010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva M., Azevedo J., Rodriguez P., Alfonso A., Botana L.M., Vasconcelos V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs. 2012;10:712–726. doi: 10.3390/md10040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguchi T., Jeon J.K., Arakawa O., Sugita H., Deguchi Y., Shida Y., Hashimoto K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J. Biochem. 1986;99:311–314. doi: 10.1093/oxfordjournals.jbchem.a135476. [DOI] [PubMed] [Google Scholar]
- 42.Kanchanapongkul J., Krittayapoositpot P. An epidemic of tetrodotoxin poisoning following ingestion of the horseshoe crab Carcinoscorpius rotundicauda. Vertigo. 1995;30:42. [PubMed] [Google Scholar]
- 43.Kungsuwan A., Suvapeepan S., Suwansakornkul P. Tetrodotoxin in the horseshoe crab Carcinoscorpius rotundicauda inhabiting Thailand. Nippon Suisan Gakkaishi. 1987;53:261–266. doi: 10.2331/suisan.53.261. [DOI] [Google Scholar]
- 44.Ngya L., Yu C.-F., Takatani T., Arakawa O. Toxicity assessment for the horseshoe crab Carcinoscorpius rotundicauda collected from Cambodia. Toxicon. 2007;49:843–847. doi: 10.1016/j.toxicon.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 45.EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) Knutsen H.K., Alexander J., Barreg ard L., Bignami M., Br€uschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., et al. Roudo 2017. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017;15:4752–4817. doi: 10.2903/j.efsa.2017.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanifin C.T., Yotsu-Yamashita M., Yasumoto T., Brodie E.D. Toxicity of dangerous prey: Variation of tetrodotoxin levels within and among populations of the newt Taricha granulosa. J. Chem. Ecol. 1999;25:2161–2175. doi: 10.1023/A:1021049125805. [DOI] [Google Scholar]
- 47.Kudo Y., Yasumoto T., Konoki K., Cho Y., Yotsu-Yamashita M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs. 2012;10:655–667. doi: 10.3390/md10030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y.H., Brown G.B., Mosher F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science. 2001;189:151–152. doi: 10.1126/science.1138374. [DOI] [PubMed] [Google Scholar]
- 49.Tanu M.B., Mahmud Y., Tsuruda K., Arakawa O., Noguchi T. Occurrence of tetrodotoxin in the skin of a rhacophoridid frog Polypedates sp. from Bangladesh. Toxicon. 2001;39:937–941. doi: 10.1016/S0041-0101(00)00231-2. [DOI] [PubMed] [Google Scholar]
- 50.Cliff J., Nicala D., Saute F., Givragy R., Azambuja G., Taela A., Chavane L., Howarth J. Konzo associated with war in Mozambique. Trop. Med. Int. Heal. 1997;2:1068–1074. doi: 10.1046/j.1365-3156.1997.d01-178.x. [DOI] [PubMed] [Google Scholar]
- 51.Yan Q., Yu P.H.-F., Li H.-Z. Detection of tetrodotoxin and bacterial production by Serratia marcescens. World J. Microbiol. Biotechnol. 2005;21:1255–1258. doi: 10.1007/s11274-005-1926-4. [DOI] [Google Scholar]
- 52.Yang G., Xu J., Liang S., Ren D., Yan X., Bao B. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon. 2010;56:324–329. doi: 10.1016/j.toxicon.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Bane V., Lehane M., Dikshit M., O’Riordan A., Furey A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang J.-H., Lee J.-S., Yotsu-Yamashita M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs. 2010;8:1049–1058. doi: 10.3390/md8041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang J., Yotsu-Yamashita M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon. 2006;48:980–987. doi: 10.1016/j.toxicon.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 56.Kudo Y., Finn J., Fukushima K., Sakugawa S., Cho Y., Konoki K., Yotsu-Yamashita M. Isolation of 6-deoxytetrodotoxin from the pufferfish, Takifugu pardalis, and a comparison of the effects of the C-6 and C-11 hydroxy groups of tetrodotoxin on its activity. J. Nat. Prod. 2014;77:1000–1004. doi: 10.1021/np401097n. [DOI] [PubMed] [Google Scholar]
- 57.Yotsu-Yamashita M., Abe Y., Kudo Y., Ritson-Williams R., Paul V.J., Konoki K., Cho Y., Adachi M., Imazu T., Nishikawa T. First identification of 5, 11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs. 2013;11:2799–2813. doi: 10.3390/md11082799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satake Y., Adachi M., Tokoro S., Yotsu-Yamashita M., Isobe M., Nishikawa T. Synthesis of 5-and 8-Deoxytetrodotoxin. Chem. Asian J. 2014;9:1922–1932. doi: 10.1002/asia.201402202. [DOI] [PubMed] [Google Scholar]
- 59.Jang J.-H., Yotsu-Yamashita M. 6, 11-Dideoxytetrodotoxin from the puffer fish, Fugu pardalis. Toxicon. 2007;50:947–951. doi: 10.1016/j.toxicon.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 60.Jan L.Y., Jan Y.N. Tracing the roots of ion channels. Cell. 1992;69:715–718. doi: 10.1016/0092-8674(92)90280-P. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh S., Hazra A.K., Banerjee S., Mukherjee B. Ecological monitoring for ascertaining the bio-safety of liver lipids from some Indian marine puffer fishes. Fish. Sci. 2005;71:29–37. doi: 10.1111/j.1444-2906.2005.00927.x. [DOI] [Google Scholar]
- 62.Indumathi S.M., Khora S.S. Toxicity assessment and screening of tetrodotoxin in the oblong blowfish (Takifugu oblongus) from the Tamil Nadu Coast of Bay of Bengal, India. Asian Pac. J. Trop. Med. 2017;10:278–284. doi: 10.1016/j.apjtm.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Mohd Nor Azman A., Samsur M., Mohammed M., Shabdin M.L., Fasihuddin B.A. Assessment of proximate composition and tetrodotoxin content in the muscle of Yellow puffer fish, Xenopterus naritus (Richardson 1848) from Sarawak, Malaysia. Int. Food Res. J. 2015;22:2280–2287. [Google Scholar]
- 64.Veeruraj A., Pugazhvendan S.R., Ajithkumar T.T., Arumugam M. Isolation and Identification of Cytotoxic and Biological Active Toxin from the Puffer Fish Arothron stellatus. Toxicol. Res. 2016;32:215. doi: 10.5487/TR.2016.32.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bragadeeswaran S., Therasa D., Prabhu K., Kathiresan K. Biomedical and pharmacological potential of tetrodotoxin-producing bacteria isolated from marine pufferfish Arothron hispidus (Muller, 1841) J. Venom. Anim Toxins Incl Trop Dis. 2010;16:421–431. doi: 10.1590/S1678-91992010000300008. [DOI] [Google Scholar]
- 66.Noguchi T., Ebesu J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001;20:1–10. doi: 10.1081/TXR-100103080. [DOI] [Google Scholar]
- 67.How C.-K., Chern C.-H., Huang Y.-C., Wang L.-M., Lee C.-H. Tetrodotoxin poisoning. Am. J. Emerg. Med. 2003;21:51–54. doi: 10.1053/ajem.2003.50008. [DOI] [PubMed] [Google Scholar]
- 68.Chua H.H., Chew L.P. Puffer fish poisoning: A family affair. Med. J. Malaysia. 2009;64:181–182. [PubMed] [Google Scholar]
- 69.Yooko A. Chemical studies on tetrodotoxin. Report III. Isolation of spheroidine. J. Chem. Soc. Jpn. 1950;71:591–592. [Google Scholar]
- 70.Nagashima Y., Maruyama N., Noguchi T., Hashimoto K. Analysis of Paralytic Shellfish Poison and Tetrodotoxin by Ion-Pairing High Performance Liquid Chromatography. Nippon suisan Gakkaishi. 1987;53:819–823. doi: 10.2331/suisan.53.819. [DOI] [Google Scholar]
- 71.Noguch T., Arakawa O. Tetrodotoxin–distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs. 2008;6:220–242. doi: 10.3390/md6020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagashima Y., Nishio S., Noguchi T., Arakawa O., Kanoh S., Hashimoto K. Detection of tetrodotoxin by thin-layer chromatography/fast atom bombardment mass spectrometry. Anal. Biochem. 1988;175:258–262. doi: 10.1016/0003-2697(88)90386-7. [DOI] [PubMed] [Google Scholar]
- 73.Mahmud Y., Arakawa O., Ichinose A., Tanu M.B., Takatani T., Tsuruda K., Kawatsu K., Hamano Y., Noguchi T. Intracellular visualization of tetrodotoxin (TTX) in the skin of a puffer Tetraodon nigroviridis by immunoenzymatic technique. Toxicon. 2003;41:605–611. doi: 10.1016/S0041-0101(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 74.Hwang D.F., Cheng C.A., Tsai H.T., Shih D.Y.C., Ko H.C., Yang R.Z., Jeng S.S. Identification of tetrodotoxin and paralytic shellfish toxins in marine gastropods implicated in food poisoning. Fish. Sci. 1995;61:675–679. doi: 10.2331/fishsci.61.675. [DOI] [Google Scholar]
- 75.Jeon J., Narita H., Nara M., Noguchi T., Maruyama J., Hashimoto K. Occurrence of tetrodotoxin in a gastropod mollusk,” araregai” Niotha clathrata. Bull. Jpn. Soc. Sci. Fish. 1984;50:2099–2102. doi: 10.2331/suisan.50.2099. [DOI] [Google Scholar]
- 76.Man C.N., Noor N.M., Harn G.L., Lajis R., Mohamad S. Screening of tetrodotoxin in puffers using gas chromatography–mass spectrometry. J. Chromatogr. A. 2010;1217:7455–7459. doi: 10.1016/j.chroma.2010.09.064. [DOI] [PubMed] [Google Scholar]
- 77.Tsuruda K., Arakawa O., Kawatsu K., Hamano Y., Takatani T., Noguchi T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon. 2002;40:131–136. doi: 10.1016/S0041-0101(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 78.Shoji Y., Yotsu-Yamashita M., Miyazawa T., Yasumoto T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001;290:10–17. doi: 10.1006/abio.2000.4953. [DOI] [PubMed] [Google Scholar]
- 79.Thuesen E.V., Kogure K., Hashimoto K., Nemoto T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Biol. Ecol. 1988;116:249–256. doi: 10.1016/0022-0981(88)90030-5. [DOI] [Google Scholar]
- 80.O’leary M.A., Schneider J.J., Isbister G.K. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon. 2004;44:549–553. doi: 10.1016/j.toxicon.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Hwang D.F., Chueh C.H., Jeng S.S. Occurrence of tetrodotoxin in the gastropod mollusk Natica lineata (lined moon shell) Toxicon. 1990;28:21–27. doi: 10.1016/0041-0101(90)90003-P. [DOI] [PubMed] [Google Scholar]
- 82.Mahmud Y., Okada K., Takatani T., Kawatsu K., Hamano Y., Arakawa O., Noguchi T. Intra-tissue distribution of tetrodotoxin in two marine puffers Takifugu vermicularis and Chelonodon patoca. Toxicon. 2003;41:13–18. doi: 10.1016/S0041-0101(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 83.Tsai Y.-H., Ho P.-H., Hwang C.-C., Hwang P.-A., Cheng C.-A., Hwang D.-F. Tetrodotoxin in several species of xanthid crabs in southern Taiwan. Food Chem. 2006;95:205–212. doi: 10.1016/j.foodchem.2004.12.032. [DOI] [Google Scholar]
- 84.Shiu Y.-C., Lu Y.-H., Tsai Y.-H., Chen S.-K., Hwang D.-F. Occurrence of tetrodotoxin in the causative gastropod Polinices didyma and another gastropod Natica lineata collected from western Taiwan. J. Food Drug Anal. 2003;11:159–163. [Google Scholar]
- 85.Rodriguez P., Alfonso A., Vale C., Alfonso C., Vale P., Tellez A., Botana L.M. First toxicity report of tetrodotoxin and 5, 6, 11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008;80:5622–5629. doi: 10.1021/ac800769e. [DOI] [PubMed] [Google Scholar]
- 86.Noguchi T., Maruyama J., Ueda Y., Hashimoto K., Harada T. Occurrence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Bull. Jpn. Soc. Sci. Fish. 1981;47:909–914. doi: 10.2331/suisan.47.909. [DOI] [Google Scholar]
- 87.Hwang D.-F., Shiu Y.-C., Hwang P.-A., Lu Y.-H. Tetrodotoxin in gastropods (snails) implicated in food poisoning in Northern Taiwan. J. Food Prot. 2002;65:1341–1344. doi: 10.4315/0362-028X-65.8.1341. [DOI] [PubMed] [Google Scholar]
- 88.Suleiman M., Muhammad J., Jelip J., William T., Chua T.H. AN OUTBREAK OF TETRODOTOXIN POISONING FROM CONSUMING HORSESHOE CRABS IN SABAH. Southeast Asian J. Trop. Med. Public Health. 2017;48:197–203. [PubMed] [Google Scholar]
- 89.Katikou P., Georgantelis D., Sinouris N., Petsi A., Fotaras T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece) Toxicon. 2009;54:50–55. doi: 10.1016/j.toxicon.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 90.Doucette G.J., Powell C.L., Do E.U., Byon C.Y., Cleves F., McClain S.G. Evaluation of 11-[3H]-tetrodotoxin use in a heterologous receptor binding assay for PSP toxins. Toxicon. 2000;38:1465–1474. doi: 10.1016/S0041-0101(99)00240-8. [DOI] [PubMed] [Google Scholar]
- 91.Bignami G.S., Raybould T.J.G., Sachinvala N.D., Grothaus P.G., Simpson S.B., Lazo C.B., Byrnes J.B., Moore R.E., Vann D.C. Monoclonal antibody-based enzyme-linked immunoassays for the measurement of palytoxin in biological samples. Toxicon. 1992;30:687–700. doi: 10.1016/0041-0101(92)90003-N. [DOI] [PubMed] [Google Scholar]
- 92.Kawatsu K., Shibata T., Hamano Y. Application of immunoaffinity chromatography for detection of tetrodotoxin from urine samples of poisoned patients. Toxicon. 1999;37:325–333. doi: 10.1016/S0041-0101(98)00116-0. [DOI] [PubMed] [Google Scholar]
- 93.Tanu M.B., Mahmud Y., Takatani T., Kawatsu K., Hamano Y., Arakawa O., Noguchi T. Localization of tetrodotoxin in the skin of a brackishwater puffer Tetraodon steindachneri on the basis of immunohistological study. Toxicon. 2002;40:103–106. doi: 10.1016/S0041-0101(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 94.Luo X., Yu R.-C., Wang X.-J., Zhou M.-J. Toxin composition and toxicity dynamics of marine gastropod Nassarius spp. collected from Lianyungang, China. Food Addit. Contam. Part A. 2012;29:117–127. doi: 10.1080/19440049.2011.615069. [DOI] [PubMed] [Google Scholar]
- 95.Hwang P.-A., Tsai Y.-H., Lu Y.-H., Hwang D.-F. Paralytic toxins in three new gastropod (Olividae) species implicated in food poisoning in southern Taiwan. Toxicon. 2003;41:529–533. doi: 10.1016/S0041-0101(02)00364-1. [DOI] [PubMed] [Google Scholar]
- 96.Chen X.W., Liu H.X., Jin Y.B., Li S.F., Bi X., Chung S., Zhang S.S., Jiang Y.Y. Separation, identification and quantification of tetrodotoxin and its analogs by LC–MS without calibration of individual analogs. Toxicon. 2011;57:938–943. doi: 10.1016/j.toxicon.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 97.Nzoughet J.K., Campbell K., Barnes P., Cooper K.M., Chevallier O.P., Elliott C.T. Comparison of sample preparation methods, validation of an UPLC–MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013;136:1584–1589. doi: 10.1016/j.foodchem.2012.01.109. [DOI] [PubMed] [Google Scholar]
- 98.Rodríguez P., Alfonso A., Otero P., Katikou P., Georgantelis D., Botana L.M. Liquid chromatography–mass spectrometry method to detect Tetrodotoxin and Its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem. 2012;132:1103–1111. doi: 10.1016/j.foodchem.2011.11.081. [DOI] [Google Scholar]
- 99.Nakagawa T., Jang J., Yotsu-Yamashita M. Hydrophilic interaction liquid chromatography–electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006;352:142–144. doi: 10.1016/j.ab.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 100.Isbister G.K., Son J., Wang F., Maclean C.J., Lin C.S., Ujma J., Balit C.R., Smith B., Milder D.G., Kiernan M.C. Puffer fish poisoning: A potentially life-threatening condition. Med. J. Aust. 2002;177:650–653. doi: 10.5694/j.1326-5377.2002.tb04999.x. [DOI] [PubMed] [Google Scholar]
- 101.Cheng C.A. Paralytic toxins of the gastropod Natica lineata in Pingtung Prefecture. Food Sci. 1996;23:845–853. [Google Scholar]
- 102.Narita T., Noguchi T., Maruyama J., Ueda Y., Hashimoto K., Watanabe Y. Occurrence of tetrodotoxin in a trumpet shell,” boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1981;47:935–941. doi: 10.2331/suisan.47.935. [DOI] [Google Scholar]
- 103.Noguchi T., Maruyama J., Narita H., Kanehisa H. Occurrence of tetrodotoxin in the gastropod mollusk Tutufa lissostoma (frog shell) Toxicon. 1984;22:219–226. doi: 10.1016/0041-0101(84)90022-9. [DOI] [PubMed] [Google Scholar]
- 104.Noguchi T., Uzu A., Koyama K., Hashimoto K. Occurrence of tetrodotoxin as the major toxin in a xanthid crab Atergatis floridus. Bull. Jpn. Soc. Sci. Fish. 1983;49:1887–1892. doi: 10.2331/suisan.49.1887. [DOI] [Google Scholar]