Abstract
Rab family proteins play a crucial role in plant developmental processes and tolerance to environmental stresses. The current study investigated whether rice Rab7 (OsRab7) overexpression could improve rice tolerance to drought and heat stress conditions. The OsRab7 gene was cloned and transformed into rice plants. The survival rate, relative water content, chlorophyll content, gas-exchange characteristics, soluble protein content, soluble sugar content, proline content, and activities of antioxidant enzymes (CAT, SOD, APX, POD) of the transgenic rice lines were significantly higher than that of the wild-type. In contrast, the levels of hydrogen peroxide, electrolyte leakage, and malondialdehyde of the transgenic lines were significantly reduced when compared to wild-type. Furthermore, the expression of four genes encoding reactive oxygen species (ROS)-scavenging enzymes (OsCATA, OsCATB, OsAPX2, OsSOD-Cu/Zn) and eight genes conferring abiotic stress tolerance (OsLEA3, OsRD29A, OsSNAC1, OsSNAC2, OsDREB2A, OsDREB2B, OsRAB16A, OsRAB16C) was significantly up-regulated in the transformed rice lines as compared to their expression in wild-type. OsRab7 overexpression also increased grain yield in rice. Taken together, the current study indicates that the OsRab7 gene improves grain yield and enhances drought and heat tolerance in transgenic rice by modulating osmolytes, antioxidants and abiotic stress-responsive genes expression. Therefore, OsRab7 gene could be exploited as a promising candidate for improving rice grain yield and stress tolerance.
Keywords: transgenic rice, OsRab7, drought and heat stress, antioxidants, gene expression
1. Introduction
Drought and heat stresses are major abiotic factors that limit crops growth and productivity worldwide, with devastating agro-economic impacts [1]. Drought stress is frequently associated with heat stress [2,3]. Those abiotic factors influence different physiological processes in plants, causing the generation of high levels of reactive oxygen species (ROS) that have negative impacts on plant antioxidant systems and biological macromolecules [4]. As a result, plants evolve different homeostatic strategies, such as physiological, biochemical, and transcriptional responses to combat the adverse abiotic stress conditions [5,6,7,8,9]. These strategies include activation of antioxidant systems and modulation of compatible solutes and proteins mediating stress tolerance pathways. The transgenic approach is getting increasingly important for enhancing abiotic stress tolerance as well as developing abiotic stress-tolerant varieties [1]. Small GTP-binding proteins are widespread in eukaryotic cells and mediate several cellular processes, such as vesicular transport, cell proliferation, and signal transduction [10,11]. Those proteins comprise five important families (Rab, Rho, Ras, Ran, and Arf/Sar) [12,13]. Rab family proteins demonstrated crucial roles in diverse plant developmental processes, tolerance to environmental stresses, membrane organization, vesicle formation, and intracellular trafficking pathways [14]. Pereira-Leal and Seabra [15] described several Rab proteins in the Arabidopsis genome. Among those Rab proteins, Rab7 demonstrated a high potential in enhancing plant tolerance to abiotic stresses. Mazel et al. [16] reported that Rab7-related proteins in Arabidopsis are localized on the vacuolar membrane and mediate the fusion of vesicle with vacuole. However, Rab7-related proteins in soybean were expressed in tonoplasts and endosomes, suggesting that Rab7 multivesicular bodies play a regulatory role in endocytic pathways. Furthermore, various Rab proteins-encoding cDNAs and genes have been cloned from diverse plants. The functions of such genes have also been studied. For instance, OsRab5a gene, cloned from Oryza sativa L., played a crucial role in nutrient uptake in roots, early endosome transport, endosperm storage protein trafficking, and endomembrane organization [17,18,19]. AtRabG3e overexpression in Arabidopsis also enhanced sodium sequestration in vacuoles, and thereby augmented plant tolerance to salt and osmotic stresses [16]. Pennisetum glaucum Rab7 overexpression augmented tobacco tolerance to salt and osmotic stresses [20]. Moreover, RabG3b gene promoted cell death during the plant senescence process and pathogen infection [21]. OsRab7B3 overexpression conferred enhanced leaf senescence in rice [11]. Overexpression of rice Rab7 (OsRab7) also improved salt tolerance in rice [22]. However, the functional role of OsRab7 in mediating other important abiotic stresses has not been reported yet.
Rice (Oryza sativa L.) is one of the most important food crops worldwide and it requires huge quantities of water during growth cycle [23]. Drought and heat stresses negatively influence crops growth and yield [23,24,25]. Several studies have reported the use of a potential transgenic approach in enhancing drought and heat stress tolerance in rice [23,26,27,28,29,30,31,32]. Wu et al. [23] reported enhanced drought and heat tolerance in the rice over-expressing OsWRKY11 transcription factor. Moreover, enhanced drought tolerance levels have been reported in rice overexpressing different genes, such as OsHsfA7 [26], LEA [27], OsNAC14 [28], OsDRAP1 [29], XERICO [30], OsLG3 [31], or OsHSP50.2 [32]. Overexpression of DPB3-1 or OsHTAS also improved heat tolerance in rice [33,34]. However, developing more transgenic stress-tolerant rice varieties is currently essential for use in breeding strategies to overcome adverse water deficit and heat stress effects and meet the increasing worldwide population demands. When considering the aforementioned remarks, the current study aimed to investigate whether the overexpression of OsRab7 gene, cloned from O. sativa L., could enhance drought and heat tolerance and improve grain yield in transgenic rice plants. In addition to the grain yield parameters, several physiological, biochemical, and transcriptional analyses were conducted to compare between the transgenics and wild type plants.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Rice (O. sativa ssp. Japonica cv. Giza 177) genotype was obtained from the Agricultural Research Center in Egypt and was used to generate transgenic lines in the present study. The wild-type and transgenic rice seeds were surface-sterilized and germinated on a wet paper for 4 days. The uniform seedlings were then transplanted into plastic pots containing mixed soil of sand, peat, and perlite (1:1:1, v/v/v) and grown in a growth chamber under conditions of 26/20 °C (day/night), 16/8 h (light/dark), and humidity of 70%. The plants were irrigated daily with sterile distilled water.
2.2. Plasmid Construction and Rice Transformation
Total RNA was isolated from three-week-old rice seedlings using RNeasy Plant Mini kit (Qiagen, Hilden, Germany), followed by the removal of contaminating DNA using RNase-Free DNase Set (Qiagen). Complementary DNA (cDNA) synthesis was carried out using Reverse Transcription kit (Qiagen). The full length cDNA of OsRab7 was then amplified using the primer pair designed by Peng et al. [22]. cDNA OsRab7 was then digested with BamHI and ligated to the modified binary vector plasmid pCU, as previously described [22,35]. The resulting constructs, pOsRab7, were introduced into Agrobacterium tumefaciens EHA105, which was then used for the transformation of rice cultivar Giza 117 (Oryza sativa L. ssp. japonica) following the Agrobacterium-mediated transformation method [36]. Transgenic plant seeds were selected on Murashige and Skoog (MS) media containing 40 mg L−1 hygromycin.
2.3. Molecular Analysis of Transgenic Plants and Transgene Expression
Putative transgenic plants survived on MS medium containing 40 mg L−1 hygromycin were further verified by polymerase chain reaction (PCR) amplification of hygromycin resistant gene (hygromycin phosphotransferase, hpt), as previously reported by Peng et al. [22]. Seedlings were then transplanted into soil to obtain T3 seeds, as this generation reveals more homozygosity and stable gene expression. The transgenic OsRab7 gene cope number was examined by southern blot analysis. In brief, genomic DNA was isolated from the leaves of the wild-type and T3 transgenic rice lines. Isolated DNA was then digested, electrophoresed on 0.8% (w/v) agarose gel, transferred onto a Hybond-N+ nylon membrane (Roche), and hybridized using the hygromycin-resistant gene.
OsRab7 gene overexpression was also examined in the wild-type and T3 transgenic rice lines using quantitative real-time PCR (qRT-PCR) analysis. As described above, RNA isolation and cDNA synthesis from wild-type and T3 transgenic lines were conducted. qRT-PCR analysis was conducted in triplicates (three biological replicates and three technical replicates), according to the manufacturer’s procedures of QuantiTect SYBR Green PCR kit (Qiagen). PCR reactions and amplification conditions were conducted, as previously reported by Peng et al. [22]. Specific primers previously designed for OsRab7 [22] was used for amplification. Actin was used as a housekeeping gene [22]. OsRab7 expression was quantified using 2−ΔΔCt method.
2.4. Drought and Heat Stress Treatments
Wild-type and 3 T3 homozygous transgenic rice lines exhibiting the highest OsRab7 expression level (OE-3, OE-4, OE-6) were used for drought and heat stress treatment experiments. The wild-type and the three T3 transgenic rice lines were germinated on a wet paper for four days. The healthy uniform seedlings were then transplanted into plastic pots containing sandy soil and grown in a growth chamber with daily irrigation with sterile distilled water for three weeks under conditions of 26/20 °C (day/night), 16/8 h (light/dark), and humidity of 70%. The 25-day-old plants were divided into three groups used for the following treatments; (i) control at 26/20 °C (day/night) with irrigation every day; (ii) drought stress at 26/20 °C (day/night) without irrigation; and, (iii) heat stress, as recommended by Aghamolki [24] at 40/32 °C (day/night) with irrigation every day. All of the treatments lasted for 10 days at which plants were collected for use in the subsequent physiological, biochemical and transcriptional analyses. Following the 10-day stress treatments, 10-day recovery was applied to calculate the survival rate. The above experiments were repeated thrice.
To evaluate the yield components of the wild-type and transgenic rice plants under normal, drought, and heat stress conditions, the wild-type and the 3 T3 homozygous transgenic rice lines were transplanted into 1-m-deep containers containing natural paddy soil located in the greenhouse at 26/20 °C (day/night). The containers were arranged in a completely randomized design, and the plants were watered daily. Approximately 10–15 days before the panicle heading stage, the following treatments were applied: (i) control, 26/20 °C (day/night) with irrigation; (ii) drought stress, 26/20 °C (day/night) without irrigation; and, (iii) heat stress, 40/32 °C (day/night) with irrigation. All of the treatments lasted for 25 days, followed by growing under normal growth conditions till harvesting. When the grains ripened, the following yield parameters were scored; panicle length (cm), number of spikelets per panicle, total number of spikelets per hill, number of filled grains per hill, filling rate (%), and total grain weight (g).
2.5. Determination of Growth Traits and Relative Water Content
Plant height, root fresh weight and shoot fresh weight of the collected plants were determined. Leaf relative water content (RWC) was estimated according to the method described by Yamasaki and Dillenburg [37].
2.6. Estimation of Oxidative Stress Biomarker Levels
Electrolyte leakage (EL) was estimated using the methodology that was described by Dionisio-Sese and Tobita [38]. Hydrogen peroxide (H2O2) was estimated by homogenizing fresh tissue (50 mg) in TCA (0.5 mL, 0.1%), followed by centrifuging the resulting homogenate and calculating H2O2 content following the methodology of Velikova et al. [39]. Malondialdehyde (MDA) content was estimated using the method reported by Rao and Sresty [40].
2.7. Estimation of Gas-Exchange Characteristics
The transpiration rate (E), net photosynthesis rate (Pn), and stomatal conductance (gs) were estimated in leaves at 10:00 a.m., as reported by Holá et al. [41], using a portable gas-exchange system LCpro+ (ADC BioScientific Ltd., Hoddesdon, UK).
2.8. Determination of Soluble Proteins, Soluble Sugars, Proline and Chlorophyll Contents
Fresh leaves were homogenized in 1 mL of 100 mM Tris buffer (pH 8.0), followed by centrifugation at 20,000× g and 4 °C for 12 min. Soluble proteins in leaves were quantified following Bradford methodology [42]. Soluble sugars were quantified, as reported by Dey [43]. Proline content was estimated using the method of Bates et al. [44] by recording the absorbance at 520 nm.
To estimate chlorophyll content, leaf disks were homogenized and extracted in 80% acetone, and absorpance was spectrophotometrically taken at 645 and 663 nm [7].
2.9. Antioxidant Enzyme Assays
Fresh leafy samples were ground in liquid N2, standardized using 0.05 M phosphate buffer (pH 7.8) and centrifuged at 14,000× g for 8 min at 5 °C. Supernatant was then used for measuring antioxidant enzymes activities. Catalase (CAT) activity was determined, as reported by Aebi [45], and absorbance was spectrophotometrically recorded at 240 nm. Peroxidase (POD) and superoxide dismutase (SOD) activities were measured following the methodology of Zhang [46]. Ascorbate peroxidase (APX) activity was measured following the methodology that was reported by Nakano and Asada [47], and absorbance was spectrophotometrically measured at 265 nm.
2.10. Stress-Related Genes Expression Analysis
Quantitative real-time PCR was carried out to measure the expression levels of four antioxidants genes (OsCATA, OsCATB, OsAPX2, OsSOD-Cu/Zn) and eight genes conferring abiotic stress tolerance (OsLEA3, OsRD29A, OsSNAC1, OsSNAC2, OsDREB2A, OsDREB2B, OsRAB16A, OsRAB16C) in the wild-type and the three T3 transgenic rice lines subjected to normal, drought, or heat stress conditions. RNA isolation and cDNA synthesis from the plant tissues were performed, as described above. qRT-PCR analysis was carried out in triplicates (three biological repeats and three technical repeats), following the manufacturer’s procedures of QuantiTect SYBR Green PCR kit (Qiagen). PCR reactions and amplification conditions for the four antioxidants genes and the eight genes conferring abiotic stress tolerance were conducted, as previously reported by Vighi et al. [48] and Cai et al. [49], respectively. Specific primers that were previously designed for the four antioxidants genes [48] and the eight genes conferring abiotic stress tolerance [49,50] were used for amplification. UBQ10 was used as an internal reference gene [48]. Genes expression levels were quantified following the 2−ΔΔCt method [51].
2.11. Statistical Analysis
One-way analysis of variance (ANOVA) was performed for the collected data using SPSS version 16.0. Values are means ±SE (n = 3), and differ significantly at p ≤ 0.05.
3. Results and Discussion
3.1. Transformation and Molecular Analysis of Transgenic Rice Lines
Genetic transformation technology has become a potential tool to enhance crop stress tolerance and accelerate breeding strategies. To investigate whether OsRab7 overexpression enhances drought and heat stress tolerance in rice, transgenic rice lines overexpressing OsRab7 were generated using Agrobacterium-mediated transformation. Sixteen independent rice transgenic lines were obtained. Five positive transgenic lines designated OE-1, OE-3, OE-4, OE-6, and OE-9 had sufficient seeds and were selected and further verified by PCR amplification of hygromycin resistant gene. The expected fragments of a size of 750 bp were amplified in the five transgenic rice lines, but not in the wild-type plants (Figure 1A). Southern blot analysis confirmed the successful integration of the transformed OsRab7 gene into the genomes of lines OE-1, OE-3, OE-4, OE-6, and OE-9, with one single copy present in each transgenic line (Figure 1B). OsRab7 transcription levels were also detected in the five T3 rice transformants (OE-1, OE-3, OE-4, OE-6, and OE-9) using qRT-PCR (Figure 1C). The three T3 transgenic rice lines (OE-3, OE-4, OE-6) exhibited the highest OsRab7 expression levels and they were therefore selected for subsequent physiological, biochemical, and transcriptional analyses.
Figure 1.
Molecular analysis of the transgenic rice lines. (A) PCR amplification of 750 bp of hpt hygromycin resistant gene in the wild-type (WT) and the 5 transgenic (OE) lines. (B) Southern blot analysis of the digested genomic DNA from the WT and the 5 transgenic lines. (C) Relative expression of OsRab7 in transgenic lines using qRT-PCR analysis. (D) Survival rate of the WT and transgenic lines following 10-day recovery after drought and heat treatments. M, 100 bp DNA ladder. Different letters above the columns indicate significant differences between lines (p ≤ 0.05).
3.2. OsRab7 Overexpression in Rice Enhances Survival Rate, Plant Growth, and Relative Water Content under Drought and Heat Stress Conditions
Following drought and heat stress treatments, a 10-day growth recovery was conducted. The survival rate of the transgenic rice lines (OE-3, OE-4, OE-6) was significantly higher than that of the wild-type (Figure 1D). Phenotypic traits serve as indicators for the identification of stress tolerance in plants. Leaf relative water content is also an important indicator of water status balance in plants [52,53]. Decrease in relative water content causes osmotic stress, which in turn affects plant growth [54]. In the present study, under normal conditions, no obvious differences were recorded in plant height, root and shoot fresh weight, or relative water content between the wild-type and the transgenic lines (Table 1). Under drought and heat stress conditions, decreases in the growth traits and relative water content were recorded for the wild-type and transgenic lines as compared to normal conditions. However, transgenic lines exhibited significantly higher growth parameters and relative water content when compared to the wild-type under drought and heat stress conditions (Table 1). These results indicated that OsRab7 overexpression in transgenic rice plants enhances their survival rate, growth, relative water content, and tolerance to drought and heat stresses.
Table 1.
Growth, biomass, and relative water content of the WT and transgenic rice seedlings grown under normal, heat, and drought stress conditions.
| Treatment | Line | Plant Height (cm) | Root Fresh Weight (mg) | Shoot Fresh Weight (mg) | Relative Water Content (%) |
|---|---|---|---|---|---|
| Normal | WT | 27.3 ± 0.94 a | 8.4 ± 0.24 a | 23.3 ± 0.56 a | 93.0 ± 6.8 a |
| OE-3 | 27.1 ± 0.78 a | 8.6 ± 0.31 a | 22.8 ± 0.51 a | 92.1 ± 4.8 a | |
| OE-4 | 26.7 ± 0.88 a | 7.9 ± 0.42 a | 23.4 ± 0.44 a | 94.4 ± 5.5 a | |
| OE-6 | 27.4 ± 0.78 a | 8.2 ± 0.32 a | 23.9 ± 0.49 a | 95.2 ± 4.9 a | |
| Drought | WT | 19.4 ± 0.66 b | 4.9 ± 0.29 b | 16.4 ± 0.51 b | 40.2 ± 5.6 b |
| OE-3 | 25.5 ± 0.84 a | 7.9 ± 0.33 a | 21.9 ± 0.62 a | 68.6 ± 4.8 a | |
| OE-4 | 25.1 ± 0.82 a | 7.8 ± 0.41 a | 22.2 ± 0.53 a | 66.2 ± 6.4 a | |
| OE-6 | 26.4 ± 0.77 a | 8.1 ± 0.37 a | 23.3 ± 0.66 a | 73.5 ± 5.3 a | |
| Heat | WT | 21.8 ± 0.83 b | 5.6 ± 0.28 b | 19.4 ± 0.56 b | 56.5 ± 4.9 b |
| OE-3 | 27.9 ± 0.88 a | 8.3 ± 0.32 a | 23.5 ± 0.52 a | 81.1 ± 5.1 a | |
| OE-4 | 27.3 ± 0.69 a | 7.7 ± 0.42 a | 23.7 ± 0.51 a | 83.6 ± 6.3 a | |
| OE-6 | 26.5 ± 0.73 a | 8.5 ± 0.39 a | 24.5 ± 0.62 a | 88.2 ± 5.2 a |
Values represent means ± SE (n = 3). Different letters next to the numbers indicate significant difference between lines under the same conditions (p ≤ 0.05). WT: Wild type; SE: Standard error.
3.3. OsRab7 Overexpression in Rice Reduces Oxidative Stress Biomarkers under Drought and Heat Stress Conditions
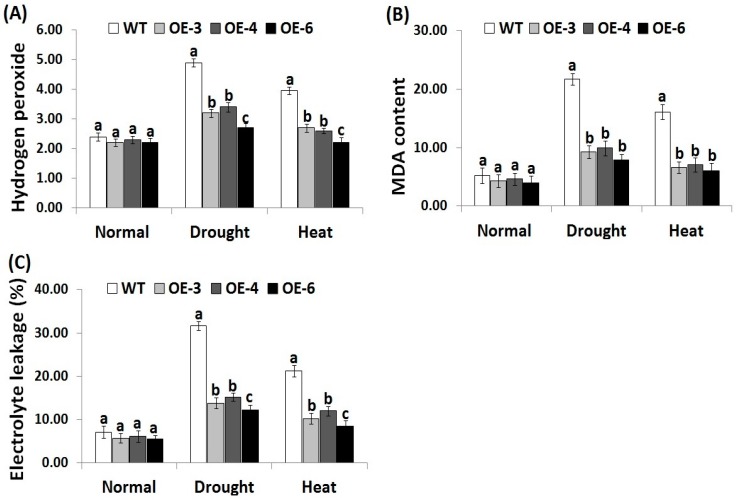
Reactive oxygen species have toxic impacts on plants [4]. Electrolyte leakage is a key indicator for the plant cell membrane damage [55]. MDA also indicates lipid peroxidation end products damage caused by free radicals [56]. Therefore, developing more stress-tolerant varieties scavenging ROS is importantly needed. In order to investigate whether the transgenic rice lines overexpressing OsRab7 could scavenge ROS, oxidative stress biomarkers, such as H2O2, MDA, and EL were estimated in the wild-type and the three transgenic lines under drought and heat stress conditions. Under normal conditions, there were no significant differences in H2O2, MDA, and EL levels between the wild-type and the three transgenic lines (Figure 2A–C). Under drought and heat stress conditions, increases in H2O2, MDA, and EL levels were recorded for the wild-type and transgenic lines as compared to normal circumstances. However, transgenic lines exhibited significantly lower levels of H2O2, MDA, and EL as compared to the wild-type under drought and heat stress conditions (Figure 2A–C). These results indicate that ROS accumulation in transgenic lines overexpressing OsRab7 is much lower than that of the wild-type. Thus, OsRab7 overexpression in transgenic rice plants counteract the toxic ROS effects and reduce the oxidative damage, conferring greater tolerance to drought and heat stresses.
Figure 2.
Hydrogen peroxide (H2O2, µmol g−1 FW) content (A), lipid peroxidation (malondialdehyde (MDA), µmol g−1 FW) level (B), and electrolyte leakage (C) of the wild-type and transgenic rice plants overexpressing OsRab7 subjected to normal, drought and heat stress conditions. Data are means ± SE (n = 3). Different letters above the columns indicate significant differences between rice lines (p ≤ 0.05). SE: Standard error.
3.4. OsRab7 Overexpression in Rice Improves Gas-Exchange Characteristics Under Drought and Heat Stress Conditions
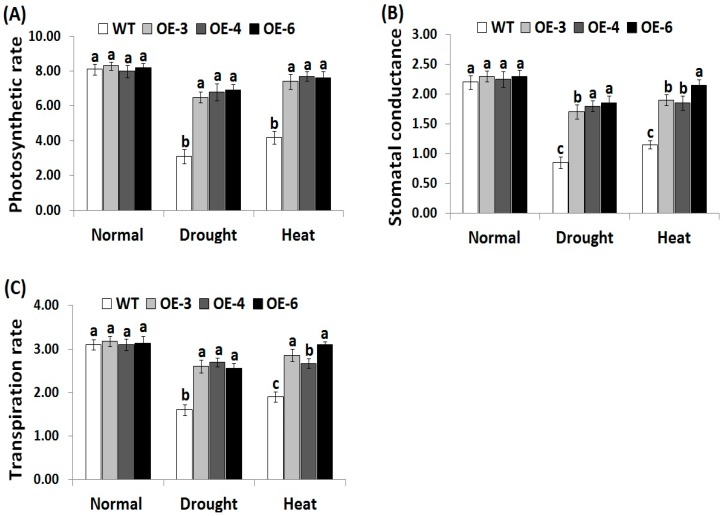
Gas-exchange parameters are significantly affected by the adverse effects of abiotic stresses. To evaluate whether OsRab7 overexpression in rice could improve gas-exchange parameters under abiotic stress, we estimated photosynthesis rate, stomatal conductance, and transpiration rate in the wild-type and the three OsRab7-overexpressing rice lines under drought and heat stress conditions. Under normal growth conditions, the transgenic rice lines did not reveal any significant difference in gas-exchange attributes when compared to the wild-type (Figure 3A–C). Under drought and heat stress conditions, decreases in gas-exchange parameters were recorded for the wild-type and transgenic lines compared to the normal growth conditions. However, transgenic lines revealed significantly higher levels of gas-exchange attributes as compared to the wild-type under drought and heat stress conditions (Figure 3A–C), indicating that the OsRab7-overexpressing rice lines exhibited greater tolerance to drought and heat stress effects through enhancing the relative water content and gas-exchange characteristics.
Figure 3.
Photosynthesis rate (Pn, μmol m2s−1) (A), stomatal conductance (gs, mol m2s−1) (B), and transpiration rate (E, mmol m2s−1) (C) of the wild-type and transgenic rice plants overexpressing OsRab7 subjected to normal, drought, and heat stress conditions. Data are means ± SE (n = 3). Different letters above the columns indicate significant differences (p ≤ 0.05).
3.5. OsRab7 Overexpression in Rice Improves Osmolytes and Chlorophyll Content under Drought and Heat Stress Conditions
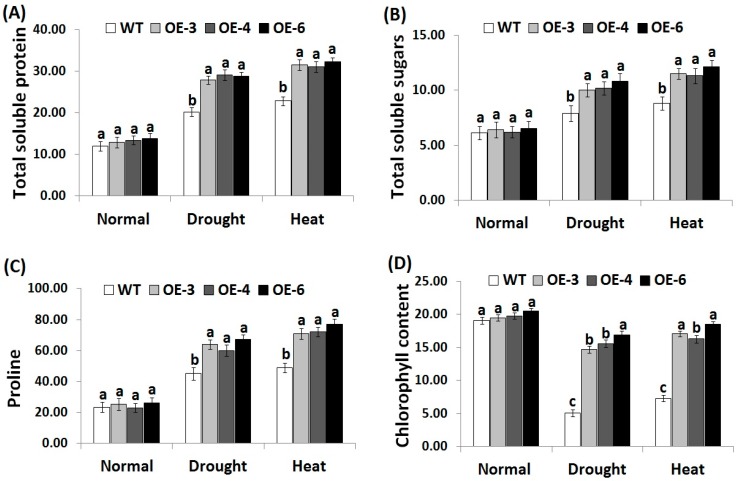
Osmotic adjustment represents one of the most important features of abiotic stress tolerance in plants, while soluble proteins, soluble sugars, proline, and other compatible solutes serve as osmoprotectants under stress conditions [57,58]. Soluble proteins and sugars also protect plant cells from dehydration and stabilize and protect the biological molecules function [59]. In the present study, the contents of soluble proteins, soluble sugars, proline, and chlorophyll were estimated in the wild-type and the three OsRab7-overexpressing rice lines under drought and heat stress conditions in order to understand the transgenic lines osmoregulation ability (Figure 4A–D). Non-significant slight differences in the contents of soluble proteins, sugars, proline, and chlorophyll were recorded between the wild-type and transgenic lines under normal growth conditions. By contrast, under drought and heat stress conditions, a remarkable increase in the contents of soluble proteins, sugars, proline, and chlorophyll was recorded for the wild-type and transgenic lines compared to normal growth conditions (Figure 4A–D). Nevertheless, transgenic lines revealed significantly higher contents of soluble proteins, sugars, proline and chlorophyll as compared to the wild-type under drought and heat stress conditions. These results reveal that OsRab7 overexpression enhanced the contents of compatible solutes and chlorophyll and promoted the osmoregulation ability in the transgenic rice lines, resulting in improved tolerance to the drought and heat stresses.
Figure 4.
Contents of total soluble protein (mg g−1 FW) (A), soluble sugars (mg g−1 FW) (B), proline (µg g−1 FW) (C) and chlorophyll (mg g−1 FW) (D) of the wild-type and transgenic rice plants overexpressing OsRab7 subjected to normal, drought and heat stress conditions. Data are means ± SE (n = 3). Different letters above the columns indicate significant differences between lines (p ≤ 0.05).
3.6. OsRab7 Overexpression in Rice Induces Antioxidant Enzyme Activities under Drought and Heat Stress Conditions
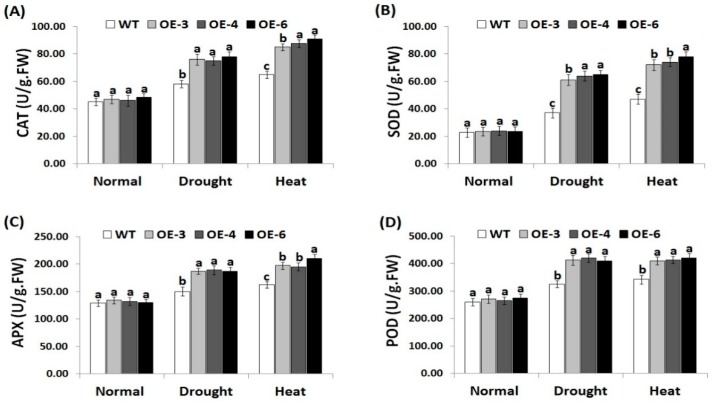
Antioxidant enzymes play a key role in scavenging ROS and improving plant tolerance to environmental stresses [8]. Therefore, we estimated the activities of CAT, SOD, APX, and POD in the wild-type and OsRab7-overexpressing rice plants (Figure 5A–D). Under normal growth conditions, there were no significant differences in the antioxidant enzymes activities between the wild-type and the three transgenic lines (Figure 5A–D). By contrast, under drought and heat stress conditions, remarkable increases in the antioxidant enzymes activities were recorded for the wild-type and transgenic lines compared to normal growth conditions. However, transgenic lines revealed significantly higher antioxidant enzymes activities when compared to the wild-type under drought and heat stress conditions. As a result, OsRab7 overexpression significantly induces the antioxidant enzymes activities of transgenic rice lines to counteract the toxic impacts of ROS, resulting in reduced oxidative damage in plant cells.
Figure 5.
Activities of catalase (CAT) (A), superoxide dismutase (SOD) (B), ascorbate peroxidase (APX) (C), and peroxidase (POD) (D) in the wild-type and transgenic rice plants overexpressing OsRab7 subjected to normal, drought, and heat stress conditions. Data are means ± SE (n = 3). Different letters above the columns indicate significant differences between rice lines (p ≤ 0.05).
3.7. OsRab7 Overexpression in Rice Induces Abiotic Stress-Related Genes Expression under Drought and Heat Stress Conditions
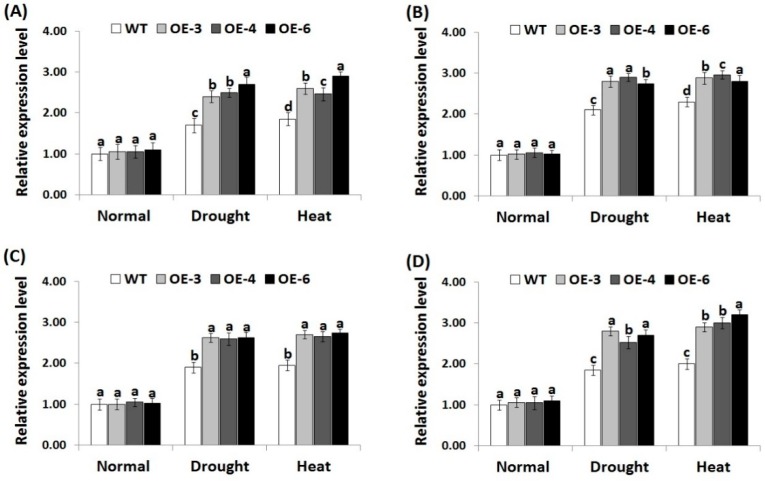
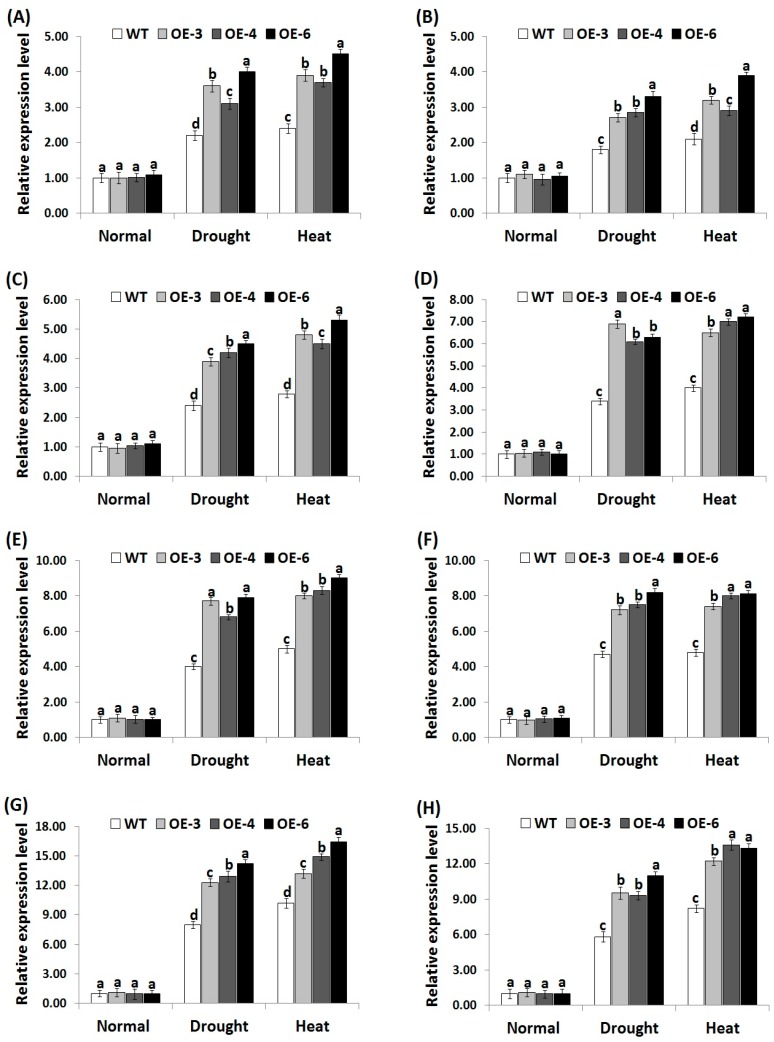
In order to investigate the signaling regulatory role of OsRab7 overexpression in stress tolerance mechanisms, we measured the expression level of four genes encoding ROS-scavenging enzymes (OsCATA, OsCATB, OsAPX2, OsSOD-Cu/Zn) and eight genes conferring abiotic stress tolerance (OsLEA3, OsRD29A, OsSNAC1, OsSNAC2, OsDREB2A, OsDREB2B, OsRAB16A, OsRAB16C) in the wild-type and the three transgenic rice lines under drought or heat stress conditions using qRT-PCR. Under normal growth conditions, non-significant slight differences in the expression of antioxidant genes and abiotic stress-related genes were recorded between the wild-type and transgenic lines (Figure 6A–D; Figure 7A–H). By contrast, under drought and heat stress conditions, a remarkable induction in the expression of all genes was recorded for the wild-type and transgenic lines as compared to normal growth conditions. Nevertheless, transgenic lines exhibited significantly higher expression levels of antioxidant genes (Figure 6A–D) and abiotic-stress related genes (Figure 7A–H) as compared to the wild-type under drought and heat stress conditions. These results indicate that OsRab7 overexpression reduces oxidative damages via inducing ROS scavenging pathways and proteins that are involved in defence mechanisms, thereby improving drought and heat tolerance.
Figure 6.
Expression levels of OsCATA (A), OsCATB (B), OsAPX2 (C), and OsSOD-Cu/Zn (D) genes in the wild-type and transgenic rice plants overexpressing OsRab7 subjected to normal, drought, and heat stress conditions. Data are means ± SE (n = 3). Different letters above the columns indicate significant differences between rice lines (p ≤ 0.05).
Figure 7.
Expression levels of OsLEA3 (A), OsRD29A (B), OsSNAC1 (C), OsSNAC2 (D), OsDREB2A (E), OsDREB2B (F), OsRAB16A (G), and OsRAB16C (H) genes in the wild-type and transgenic rice plants overexpressing OsRab7 under normal, drought, and heat stress conditions. Data are means ± SE (n = 3). Different letters above columns indicate significant difference between rice lines (p ≤ 0.05).
In consistent with the results of antioxidant enzyme assays, the expression level of the antioxidant genes and abiotic stress-related genes was significantly induced in transgenic rice lines under stress conditions. These results were in harmony with previous report [49], which indicated that rice overexpressing rat neuronal NO synthase (nNOS) exhibited much higher expression levels of antioxidant genes (OsCATA, OsCATB, OsPOX1) and abiotic stress-responsive genes (OsLEA3, OsDREB2A, OsDREB2B, OsRD29A, OsSNAC1, OsSNAC2) as compared to the wild-type under drought and salt stress conditions. Moreover, the results were in harmony with the previous findings of Yu et al. [50], who reported that rice plants overexpressing OsEm1 exhibited higher expression levels of late embryogenesis abundant proteins (OsLEA3, OsRAB16A, OsRAB16C) involved in abiotic stress tolerance as compared to the wild-type under drought stress. The results are also in harmony with the previous reports that also indicated the key role of Rab proteins in enhancing plant tolerance to abiotic stress [16,22]. Rab7 proteins are involved in vesicle trafficking and localized in the tonoplast in Arabidopsis and rice [60,61,62]. Enhanced vesicle trafficking was reported in soybean plants overexpressing Rab7 [63]. Peng et al. [22] also reported that OsRab7 overexpression enhanced rice salt tolerance through enhancing vesicle trafficking. Moreover, previous studies reported that osmotic stress signaling transduction could be mediated by the intracellular vesicle trafficking, as demonstrated by the role of phosphatidylinositol signaling in vesicle trafficking and stress tolerance [64,65]. Therefore, together with the previously demonstrated role of OsRab7 in inducing vesicle trafficking, the present study indicates that OsRab7 overexpression might induce drought and heat tolerance via enhancing vesicle trafficking in rice.
3.8. OsRab7 Overexpression in Rice Increases Grain Yield under Drought and Heat Stress Conditions
Rice grain yield is severely influenced upon exposure to drought and heat stress. Therefore, it was important to evaluate the adverse impacts of drought and heat stress on grain yield of the wild-type and the three transgenic rice lines overexpressing OsRab7 by recording various yield parameters, such as panicle length, number of spikelets per panicle, total number of spikelets per hill, number of filled grains per hill, filling rate, and total grain weight. The evaluation of these yield components showed that there were no obvious differences in the grain yield between the wild-type and the transgenic lines (Table 2). However, under drought and heat stress conditions, reductions in the grain yield parameters were recorded for the wild-type and transgenic lines as compared to normal growth conditions. Nevertheless, transgenic lines exhibited significantly lower decreases in grain yield when compared to the wild-type under drought and heat stress conditions (Table 2). For example, the transgenic lines had a higher filling rate than that of the wild type plants under drought and heat stress conditions, resulting in increases in the total grain weight. These results were in consistent with that of previous studies which reported higher rice grain yield in rice plants overexpressing AP37 [66], TIFY [67], OsNAC2 [68], and OsNRT2.1 [69].
Table 2.
Yield traits of WT and transgenic rice lines under normal and stress conditions.
| Treatment | Line | Panicle Length (cm) | Number of Spikelets per Panicle | Total no. of Spikelets per Hill | Number of Filled Grains Per Hill | Filling Rate (%) | Total Grain Weight (g) |
|---|---|---|---|---|---|---|---|
| Normal | WT | 17.8 ± 1.11 a | 89.8 ± 8.52 a | 1188.4 ± 132.3 a | 1042.6 ± 141.3 a | 87.3 ± 12.11 a | 18.2 ± 1.32 a |
| OE-3 | 18.2 ± 1.07 a | 91.3 ± 7.61 a | 1166.8 ± 143.4 a | 1044.9 ± 113.5 a | 89.2 ± 10.14 a | 17.1 ± 1.41 a | |
| OE-4 | 18.1 ± 1.18 a | 88.6 ± 9.44 a | 1180.6 ± 128.7 a | 1068.7 ± 131.7 a | 90.2 ± 12.73 a | 18.7 ± 1.36 a | |
| OE-6 | 17.8 ± 1.22 a | 92.4 ± 8.66 a | 1201.9 ± 144.5 a | 1076.9 ± 122.8 a | 89.7 ± 10.52 a | 13.9 ± 1.45 a | |
| Drought | WT | 13.1 ± 1.34 b | 79.7 ± 7.94 b | 1020.2 ± 125.7 b | 698.30 ± 133.5 b | 68.1 ± 13.46 b | 17.8 ± 1.41 b |
| OE-3 | 16.9 ± 1.52 a | 87.2 ± 8.14 a | 1178.8 ± 112.6 a | 993.7 ± 124.5 a | 84.5 ± 10.32 a | 17.3 ± 1.38 a | |
| OE-4 | 17.1 ± 1.44 a | 88.9 ± 7.91 a | 1163.9 ± 118.9 a | 982.4 ± 121.5 a | 84.7 ± 11.43 a | 18.1 ± 1.32 a | |
| OE-6 | 17.7 ± 1.56 a | 90.3 ± 8.11 a | 1188.3 ± 155.3 a | 1028.7 ± 118.6 a | 86.6 ± 13.37 a | 17.4 ± 1.31 a | |
| Heat | WT | 14.2 ± 1.39 b | 81.4 ± 7.92 b | 1076.9 ± 162.3 b | 734.51 ± 109.2 b | 68.9 ± 15.62 b | 14.2 ± 1.28 b |
| OE-3 | 17.8 ± 1.68 a | 90.6 ± 8.55 a | 1192.7 ± 133.5 a | 982.6 ± 111.5 a | 82.8 ± 16.74 a | 17.9 ± 1.31 a | |
| OE-4 | 17.5 ± 1.12 a | 88.5 ± 9.12 a | 1203.9 ± 126.7 a | 964.4 ± 108.4 a | 80.6 ± 14.77 a | 18.3 ± 1.28 a | |
| OE-6 | 18.3 ± 1.88 a | 93.1 ± 8.32 a | 1201.2 ± 152.5 a | 1048.9 ± 126.8 a | 87.4 ± 13.56 a | 18.1 ± 1.31 a |
Values represent means ± SE (n = 3). Different letters next to the numbers indicate significant difference between lines under the same conditions (p ≤ 0.05).
4. Conclusions
Drought and heat stress affects rice growth and yield. To improve rice tolerance to those adverse stresses, OsRab7 was cloned and overexpressed in rice plants. The wild-type and three generated transgenic rice lines were included in stress treatments. The results showed that OsRab7 overexpression improves rice tolerance to drought and heat stress by modulating gas-exchange attributes, osmolytes, photosynthesis, antioxidant machinery, and expression of genes that are involved in defence pathways. The present study also demonstrated that OsRab7 overexpression increases grain yield in transgenic rice. Therefore, OsRab7 gene could serve as a candidate for enhancing stress tolerance and grain yield in rice. Future work should explore the regulatory network and function of OsRab7 in greater depth to unravel the mechanisms controlling stress tolerance in rice.
Author Contributions
M.A.E.-E. designed the study, performed the experiments and data analysis, and wrote, revised and approved the manuscript. A.A.A. revised and approved the manuscript.
Funding
This research has been financially supported by Tanta University in Egypt. The authors would also like to acknowledge Tanta University labs for facilitating performing this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zang X., Geng X., He K., Wang F., Tian X., Xin M., Yao Y., Hu Z., Ni Z., Sun Q., et al. Overexpression of the Wheat (Triticum aestivum L.) TaPEPKR2 gene enhances heat and dehydration tolerance in both wheat and Arabidopsis. Front. Plant Sci. 2018;9:1710. doi: 10.3389/fpls.2018.01710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja I., de Vos R.C., Bones A.M., Hall R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010;15:664–674. doi: 10.1016/j.tplants.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhou R., Yu X., Ottosen C., Rosenqvist E., Zhao L., Wang Y., Yu W., Zhao T., Wu Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017;17:24. doi: 10.1186/s12870-017-0974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Wang Z., Ke Q., Ji C.Y., Jeong J.C., Lee H.S., Lim Y.P., Xu B., Deng X., Kwak S.S. Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiol. Biochem. 2014;85:31–40. doi: 10.1016/j.plaphy.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 5.El-Esawi M.A., Al-Ghamdi A.A., Ali H.M., Alayafi A.A., Witczak J., Ahmad M. Analysis of genetic variation and enhancement of salt tolerance in French pea. Int. J. Mol. Sci. 2018;19:2433. doi: 10.3390/ijms19082433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Esawi M.A., Alaraidh I.A., Alsahli A.A., Ali H.M., Alayafi A.A., Witczak J., Ahmad M. Genetic variation and alleviation of salinity stress in barley. Molecules. 2018;23:2488. doi: 10.3390/molecules23102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Esawi M.A., Alaraidh I.A., Alsahli A.A., Alamri S.A., Ali H.M., Alayafi A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018;132:375–384. doi: 10.1016/j.plaphy.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 8.El-Esawi M.A., Alaraidh I.A., Alsahli A.A., Alzahrani S.M., Ali H.M., Alayafi A.A., Ahmad M. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018;19:3310. doi: 10.3390/ijms19113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Esawi M.A., Al-Ghamdi A.A., Ali H.M., Alayafi A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019;159:55–65. doi: 10.1016/j.envexpbot.2018.12.001. [DOI] [Google Scholar]
- 10.Nuoffer C., Balch W.E. GTPases: Multifunctional molecular switches regulating/vesicular traffic. Annu. Rev. Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- 11.Pitakrattananukool S., Kawakatsu T., Anuntalabhochal S., Takaiwa F. Overexpression of OsRab7B3, a small GTP binding protein gene, enhances leaf senescence in transgenic rice. Biosci. Biotechnol. Biochem. 2012;76:1296–1302. doi: 10.1271/bbb.120050. [DOI] [PubMed] [Google Scholar]
- 12.Takai Y., Sasaki T., Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Sohn E.J., Kim E.S., Zhao M., Kim S.J., Kim H., Kim Y.W., Lee Y.J., Hillmer S., Sohn U., Jiang L., et al. Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell. 2003;15:1057–1070. doi: 10.1105/tpc.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal P., Reddy M.K., Sopory S.K., Agarwal P.K. Plant rabs: Characterization, functional diversity and role in stress tolerance. Plant Mol. Biol. Rep. 2009;27:417–430. doi: 10.1007/s11105-009-0100-9. [DOI] [Google Scholar]
- 15.Pereira-Leal J.B., Seabra M.C. Evolution of the Rab family of small GTP-binding Proteins. J. Mol. Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 16.Mazel A., Leshem Y., Tiwari B.S., Levine A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e) Plant Physiol. 2004;134:118–128. doi: 10.1104/pp.103.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Xia M., Chen Q., Wu Z., Wu P. Identification of a new small GTP-binding protein gene OsRab5a, genomic organization, and expression pattern analysis during nitrate supply and early nutrient starvation in rice (Oryza sativa L.) root. Plant Sci. 2002;163:273–280. doi: 10.1016/S0168-9452(02)00096-1. [DOI] [Google Scholar]
- 18.Wang Y., Ren Y., Liu X., Jiang L., Chen L., Han X., Jin M., Liu S., Liu F., Lu J., et al. OsRab5a regulates endomembrane organization and storage protein trafficking in rice endosperm cells. Plant J. 2010;64:812–824. doi: 10.1111/j.1365-313X.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M., Satoh-Cruz M., Wen L., Crofts A.J., Sugino A., Washida H., Okita T.W., Ogawa M., Kawagoe Y., Maeshima M., et al. The small GTPase Rab5a is essential for intracellular transport of proglutelin from the Golgi apparatus to the protein storage vacuole and endosomal membrane organization in developing rice endosperm. Plant Physiol. 2011;157:632–644. doi: 10.1104/pp.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal P.K., Agarwal P., Jain P., Jha B., Reddy M.K., Sopory S.K. Constitutive overexpression of a stress-inducible small GTP-binding protein PgRab7 from Pennisetum glaucum enhances abiotic stress tolerance in transgenic tobacco. Plant Cell Rep. 2008;27:105–115. doi: 10.1007/s00299-007-0446-0. [DOI] [PubMed] [Google Scholar]
- 21.Kwon S., Cho H.J., Bae K., Jung J.H., Jin H.C., Park O.K. Role of an Arabidopsis Rab GTPase RabG3b in pathogen response and leaf senescence. J. Plant Biol. 2009;52:79–87. doi: 10.1007/s12374-009-9011-4. [DOI] [Google Scholar]
- 22.Peng X., Ding X., Chang T., Wang Z., Liu R., Zeng X., Cai Y., Zhu Y. Overexpression of a vesicle trafficking gene, OsRab7, enhances salt tolerance in rice. Sci. World J. 2014;2014:483526. doi: 10.1155/2014/483526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X., Shiroto Y., Kishitani S., Ito Y., Toriyama K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009;28:21–30. doi: 10.1007/s00299-008-0614-x. [DOI] [PubMed] [Google Scholar]
- 24.Aghamolki M.T.K., Yusop M.K., Oad F.C., Zakikhani H., Jaafar H.Z., Kharidah S., Musa M.H. Heat stress effects on yield parameters of selected rice cultivars at reproductive growth stages. J. Food Agric. Environ. 2014;12:741–746. [Google Scholar]
- 25.Kilasi N.L., Singh J., Vallejos C.E., Ye C., Jagadish S.V.K., Kusolwa P., Rathinasabapathi B. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018;9:1578. doi: 10.3389/fpls.2018.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu A.L., Zou J., Liu C.F., Zhou X.Y., Zhang X.W., Luo G.Y., Chen X.B. Over-expression of OsHsfA7 enhanced salt and drought tolerance in transgenic rice. BMB Rep. 2013;46:31–36. doi: 10.5483/BMBRep.2013.46.1.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao B., Huang Y., Tang N., Xiong L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor. Appl. Genet. 2007;115:35–46. doi: 10.1007/s00122-007-0538-9. [DOI] [PubMed] [Google Scholar]
- 28.Shim J.S., Oh N., Chung P.J., Kim Y.S., Choi Y.D., Kim J.-K. Overexpression of OsNAC14 improves drought tolerance in rice. Front. Plant Sci. 2018;9:310. doi: 10.3389/fpls.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L., Wang Y., Wang W., Zhao X., Qin Q., Sun F., Hu F., Zhao Y., Li Z., Fu B., et al. Characterization of transcription factor gene OsDRAP1 conferring drought tolerance in rice. Front. Plant Sci. 2018;9:94. doi: 10.3389/fpls.2018.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng D.E., Hou P., Xiao F.L.Y. Overexpression of Arabidopsis XERICO gene confers enhanced drought and salt stress tolerance in rice (Oryza Sativa L.) J. Plant Biochem. Biotechnol. 2013;24:56–64. doi: 10.1007/s13562-013-0236-4. [DOI] [Google Scholar]
- 31.Xiong H., Yu J., Miao J., Li J., Zhang H., Wang X., Liu P., Zhao Y., Jiang C., Yin Z. Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol. 2018;178:451–467. doi: 10.1104/pp.17.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang J., Chen X., Hu W., Xiang Y., Yan M., Wang J. Overexpressing heat-shock protein OsHSP50.2 improves drought tolerance in rice. Plant Cell Rep. 2018;37:1585–1595. doi: 10.1007/s00299-018-2331-4. [DOI] [PubMed] [Google Scholar]
- 33.Sato H., Todaka D., Kudo M. The Arabidopsis transcriptional regulator DPB3-1 enhances heat stress tolerance without growth retardation in rice. Plant Biotechnol. J. 2016;14:1756–1767. doi: 10.1111/pbi.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J., Zhang C., Wei C., Liu X., Wang M., Yu F., Xie Q., Tu J. The RING finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2-induced stomatal closure in rice. Plant Physiol. 2016;170:429–443. doi: 10.1104/pp.15.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R., Zhao X., Shao Z., Wei Z., Wang Y., Zhu L., Zhao J., Sun M., He R., He G. Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell. 2007;19:847–861. doi: 10.1105/tpc.106.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Z., Ma D., Tang L., Hong Y., Luo A., Zhou J., Dai X. Expression of the spinach betaine aldehyde dehydrogenase (BADH) gene in transgenic tobacco plants. Chin. J. Biotechnol. 1997;13:153. [PubMed] [Google Scholar]
- 37.Yamasaki S., Dillenburg L.C. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999;11:69–75. [Google Scholar]
- 38.Dionisio-Sese M.L., Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- 39.Velikova V., Yordanov I., Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 40.Rao K.V.M., Sresty T.V.S. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 41.Holá D., Benešová M., Honnerová J., Hnilička F., Rothová O., Kočová M., Hniličková H. The evaluation of photosynthetic parameters in maize inbred lines subjected to water deficiency: Can these parameters be used for the prediction of performance of hybrid progeny? Photosynthetica. 2010;4:545–558. doi: 10.1007/s11099-010-0072-x. [DOI] [Google Scholar]
- 42.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 43.Dey P.M. Oligosaccharides. In: Dey P.M., editor. Methods in Plant Biochemistry, Carbohydrates. Volume 2. Academic Press; London, UK: 1990. pp. 189–218. [Google Scholar]
- 44.Bates L., Waldren P.P., Teare J.D. Rapid determination of free proline of water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 45.Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X.Z. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang X.Z., editor. Research Methodology of Crop Physiology. Agriculture Press; Beijing, China: 1992. pp. 208–211. [Google Scholar]
- 47.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 48.Vighi I.L., Benitez L.C., Amaral M.N., Moraes G.P., Auler P.A., Rodrigues G.S., Deuner S., Maia L.C., Braga E.J.B. Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol. Plant. 2017;61:1–11. doi: 10.1007/s10535-017-0727-6. [DOI] [Google Scholar]
- 49.Cai W., Liu W., Wang W.S., Fu Z.W., Han T.T., Lu Y.T. Overexpression of rat neurons nitric oxide synthase in rice enhances drought and salt tolerance. PLoS ONE. 2015;10:e0131599. doi: 10.1371/journal.pone.0131599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J., Lai Y., Wu X., Wu G., Guo C. Overexpression of OsEm1 encoding a group I LEA protein confers enhanced drought tolerance in rice. Biochem. Biophys. Res. Commun. 2016;478:703–709. doi: 10.1016/j.bbrc.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 52.Siddiqui M.H., Al-Khaishany M.Y., Al-Qutami M.A., Al-Whaibi M.H., Grover A., Ali H.M., Al-Wahibi M.S. Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J. Biol. Sci. 2015;22:656–663. doi: 10.1016/j.sjbs.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekmen A.H., Ozgur R., Uzilday B., Turkan I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 2014;99:141–149. doi: 10.1016/j.envexpbot.2013.11.010. [DOI] [Google Scholar]
- 54.Alexieva V., Sergiev I., Mapelli S., Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- 55.Bajji M., Kinet J., Lutts S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002;36:61–70. doi: 10.1023/A:1014732714549. [DOI] [Google Scholar]
- 56.Ganguli L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. Fundam. Mol. Mech. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 57.Ben K.R., Abdelly C., Savouré A. Proline, a multifunctional amino-acid involved in plant adaptation to environmental constraints. Biol. Aujourdhui. 2012;206:291. doi: 10.1051/jbio/2012030. [DOI] [PubMed] [Google Scholar]
- 58.Chaleff R.S. Further characterization of picloram tolerant mutance of Nicotinana tabacum. Theor. Appl. Genet. 1980;58:91–95. doi: 10.1007/BF00277772. [DOI] [PubMed] [Google Scholar]
- 59.Wang F., Liu P., Zhu J. Effect of magnesium (Mg) on contents of free proline, soluble sugar and protein in soybean leaves. J. Henan Agric. Sci. 2004;6:35–38. [Google Scholar]
- 60.Nahm M.Y., Kim S.W., Yun D., Lee S.Y., Cho M.J., Bahk J.D. Molecular and biochemical analyses of OsRab7, a rice Rab7 homolog. Plant Cell Physiol. 2003;44:1341–1349. doi: 10.1093/pcp/pcg163. [DOI] [PubMed] [Google Scholar]
- 61.Saito C., Ueda T., Abe H., Wada Y., Kuroiwa T., Hisada A., Furuya M., Nakano A. A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J. 2002;29:245–255. doi: 10.1046/j.0960-7412.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 62.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 63.Limpens E., Lvanov S., van Esse W., Voets G., Fedorova E., Bisseling T. Medicago N2-Fixing symbiosomes acquire the endocytic identity marker Rab7 but delay the acquisition of vacuolar identity. Plant Cell. 2009;21:2811–2828. doi: 10.1105/tpc.108.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine A. Regulation of stress responses by intracellular vesicle trafficking? Plant Physiol. Biochem. 2002;40:531–535. doi: 10.1016/S0981-9428(02)01398-0. [DOI] [Google Scholar]
- 65.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oh S.J., Kim Y.S., Kwon C.W., Park H.K., Jeong J.S., Kim J.K. Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol. 2009;150:1368–1379. doi: 10.1104/pp.109.137554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hakata M., Kuroda M., Ohsumi A., Hirose T., Nakamura H., Muramatsu M., Ichikawa H., Yamakawa H. Overexpression of a rice TIFY gene increases grain size through enhanced accumulation of carbohydrates in the stem. Biosci. Biotechnol. Biochem. 2012;76:2129–2134. doi: 10.1271/bbb.120545. [DOI] [PubMed] [Google Scholar]
- 68.Jiang D., Chen W., Dong J., Li J., Yang F., Wu Z., Zhou H. Overexpression of OsmiR164b-resistant OsNAC2 improves plant architecture and grain yield in rice. J. Exp. Bot. 2018;69:1533–1543. doi: 10.1093/jxb/ery017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo B., Chen J., Zhu L., Liu S., Li B., Lu H., Ye G., Xu G., Fan X. Overexpression of a high-affinity nitrate transporter OsNRT2.1 increases yield and manganese accumulation in rice under alternating wet and dry condition. Front. Plant Sci. 2018;9:1192. doi: 10.3389/fpls.2018.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]