Abstract
Background
Being devoid of de novo protein synthesis capacity, red blood cells (RBCs) have evolved to recycle oxidatively damaged proteins via mechanisms that involve methylation of dehydrated and deamidated aspartate and asparagine residues. Here we hypothesize that such mechanisms are relevant to routine storage in the blood bank.
Study design and Methods
Within the framework of the REDS-III RBC-Omics study, packed RBC units (n=599) were stored under blood bank conditions for 10, 23 and 42 days and profiled for oxidative hemolysis and time-dependent metabolic dysregulation of the trans-sulfuration pathway.
Results
In these units methionine consumption positively correlated with storage age and oxidative hemolysis. Mechanistic studies show that this phenomenon is favored by oxidative stress or hyperoxic storage (SO2 >95%), and prevented by hypoxia or methyltransferase inhibition. Through a combination of proteomics approaches and 13C-methionine tracing, we observed oxidation-induced increases in both Asn deamidation to Asp and formation of methyl-Asp on key structural proteins and enzymes, including band 3, hemoglobin, ankyrin, 4.1, spectrin beta, aldolase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), biphosphoglycerate mutase, lactate dehydrogenase and catalase. Methylated regions tended to map proximal to the active site (e.g. N316 of GAPDH) and/or residues interacting with the N-terminal cytosolic domain of band 3.
Conclusion
While methylation of basic amino acid residues serves as an epigenetic modification in nucleated cells, protein methylation at carboxylate side chains and deamidated asparagines is a non-epigenetic post-translational sensor of oxidative stress and refrigerated storage in anucleated human RBCs.
Keywords: Mass spectrometry, 13C metabolic labeling, blood storage, transfusion medicine
Introduction
Red blood cells (RBCs) are the most abundant host cell in the human body, accounting for ~83% of cells in a 75 kg adult.1 To maximize oxygen-carrying capacity, mammalian RBCs lose their nuclei and organelles during the maturation process, allowing maximum cell loading with hemoglobins and thus oxygen. While carrying and delivering oxygen to tissues during their ~120 day lifespan, RBCs are constantly challenged by oxidative stress. However, though devoid of de novo protein synthesis capacity, RBCs preserve redox homeostasis through antioxidant defenses at the protein and metabolic level. Thus, RBCs present both one of the simplest and most fascinating models for biochemical studies of oxidative stress responses.
Tissue reoxygenation through RBC transfusion is the most frequent medical procedure worldwide to treat hypoxia secondary to injury and disease. Each year ~11 million units are transfused in the United States alone,2 a feat made possible by over 100 years3 of investigations on RBC preservation strategies that afford refrigerated storage for up to 42 days. While necessary, cold liquid storage induces biochemical and morphological alterations termed the “storage lesion(s)”.4 The recent introduction of omics technologies in transfusion medicine has helped elucidate the extent and progression of the storage lesion(s).5,6 Mass spectrometry (MS)-based approaches have highlighted an impairment in energy and redox homeostasis after storage week 2, resulting in the decrease of adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (DPG), both relevant to binding and off-loading of oxygen by hemoglobin. In addition, oxygen saturation and accumulation of reactive oxygen species (ROS)7 are observed between storage week 2 and 3, ultimately resulting in irreversible oxidation of purines,8 lipids9 and proteins (including carbonylation,10,11 non-enzymatic glycation12 and thiol oxidation13–19). These considerations, combined with systems biology-based elaborations of omics data,20–22 reveal the potential of blood bank storage as a medically relevant model of oxidative stress to RBCs.
While the clinical impacts of the storage lesion(s) are under investigation, elegant studies in animals9,23,24 and preliminary data in humans8,25–28 suggest that such alterations may impact RBC post-transfusion recovery and in vivo extravascular hemolysis. However, randomized clinical trials29–32 have shown that current standards of practice are non-inferior to administration of the freshest units available. This apparent disconnect may underscore biological variability as a critical factor in RBC biology, storability, post-transfusion recoveries and metabolic responses to oxidative stress and hemolysis.33–35 To examine donor variability in a large prospective clinical cohort, the Recipient Epidemiology and Donor Evaluation Study (REDS-III) RBC-Omics protocol enrolled ~13,403 blood donors of different race-ethnicities, gender, age and prior donation intensity to determine how donor biology impacts propensity to hemolysis at the end of in vitro storage following a pro-oxidant insult with 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH).36 Donors showing extreme oxidative hemolysis measurements (as well as other spontaneous and stress hemolysis phenotypes not reported in this manuscript) at storage day 42 (<5 and >95 percentile) were recalled and invited to donate a second unit, which was stored under routine blood bank conditions and sampled at storage day 10, 23 and 42 to test oxidative hemolysis as well as to perform metabolomics analyses on a subset of these. Leveraging the power of the RBC-Omics study, here we report the results of a quantitative measurement of methionine, one of the most significant metabolites consumed in stored RBCs in different additives.37 We report that methionine consumption rates correlate with storage duration but also with donor-dependent RBC propensity to hemolyze when exposed to oxidative insults. Since previous tracing experiments have shown that glutathione synthesis is significantly down-regulated in RBCs under refrigerated storage conditions,37,38 we hypothesized an alternative fate for RBC methionine as a critical methyl donor in stored RBCs.
Pioneering work by Clarke, Ingrosso and colleagues identified aspartate methylation of RBC membrane proteins in response to aging or pathological oxidative stress, such as in glucose 6-phosphate dehydrogenase deficiency or Down syndrome.39–44 While methylation of histones impacting RNA transcription is a key epigenetic modification in nucleated cells, it is interesting to note that specific methyltransferase enzymes exist in human RBCs to methylate oxidized isoaspartate moieties formed from the deamidation/dehydration of asparagine/aspartate residues following oxidative insults.43 Here, we build on these classical studies to demonstrate that oxidative stress promoted by RBC storage in the blood bank is sufficient to induce methylation of membrane proteins and previously unidentified targets. 13C-Methionine tracing experiments in normoxia, hypoxia and hyperoxia/oxidative-stress reveal a dependency of isoaspartate methylation on SO2 and oxidative stress status. Finally, structural modeling of methylated isoaspartate residues is utilized to discuss the implications on stored RBC metabolism and the potential advantages of alternative storage strategies that attenuate the oxidative injury, such as anaerobic storage.45
Materials and Methods
Commercial reagents were purchased from Sigma-Aldrich unless otherwise noted.
REDS-III RBC-Omics Samples:
Donor selection and recruitment for the RBC-Omics study was described by Kanias et al.36 Donors were enrolled at the four participating REDS-III US blood centers. Overall, 97% (13,403) of the whole blood donations provided by 13,758 donors age 18+ who provided informed consent were evaluable for hemolysis parameters, including oxidative hemolysis analysis on ~42-day stored RBC derived from 8,502 whole blood donations. Blood collection, sample processing and other aspects of the screening and recall phases of the RBC-Omics Study are detailed in the Supporting Information (SI).
Oxidative hemolysis
Oxidative hemolysis was determined at the University of Pittsburgh and Blood Systems Research Institute as reported and further detailed in the SI, as described
Human RBC SO2 levels at 8h post-donation
CP2D-AS-3 RBCs with controlled SO2 (i.e., >95% (hyperoxic), normoxic (untreated) to <20%, <10%, <5% or <3% (hypoxic)) on Day 0 were prepared in vented chambers (Difco BLL) by Hemanext inc (Lexington, MA).
Human RBC incubation with 13C-methionine and inhibitor of methyltransferase in normoxia, hypoxia or H2O2
Freshly donated leukocyte-filtered RBCs (Bonfils Blood Center, Denver, CO) were incubated for 0, 1 and 16 h in additive solution 3 supplemented with 1 mM [13C5,15N]-methionine at 4°C under normoxic or hypoxic conditions in the presence or absence of 1 μM adenosine dialdehyde (Cayman Chemical). In separate experiments, fresh RBCs were exposed to H2O2 at 0.1% and 0.5% v/v for 0, 1, and 16 h, either under normoxic or hypoxic conditions (for 16 h). A volume of 50 μL was collected at each time point for metabolic tracing experiments via UHPLC-MS. See SI for additional experimental details.
Fractionation and nanoUHPLC-MS/MS proteomics
Leukocyte-reduced human RBCs were washed 5 times in PBS prior to lysis in distilled water with sonication. Samples were digested, fractionated, and analyzed by nanoUHPLC-MS/MS proteomics as detailed in the SI.
Sample processing and metabolite extraction
RBCs were separated by centrifugation (10 min at 4°C and 2500 g) then 50 μL was extracted 1:10 in ice cold lysis buffer (methanol:acetonitrile:water 5:3:2) in presence of a mix of fully 13C,15N-labeled amino acid standards (also including [13C5,15N]methionine) at 1 μM final concentration. Samples were vortexed and insoluble material pelleted as described.46,47
UHPLC-MS metabolomics
Analyses were performed using a Vanquish UHPLC coupled online to a Q Exactive mass spectrometer (Thermo Fisher, Bremen, Germany). Samples were analyzed using a 3 minute isocratic condition48 or a 9 min gradient as described.47 Solvents were supplemented with 0.1% formic acid for positive mode runs and 1 mM ammonium acetate for negative mode runs. MS acquisition, data analysis and elaboration was performed as described.47,48 Additional analyses, including untargeted analyses and Fish score calculation via MS/MS were calculated against the ChemSpider database with Compound Discoverer 2.0(Thermo Fisher, Bremen, Germany). Graphs and statistical analyses (either t-test or repeated measures ANOVA) were prepared with GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA).
Structural modeling
was performed with PyMol by mapping methylated D, E or N → D-methyl residues on proteins of interest. Structures were obtained from the Protein DataBank as noted in the results section.
Results
Methionine consumption correlates with oxidative hemolysis and fuels SAM/SAH-dependent methyltransferases in samples from the REDS-III cohort
Small scale metabolomics studies of stored RBCs have shown a time-dependent accumulation of all amino acids in the supernatant, with the notable exceptions of glutamine and methionine, both building blocks for glutathione biosynthesis.49 In particular, methionine metabolism generates cysteine via the trans-sulfuration pathway. In previous studies we utilized metabolic tracing of stable isotope-labeled glutamine in stored RBCs,37,50 while here we focused on the role of methionine in RBC responses to storage-induced oxidative stress. In the RBC-Omics study, end-of-storage leukoreduced RBC from 8,502 US blood donors were screened for their hemolytic propensity following oxidative insult with AAPH. Extreme hemolyzers (<5 and >95% percentile)as well as donors with mid-range findings on end-of-storage hemolysis parameters were recalled to donate second whole blood units approximately 2 – 6 months later, which were processed into leukocyte reduced RBC components and stored for 10, 23 and 42 days prior to sampling and new measurements of oxidative (and spontaneous and other stress-induced) hemolysis on intact RBC; targeted quantitative measurement of methionine was performed on selected cryopreserved aliquots of RBC derived from 200 recalled donations (599 total samples tested - Figure 1.A). A significant but moderate positive correlation was observed between oxidative hemolysis measurements during the first phase and the recall phase of the study (described by Lanteri et al. Transfusion 2018; in press), indicating the stability and donor-dependency of this parameter. As shown in Figure 1.B, methionine levels decreased significantly during storage of samples obtained from the RBC-Omics recalled donors, from 5.8 + 2.9 μM at storage day 10 to 1.2 + 0.9 μM at storage day 42. However, when focusing on the 20 extreme highest and lowest hemolyzers among the recalled donors, methionine levels differed significantly at day 10, while they did not at later time points (Figure 1.C), indicative of significantly higher methionine consumption in high oxidative hemolyzers (Figure 1.D). Low oxidative hemolyzers were characterized by significantly higher levels of methionine derivatives S-adenosylhomocysteine (SAH) and S-adenosylmethionine (SAM), but lower levels of homocysteine and cysteine (Supplementary Figure 1), suggesting that methionine catabolism fueled biochemical pathways aside from glutathione homeostasis (Figure 1.E), consistent with previous findings of impaired glutathione synthesis in stored erythrocytes.37,38 Given the elevated levels of SAH and SAM, we next turned our focus to protein methylation as a possible consequence of increased methionine consumption.
Figure 1 – The rate of methionine consumption correlates with oxidative hemolysis in the REDS III Omics study.
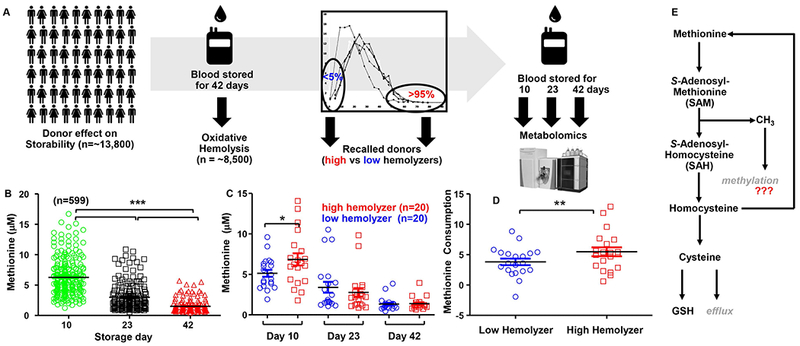
In A, ~13.8 thousand donors were enrolled within the framework of the REDS III-Omics study. A single unit of blood was donated, leukofiltered and stored at 4°C for 42 days (standard blood bank conditions). At day 42, ~8,500 eligible units were tested for oxidative hemolysis (AAPH assay) and extreme hemolyzers (<5 and >95% percentile) recalled to donate a second unit. Subsequent units were processed as in phase I, though sampling occurred at storage days 10, 23 and 42 for a second measurement of oxidative hemolysis and targeted quantitation of methionine via standard UHPLC-MS metabolomics approaches. Methionine levels decreased significantly over the storage duration (n = 599; p <0.00001 ANOVA - B). In particular, methionine measurements in extreme 20 high vs low hemolzyers in the recalled cohort showed that methionine levels were the highest in high oxidative hemolyzers at storage day 10, but not in subsequent time points (C). Total methionine consumption (ΔMethionine at day 42 – day 10) was significantly higher in high oxidative hemolyzers (D). An overview of methionine metabolism is shown in E. * p < 0.05; ** p < 0.01 T-test.
Human RBCs contain several protein methyltransferases that catalyze the esterification of side chain carboxylates
Protein methylation is a well-established epigenetic modification in nucleated cells. However, owing to the lack of nuclei and the apparent simplicity of the RBC proteome, we questioned whether RBCs were equipped with the protein machinery to introduce this post-translational modification. Through a combination of protein-level gel electrophoresis-based fractionation51 and peptide-level high pH reverse phase preparative scale chromatography, each followed by nanoUHPLC-MS/MS, we identified a series of protein methyltransferases (Supplementary Table 1), the most abundant across datasets consistently being protein L-isoaspartate O-methyltransferase (PCMT1 – Supplementary Figure 2). PCMT1 methylates isoaspartates formed via dehydration and deamidation of aspartate and asparagine residues, respectively (Supplementary Figure 2.A). Isoaspartate formation has been noted in RBC aging52 and is elevated in membrane proteins in response to oxidative stress.53 A similar activity is performed by the protein glutamate O-methyltransferase (ARMT1), confidently identified in the cytosol of mature RBCs in our proteomics analysis (Supplementary Figure 3).
Isoaspartate and glutamate O-methyltransferases in mature human erythrocytes catalyze widespread protein methylation, especially of the band 3 N-terminus
After confirming the presence in human RBCs of methyltransferases that esterify side chain carboxylates, we mined our deep proteomics data of cytosolic and membrane RBC proteins to map methylated aspartate and glutamate residues, as well as deamidated → methylated asparagine residues. Results (extensively reported in Supplementary Table 2) included a long series of proteins involved in structural integrity, RBC vesiculation and ion homeostasis, redox reactions and protein degradation, glycolysis, (calcium) signaling components and hemoglobins (Figure 2.A enlists abbreviated Uniprot names for top hits in each group). In particular, we noted that the anion exchanger 1, also known as band 3 protein (SLC4A1) was extensively methylated in the N-terminal cytosolic domain on D, E and deamidated N residues (Figure 2.B). Many of the methylated residues are located on amino acid sequences previously reported to mediate the interface of this very acidic N-terminus with structural proteins (including ankyrin) and functional proteins (including deoxyhemoglobin and glycolytic enzymes – such as glyceraldehyde 3-phosphate dehydrogenase – GADPH; phosphofructokinase – PFK; and aldolase – ALDOA – Figure 2.B).54–58 Of note, the very N-term of band 3 is usually not observed in routine proteomics workflows, owing to the absence of trypsin cleavage sites resulting in a 56 residue peptide. Here, identification and acquisition of high-resolution spectra of peptides in this region – including methylation of residues D6/D7 (Figure 3.A) and D38 (Figure 3.B) was made possible by extensive offline pre-fractionation of peptides. Of note, this N-terminal band 3 peptide (an intrinsically disordered region of the protein that has hitherto eluded structural characterization) has been modeled to fold next to the N-term residues 330-391,58 proximal to the trans-membrane domain, both regions appearing to be extensively methylated in our dataset (Figure 3.C and D; Supplementary Figure 4).
Figure 2 – Highlights of proteins with high-confidence assignment of D-methylation or N-deamidation (→methylation) in human RBCs.
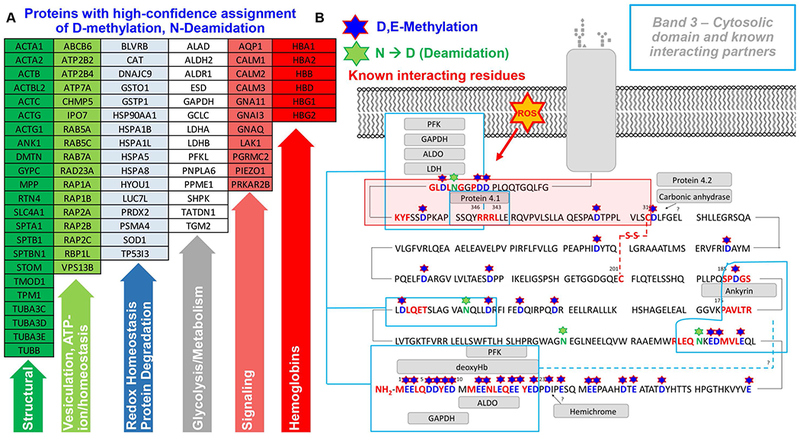
(UniProt abbreviations are listed – A; full list is provided in Supplementary Table 2, along with peptide sequences). In B, an overview of the cytosolic domain of band 3, from residues N-term (1) through residue 380 (SLC4A1_HUMAN; P02730). Stars highlight methylated aspartate and glutamate residues (single letter code D and E, respectively – in blue) as well as deamidated and deamidated → methylated residues (N – in green) on the N-term cytosolic domain of band 3. Rectangles also show the sequences on the N-term of band 3 that have been previously identified54–58 as essential mediators of the interaction between this membrane protein and structural proteins and glycolytic enzymes.
Figure 3 – State of the art proteomics characterizes extensive methylation of N-term of Band 3.
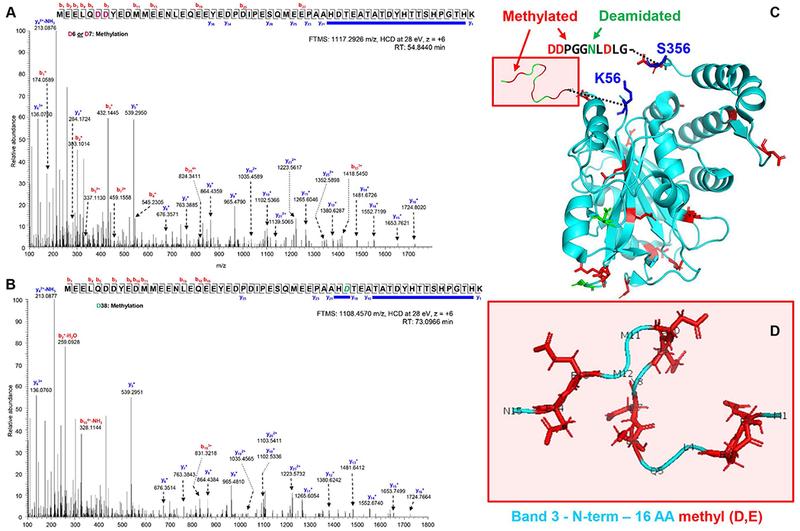
Two representative spectra with extensive coverage of fragment ions (including those covering disambiguation of methylated residues D6/7 or D38) are provided in A and B. Additional spectra are shown in Supplementary Figure 3 and all peptides with Mascot ion scores and Δscores are reported in Supplementary Table 2. In C, an overview of the crystal structure of band 3 (N-term cytosolic domain, based on pdb: 1hyn). In D, the very N-term (1-16 residues of band 3 are highlighted (based on pdb: 3btb). D-methyl and N-deamidated residues are highlighted in red and green, respectively.
Extensive methylation of the “transport metabolon” and interacting partners, including hemoglobin, ankyrin, GAPDH, and ALDOA
Elegant work from Low’s group has identified the N-term of band 3 as a “transport metabolon”, i.e. a docking site for key functional and structural proteins with functional implications on structural and glycolytic homeostasis.54–58 Notable entries among these interacting partners are deoxyhemoglobin and peroxidized hemoglobin (hemichrome), two key players in RBC oxygen transport and mediators of oxidative stress to membrane components. Here we map for the first time that a series of D, E and deamidated N residues on hemoglobin alpha and beta chains are methylated in human RBCs (Figure 4.A), with most residues mapping on the surface of the globin tetramer, particularly on residues flanking the prosthetic group (Figure 4.B and C). Of note, the negative charge of the very N-term of band 3 has been proposed to mediate the interaction with deoxyhemoglobin by stabilizing its T-state, in like fashion to ATP and DPG. Here, we show that residues in both the N-term and in a band 3 region that interfaces with the N-term are extensively methylated.
Figure 4 – Methylation of isoaspartate and deamidated asparagine residues in human hemoglobin alpha and beta,
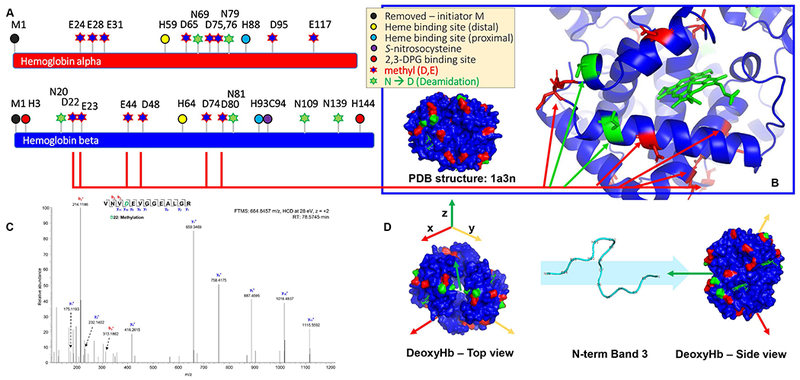
as mapped on their sequence (A), highlighted in the deoxyhemoglobin structure (based on pdb: 1a3n – B) and shown in a representative MS/MS spectrum (methylation of D22 – C). A top and side view of deoxyhemoglobin, along with the N-term of band 3 that has been shown to interact with the deoxyhemoglobin tetramer is shown in D. D-methyl and N-deamidated residues are highlighted in red and green, respectively.
Similarly, the negatively charged very N-term of band 3 is known to interact with glycolytic enzymes, including GAPDH, an interaction that has been modeled to occur in the active site pocket of the GAPDH tetramer (Supplementary Figure 5).59 Methylation of D and deamidated N in GAPDH were here confidently observed, particularly in residues facing the active site of the enzyme and flanking the active C152 (e.g. N155 - Figure 5.A-D; Supplementary Figure 5.A-C). In particular, residues like N316 (identified to be deamidated and methylated) are separated by <3.0 Å from the bound substrate (Figure 5). An estimated linear length for the methyl ester (-OCH3) of >2.5 Å is considerably longer than the 0.96 Å O-H bond length for this unmethylated (and protonated) side chain. Thus, methylation of negatively charged residues facing the active site is predicted to alter microenvironment polarity and/or facilitate access to the active site for coenzyme NAD+ and substrate glyceraldehyde 3-phosphate (Figure 5).
Figure 5 – Methylation of isoaspartate and deamidated asparagine residues in human glyceraldehyde 3-phosphate dehydrogenase (GAPDH),
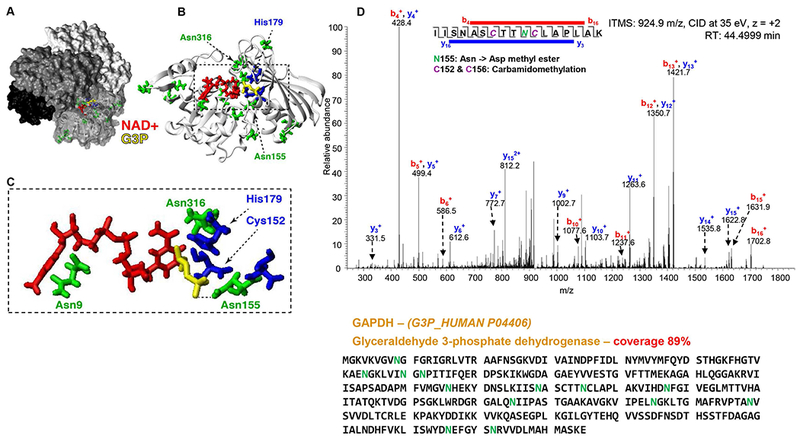
as highlighted in a 3D structural model of the human ternary complex. (A-B) Surface representation of the holo GAPDH tetramer (PDB accession 1WNC) with coenzyme (red) and substrate (yellow). (C) Modified Asn residues (green) and oxidized residues (blue). Asn316 is within 3 Å of the coenzyme. The substrate was modeled based on a superposition with the bacterial ternary complex (PDB accession 1DC4). (D) Representative MS/MS spectrum (methylation of deamidated N155 in the active site of GAPDH) and the protein sequence.
In addition to band 3-interacting partners, we identified extensive methylation and deamidation → methylation of several structural proteins that preserve the integrity of the erythrocyte membrane skeleton, such as Ankyrin, spectrin beta, protein 4.1 and erythrocyte membrane protein 55 kDa (Supplementary Table 2; Supplementary Figures 6–8). Similarly, glycolytic enzymes – some of which are known to interact with band 3 – were methylated in D, E, R and deamidated N residues, mapping to the active site of many enzymes (e.g. R56 and N361 in ALDOA; N17 and N190 in BPGM; N108 and N138 of LDHA – Supplementary Figures 9–11). Of note, key antioxidant enzymes or enzymes involved in glutathione homeostasis like catalase were found to be extensively methylated proximal to their catalytic site (Supplementary Figure 12).
Hemoglobin oxygen saturation modulates methionine consumption in stored RBCs
Modulation of hemoglobin SO2 has been shown to mitigate (via hypoxia) or promote (via hyperoxia) oxidative stress in stored RBCs, as determined by direct measurements of glutathione homeostasis60 and oxidation of thiol groups in cysteine residues involved in key enzymatic functions: in hemoglobin (e.g. hemoglobin beta C93), GAPDH (C152 and C156), and peroxiredoxin 2 (C51 and C172).14,17–19,61 Here, we hypothesized that methionine consumption promoted by oxidative stress would be attenuated or promoted by hypoxic or hyperoxic storage, respectively (Figure 6.A). Leukocyte-filtered packed RBCs (n = 4) were stored at 4°C for up to 42 days under normoxic (no control of oxygen tension), or controlled hyperoxic (SO2 >95%) or hypoxic conditions (SO2 = 20, 10, 5 or < 3% - Figure 6.A). Consistent with the hypothesis, methionine consumption was significantly attenuated by hypoxia, with SAM conversion to SAH reduced in hypoxic storage and promoted by hyperoxic storage. Dose response-like distributions were observed only for SAM, whose consumption decreased proportionally to the degree of hypoxia (Figure 6.B). While no significant increases were observed in extracellular levels of homocysteine across groups, intracellular levels of homocysteine, cysteine and folate metabolism intermediates (methenyltetrahydrofolate and methylenetetrahydrofolate) were observed in hypoxic RBCs proportionally to hypoxia (Supplementary Figure 13).
Figure 6 – Storage of leukocyte-filtered packed RBCs under normoxic, hyperoxic (SO2>95%) or hypoxic conditions (SO2 = 20, 10, 5 or <3 % - A) generated weekly samples for (B) metabolomics analysis of methionine, S-adenosylmethionine, and S-adenosylhomocysteine.
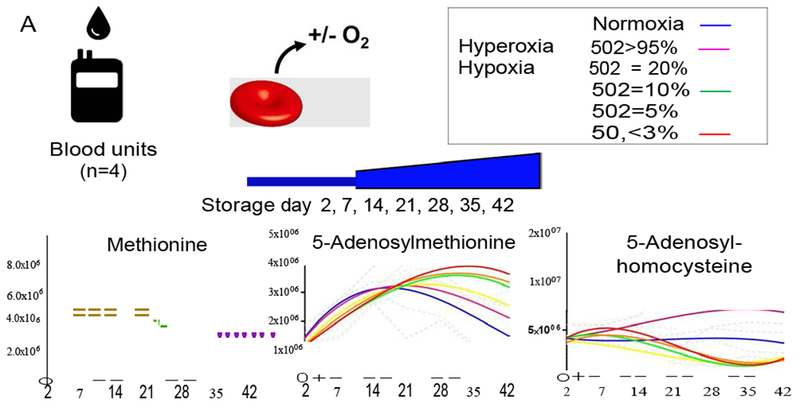
Continuous lines indicate interpolation by third degree polynomial of medians values for weekly measurements in 4 biological replicates (+ ranges – dotted lines). Y-axis values are intensity in arbitrary units. Color code for each condition is indicated in A: normoxia = blue; hyperoxia (SO2>95%) = purple; Hypoxia: SO2 = 20% (yellow); 10% (green); 5% (orange); <3% (red).
Oxidative stress promotes methionine consumption, as gleaned with 13C,15N-methionine tracing metabolomics
To determine whether increased methionine consumption in RBCs stored under hyperoxia (SO2 >95%) was indeed due to oxidative stress, we incubated fresh RBCs (n = 4) with pro-oxidants methylene blue or hypoxanthine (100 μM) + xanthine oxidase (Supplementary Figure 14). These treatments promote oxidative stress either indirectly or directly: (i) methylene blue conversion to leukomethylene blue by biliverdin reductase B (BLVRB) consumes the main reducing cofactor in antioxidant reactions, NADPH62; (ii) hypoxanthine oxidation to urate by xanthine oxidase generates hydrogen peroxide (H2O2).8 Incubation for 1 and 3 h of each pro-oxidant significantly promoted methionine consumption (Supplementary Figure 14).
In the light of these observations, we incubated leukocyte-filtered fresh packed human RBCs in additive solution-3 supplemented with 1 mM [13C5,15N]methionine. This approach allows the tracing of methionine catabolism through the SAM/SAH pathway, the trans-sulfuration pathway (Figure 7.A) and alternative pathways (including direct methionine oxidation, the transamination pathway, and purine catabolism/polyamine synthesis – Supplementary Figure 15.A-F). Upon incubation with heavy methionine, RBCs were stored at 4°C for 0, 1, and 16 h under normoxic or hypoxic conditions, in the presence or absence of the methyltransferase inhibitor adenosine dialdehyde. Additionally, RBCs were exposed to H2O2 (0.1% and 0.5%) under normoxic or hypoxic conditions. Incubation with H2O2 > 0.5% caused excess lysis within the time window tested here (data not shown). Consistent with previous studies,60 hypoxia preserved glutathione homeostasis and increased GSH/GSSG ratios over normoxic RBCs starting from 1h of incubation, whereas H2O2 stress promoted GSH exhaustion, proportionally to H2O2 % (Supplementary Figure 16). Hypoxia decreased methionine uptake and consumption, respectively, while an opposite effect was observed in response to H2O2 (Figure 7.B and C). SAM labeling exceeded 90% of the total metabolite, suggesting that SAM synthesis is very active in RBCs, either via the donation of a methyl group (13C-methyl-SAM) and transfer of [13C5,15N]methionine to an adenosyl moiety (Supplementary Figure 15.A). Inhibition of methyltransferases decreased consumption of methionine and labeled S-adenosylmethionine (SAM) (Figure 7.D). Labeled SAM/SAH ratios increased with methyltransferase inhibition, and decreased in normoxic RBCs exposed to H2O2 (Figure 7.E). Methionine consumption (Figure 7.B and C) and oxidation to methionine sulfoxide (Supplementary Figure 15.D) were comparable in hypoxic and normoxic RBCs incubated with H2O2, suggesting that the benefits observed in untreated hypoxic RBCs are attributable to decreased oxidative stress in this group in the absence of pro-oxidant stressors. Of note, hypoxia promoted the transamination pathway (HMTBA and glutamate, though accounting for <1% of total RBC glutamate – Supplementary Figure 15.F). Consistent with previous studies on adenine tracing in stored RBCs,63 >90% of methylthioadenosine was labeled at 16h of incubation with heavy methionine in all groups, especially in response to inhibition of methyltransferases (Supplementary Figure 15.C), though the contribution of this pathway to total polyamine synthesis was < 5% (Supplementary Figure 15.A).
Figure 7 – [13C5,15N]methionine tracing experiments (A) were performed in fresh human RBCs, stored at 4°C for 0, 1 and 16h under normoxic or hypoxic condtions, in presence or absence of the methyltransferase inhibitor adenosine dialdehyde.
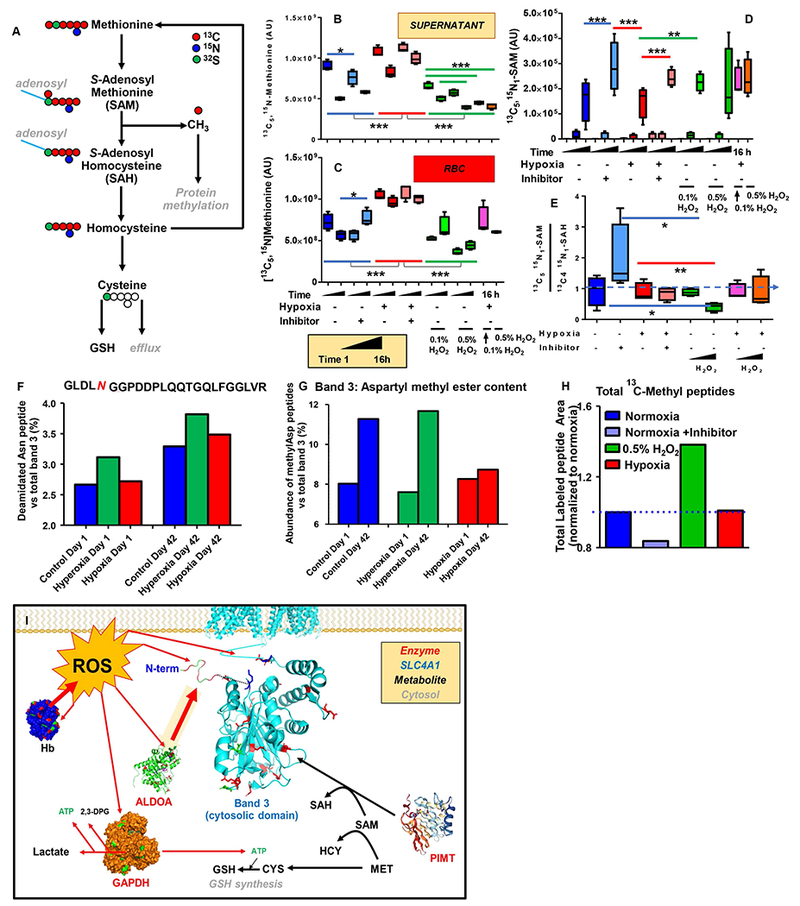
Additionally, RBCs were exposed to H2O2 (0.1% and 0.5%) under normoxic or hypoxic conditions for 1 to 16 h (time course). Hypoxia decreased methionine uptake (B) and consumption (C), respectively, while an opposite effect was observed in response to H2O2. Inhibition of methyltransferases decreased methionine consumption and labeled S-adenosylmethionine (SAM) consumption (D). Labeled SAM/SAH ratios were increased in response to the inhibition of methyltransferases, while these ratios decreased in normoxic RBCs exposed to H2O2 (E). Asterisks in panels B-E indicate significance by ANOVA with Tukey post-hoc test for multiple column comparisons (* p< 0.05; ** p < 0.01; *** p < 0.001). Relative quantitation of a representative deamidated (F) and total deamidated → methylated peptides (G) in the N-term of band 3 in RBCs stored under normoxic, hyperoxic (SO2>95%) or hypoxic conditions (SO2<5%) at storage day 1 and 42. In H, fold changes of total 13C-methyl-D labeled peptides (peak areas normalized to normoxic RBCs) in normoxic RBCs (+/− methyltransferase inhibitor – dark/light blue, respectively), hydrogen peroxide (0.5% - green) and hypoxia (red) upon 16h incubation with [13C5,15N]methionine (1 mM). In I, a summary of the model proposed here.
Hypoxia limits storage- or oxidative stress-induced protein methylation
Since methionine consumption was decreased in hypoxic storage while promoted in hyperoxic storage (Figure 6.B), we hypothesized that protein methylation was decreased in the former group and increased in the latter in comparison to normoxic RBCs. Indeed, asparagine deamidation in the N-term cytosolic domain of band 3 was elevated in hyperoxic RBCs at storage day 1 relative to normoxia and increased over storage in normoxic, hypoxic and hyperoxic RBCs, especially in the hyperoxic group (n=4; Figure 7.F). While minor differences were observed across groups in terms of N deamidation, quantitation of methylated D or deamidated → methylated N residues of all proteins, including band 3 (Figure 7.G) revealed significantly increased methylation by storage day 42 in normoxic and hyperoxic RBCs, but not in hypoxic RBCs – which showed levels that were comparable to day 1 controls at the end of the storage period.
Finally, we calculated the total 13C-methyl accumulation on aspartates and deamidated asparagines from the [13C5,15N]methionine tracing experiments. Results clearly show that 16h of incubation with 0.5% H2O2 are sufficient to induce a 1.5-fold increase in the methylation of total proteins when compared to normoxic RBCs, while inhibition of methyltransferases decreased the total levels of 13C-methylated peptides detected (Figure 7.H). No significant changes were observed between normoxic and hypoxic RBCs within a 16h time window, consistent with the observation that changes between these two groups are observed only after prolonged storage (up to 42 days in Figure 7.G). Results are tabulated extensively in Supplementary Table 3, along with quantitative measurements as peak areas of precursor ions for 13C-methylated peptides.
Discussion
Non-epigenetic methylation of proteins at carboxylic acid side chains was first described in the 1960s.64 More recently, studies have documented increased asparagine deamidation and aspartate methylation in RBCs in response to senescence, aging, Down syndrome and glucose 6-phosphate dehydrogenase deficiency.39–44 In RBCs, where de novo protein synthesis is impeded by the lack of translation machinery, oxidative stress-induced protein methylation is a recycling strategy to convert a fraction of isoaspartate moieties back to the L-aspartate form.53 Being devoid of nuclei, RBCs are an ideal model to study non-epigenetic methylation of human proteins. Despite the groundbreaking nature of earlier studies, only with the development of recent technologies has extensive mapping of methylated D and deamidated N residues become possible. Here we used a combination of sophisticated proteomics and metabolic tracing techniques to provide temporal and spatial resolution of methylation and to infer potential functional relevance.
For example, we report that the N-terminal cytosolic domain of band 3 is methylated along with other crucial regions of band 3 that interact with deoxyhemoglobin, glycolytic enzymes and structural proteins.54–58 Fine tuning of such interactions modulates metabolic responses to hypoxia, such as at high altitude.65–67 While the full picture of how such methylation impacts structure and protein-protein interactions is not yet known, it is interesting to speculate on the basis of computational models59 that esterification ablates negative charges in the N-term of band 3, perhaps destabilizing interactions with glycolytic enzymes and others.54 Focusing on GAPDH, several methylated residues identified here mapped as close as < 3 Ångström to the substrate binding site. Similar observations were made for a long series of glycolytic (e.g. bisphosphoglycerate mutase, LDHA and ALDOA) and antioxidant enzymes (e.g. catalase and glutamate-cysteine ligase). Structural proteins, especially band 3-interacting Ankyrin, spectrin beta and protein 4.1, were extensively methylated at D and deamidated N residues, expanding on previous reports of increased total aspartate methylation of these proteins.43,44 Notably, we identified 11 deamidated residues (N, Q) and 14 methylated residues (D, E, K, R) on protein 4.1, an observation underlying the change in electrophoretic mobility that gives rise to two gel bands historically identified as 4.1a and 4.1b, a marker of RBC senescence historically attributed to deamidation of residues 478 and 502.68
If RBCs are an optimal model to investigate non-epigenetic modifications, their storage in the blood bank offers a clinically relevant scenario where these modifications may impact the efficacy of current transfusion practices. Access to ~600 stored RBC samples collected in the REDS-III RBC-Omics study allowed for evaluations of donors whose RBCs reproducibly hemolyzed more or less in response to oxidative stress. Despite the lack of a day 0 baseline, in RBCs from these donors, oxidative hemolysis correlated with methionine consumption (and SAM conversion to SAH) from storage day 10 to 23 and 42. We thus combined mechanistically focused experiments (in presence of oxidative insults or controlled hypoxic vs hyperoxic storage conditions) to show that the redox status impacts methionine uptake, consumption and subsequent protein methylation. Of note, since methionine is not present in common storage additives (here additive solution 1 and 3), only consumption of endogenous methionine was observed in the RBC-Omics cohort and the controlled SO2-storage samples. Here, storage in the presence of heavy methionine indicated that methionine supplementation to stored RBCs may boost protein recycling and aid in ROS scavenging. However, excess methionine metabolism via the trans-sulfuration pathway could negatively impact recipients with accumulation of homocysteine, a metabolite associated with thromboembolic complications in adults with homocysteinuria.69 On the other hand, hypoxic storage represented a viable strategy to prevent oxidative stress, reducing methionine consumption and ultimately, protein methylation.
Conclusion
In the present study, we mapped methylation of D, E and deamidated N and Q residues in structural RBC proteins and glycolytic/antioxidant enzymes as a function of oxidative stress or refrigerated storage under blood bank conditions (Figure 7.I). The results are suggestive of a widespread mechanism that RBCs exploit to attempt recycling of oxidatively modified residues. This phenomenon had been previously described as a function of human aging, RBC senescence and pathologies associated with oxidative stress, but never before with respect to RBC storage in the blood bank. Mapping of methylated residues and tracing with stable isotope tracers allowed for the first time to infer structural and functional implications of this post-translational modification in key structural (Ankyrin, protein 4.1, spectrin) and functional (band 3, GAPDH, ALDOA, BPGM, LDHA, CAT) proteins in human RBCs and, predictably, in other more complex cell types.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by funds from the Boettcher Webb-Waring Investigator Award (ADA), the Shared Instrument grant by the National Institute of Health (S10OD021641 to KCH). Trainee support was provided by NIH T32 HL007171 (TN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to acknowledge NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI contracts NHLBI HHSN2682011-00001I, -00002I, -00003I, -00004I, -00005I, -00006I, -00007I, -00008I, and -00009I. The authors would like to express their deep gratitude Dr. Simone Glynn of NHLBI for her support throughout this study, the RBC-Omics research staff at all participating blood centers and testing labs for their contribution to this project, and to all blood donors who agreed to participate in this study.
RBC-OMICS STUDY GROUP MEMBERS
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), Red Blood Cell (RBC)-Omics Study, is the responsibility of the following persons: Hubs: A. E. Mast, J. L. Gottschall, W. Bialkowski, L. Anderson, J. Miller, A. Hall, Z. Udee, and V. Johnson, BloodCenter of Wisconsin, Milwaukee, WI; D. J. Triulzi, J. E. Kiss, and P. A. D’Andrea, The Institute for Transfusion Medicine (ITXM), Pittsburgh, PA; E. L. Murphy and A. M. Guiltinan, University of California, San Francisco, San Francisco, CA; R. G. Cable, B. R. Spencer, and S. T. Johnson, American Red Cross Blood Services, Farmington, CT; Data coordinating center: D. J. Brambilla, M. T. Sullivan, S. M. Endres, G. P. Page, Y. Guo, N. Haywood, D. Ringer, and B. C. Siege, RTI International, Rockville, MD; Central and testing laboratories: M. P. Busch, M. C. Lanteri, M. Stone, and S. Keating, Blood Systems Research Institute, San Francisco, CA; T. Kanias and M. Gladwin, Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA; Steering committee chairman: S. H. Kleinman, University of British Columbia, Victoria, BC, Canada; National Heart, Lung, and Blood Institute, National Institutes of Health: S. A. Glynn, K. B. Malkin, and A. M. Cristman
Footnotes
Disclosure of Conflict of interest Tatsuro Yoshida and Andrew Dunham are employed by Hemanext Inc. Angelo D’Alessandro is a consultant for Hemanext Inc. Travis Nemkov, Kirk C. Hansen, Angelo D’Alessandro are founders of Omix Technologies Inc. James C Zimring serves on the scientific advisory board for Rubius Therapeutics. All the other authors disclose no conflicts of interest relevant to this study.
References
- 1.D’Alessandro A, Zolla L. Proteomic analysis of red blood cells and the potential for the clinic: what have we learned so far? Expert Rev. Proteomics. 2017;14(3):243–252. [DOI] [PubMed] [Google Scholar]
- 2.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion . 2017;57 Suppl 2:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwalt TJ A short history of transfusion medicine. Transfusion . 2003;37(5):550–563. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion . 2015;55(1):205–219. [DOI] [PubMed] [Google Scholar]
- 5.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion . 2016;56(4):980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimring James C Widening our gaze of red blood storage haze: a role for metabolomics. Transfusion . 2015;55(6):1139–1142. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103(2):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion . 2016;56(10):2560–2570. [DOI] [PubMed] [Google Scholar]
- 10.Delobel J, Prudent M, Tissot J-D, Lion N. Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Proteomics Clin. Appl 2016;10(3):257–266. [DOI] [PubMed] [Google Scholar]
- 11.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J. Cell. Mol. Med 2007;11(1):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Mirasole C, Zolla L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a MALDI-TOF mass-spectrometry-based investigation. Vox Sang. 2013;105(2):177–180. [DOI] [PubMed] [Google Scholar]
- 13.Delobel J, Prudent M, Crettaz D, et al. Cysteine redox proteomics of the hemoglobin-depleted cytosolic fraction of stored red blood cells. Proteomics Clin. Appl 2016;10(8):883–893. [DOI] [PubMed] [Google Scholar]
- 14.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion . 2011;51(7):1439–1449. [DOI] [PubMed] [Google Scholar]
- 15.Rinalducci S, D’Amici GM, Blasi B, Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie. 2011;93(5):845–853. [DOI] [PubMed] [Google Scholar]
- 16.Rinalducci S, Ferru E, Blasi B, Turrini F, Zolla L. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012;10 Suppl 2:s55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion . 2016;56(2):421–426. [DOI] [PubMed] [Google Scholar]
- 18.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):e32–42. [DOI] [PubMed] [Google Scholar]
- 19.Harper VM, Oh JY, Stapley R, et al. Peroxiredoxin-2 Recycling Is Inhibited During Erythrocyte Storage. Antioxid. Redox Signal. 2015;22(4):294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion . 2016;56(4):852–862. [DOI] [PubMed] [Google Scholar]
- 21.Paglia G, D’Alessandro A, Rolfsson Ó, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128(13):e43–50. [DOI] [PubMed] [Google Scholar]
- 22.Yurkovich JT, Bordbar A, Sigurjónsson ÓE, Palsson BO. Systems biology as an emerging paradigm in transfusion medicine. BMC Syst. Biol 2018;12:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion . 1997;37(7):719–726. [DOI] [PubMed] [Google Scholar]
- 24.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Invest 2017;127(1):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: The experimental evidence. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis. 2010;43(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Xiong Y, Wang R, Tang F, Wang X. Blood banking-induced alteration of red blood cell oxygen release ability. Blood Transfus. 2016;14(3):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel R, Johnson DJ, Scott AV, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion . 2016;56(7):1690–1698. [DOI] [PubMed] [Google Scholar]
- 29.Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus. 2017;15(2):112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA. 2015;314(23):2514–2523. [DOI] [PubMed] [Google Scholar]
- 31.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med 2015;372(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N. Engl. J. Med 2016;375(20):1937–1945. [DOI] [PubMed] [Google Scholar]
- 33.van ‘t Erve TJ, Doskey CM, Wagner BA, et al. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic. Biol. Med 2014;76:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van ‘t Erve TJ, Wagner BA, Martin SM, et al. The heritability of hemolysis in stored human red blood cells. Transfusion . 2015;55(6):1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion . 2008;48(6):1053–1060. [DOI] [PubMed] [Google Scholar]
- 36.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1(15):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion . 2017;57(2):325–336. [DOI] [PubMed] [Google Scholar]
- 38.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion . 2011;51(7):1450–1459. [DOI] [PubMed] [Google Scholar]
- 39.Lou LL, Clarke S. Enzymatic methylation of band 3 anion transporter in intact human erythrocytes. Biochemistry (Mosc.). 1987;26(1):52–59. [DOI] [PubMed] [Google Scholar]
- 40.Ingrosso D, Cimmino A, D’Angelo S, et al. Protein methylation as a marker of aspartate damage in glucose-6-phosphate dehydrogenase-deficient erythrocytes. FEBS J 2002;269(8):2032–2039. [DOI] [PubMed] [Google Scholar]
- 41.Barber JR, Clarke S. Membrane protein carboxyl methylation increases with human erythrocyte age. Evidence for an increase in the number of methylatable sites. J. Biol. Chem 1983;258(2):1189–1196. [PubMed] [Google Scholar]
- 42.Galletti P, De Bonis ML, Sorrentino A, et al. Accumulation of altered aspartyl residues in erythrocyte proteins from patients with Down’s syndrome. FEBS J 2007;274(20):5263–5277. [DOI] [PubMed] [Google Scholar]
- 43.Janson CA, Clarke S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J. Biol. Chem 1980;255(24):11640–11643. [PubMed] [Google Scholar]
- 44.McFadden PN, Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc. Natl. Acad. Sci. U. S. A 1982;79(8):2460–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8(4):220–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion . 2016;56(4):980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion . 2017;57(2):325–336. [DOI] [PubMed] [Google Scholar]
- 48.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. RCM. 2017;31(8):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Alessandro A, Nemkov T, Kelher M, et al. Routine Storage of Red Blood Cell Units in Additive Solution-3: a comprehensive investigation of the RBC metabolome. Transfusion . 2015;55(6):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemkov T, Sun K, Reisz JA, et al. Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage. Front. Med 2017;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dzieciatkowska M, Hill R, Hansen KC. GeLC-MS/MS analysis of complex protein mixtures. Methods Mol. Biol. Clifton NJ. 2014;1156:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galletti P, Ingrosso D, Nappi A, et al. Increased methyl esterification of membrane proteins in aged red-blood cells. Preferential esterification of ankyrin and band-4.1 cytoskeletal proteins. Eur. J. Biochem 1983;135(1):25–31. [DOI] [PubMed] [Google Scholar]
- 53.Ingrosso D, D’angelo S, di Carlo E, et al. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur. J. Biochem 2000;267(14):4397–4405. [DOI] [PubMed] [Google Scholar]
- 54.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. U. S. A 2005;102(7):2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campanella ME, Chu H, Wandersee NJ, et al. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood. 2008;112(9):3900–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu H, Low PS. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem. J 2006;400(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu L, Morrison M. The interaction of human erythrocyte Band 3 with cytoskeletal components. Arch. Biochem. Biophys 1983;227(1):31–38. [DOI] [PubMed] [Google Scholar]
- 58.Puchulu-Campanella E, Chu H, Anstee DJ, et al. Identification of the Components of a Glycolytic Enzyme Metabolon on the Human Red Blood Cell Membrane. J. Biol. Chem 2013;288(2):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisenmesser EZ, Post CB. Insights into tyrosine phosphorylation control of protein-protein association from the NMR structure of a band 3 peptide inhibitor bound to glyceraldehyde-3-phosphate dehydrogenase. Biochemistry (Mosc.). 1998;37(3):867–877. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida T, Blair A, D’alessandro A, et al. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus. 2017;15(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Longo V, D’Alessandro A, Zolla L. Deoxygenation of leucofiltered erythrocyte concentrates preserves proteome stability during storage in the blood bank. Blood Transfus. 2014;12(4):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonagh EM, Bautista JM, Youngster I, Altman RB, Klein TE. PharmGKB summary: methylene blue pathway. Pharmacogenet. Genomics. 2013;23(9):498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paglia G, Sigurjónsson ÓE, Bordbar A, et al. Metabolic fate of adenine in red blood cells during storage in SAGM solution. Transfusion . 2016;56(10):2538–2547. [DOI] [PubMed] [Google Scholar]
- 64.Paik WK, Paik DC, Kim S. Historical review: the field of protein methylation. Trends Biochem. Sci 2007;32(3):146–152. [DOI] [PubMed] [Google Scholar]
- 65.D’Alessandro A, Nemkov T, Sun K, et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res 2016;15(10):3883–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu H, Zhang Y, Wu H, et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulation. 2016;134(5):405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun K, Zhang Y, D’Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun 2016;7:12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inaba M, Gupta KC, Kuwabara M, et al. Deamidation of human erythrocyte protein 4.1: possible role in aging. Blood. 1992;79(12):3355–3361. [PubMed] [Google Scholar]
- 69.Sacharow SJ, Picker JD, Levy HL. Homocystinuria Caused by Cystathionine Beta-Synthase Deficiency. GeneReviews®. 1993; [PubMed] [Google Scholar]
- 70.Perry JJP, Ballard GD, Albert AE, et al. Human C6orf211 encodes Armt1, a protein carboxyl methyltransferase that targets PCNA and is linked to the DNA damage response. Cell Rep 2015;10(8):1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


