Abstract
Aims
We conducted a national survey to clarify the characteristics and clinical course of type 1 diabetes related to anti-programmed cell death-1 therapy.
Methods
We analyzed the detailed data of 22 patients that were collected using a Japan Diabetes Society survey and a literature database search.
Results
Among the 22 patients, 11 (50.0%) met the criteria for fulminant type 1 diabetes and 11 (50.0%) met the criteria for acute-onset type 1 diabetes. The average patient age was 63 years. The mean duration between the date of the first anti-PD-1 antibody injection and development of type 1 diabetes was 155 days and ranged from 13 to 504 days. Flu-like symptoms, abdominal symptoms, and drowsiness were observed in 27.8, 31.6, and 16.7% patients, respectively. Mean ± standard deviation or median (first quartile–third quartile) glucose levels, HbA1c levels, urinary C-peptide immunoreactivity levels, and fasting serum C-peptide immunoreactivity levels were 617 ± 248 mg/dl, 8.1 ± 1.3%, 4.1 (1.4–9.4) μg/day, and 0.46 (0.20–0.70) ng/ml, respectively. Seventeen of 20 patients (85.0%) developed ketosis, and 7 of 18 patients (38.9%) developed diabetic ketoacidosis. Ten of 19 patients (52.6%) showed at least one elevated pancreatic enzyme level at the onset and two of seven patients showed this elevation before diabetes onset. Only one of 21 patients was anti-glutamic acid decarboxylase antibody positive.
Conclusions
Anti-programmed cell death-1 antibody-related type 1 diabetes varies from typical fulminant type 1 diabetes to acute-onset type 1 diabetes. However, diabetic ketoacidosis was frequently observed at the onset of diabetes. An appropriate diagnosis and treatment should be provided to avoid life-threatening metabolic alterations.
Keywords: Nivolumab, Pembrolizumab, Anti-PD-1 antibody, Fulminant type 1 diabetes, Type 1 diabetes, Immune-checkpoint inhibitors
Introduction
Type 1 diabetes is a disease that is characterized by the destruction of pancreatic beta cells, which leads to a deficiency in insulin secretion. Most patients with type 1 diabetes have an immunological component of their disease pathogenesis, such as autoantibodies or genetic associations with genes controlling immune responses [1]. Besides autoimmune type 1 diabetes patients, there are many fulminant type 1 diabetes patients in Japan and other East Asian countries. Fulminant type 1 diabetes is a severe subtype of type 1 diabetes that was discovered in Japan and characterized by extremely rapid progression of beta-cell destruction and severe ketoacidosis [2, 3]. Islet autoantibodies were usually negative in this category. Some patients with severe fulminant type 1 diabetes experienced cardiac arrest or sudden death [4].
Immune-checkpoint inhibitors including anti-programmed cell death-1 (PD-1) antibodies such as nivolumab and pembrolizumab selectively block interaction with the PD-1 receptor on T cells and restore T cell activation and proliferation [5]. Thus, PD-1 antibodies induce an anti-tumor response, and recently, they have been approved for the treatment of many types of cancer, such as melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin’s lymphoma, head and neck cancer, and stomach cancer in Japan and also for urothelial carcinoma, colorectal cancer, and hepatocellular carcinoma in addition to above cancers in Western countries. However, as the use of immune-checkpoint inhibitors increases, the incidence of autoimmune side effects, which are called immune-related adverse events (irAE), is expected to increase. Interstitial pneumonia, colitis, hypothyroidism, hepatitis, skin rash, vitiligo, myasthenia gravis, neuropathy, myositis, uveitis, and type 1 diabetes are known autoimmune side effects [6]. Among them, type 1 diabetes is a severe side effect, because it progresses rapidly, often presents as ketoacidosis, and would be fatal without appropriate treatment [7]. Some patients with diabetes associated with anti-PD-1 antibodies meet the criteria for fulminant type 1 diabetes, and need prompt diagnosis and treatment. Fulminant type 1 diabetes has mainly been reported in East Asia and it accounts for approximately 20% of type 1 diabetes cases with a classical acute-onset pattern in Japan [8]. Thus, it is important to clarify the clinical characteristics of patients who developed anti-PD-1 antibody-related type 1 diabetes, including the classification of this clinical subtype, in Japan.
All nivolumab-induced adverse events were collected and they were publicly available in the safety databases created by Ono Pharmaceutical Company and Bristol-Myers Squibb [9], and all pembrolizumab-induced adverse events were also collected by MSD [10]. The diagnosis of adverse events and classification of type 1 diabetes or fulminant type 1 diabetes was provided by the patients’ attending doctors. According to the safety database, 20,600 patients were treated with nivolumab from July 4, 2014 to August 15, 2017 in Japan, and 67 patients (0.33%) developed “type 1 diabetes” or “fulminant type 1 diabetes”. Among them, 40 patients (0.19%) were reported as having type 1 diabetes (10 patients had melanoma, 24 patients had non-small cell lung cancer, three patients had renal cell carcinoma, and the diagnosis for three patients was unknown) and 27 patients (0.13%) were reported to have fulminant type 1 diabetes (14 patients had melanoma, 12 patients had non-small cell lung cancer, and the diagnosis for one patient was unknown). Similarly, 3,603 patients were treated with pembrolizumab from December 19, 2016 to August 14, 2017 in Japan, and five patients (0.14%) developed “type 1 diabetes” or “fulminant type 1 diabetes”. Among them, four patients (0.11%) were reported as having type 1 diabetes (one patient had melanoma, three patients had non-small cell lung cancer) and one patient (0.03%) was reported as having fulminant type 1 diabetes (non-small cell lung cancer). In the Ehime Study, a regional survey of type 1 diabetes in Japan and nine fulminant type 1 diabetes patients were observed out of 4980 diabetic patients and the frequency of fulminant type 1 diabetes in the general Japanese population was estimated to be 0.01% [11]. Therefore, incidence rates of type 1 diabetes related to nivolumab and pembrolizumab are 33 and 14 times higher than that of typical fulminant type 1 diabetes, respectively. In addition, there was no particular relationship between primary tumors and developed anti-PD-1 antibody-related type 1 diabetes. These results confirm that the blockade of binding between PD-1 and PD-L1 molecules by the anti-PD-1 antibody, not by a particular medicine, causes anti-PD-1 antibody-related type 1 diabetes.
There are patients who were reported to develop “fulminant type 1 diabetes” or “type 1 diabetes”. However, there are too few of these case reports to reveal the entire clinical picture. In addition, type 1 diabetes, including fulminant type 1 diabetes, has racial differences in frequency and feature. Therefore, the Japanese analysis to clarify the characteristics and clinical course of anti-PD1 antibody-related type 1 diabetes is required.
In this study, we conducted a literature search and a national survey among the Japan Diabetes Society (JDS) members to collect data from patients who developed anti-PD-1 antibody-related type 1 diabetes. In addition to the 10 reported cases, we found an additional 12 unpublished cases. We have clarified the detailed characteristics and the clinical course in these 22 patients.
Methods
This study was performed under the auspices of the Japan Diabetes Society (JDS).
We conducted a national survey among JDS members and they reported information about patients who developed type 1 diabetes after anti-PD-1 therapy. We also performed a literature search using the key words “nivolumab”, “pembrolizumab”, “type 1 diabetes”, and/or “PD-1” in PubMed and Ichushi (a Japanese article database) to identify articles published up to August 31, 2017 that described patients who developed type 1 diabetes after anti-PD-1 therapy. A questionnaire survey was administered to the members of the JDS and authors of the articles, and data from 22 patients were analyzed. The questionnaire included information on age, sex, primary tumor, metastasis, past history, family history, date of diabetes onset, flu-like symptom, digestive symptoms, hyperglycemic symptoms, consciousness, weight reduction, or other complications with or without drug withdrawal, and pancreatic imaging findings. We also examined body size measurements, laboratory findings, and islet autoantibodies, which were determined at 1 month before onset, the day of onset, and 1 month after onset at each hospital. The onset date is defined as the day that patients were diagnosed with anti-PD-1 antibody-related type 1 diabetes and their doctors started treatment for diabetes. As a reference value, we used data from elderly onset fulminant type 1 diabetes patients (their age range was 54–80 years) who had already registered to the database created by the JDS Committee on Type 1 Diabetes Mellitus Research, and whose data had been reported by the committee of the JDS between July 15, 2000 and June 30, 2006 [12].
Diagnosis of acute-onset type 1 diabetes was made based on the criteria set by the Committee of the JDS [13]. Briefly, among patients who developed diabetic ketosis or ketoacidosis within 3 months after the onset of hyperglycemic symptoms and who needed insulin treatment continuously after their diabetes diagnosis, patients with islet autoantibodies are diagnosed with “acute-onset type 1 diabetes (autoimmune)”, and those whose endogenous insulin secretion is depleted (fasting serum C-peptide immunoreactivity < 0.6 ng/ml) without verifiable islet autoantibodies are diagnosed with “acute-onset type 1 diabetes”. Both diagnoses are called acute-onset type 1 diabetes in this study. A diagnosis of fulminant type 1 diabetes was based on the following criteria from the Report of the JDS Committee on type 1 diabetes mellitus research: (1) ketosis or ketoacidosis within 1 week after the onset of hyperglycemic symptoms; (2) urinary C-peptide below 10 μg/day or fasting serum C-peptide below 0.3 ng/ml and serum C-peptide below 0.5 ng/ml after glucagon injection or meal load soon after the onset of disease; and (3) plasma glucose (PG) level above 288 mg/dl and HbA1c below 8.7% at the first visit [14, 15]. To diagnose the patient’s clinical entity, we used the lowest serum C-peptide value within 2 weeks after their onset of anti-PD-1 antibody-related type 1 diabetes, because duration of the disease before the start of insulin treatment can be 1–2 weeks for the development of fulminant type 1 diabetes. Diagnosis of diabetic ketoacidosis was based on criteria from the American Diabetes Association [16].
The value of HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value, which was calculated using the formula reported by the Committee of the JDS [17].
Values are expressed as the mean ± standard deviation (SD).
Results
Analysis of 22 patients with anti-PD-1 antibody-related type 1 diabetes
We analyzed data from 22 Japanese patients with anti-PD-1 antibody-related type 1 diabetes. Among them, 10 patients had been already reported as case reports [18–26]. Of the 22 patients, 11 (50.0%) met the criteria for fulminant type 1 diabetes [14, 15], 11 (50.0%) met the criteria for acute-onset type 1 diabetes [13]. Although patients who met the criteria for fulminant type 1 diabetes exhibited comparable plasma glucose levels with those who met the criteria for acute-onset type 1 diabetes (642 ± 263 vs. 597 ± 246 mg/dl, respectively, P = 0.90), they tended to show lower HbA1c (7.4 ± 0.8 vs. 8.7 ± 1.4%, respectively, P = 0.42). All patients were reported to have developed type 1 diabetes after nivolumab treatment.
Background, clinical, and biological characteristics of anti-PD-1 antibody-related type 1 diabetes
The medical history of malignancy for the 22 patients is shown in Table 1. Among these 22 patients, eight (36.4%) had melanoma, 10 (45.5%) had non-small cell lung cancer, two (9.1%) had renal cell carcinoma, one (4.5%) had Hodgkin’s lymphoma, and one (4.5%) had breast cancer (off-label use). No patient had pancreatic metastasis. One breast cancer patient underwent four courses of pembrolizumab (PD-1 therapy) 196 days before nivolumab treatment [20], and one lung cancer patient received 10 courses of durvalumab (programmed cell death ligand-1, PD-L1 antibody) 128 days before nivolumab treatment. The rate of adverse events reported for the patients in this study was consistent with the adverse event rate in the safety databases.
Table 1.
Background of 22 patients with nivolumab-related type 1 diabetes
| N (%) | |
|---|---|
| Primary Tumor | |
| Melanoma | 8 (36.4) |
| Non-small cell lung cancer | 10 (45.5) |
| Renal cell carcinoma | 2 (9.1) |
| Hodgkin’s lymphoma | 1 (4.5) |
| Breast cancer (off-label) | 1 (4.5) |
| Pancreatic metastasis (−/+) | 14/0 |
| Other immune-checkpoint inhibitors | |
| Pembrolizumab (PD-1 antibody) | 1 (5.6) |
| Durvalumab (PD-L1 antibody) | 1 (5.6) |
| Other adverse events | |
| Thyroid disease | 2 (11.1) |
| Pituitary disease | 2 (11.1) |
| Interstitial pneumonia | 1 (5.6) |
| Enteritis | 3 (16.7) |
| Vitiligo | 1 (5.6) |
| Dry eye | 1 (5.6) |
PD-1 programmed cell death-1, PD-L1 programmed cell death ligand-1
Clinical and biological characteristics of 22 anti-PD-1 antibody-related type 1 diabetes patients are shown in Table 2. Data from 63 elderly onset patients with fulminant type 1 diabetes, which had already been reported [12], were used as a reference. Subjective symptoms such as flu-like symptoms, abdominal symptoms, and drowsiness were less likely to occur in anti-PD-1 antibody-related type 1 diabetes than in fulminant type 1 diabetes patients. Similarly, at the time of type 1 diabetes diagnosis, anti-PD-1 antibody-related type 1 diabetes tended to show lower plasma glucose levels (617 ± 248 vs. 853 ± 362 mg/dl), higher HbA1c levels (8.1 ± 1.3 vs. 7.0 ± 0.7%), and higher arterial pH (7.26 ± 0.15 vs. 7.12 ± 0.20) compared with fulminant type 1 diabetes patients. Seventeen of 20 patients (85.0%) showed ketosis, and seven of 18 patients (38.9%) developed diabetic ketoacidosis. Hepatic enzymes were not elevated in any anti-PD-1 antibody-related type 1 diabetes patient, but 10 of 19 patients (52.6%) showed at least one elevated exocrine pancreatic enzyme levels at the onset; seven of 16 patients (43.8%) showed elevated amylase levels, 11 of 16 patients (68.8%) showed elevated lipase levels, and four of 10 patients (40%) showed elevated elastase-1 levels. Moreover, two of seven patients (28.6%) showed elevated amylase or lipase levels before onset; two patients (28.6%) showed elevated amylase levels, and one patient (only one patient’s data were available for lipase) showed an elevated lipase level. The elevations of liver and pancreatic enzymes were determined according to normal ranges of tests adopted by each hospital. Only one patient was anti-glutamic acid decarboxylase (GAD) antibody positive. One other patient showed an increase in anti-cytomegalovirus IgM (1.52 enzyme immunoassay, EIA, titer at the first time point and 1.76 EIA titer 4 weeks later, normal limit < 0.80 EIA titer) and anti-cytomegalovirus IgG (107 EIA titer at the first time point and over 128 EIA titer at 4 weeks later, normal limit < 2.0 EIA titer), and other patients showed no blood examination findings suggestive of acute viral infection. Pancreatic imaging findings were not analyzed, because there was little information. As other endocrinological irAEs, two patients also developed thyroid-associated irAEs and two patients developed pituitary-related irAEs.
Table 2.
Clinical and biological characteristics of patients
| Nivolumab-related T1DM | Elderly onset fulminant T1DM [12] | P a | |
|---|---|---|---|
| N | 22 | 63 | |
| Age (years) | 63 ± 12 (21) (31-83) | 54–80 | |
| Sex (male/female) | 13/9 | 43/20 | 0.44 |
| BMI (kg/m2) | 20.7 ± 3.8 (18) | 21.6 ± 2.8 | 0.87 |
| History of diabetes (−/+) | 15/3 | ||
| Family history of diabetes (−/+) | 12/5 | 47/14 | 0.58 |
| Days before development (days) | 121 (71–190) (21) | ||
| Symptoms | |||
| Thirst (−/+) | 3/17 | 7/54 | 0.68 |
| Flu-like symptoms (−/+) | 13/5 | 20/42 | < 0.01 |
| Abdominal symptoms (−/+) | 13/6 | 23/40 | 0.01 |
| Drowsiness (−/+) | 15/3 | 34/26 | 0.04 |
| Weight loss (−/+) | 5/11 | ||
| Diabetic ketoacidosis (−/+) | 11/7 | ||
| Ketosis (−/+) | 3/17 | ||
| Laboratory data | |||
| Plasma glucose (mg/dl) | 617 ± 248 (20) | 853 ± 362 | 0.72 |
| HbA1c (%, NGSP) | 8.1 ± 1.3 (21) | 7.0 ± 0.7 | 0.44 |
| Arterial ph | 7.26 ± 0.14 (15) | 7.12 ± 0.20 | 0.74 |
| Urinary CPR (μg/day) | 4.1 (1.4–9.4) (9) | 4.3 ± 3.9 | N/A |
| Fasting serum CPR (ng/ml) | 0.46 (0.20–0.70) (14) | 0.21 ± 0.18 | N/A |
| Na (mEq/l) | 130 ± 6 (15) | 129 ± 9 | 0.96 |
| K (mEq/l) | 5.4 ± 1.2 (15) | 5.8 ± 1.0 | 0.85 |
| Cl (mEq/l) | 94 ± 7 (15) | 92 ± 10 | 0.92 |
| AST (IU/l) | 22 ± 9 (16) | 49 ± 55 | N/A |
| ALT (IU/l) | 22 ± 8 (16) | 55 ± 53 | N/A |
| BUN (mg/dl) | 25 (15–38) (15) | ||
| Cr (mg/dl) | 1.21 ± 0.76 (16) | ||
| Increase of exocrine pancreatic enzyme | |||
| Amylase (−/+) | 9/7 | 16/30 | 0.13 |
| Lipase (−/+) | 5/11 | 5/28 | 0.19 |
| Elastase-1 (−/+) | 6/4 | 1/32 | < 0.001 |
| Anti-GAD antibody (−/+) | 20/1 | 56/6 | 0.48 |
| Anti-IA-2 antibody (−/+) | 17/0 | 21/0 | N/A |
| ICA (−/+) | 9/0 | ||
| IAA (−/+) | 9/0 | ||
| Anti-ZnT8 antibody (−/+) | 5/0 | ||
Values are expressed as the mean ± standard deviation or median (first quartile–third quartile). The number in parenthesis is available number of patient cases. Days before development are the time between the first infusion of nivolumab and development of T1DM
T1DM type 1 diabetes mellitus, BMI body mass index, CPR C-peptide immunoreactivity, AST aspartate transaminase, ALT alanine aminotransferase, BUN blood urea nitrogen, Cr creatinine, GAD glutamic acid decarboxylase, IA-2 insulinoma associated protein-2, ICA islet cell antibody, IAA insulin auto-antibody, ZnT8 zinc transporter 8, ND not determined, N/A not applicable
at test and Chi-square test, p significance probability
The mean duration between the date of the first anti-PD-1 antibody injection and development of type 1 diabetes was 155 ± 123 days, ranging from 13 to 504 days. The distribution of the period is shown in Fig. 1. All reported patients continued to receive insulin therapy (data for five patients are unknown) 1 month after the development of type 1 diabetes. Of 22 patients, one patient continued nivolumab treatment after the development of type 1 diabetes, eight patients stopped, and nine patients interrupted their treatments for 7–44 days before restarting.
Fig. 1.
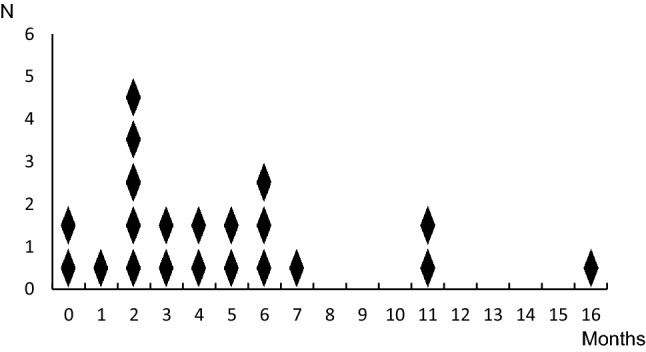
Distribution within the period between the first anti-PD-1 antibody injection and development of type 1 diabetes. The vertical axis shows the number of anti-PD-1 antibody-related type 1 diabetes patients, and the horizontal axis shows the period (months) when patients developed type 1 diabetes after they started anti-PD-1 antibody therapy
The changes in patients’ serum C-peptide levels after they were diagnosed with diabetes are shown in Fig. 2. All data were measured before they restarted anti-PD-1 antibody treatment. For most patients, their serum C-peptide levels decreased over a period of 2–3 weeks after the development of diabetes. In three patients, whose serum C-peptide levels were maintained to some extent, one patient’s serum C-peptide level was increased 1 week after stopping nivolumab, and two patients’ C-peptide levels tended to increase 2–3 weeks after stopping nivolumab. One of them (noted by an asterisk) received steroid injections as a part of the chemotherapy at 21–23 days after the development of type 1 diabetes. The changes of serum C-peptide levels after restarting anti-PD-1 antibody treatment could not be analyzed because of a lack of information.
Fig. 2.
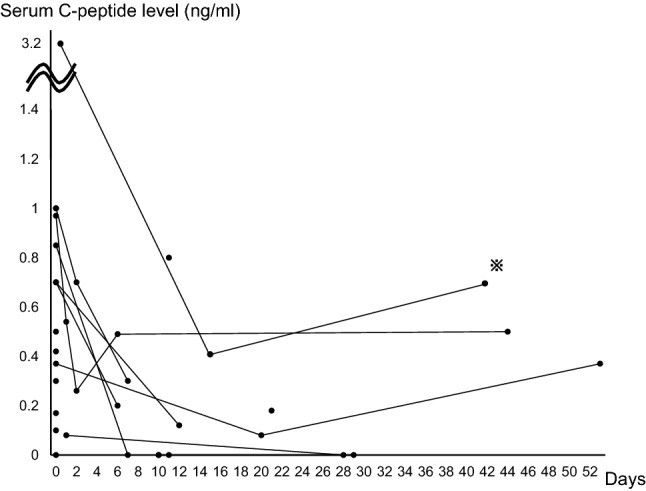
Changes in patients’ serum C-peptide levels. The vertical axis shows serum C-peptide levels, and the horizontal axis shows the number of days after patients were diagnosed with anti-PD-1 antibody-related type 1 diabetes. The patient noted by the asterisk received steroid injections as a part of chemotherapy at 21–23 days after the development of type 1 diabetes
Discussion
In the present study, which was conducted in Japan, we clarified the characteristics of anti-PD-1 antibody-related type 1 diabetes, regarding the clinical symptoms, status of islet antibodies, and clinical entity as a subtype of (type 1) diabetes. A national survey among JDS members was effective in collecting and analyzing unpublished cases.
For clinical symptoms, patients with anti-PD-1 antibody-related type 1 diabetes were generally less likely to show abdominal symptoms and drowsiness compared with patients with fulminant type 1 diabetes patients. This might be because of the milder onset of anti-PD-1 antibody-related type 1 diabetes compared with fulminant type 1 diabetes. In addition, our patients were less likely to show flu-like symptoms compared with fulminant type 1 diabetes patients. Seventy-two percent of typical fulminant type 1 diabetes patients had a history of flu-like symptoms before onset, and anti-enterovirus, anti-human herpesvirus 6, and anti-cytomegalovirus antibody levels are reported to increase in some patients [8, 27]. Most patients, except one who showed elevated cytomegalovirus antibodies, showed negative symptoms and blood examination results for an acute viral infection in this study. We can speculate that injection of anti-PD-1 antibody activates T cells instead of a viral infection activating these cells, and the activated T cells, particularly in patients whose T cells tended to attack their pancreatic beta cells, may destroy beta cells to contribute to development of type 1 diabetes.
For islet autoantibodies, only one patient was positive for anti-GAD-antibody, and no patients were positive for any other antibodies. Of the 24 anti-PD-1 antibody-related type 1 diabetes patients including Japanese and Westerners (six Japanese patients were also included in our study), 50% of them were positive for islet autoantibodies and almost all of the positive patients were Western patients [28]. Considering that fulminant type 1 diabetes develops mainly in East Asia and rarely in Western countries [3], it is possible that there is a difference in pathogenesis between Asian patients who develop anti-PD-1 antibody-related type 1 diabetes without islet autoantibodies and Western patients with antibodies or that a difference in the human leukocyte antigen (HLA) genotypes between Asian and Western patients influences the tendency to produce antibodies. Because few detailed data are available on the incidence of anti-PD-1 antibody-related type 1 diabetes in Western countries [29], and the data on HLA were not enough to evaluate also in this study, further data accumulation is recommended.
Among our anti-PD-1 antibody-related type 1 diabetes patients, we found that 50.0% of the patients fulfilled the criteria of fulminant type 1 diabetes in the national survey. Because patients visited their clinics regularly for their cancer treatment in every 2–3 weeks, it was possible to detect the development of diabetes earlier than ordinary fulminant type 1 diabetes. In addition, the diagnostic criteria of fulminant type 1 diabetes reported that the duration between the onset of hyperglycemic symptoms and ketosis or ketoacidosis is usually within 1 week but sometimes between 1 to 2 weeks. Therefore, we used the lowest serum C-peptide level within 2 weeks after the onset of anti-PD-1 antibody-related type 1 diabetes to diagnose their clinical entity. Among patients who were diagnosed with acute-onset type 1 diabetes, one patient’s serum C-peptide was exhausted 18 days after the onset of diabetes. The prevalence of subtypes within PD-1 antibody-related type 1 diabetes might change by timing when serum C-peptide is measured, and further study is needed, because there is not enough information on subsequent serum C-peptide levels. In this study, patients’ arterial pH and HbA1c levels at onset tended to be higher compared with that of elderly onset fulminant type 1 diabetes patients, and plasma glucose levels were lower than in the elderly onset fulminant type 1 diabetes patients [12], suggesting that anti-PD-1 antibody-related type 1 diabetes develops somewhat less rapidly compared with typical fulminant type 1 diabetes. However, the patients’ plasma glucose levels at disease onset (overall, 617 ± 248 mg/dl) were higher than that of acute-onset type 1 diabetes patients in Japan (434 ± 213 mg/dl) [8] and type 1 diabetes patients in Western countries [385 (95% confidence interval, CI 371–401) mg/dl] [30]. Moreover, our patients frequently developed diabetic ketoacidosis (38.9%) at the onset, which is higher than the incidence rate of diabetic ketoacidosis at the onset of type 1 diabetes in Germany and Austria (21.1%) [31]. Although their mean serum C-peptide level at the onset, 0.46 (0.20–0.70) ng/ml, is almost the same as that of both acute-onset type 1 diabetes patients in Japan [13] and type 1 diabetes in the United States [32], the insulin-secreting capacity in most of the patients was exhausted in about 2–3 weeks after disease development, which is slower than in patients with fulminant type 1 diabetes but faster than in both patients with acute-onset type 1 diabetes in Japan [13] and those with type 1 diabetes in the Unites States [33]. These results suggest that the speed of the progression of beta-cell destruction related to anti-PD-1 antibody treatment is slower than fulminant type 1 diabetes but faster than acute-onset type 1 diabetes in Japan. Based on the above-mentioned results and considering many differences in characteristics between anti-PD-1 antibody-related type 1 diabetes and typical fulminant or acute-onset type 1 diabetes, it would be appropriate to consider anti-PD-1 antibody-related type 1 diabetes as a distinct entity and to introduce a newly coined name for this entity. We suggest ‘anti-PD-1 antibody-related (type 1) diabetes’ as an appropriate name for this entity. This subtype may be classified in type 1 diabetes or “other specific types of diabetes”.
The JDS recommends to check patients’ plasma glucose levels at every visit (2–3 weeks), and the American society of Clinical Oncology recommends to measure glucose levels with each treatment cycle during induction for 12 weeks, then every 3–6 weeks thereafter [34]. The speed of the progression of beta-cell destruction in this study can support this recommendation. In addition, the result of this study revealed that most patients developed anti-PD-1 antibody-related type 1 diabetes within 7 months from the first dose of anti-PD-1 antibody, and we suggest that careful monitoring of plasma glucose levels should be delivered especially during these 7 months. However, anti-PD-1 antibody-related (type 1) diabetes has a broad disease spectrum. Patients can present with both fulminant type 1 diabetes and acute-onset type 1 diabetes. Because we could not analyze the factor that causes severe disease presentation or death because of the lack of information, further study is needed.
Another exceptional result is that in three patients, the serum C-peptide level tended to increase after withdrawal of anti-PD-1 therapy [23, 24, 26]. However, in many other patients, the serum C-peptide levels were either undetectable from the beginning or declined and became undetectable (data not shown) [18, 22, 28]. Because the three patients’ serum C-peptide levels were maintained to some extent, prompt diagnosis and treatment might contribute to avoiding complete exhaustion of insulin secretion. Moreover, 10 of 19 patients (52.6%) showed at least one elevated exocrine pancreatic enzyme result at the onset of type 1 diabetes and two of seven patients (28.6%) showed elevated exocrine pancreatic enzyme levels 1 month before onset. Careful monitoring of exocrine pancreatic enzyme levels may contribute to early detection of the development of beta-cell destruction in anti-PD-1 antibody-related type 1 diabetes.
As a limitation, in this study, we excluded cases of anti-PD1 antibody-related type 1 diabetes during the drug trials and it might cause a selection bias. In addition, significant differences in Table 2 should be regarded only as a guide, because the period and method of collecting patients are different between two studies and there seems to be some bias. In addition, we analyzed mainly the data for anti-PD-1 antibody-related type 1 diabetes at the onset and before and after 1 month. Future follow-up and analysis of the resulting long-term data are recommended.
In conclusion, we found that anti-PD-1 antibody-related type 1 diabetes varied from typical fulminant type 1 diabetes to acute-onset type 1 diabetes, but diabetic ketoacidosis was frequently observed at the onset of diabetes. An appropriate diagnosis and treatment should be provided to avoid life-threatening metabolic alterations.
Acknowledgements
The other member of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research is Seiho Nagafuchi (Faculty of Medicine, Saga University). The authors express their sincere gratitude to the authors of the referenced cases, attending doctors, and diabetes specialists affiliated with the JDS for answering the questionnaire: Drs. Masahide Okamoto (Oita University), Osamu Ogawa (Kameda Medical Center), Emi Itateyama (Oita Red Cross Hospital), Hiroyuki Ohtake (Saitama Medical Center), Yushi Hirota (Kobe University), Masako Tomoyasu (Tokyu Hospital), Yasushi Nakajima (Nippon Medical School Hospital), Seiichi Yano (Kyushu University), Kenji Ashida (Kyushu University), Ryo Kumagai (Mito Kyodo General Hospital), Hiroaki Yagyu (Mito Kyodo General Hospital), Nobuyuki Koriyama (Kagoshima Medical Center), Michiaki Fukui (Kyoto Prefectural University of Medicine), Harumi Daikoku (Showa General Hospital), Shiko Asai (Kawasaki Municipal Tama Hospital), Akihiro Mochida (Juntendo University Urayasu Hospital), Fumitaka Okajima (Nippon Medical School Chiba Hokusoh Hospital), Satoshi Takagi (Tokyo Women’s Medical University), Kaoru Nagasawa (Toranomon Hospital), and Yasunori Taketomo (Kindai University). The authors also sincerely thank the doctors at Osaka University for their assistance with this study: Drs. Chisaki Ishibashi, Kenji Fukui, Hiromi Iwahashi, and Iichiro Shimomura.
Conflict of interest
Akihisa Imagawa received scholarship donations from Ono Pharmaceutical Co., Ltd. and MSD and honoraria for lectures from Ono Pharmaceutical Co., Ltd. Norio Abiru received research grants from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb. Hiroshi Ikegami received a scholarship grant from Ono Pharmaceutical Co., Ltd. Yumiko Kawabata received a scholarship grant from Ono Pharmaceutical Co., Ltd. Other authors declare that they have no conflicts of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (The Ethics Committees of the JDS, date of approval: 26 August 2016, approval number: 28-003-(2), Osaka University Hospital, date of approval: 27 April 2016, approval number: 15589, and Osaka Medical College, date of approval: 10 January 2017, approval number: 2098) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 3.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3:36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- 4.Baden MY, Imagawa A, Iwahashi H, Shimomura I, Awata T, Ikegami H, Uchigata Y, Osawa H, Kajio H, Kawasaki E, Kawabata Y, Shimada A, Takahashi K, Tanaka S, Yasuda K, Yasuda H, Kobayashi T, Hanafusa T. Risk factors for sudden death and cardiac arrest at the onset of fulminant type 1 diabetes mellitus. Diabetol Int. 2016;7:281–288. doi: 10.1007/s13340-015-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 6.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Ikegami H, Kawabata Y, Noso S. Immune checkpoint therapy and type 1 diabetes. Diabetol Int. 2016;7:221–227. doi: 10.1007/s13340-016-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Toyoda T, Maruyama T, Makino H. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26:2345–2352. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 9.Ono Pharmaceutical Co., Ltd. Safety appropriate use information. https://www.opdivo.jp/basic-info/report/(article in Japanese). Accessed 9 Feb 2018
- 10.MSD. Side effect. https://www.msdconnect.jp/products/keytruda/safety.xhtml(article in Japanese). Accessed 9 Feb 2018
- 11.Murao S, Makino H, Kaino Y, Konoue E, Ohashi J, Kida K, Fujii Y, Shimizu I, Kawasaki E, Fujiyama M, Kondo S, Tanaka K, Tarumi Y, Seto I, Kato K, Ohno K, Kusunoki Y, Ebisui O, Takada Y, Tanabe K, Takemoto K, Onuma H, Nishimiya T, Osawa H. Differences in the contribution of HLA-DR and -DQ haplotypes to susceptibility to adult- and childhood-onset type 1 diabetes in Japanese patients. Diabetes. 2004;53:2684–2690. doi: 10.2337/diabetes.53.10.2684. [DOI] [PubMed] [Google Scholar]
- 12.Imagawa A, Hanafusa T, Iwahashi H, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H. Uniformity in clinical and HLA-DR status regardless of age and gender within fulminant type 1 diabetes. Diabetes Res Clin Pract. 2008;82:233–237. doi: 10.1016/j.diabres.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki E, Maruyama T, Imagawa A, Awata T, Ikegami H, Uchigata Y, Osawa H, Kawabata Y, Kobayashi T, Shimada A, Shimizu I, Takahashi K, Nagata M, Makino H, Hanafusa T. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012) Diabetol Int. 2013;4:221–225. doi: 10.1007/s13340-013-0122-2. [DOI] [Google Scholar]
- 14.Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, Kawasaki E, Kawabata Y, Kobayashi T, Shimada A, Shimizu I, Takahashi K, Nagata M, Makino H, Maruyama T. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. Diabetol Int. 2012;3:179–183. doi: 10.1007/s13340-012-0098-3. [DOI] [Google Scholar]
- 15.Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, Kawasaki E, Kawabata Y, Kobayashi T, Shimada A, Shimizu I, Takahashi K, Nagata M, Makino H, Maruyama T. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, Kakuma T, Shibata H. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915–918. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239:155–158. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 20.Daikoku H, Shigeta M, Minakata M, Hidaka N. A case of acute-onset type 1 diabetes mellitus developed after the administration of anti-PD-1 antibody. J Jpn Diabetes Soc. 2016;59:811–818. [Google Scholar]
- 21.Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol. 2017;105:383–386. doi: 10.1007/s12185-016-2101-4. [DOI] [PubMed] [Google Scholar]
- 22.Usui Y, Udagawa H, Matsumoto S, Imai K, Ohashi K, Ishibashi M, Kirita K, Umemura S, Yoh K, Niho S, Osame K, Goto K. Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J Thorac Oncol. 2017;12:e41–e43. doi: 10.1016/j.jtho.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Teramoto Y, Nakamura Y, Asami Y, Imamura T, Takahira S, Nemoto M, Sakai G, Shimada A, Noda M, Yamamoto A. Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient. J Dermatol. 2017;44:605–606. doi: 10.1111/1346-8138.13486. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai R, Muramatsu A, Nakajima R, Fujii M, Kaino K, Katakura Y, Okumura N, Ohara G, Kagohashi K, Satoh H, Yagyu H. Acute-onset type 1 diabetes mellitus caused by nivolumab in a patient with advanced pulmonary adenocarcinoma. J Diabetes Investig. 2017;8:798–799. doi: 10.1111/jdi.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asai S, Katabami T, Kawanabe S, Igarashi K, Fukuda H, Tanaka Y. A case of fulminant type 1 diabates mellitus developed after the administration of nibormab, an immune checkpoint inhibitor. J Jpn Diabetes Soc. 2017;60:237–243. [Google Scholar]
- 26.Matsumura K, Nagasawa K, Oshima Y, Kikuno S, Hayashi K, Nishimura A, Okubo M, Uruga H, Kishi K, Kobayashi T, Mori Y. Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with nivolumab. J Diabetes Investig. 2018;9:438–441. doi: 10.1111/jdi.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanafusa T, Imagawa A, Iwahashi H, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H. Report of the Japan Diabetes Society’s Committee on research on fulminant type 1 diabetes mellitus: analysis of antiviral antibodies at disease onset. J Jpn Diabetes Soc. 2008;51:531–536. [Google Scholar]
- 28.Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, Da Meda L, Madelaine-Chambrin I, Bagot M, Basset-Seguin N, Pages C, Mourah S, Boudou P, Lebbe C, Gautier JF. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. 2017;66:1399–1410. doi: 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Hekkala A, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care. 2007;30:861–866. doi: 10.2337/dc06-2281. [DOI] [PubMed] [Google Scholar]
- 31.Neu A, Hofer SE, Karges B, Oeverink R, Rosenbauer J, Holl RW. Ketoacidosis at diabetes onset is still frequent in children and adolescents: a multicenter analysis of 14,664 patients from 106 institutions. Diabetes Care. 2009;32:1647–1648. doi: 10.2337/dc09-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gjessing HJ, Matzen LE, Faber OK, Froland A. Fasting plasma C-peptide, glucagon stimulated plasma C-peptide, and urinary C-peptide in relation to clinical type of diabetes. Diabetologia. 1989;32:305–311. doi: 10.1007/BF00265547. [DOI] [PubMed] [Google Scholar]
- 33.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]


